Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review
Abstract
1. Introduction
2. Theoretical Background
Kinetic Model
- Step (a) adsorption of a gaseous component,
- Step (b) dissociation of the gaseous molecule and transfer of electrons,
- Step (c) nucleation and growth of crystals,
- Step (d) diffusion and transport of cations, anions, and electrons through the oxide layer.
- Step (a) diffusion of fluid reactants through the fluid film surrounding the solid.
- Step (b) diffusion of the fluid reagents through the porous solid layer,
- Step (c) adsorption of the fluid reagents on the surface of the solid reagent,
- Step (d) chemical reaction with the solid surface,
- Step (e) desorption of the fluid products from the solid reaction surface,
- Step (f) diffusion of the product far from the reaction surface through the porous surface, the solid media, and the fluid film surrounding the solid.
3. Mechanisms of High-Temperature Oxidation
4. Effect of pH on Oxidation and Temperature
5. Fluidized Bed Reactors for Thermochemical Water Splitting and Their Materials
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Budama, V.K.; Duarte, J.P.R.; Roeb, M.; Sattler, C. Potential of Solar Thermochemical Water-Splitting Cycles: A Review. Sol. Energy 2023, 249, 353–366. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.; Song, H.; Zheng, K.; Wang, J.; Wang, H.; Kong, H. A Review of Solar Thermochemical Cycles for Fuel Production. Appl. Energy 2024, 357, 122499. [Google Scholar] [CrossRef]
- Siegel, N.P.; Miller, J.E.; Ermanoski, I.; Diver, R.B.; Stechel, E.B. Factors Affecting the Efficiency of Solar Driven Metal Oxide Thermochemical Cycles. Ind. Eng. Chem. Res. 2013, 52, 3276–3286. [Google Scholar] [CrossRef]
- Monnerie, N. Green Hydrogen Production at DLR; German Aerospace Center: Cologne, Germany, 2023. [Google Scholar]
- Poudel, M.B.; Lohani, P.C.; Acharya, D.; Kandel, D.R.; Kim, A.A.; Yoo, D.J. MOF Derived Hierarchical ZnNiCo-LDH on Vapor Solid Phase Grown CuxO Nanowire Array as High Energy Density Asymmetric Supercapacitors. J. Energy Storage 2023, 72, 108220. [Google Scholar] [CrossRef]
- Elayappan, V.; Shanmugam, R.; Chinnusamy, S.; Yoo, D.J.; Mayakrishnan, G.; Kim, K.; Noh, H.S.; Kim, M.K.; Lee, H. Three-Dimensional Bimetal TMO Supported Carbon Based Electrocatalyst Developed via Dry Synthesis for Hydrogen and Oxygen Evolution. Appl. Surf. Sci. 2020, 505, 144642. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Song, J.; Lee, S. Photoelectrochemical Device Designs toward Practical Solar Water Splitting: A Review on the Recent Progress of BiVO4 and BiFeO3 Photoanodes. Appl. Sci. 2018, 8, 1388. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2022; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Noda, S.; Yamaguchi, Y. Estimation of Surface Iron Oxide Abundance with Suppression of Grain Size and Topography Effects. Ore Geol. Rev. 2017, 83, 312–320. [Google Scholar] [CrossRef]
- Whitney, W.R. The Corrosion of Iron. J. Am. Chem. Soc. 1903, 25, 394–406. [Google Scholar] [CrossRef]
- Dunn, J.S. The High Temperature Oxidation of Metals. Proc. R. Soc. London. Ser. A Contain. Pap. A Math. Phys. Character 1926, 111, 203–209. [Google Scholar] [CrossRef]
- Tuck, C.W.; Odgers, M.; Sachs, K. The oxidation of iron at 950 °C in oxygen/water vapour mixtures. Corros. Sci. 1969, 9, 271–285. [Google Scholar] [CrossRef]
- Harikrishna, R.B.; Sharma, S.; Deka, H.; Sundararajan, T.; Rao, G.R. Thermochemical Production of Green Hydrogen Using Ferrous Scrap Materials. Int. J. Hydrogen Energy 2024, 52, 1488–1497. [Google Scholar] [CrossRef]
- Nakamura, T. Hydrogen Production from Water Utilizing Solar Heat at High Temperatures. Sol. Energy 1977, 19, 467–475. [Google Scholar] [CrossRef]
- Steinfeld, A.; Sanders, S.; Palumbo, R. Design Aspects of Solar Thermochemical Engineering-a Case Study: Two-Step Water-Splitting Cycle Using the Fe3O4/FeO Redox System. Sol. Energy 1999, 65, 43–53. [Google Scholar] [CrossRef]
- Lede, J.; Lapicque, F.; Villermaux, J. Production of Hydrogen by Direct Thermal Decomposition of Water. Int. J. Hydrogen Energy 1983, 8, 675–679. [Google Scholar] [CrossRef]
- Fernandez Saavedra, R. Bibliographic Review about Solar Hydrogen Production Through Thermochemical Cycles. In Revision Bibliografica sobre la Produccion de Hidrogeno Solar Mediante Ciclos Termoquimicos; CIEMAT: Madrid, Spain, 2008. [Google Scholar]
- Scheffe, J.R.; Steinfeld, A. Oxygen Exchange Materials for Solar Thermochemical Splitting of H2O and CO2: A Review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Perez, N. Electrochemistry and Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Etievant, C. Solar Energy Materials Solar High-Temperature Direct Water Splitting a Review of Experiments in France. Sol. Energy Mater. 1991, 24, 413–440. [Google Scholar] [CrossRef]
- Meredig, B.; Wolverton, C. First-Principles Thermodynamic Framework for the Evaluation of Thermochemical H2O-Or CO2-Splitting Materials. Phys. Rev. B Condens. Matter Mater. Phys. 2009, 80, 245119. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N. Thermochemical Cycles for High-Temperature Solar Hydrogen Production. Chem. Rev. 2007, 107, 4048–4077. [Google Scholar] [CrossRef]
- Barustan, M.I.A.; Jung, S.-M. Morphology of Iron and Agglomeration Behaviour during Reduction of Iron Oxide Fines. Met. Mater. Int. 2019, 25, 1083–1097. [Google Scholar] [CrossRef]
- Gao, Z.; Fu, F.; Niu, L.; Jin, M.; Wang, X. Effect of Support on Hydrogen Generation over Iron Oxides in the Chemical Looping Process. RSC Adv. 2021, 11, 37552–37558. [Google Scholar] [CrossRef] [PubMed]
- Young, J. Effects of Water Vapour on Oxidation. Corros. Ser. 2008, 1, 455–495. [Google Scholar]
- Saunders, S.R.J.; Monteiro, M.; Rizzo, F. The Oxidation Behaviour of Metals and Alloys at High Temperatures in Atmospheres Containing Water Vapour: A Review. Prog. Mater. Sci. 2008, 53, 775–837. [Google Scholar] [CrossRef]
- Belton, G.R.; Richardson, F.D. A Volatile Iron Hydroxide. Trans. Faraday Soc. 1962, 58, 1562–1572. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen Production via a Two-Step Water Splitting Thermochemical Cycle Based on Metal Oxide—A Review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Arshad, F.; Hassan, I.U.; Tabook, M.A.; Pedram, M.Z.; Mustaqeem, M.; Tabassum, H.; Ahmed, W.; Rezakazemi, M. Thermocatalytic Hydrogen Production through Decomposition of Methane-A Review. Front. Chem. 2021, 9, 736801. [Google Scholar] [CrossRef]
- Robie, R.A.; Hemingway, B.S. Thermodynamic Properties of Minerals and Related Substances at 298.15 K and 1 Bar (105 Pascals) Pressure and at Higher Temperatures (No. 2131); U.S. G.P.O.: Washington, DC, USA, 1995.
- Barin, I.; Platzhi, G. Thermochemical Data of Pure Substances; VCh: Weinheim, Germany, 1989; Volume 304, ISBN 3527287450. [Google Scholar]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-Driven Structural and Thermodynamic Complexity in Iron Oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef]
- Svoboda, K.; Slowinski, G.; Rogut, J.; Baxter, D. Thermodynamic Possibilities and Constraints for Pure Hydrogen Production by Iron Based Chemical Looping Process at Lower Temperatures. Energy Convers. Manag. 2007, 48, 3063–3073. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. High-Temperature Corrosion Resistance. JOM 1987, 39, 28–31. [Google Scholar] [CrossRef]
- Jeong, M.H.; Lee, D.H.; Bae, J.W. Reduction and Oxidation Kinetics of Different Phases of Iron Oxides. Int. J. Hydrogen Energy 2015, 40, 2613–2620. [Google Scholar] [CrossRef]
- Schuetze, M. Fundamentals of High Temperature Corrosion; Materials Science and Technology: A Comprehensive Treatment: Corrosion and Environmental Degradation; Wiley: Hoboken, NJ, USA, 2000; Volume I+II, pp. 67–130. [Google Scholar] [CrossRef]
- Kodama, T.; Bellan, S.; Gokon, N.; Cho, H.S. Particle Reactors for Solar Thermochemical Processes. Sol. Energy 2017, 156, 113–132. [Google Scholar] [CrossRef]
- Gokon, N.; Mataga, T.; Kondo, N.; Kodama, T. Thermochemical Two-Step Water Splitting by Internally Circulating Fluidized Bed of NiFe2O4 Particles: Successive Reaction of Thermal-Reduction and Water-Decomposition Steps. Int. J. Hydrogen Energy 2011, 36, 4757–4767. [Google Scholar] [CrossRef]
- Palumbo, R.; Diver, R.B.; Larson, C.; Coker, E.N.; Miller, J.E.; Guertin, J.; Schoer, J.; Meyer, M.; Siegel, N.P. Solar Thermal Decoupled Water Electrolysis Process I: Proof of Concept. Chem. Eng. Sci. 2012, 84, 372–380. [Google Scholar] [CrossRef]
- Genescá, J.; Meas, Y.; Rodríguez, F.; Mendoza, J.; Durán, R.; Uruchurtu, J.; González, J.G. Técnicas Electroquímicas Para el Control y Estudio de la Corrosión; Faculty of Chemistry, UNAM: Mexico City, Mexico, 2002; Volume 244. [Google Scholar]
- Bircumshaw, L.L.; Riddiford, A.C. Transport Control in Heterogeneous Reactions. Q. Rev. Chem. Soc. 1952, 6, 157–185. [Google Scholar] [CrossRef]
- Hougen, O.A. Diffusion and Heat Exchange in Chemical Kinetics. J. Am. Chem. Soc. 1956, 78, 885–886. [Google Scholar] [CrossRef]
- Wen, C.Y. Noncatalytic Heterogeneous Solid-Fluid Reaction Models. Ind. Eng. Chem. 1968, 60, 34–54. [Google Scholar] [CrossRef]
- Surman, P.L. The oxidation of iron at controlled oxygen partial pressures. hydrogen/water vapour. Corros. Sci. 1973, 13, 113–124. [Google Scholar] [CrossRef]
- Park, H.C.; Sakai, Y.; Kimura, S.; Tone, S.; Otake, T. A Rate Analysis for Oxidation of Porous Reduced Iron with Water Vapor. J. Chem. Eng. Jpn. 1984, 17, 395–399. [Google Scholar] [CrossRef]
- Donovan, R.J.; Berra, Y. External Diffusion Effects on Heterogeneous Reactions. In Elements of Chemical Reaction Engineering; Pearson: London, UK, 1999; pp. 686–738. [Google Scholar]
- Levenspiel, O. Ingeniería de Las Reacciones Químicas, 3rd ed.; Editorial Limusa Wiley: Mexico City, Mexico, 2010. [Google Scholar]
- Klaewkla, R.; Arend, M.; Hoelderich, W.F. A Review of Mass Transfer Controlling the Reaction Rate in Heterogeneous Catalytic Systems; INTECH Open Access Publisher: Rijeka, Croatia, 2011; Volume 5. [Google Scholar]
- Wen, C.Y.; Wang, S.C. Thermal and Diffusional Effects in Noncatalytic Solid Gas Reactions. Ind. Eng. Chem. 1970, 62, 30–51. [Google Scholar] [CrossRef]
- Valipour, M.S.; Saboohi, Y. Modeling of Multiple Noncatalytic Gas-Solid Reactions in a Moving Bed of Porous Pellets Based on Finite Volume Method. Heat Mass Transf. Waerme Stoffuebertragung 2007, 43, 881–894. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, W.; Zhu, S.; Wang, F. Comparison between the Oxidation of Iron in Oxygen and in Steam at 650–750 °C. Corros. Sci. 2013, 75, 309–317. [Google Scholar] [CrossRef]
- Young, D.J.; Watson, S. High-Temperature Corrosion in Mixed Gas Environments. Oxid. Met. 1995, 44, 239–264. [Google Scholar] [CrossRef]
- Go, K.S.; Son, S.R.; Kim, S.D. Reaction Kinetics of Reduction and Oxidation of Metal Oxides for Hydrogen Production. Int. J. Hydrogen Energy 2008, 33, 5986–5995. [Google Scholar] [CrossRef]
- Li, P.; Guo, H.; Gao, J.; Min, J.; Yan, B.; Chen, D.; Seetharaman, S. Novel Concept of Steam Modification towards Energy and Iron Recovery from Steel Slag: Oxidation Mechanism and Process Evaluation. J. Clean. Prod. 2020, 254, 119952. [Google Scholar] [CrossRef]
- Fujji, C.T.; Meussner, R.A. The Mechanism of the High-Temperature Oxidation of Iron-Chromium Alloys in Water Vapor. J. Electrochem. Soc. 1964, 111, 1215. [Google Scholar] [CrossRef]
- Bauer, S.H.; Schott, G.L.; Duff, R.E. Kinetic Studies of Hydroxyl Radicals in Shock Waves. I. The Decomposition of Water between 2400° and 3200 °K. J. Chem. Phys. 1958, 28, 1089–1096. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Yang, D.; Shuai, Y.; Lougou, B.G.; Pan, Q.; Wang, F. Selection of Iron-Based Oxygen Carriers for Two-Step Solar Thermochemical Splitting of Carbon Dioxide. Energy Convers. Manag. 2023, 279, 116772. [Google Scholar] [CrossRef]
- Srinivasan, N.K.; Michael, J.V. The Thermal Decomposition of Water. Int. J. Chem. Kinet. 2006, 38, 211–219. [Google Scholar] [CrossRef]
- Homer, J.B.; Hurle, I.R. The Dissociation of Water Vapour behind Shock Waves. Proc. R. Soc. London. A. Math. Phys. Sci. 1970, 314, 585–598. [Google Scholar] [CrossRef]
- Brockris, J.O.; Reddy, A.K.N. Electroquimica Moderna; Editorial Reverté: Barcelona, Spain, 2003; Volume 2. [Google Scholar]
- Rahmel, A.; Tobolsk, J. Einfluss von wasserdampf und kohlendioxyd auf die oxydation von eisen in sauerstoff bei hohen temperaturen. Corros. Sci. 1965, 5, 333. [Google Scholar] [CrossRef]
- Gareev, K.G. Diversity of Iron Oxides: Mechanisms of Formation, Physical Properties and Applications. Magnetochemistry 2023, 9, 119. [Google Scholar] [CrossRef]
- Khanna, A.S. Introduction to High Temperature Oxidation and Corrosion; ASM International: Detroit, MI, USA, 2002; ISBN 0871707624. [Google Scholar]
- Sanchez-Caldera, L.E.; Griffith, P.; Asm, F.; Rabinowicz, E.E.; Asme, F. The Mechanism of Corrosion-Erosion in Steam Extraction Lines of Power Stations. J. Eng. Gas Turbines Power 1988, 110, 180–184. [Google Scholar] [CrossRef]
- Lawless, K.R. The Oxidation of Metals. Rep. Prog. Phys. 1974, 37, 231. [Google Scholar] [CrossRef]
- Stehle, R.C.; Bobek, M.M.; Hooper, R.; Hahn, D.W. Oxidation Reaction Kinetics for the Steam-Iron Process in Support of Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 15125–15135. [Google Scholar] [CrossRef]
- Stehle, R.C.; Bobek, M.M.; Hahn, D.W. Iron Oxidation Kinetics for H2 and CO Production via Chemical Looping. Int. J. Hydrogen Energy 2015, 40, 1675–1689. [Google Scholar] [CrossRef]
- Ávila, J.; Genescá, J. Más Allá de La Herrumbre 1; Fondo de Cultura Económica: Mexico City, Mexico, 2013; ISBN 6071603196. [Google Scholar]
- Kosaka, F.; Hatano, H.; Oshima, Y.; Otomo, J. Iron Oxide Redox Reaction with Oxide Ion Conducting Supports for Hydrogen Production and Storage Systems. Chem. Eng. Sci. 2015, 123, 380–387. [Google Scholar] [CrossRef]
- McCafferty, E. Thermodynamics of Corrosion: Pourbaix Diagrams. In Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; pp. 95–117. [Google Scholar]
- Pignocco, A.J.; Pellissier, G.E. Leed studies of oxygen adsorption and oxide formation on an (011) iron surface. Surf. Sci. 1967, 7, 261–278. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal splitting of water and on-site separation of the products. experimental feasibility study. J. Hydrogen Energy 1998, 23, 9–98. [Google Scholar] [CrossRef]
- Kodama, T. High-Temperature Solar Chemistry for Converting Solar Heat to Chemical Fuels. Prog. Energy Combust. Sci. 2003, 29, 567–597. [Google Scholar] [CrossRef]
- Ehlers, J.; Young, D.J.; Smaardijk, E.J.; Tyagi, A.K.; Penkalla, H.J.; Singheiser, L.; Quadakkers, W.J. Enhanced Oxidation of the 9%Cr Steel P91 in Water Vapour Containing Environments. Corros. Sci. 2006, 48, 3428–3454. [Google Scholar] [CrossRef]
- Kritzer, P. Corrosion in High-Temperature and Supercritical Water and Aqueous Solutions: A Review. J. Supercrit. Fluids 2004, 29, 1–29. [Google Scholar] [CrossRef]
- Siegel, N.P.; Ermanoski, I. A Beam-Down Central Receiver for Solar Thermochemical Hydrogen Production; American Solar Energy Society: Baltimore, MD, USA, 2013. [Google Scholar]
- Miyaoka, H. Thermochemical Water Splitting by Concentrated Solar Power. In Lecture Notes in Energy; Springer: Berlin/Heidelberg, Germany, 2016; Volume 32, pp. 137–151. [Google Scholar]
- Kodama, T.; Gokon, N.; Matsubara, K.; Yoshida, K.; Koikari, S.; Nagase, Y.; Nakamura, K. Flux Measurement of a New Beam-down Solar Concentrating System in Miyazaki for Demonstration of Thermochemical Water Splitting Reactors. In Proceedings of the Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 49, pp. 1990–1998. [Google Scholar]
- Kodama, T.; Gokon, N.; Enomoto, S.I.; Itoh, S.; Hatamachi, T. Coal Coke Gasification in a Windowed Solar Chemical Reactor for Beam-down Optics. J. Sol. Energy Eng. Trans. ASME 2010, 132, 041004. [Google Scholar] [CrossRef]
- Gokon, N.; Izawa, T.; Kodama, T. Steam Gasification of Coal Cokes by Internally Circulating Fluidized-Bed Reactor by Concentrated Xe-Light Radiation for Solar Syngas Production. Energy 2015, 79, 264–272. [Google Scholar] [CrossRef]
- Bellan, S.; Gokon, N.; Matsubara, K.; Cho, H.S.; Kodama, T. Heat Transfer Analysis of 5kWth Circulating Fluidized Bed Reactor for Solar Gasification Using Concentrated Xe Light Radiation. Energy 2018, 160, 245–256. [Google Scholar] [CrossRef]
- Koepf, E.; Advani, S.G.; Steinfeld, A.; Prasad, A.K. A Novel Beam-down, Gravity-Fed, Solar Thermochemical Receiver/Reactor for Direct Solid Particle Decomposition: Design, Modeling, and Experimentation. Int. J. Hydrogen Energy 2012, 37, 16871–16887. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Cho, H.S.; Bellan, S.; Matsubara, K.; Inoue, K. Particle Fluidized Bed Receiver/Reactor with a Beam-down Solar Concentrating Optics: Performance Test of Two-Step Water Splitting with Ceria Particles Using 30-KWth Sun-Simulator. In Proceedings of the AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2018; Volume 2033. [Google Scholar]
- Bellan, S.; Kodama, T.; Seok Cho, H.; Kim, J.S. Hydrogen Production by Solar Fluidized Bed Reactor Using Ceria: Euler-Lagrange Modelling of Gas-Solid Flow to Optimize the Internally Circulating Fluidized Bed. J. Therm. Sci. Technol. 2022, 17, 22-00076. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, T.; Takahashi, S.; Kodama, T. Thermochemical Two-Step Water-Splitting for Hydrogen Production Using Fe-YSZ Particles and a Ceramic Foam Device. Energy 2008, 33, 1407–1416. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Cho, H.S.; Matsubara, K.; Kaneko, H.; Senuma, K.; Itoh, S.; Yokota, S.N. Particles Fluidized Bed Receiver/Reactor Tests with Quartz Sand Particles Using a 100-KWth Beam-down Solar Concentrating System at Miyazaki. In Proceedings of the AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2017; Volume 1850. [Google Scholar]
- Gokon, N.; Kumaki, S.; Miyaguchi, Y.; Bellan, S.; Kodama, T.; Cho, H. Development of a 5kWth Internally Circulating Fluidized Bed Reactor Containing Quartz Sand for Continuously-Fed Coal-Coke Gasification and a Beam-down Solar Concentrating System. Energy 2019, 166, 1–16. [Google Scholar] [CrossRef]
- Kiwan, S.; Soud, Q.R. Numerical Investigation of Sand-Basalt Heat Storage System for Beam-down Solar Concentrators. Case Stud. Therm. Eng. 2019, 13, 100372. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Lund, P.D.; Jiang, C.; Li, X. High Performance Integrated Receiver-Storage System for Concentrating Solar Power Beam-down System. Sol. Energy 2019, 187, 85–94. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Lund, P.D.; Jiang, C.; Li, X. Modelling and Performance Evaluation of an Integrated Receiver-Storage for Concentrating Solar Power Beam-down System under Heterogeneous Radiative Conditions. Sol. Energy 2019, 188, 1264–1273. [Google Scholar] [CrossRef]
- Díaz-Heras, M.; Barreneche, C.; Belmonte, J.F.; Calderón, A.; Fernández, A.I.; Almendros-Ibáñez, J.A. Experimental Study of Different Materials in Fluidized Beds with a Beam-down Solar Reflector for CSP Applications. Sol. Energy 2020, 211, 683–699. [Google Scholar] [CrossRef]
- Fresno, F.; Yoshida, T.; Gokon, N.; Fernández-Saavedra, R.; Kodama, T. Comparative Study of the Activity of Nickel Ferrites for Solar Hydrogen Production by Two-Step Thermochemical Cycles. Int. J. Hydrogen Energy 2010, 35, 8503–8510. [Google Scholar] [CrossRef]
- Taylor, R.W.; Berjoan, R.; Coutures, P. Solar gasification of carbonaceous materials. Sol. Energy 1983, 30, 513–525. [Google Scholar] [CrossRef]
- Flamant, G.; Olalde, G. High temperature solar gas heating comparison between packed and fluidized bed receivers. Sol. Energy 1983, 31, 463–471. [Google Scholar] [CrossRef]
- Flamant, G.; Gauthier, D.; Boudhari, C.; Flitris, Y. A 50 KW Fluidized Bed High Temperature Solar Receiver: Heat Transfer Analysis. J. Sol. Energy Eng. 1988, 110, 313–320. [Google Scholar] [CrossRef]
- Steinfeld, A.; Frei, A.; Kuhn, P.; Wuillemin, D. solar thermal production of zinc and syngas via combined zno-reduction and CH4 reforming processes. J. Hydrogen Energy 1995, 20, 793–804. [Google Scholar] [CrossRef]
- Gokon, N.; Takahashi, S.; Yamamoto, H.; Kodama, T. Thermochemical Two-Step Water-Splitting Reactor with Internally Circulating Fluidized Bed for Thermal Reduction of Ferrite Particles. Int. J. Hydrogen Energy 2008, 33, 2189–2199. [Google Scholar] [CrossRef]
- Nikulshina, V.; Gebald, C.; Steinfeld, A. CO2 Capture from Atmospheric Air via Consecutive CaO-Carbonation and CaCO3-Calcination Cycles in a Fluidized-Bed Solar Reactor. Chem. Eng. J. 2009, 146, 244–248. [Google Scholar] [CrossRef]
- Kodama, T.; Gokon, N.; Cho, H.S.; Matsubara, K.; Etori, T.; Takeuchi, A.; Yokota, S.N.; Ito, S. Particles Fluidized Bed Receiver/Reactor with a Beam-down Solar Concentrating Optics: 30-KWth Performance Test Using a Big Sun-Simulator. In Proceedings of the AIP Conference Proceedings; American Institute of Physics Inc.: College Park, MD, USA, 2016; Volume 1734. [Google Scholar]
- Bellan, S.; Gokon, N.; Matsubara, K.; Cho, H.S.; Kodama, T. Numerical and Experimental Study on Granular Flow and Heat Transfer Characteristics of Directly-Irradiated Fluidized Bed Reactor for Solar Gasification. Int. J. Hydrogen Energy 2018, 43, 16443–16457. [Google Scholar] [CrossRef]
- Hoskins, A.L.; Millican, S.L.; Czernik, C.E.; Alshankiti, I.; Netter, J.C.; Wendelin, T.J.; Musgrave, C.B.; Weimer, A.W. Continuous On-Sun Solar Thermochemical Hydrogen Production via an Isothermal Redox Cycle. Appl. Energy 2019, 249, 368–376. [Google Scholar] [CrossRef]
- Jiang, K.; Kong, Y.; Xu, C.; Ge, Z.; Du, X. Experimental Performance of Gas-Solid Countercurrent Fluidized Bed Particle Solar Receiver with High-Density Suspension. Appl. Therm. Eng. 2022, 213, 118661. [Google Scholar] [CrossRef]


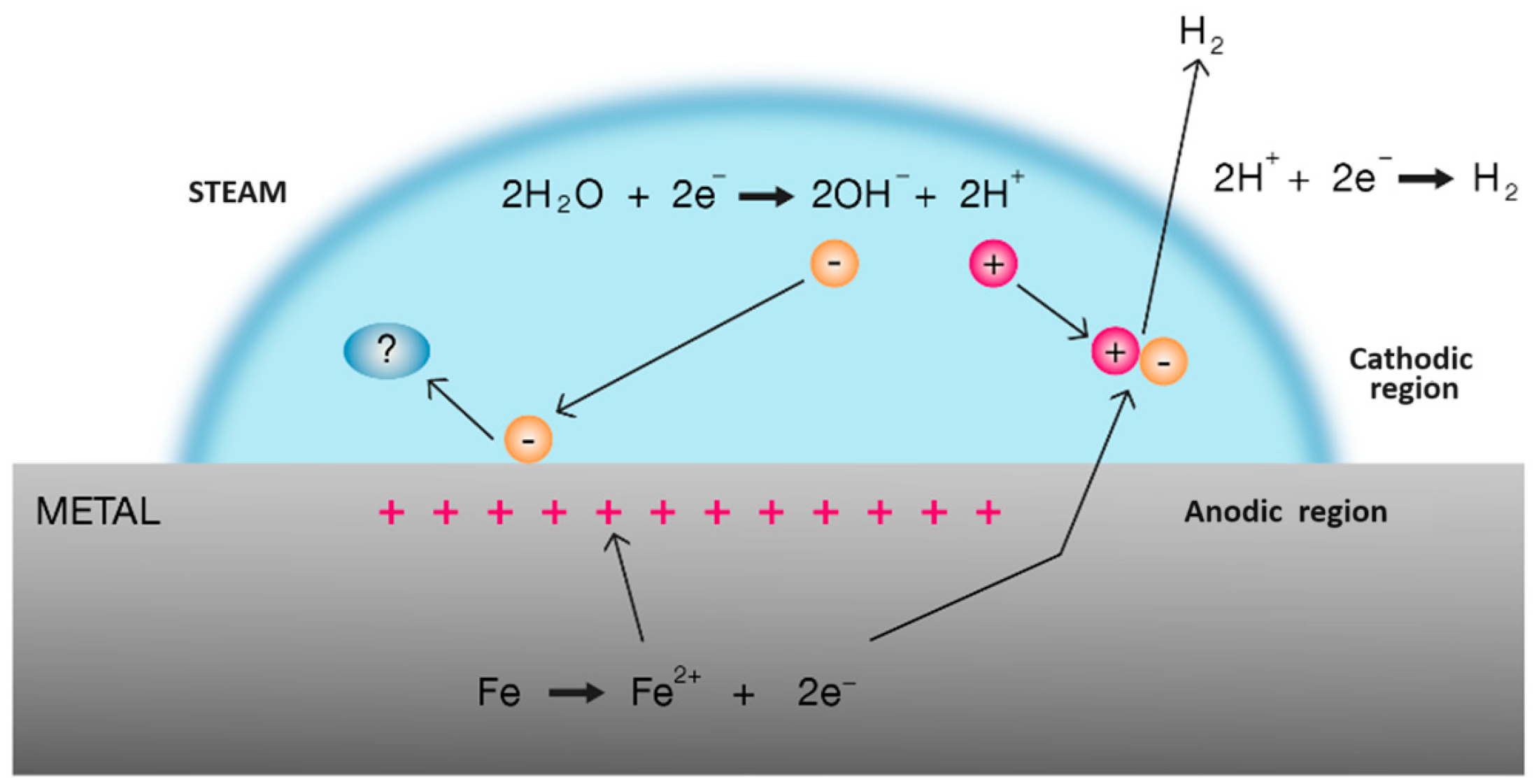


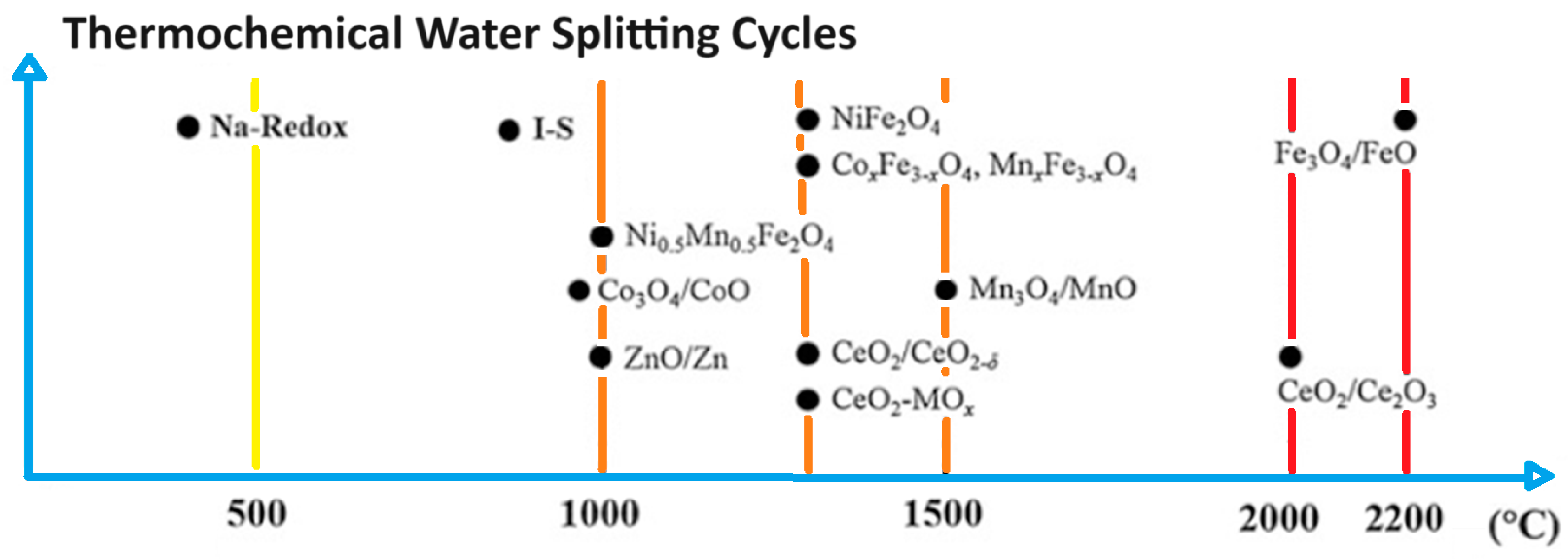
| Chemical Species | Gibbs Free Energy ΔG (kJ/mol) | Enthalpy ΔH (kJ/mol) | Entropy S (kJ/mol) | Temp. K (1 atm) | Refs. |
|---|---|---|---|---|---|
| H2O(l) | −237.1 | −285.8 | 70.0 | 298 | [31,32] |
| H2O(g) | −228.6 | −241.8 | 188.8 | 298 | [31,32] |
| −214.1 | −244.7 | 213.8 | 600 | [32] | |
| −198.2 | −247.1 | 228.6 | 900 | ||
| H2(g) | 0 | 0 | 130.6 | 298 | [31,32] |
| 0 | 0 | 151.0 | 600 | [32] | |
| 0 | 0 | 163.1 | 900 | ||
| OH− (aqueous ion) | −157.3 | −230.0 | −10.7 | 298 | [31] |
| OH−(g) | 34.7 | 38.9 | 183.7 | 298 | [32] |
| 29.5 | 38.9 | 204.4 | 600 | ||
| 24.9 | 38.4 | 216.5 | 900 | ||
| Fe0 | 0 | 0 | 27.09 | 298 | [31] |
| Fe2+ | −90.0 | −91.1 | −107.1 | 298 | [31] |
| Fe3+ | −16.7 | −49.9 | −280.0 | 298 | [31] |
| Fe.947O | −244.9 | −266.3 | 56.6 | 298 | [31] |
| −245.1 | −266.3 | 57.5 | 298 | [32] | |
| −224.8 | −263.7 | 93.0 | 600 | ||
| −205.8 | −262.7 | 115.1 | 900 | ||
| FeO | −251.4 | −272.0 | 60.6 | 298 | [31,32] |
| −231.7 | −269.4 | 97.3 | 600 | [32] | |
| −213.1 | −268.4 | 120.2 | 900 | ||
| −774.4 | −826.2 | 87.4 | 298 | [31,32] | |
| −742.3 | −824.2 | 87.4 | 298 | [32] | |
| −661.4 | −817.6 | 173.3 | 600 | ||
| −585.3 | −808.4 | 235.4 | 900 | ||
| −731.4 | −811.6 | 93.0 | 298 | [33] | |
| −1012.7 | −1115.7 | 146.1 | 298 | [31] | |
| −1015.2 | −1118.4 | 146.1 | 298 | [32] | |
| −914.4 | −1107.4 | 272.1 | 600 | ||
| −821.8 | −1088.6 | 368.3 | 900 | ||
| FeO(OH) | −491.8 | −562.6 | 60.4 | 298 | [31] |
| Fe(OH)2 | −486.9 | −568.9 | 87.9 | 298 | [32] |
| −405.7 | −564.1 | 160.4 | 600 | ||
| −327.6 | −558.9 | 207.7 | 900 |
| Reaction | Gibbs Free Energy ΔG (kJ/mol) | Enthalpy ΔH (kJ/mol) | Temp. K (pres. atm) | Refs. |
|---|---|---|---|---|
| −14.52 | −32.09 | 600 | [34] | |
| −199.3 | - | 1000 | [35] | |
| 38.9 + 5 × 80T (cal) | - | - | [28] | |
| 48.9 | - | 973–1123 | [36] | |
| 11.0 | - | 973–1123 | [36,37] | |
| - | −33.6 | 873 | [22,23,38,39] | |
| 10.39 | −47.54 | 400 | [34] | |
| 41.02 | −79.23 | 400 | [34] | |
| 228.22 | −120.48 | 600 | [34] | |
| 40.5 | - | - | [40] | |
| 44.4 | - | - | [40] |
| Equation | Observation | Refs. |
|---|---|---|
| Logarithmic reaction rate: | ||
| It represents the initial oxidation states at low temperatures. | [41] | |
| - | [41] | |
| Linear reaction rate: | ||
| It represents a constant rate of oxide growth applicable at very high temperatures. | [41] | |
| - | [41] | |
| - | [52] | |
| Parabolic reaction rate: | ||
| It adjusts to the processes controlled by the diffusion of species. | [41] | |
| - | [41,53] | |
| - | [52] | |
| The function f(X) depends on the reaction mechanism for diffusion in one, two, or three dimensions. | [54] | |
| The reaction rate of a sample of iron slag in water vapour. | [55] | |
| Half Reaction | Standard Reduction Potential, E° (V) | Ref. |
|---|---|---|
| E° = 0.000 (V) | [69] | |
| E° = −0.447 (V) | [69] | |
| Reaction | ||
| - | [55] | |
| E° = 0.356 (V) | [55] | |
| E° = 0.210 (V) | [40] | |
| E° = 0.230 (V) | [40] | |
| Thermal Power | Temperatures Reached or Required | Thermal Fluid | Fluidized Bed Material | Particle Size (µm) | Radiation or Concentration Ratio | Refs. |
|---|---|---|---|---|---|---|
| 1 kWth | 1300 °C | Water/Steam | Coal-coke | 140 (200–300) (300–500) (500–710) | 477 W/cm2 | [74,80,81,82] |
| 10 to 20 kWth | 2200 °C | Water/Steam | ZnO reagent powder | 1–5 | - | [83] |
| 3 MWth | 1500 °C | Water/Steam | Cerium oxide material | <300 (100–300) | 1.5 kW/m2 | [38,77,84,85] |
| 110 kWth | 1400 °C | Gases | Non-stoichiometric cerium oxide particles | 10–210 | - | [79,86] |
| 100 kWth | 960–1100 °C | Air | Quartz sand particles | 100–500 | - | [87] |
| 250 kWth | 800–1000 °C | Air | A mixture of coal-coke and quartz sand | 100-300 (coal) 100–700 (sand) | - | [88] |
| 30 kWth | 560 °C | Air | A mixture of sand and basalt | - | [89] | |
| 450 kWth | 770 °C | Air | Isotropic materials | 4.8 kWh/m2/900 suns | [90,91] | |
| 140.63 Wth (2 kWe) 231.32 Wth (4 kWe) | 250 °C | Air | Sand, ceramic casting media (carbo Accucast ID50) y SiC | 65 kW/m2 (2kWe) 115 kW/m2 (4 kWe) | [92] |
| Iron Oxides Material | Sample/Particle Size (µm) | Temp. (°C) (Time) | Partial Vapour Pressure | Thermal Fluid (Flow) | Refs. | |
|---|---|---|---|---|---|---|
| Magnetite | Fe3O4 | 30–50 100–125 | 575 673 | - | - | [23,74] |
| The partial substitution of iron in Fe3O4 by Ni, Co, and Zr, to form mixed metal oxides. | (Fe(1−x) Mx)3O4 NiFe2O4 NiFe2O4/m-ZrO2 | Sample in a quartz tube | 1000 | - | - | [23,38,74] |
| Magnetite supported on zirconium, stabilized with cubic yttria | Fe3O4/c-YSZ | Ceramic foam | 1100 (80 min) | 75% of the steam pressure at 90 °C, 1 bar | H2O/N2 (10 Ncm3/min) | [86] |
| Commercial nickel ferrite supported on zirconium oxide | NiFe2O4/ ZrO2 | Sample on Pt cup | 1000 (60 min) | steam pressure at 80 °C, 1 bar | H2O/N2 (4 mL/min) | [93] |
| Unsupported commercial nickel ferrite | NiFe2O4 | 212–710 | 1.6–1.7 kWth (10–92 min) | 51% (0.51 atm) of the steam pressure at 82 °C, 1 atm | H2O/N2 (0.24 Ndm3/min) | [39] |
| Monoclinic magnetite supported on zirconium substrates | Fe3O4/m-ZrO2 | Polished Fe bar without oxidation | 397–602 (180 min) | - | Nitrogen (100 cc/min) Argon (200 cc/min) Liquid water (12.5 µL/min) | [67] |
| Iron oxide with various support materials, ZrO2, CeO2, yttria-stabilized zirconia (YSZ), and gadolinia-doped ceria (GDC) | Fe2O3/ZrO2 Fe2O3/CeO2 Fe2O3/YSZ Fe2O4/GDC | 150–300 | 550 | - | H2O/Ar (prop. 5:95) (300 mL/min) | [70] |
| Reactor Geometry | Bed/Gas | Material | Power/Radiation | Reactor | Year/Refs. |
|---|---|---|---|---|---|
| Diameter = 5 cm Height = 32 cm | Charcoal/CO2 | Silice glass tube | 2 kW/ 400 W/cm2 |  | 1983 [94] |
| ZrO2, SiC/air, N2, CO2 | Clear quartz tube | 6.5 kW/ 1000 W/m2 |  | 1983 [95] | |
| 0.90 m high, 0.78 m wide, with a radius of curvature of 0.78 m | Alumina particles/air | Refractory stainless steel (AISI 310) | 30–45 kW/ 1 kW/m2 |  | 1988 [96] |
| Diameter = 2 cm | ZnO + Al2O3/ CH4 + Ar | Quartz tube | 15 kW/ 4000 suns | 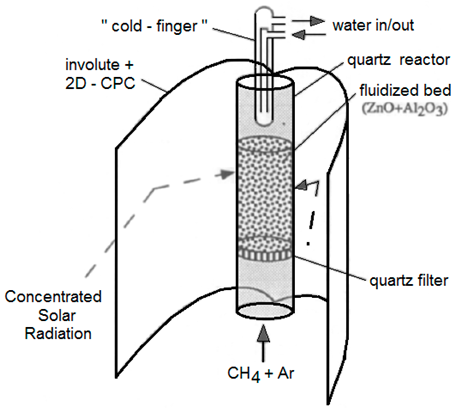 | 1995 [97] |
| Diameter = 45 mm, thickness 2.5 mm | NiFe2O4—ZrO2/N2 | Quartz tube | 6 kW |  | 2008 [98] |
| 25 mm in diameter, thickness 1.5 mm, height 25 cm. | CaO or CaCO3/H2O, Ar, CO2 | Quartz tube | 75 kW/ 4250 suns | 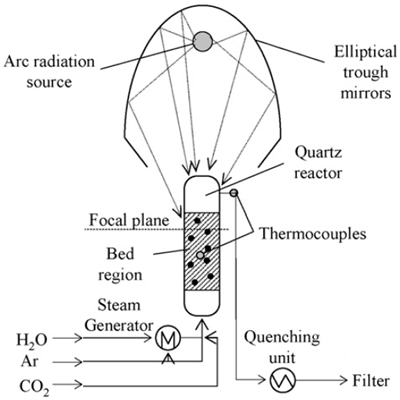 | 2009 [99] |
| 420 mm in length, 62.3 mm in inner diameter, and 7 mm in thickness | Coke/CO2 | Stainless steel with a quartz window | 6 kW/ 477 W/cm2 | 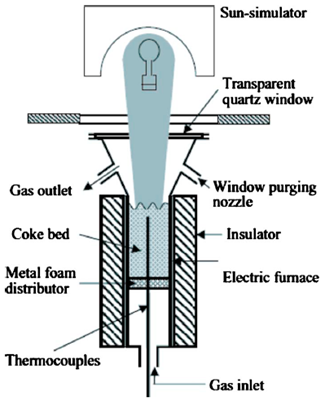 | 2010 [80] |
| The inner diameter was 45 mm, and the thickness was 2.5 mm. | NiFe2O4/vapour | Stainless-steel tube with a quartz cap | 3 × 6 kW/ 637 W/cm2 | 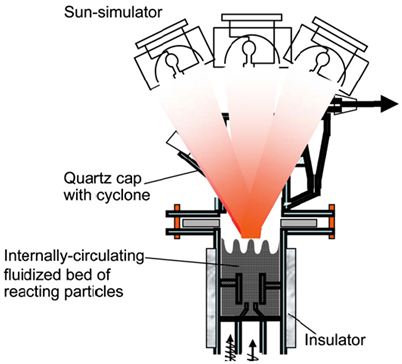 | 2011 [39] |
| Length 420 mm, inner diameter 62.3 mm, thickness 7 mm | Coke/Ar-vapour CeO2/N2 | stainless-steel tube (SUS310S) with a quartz window | 18 kW/ 637 W/cm2 | 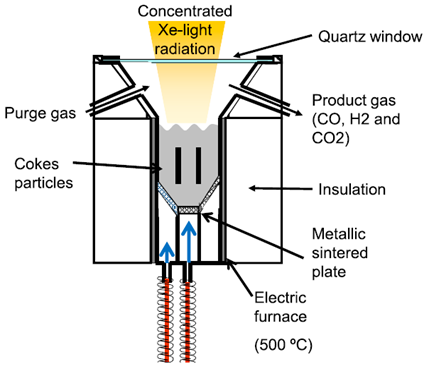 | 2015 [81,84] |
| 42 cm diameter at the top | Quartz sand particles/air | Inconel and stainless steel, with a quartz window | 133 kW/ 950 kW/m2 | 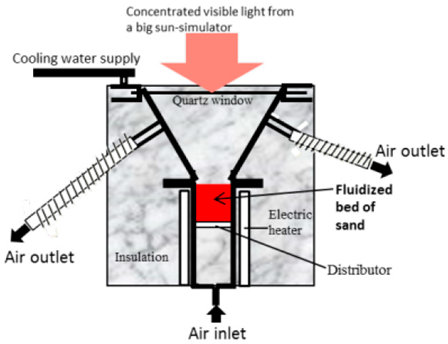 | 2016 [100] |
| 0.2 m long and 0.3 m internal diameter at the top and 0.007 m thick | Quartz particles/N2 | Stainless steel tube with quartz window (5 mm thick) | 21 kW/ 903 kW/m2 | 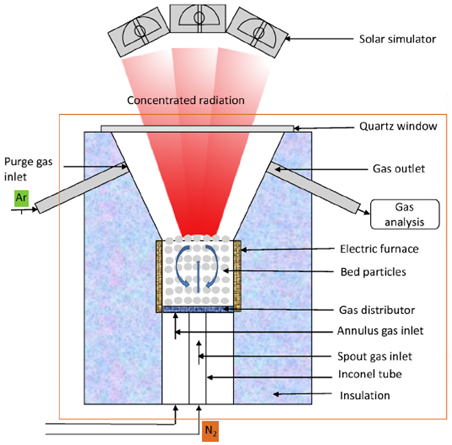 | 2018 [101] |
| 35.6 cm long and 2.54 cm outer diameter | Iron aluminate (FeAl2O4)/N2 | Silicon carbide cylindrical tubes (SiC) | 10 kW | 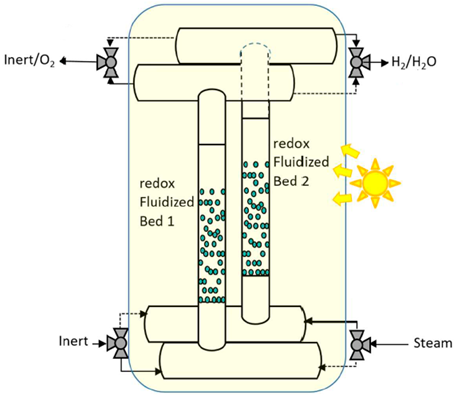 | 2019 [102] |
| Inside diameter of 7.62 cm and a height of 8 cm | Sand, carbon Accucast ID50 y SiC/air | Stainless-steel tube (304) | 2kWe/ 65 kW/m2 4 kWe/ 115 kW/m2 | 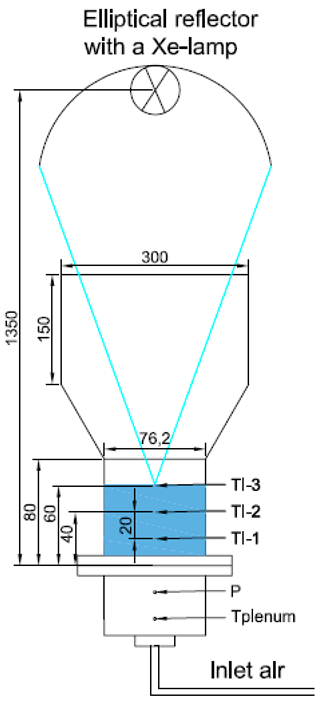 | 2020 [92] |
| Tube with 40 mm inner diameter, 10 mm wall thickness, and 1780 mm length | Quartz sand/air | Pure iron metal tube, hot particle container made of stainless steel AISI 304 | 10–40 kWe | 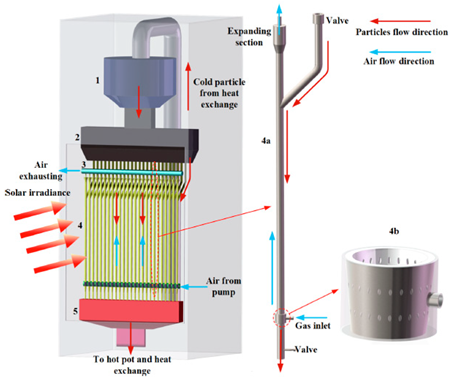 | 2022 [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, M.; Pulido, D.; Fuentealba, E.; Soliz, A.; Toro, N.; Sagade, A.; Galleguillos Madrid, F.M. Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review. Appl. Sci. 2024, 14, 7056. https://doi.org/10.3390/app14167056
Fuentes M, Pulido D, Fuentealba E, Soliz A, Toro N, Sagade A, Galleguillos Madrid FM. Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review. Applied Sciences. 2024; 14(16):7056. https://doi.org/10.3390/app14167056
Chicago/Turabian StyleFuentes, Manuel, Diego Pulido, Edward Fuentealba, Alvaro Soliz, Norman Toro, Atul Sagade, and Felipe M. Galleguillos Madrid. 2024. "Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review" Applied Sciences 14, no. 16: 7056. https://doi.org/10.3390/app14167056
APA StyleFuentes, M., Pulido, D., Fuentealba, E., Soliz, A., Toro, N., Sagade, A., & Galleguillos Madrid, F. M. (2024). Direct Solar Thermal Water-Splitting Using Iron and Iron Oxides at High Temperatures: A Review. Applied Sciences, 14(16), 7056. https://doi.org/10.3390/app14167056








