Electrocatalytic Nitrate Reduction for Brackish Groundwater Treatment: From Engineering Aspects to Implementation
Abstract
1. Introduction
2. Reaction Mechanisms and Kinetics of Electrocatalytic Nitrate Reduction
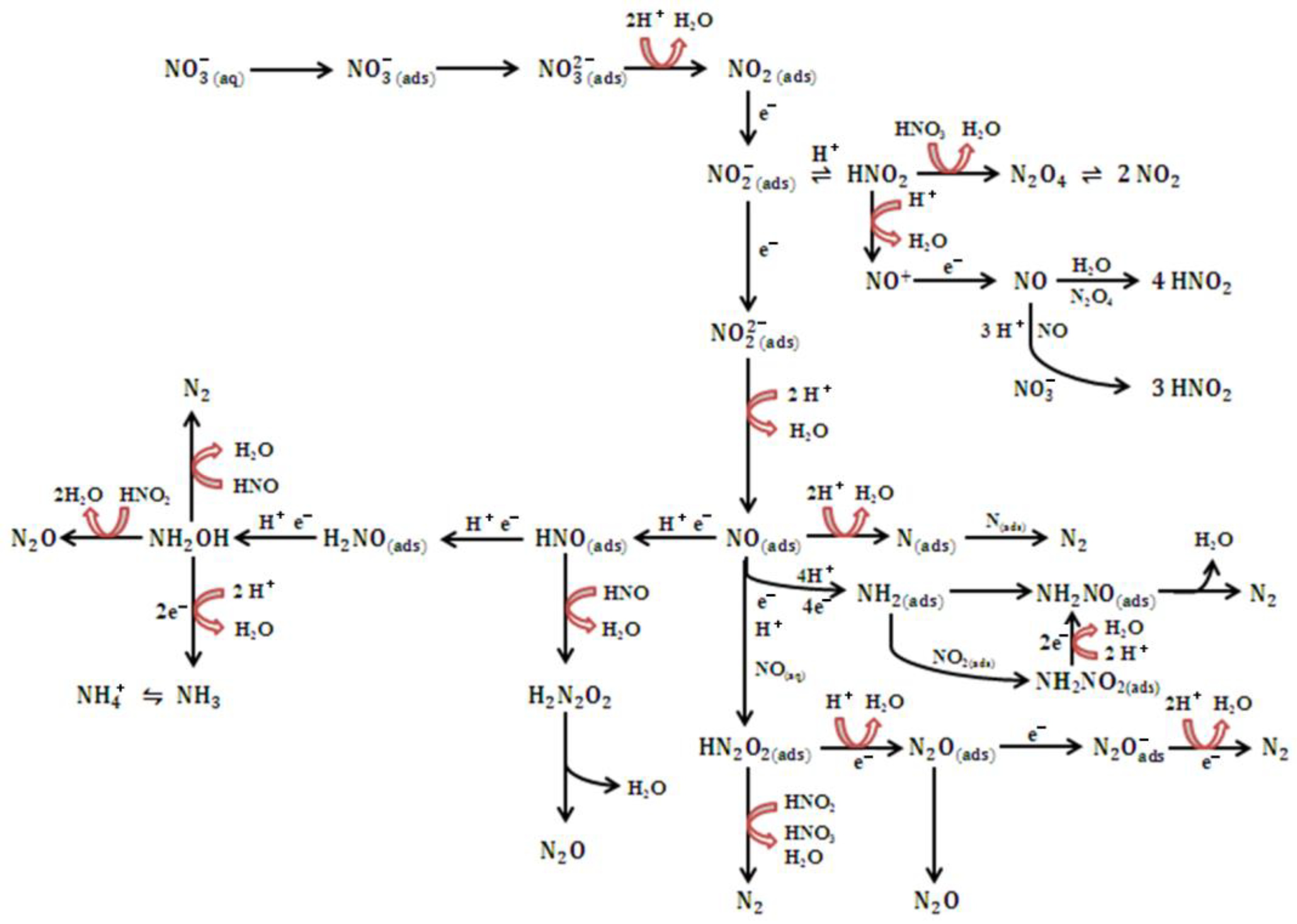
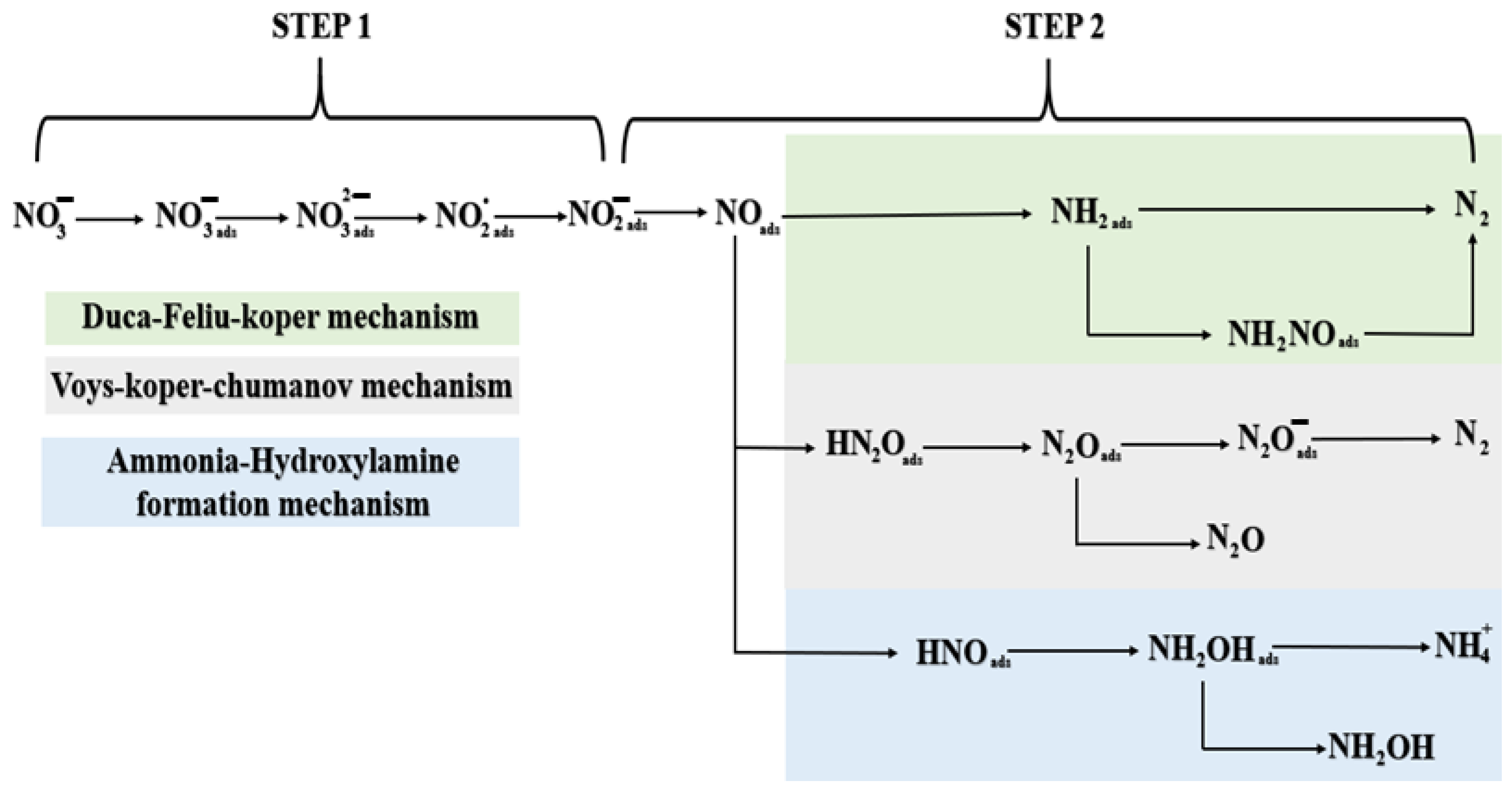
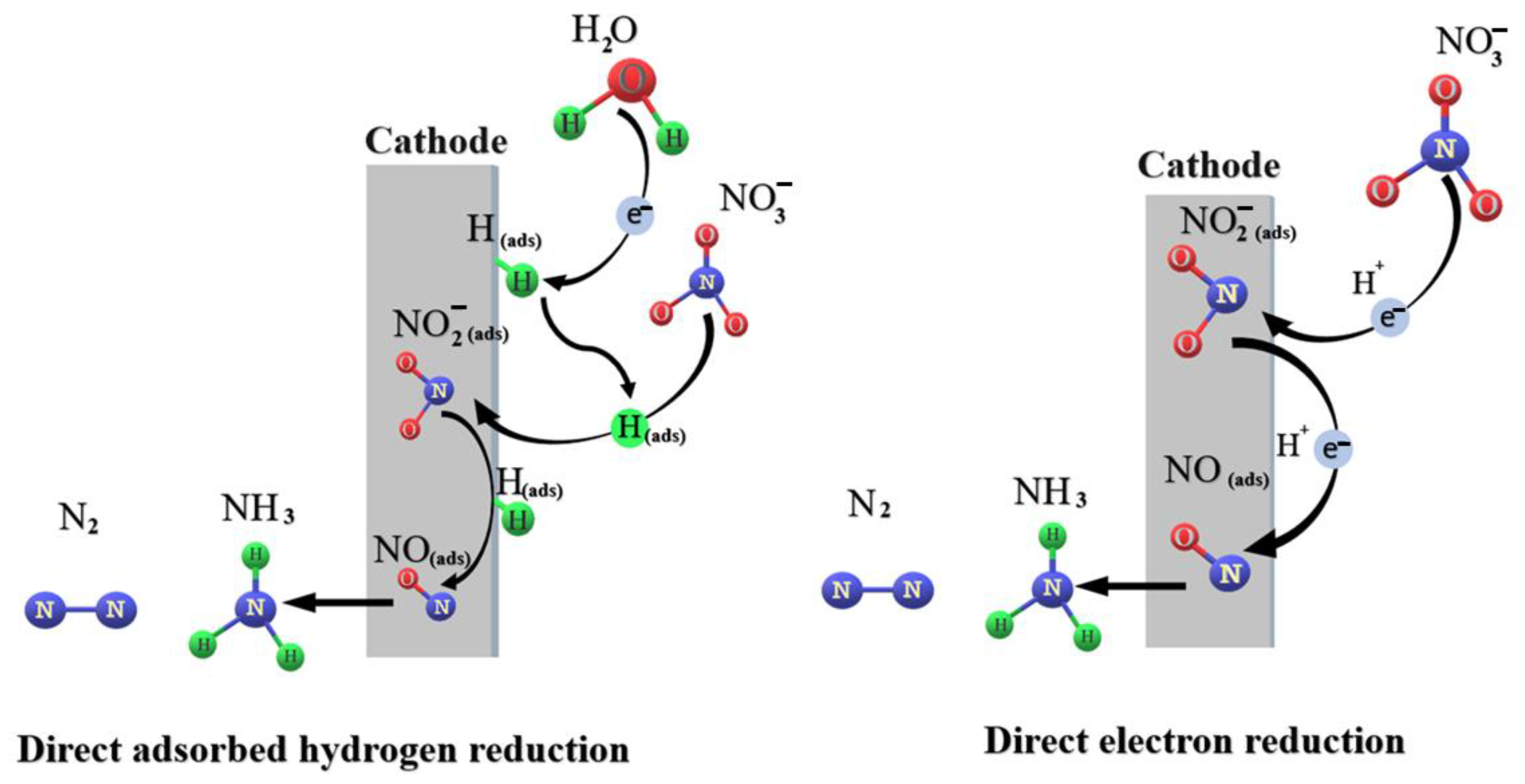
2.1. Mechanistic Insight into the Electrochemical Nitrate Reduction
2.2. DFT Calculation as an Advanced Tool for eNO3R
2.2.1. Insights into eNO3R: Descriptors and Rate Determining Steps (RDS)
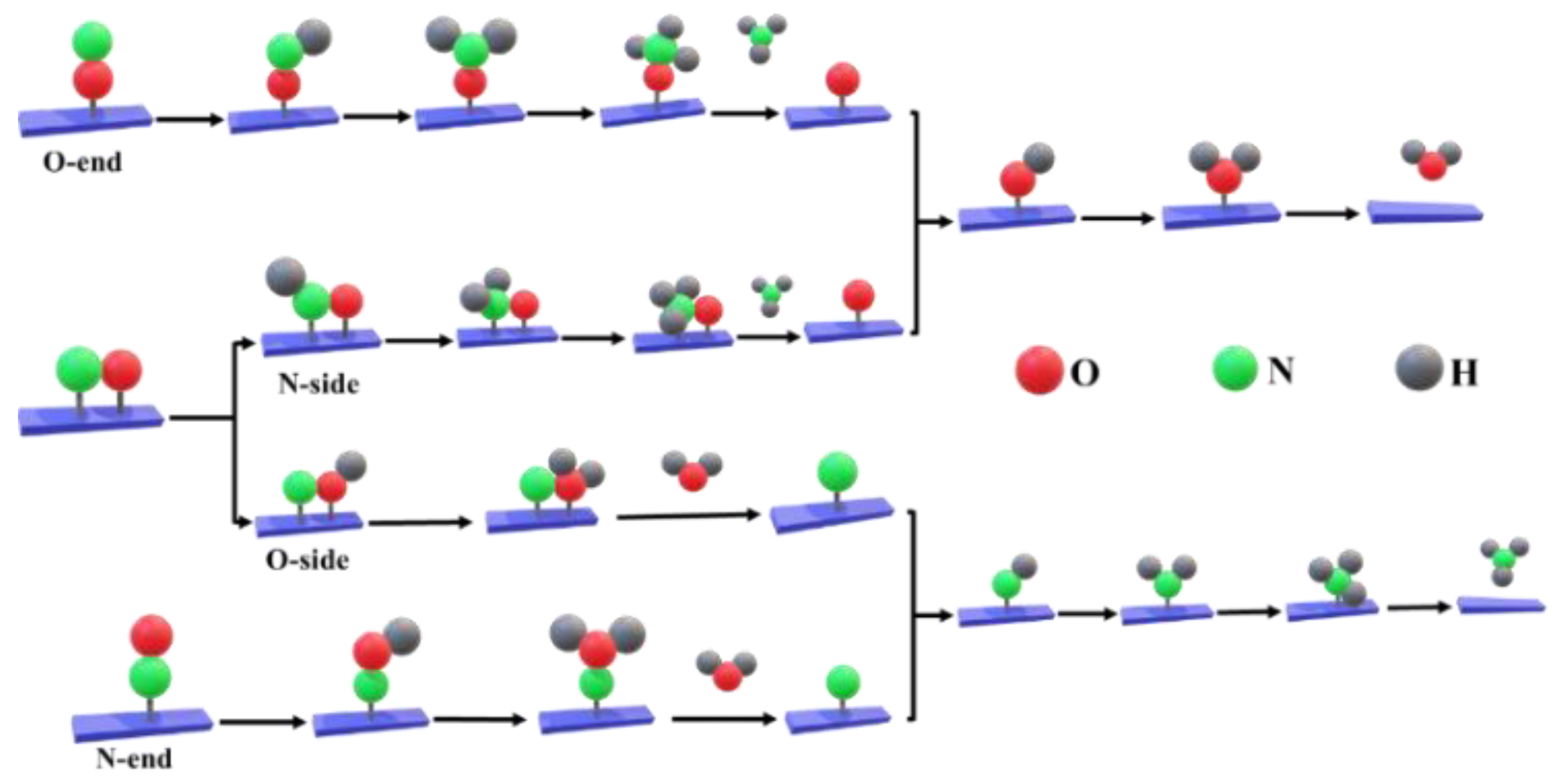
2.2.2. Nitrate to Ammonium
2.2.3. Nitrate to Nitrogen
2.2.4. eNO3R vs. HER
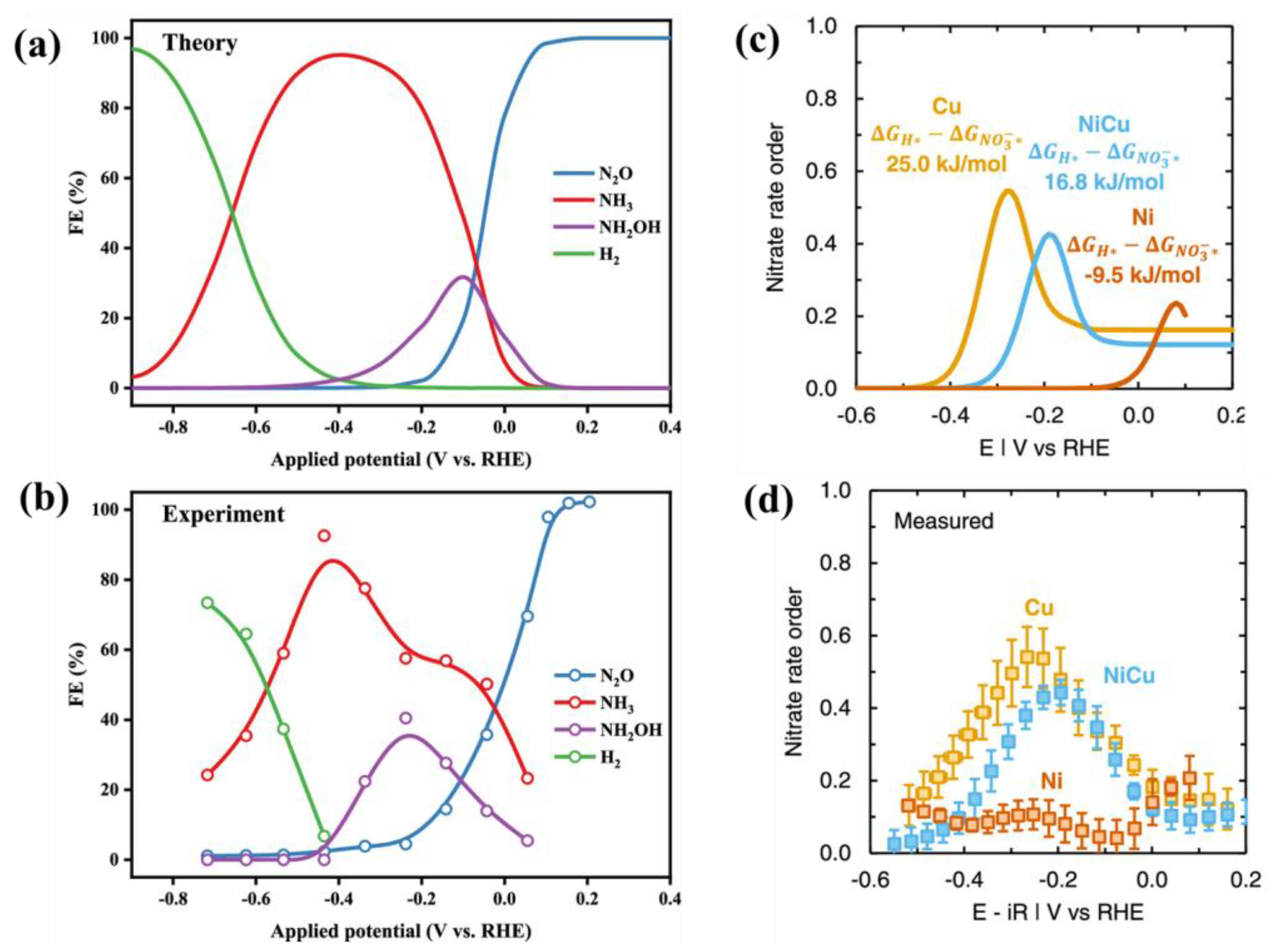
2.2.5. Analyzing Experimental Data vs. DFT for Nitrate Reduction
2.3. Machine Learning and Artificial Intelligence for Efficient eNO3R
3. Recent Developments in Electrocatalyst Fabrication and Characterization Techniques for Effective eNO3R Process
3.1. Advances in Electrocatalyst Preparation Techniques for Efficient Nitrate Reduction
3.1.1. Electrodeposition
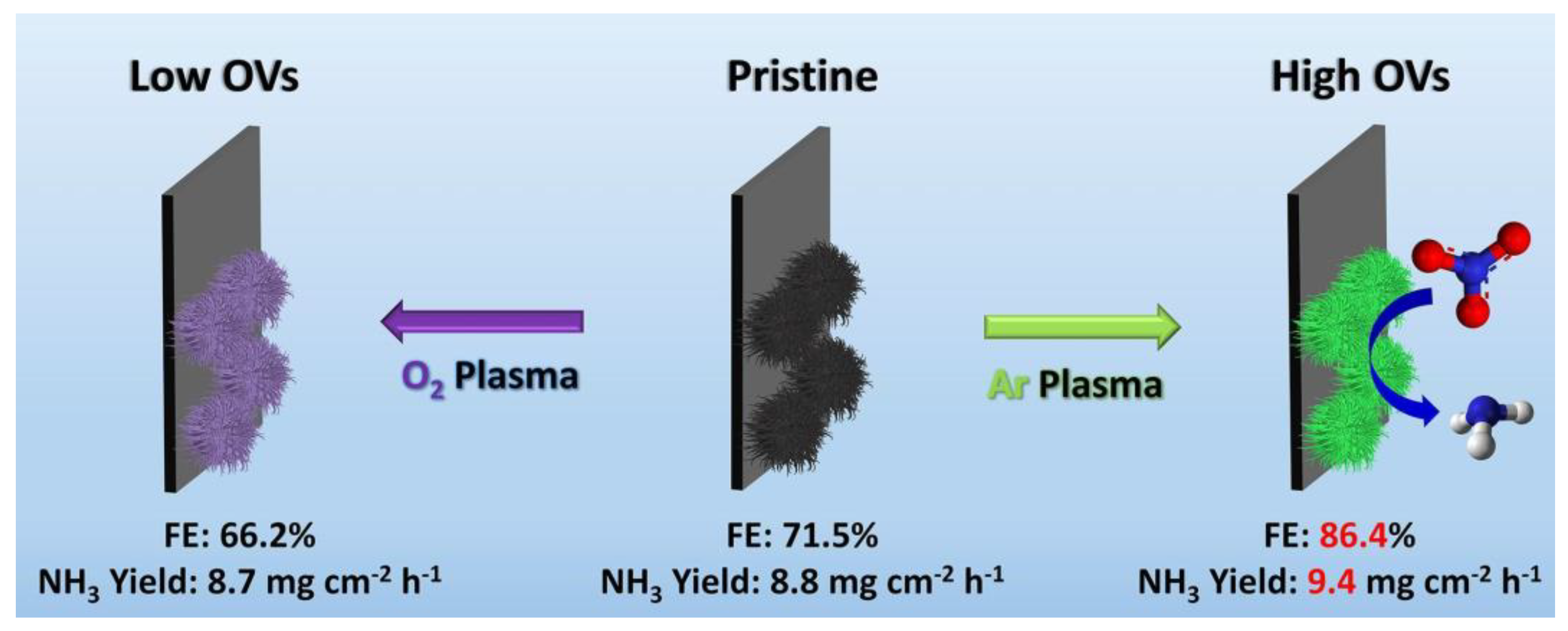
3.1.2. Plasma Treatment
3.1.3. Thermal Treatment (Calcination)
3.1.4. Thermal Treatment (Annealing)
3.1.5. Hydrothermal Treatment
3.1.6. Impregnation Method
3.2. Advanced in Situ Characterization Techniques
4. Advanced Strategies for eNO3R Toward Ammonia and Nitrogen
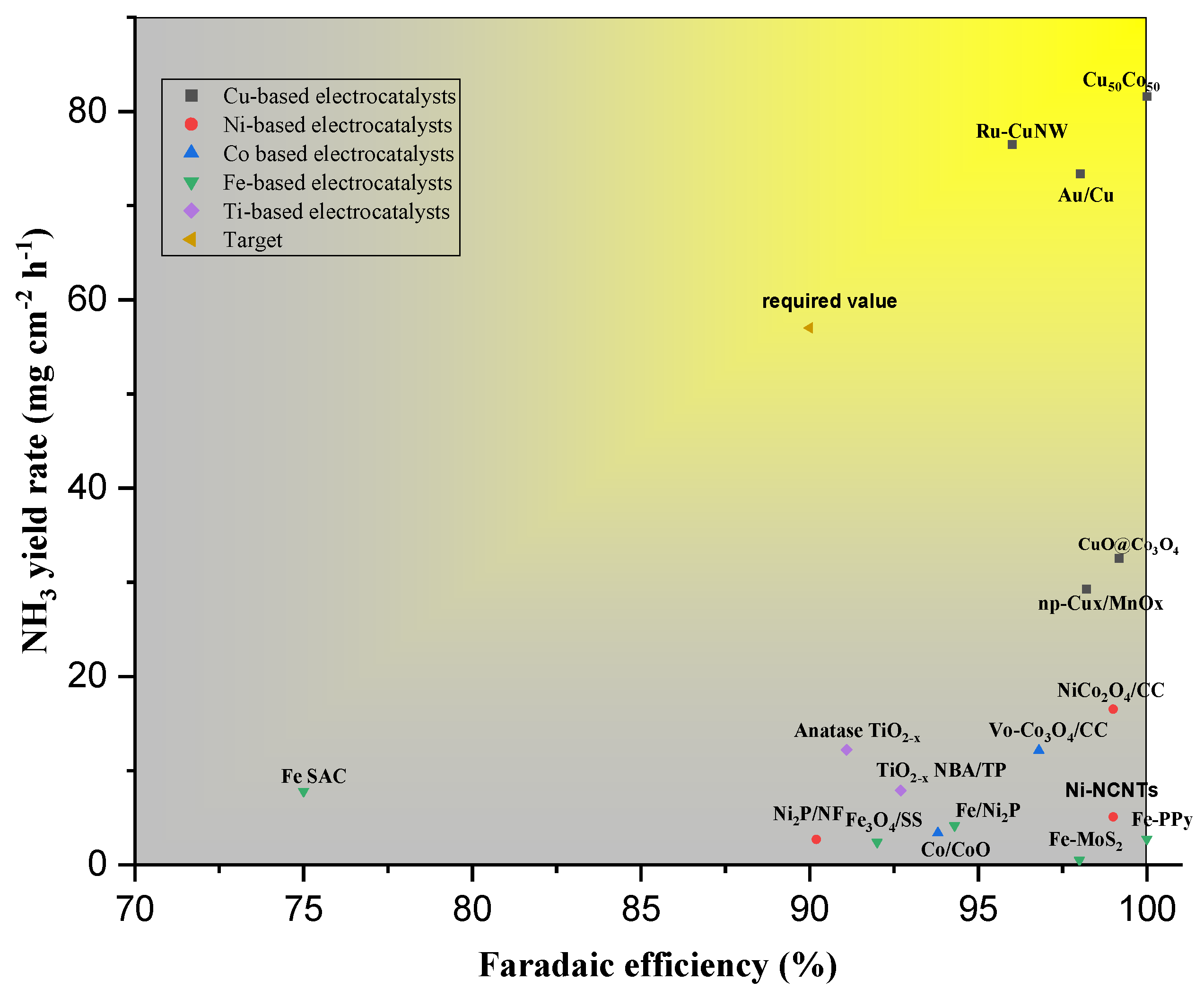
4.1. Copper-Based Electrocatalyst for the eNO3R: A Case Study for Ammonia Production
4.1.1. Copper-Based Single Atom Catalyst (Cu-Based SACs)
4.1.2. Facet Engineering
4.1.3. Copper-Based Bimetallic Catalysts
4.1.4. Copper Oxide
4.1.5. Cu-Based Heterostructure Engineering
4.1.6. Copper-Based Transition Metal Oxide Composite
4.1.7. Copper-Based Inorganic/Organic Material
4.1.8. Copper-Based Nanomaterials
4.2. Advanced Strategies Toward eNO3R to Nitrogen
4.2.1. Nanoscale Zero-Valent Iron (nZVI)
4.2.2. Indirect eNO3R to Nitrogen via Cl-Electrochemical Advanced Oxidation
5. Application of eNO3R to Brackish Groundwater: A Case Study
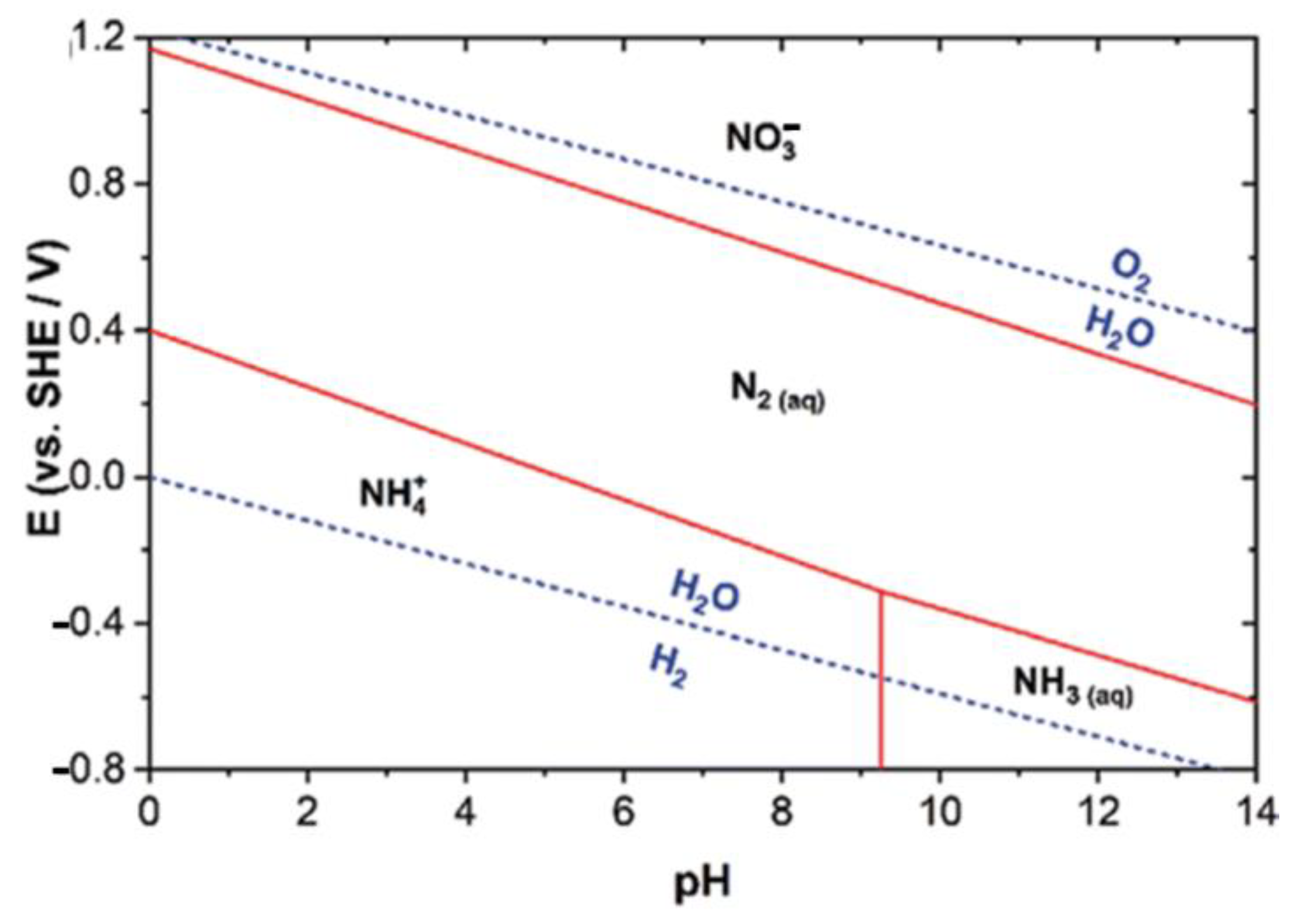
5.1. pH
5.2. Effect of Chloride Anions
5.3. Coexistence Ions
5.4. Cathode/Anode Passivation
5.5. Nitrate Concentration
5.6. Influence of Applied Voltage and Current Density
5.7. Challenge to Move from Lab Scale to Pilot eNO3R
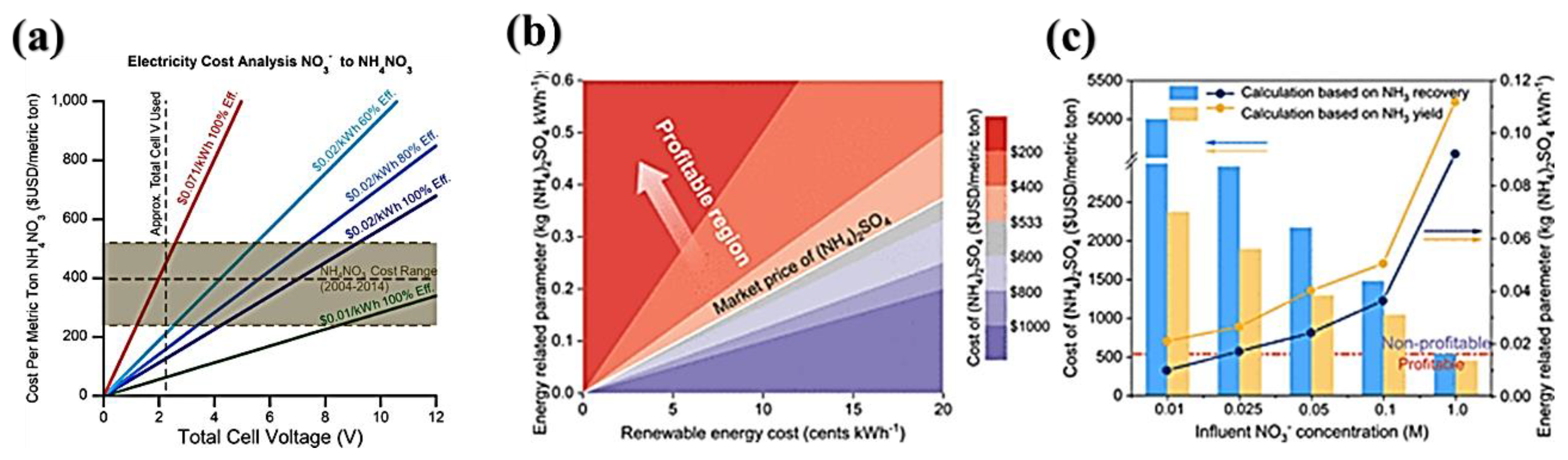
5.8. Economic Analysis
5.9. Sustainability Analysis
6. Summary and Outlook
- The mechanism for the eNO3R process is not well understood, which could be a reason to continue using DFT calculations. Farther, the complexity of the nitrate reduction process may make it difficult to accurately model using DFT. On the other hand, the DFT calculations require significant computational resources, which can be time-consuming and expensive. Additionally, DFT calculations rely on several approximations and assumptions, which may not always accurately reflect the real-world system. Despite these challenges, the use of DFT and microkinetic modeling can still provide valuable insights into the mechanism of electrocatalytic nitrate reduction and guide the tailored design of more efficient and selective electrode materials.
- Machine learning (ML) and artificial intelligence (AI) play an increasingly important role in optimizing catalyst design, by enhancing the efficiency of DFT. These tools reduce the complexity of high-dimensional catalytic activity maps by identifying key descriptors, such as adsorption energies of critical species. This approach allows for more efficient screening of potential catalysts across vast chemical spaces, accelerating material discovery while significantly lowering the computational cost of DFT calculations.
- Developing more efficient electrocatalysts for eNO3R is needed to find non-precious metal electrode materials that are highly efficient and durable, as the scarcity and cost of noble metals like Pd make them unsuitable for practical use. This requires extensive research and testing of various materials and modifications to improve their stability and durability, as well as their selectivity and activity for electrochemical nitrate reduction. Furthermore, advanced research in nanomaterials for Cu-nanomaterials or Fe-nanomaterials is required due to their operational low potential which reduces energy consumption for the process.
- Brackish groundwater with high chloride concentration can be advantageous for nitrate conversion to nitrogen; an efficient anode of Ti/Ru-Ir is widely used for this purpose. However, by-product formation such as chlorine-containing byproducts (CBPs) can be the primary drawback, especially with water-containing organic compounds. Focusing on a future hybrid process that includes photo-electrocatalysis or electrocoagulation may be an effective strategy for addressing this special issue.
- Optimizing operational parameters such as pH, current density, and applied voltage is crucial to improve eNO3R and their effects should be examined in real environments as in the case of brackish groundwater. Additionally, further studies are needed, especially on the challenge of anode and cathode passivation which results in fouling of the electrodes and seriously decrease the efficiency of nitrate reduction, notably on the application of electric pulses and the specific type to ensure the performance of the process and prevent metal leaching. This can enhance nitrate removal efficiency, minimize by-product formation, and promote a sustainable process.
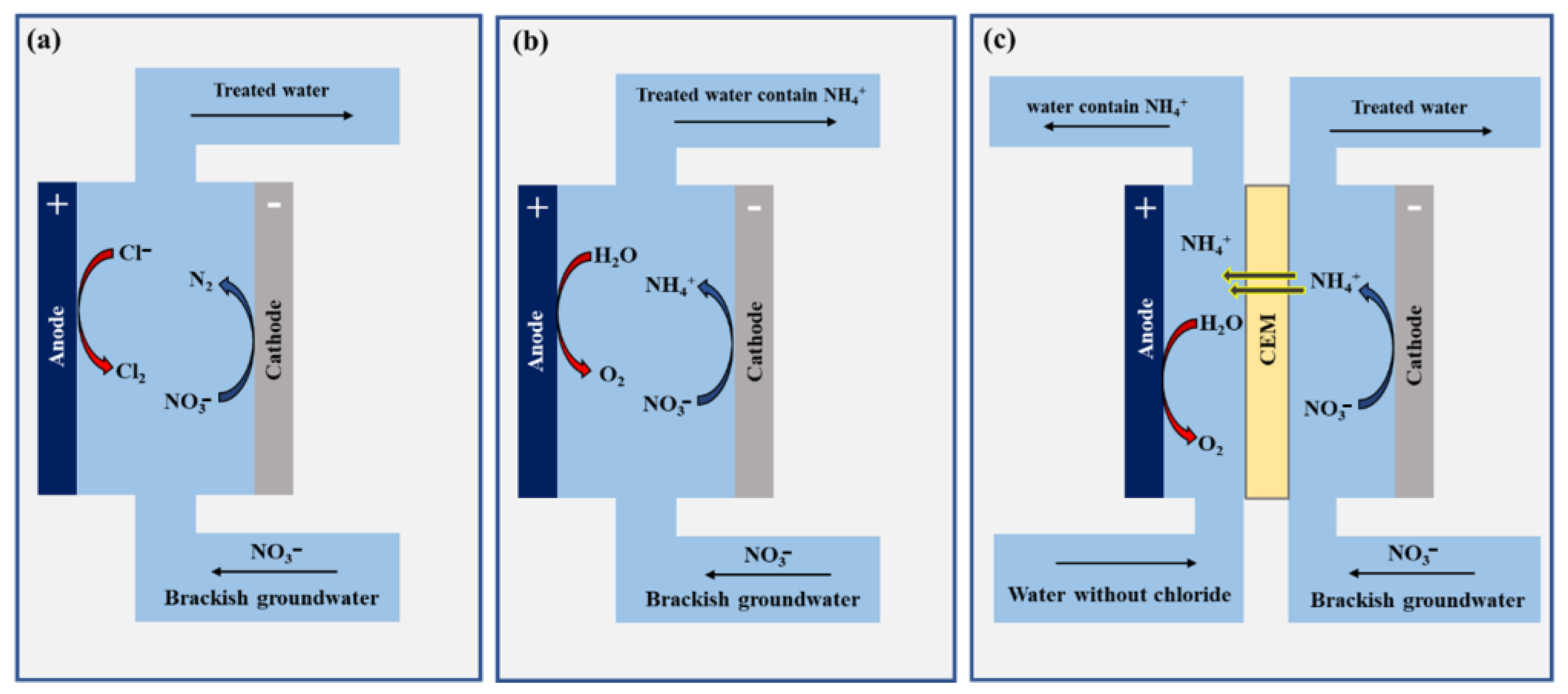
- Selecting the configuration type for the cell, divided or undivided, can greatly impact the efficiency and selectivity of the reduction process. Divided cells are more effective at nitrate reduction, and they could be the suitable choice for ammonia generation as they prevent re-oxidation of the reduced nitrate in the anode and concurrently, we recover the ammonium that migrates through the cation exchange membrane (CEM). However, the drawbacks associated with divided cells are fouling and scaling of the membrane in complex water matrices as the case in brackish groundwater, additionally, the limited availability of ammonium-selective membranes needs to be addressed in future research. The alternative pathway for eNO3R to ammonium in brackish groundwater can be employed in undivided cells, providing a specific anode that prevents the production of chlorine radicals and active chlorine. These anodes are currently being developed, especially for large-scale applications. On the other hand, undivided cells remain the optimal choice for eNO3R to due to their effectiveness in minimizing energy consumption and operational costs, while also simplifying system design and maintenance.
- Reactor design and the components of the cells, such as shapes, dimensions of electrodes, and their placement in the flow channel are essential for the success of the process due to their role in ensuring fluid dispersion, current distribution, and mass transport. Mathematical approaches to designing electrochemical reactors have been adopted for different technology using numerical tools such as computational fluids dynamics (CFD) since we can test the various reactors with different components before the experimental characterization in laboratory-scale cells and then, during the scale-up of pilot plants. Therefore, the use of CFD simulation to visualize surface-phase changes during electrode reactions, including the mass transport and current distribution during eNO3R is crucial to increase the knowledge and performance of the process. Furthermore, designing a new reactor to lower the ohmic resistance and thus reduce unnecessary energy consumption is also a desirable approach.
- Researchers who focus on the conversion of eNO3R for ammonia production to pursue subsequent utilization should consider the concentration of nitrate in water. Using renewable energies for cost minimization remains advantageous. However, in waters with low nitrate concentrations, it is preferable to prioritize the production of drinking water, specifically through the pathway of converting nitrates into nitrogen. Nevertheless, the generation of ammonium from chemical industry waste, sewage, and the site of caliche ore with high nitrate concentration is the most effective approach to address “two birds in one stone”, removing nitrate and producing ammonia.
- The operating cost requires special consideration for large-scale applications, especially raw materials, transportation, and post-treatment costs. Also, the electrical energy consumed during nitrate electroreduction represents a large proportion of the total cost. In fact, coupling thermodynamically favorable reactions such as the oxidation of organics is promising, which can not only decrease the electrical energy needed but can also raise the additional value of electrochemical water treatment.
- The inclusion of renewable energies, notably solar power, in electrocatalytic nitrate reduction (eNO3R) promotes sustainable development by minimizing dependence on non-renewable sources and minimizing the carbon footprint. Moreover, the use of Life Cycle Assessment (LCA) is essential to assess the environmental impact of eNO3R compared to conventional methods, concentrating on energy consumption, emissions, and waste. Yet in-depth research studies are called for to address challenges such as scale-up, high initial costs, and the requirement for technological innovation for sustainable implementation, with the potential support of economic incentives and emerging renewable energy trends.
Author Contributions
Funding
Conflicts of Interest
References
- Ighalo, J.O.; Adeniyi, A.G. A Comprehensive Review of Water Quality Monitoring and Assessment in Nigeria. Chemosphere 2020, 260, 127569. [Google Scholar] [CrossRef] [PubMed]
- Ekere, N.R.; Agbazue, V.E.; Ngang, B.U.; Ihedioha, J.N. Hydrochemistry and Water Quality Index of Groundwater Resources in Enugu North District, Enugu, Nigeria. Environ. Monit. Assess. 2019, 191, 150. [Google Scholar] [CrossRef] [PubMed]
- Howe, C.W.; Hecht, A. Book Reviews. Clim. Chang. 1995, 31, 119–126. [Google Scholar] [CrossRef]
- Malik, M.S.; Shukla, J.P. GIS Modeling Approach for Assessment of Groundwater Vulnerability in Parts of Tawa River Catchment Area, Hoshangabad, Madhya Pradesh, India. Groundw. Sustain. Dev. 2019, 9, 100249. [Google Scholar] [CrossRef]
- Heiß, L.; Bouchaou, L.; Tadoumant, S.; Reichert, B. Index-Based Groundwater Vulnerability and Water Quality Assessment in the Arid Region of Tata City (Morocco). Groundw. Sustain. Dev. 2020, 10, 100344. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the Environment: A Critical Review of Their Distribution, Sensing Techniques, Ecological Effects and Remediation. Chemosphere 2022, 287, 131996. [Google Scholar] [CrossRef] [PubMed]
- Zahra HAFIANE, F.; Tahri, L.; Nouayti, N.; El Jarmouni, M.; Arifi, K.; Elamrani Idrissi, A.; Fekhaoui, M. Assessment of spatial and seasonal nitrate variation of groundwater in the irrigated perimeter (Tadla Plain-Morocco). Agric. For. 2020, 66, 203–214. [Google Scholar] [CrossRef]
- Lahjouj, A.; El Hmaidi, A.; Bouhafa, K.; Boufala, M. Mapping Specific Groundwater Vulnerability to Nitrate Using Random Forest: Case of Sais Basin, Morocco. Model. Earth Syst. Environ. 2020, 6, 1451–1466. [Google Scholar] [CrossRef]
- Wagh, V.; Panaskar, D.; Muley, A.; Mukate, S.; Gaikwad, S. Neural Network Modelling for Nitrate Concentration in Groundwater of Kadava River Basin, Nashik, Maharashtra, India. Groundw. Sustain. Dev. 2018, 7, 436–445. [Google Scholar] [CrossRef]
- Adimalla, N.; Taloor, A.K. Hydrogeochemical Investigation of Groundwater Quality in the Hard Rock Terrain of South India Using Geographic Information System (GIS) and Groundwater Quality Index (GWQI) Techniques. Groundw. Sustain. Dev. 2020, 10, 100288. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; World Health Organization Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer; Distributed by WHO Press: Lyon, France; Geneva, The Netherland, 2010; ISBN 978-92-832-1294-2. [Google Scholar]
- Greer, F.R. Infant Methemoglobinemia: The Role of Dietary Nitrate in Food and Water. Pediatrics 2005, 116, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Nitrate Contamination: Exposure, Consequence, and Control; Bogárdi, I., Kuzelka, R.D., Ennenga, W.G., Eds.; NATO ASI Series; Series G, Ecological Sciences; Springer-Verlag: New York, NY, USA, 1991; ISBN 978-0-387-53088-8. [Google Scholar]
- World Health Organization (WHO). Nitrate and Nitrite in Drinking-Water: Background Document for Development of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Zhang, Z.; Huang, G.; Zhang, P.; Shen, J.; Wang, S.; Li, Y. Development of Iron-Based Biochar for Enhancing Nitrate Adsorption: Effects of Specific Surface Area, Electrostatic Force, and Functional Groups. Sci. Total Environ. 2023, 856, 159037. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J. Enhanced Removal of Nitrate from Water Using Surface Modification of Adsorbents—A Review. J. Environ. Manag. 2013, 131, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Samatya, S.; Kabay, N.; Yüksel, Ü.; Arda, M.; Yüksel, M. Removal of Nitrate from Aqueous Solution by Nitrate Selective Ion Exchange Resins. React. Funct. Polym. 2006, 66, 1206–1214. [Google Scholar] [CrossRef]
- Korak, J.A.; Mungan, A.L.; Watts, L.T. Critical Review of Waste Brine Management Strategies for Drinking Water Treatment Using Strong Base Ion Exchange. J. Hazard. Mater. 2023, 441, 129473. [Google Scholar] [CrossRef]
- Ding, X.; Wei, D.; Guo, W.; Wang, B.; Meng, Z.; Feng, R.; Du, B.; Wei, Q. Biological Denitrification in an Anoxic Sequencing Batch Biofilm Reactor: Performance Evaluation, Nitrous Oxide Emission and Microbial Community. Bioresour. Technol. 2019, 285, 121359. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, E.; Yang, L.; Liu, S.; Rensing, C.; Zhou, S. Combining Biological Denitrification and Electricity Generation in Methane-Powered Microbial Fuel Cells. J. Environ. Sci. 2023, 130, 212–222. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, Y. New Direction in Biological Nitrogen Removal from Industrial Nitrate Wastewater via Anammox. Appl. Microbiol. Biotechnol. 2019, 103, 7459–7466. [Google Scholar] [CrossRef]
- Pang, S.; Zhang, G.; Hu, Y.; Luo, X.; Li, N.; Zhu, J.; Jiang, J.; Han, B.; Shen, T. Enhancing the Total Nitrogen Removal Efficiency of Anaerobic Ammonium Oxidation (Anammox) with Two Types of Biochars. J. Environ. Chem. Eng. 2023, 11, 109108. [Google Scholar] [CrossRef]
- Vilardi Giorgio Bimetallic Nzvi-Induced Chemical Denitrification Modelling Using the Shrinking Core Model. Chem. Eng. Trans. 2018, 70, 235–240. [CrossRef]
- Jiang, M.; Wu, Y.; He, P.; Hu, S.; Li, Q.; Chen, S. Assembled Denitrifying Consortia for Efficient Nitrate Removal under Low-COD/N Conditions. Chem. Eng. J. 2023, 460, 141655. [Google Scholar] [CrossRef]
- Yun, Y.; Wen, X. High Catalytic Activity and N2 Selectivity on Catalytic Denitrification by Fe0 and Bimetallic Catalyst. J. Environ. Eng. 2020, 146, 04020104. [Google Scholar] [CrossRef]
- Xue, S.; Wen, X.; Yun, Y. Pathway and Mechanism Study on Improvement of N2 Selectivity of Catalytic Denitrification. J. Saudi Chem. Soc. 2023, 27, 101577. [Google Scholar] [CrossRef]
- Patra, S.; Pranudta, A.; Chanlek, N.; Nguyen, T.T.; Nhat, N.H.; El-Moselhy, M.M.; Padungthon, S. Denitrification of Nitrate in Regeneration Waste Brine Using Hybrid Cation Exchanger Supported Nanoscale Zero-Valent Iron with/without Palladium Nanoparticles. Chemosphere 2023, 310, 136851. [Google Scholar] [CrossRef] [PubMed]
- Epsztein, R.; Nir, O.; Lahav, O.; Green, M. Selective Nitrate Removal from Groundwater Using a Hybrid Nanofiltration–Reverse Osmosis Filtration Scheme. Chem. Eng. J. 2015, 279, 372–378. [Google Scholar] [CrossRef]
- Mehenktaş, C.; Arar, Ö. Application of Membrane Processes for Nitrate (NO3-) Removal. Curr. Chin. Sci. 2023, 3, 42–56. [Google Scholar] [CrossRef]
- Tyagi, S.; Rawtani, D.; Khatri, N.; Tharmavaram, M. Strategies for Nitrate Removal from Aqueous Environment Using Nanotechnology: A Review. J. Water Process Eng. 2018, 21, 84–95. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, M.; Zeng, G.; Liu, X.; Fang, C.; Li, C. A Three-Dimensional Cu Nanobelt Cathode for Highly Efficient Electrocatalytic Nitrate Reduction. Nanoscale 2020, 12, 9385–9391. [Google Scholar] [CrossRef]
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A Review on Industrial Wastewater Treatment via Electrocoagulation Processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A Comprehensive Review of Electrocoagulation for Water Treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef]
- Zou, S.; Guan, L.; Taylor, D.P.; Kuhn, D.; He, Z. Nitrogen Removal from Water of Recirculating Aquaculture System by a Microbial Fuel Cell. Aquaculture 2018, 497, 74–81. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A. Haber-Bosch Process Intensification: A First Step towards Small-Scale Distributed Ammonia Production. Chem. Eng. Res. Des. 2023, 195, 651–661. [Google Scholar] [CrossRef]
- Nie, S.; Mo, S.; Gao, T.; Yan, B.; Shen, P.; Kashif, M.; Zhang, Z.; Li, J.; Jiang, C. Coupling Effects of Nitrate Reduction and Sulfur Oxidation in a Subtropical Marine Mangrove Ecosystem with Spartina Alterniflora Invasion. Sci. Total Environ. 2023, 862, 160930. [Google Scholar] [CrossRef] [PubMed]
- Katsounaros, I. On the Assessment of Electrocatalysts for Nitrate Reduction. Curr. Opin. Electrochem. 2021, 28, 100721. [Google Scholar] [CrossRef]
- Anastasiadou, D.; van Beek, Y.; Hensen, E.J.M.; Costa Figueiredo, M. Ammonia Electrocatalytic Synthesis from Nitrate. Electrochem. Sci. Adv. 2022, 3, e2100220. [Google Scholar] [CrossRef]
- Dima, G.E.; de Vooys, A.C.A.; Koper, M.T.M. Electrocatalytic Reduction of Nitrate at Low Concentration on Coinage and Transition-Metal Electrodes in Acid Solutions. J. Electroanal. Chem. 2003, 554–555, 15–23. [Google Scholar] [CrossRef]
- de Vooys, A.C.A.; Beltramo, G.L.; van Riet, B.; van Veen, J.A.R.; Koper, M.T.M. Mechanisms of Electrochemical Reduction and Oxidation of Nitric Oxide. Electrochim. Acta 2004, 49, 1307–1314. [Google Scholar] [CrossRef]
- Duca, M.; Figueiredo, M.C.; Climent, V.; Rodriguez, P.; Feliu, J.M.; Koper, M.T.M. Selective Catalytic Reduction at Quasi-Perfect Pt(100) Domains: A Universal Low-Temperature Pathway from Nitrite to N2. J. Am. Chem. Soc. 2011, 133, 10928–10939. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, T.; Cotton, T.M.; Chumanov, G. Photoinduced Electrochemical Reduction of Nitrite at an Electrochemically Roughened Silver Surface. J. Phys. Chem. B 1999, 103, 6567–6572. [Google Scholar] [CrossRef]
- Bartberger, M.D.; Liu, W.; Ford, E.; Miranda, K.M.; Switzer, C.; Fukuto, J.M.; Farmer, P.J.; Wink, D.A.; Houk, K.N. The Reduction Potential of Nitric Oxide (NO) and Its Importance to NO Biochemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 10958–10963. [Google Scholar] [CrossRef]
- Katsounaros, I.; Kyriacou, G. Influence of Nitrate Concentration on Its Electrochemical Reduction on Tin Cathode: Identification of Reaction Intermediates. Electrochim. Acta 2008, 53, 5477–5484. [Google Scholar] [CrossRef]
- Kuwabata, S.; Uezumi, S.; Tanaka, K.; Tanaka, T. Assimilatory and Dissimilatory Reduction of Nitrate and Nitrite with a Tris(Tetrabutylammonium) Nonakis(Benzenethiolato)Octasulfidohexaferratedimolybdate(3-) Modified Glassy-Carbon Electrode in Water. Inorg. Chem. 1986, 25, 3018–3022. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Elaboration by High-Energy Ball Milling of Copper/Palladium Composite Materials—Characterization and Electrocatalytic Activity for the Reduction of Nitrate in Alkaline Medium. J. Electroanal. Chem. 2008, 622, 64–72. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Elaboration of Cu−Pd Films by Coelectrodeposition: Application to Nitrate Electroreduction. J. Phys. Chem. C 2009, 113, 290–297. [Google Scholar] [CrossRef]
- Lange, R.; Maisonhaute, E.; Robin, R.; Vivier, V. On the Kinetics of the Nitrate Reduction in Concentrated Nitric Acid. Electrochem. Commun. 2013, 29, 25–28. [Google Scholar] [CrossRef]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Bi, X. Enhanced Reduction of Nitrate by Noble Metal-Free Electrocatalysis on P Doped Three-Dimensional Co3O4 Cathode: Mechanism Exploration from Both Experimental and DFT Studies. Chem. Eng. J. 2020, 382, 123034. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Hui, K.S.; Dinh, D.A.; Lu, Z.; Zhang, Q.; Hui, K.N. Non−noble Single−atom Alloy for Electrocatalytic Nitrate Reduction Using Hierarchical High−throughput Screening. Nano Energy 2023, 113, 108543. [Google Scholar] [CrossRef]
- Hu, T.; Wang, C.; Wang, M.; Li, C.M.; Guo, C. Theoretical Insights into Superior Nitrate Reduction to Ammonia Performance of Copper Catalysts. ACS Catal. 2021, 11, 14417–14427. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, W.; Dai, G.; Yang, X.; Xia, R.; Xu, Y.; Yang, X.; Tu, W. Electroreduction of Nitrate to Ammonia on Atomically-Dispersed Cu-N4 Active Sites with High Efficiency and Stability. Fuel 2023, 332, 126106. [Google Scholar] [CrossRef]
- Zheng, X.; Yan, Y.; Li, X.; Liu, Y.; Yao, Y. Theoretical Insights into Dissociative-Associative Mechanism for Enhanced Electrochemical Nitrate Reduction to Ammonia. J. Hazard. Mater. 2023, 446, 130679. [Google Scholar] [CrossRef]
- Li, J.; Zhao, D.; Zhang, L.; Ren, Y.; Yue, L.; Li, Z.; Sun, S.; Luo, Y.; Chen, Q.; Li, T.; et al. Boosting Electrochemical Nitrate-to-Ammonia Conversion by Self-Supported MnCo2O4 Nanowire Array. J. Colloid Interface Sci. 2023, 629, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, C.; Jiang, X.; Guo, J.-Y.; Wei, Y.; Li, J.-W.; Sheng, T.; Zhao, X.; Wei, L. High-Index Surface Structure Engineering of Au–Pd Concave Triple-Octahedrons for Boosting Electrocatalytic Nitrate Reduction to Ammonia. ACS Sustain. Chem. Eng. 2023, 11, 1631–1637. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Jiang, P.; Qu, F.; Yu, D.; Hao, X.; Zhang, J. Electrochemical Denitrification by a Recyclable Nanoporous Co3o4/Co Cathode: Rapid Recovery and Selective Catalysis. Available online: https://ssrn.com/abstract=4327262 (accessed on 20 August 2024).
- Li, X.; Zhang, G.; Zhang, N.; Luo, Y.; Shen, P.; Li, X.; Chu, K. Regulating Pd Nanosheets by W-Doping for Electrochemical Nitrate Reduction to Ammonia. New J. Chem. 2022, 46, 14724–14730. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Tao, W.; Wang, P.; Li, H.; Huang, R.; Zhou, G. Engineering Sulfur Vacancies Optimization in Ni3Co6S8 Nanospheres toward Extraordinarily Efficient Nitrate Electroreduction to Ammonia. Appl. Catal. B Environ. 2023, 324, 122193. [Google Scholar] [CrossRef]
- Lei, F.; Xu, W.; Yu, J.; Li, K.; Xie, J.; Hao, P.; Cui, G.; Tang, B. Electrochemical Synthesis of Ammonia by Nitrate Reduction on Indium Incorporated in Sulfur Doped Graphene. Chem. Eng. J. 2021, 426, 131317. [Google Scholar] [CrossRef]
- Gou, F.; Wang, H.; Fu, M.; Jiang, Y.; Shen, W.; He, R.; Li, M. Boron-Induced Electron Localization in Cu Nanowires Promotes Efficient Nitrate Reduction to Ammonia in Neutral Media. Appl. Surf. Sci. 2023, 612, 155872. [Google Scholar] [CrossRef]
- Niu, Z.; Fan, S.; Li, X.; Duan, J.; Chen, A. Interfacial Engineering of CoMn2O4/NC Induced Electronic Delocalization Boosts Electrocatalytic Nitrogen Oxyanions Reduction to Ammonia. Appl. Catal. B Environ. 2023, 322, 122090. [Google Scholar] [CrossRef]
- Fan, J.; Chen, Y.; Chen, X.; Wu, Z.; Teng, W.; Zhang, W. Atomically Dispersed Iron Enables High-Efficiency Electrocatalytic Conversion of Nitrate to Dinitrogen on a N-Coordinated Mesoporous Carbon Architecture. Appl. Catal. B Environ. 2023, 320, 121983. [Google Scholar] [CrossRef]
- Calle-Vallejo, F.; Koper, M.T.M.; Bandarenka, A.S. Tailoring the Catalytic Activity of Electrodes with Monolayer Amounts of Foreign Metals. Chem. Soc. Rev. 2013, 42, 5210. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.-H.; Tan, C.-S.; et al. Enhanced Nitrate-to-Ammonia Activity on Copper–Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef] [PubMed]
- Karamad, M.; Goncalves, T.J.; Jimenez-Villegas, S.; Gates, I.D.; Siahrostami, S. Why Copper Catalyzes Electrochemical Reduction of Nitrate to Ammonia. Faraday Discuss. 2023, 243, 502–519. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Richards, D.; Singh, N.; Goldsmith, B.R. Activity and Selectivity Trends in Electrocatalytic Nitrate Reduction on Transition Metals. ACS Catal. 2019, 9, 7052–7064. [Google Scholar] [CrossRef]
- Sathishkumar, N.; Chen, H.-T. Mechanistic Understanding of the Electrocatalytic Nitrate Reduction Activity of Double-Atom Catalysts. J. Phys. Chem. C 2023, 127, 994–1005. [Google Scholar] [CrossRef]
- McIntyre, S.M.; Ennis, C.; Garden, A.L. A Computational Investigation into the Hydrogenation of NO on Water Ice Surfaces to Rationalize the NO:HNO:NOH Disparity in Space. ACS Earth Space Chem. 2023, 7, 1195–1206. [Google Scholar] [CrossRef]
- Lim, J.; Liu, C.-Y.; Park, J.; Liu, Y.-H.; Senftle, T.P.; Lee, S.W.; Hatzell, M.C. Structure Sensitivity of Pd Facets for Enhanced Electrochemical Nitrate Reduction to Ammonia. ACS Catal. 2021, 11, 7568–7577. [Google Scholar] [CrossRef]
- Chun, H.-J.; Zeng, Z.; Greeley, J. DFT Insights into NO Electrochemical Reduction: A Case Study of Pt(211) and Cu(211) Surfaces. ACS Catal. 2022, 12, 1394–1402. [Google Scholar] [CrossRef]
- Yu, P.; Wang, F.; Shifa, T.A.; Zhan, X.; Lou, X.; Xia, F.; He, J. Earth Abundant Materials beyond Transition Metal Dichalcogenides: A Focus on Electrocatalyzing Hydrogen Evolution Reaction. Nano Energy 2019, 58, 244–276. [Google Scholar] [CrossRef]
- Long, J.; Guo, C.; Fu, X.; Jing, H.; Qin, G.; Li, H.; Xiao, J. Unveiling Potential Dependence in NO Electroreduction to Ammonia. J. Phys. Chem. Lett. 2021, 12, 6988–6995. [Google Scholar] [CrossRef]
- Carvalho, O.Q.; Marks, R.; Nguyen, H.K.K.; Vitale-Sullivan, M.E.; Martinez, S.C.; Árnadóttir, L.; Stoerzinger, K.A. Role of Electronic Structure on Nitrate Reduction to Ammonium: A Periodic Journey. J. Am. Chem. Soc. 2022, 144, 14809–14818. [Google Scholar] [CrossRef]
- Xin, H.; Mou, T.; Pillai, H.S.; Wang, S.-H.; Huang, Y. Interpretable Machine Learning for Catalytic Materials Design toward Sustainability. Acc. Mater. Res. 2024, 5, 22–34. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, B.; Li, Z.; Li, W.; Li, Q.; Yu, X. High Throughput Screening for Electrocatalysts for Nitrogen Reduction Reaction Using Metal-Doped Bilayer Borophene: A Combined Approach of DFT and Machine Learning. Mol. Catal. 2024, 557, 113972. [Google Scholar] [CrossRef]
- Liu, J.; Guo, H.; Xiong, Y.; Chen, X.; Yu, Y.; Wang, C. Rational Design of Pt-Anchored Single-Atom Alloy Electrocatalysts for NO-to-NH3 Conversion by Density Functional Theory and Machine Learning. Chin. J. Catal. 2024, 62, 243–253. [Google Scholar] [CrossRef]
- Yang, L.; Fan, J.; Xiao, B.; Zhu, W. Unveiling “Sabatier Principle” for Electrocatalytic Nitric Oxide Reduction on Single Cluster Catalysts: A DFT and Machine Learning Guideline. Chem. Eng. J. 2023, 468, 143823. [Google Scholar] [CrossRef]
- Gao, Q.; Pillai, H.S.; Huang, Y.; Liu, S.; Mu, Q.; Han, X.; Yan, Z.; Zhou, H.; He, Q.; Xin, H.; et al. Breaking Adsorption-Energy Scaling Limitations of Electrocatalytic Nitrate Reduction on Intermetallic CuPd Nanocubes by Machine-Learned Insights. Nat. Commun. 2022, 13, 2338. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Z.; Yu, W.; Guo, Y. A Density-Functional-Theory-Based and Machine-Learning-Accelerated Hybrid Method for Intricate System Catalysis. Mater. Rep. Energy 2021, 1, 100046. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The Electrodeposition of Composite Coatings: Diversity, Applications and Challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Zurita, N.; García, S.G. Comparative Study of Electrodeposited Copper Nanoparticles on Different Substrates for Their Use in the Reduction of Nitrate Ions. Results Eng. 2023, 17, 100800. [Google Scholar] [CrossRef]
- Kuang, P.; Natsui, K.; Einaga, Y.; Feng, C.; Cui, Y.; Zhang, W.; Deng, Y. Annealing Enhancement in Stability and Performance of Copper Modified Boron-Doped Diamond (Cu-BDD) Electrode for Electrochemical Nitrate Reduction. Diam. Relat. Mater. 2021, 114, 108310. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Chen, Z.; Wang, L.; Wu, P.; Wang, F. Electrochemical Reduction of Nitrate via Cu/Ni Composite Cathode Paired with Ir-Ru/Ti Anode: High Efficiency and N2 Selectivity. Electrochim. Acta 2018, 291, 151–160. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xie, A.-Y.; Lou, Y.-Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Electrochemical Synthesis of Tetrahexahedral Cu Nanocrystals with High-Index Facets for Efficient Nitrate Electroreduction. J. Electroanal. Chem. 2022, 907, 116022. [Google Scholar] [CrossRef]
- Chen, K.-L.; Ahmad, M.S.; Chen, C.-L. Enhanced Nitrate Reduction over Functionalized Pd/Cu Electrode with Tunable Conversion to Nitrogen and Sodium Hydroxide Recovery. Sci. Total Environ. 2023, 869, 161849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.; Geng, X.; Wu, K.; Debliquy, M. Metal Oxide Semiconductors with Highly Concentrated Oxygen Vacancies for Gas Sensing Materials: A Review. Sens. Actuators A Phys. 2020, 309, 112026. [Google Scholar] [CrossRef]
- Jang, D.; Maeng, J.; Kim, J.; Han, H.; Park, G.H.; Ha, J.; Shin, D.; Hwang, Y.J.; Kim, W.B. Boosting Electrocatalytic Nitrate Reduction Reaction for Ammonia Synthesis by Plasma-Induced Oxygen Vacancies over MnCuOx. Appl. Surf. Sci. 2023, 610, 155521. [Google Scholar] [CrossRef]
- Kawashima, K.; Márquez, R.A.; Smith, L.A.; Vaidyula, R.R.; Carrasco-Jaim, O.A.; Wang, Z.; Son, Y.J.; Cao, C.L.; Mullins, C.B. A Review of Transition Metal Boride, Carbide, Pnictide, and Chalcogenide Water Oxidation Electrocatalysts. Chem. Rev. 2023, 123, 12795–13208. [Google Scholar] [CrossRef]
- Zang, B.; Zhang, Z.; Yuan, H.; Li, R.; Li, S.; Lu, J. Electrochemical Reduction of Nitrate in an Electric Kettle Equipped with Copper-Modified Titanate Cathode under High-Temperature. J. Water Process Eng. 2023, 56, 104415. [Google Scholar] [CrossRef]
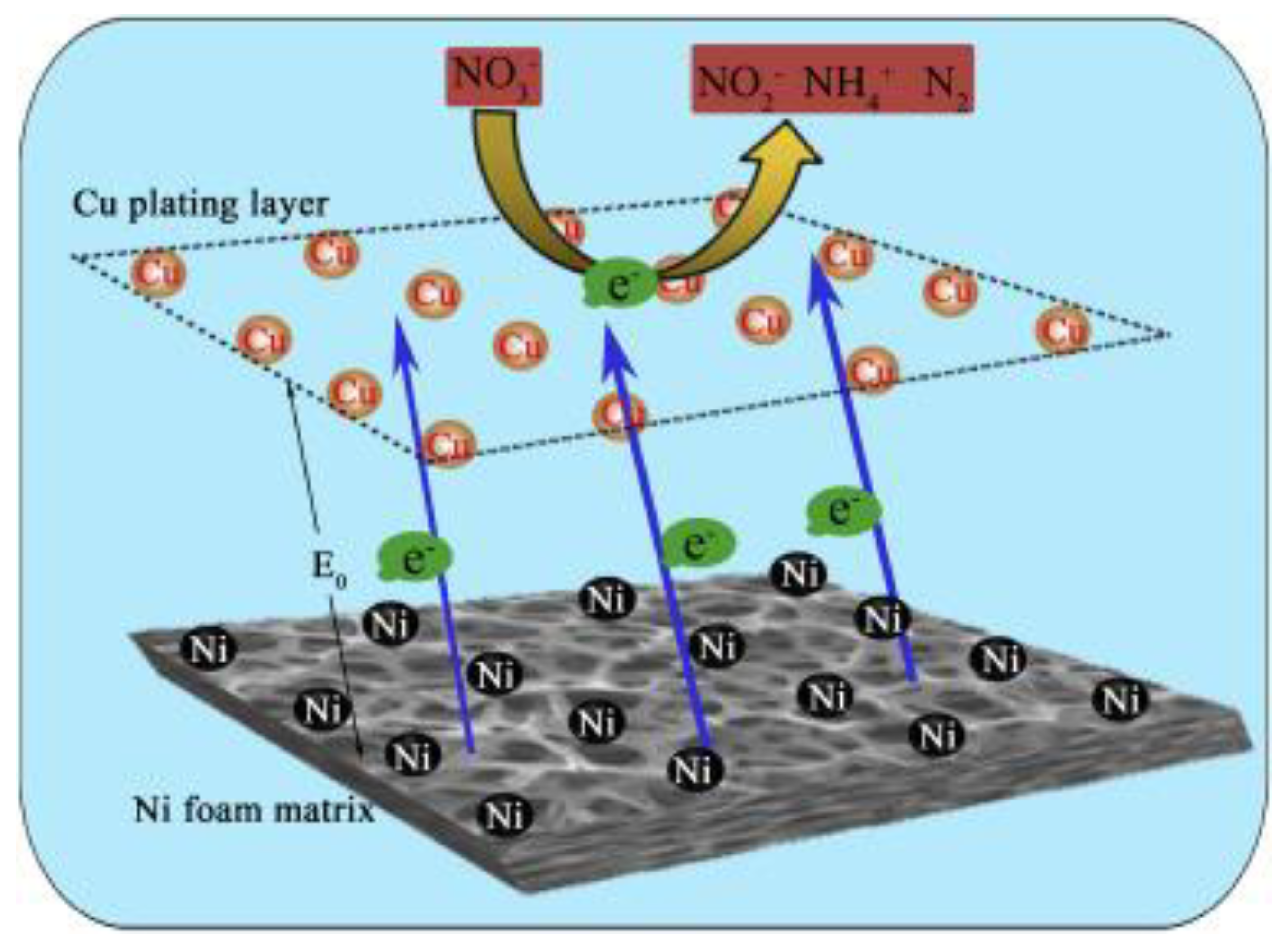
- Liu, X.; Guo, R.; Qin, X.; Xu, C. Enhanced Selective Nitrate-to-Nitrogen Electrocatalytic Reduction by CNTs Doped Ni Foam/Cu Electrode Coupled with Cl−. J. Water Process Eng. 2023, 54, 104067. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Du, F.; Yang, L.; Huang, S.; Gao, J.; Li, C.; Guo, C. A Core–Shell Copper Oxides-Cobalt Oxides Heterostructure Nanowire Arrays for Nitrate Reduction to Ammonia with High Yield Rate. Green Energy Environ. 2023, 8, 1619–1629. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-Based Fenton-like Catalysts for Water Treatment: Preparation, Characterization and Modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef]
- Chen, M.; Huang, X.; Wang, N.; Wang, T.; Yang, J.; Wei, Y.; Dao, X.; Zhou, L.; Hao, H. Cu2O Nanoparticles Modified BiO2-x Nanosheets for Efficient Electrochemical Reduction of Nitrate-N and Nitrobenzene from Wastewater. Sep. Purif. Technol. 2022, 289, 120728. [Google Scholar] [CrossRef]
- Pham, X.-H.; Ashik, U.P.M.; Hayashi, J.-I.; Pérez Alonso, A.; Pla, D.; Gómez, M.; Pham Minh, D. Review on the Catalytic Tri-Reforming of Methane—Part II: Catalyst Development. Appl. Catal. A Gen. 2021, 623, 118286. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, Y.; Shi, W.; Wang, W.; Zhang, R.; Bao, X.; Zhang, B.; Li, L.; Cui, F. Electrochemical-Catalytic Reduction of Nitrate over Pd–Cu/γAl2O3 Catalyst in Cathode Chamber: Enhanced Removal Efficiency and N2 Selectivity. Chem. Eng. J. 2016, 290, 201–208. [Google Scholar] [CrossRef]
- Liu, M.J.; Guo, J.; Hoffman, A.S.; Stenlid, J.H.; Tang, M.T.; Corson, E.R.; Stone, K.H.; Abild-Pedersen, F.; Bare, S.R.; Tarpeh, W.A. Catalytic Performance and Near-Surface X-Ray Characterization of Titanium Hydride Electrodes for the Electrochemical Nitrate Reduction Reaction. J. Am. Chem. Soc. 2022, 144, 5739–5744. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, R.; Wang, S.; Song, C.; Lu, S.; Fang, L.; Yin, F.; Liu, H. Sequential Active-Site Switches in Integrated Cu/Fe-TiO2 for Efficient Electroreduction from Nitrate into Ammonia. Appl. Catal. B Environ. 2023, 325, 122360. [Google Scholar] [CrossRef]
- Braley, S.E.; Xie, J.; Losovyj, Y.; Smith, J.M. Graphite Conjugation of a Macrocyclic Cobalt Complex Enhances Nitrite Electroreduction to Ammonia. J. Am. Chem. Soc. 2021, 143, 7203–7208. [Google Scholar] [CrossRef]
- Li, J.; Yue, M.-F.; Wei, Y.-M.; Li, J.-F. Synthetic Strategies of Single-Atoms Catalysts and Applications in Electrocatalysis. Electrochim. Acta 2022, 409, 139835. [Google Scholar] [CrossRef]
- Li, P.; Li, R.; Liu, Y.; Xie, M.; Jin, Z.; Yu, G. Pulsed Nitrate-to-Ammonia Electroreduction Facilitated by Tandem Catalysis of Nitrite Intermediates. J. Am. Chem. Soc. 2023, 145, 6471–6479. [Google Scholar] [CrossRef]
- Wang, C.; Ye, F.; Shen, J.; Xue, K.-H.; Zhu, Y.; Li, C. In Situ Loading of Cu 2 O Active Sites on Island-like Copper for Efficient Electrochemical Reduction of Nitrate to Ammonia. ACS Appl. Mater. Interfaces 2022, 14, 6680–6688. [Google Scholar] [CrossRef]
- Butcher, D.P.; Gewirth, A.A. Nitrate Reduction Pathways on Cu Single Crystal Surfaces: Effect of Oxide and Cl−. Nano Energy 2016, 29, 457–465. [Google Scholar] [CrossRef]
- Gong, Z.; Zhong, W.; He, Z.; Jia, C.; Zhou, D.; Zhang, N.; Kang, X.; Chen, Y. Improving Electrochemical Nitrate Reduction Activity of Layered Perovskite Oxide La2CuO4 via B-Site Doping. Catal. Today 2022, 402, 259–265. [Google Scholar] [CrossRef]
- Yao, J.; Mei, Y.; Yuan, T.; Chen, J.; Pan, H.; Wang, J. Electrochemical Removal of Nitrate from Wastewater with a Ti Cathode and Pt Anode for High Efficiency and N2 Selectivity. J. Electroanal. Chem. 2021, 882, 115019. [Google Scholar] [CrossRef]
- Dutta, A. Fourier Transform Infrared Spectroscopy. In Spectroscopic Methods for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 73–93. ISBN 978-0-323-46140-5. [Google Scholar]
- Yang, Q.; Yang, C.-C.; Lin, C.-H.; Jiang, H.-L. Metal-Organic-Framework-Derived Hollow N-Doped Porous Carbon with Ultrahigh Concentrations of Single Zn Atoms for Efficient Carbon Dioxide Conversion. Angew. Chem. Int. Ed. 2019, 58, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- Timoshenko, J.; Roldan Cuenya, B. In Situ/Operando Electrocatalyst Characterization by X-Ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Seifert, S.; Winans, R.E.; Li, T. Understanding Synthesis and Structural Variation of Nanomaterials through In Situ/Operando XAS and SAXS. Small 2022, 18, 2106017. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Lee, L.Y.S. Observing Electrocatalytic Processes via In Situ Electrochemical Scanning Tunneling Microscopy: Latest Advances. Chem. Asian J. 2022, 17, e202200384. [Google Scholar] [CrossRef]
- Ma, X.; Yu, Z.; Han, Y.; Song, X.; Sun, Q. In Situ Scanning Electron Microscopy Observation of Deformation and Fracture Behavior of Ti-3Zr-2Sn-3Mo-25Nb Alloy. Rare Met. 2012, 31, 318–322. [Google Scholar] [CrossRef]
- Petrii, O.A.; Safonova, T.Y. Electroreduction of Nitrate and Nitrite Anions on Platinum Metals: A Model Process for Elucidating the Nature of the Passivation by Hydrogen Adsorption. J. Electroanal. Chem. 1992, 331, 897–912. [Google Scholar] [CrossRef]
- de Groot, M.T.; Koper, M.T.M. The Influence of Nitrate Concentration and Acidity on the Electrocatalytic Reduction of Nitrate on Platinum. J. Electroanal. Chem. 2004, 562, 81–94. [Google Scholar] [CrossRef]
- Safonova, T.Y.; Petrii, O.A. Effect of Inorganic Cations on the Electroreduction of Nitrate Anions on Pt|Pt Electrodes in Sulfuric Acid Solutions. J. Electroanal. Chem. 1998, 448, 211–216. [Google Scholar] [CrossRef]
- Li, H.; Robertson, D.H.; Chambers, J.Q.; Hobbs, D.T. Electrochemical Reduction of Nitrate and Nitrite in Concentrated Sodium Hydroxide at Platinum and Nickel Electrodes. J. Electrochem. Soc. 1988, 135, 1154–1158. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Wang, B.; Lei, F.; Li, L.; Chan, A.W.M.; et al. Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Chambers, J.Q.; Hobbs, D.T. Electroreduction of Nitrate Ions in Concentrated Sodium Hydroxide Solutions at Lead, Zinc, Nickel and Phthalocyanine-Modified Electrodes. J. Appl. Electrochem. 1988, 18, 454–458. [Google Scholar] [CrossRef]
- Yang, J.; Sebastian, P.; Duca, M.; Hoogenboom, T.; Koper, M.T.M. pH Dependence of the Electroreduction of Nitrate on Rh and Pt Polycrystalline Electrodes. Chem. Commun. 2014, 50, 2148–2151. [Google Scholar] [CrossRef] [PubMed]
- Wasberg, M.; Horányi, G. Electrocatalytic Reduction of Nitric Acid at Rhodized Electrodes and Its Inhibition by Chloride Ions. Electrochim. Acta 1995, 40, 615–623. [Google Scholar] [CrossRef]
- Ureta-Zañartu, S.; Yáñez, C. Electroreduction of Nitrate Ion on Pt, Ir and on 70:30 Pt:Ir Alloy. Electrochim. Acta 1997, 42, 1725–1731. [Google Scholar] [CrossRef]
- Katsounaros, I.; Ipsakis, D.; Polatides, C.; Kyriacou, G. Efficient Electrochemical Reduction of Nitrate to Nitrogen on Tin Cathode at Very High Cathodic Potentials. Electrochim. Acta 2006, 52, 1329–1338. [Google Scholar] [CrossRef]
- Katsounaros, I.; Kyriacou, G. Influence of the Concentration and the Nature of the Supporting Electrolyte on the Electrochemical Reduction of Nitrate on Tin Cathode. Electrochim. Acta 2007, 52, 6412–6420. [Google Scholar] [CrossRef]
- Tada, K.; Shimazu, K. Kinetic Studies of Reduction of Nitrate Ions at Sn-Modified Pt Electrodes Using a Quartz Crystal Microbalance. J. Electroanal. Chem. 2005, 577, 303–309. [Google Scholar] [CrossRef]
- Shimazu, K.; Kawaguchi, T.; Tada, K. Preparation of Binary Metal Electrocatalysts by Self-Assembly of Precursor Ionic Species on Gold and Reduction of Nitrate Ions. J. Electroanal. Chem. 2002, 529, 20–27. [Google Scholar] [CrossRef]
- Shimazu, K.; Goto, R.; Tada, K. Electrochemical Reduction of Nitrate Ions on Tin-Modified Platinum and Palladium Electrodes. Chem. Lett. 2002, 31, 204–205. [Google Scholar] [CrossRef]
- Shimazu, K.; Goto, R.; Piao, S.; Kayama, R.; Nakata, K.; Yoshinaga, Y. Reduction of Nitrate Ions on Tin-Modified Palladium Thin Film Electrodes. J. Electroanal. Chem. 2007, 601, 161–168. [Google Scholar] [CrossRef]
- Casella, I.G.; Contursi, M. An Electrochemical and XPS Study of the Electrodeposited Binary Pd–Sn Catalyst: The Electroreduction of Nitrate Ions in Acid Medium. J. Electroanal. Chem. 2006, 588, 147–154. [Google Scholar] [CrossRef]
- da Cunha, M.C.P.M.; De Souza, J.P.I.; Nart, F.C. Reaction Pathways for Reduction of Nitrate Ions on Platinum, Rhodium, and Platinum−Rhodium Alloy Electrodes. Langmuir 2000, 16, 771–777. [Google Scholar] [CrossRef]
- Brylev, O.; Sarrazin, M.; Belanger, D.; Roue, L. Rhodium Deposits on Pyrolytic Graphite Substrate: Physico-Chemical Properties and Electrocatalytic Activity towards Nitrate Reduction in Neutral Medium. Appl. Catal. B Environ. 2006, 11, 243–253. [Google Scholar] [CrossRef]
- Brylev, O.; Sarrazin, M.; Roue, L.; Belanger, D. Nitrate and Nitrite Electrocatalytic Reduction on Rh-Modified Pyrolytic Graphite Electrodes. Electrochim. Acta 2007, 52, 6237–6247. [Google Scholar] [CrossRef]
- De, D.; Kalu, E.E.; Tarjan, P.P.; Englehardt, J.D. Kinetic Studies of the Electrochemical Treatment of Nitrate and Nitrite Ions on Iridium-Modified Carbon Fiber Electrodes. Chem. Eng. Technol. 2004, 27, 56–64. [Google Scholar] [CrossRef]
- Georgeaud, V.; Diamand, A.; Borrut, D.; Grange, D.; Coste, M. Electrochemical Treatment of Wastewater Polluted by Nitrate: Selective Reduction to N2 on Boron-Doped Diamond Cathode. Water Sci. Technol. 2011, 63, 206–212. [Google Scholar] [CrossRef]
- Ghazouani, M.; Akrout, H.; Bousselmi, L. Nitrate and Carbon Matter Removals from Real Effluents Using Si/BDD Electrode. Environ. Sci. Pollut. Res. 2017, 24, 9895–9906. [Google Scholar] [CrossRef]
- Ye, L.; Nayak-Luke, R.; Bañares-Alcántara, R.; Tsang, E. Reaction: “Green” Ammonia Production. Chem 2017, 3, 712–714. [Google Scholar] [CrossRef]
- Yang, J.; Qi, H.; Li, A.; Liu, X.; Yang, X.; Zhang, S.; Zhao, Q.; Jiang, Q.; Su, Y.; Zhang, L.; et al. Potential-Driven Restructuring of Cu Single Atoms to Nanoparticles for Boosting the Electrochemical Reduction of Nitrate to Ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. [Google Scholar] [CrossRef]
- Wu, K.; Sun, C.; Wang, Z.; Song, Q.; Bai, X.; Yu, X.; Li, Q.; Wang, Z.; Zhang, H.; Zhang, J.; et al. Surface Reconstruction on Uniform Cu Nanodisks Boosted Electrochemical Nitrate Reduction to Ammonia. ACS Mater. Lett. 2022, 4, 650–656. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Hu, X.; He, K.; Yu, Y.; Li, T.; Tu, Q.; Qian, X.; Yue, Q.; Wasielewski, M.R.; et al. Alternative Route for Electrochemical Ammonia Synthesis by Reduction of Nitrate on Copper Nanosheets. Appl. Mater. Today 2020, 19, 100620. [Google Scholar] [CrossRef]
- Bi, B.; Dong, A.-Q.; Shi, M.-M.; Sun, X.-F.; Li, H.-R.; Kang, X.; Gao, R.; Meng, Z.; Chen, Z.-Y.; Xu, T.-W.; et al. Efficient Ammonia Synthesis from Nitrate Catalyzed by Au/Cu with Enhanced Adsorption Ability. Small Struct. 2023, 4, 2200308. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Zheng, Q.-Z.; Lou, Y.-Y.; Zhao, K.-M.; Hu, S.-N.; Li, G.; Akdim, O.; Huang, X.-Y.; Sun, S.-G. Ampere-Level Current Density Ammonia Electrochemical Synthesis Using CuCo Nanosheets Simulating Nitrite Reductase Bifunctional Nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef] [PubMed]
- Chavez, M.E.; Biset-Peiró, M.; Murcia-López, S.; Morante, J.R. Cu2O–Cu@Titanium Surface with Synergistic Performance for Nitrate-to-Ammonia Electrochemical Reduction. ACS Sustain. Chem. Eng. 2023, 11, 3633–3643. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, J.; Dieckhöfer, S.; Varhade, S.; Brix, A.C.; Lielpetere, A.; Seisel, S.; Junqueira, J.R.C.; Schuhmann, W. Splicing the Active Phases of Copper/Cobalt-Based Catalysts Achieves High-Rate Tandem Electroreduction of Nitrate to Ammonia. Nat. Commun. 2022, 13, 1129. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Zhang, Z.; Sugiura, N. Efficient Electrochemical Reduction of Nitrate to Nitrogen Using Ti/IrO2–Pt Anode and Different Cathodes. Electrochim. Acta 2009, 54, 4600–4606. [Google Scholar] [CrossRef]
- Teng, W.; Bai, N.; Liu, Y.; Liu, Y.; Fan, J.; Zhang, W. Selective Nitrate Reduction to Dinitrogen by Electrocatalysis on Nanoscale Iron Encapsulated in Mesoporous Carbon. Environ. Sci. Technol. 2018, 52, 230–236. [Google Scholar] [CrossRef]
- Liu, Z.; Dong, S.; Zou, D.; Ding, J.; Yu, A.; Zhang, J.; Shan, C.; Gao, G.; Pan, B. Electrochemically Mediated Nitrate Reduction on Nanoconfined Zerovalent Iron: Properties and Mechanism. Water Res. 2020, 173, 115596. [Google Scholar] [CrossRef]
- Teng, W.; Fan, J.; Zhang, W. Iron-Catalyzed Selective Denitrification over N-Doped Mesoporous Carbon. ACS Appl. Mater. Interfaces 2020, 12, 28091–28099. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Smith, R.L.; Liu, L.; Qi, X. Synthesis of Self-Renewing Fe(0)-Dispersed Ordered Mesoporous Carbon for Electrocatalytic Reduction of Nitrates to Nitrogen. Sci. Total Environ. 2022, 836, 155640. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Chen, J.; Tai, Z.; Wang, L.; Liu, L.; Yang, J. Iron Nanoparticles Confined in Periodic Mesoporous Organosilicon as Nanoreactors for Efficient Nitrate Reduction. ACS Appl. Nano Mater. 2022, 5, 5149–5157. [Google Scholar] [CrossRef]
- Wang, J.; Ling, L.; Deng, Z.; Zhang, W. Nitrogen-Doped Iron for Selective Catalytic Reduction of Nitrate to Dinitrogen. Sci. Bull. 2020, 65, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Li, G.; Lei, Z.; Zhu, T.; Xue, Y.; Wei, C.; Feng, C. Highly Active and Durable Carbon Electrocatalyst for Nitrate Reduction Reaction. Water Res. 2019, 161, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, T.; Kan, M.; Song, D.; Jia, J.; Zhao, Y.; Qian, X. Binderless and Oxygen Vacancies Rich FeNi/Graphitized Mesoporous Carbon/Ni Foam for Electrocatalytic Reduction of Nitrate. Environ. Sci. Technol. 2020, 54, 13344–13353. [Google Scholar] [CrossRef]
- Sun, J.; Gao, W.; Fei, H.; Zhao, G. Efficient and Selective Electrochemical Reduction of Nitrate to N2 by Relay Catalytic Effects of Fe-Ni Bimetallic Sites on MOF-Derived Structure. Appl. Catal. B Environ. 2022, 301, 120829. [Google Scholar] [CrossRef]
- Niu, Q.; Yang, S.; Song, Z.; Liu, C.; Li, Z.; Wang, X.; Song, L. Fabrication of Efficient and Robust Fe0/Ni2P/CC Composite and the Employment for Electrochemical Reduction of Nitrate. J. Environ. Chem. Eng. 2021, 9, 106412. [Google Scholar] [CrossRef]
- Jonoush, Z.A.; Rezaee, A.; Ghaffarinejad, A. Electrocatalytic Nitrate Reduction Using Fe0/Fe3O4 Nanoparticles Immobilized on Nickel Foam: Selectivity and Energy Consumption Studies. J. Clean. Prod. 2020, 242, 118569. [Google Scholar] [CrossRef]
- Su, L.; Han, D.; Zhu, G.; Xu, H.; Luo, W.; Wang, L.; Jiang, W.; Dong, A.; Yang, J. Tailoring the Assembly of Iron Nanoparticles in Carbon Microspheres toward High-Performance Electrocatalytic Denitrification. Nano Lett. 2019, 19, 5423–5430. [Google Scholar] [CrossRef]
- Su, L.; Zhang, F.; Wang, L.; Fang, X.; Jiang, W.; Yang, J. Flexible Electrocatalysts: Interfacial-Assembly of Iron Nanoparticles for Nitrate Reduction. Chem. Commun. 2021, 57, 6740–6743. [Google Scholar] [CrossRef]
- Ni, F.; Ma, Y.; Chen, J.; Luo, W.; Yang, J. Boron-Iron Nanochains for Selective Electrocatalytic Reduction of Nitrate. Chin. Chem. Lett. 2021, 32, 2073–2078. [Google Scholar] [CrossRef]
- Hong, W.; Su, L.; Wang, J.; Jiang, M.; Ma, Y.; Yang, J. Boosting the Electrocatalysis of Nitrate to Nitrogen with Iron Nanoparticles Embedded in Carbon Microspheres. Chem. Commun. 2020, 56, 14685–14688. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Chen, J.; Zhang, H.; Zhang, W.; Yang, J. Fe/Fe3C Nanoparticle-Decorated N-Doped Carbon Nanofibers for Improving the Nitrogen Selectivity of Electrocatalytic Nitrate Reduction. J. Mater. Chem. A 2020, 8, 15853–15863. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical Reduction of Nitrate to Ammonia via Direct Eight-Electron Transfer Using a Copper–Molecular Solid Catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Q.; Liao, P.; Duan, W.; Liang, S.; Yan, Z.; Feng, C. Single-Atom Cu Catalysts for Enhanced Electrocatalytic Nitrate Reduction with Significant Alleviation of Nitrite Production. Small 2020, 16, 2004526. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Karamad, M.; Yong, X.; Huang, Q.; Cullen, D.A.; Zhu, P.; Xia, C.; Xiao, Q.; Shakouri, M.; Chen, F.-Y.; et al. Electrochemical Ammonia Synthesis via Nitrate Reduction on Fe Single Atom Catalyst. Nat. Commun. 2021, 12, 2870. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic Reduction of Nitrate on Copper Single Crystals in Acidic and Alkaline Solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Hu, Q.; Qin, Y.; Wang, X.; Wang, Z.; Huang, X.; Zheng, H.; Gao, K.; Yang, H.; Zhang, P.; Shao, M.; et al. Reaction Intermediate-Mediated Electrocatalyst Synthesis Favors Specified Facet and Defect Exposure for Efficient Nitrate–Ammonia Conversion. Energy Environ. Sci. 2021, 14, 4989–4997. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Wu, Z.-L.; Lin, C.-Y.; Huang, Y.-H.; Huang, C.-P. Manipulating the Crystalline Morphology and Facet Orientation of Copper and Copper-Palladium Nanocatalysts Supported on Stainless Steel Mesh with the Aid of Cationic Surfactant to Improve the Electrochemical Reduction of Nitrate and N2 Selectivity. Appl. Catal. B Environ. 2020, 273, 119053. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, Z.; Yu, J.; Guo, Y.; Mushtaq, M.A.; Ding, Y.; Song, Z.; Zhang, W.; Huang, X.; Li, Y.; et al. Constructing Cu-CuO Heterostructured Skin on Cu Cubes to Promote Electrocatalytic Ammonia Production from Nitrate Wastewater. J. Hazard. Mater. 2022, 439, 129653. [Google Scholar] [CrossRef]
- Bae, S.-E.; Gewirth, A.A. Differential Reactivity of Cu(111) and Cu(100) during Nitratereduction in Acid Electrolyte. Faraday Discuss. 2009, 140, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Wu, Z.-L.; Huang, Y.-H.; Huang, C.-P. Electrochemical Nitrate Reduction as Affected by the Crystal Morphology and Facet of Copper Nanoparticles Supported on Nickel Foam Electrodes (Cu/Ni). Chem. Eng. J. 2020, 383, 123157. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Electrochemical Reduction of Nitrate and Nitrite in Alkaline Media at CuNi Alloy Electrodes. Electrochim. Acta 2013, 89, 488–496. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gambirasi, A.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Hydrogen Evolution Assisted Electrodeposition of Porous Cu-Ni Alloy Electrodes and Their Use for Nitrate Reduction in Alkali. Electrochim. Acta 2014, 140, 337–344. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, R.; Shao, Q.; Zhu, T.; Huang, X. Oxygen Vacancies in Amorphous InOx Nanoribbons Enhance CO2 Adsorption and Activation for CO2 Electroreduction. Angew. Chem. Int. Ed. 2019, 58, 5609–5613. [Google Scholar] [CrossRef]
- Daiyan, R.; Tran-Phu, T.; Kumar, P.; Iputera, K.; Tong, Z.; Leverett, J.; Khan, M.H.A.; Asghar Esmailpour, A.; Jalili, A.; Lim, M.; et al. Nitrate Reduction to Ammonium: From CuO Defect Engineering to Waste NOx-to-NH3 Economic Feasibility. Energy Environ. Sci. 2021, 14, 3588–3598. [Google Scholar] [CrossRef]
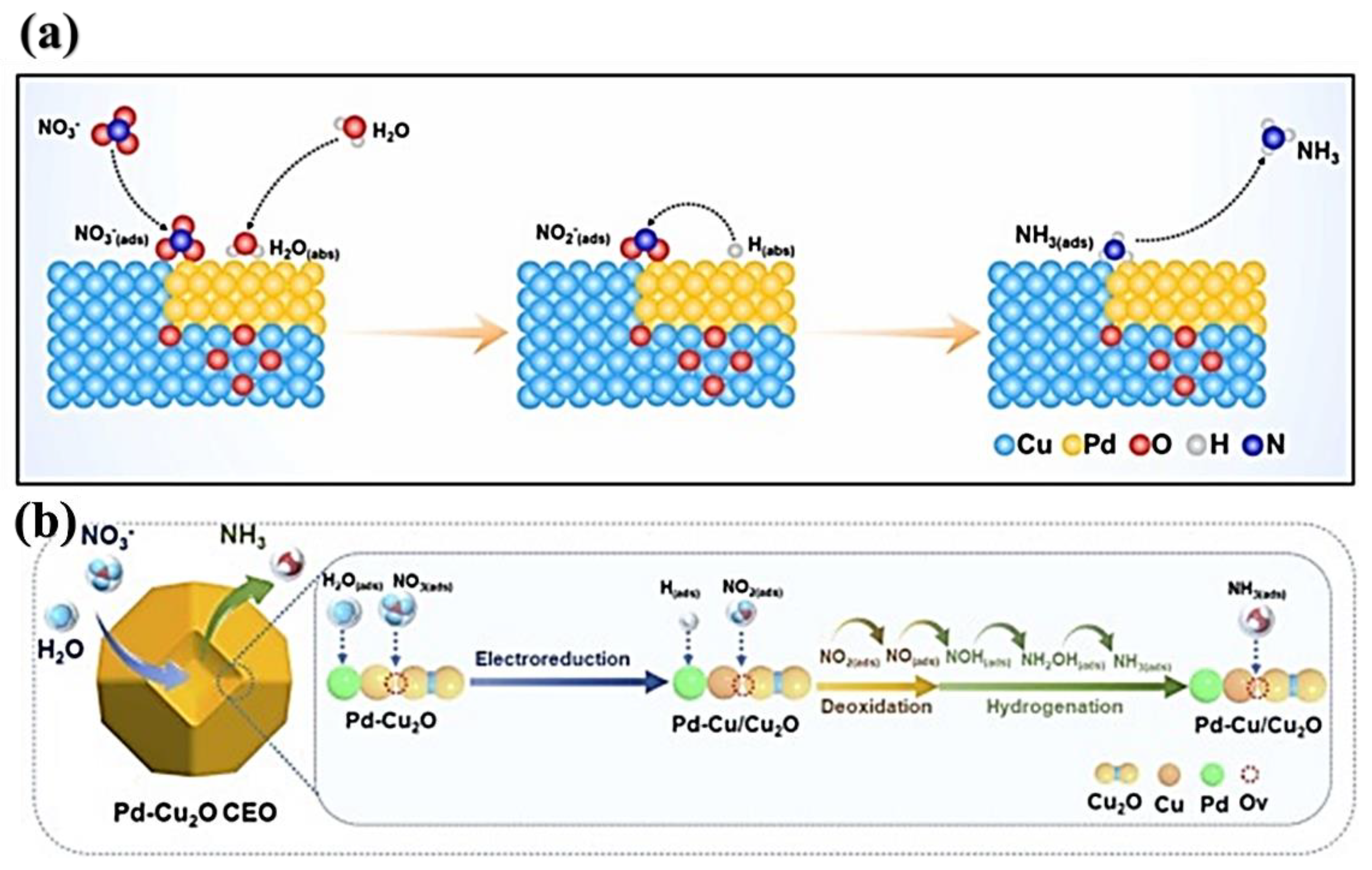
- Ren, T.; Yu, Z.; Yu, H.; Deng, K.; Wang, Z.; Li, X.; Wang, H.; Wang, L.; Xu, Y. Interfacial Polarization in Metal-Organic Framework Reconstructed Cu/Pd/CuOx Multi-Phase Heterostructures for Electrocatalytic Nitrate Reduction to Ammonia. Appl. Catal. B Environ. 2022, 318, 121805. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Ren, T.; Wang, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Ultralow-Content Pd in-Situ Incorporation Mediated Hierarchical Defects in Corner-Etched Cu2O Octahedra for Enhanced Electrocatalytic Nitrate Reduction to Ammonia. Appl. Catal. B Environ. 2022, 306, 121094. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Shuang, C.; Li, A. The Improvement on Total Nitrogen Removal in Nitrate Reduction by Using a Prepared CuO–Co3O4/Ti Cathode. Chemosphere 2020, 255, 126970. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L.; Jiao, X.; Li, P. Electrochemical Reduction of Nitrate on Different Cu-Zn Oxide Composite Cathodes. Int. J. Electrochem. Sci. 2017, 12, 4370–4383. [Google Scholar] [CrossRef]
- Öznülüer, T.; Özdurak, B.; Öztürk Doğan, H. Electrochemical Reduction of Nitrate on Graphene Modified Copper Electrodes in Alkaline Media. J. Electroanal. Chem. 2013, 699, 1–5. [Google Scholar] [CrossRef]
- Çirmi, D.; Aydın, R.; Köleli, F. The Electrochemical Reduction of Nitrate Ion on Polypyrrole Coated Copper Electrode. J. Electroanal. Chem. 2015, 736, 101–106. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhang, Z.; Mo, Z.; Wang, C.; Gao, S. Flower-like Open-Structured Polycrystalline Copper with Synergistic Multi-Crystal Plane for Efficient Electrocatalytic Reduction of Nitrate to Ammonia. Nano Energy 2022, 97, 107124. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Gupta, S.; Rivera, D.J.; Lambeets, S.V.; Pecaut, S.; Kim, J.Y.T.; Zhu, P.; Finfrock, Y.Z.; Meira, D.M.; et al. Efficient Conversion of Low-Concentration Nitrate Sources into Ammonia on a Ru-Dispersed Cu Nanowire Electrocatalyst. Nat. Nanotechnol. 2022, 17, 759–767. [Google Scholar] [CrossRef]
- Lubphoo, Y.; Chyan, J.; Grisdanurak, N.; Liao, C. Influence of Pd–Cu on Nanoscale Zero–Valent Iron Supported for Selective Reduction of Nitrate. J. Taiwan Inst. Chem. Eng. 2016, 59, 285–294. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Luo, H.; Chen, J.; Kuang, M.; Yang, J. Iron Nanoparticles Protected by Chainmail-structured Graphene for Durable Electrocatalytic Nitrate Reduction to Nitrogen. Angew. Chem. 2023, 135, e202217071. [Google Scholar] [CrossRef]
- Yan, C.; Liu, L. Oxidation of Gas Phase Ammonia via Accelerated Generation of Radical Species and Synergy of Photo Electrochemical Catalysis with Persulfate Activation by CuO-Co3O4 on Cathode Electrode. J. Hazard. Mater. 2020, 388, 121793. [Google Scholar] [CrossRef]
- Li, F.; Peng, X.; Liu, Y.; Mei, J.; Sun, L.; Shen, C.; Ma, C.; Huang, M.; Wang, Z.; Sand, W. A Chloride-Radical-Mediated Electrochemical Filtration System for Rapid and Effective Transformation of Ammonia to Nitrogen. Chemosphere 2019, 229, 383–391. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, L.; Liang, S.; Ye, J.; Yang, X.; Lei, Z.; Yan, Z.; Li, Y.; Wei, C.; Feng, C. Discovering the Importance of ClO• in a Coupled Electrochemical System for the Simultaneous Removal of Carbon and Nitrogen from Secondary Coking Wastewater Effluent. Environ. Sci. Technol. 2020, 54, 9015–9024. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Bai, J.; Li, J.; Li, L.; Zhou, T.; Chen, S.; Wang, J.; Rahim, M.; Guan, X.; et al. Efficient Degradation of N-Containing Organic Wastewater via Chlorine Oxide Radical Generated by a Photoelectrochemical System. Chem. Eng. J. 2020, 392, 123695. [Google Scholar] [CrossRef]
- Li, F.; Sun, L.; Liu, Y.; Fang, X.; Shen, C.; Huang, M.; Wang, Z.; Dionysiou, D.D. A ClO -Mediated Photoelectrochemical Filtration System for Highly-Efficient and Complete Ammonia Conversion. J. Hazard. Mater. 2020, 400, 123246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active Chlorine Mediated Ammonia Oxidation Revisited: Reaction Mechanism, Kinetic Modelling and Implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chen, R.; Wen, Y.; Li, Y.; Zhan, W.; Ma, F.; Jiang, X.; He, W.; Ni, H. Trace Amount of RuO2 Loaded on TiO2 Nanowires for Efficient Electrocatalytic Degradation of Ammonia Nitrogen in Wastewater. J. Alloys Compd. 2022, 928, 167058. [Google Scholar] [CrossRef]
- Hansen, H.A.; Man, I.C.; Studt, F.; Abild-Pedersen, F.; Bligaard, T.; Rossmeisl, J. Electrochemical Chlorine Evolution at Rutile Oxide (110) Surfaces. Phys. Chem. Chem. Phys. 2010, 12, 283–290. [Google Scholar] [CrossRef]
- Song, X.; Chen, X.; Zhang, S.; Wu, D. Treatment of High Chlorine-Containing Composting Leachate Biochemical Effluent by Ti/RuO2-IrO2 Anodic Electrochemical Oxidation: Optimization and Evolution of Pollutants. J. Environ. Chem. Eng. 2023, 11, 109674. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic Reduction of Nitrate: Fundamentals to Full-Scale Water Treatment Applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Mandal, P.; Yadav, M.K.; Gupta, A.K.; Dubey, B.K. Chlorine Mediated Indirect Electro-Oxidation of Ammonia Using Non-Active PbO2 Anode: Influencing Parameters and Mechanism Identification. Sep. Purif. Technol. 2020, 247, 116910. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Ai, H.; Chen, A.; Xu, L.; Labiadh, L.; Fu, M.-L.; Yuan, B. Construction of the Multi-Layer TiO2-NTs/Sb-SnO2/PbO2 Electrode for the Highly Efficient and Selective Oxidation of Ammonia in Aqueous Solution: Characterization, Performance and Mechanism. J. Environ. Chem. Eng. 2023, 11, 109834. [Google Scholar] [CrossRef]
- Romano, A.; Ortiz, I.; Urtiaga, A.M. Comprehensive Kinetics of Electrochemically Assisted Ammonia Removal in Marine Aquaculture Recirculating Systems. J. Electroanal. Chem. 2021, 897, 115619. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, T.; Wang, J.; Xia, L.; Xu, Q.; Zhou, B. Extremely Efficient Decomposition of Ammonia N to N2 Using ClO• from Reactions of HO• and HOCl Generated in Situ on a Novel Bifacial Photoelectroanode. Environ. Sci. Technol. 2019, 53, 6945–6953. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Mostafa, E.; Baltruschat, H. Electrogeneration of Inorganic Chloramines on Boron-Doped Diamond Anodes during Electrochemical Oxidation of Ammonium Chloride, Urea and Synthetic Urine Matrix. Water Res. 2019, 160, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, L.; Yang, F. Electro-Enhanced Chlorine-Mediated Ammonium Nitrogen Removal Triggered by an Optimized Catalytic Anode for Sustainable Saline Wastewater Treatment. Sci. Total Environ. 2021, 776, 146035. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Sun, W.; Zhu, L. The Impact of New Urbanization and Industrial Structural Changes on Regional Water Stress Based on Water Footprints. Sustain. Cities Soc. 2022, 79, 103686. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, J.; Qian, H.; Wang, H.; Ren, W.; Qu, W. Hydrogeochemical Characteristics and Processes of Groundwater in an over 2260 Year Irrigation District: A Comparison between Irrigated and Nonirrigated Areas. J. Hydrol. 2022, 606, 127437. [Google Scholar] [CrossRef]
- Yao, F.; Yang, Q.; Zhong, Y.; Shu, X.; Chen, F.; Sun, J.; Ma, Y.; Fu, Z.; Wang, D.; Li, X. Indirect Electrochemical Reduction of Nitrate in Water Using Zero-Valent Titanium Anode: Factors, Kinetics, and Mechanism. Water Res. 2019, 157, 191–200. [Google Scholar] [CrossRef]
- Beltrame, T.F.; Carvalho, D.; Marder, L.; Ulla, M.A.; Marchesini, F.A.; Bernardes, A.M. Comparison of Different Electrode Materials for the Nitrate Electrocatalytic Reduction in a Dual-Chamber Cell. J. Environ. Chem. Eng. 2020, 8, 104120. [Google Scholar] [CrossRef]
- Paul, S.; Adalder, A.; Ghorai, U.K. Progress of Electrocatalytic Urea Synthesis: Strategic Design, Reactor Engineering, Mechanistic Details and Techno-Commercial Study. Mater. Chem. Front. 2023, 7, 3820–3854. [Google Scholar] [CrossRef]
- Mao, Q.; Deng, K.; Yu, H.; Xu, Y.; Wang, Z.; Li, X.; Wang, L.; Wang, H. In Situ Reconstruction of Partially Hydroxylated Porous Rh Metallene for Ethylene Glycol-Assisted Seawater Splitting. Adv. Funct. Mater. 2022, 32, 2201081. [Google Scholar] [CrossRef]
- Avila, Y.; Acevedo-Peña, P.; Reguera, L.; Reguera, E. Recent Progress in Transition Metal Hexacyanometallates: From Structure to Properties and Functionality. Coord. Chem. Rev. 2022, 453, 214274. [Google Scholar] [CrossRef]
- Hoa, V.H.; Austeria, M.; Thi Dao, H.; Mai, M.; Kim, D.H. Dual-Phase Cobalt Phosphide/Phosphate Hybrid Interactions via Iridium Nanocluster Interfacial Engineering toward Efficient Overall Seawater Splitting. Appl. Catal. B Environ. 2023, 327, 122467. [Google Scholar] [CrossRef]
- Sun, J.; Li, J.; Li, Z.; Li, C.; Ren, G.; Zhang, Z.; Meng, X. Modulating the Electronic Structure on Cobalt Sites by Compatible Heterojunction Fabrication for Greatly Improved Overall Water/Seawater Electrolysis. ACS Sustain. Chem. Eng. 2022, 10, 9980–9990. [Google Scholar] [CrossRef]
- Fan, J.; Fu, C.; Liang, R.; Lv, H.; Fang, C.; Guo, Y.; Hao, W. Mild Construction of “Midas Touch” Metal–Organic Framework-Based Catalytic Electrodes for Highly Efficient Overall Seawater Splitting. Small 2022, 18, 2203588. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yu, C.; Wang, H.; Chen, L.; Huang, H.; Han, Y.; Wei, Q.; Qiu, J. A Robust & Weak-Nucleophilicity Electrocatalyst with an Inert Response for Chlorine Ion Oxidation in Large-Current Seawater Electrolysis. J. Energy Chem. 2024, 90, 486–495. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, D.; Peng, G.; Ma, Y.; Li, J.; Shi, J.; Peng, J.; Ding, L. Electrocatalytic Reduction of Nitrate in Water Using Cu/Pd Modified Ni Foam Cathode: High Nitrate Removal Efficiency and N2-Selectivity. Sep. Purif. Technol. 2020, 241, 116743. [Google Scholar] [CrossRef]
- Manzo-Robledo, A.; Lévy-Clément, C.; Alonso-Vante, N. The Interplay between Hydrogen Evolution Reaction and Nitrate Reduction on Boron-Doped Diamond in Aqueous Solution: The Effect of Alkali Cations. Electrochim. Acta 2014, 117, 420–425. [Google Scholar] [CrossRef]
- Fedurco, M.; Kedzierzawski, P.; Augustynski, J. Effect of Multivalent Cations upon Reduction of Nitrate Ions at the Ag Electrode. J. Electrochem. Soc. 1999, 146, 2569–2572. [Google Scholar] [CrossRef]
- Forero-Saboya, J.; Davoisne, C.; Dedryvère, R.; Yousef, I.; Canepa, P.; Ponrouch, A. Understanding the Nature of the Passivation Layer Enabling Reversible Calcium Plating. Energy Environ. Sci. 2020, 13, 3423–3431. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, R.; Zhang, C.; Wu, Z.; Ren, H.; Ng, H.Y. Critical Review on the Pulsed Electrochemical Technologies for Wastewater Treatment: Fundamentals, Current Trends, and Future Studies. Chem. Eng. J. 2024, 479, 147588. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, Z.; Yan, C.; Zhang, X.; Tian, Y.; Zhang, X.; Quan, X. Simultaneous Removal of Nitrogen and Refractory Organics from a Biologically Treated Leachate by Pulse Electrochemical Oxidation in a Multi-Channel Flow Reactor. ACS Omega 2021, 6, 25539–25550. [Google Scholar] [CrossRef]
- Taguchi, S.; Feliu, J.M. Electrochemical Reduction of Nitrate on Pt(S)[n(111)×(111)] Electrodes in Perchloric Acid Solution. Electrochim. Acta 2007, 52, 6023–6033. [Google Scholar] [CrossRef]
- Ding, J.; Li, W.; Zhao, Q.-L.; Wang, K.; Zheng, Z.; Gao, Y.-Z. Electroreduction of Nitrate in Water: Role of Cathode and Cell Configuration. Chem. Eng. J. 2015, 271, 252–259. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Nitrate Removal by a Paired Electrolysis on Copper and Ti/IrO2 Coupled Electrodes—Influence of the Anode/Cathode Surface Area Ratio. Water Res. 2010, 44, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Tang, Z.; Chen, B.; Zhu, C.; Tang, H.; Meng, G. Efficient Electrocatalytic Reduction of Nitrate to Nitrogen Gas by a Cubic Cu2O Film with Predominant (111) Orientation. Chem. Commun. 2022, 58, 3613–3616. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Zhang, Y.; Oturan, N.; Oturan, M.A. Non-Precious Co3O4-TiO2/Ti Cathode Based Electrocatalytic Nitrate Reduction: Preparation, Performance and Mechanism. Appl. Catal. B Environ. 2019, 254, 391–402. [Google Scholar] [CrossRef]
- Abdallah, R.; Geneste, F.; Labasque, T.; Djelal, H.; Fourcade, F.; Amrane, A.; Taha, S.; Floner, D. Selective and Quantitative Nitrate Electroreduction to Ammonium Using a Porous Copper Electrode in an Electrochemical Flow Cell. J. Electroanal. Chem. 2014, 727, 148–153. [Google Scholar] [CrossRef]
- Makover, J.; Hasson, D.; Semiat, R.; Shemer, H. Electrochemical Removal of Nitrate from High Salinity Waste Stream in a Continuous Flow Reactor. J. Environ. Chem. Eng. 2020, 8, 103727. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, L.; Yan, Z.; Lin, Z.; Lei, Z.; Zhang, Y.; Xu, H.; Dang, Z.; Wei, C.; Feng, C. Self-Activated Ni Cathode for Electrocatalytic Nitrate Reduction to Ammonia: From Fundamentals to Scale-Up for Treatment of Industrial Wastewater. Environ. Sci. Technol. 2021, 55, 13231–13243. [Google Scholar] [CrossRef]
- Rivera, F.F.; Pérez, T.; Castañeda, L.F.; Nava, J.L. Mathematical Modeling and Simulation of Electrochemical Reactors: A Critical Review. Chem. Eng. Sci. 2021, 239, 116622. [Google Scholar] [CrossRef]
- Oriol, R.; Nava, J.L.; Brillas, E.; Cornejo, O.M.; Sirés, I. Modeling the Electrocatalytic Nitrate Removal in a Rotating Cylinder Electrode Reactor. Sep. Purif. Technol. 2024, 340, 126714. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, Y.; Wen, K.; Pan, F.; Ma, H.; Niu, J.; Wang, C.; Zhao, J. Efficient and Selective Electrochemical Nitrate Reduction to N2 Using a Flow-Through Zero-Gap Electrochemical Reactor with a Reconstructed Cu(OH)2 Cathode: Insights into the Importance of Inter-Electrode Distance. Environ. Sci. Technol. 2024, 58, 4824–4836. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Qu, X.; Alvarez, P.J.J.; Chaplin, B.P.; Chen, W.; Crittenden, J.C.; Feng, Y.; Gao, G.; He, Z.; Hou, C.-H.; et al. Opportunities for Nanotechnology to Enhance Electrochemical Treatment of Pollutants in Potable Water and Industrial Wastewater—A Perspective. Environ. Sci. Nano 2020, 7, 2178–2194. [Google Scholar] [CrossRef]
- Qiu, W.; Liu, Y.; Xie, M.; Jin, Z.; Li, P.; Yu, G. Structural Engineering of Catalysts for Ammonia Electrosynthesis from Nitrate: Recent Advances and Challenges. EES. Catal. 2024, 2, 202–219. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Li, M.; Yu, Y.; Zhang, B. Nitrate Electroreduction: Mechanism Insight, in Situ Characterization, Performance Evaluation, and Challenges. Chem. Soc. Rev. 2021, 50, 6720–6733. [Google Scholar] [CrossRef] [PubMed]
- Shahid, U.B.; Siddharth, K.; Shao, M. Electrifying the Nitrogen Cycle: An Electrochemical Endeavor. Curr. Opin. Electrochem. 2021, 30, 100790. [Google Scholar] [CrossRef]
- McEnaney, J.M.; Blair, S.J.; Nielander, A.C.; Schwalbe, J.A.; Koshy, D.M.; Cargnello, M.; Jaramillo, T.F. Electrolyte Engineering for Efficient Electrochemical Nitrate Reduction to Ammonia on a Titanium Electrode. ACS Sustain. Chem. Eng. 2020, 8, 2672–2681. [Google Scholar] [CrossRef]
- Gao, J.; Shi, N.; Guo, X.; Li, Y.; Bi, X.; Qi, Y.; Guan, J.; Jiang, B. Electrochemically Selective Ammonia Extraction from Nitrate by Coupling Electron- and Phase-Transfer Reactions at a Three-Phase Interface. Environ. Sci. Technol. 2021, 55, 10684–10694. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A. The Use of Renewable Energies Driving Electrochemical Technologies for Environmental Applications. Curr. Opin. Electrochem. 2020, 22, 211–220. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Scialdone, O. (Eds.) Electrochemical Water and Wastewater Treatment; Butterworth-Heinemann: Oxford, UK; Cambridge, MA, USA, 2018; ISBN 978-0-12-813160-2. [Google Scholar]
- Lacasa, E.; Cotillas, S.; Saez, C.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Environmental Applications of Electrochemical Technology. What Is Needed to Enable Full-Scale Applications? Curr. Opin. Electrochem. 2019, 16, 149–156. [Google Scholar] [CrossRef]
- Millán, M.; Fernández-Marchante, C.M.; Lobato, J.; Cañizares, P.; Rodrigo, M.A. Management of Solar Energy to Power Electrochemical Wastewater Treatments. J. Water Process Eng. 2021, 41, 102056. [Google Scholar] [CrossRef]
- Kani, N.C.; Gauthier, J.A.; Prajapati, A.; Edgington, J.; Bordawekar, I.; Shields, W.; Shields, M.; Seitz, L.C.; Singh, A.R.; Singh, M.R. Solar-Driven Electrochemical Synthesis of Ammonia Using Nitrate with 11% Solar-to-Fuel Efficiency at Ambient Conditions. Energy Environ. Sci. 2021, 14, 6349–6359. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Al-Othman, A.; Kafiah, F.; Abdelsalam, E.; Almomani, F.; Alkasrawi, M. Environmental Impacts of Solar Photovoltaic Systems: A Critical Review of Recent Progress and Future Outlook. Sci. Total Environ. 2021, 759, 143528. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Rodrigues, T.P.; Chagas, A.L.S. The Impact of Wind Power on the Brazilian Labor Market. Renew. Sustain. Energy Rev. 2020, 128, 109887. [Google Scholar] [CrossRef]
- Strielkowski, W.; Civín, L.; Tarkhanova, E.; Tvaronavičienė, M.; Petrenko, Y. Renewable Energy in the Sustainable Development of Electrical Power Sector: A Review. Energies 2021, 14, 8240. [Google Scholar] [CrossRef]
- Shahsavari, A.; Akbari, M. Potential of Solar Energy in Developing Countries for Reducing Energy-Related Emissions. Renew. Sustain. Energy Rev. 2018, 90, 275–291. [Google Scholar] [CrossRef]
- Sun, Y.; Bai, S.; Wang, X.; Ren, N.; You, S. Prospective Life Cycle Assessment for the Electrochemical Oxidation Wastewater Treatment Process: From Laboratory to Industrial Scale. Environ. Sci. Technol. 2023, 57, 1456–1466. [Google Scholar] [CrossRef]
- Nath, S. Electrochemical Wastewater Treatment Technologies Through Life Cycle Assessment: A Review. ChemBioEng Rev. 2024, 11, e202400016. [Google Scholar] [CrossRef]
- Mosalpuri, M.; Li, W.; Mba Wright, M. Techno-Economic Analysis and Life Cycle Assessment of Hydroxylamine Eco-Manufacturing via Wastewater Electrochemical Reduction. ACS Sustain. Chem. Eng. 2023, 11, 13636–13645. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a Century of Ammonia Synthesis Changed the World. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Liu, H. Ammonia Synthesis Catalyst 100 Years: Practice, Enlightenment and Challenge. Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]





| Reduction Reaction | Standard Redox Potential |
|---|---|
| 2(aq) + 12 H+ + 10 e− ⇌ (g) + 6 H2O(l) | = + 1.25 V |
| (aq) + 10 H+ + 8 e− ⇌ (aq) + 3 H2O(l) | = + 1.20 V |
| 2(aq) + 10 H+ + 8 e− ⇌ O(g) + 5 H2O(l) | = + 1.12 V |
| (aq) + 4 H+ + 3 e− ⇌ NO(g) + 2 H2O(l) | = + 0.96 V |
| (aq) + 10 H+ + 8 e− ⇌ (g) + 4 H2O(l) | = + 0.90 V |
| (aq) + 2 H+ + 2 e− ⇌ (aq) + H2O(l) | = + 0.89 V |
| (aq)− + 9 H+ + 8e− ⇌ (aq) + 3H2O(l) | = + 0.82 V |
| (aq) + 2 H+ + e− ⇌ (g) + H2O(l) | = + 0.80 V |
| (aq) + 7 H+ + 6 e− ⇌ (aq) + 2 H2O(l) | = + 0.67 V |
| (aq) + 7 H2O + 8 e− ⇌ (aq) + 9(l) | = −0.12 V |
| (aq) + 10 H2O + 14 e− ⇌ (aq) + 16(l) | = −0.15 V |
| Electrode Materials | Free Energy Diagram | Rate Determining Steps | Refs. |
|---|---|---|---|
| Cu catalyst anchored on porous N-doped carbon. (Cu−NC) | 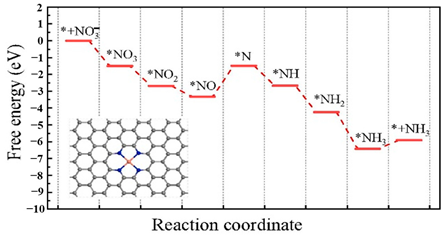 | *NO to *N | [52] |
| CuO(111) surface with two oxygen vacancy (OVs) (CuO−2OVs) | 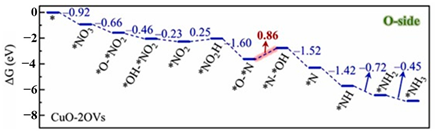 | The protonation of *O-*N to*N-*OH | [53] |
| (3 1 1) facet |  | *NO to *NOH | [54] |
| (2 2 0) facet | 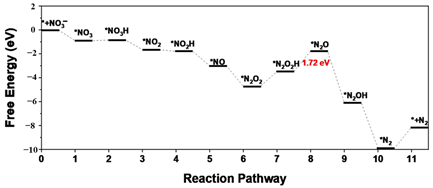 | * to * | [54] |
| Au (111) (in yellow), Au (331) (in blue), Pd modified Au(331) (in red). |  | (ads) → (ads) + O(ads) Au (111) = 0.72 eV Au (331) = 0. 39 eV Pd-Au (331) = 0.37 eV (ads) → (ads) + O(ads) Au (111) = 1.74 eV Au (331) = 0.83 eV Pd-Au (331) = 0.23 eV | [55] |
| nanoporous |  | * to * | [56] |
| Pd (in blue), (in red). |  | *N to *NH Pd = 3.38 eV * to * = 2.99 eV | [57] |
| Cu NWAs (in red), NWAs (in yellow) | 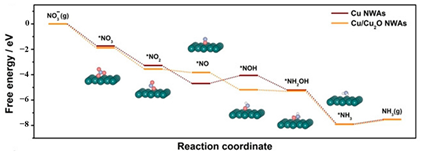 | The protonation of *O-*N to*N-*OH | [58] |
| (NCS) (in blue) nanospheres with sulfur-rich vacancies (NCS-2) (in red) | 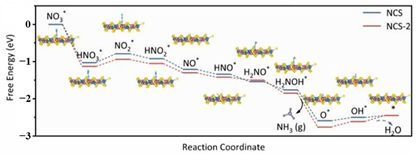 | * to * NCS-2 = 0.24 eV NCS-2 = 0.19 eV | [59] |
| The incorporation of indium in sulfur-doped graphene (In-S-G) | 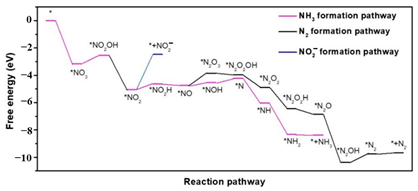 | formation pathway *NO to * (0.90 eV) production * to * (0.63 eV) | [60] |
| Boron-doped copper nanowire electrocatalyst (B-Cu) |  | The hydrogenation of *NO to form H*NO | [61] |
| Cobalt manganese spinel nanoparticles ( | 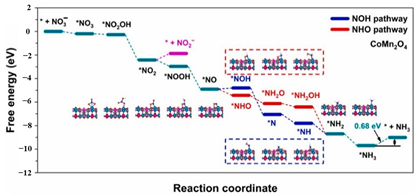 | The desorption of * | [62] |
| Cobalt manganese spinel nanoparticles embedded in multichannel carbon fibers () | 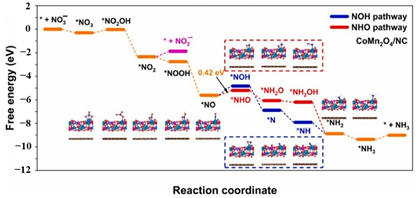 | *NO-to-*NHO | [62] |
| Iron coordinated with nitrogen on an ordered mesoporous carbon framework (Meso-Fe-N-C) | 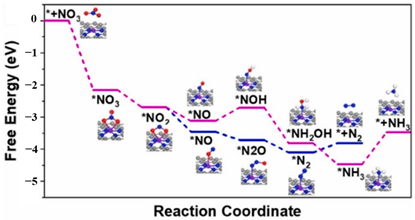 | formation pathway desorption production *NO-to-*NHO | [63] |
| Characterization Techniques | Detected Products and Intermediates | Principles | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|
| Shell isolated nanoparticle enhanced Raman spectroscopy (SHINERS) | All the intermediates adsorbed on different faces of catalyst material. | The principle is based on measuring the inelastic scattering of light caused by molecular vibrations involving changes in the polarization of the molecule. It consisted of a gold nanoparticle with an insulating thin SiO2 shell (Au/SiO2) to eliminate the interference of deposited Au or other substrates (Ag, Au), which have a surface plasmon resonance effect, and to inhibit the signal from the analytes adsorbed on the Au core. | High sensitivity, rapid, less destructive, and noninvasive technique. | It cannot be used for the detection of atomic cations and anions directly, complicated process, and has a weak pH tolerance. | [102,103] |
| Fourier-transformed infrared spectroscopy (FT-IR) | All intermediates formed at the surface of the electrode. | The incident infrared ray is guided by the prism and optical stage toward the chemical bonds of intermediates formed at the surface of the electrode, which was then diffused and reflected in the detector. The FT-IR measurements were performed on a spectrometer equipped with a liquid-nitrogen-cooled detector. | Rapid determination of adsorbed intermediates, applied in wide concentration ranges, nondestructive, and repeatable. | FTIR instruments have only a single beam. | [104,105,106] |
| X-Ray Absorption Spectroscopy | Active sites at the surface of the electrode. | The method involves selecting the desired energy of X-ray radiation using a monochromator and measuring the resulting XAS spectrum, which is composed of two parts: the X-ray absorption near-edge structure (XANES) and the extended X-ray absorption fine structure (EXAFS). The XANES spectrum provides information on the electronic structure of the element being studied, while the EXAFS spectra give information on the local geometry of the atom. This technique is well suited for identifying active sites in multi-element systems and can measure elements at low concentrations due to their high flux properties. | No crystal and ordered structure is needed for the sample additionally the method works for single-atom materials too. | XAS data represents an average of the entire sample and Soft XAS requires an ultra-high or high vacuum environment. XAS may not provide detailed information about specific regions of the sample. | [107,108,109] |
| Electrochemical Scanning Tunneling Microscopy | surface morphology | In situ, electrochemical scanning tunneling microscopy (EC-STM) is a form of scanning probe microscopy that uses a metal needle tip and an electrochemical control circuit to acquire high-resolution images of a sample surface. It operates on the quantum tunneling effect and can work in various environments, including ultra-high vacuum and liquid environments. | Applicable to a wide range of environments, Wide working temperature range, and high resolution. | Damage risk in constant height mode on undulating surfaces, cannot detect bulk structure information of the sample, cannot directly observe insulator materials, and the resolution of the image to the real surface is limited if the sample surface is covered with a conductive layer. | [110,111] |
| Cathode | Ammonia Production | Nitrogen Selectivity | Refs. | ||
|---|---|---|---|---|---|
| Conditions | Performance | Conditions | Performance | ||
| Copper-Based Electrocatalysts | |||||
| Cu Single Atom Gel (SAG) | 0.1 M phosphate buffer solution (PBS), pH 7, 20 mM KNO3, and Overpotential −0.9 V vs. Reference Hydrogen Electrode (RHE) | yield rate: 0.53 mg cm−2 h−1; FE: 95.3% | - | - | [101] |
| Cu-N-C SAC Copper into porous nitrogen-doped carbon | 0.1 M KOH, 0.1 M KNO3, and Overpotential − 1.00 V vs. RHE | yield rate: 4.5 mg cm−2 h−¹; FE: 84.7% | - | - | [135] |
| Cu nanodisks (111) | 0.1 M KOH, 10 mM KNO3, 10 mM KNO2 and Overpotential −0.5 V vs. RHE | yield rate: 2.18 mg mgcat−1 h−¹; FE: 81.1% | [136] | ||
| Cu nanosheets | 0.1 M KOH 170 ppm NO3—N and Overpotential −0.15 V vs. RHE | production rate: 0.39 mg mgcat−1 h−¹; FE: 99.7% | - | - | [137] |
| Au/Cu | 0.5 M NaNO3, 1 M NaOH, and Overpotential −0.7 V vs. RHE | yield rate: 73.4 mg cm−2 h−1; FE: 98.02% | - | - | [138] |
| Cu50Co50 | 1 M KOH, 100 mM KNO3, and Overpotential −0.2 V vs. RHE | yield rate: 81.7 mg cm−2 h−¹; FE: 100 ± 1% | - | - | [139] |
| Cu/Cu2O | 0.5 M Na2SO4 14.3 mM and Overpotential −0.85 V vs. RHE | production rate: 4.088 mg cm−2 h−1; FE: 95.8% | - | - | [58] |
| Cu2O-Cu/Ti | 0.1 M KNO3, 1 M KOH, and Overpotential 0.5 V vs. RHE | yield rate: 4.76 mg cm−2 h−1; FE: 92% | - | - | [140] |
| Cu/CuOx/Co/CoO | 0.1 M , pH 13, 0.1 M KNO3, and Overpotential −0.175 V RHE | yield rate: 19.94 mg cm−2 h−1; FE: 93.3% | - | - | [141] |
| Iron-based electrocatalysts | |||||
| Fe | - | - | 3.5 mM Na2SO4 + 7 mM NaNO3 electrolysis duration of 3 h. | 87% nitrate reduction efficiency with 100% nitrogen selectivity. | [142] |
| nZVI@OMC mesoporous carbon supported nanoscale zerovalent iron | - | - | 50 mg/L – N;0.02 M NaCl and 24 h of electrolysis. | 65% nitrate conversion with 74% nitrogen selectivity. | [143] |
| nZVI@D201 polymeric beads supported by nZVI | - | - | 50 mg/L –N; 1.0 M NaCl and 60 h of electrolysis. | 80% nitrate conversion with 95% nitrogen selectivity. | [144] |
| FeNC/MC Iron over N-doped mesoporous Carbon | - | - | 100 mg/L –N; 0.1 M Na2SO4 and 24 h of electrolysis. | 87% nitrate conversion with 81% nitrogen selectivity. | [145] |
| Fe#OMC Fe(0)-dispersed ordered mesoporous carbon | - | - | 50 mg/L –N; 0.1 M Na2SO4; 0.02 M NaCl and 24 h of electrolysis. | 86.9% nitrate conversion with 100% nitrogen selectivity. | [146] |
| Fe@PMO Iron Nanoparticles Confined in Periodic Mesoporous Organosilicon | - | - | 100 mg/L –N; 0.02 M Na2SO4; 0.02 M NaCl and 24 h of electrolysis. | 90% nitrate conversion with 99% nitrogen selectivity. | [147] |
| FeN-NC N-doped Fe nanoparticles | - | - | 100 mg/L –N; 0.1 M Na2SO4; 0.02 M NaCl and 24 h of electrolysis. | 91% nitrogen selectivity | [148] |
| Fe@N-C N-doped graphitic carbon-encapsulated iron nanoparticles | - | - | 50 mg/L –N; 1.0 g/L NaCl and 24 h of electrolysis. | 83% nitrate conversion with 100% nitrogen selectivity | [149] |
| FeNi/g-mesoC/NF binderless FeNi/graphitized mesoporous carbon on Ni Foam | - | - | 50 mg/L –N; 0.05 M Na2SO4; 0.02 M NaCl and 24 h of electrolysis. | 75% nitrate conversion with 100% nitrogen selectivity | [150] |
| Fe3Ni-N-C Fe/Ni bimetallic nitrogen-doped porous carbon | - | - | 100 ppm -N; 0.1 M Na2SO4 and 0.5 h of electrolysis. | 97.9% nitrate conversion with 99.3% nitrogen selectivity. | [151] |
| Fe0/Ni2P/CC | - | - | 15 mg/L –N; 0.3 M Na2SO4 and 4 h of electrolysis. | 89.81% nitrate conversion with 95.55% nitrogen selectivity. | [152] |
| Ni-Fe0@Fe3O4 Fe0@Fe3O4 nanoparticles immobilized on nickel foam | - | - | 0.5 g/L Cl-, 50 mg/L , Current density of 5 mA/cm2, electrolysis duration of 4 h, and pH of 6.2. | 90.19% nitrate conversion with 88.09% nitrogen selectivity. | [153] |
| CL-Fe@C Iron Nanoparticles in Carbon Microspheres | - | - | 100 mg/L –N; 0.02 M NaCl and 48 h of electrolysis. | 54% nitrate conversion with 98% nitrogen selectivity. | [154] |
| CC/Fe@C carbon-coated iron nanoparticles on a functionalized carbon cloth | - | - | 50 mg/L –N; 0.01 M Na2SO4; 0.01 M NaCl and 24 h of electrolysis. | 92% nitrate conversion with 82% nitrogen selectivity. | [155] |
| B-Fe NCs boron-iron nanochains | - | - | 100 mg/L –N; 0.02 M Na2SO4; 0.02 M NaCl and 24 h of electrolysis. | 80% nitrate conversion with 99% nitrogen selectivity. | [156] |
| Fe@C-1 iron nanoparticles uniformly embedded in the carbon microsphere | - | - | 100 mg/L –N; 0.02 M NaCl and 48 h of electrolysis. | 75.9% nitrate conversion with 98% nitrogen selectivity | [157] |
| Fe/Fe3C-NCNF-2 Fe/Fe3C nanoparticle-decorated N-doped carbon nanofibers | 100 mg/L –N; 0.02 M NaCl and 24 h of electrolysis. | 95% nitrate conversion with 100% nitrogen selectivity. | [158] | ||
| Influence Factors | Descriptions | Refs. |
|---|---|---|
| Initial chloride concentration | high initial concentration of chlorides increases the production of active chlorine and , thereby enhancing Ammonia oxidation | [185,190,191] |
| pH | pH decreases during the process. Acidic and neutral media are preferable for ammonia oxidation. Alkaline media decrease ammonia oxidation efficiency | [185,192,193] |
| Current density | Increasing current density improves ammonia oxidation efficiency (rule applied for lower current density than 37.5 mA·cm−2) | [186,192] |
| Ammonia concentration | High ammonia in solution inhibits the formation of active chlorine and | [194,195] |
| Temperature | Increasing temperature promotes the oxidation of ammonia. However, at temperatures higher than 55 °C the efficiency decreases due to the lose of active chlorine. | [193] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Outaleb, H.; Kouzbour, S.; Audonnet, F.; Vial, C.; Gourich, B. Electrocatalytic Nitrate Reduction for Brackish Groundwater Treatment: From Engineering Aspects to Implementation. Appl. Sci. 2024, 14, 8986. https://doi.org/10.3390/app14198986
Outaleb H, Kouzbour S, Audonnet F, Vial C, Gourich B. Electrocatalytic Nitrate Reduction for Brackish Groundwater Treatment: From Engineering Aspects to Implementation. Applied Sciences. 2024; 14(19):8986. https://doi.org/10.3390/app14198986
Chicago/Turabian StyleOutaleb, Hamza, Sanaa Kouzbour, Fabrice Audonnet, Christophe Vial, and Bouchaib Gourich. 2024. "Electrocatalytic Nitrate Reduction for Brackish Groundwater Treatment: From Engineering Aspects to Implementation" Applied Sciences 14, no. 19: 8986. https://doi.org/10.3390/app14198986
APA StyleOutaleb, H., Kouzbour, S., Audonnet, F., Vial, C., & Gourich, B. (2024). Electrocatalytic Nitrate Reduction for Brackish Groundwater Treatment: From Engineering Aspects to Implementation. Applied Sciences, 14(19), 8986. https://doi.org/10.3390/app14198986







