Effect of Blood on Synovial Joint Tissues: Potential Role of Ferroptosis
Abstract
1. Introduction
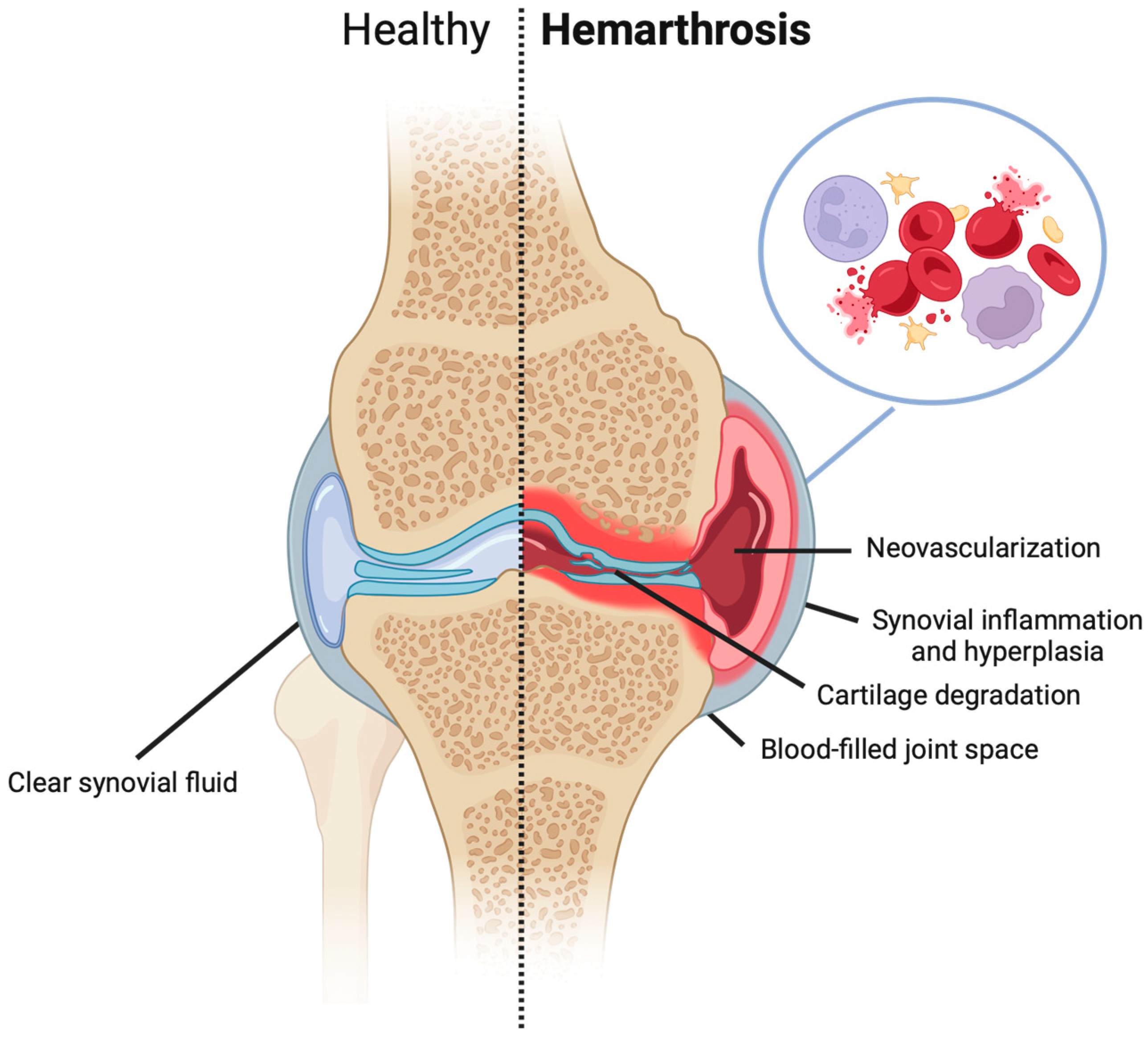
2. The Etiology of Hemarthrosis
3. Interplay of Erythrocytes, Hemoglobin/Iron, and Synovium
3.1. Oxidative Stress
3.2. Role of Iron
3.3. Ferroptosis Pathway
3.4. Hemarthrosis-Induced Cytokines
3.5. Fibrosis
3.6. Role of Blood Components
4. In Vitro Models
4.1. Cartilage
4.2. Synovium
4.3. Meniscus
4.4. Bone
4.5. ACL
5. In Vivo Models of Hemarthrosis
| Species | Advantages | Disadvantages | Applications |
|---|---|---|---|
Canine Ref: [7,24,53,55,67,73,95,99,101,102,103,104,105,106,107,108,109] |
|
|
|
Lapine Ref: [70,74,81,94,110,111,112,113] |
|
|
|
Rodent Ref: [59,76,93,98,114,115,116,117,118] |
|
|
|
5.1. Canine
5.2. Lapine/Rabbit
5.3. Rodent
6. Strategies to Promote Joint Repair
6.1. Surgical Intervention & Current Repair Techniques
Evacuation of Blood Post-Injury (or Surgery)
6.2. Platelet-Rich Plasma (PRP)
7. Potential Therapeutics
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Schurz, J.; Ribitsch, V. Rheology of synovial fluid. Biorheology 1987, 24, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Sakane, T.; Shingu, M.; Yokoyama, M.M. Effect of stimulated neutrophils from the synovial fluid of patients with rheumatoid arthritis on lymphocytes—A possible role of increased oxygen radicals generated by the neutrophils. J. Clin. Immunol. 1983, 3, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Knobe, K.; Berntorp, E. Haemophilia and joint disease: Pathophysiology, evaluation, and management. J. Comorbidity 2011, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.R.; Gallagher, P.J.; Potter, A.R.; Bell, M.J.; Bacon, P.A. The effect of synovial iron on the progression of rheumatoid disease. A histologic assessment of patients with early rheumatoid synovitis. Arthritis Rheum. 1984, 27, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Blake, D.R.; Wainwright, A.C.; Steven, M.M. Relationship between iron deposits and tissue damage in the synovium: An ultrastructural study. Ann. Rheum. Dis. 1986, 45, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Li, S. The hidden blood loss and its factors in patients undergoing minimally invasive knee arthroscopy. Front. Surg. 2022, 9, 944481. [Google Scholar] [CrossRef] [PubMed]
- Potpally, N.; Rodeo, S.; So, P.; Mautner, K.; Baria, M.; Malanga, G.A. A Review of Current Management of Knee Hemarthrosis in the Non-Hemophilic Population. Cartilage 2021, 13 (Suppl. S1), 116S–121S. [Google Scholar] [CrossRef] [PubMed]
- Evers, B.J.; Van Den Bosch, M.H.J.; Blom, A.B.; van der Kraan, P.M.; Koëter, S.; Thurlings, R.M. Post-traumatic knee osteoarthritis; the role of inflammation and hemarthrosis on disease progression. Front. Med. 2022, 9, 973870. [Google Scholar] [CrossRef]
- van Meegeren, M.E.R.; Roosendaal, G.; Jansen, N.W.D.; Lafeber, F.P.J.G.; Mastbergen, S.C. Blood-Induced Joint Damage: The Devastating Effects of Acute Joint Bleeds versus Micro-Bleeds. Cartilage 2013, 4, 313–320. [Google Scholar]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Hooiveld, M.; Roosendaal, G.; Wenting, M.; van den Berg, M.; Bijlsma, J.; Lafeber, F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am. J. Pathol. 2003, 162, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Gangi, L.R.; Zandkarimi, F.; Stockwell, B.R.; Hung, C.T. Red blood cell exposure increases chondrocyte susceptibility to oxidative stress following hemarthrosis. Osteoarthr. Cartil. 2023, 31, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Lyons, L.P.; Weinberg, J.B.; Wittstein, J.R.; McNulty, A.L. Blood in the Joint: Effects of Hemarthrosis on Meniscus Health and Repair Techniques. Osteoarthr. Cartil. 2021, 29, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.L.; Vavken, P.; Murray, M.M. Erythrocytes inhibit ligament fibroblast proliferation in a collagen scaffold. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.; Fufa, D.; Abreu, E.L.; Kevy, S.; Murray, M.M. Platelets, but not erythrocytes, significantly affect cytokine release and scaffold contraction in a provisional scaffold model. Wound Repair Regen. 2008, 16, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Han, Z.; Wang, Y.; Su, J.; Lin, Y.; Cheng, X.; Liu, W.; He, J.; Fan, Y.; Chen, L.; et al. Targeting Ferroptosis in Bone-Related Diseases: Facts and Perspectives. J. Inflamm. Res. 2023, 16, 4661–4677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Wong, J.H.; Berman, Z.T.; Lombardi, A.F.; Chang, E.Y.; von Drygalski, A. Bleeding with iron deposition and vascular remodelling in subchondral cysts: A newly discovered feature unique to haemophilic arthropathy. Haemophilia 2021, 27, e730–e738. [Google Scholar] [CrossRef]
- Forsyth, A.L.; Rivard, G.É.; Valentino, L.A.; Zourikian, N.; Hoffman, M.; Monahan, P.E.; Van Meegeren, M.E.R.; Forriol, F. Consequences of intra-articular bleeding in haemophilia: Science to clinical practice and beyond. Haemophilia 2012, 18 (Suppl. S4), 112–119. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Nauman, P.; Mandat, T.; Paradowska-Gorycka, A.; Romanowska-Próchnicka, K.; Szukiewicz, D.; Kotela, A.; Kubaszewski, Ł. Kotela, I.; et al. Cytokines in the pathogenesis of hemophilic arthropathy. Cytokine Growth Factor. Rev. 2018, 39, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Monahan, P.E.; Doria, A.S.; Ljung, R.; Jiménez-Yuste, V. Optimizing joint function: New knowledge and novel tools and treatments. Haemophilia 2012, 18 (Suppl. S5), 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, M.; Maśliński, W.; Prochorec-Sobieszek, M.; Nieciecki, M.; Sudoł-Szopińska, I. Cartilage and bone damage in rheumatoid arthritis. Reumatologia 2018, 56, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Roosendaal, G.; TeKoppele, J.M.; Vianen, M.E.; van den Berg, H.M.; Lafeber, F.P.; Bijlsma, J.W. Blood-induced joint damage: A canine in vivo study. Arthritis Rheum. 1999, 42, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, M.; Cardenas, A.C. Hemarthrosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK525999/ (accessed on 24 April 2024).
- Maffulli, N.; Binfield, P.M.; King, J.B.; Good, C.J. Acute haemarthrosis of the knee in athletes. A prospective study of 106 cases. J. Bone Jt. Surg. Br. 1993, 75, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Khella, C.M.; Asgarian, R.; Horvath, J.M.; Rolauffs, B.; Hart, M.L. An Evidence-Based Systematic Review of Human Knee Post-Traumatic Osteoarthritis (PTOA): Timeline of Clinical Presentation and Disease Markers, Comparison of Knee Joint PTOA Models and Early Disease Implications. Int. J. Mol. Sci. 2021, 22, 1996. [Google Scholar] [CrossRef] [PubMed]
- Hardaker, W.T.; Garrett, W.E.; Bassett, F.H. Evaluation of acute traumatic hemarthrosis of the knee joint. South. Med. J. 1990, 83, 640–644. [Google Scholar] [CrossRef]
- DeHaven, K.E. Diagnosis of acute knee injuries with hemarthrosis. Am. J. Sports Med. 1980, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Swärd, P.; Frobell, R.; Englund, M.; Roos, H.; Struglics, A. Cartilage and bone markers and inflammatory cytokines are increased in synovial fluid in the acute phase of knee injury (hemarthrosis)—A cross-sectional analysis. Osteoarthr. Cartil. 2012, 20, 1302–1308. [Google Scholar] [CrossRef]
- Watt, F.E.; Paterson, E.; Freidin, A.; Kenny, M.; Judge, A.; Saklatvala, J.; Williams, A.; Vincent, T.L. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association with Early Clinical Outcomes. Arthritis Rheumatol. 2016, 68, 2129–2140. [Google Scholar] [CrossRef]
- Higuchi, H.; Shirakura, K.; Kimura, M.; Terauchi, M.; Shinozaki, T.; Watanabe, H.; Takagishi, K. Changes in biochemical parameters after anterior cruciate ligament injury. Int. Orthop. 2006, 30, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Lohmander, L.S.; Englund, P.M.; Dahl, L.L.; Roos, E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am. J. Sports Med. 2007, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Ames, P.R.; Maffulli, N.; Capasso, G.; Brancaccio, V. Minimal trauma knee hemarthrosis. Bull. Hosp. Jt. Dis. 1996, 54, 258–260. [Google Scholar] [PubMed]
- Bamford, D.J.; Paul, A.S.; Noble, J.; Davies, D.R. Avoidable complications of arthroscopic surgery. J. R. Coll. Surg. Edinb. 1993, 38, 92–95. [Google Scholar] [PubMed]
- Small, N.C. Complications in arthroscopic surgery of the knee and shoulder. Orthopedics 1993, 16, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Saksena, J.; Platts, A.D.; Dowd, G.S.E. Recurrent haemarthrosis following total knee replacement. Knee 2010, 17, 7–14. [Google Scholar] [CrossRef]
- Liddle, A.D.; Rodriguez-Merchan, E.C. Evidence-Based Management of the Knee in Hemophilia. JBJS Rev. 2017, 5, e12. [Google Scholar] [CrossRef]
- Gualtierotti, R.; Solimeno, L.P.; Peyvandi, F. Hemophilic arthropathy: Current knowledge and future perspectives. J. Thromb. Haemost. 2021, 19, 2112–2121. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, T.; Shikichi, M.; Kitamura, H.; Yanase, H.; Nozawa-Inoue, K. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef]
- Madhok, R.; York, J.; Sturrock, R.D. Haemophilic arthritis. Ann. Rheum. Dis. 1991, 50, 588–591. [Google Scholar] [CrossRef]
- Calcaterra, I.; Iannuzzo, G.; Dell’Aquila, F.; Di Minno, M.N.D. Pathophysiological Role of Synovitis in Hemophilic Arthropathy Development: A Two-Hit Hypothesis. Front. Physiol. 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Valentino, L.A. Blood-induced joint disease: The pathophysiology of hemophilic arthropathy. J. Thromb. Haemost. 2010, 8, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Wyseure, T.; Mosnier, L.O.; von Drygalski, A. Advances and challenges in hemophilic arthropathy. Semin. Hematol. 2016, 53, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Karsten, E.; Breen, E.; Herbert, B.R. Red blood cells are dynamic reservoirs of cytokines. Sci. Rep. 2018, 8, 3101. [Google Scholar] [CrossRef]
- Horobin, J.T.; Sabapathy, S.; Kuck, L.; Simmonds, M.J. Shear Stress and RBC-NOS Serine1177 Phosphorylation in Humans: A Dose Response. Life 2021, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Muirden, K.D.; Fraser, J.R.; Clarris, B. Ferritin formation by synovial cells exposed to haemoglobin in vitro. Ann. Rheum. Dis. 1967, 26, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Ghadially, F.N. Pathology of experimental haemarthrosis. Ann. Rheum. Dis. 1966, 25, 402–415. [Google Scholar] [CrossRef]
- Jansen, N.W.D.; Roosendaal, G.; Lafeber, F.P.J.G. Understanding haemophilic arthropathy: An exploration of current open issues. Br. J. Haematol. 2008, 143, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Tajima, T.; Sekimoto, T.; Yamaguchi, N.; Taniguchi, N.; Kurogi, S.; Maruyama, M.; Chosa, E. Hemoglobin stimulates the expression of ADAMTS-5 and ADAMTS-9 by synovial cells: A possible cause of articular cartilage damage after intra-articular hemorrhage. BMC Musculoskelet. Disord. 2017, 18, 449. [Google Scholar] [CrossRef]
- Senator, G.B.; Muirden, K.D. Concentration of iron in synovial membrane, synovial fluid, and serum in rheumatoid arthritis and other joint diseases. Ann. Rheum. Dis. 1968, 27, 49–54. [Google Scholar] [CrossRef]
- Roosendaal, G.; Vianen, M.E.; Marx, J.J.; van den Berg, H.M.; Lafeber, F.P.; Bijlsma, J.W. Blood-induced joint damage: A human in vitro study. Arthritis Rheum. 1999, 42, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Hoaglund, F.T. Experimental hemarthrosis. The response of canine knees to injections of autologous blood. J. Bone Jt. Surg. Am. 1967, 49, 285–298. [Google Scholar] [CrossRef]
- Ostalowska, A.; Birkner, E.; Wiecha, M.; Kasperczyk, S.; Kasperczyk, A.; Kapolka, D.; Zon-Giebel, A. Lipid peroxidation and antioxidant enzymes in synovial fluid of patients with primary and secondary osteoarthritis of the knee joint. Osteoarthr. Cartil. 2006, 14, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Convery, F.R.; Woo, S.L.; Akeson, W.H.; Amiel, D.; Malcom, L.L. Experimental hemarthrosis in the knee of the mature canine. Arthritis Rheum. 1976, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, R.; Liu, S.; Duan, T.; Zhai, L.; Zhang, M.; Han, X.; Xiang, Y.; Huang, X.; Lin, H.; et al. RSL3 Drives Ferroptosis through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front. Pharmacol. 2018, 9, 1371. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zilka, O.; Shah, R.; Li, B.; Friedmann Angeli, J.P.; Griesser, M.; Conrad, M.; Pratt, D.A. On the Mechanism of Cytoprotection by Ferrostatin-1 and Liproxstatin-1 and the Role of Lipid Peroxidation in Ferroptotic Cell Death. ACS Cent. Sci. 2017, 3, 232–243. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. [Google Scholar] [CrossRef]
- Rodriguez-Merchan, E.C. The Current Role of Disease-modifying Osteoarthritis Drugs. Arch. Bone Jt. Surg. 2023, 11, 11–22. [Google Scholar]
- Hooiveld, M.J.J.; Roosendaal, G.; van den Berg, H.M.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. Haemoglobin-derived iron-dependent hydroxyl radical formation in blood-induced joint damage: An in vitro study. Rheumatology 2003, 42, 784–790. [Google Scholar] [CrossRef]
- Moradi, B.; Rosshirt, N.; Tripel, E.; Kirsch, J.; Barié, A.; Zeifang, F.; Gotterbarm, T.; Hagmann, S. Unicompartmental and bicompartmental knee osteoarthritis show different patterns of mononuclear cell infiltration and cytokine release in the affected joints. Clin. Exp. Immunol. 2015, 180, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Pessler, F.; Chen, L.X.; Dai, L.; Gomez-Vaquero, C.; Diaz-Torne, C.; Paessler, M.E.; Scanzello, C.; Cakir, N.; Einhorn, E.; Schumacher, H.R. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans’ Illness and joint pain compared to normal and osteoarthritis synovium. Clin. Rheumatol. 2008, 27, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Stramer, B.M.; Mori, R.; Martin, P. The inflammation-fibrosis link? A Jekyll and Hyde role for blood cells during wound repair. J. Investig. Dermatol. 2007, 127, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int. Rev. Cytol. 2007, 257, 143–179. [Google Scholar] [PubMed]
- Kovacs, E.J.; DiPietro, L.A. Fibrogenic cytokines and connective tissue production. FASEB J. 1994, 8, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Smith, P.A.; Stannard, J.P.; Pfeiffer, F.M.; Kuroki, K.; Bozynski, C.C.; Cook, C.R. A canine hybrid double-bundle model for study of arthroscopic ACL reconstruction. J. Orthop. Res. 2015, 33, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Hooiveld, M.J.J.; Roosendaal, G.; Jacobs, K.M.G.; Vianen, M.E.; van den Berg, H.M.; Bijlsma, J.W.J.; Lafeber, F.P.J.G. Initiation of degenerative joint damage by experimental bleeding combined with loading of the joint: A possible mechanism of hemophilic arthropathy. Arthritis Rheum. 2004, 50, 2024–2031. [Google Scholar] [CrossRef]
- Thomas, N.P.; Wu, W.J.; Fleming, B.C.; Wei, F.; Chen, Q.; Wei, L. Synovial inflammation plays a greater role in post-traumatic osteoarthritis compared to idiopathic osteoarthritis in the Hartley guinea pig knee. BMC Musculoskelet. Disord. 2017, 18, 556. [Google Scholar] [CrossRef]
- Muirden, K.D. Clearnace of Fe59-labelled erythrocytes from normal and inflamed rabbit knee joints. I. Relationship to the anaemia of rheumatoid arthritis. Ann. Rheum. Dis. 1969, 28, 548–551. [Google Scholar] [CrossRef]
- Hakobyan, N.; Enockson, C.; Cole, A.A.; Sumner, D.R.; Valentino, L.A. Experimental haemophilic arthropathy in a mouse model of a massive haemarthrosis: Gross, radiological and histological changes. Haemoph. Off. J. World Fed. Hemoph. 2008, 14, 804–809. [Google Scholar] [CrossRef]
- Jansen, N.W.D.; Roosendaal, G.; Bijlsma, J.W.J.; Degroot, J.; Lafeber, F.P.J.G. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: An in vitro study. Arthritis Rheum. 2007, 56, 199–207. [Google Scholar] [CrossRef]
- van Meegeren, M.E.R.; Roosendaal, G.; Barten-van Rijbroek, A.D.; Schutgens, R.E.G.; Lafeber, F.P.J.G.; Mastbergen, S.C. Coagulation aggravates blood-induced joint damage in dogs. Arthritis Rheum. 2012, 64, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Huebner, K.D.; Shrive, N.G.; Frank, C.B. New surgical model of post-traumatic osteoarthritis: Isolated intra-articular bone injury in the rabbit. J. Orthop. Res. 2013, 31, 914–920. [Google Scholar] [CrossRef]
- Bajpayee, A.G.; De la Vega, R.E.; Scheu, M.; Varady, N.H.; Yannatos, I.A.; Brown, L.A.; Krishnan, Y.; Fitzsimons, T.J.; Bhattacharya, P.; Frank, E.H.; et al. Sustained intra-cartilage delivery of low dose dexamethasone using a cationic carrier for treatment of post traumatic osteoarthritis. Eur. Cells Mater. 2017, 34, 341–364. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, K.; Yu, S.; Luo, J.; Guo, J.; Lin, J.; Wang, G.; Guo, Z.; Ye, Y.; Guo, F. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 2020, 27, 33–43. [Google Scholar] [CrossRef] [PubMed]
- McCarty, W.J.; Luan, A.; Siddiqui, M.; Hansen, B.C.; Masuda, K.; Sah, R.L. The Biomechanical Properties of Mixtures of Blood and Synovial Fluid. J. Orthop. Res. 2011, 29, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Mapp, P.I.; Walsh, D.A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011, 63, 2700–2710. [Google Scholar] [CrossRef]
- Stefani, R.M.; Halder, S.S.; Estell, E.G.; Lee, A.J.; Silverstein, A.M.; Sobczak, E.; Chahine, N.O.; Ateshian, G.A.; Shah, R.P.; Hung, C.T. A Functional Tissue-Engineered Synovium Model to Study Osteoarthritis Progression and Treatment. Tissue Eng. Part A 2019, 25, 538–553. [Google Scholar] [CrossRef]
- Machado, T.S.L.; Massoco, C.O.; Silva, L.C.L.C.; Fülber, J.; Moreira, J.J.; Baccarin, R.Y.A. Effects of blood-derived products and sodium hyaluronate on equine synovial fluid cells and on synovial fluid from osteochondrotic joints of horses after arthroscopy and administration of treatment. Am. J. Vet. Res. 2019, 80, 646–656. [Google Scholar] [CrossRef]
- Heard, B.J.; Barton, K.I.; Agbojo, O.M.; Chung, M.; Sevick, J.L.; Bader, T.J.; Martin, C.R.; Shrive, N.G.; Hart, D.A. Molecular Response of Rabbit Menisci to Surgically Induced Hemarthrosis and a Single Intra-Articular Dexamethasone Treatment. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2019, 37, 2043–2052. [Google Scholar] [CrossRef]
- Betsch, K.; Martinez, V.G.; Lyons, L.P.; Brice Weinberg, J.; Wittstein, J.R.; McNulty, A.L. Shedding light on the effects of blood on meniscus tissue: The role of mononuclear leukocytes in mediating meniscus catabolism. Osteoarthr. Cartil. 2024, 32, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Niikura, T.; Miwa, M.; Sakai, Y.; Lee, S.Y.; Kuroda, R.; Fujishiro, T.; Kubo, S.; Doita, M.; Kurosaka, M. Human hemarthrosis-derived progenitor cells can differentiate into osteoblast-like cells in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Maerz, T.; Fleischer, M.; Newton, M.D.; Davidson, A.; Salisbury, M.; Altman, P.; Kurdziel, M.D.; Anderson, K.; Bedi, A.; Baker, K.C. Acute mobilization and migration of bone marrow-derived stem cells following anterior cruciate ligament rupture. Osteoarthr. Cartil. 2017, 25, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Murray, M.M. Peripheral Blood Mononuclear Cells Enhance the Anabolic Effects of Platelet-Rich Plasma on Anterior Cruciate Ligament Fibroblasts. J. Orthop. Res. 2013, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hettinga, D.L.I.I. Normal Joint Structures and Their Reaction to Injury. J. Orthop. Sports Phys. Ther. 1979, 1, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pulles, A.E.; Mastbergen, S.C.; Schutgens, R.E.G.; Lafeber, F.P.J.G.; van Vulpen, L.F.D. Pathophysiology of hemophilic arthropathy and potential targets for therapy. Pharmacol. Res. 2017, 115, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Oh, H.C.; Park, S.H.; Lee, S.; Lee, Y.; Kim, S.H. Treatment of Recurrent Hemarthrosis after Total Knee Arthroplasty. Knee Surg. Relat. Res. 2018, 30, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Q.Y.; Teng, X.H.; Li, Z.P.; Zhu, M.T.; Gu, C.J.; Chen, B.J.; Xie, Q.Q.; LuO, X.J. The pathogenesis and regulatory role of HIF-1 in rheumatoid arthritis. Cent-Eur. J. Immunol. 2023, 48, 338–345. [Google Scholar] [CrossRef]
- Pettersson, H.; Ahlberg, A.; Nilsson, I.M. A radiologic classification of hemophilic arthropathy. Clin. Orthop. 1980, 149, 153–159. [Google Scholar] [CrossRef]
- Fritschy, D.; Daniel, D.M.; Rossman, D.; Rangger, C. Bone imaging after acute knee hemarthrosis. Knee Surg. Sports Traumatol. Arthrosc. 1993, 1, 20–27. [Google Scholar] [CrossRef]
- Mizuno, K.; Mineo, K.; Tachibana, T.; Sumi, M.; Matsubara, T.; Hirohata, K. The osteogenetic potential of fracture haematoma. Subperiosteal and intramuscular transplantation of the haematoma. J. Bone Jt. Surg. Br. 1990, 72, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Boettger, M.K.; Krucker, S.; Gajda, M.; Schaible, H.G.; Hilberg, T. Repeated autologous intraarticular blood injections as an animal model for joint pain in haemophilic arthropathy. Arthritis Res. Ther. 2013, 15, R148. [Google Scholar] [CrossRef] [PubMed]
- Madhok, R.; Bennett, D.; Sturrock, R.D.; Forbes, C.D. Mechanisms of joint damage in an experimental model of hemophilic arthritis. Arthritis Rheum. 1988, 31, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Tessier, J.E.; Salimi, Z.; Restrepo, G.L.; Samuels, L.D.; Joist, J.H. Experimental hemarthrosis in dogs: Progressive synovitis monitored with technetium-99m pyrophosphate joint imaging. Haemostasis 1988, 18, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Hung, C.T.; Kuroki, K.; Stoker, A.M.; Cook, C.R.; Pfeiffer, F.M.; Sherman, S.L.; Stannard, J.P. Animal models of cartilage repair. Bone Jt. Res. 2014, 3, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.H.; Capito, N.; Kuroki, K.; Stoker, A.M.; Cook, J.L.; Sherman, S.L. A Review of Translational Animal Models for Knee Osteoarthritis. Arthritis 2012, 2012, 764621. [Google Scholar] [CrossRef]
- Lv, Z.; Han, J.; Li, J.; Guo, H.; Fei, Y.; Sun, Z.; Dong, J.; Wang, M.; Fan, C.; Li, W.; et al. Single cell RNA-seq analysis identifies ferroptotic chondrocyte cluster and reveals TRPV1 as an anti-ferroptotic target in osteoarthritis. eBioMedicine 2022, 84, 104258. [Google Scholar] [CrossRef]
- Bozynski, C.C.; Stannard, J.P.; Smith, P.; Hanypsiak, B.T.; Kuroki, K.; Stoker, A.; Cook, C.; Cook, J.L. Acute Management of Anterior Cruciate Ligament Injuries Using Novel Canine Models. J. Knee Surg. 2016, 29, 594–603. [Google Scholar] [CrossRef]
- Vavken, P.; Murray, M.M. The potential for primary repair of the ACL. Sports Med. Arthrosc. Rev. 2011, 19, 44–49. [Google Scholar] [CrossRef]
- Jansen, N.W.D.; Roosendaal, G.; Wenting, M.J.G.; Bijlsma, J.W.J.; Theobald, M.; Hazewinkel, H.A.W.; Lafeber, F.P.J.G. Very rapid clearance after a joint bleed in the canine knee cannot prevent adverse effects on cartilage and synovial tissue. Osteoarthr. Cartil. 2009, 17, 433–440. [Google Scholar] [CrossRef]
- Bozynski, C.C.; Kuroki, K.; Stannard, J.P.; Smith, P.A.; Stoker, A.M.; Cook, C.R.; Cook, J.L. Evaluation of Partial Transection versus Synovial Debridement of the ACL as Novel Canine Models for Management of ACL Injuries. J. Knee Surg. 2015, 28, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.L.; Brandt, K.D.; O’Connor, B.L.; Visco, D.M.; Albrecht, M.E. Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum. 1990, 33, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Lima, E.G.; Bian, L.; O’Conor, C.J.; Jayabalan, P.S.; Stoker, A.M.; Kuroki, K.; Cook, C.R.; Ateshian, G.A.; Cook, J.L.; et al. Passaged Adult Chondrocytes Can Form Engineered Cartilage with Functional Mechanical Properties: A Canine Model. Tissue Eng. Part A 2010, 16, 1041–1051. [Google Scholar] [CrossRef]
- Stefani, R.M.; Barbosa, S.; Tan, A.R.; Setti, S.; Stoker, A.M.; Ateshian, G.A.; Cadossi, R.; Vunjak-Novakovic, G.; Aaron, R.K.; Cook, J.L.; et al. Pulsed electromagnetic fields promote repair of focal articular cartilage defects with engineered osteochondral constructs. Biotechnol. Bioeng. 2020, 117, 1584–1596. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained Low-dose Dexamethasone Delivery Via a PLGA Microsphere-embedded Agarose Implant for Enhanced Osteochondral Repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Fox, D.B.; Malaviya, P.; Tomlinson, J.L.; Farr, J.; Kuroki, K.; Cook, C.R. Evaluation of small intestinal submucosa grafts for meniscal regeneration in a clinically relevant posterior meniscectomy model in dogs. J. Knee Surg. 2006, 19, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.L.; Stoker, A.M.; Stannard, J.P.; Kuroki, K.; Cook, C.R.; Pfeiffer, F.M.; Bozynski, C.; Hung, C.T. A novel system improves preservation of osteochondral allografts. Clin. Orthop. 2014, 472, 3404–3414. [Google Scholar] [CrossRef]
- Cook, J.L.; Smith, P.; Stannard, J.P.; Pfeiffer, F.; Kuroki, K.; Bozynski, C.C.; Cook, C. A Canine Arthroscopic Anterior Cruciate Ligament Reconstruction Model for Study of Synthetic Augmentation of Tendon Allografts. J. Knee Surg. 2017, 30, 704–711. [Google Scholar]
- Heard, B.J.; Barton, K.I.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. Single intra-articular dexamethasone injection immediately post-surgery in a rabbit model mitigates early inflammatory responses and post-traumatic osteoarthritis-like alterations. J. Orthop. Res. 2015, 33, 1826–1834. [Google Scholar] [CrossRef]
- Heard, B.J.; Solbak, N.M.; Chung, M.; Achari, Y.; Shrive, N.G.; Frank, C.B.; Hart, D.A. The infrapatellar fat pad is affected by injury induced inflammation in the rabbit knee: Use of dexamethasone to mitigate damage. Inflamm. Res. 2016, 65, 459–470. [Google Scholar] [CrossRef]
- Ishizue, K.K.; Lyon, R.M.; Amiel, D.; Woo, S.L. Acute hemarthrosis: A histological, biochemical, and biomechanical correlation of early effects on the anterior cruciate ligament in a rabbit model. J. Orthop. Res. 1990, 8, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, C.; Zhong, Y.; He, J.; Cao, X.; Xia, H.; Ba, H.; Li, P.; Wu, S.; Peng, C. Interleukin-17 Can Induce Osteoarthritis in Rabbit Knee Joints Similar to Hulth’s Method. BioMed Res. Int. 2017, 2017, 2091325. [Google Scholar] [CrossRef]
- Ikeda, Y.; Hamano, H.; Horinouchi, Y.; Miyamoto, L.; Hirayama, T.; Nagasawa, H.; Tamaki, T.; Tsuchiya, K. Role of ferroptosis in cisplatin-induced acute nephrotoxicity in mice. J. Trace Elem. Med. Biol. 2021, 67, 126798. [Google Scholar] [CrossRef]
- Proffen, B.L.; Sieker, J.T.; Murray, M.M.; Akelman, M.R.; Chin, K.E.; Perrone, G.S.; Patel, T.K.; Fleming, B.C. Extracellular Matrix-Blood Composite Injection Reduces Post-Traumatic Osteoarthritis after Anterior Cruciate Ligament Injury in the Rat. J. Orthop. Res. 2016, 34, 995–1003. [Google Scholar] [CrossRef]
- Li, Q.; Han, X.; Lan, X.; Gao, Y.; Wan, J.; Durham, F.; Cheng, T.; Yang, J.; Wang, Z.; Jiang, C. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2017, 2, e90777. [Google Scholar] [CrossRef]
- Miao, Y.; Chen, Y.; Xue, F.; Liu, K.; Zhu, B.; Gao, J.; Yin, J.; Zhang, C.; Li, G. Contribution of ferroptosis and GPX4′s dual functions to osteoarthritis progression. eBioMedicine 2022, 76, 103847. [Google Scholar] [CrossRef] [PubMed]
- Ankay Yilbas, A.; Akca, B.; Buyukakkus, B.; Bahador Zirh, E.; Zeybek, D.; Uzumcugil, F.; Saricaoglu, F. Procaine and saline have similar effects on articular cartilage and synovium in rat knee. BMC Anesthesiol. 2018, 18, 51. [Google Scholar] [CrossRef]
- Magarian, E.M.; Fleming, B.C.; Harrison, S.L.; Mastrangelo, A.N.; Badger, G.J.; Murray, M.M. Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am. J. Sports Med. 2010, 38, 2528–2534. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; DiLallo, M.; Feeley, B.T.; Lansdown, D.A. Osteoarthritis and ACL Reconstruction-Myths and Risks. Curr. Rev. Musculoskelet. Med. 2020, 13, 115–122. [Google Scholar] [CrossRef]
- Betre, H.; Liu, W.; Zalutsky, M.R.; Chilkoti, A.; Kraus, V.B.; Setton, L.A. A thermally responsive biopolymer for intra-articular drug delivery. J. Control Release 2006, 115, 175–182. [Google Scholar] [CrossRef]
- Mapara, M.; Thomas, B.S.; Bhat, K.M. Rabbit as an animal model for experimental research. Dent. Res. J. 2012, 9, 111–118. [Google Scholar]
- Cook, J.L.; Hudson, C.C.; Kuroki, K. Autogenous osteochondral grafting for treatment of stifle osteochondrosis in dogs. Vet. Surg. 2008, 37, 311–321. [Google Scholar] [CrossRef]
- Punzi, L.; Galozzi, P.; Luisetto, R.; Favero, M.; Ramonda, R.; Oliviero, F.; Scanu, A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open 2016, 2, e000279. [Google Scholar] [CrossRef]
- Diermeier, T.A.; Rothrauff, B.B.; Engebretsen, L.; Lynch, A.; Svantesson, E.; Hamrin Senorski, E.A.; Meredith, S.J.; Rauer, T.; Ayeni, O.R.; Paterno, M.; et al. Treatment after ACL injury: Panther Symposium ACL Treatment Consensus Group. Br. J. Sports Med. 2021, 55, 14–22. [Google Scholar] [CrossRef]
- Kraus, V.B.; Stabler, T.V.; Kong, S.Y.; Varju, G.; McDaniel, G. Measurement of synovial fluid volume using urea. Osteoarthr. Cartil. 2007, 15, 1217–1220. [Google Scholar] [CrossRef]
- Anz, A.W.; Branch, E.A.; Rodriguez, J.; Chillemi, F.; Bruce, J.R.; Murphy, M.B.; Suzuki, R.K.; Andrews, J.R. Viable Stem Cells Are in the Injury Effusion Fluid and Arthroscopic Byproducts from Knee Cruciate Ligament Surgery: An In Vivo Analysis. Arthrosc. J. Arthrosc. Relat. Surg. 2017, 33, 790–797. [Google Scholar] [CrossRef]
- Sinkler, M.A.; Furdock, R.J.; McMellen, C.J.; Calcei, J.G.; Voos, J.E. Biologics, Stem Cells, Growth Factors, Platelet-Rich Plasma, Hemarthrosis, and Scaffolds May Enhance Anterior Cruciate Ligament Surgical Treatment. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 39, 166–175. [Google Scholar] [CrossRef]
- Wu, P.I.K.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. 2016, 27, 825–853. [Google Scholar] [CrossRef]
- Malanga, G.A.; Goldin, M. PRP: Review of the current evidence for musculoskeletal conditions. Curr. Phys. Med. Rehabil. Rep. 2014, 2, 1–15. [Google Scholar] [CrossRef]
- Davis, V.L.; Abukabda, A.B.; Radio, N.M.; Witt-Enderby, P.A.; Clafshenkel, W.P.; Cairone, J.V.; Rutkowski, J.L. Platelet-rich preparations to improve healing. Part I: Workable options for every size practice. J. Oral. Implantol. 2014, 40, 500–510. [Google Scholar] [CrossRef]
- Fufa, D.; Shealy, B.; Jacobson, M.; Kevy, S.; Murray, M.M. Activation of platelet-rich plasma using soluble type I collagen. J. Oral. Maxillofac. Surg. 2008, 66, 684–690. [Google Scholar] [CrossRef]
- Kerwar, S.S.; Englert, M.E.; McReynolds, R.A.; Landes, M.J.; Lloyd, J.M.; Oronsky, A.L.; Wilson, F.J. Type II collagen-induced arthritis. Studies with purified anticollagen immunoglobulin. Arthritis Rheum. 1983, 26, 1120–1131. [Google Scholar] [CrossRef]
- Figueroa, D.; Figueroa, F.; Calvo, R.; Vaisman, A.; Ahumada, X.; Arellano, S. Platelet-rich plasma use in anterior cruciate ligament surgery: Systematic review of the literature. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 981–988. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef]
- Mishima, E.; Ito, J.; Wu, Z.; Nakamura, T.; Wahida, A.; Doll, S.; Tonnus, W.; Nepachalovich, P.; Eggenhofer, E.; Aldrovandi, M.; et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 2022, 608, 778–783. [Google Scholar] [CrossRef]
- Li, J.; Xu, Z.; OuYang, C.; Wu, X.; Xie, Y.; Xie, J. Protopine alleviates lipopolysaccharide-triggered intestinal epithelial cell injury through retarding the NLRP3 and NF-κB signaling pathways to reduce inflammation and oxidative stress. Allergol. Immunopathol. 2022, 50, 84–92. [Google Scholar]
- Yue, M.; Huang, J.; Ma, X.; Huang, P.; Liu, Y.; Zeng, J. Protopine Alleviates Dextran Sodium Sulfate-Induced Ulcerative Colitis by Improving Intestinal Barrier Function and Regulating Intestinal Microbiota. Molecules 2023, 28, 5277. [Google Scholar] [CrossRef]
- Hiller, K.O.; Ghorbani, M.; Schilcher, H. Antispasmodic and relaxant activity of chelidonine, protopine, coptisine, and Chelidonium majus extracts on isolated guinea-pig ileum. Planta Med. 1998, 64, 758–760. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Y.; Sang, W.; Wang, C.; Lu, H.; Lai, P.; Zhu, L.; Ma, J. Protopine protects chondrocytes from undergoing ferroptosis by activating Nrf2 pathway. Biochem. Biophys. Res. Commun. 2024, 710, 149599. [Google Scholar] [CrossRef]
- Li, Z.A.; Sant, S.; Cho, S.K.; Goodman, S.B.; Bunnell, B.A.; Tuan, R.S.; Gold, M.S.; Lin, H. Synovial joint-on-a-chip for modeling arthritis: Progress, pitfalls, and potential. Trends Biotechnol. 2023, 41, 511–527. [Google Scholar] [CrossRef]
- Guilak, F.A.; Hung, C.T. Physical Regulation of Cartilage Metabolism. Basic Orthop. Biomech. 2005, 259–300. [Google Scholar]
- Mauck, R.L.; Hung, C.T.; Ateshian, G.A. Modeling of Neutral Solute Transport in a Dynamically Loaded Porous Permeable Gel: Implications for Articular Cartilage Biosynthesis and Tissue Engineering. J. Biomech. Eng. 2003, 125, 602–614. [Google Scholar] [CrossRef]
- Gangi, L.R.; Petersen, C.A.; Oungoulian, S.R.; Estell, E.G.; Durney, K.M.; Suh, J.T.; Ateshian, G.A.; Hung, C.T. A Friction Testing-Bioreactor Device for Study of Synovial Joint Biomechanics, Mechanobiology, and Physical Regulation. J. Vis. Exp. 2022, 184, e63880. [Google Scholar]
- Sakhrani, N.; Stefani, R.M.; Setti, S.; Cadossi, R.; Ateshian, G.A.; Hung, C.T. Pulsed Electromagnetic Field Therapy and Direct Current Electric Field Modulation Promote the Migration of Fibroblast-like Synoviocytes to Accelerate Cartilage Repair In Vitro. Appl. Sci. 2022, 12, 12406. [Google Scholar] [CrossRef]



| Reference | Experimental Design | Research Findings | |
|---|---|---|---|
| CARTILAGE | Convery et al., 1976 [55] | Biochemical and biomechanical analyses of mongrel dogs subject to continuous hemarthrosis [55] | Significant decrease in GAG content observed as early as 4 weeks. Total collagen content was not significantly affected until after 12 and 16 weeks. Experimental cartilage lost significant amount of mechanical integrity as a result of continuous hemarthrosis [55]. |
| Roosendaal et al., 1999 [52] | Co-culture of human cartilage tissue with RBC or mononuclear cells (MNC) + RBC followed by a 12-day recovery period [52] | Acute MNC + RBC exposure resulted in prolonged dose-dependent inhibition of proteoglycan synthesis and chondrocyte death [52]. | |
| Hooiveld et al., 2003 [9] | Co-culture of human cartilage tissue with whole blood, mononuclear cells + RBC, or caspase inhibitors followed by a 12-day recovery period [9] | After 4-day exposure of blood treatment, apoptosis occurred in chondrocytes. Caspase inhibitors partially restored proteoglycan synthesis after blood exposure [9] | |
| Hooiveld et al., 2003 [61] | Co-culture of human cartilage tissue with RBC, mononuclear cells + RBC, CD14+ cells + RBC, or lysed RBC with IL-1 followed by a 12-day recovery period [61] | Lysed RBC + IL-1 produced by monocytes inhibited proteoglycan synthesis and increased production of hydrogen peroxide. Hemoglobin-derived iron + hydrogen peroxide resulted in formation of hydroxyl radicals that lead to further chondrocyte damage. DMSO and hydroxyl radicals can restore cartilage proteoglycan synthesis [61]. | |
| Jansen et al., 2007 [72] | Healthy human articular cartilage explants cultured in the presence or absence of varying blood concentrations for different time intervals followed by a 12-day recovery period after withdrawal of blood [72] | 50% v/v blood exposure to cartilage led to detrimental effects independent of time. Blood concentration-dependent effects were observed and after 2 days of exposure of at least 10% v/v, effects were irreversible [72]. | |
| van Meegeren et al., 2012 [73] | Human cartilage explants exposed to coagulating and anticoagulated blood, plasma and serum [73] | Exposure of coagulating blood resulted in more damage than anticoagulated blood. In absence of mononuclear cells and RBC, plasma and serum exposure did not alter cartilage matrix turnover [73]. | |
| Huebner et al., 2013 [74] | Gene expression analyses of cartilage from a rabbit bone drill PTOA model [74] | Upregulation of cartilage degradation markers including TGF and MMP13, suggesting that intra-articular bone injury contributes to progressive cartilage damage consistent with OA in adult rabbits [74]. | |
| van Meegeren et al., 2013 [7] | Evaluation of biochemical properties of canine explants comparing effects of acute blood exposure vs. intermittent intra-articular blood injections [7] | Explants exposed to acute and micro bleeds saw increased proteoglycan synthesis rates. Release of newly formed GAGs was significantly increased following acute blood exposure [7] | |
| Bajpayee et al., 2017 [75] | Gene expression and biochemical analysis of rabbit cartilage following intra-articular DEX administration in a rabbit ACL transection model [75] | No significant differences observed in collagen content between ACLT and control. Significant decrease in GAG content observed in cartilage of ACLT in comparison to control. Avidin-DEX treatment suppressed catabolic gene expression and joint swelling significantly more than free DEX treatment [75]. | |
| Yao et al., 2020 [76] | Simulate inflammation and iron overload via administration of IL-1 and ferric ammonium citrate [76] | Under inflammation and iron overload, chondrocytes undergo ferroptosis. Ferroptotic induction via erastin caused increased cartilage degradation enzymes and decreased collagen II expression [76]. | |
| Lee et al., 2023 [11] | Co-culture of human chondrocyte-based tissue engineered cartilage and human chondrocyte monolayer with intact RBC and RBC lysates [11] | Markers of tissue breakdown were observed in cartilage constructs without parallel losses in DNA. Dose-dependent loss of viability observed in chondrocyte monolayers, with greater toxicity observed with lysates; intact RBCs induced changes to lipid profiles and upregulated highly oxidizable fatty acids; RBC lysates induced cell death via ferroptosis [11] | |
| SYNOVIUM | Muirden et al., 1967 [47] | Assess ferritin formation by synovial cells exposed to hemoglobin [47] | Findings suggest that synovial cells release iron from hemoglobin thereby synthesizing apoferritin without other cell intervention [47] |
| Roosendaal et al., 1999 [24] | Analysis of catabolic activity of supernatants of synovial tissue after intraarticular blood exposure in canine model [24] | After 4 days of blood exposure, synovial tissue had no significant catabolic activity. After 16 days, synovial tissue had significant cartilage destructive properties. The supernatant of synovial tissue decreased total proteoglycan content and lowered proteoglycan synthesis [24]. | |
| Hooiveld et al., 2004 [69] | Biochemical analysis of synovial fluid of PTOA guinea pig model [69] | Stromal cell-derived factor-1 (SDF-1) and MMP13 synovial fluid concentration were statistically similar between idiopathic and PTOA groups suggesting that other inflammatory factors may be involved [69] | |
| McCarty et al., 2011 [77] | Characterize composition, coagulation and mechanical properties of blood and synovial fluid mixtures [77] | Dilution of blood with SF decreased mechanical stiffness of the final clot structure, altered coagulation torque profile over time, and increased fluid permeability. In comparison to control, SF had a lesser effect on mechanical properties of clot possibly due to presence of HA [77] | |
| Ashraf et al., 2011 [78] | Measure synovial inflammation and angiogenesis as a result of meniscal transection in a rat OA model [78] | Synovial inflammation increased 24 h after drug-induced synovitis. Increase of macrophage infiltration and endothelial cell proliferation confirmed synovitis and angiogenesis after meniscal transection. Anti-inflammatory drugs inhibited synovitis and synovial angiogenesis [78]. | |
| Stefani et al., 2019 [79] | Investigation of solute transport measures in bovine tissue-engineered synovium model [79] | NO and hyaluronic acid (HA) secretion of engineered synovium was similar to native synovial tissue. IL-treated engineered synovium displayed characteristics of human OA synovium [79]. | |
| Machado et al., 2019 [80] | Compare effects of blood-derived products and sodium hyaluronate on equine synovial fluid cells from osteochondrotic joint [80] | All treatments decreased production of reactive oxygen species. PRP increased prostaglandin (PG) E2 concentrations and PRP, APP, and IRAP increased IL-1 receptor antagonist proteins [80] | |
| MENISCUS | Heard et al., 2019 [81] | Acute controlled response of meniscal cell to blood and blood + DEX [81] | Autologous blood exposure did not affect meniscal cell viability but blood + DEX treatment reduced cell viability suggesting that meniscal cells may be susceptible to glucocorticoid-mediated apoptosis [81]. |
| Betsch et al., 2024 [82] | Porcine explants and primary meniscus cells exposed to various concentrations of whole blood for 3 days to simulate injury [82] | Acute whole blood exposure increased MMP activity. Blood-derived mononuclear leukocytes increased NO release and MMP activity but decreased GAG content suggesting that they activate catabolic pathways that can lead to meniscal degradation. Isolated intact RBC did not affect meniscus catabolism [82]. | |
| BONE | Niikura et al., 2005 [83] | Assess potential of hemarthrosis-derived cells to differentiate into osteoblast-like cells via gene expression and osteoblastic markers [83] | Osteoblastic gene markers significantly increased in hemarthrosis-derived cells in comparison to control and cell morphology was cuboidal shaped. Human hemarthrosis-derived cells contain osteoprogenitor cells and can be used for bone regeneration [83] |
| Maerz et al., 2017 [84] | Rats were subjected to noninvasive ACL rupture. Whole blood MSC concentration was assessed using flow cytometry and synovial fluid and serum were assayed for stromal cell-derived factor (SDF-1) [84] | Following ACL rupture, there was a significant increase in bone marrow-derived MSC concentration and SDF-1. Cell tracking indicated active recruitment of MSCs to the injured joint [84]. | |
| ACL | Jacobson et al., 2008 [14] | Measure effect of platelets and erythrocytes on cytokine release and scaffold contraction in a 3D fibroblast model [14] | Platelet concentration significantly affects fibroblast proliferation, cytokine release, and gel contraction [14] |
| Harrison et al., 2011 [13] | Analyze effect of erythrocyte concentration on wound healing capacity of an ACL fibroblast collagen scaffold [13] | Greater scaffold contraction and fibroblast proliferation of samples with RBC concentrations lower than that in whole blood. Increasing RBC concentration over amount in whole blood stimulates fibroblast collagen production and decreases scaffold contraction [13]. | |
| Yoshida et al., 2013 [85] | Examine effect of peripheral blood mononuclear cells (PBMCs) on 3D ACL fibroblast collagen scaffold [85] | Co-culture of PBMCs and ACL fibroblasts without presence of platelets had no effect. PBMCs + platelet exposure led to an increase in collagen gene and protein expression as well as increased IL-6 expression [85]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicholson, H.J., III; Sakhrani, N.; Rogot, J.; Lee, A.J.; Ojediran, I.G.; Sharma, R.; Chahine, N.O.; Ateshian, G.A.; Shah, R.P.; Hung, C.T. Effect of Blood on Synovial Joint Tissues: Potential Role of Ferroptosis. Appl. Sci. 2024, 14, 6292. https://doi.org/10.3390/app14146292
Nicholson HJ III, Sakhrani N, Rogot J, Lee AJ, Ojediran IG, Sharma R, Chahine NO, Ateshian GA, Shah RP, Hung CT. Effect of Blood on Synovial Joint Tissues: Potential Role of Ferroptosis. Applied Sciences. 2024; 14(14):6292. https://doi.org/10.3390/app14146292
Chicago/Turabian StyleNicholson, Howard J., III, Neeraj Sakhrani, James Rogot, Andy J. Lee, Inioluwa G. Ojediran, Ratna Sharma, Nadeen O. Chahine, Gerard A. Ateshian, Roshan P. Shah, and Clark T. Hung. 2024. "Effect of Blood on Synovial Joint Tissues: Potential Role of Ferroptosis" Applied Sciences 14, no. 14: 6292. https://doi.org/10.3390/app14146292
APA StyleNicholson, H. J., III, Sakhrani, N., Rogot, J., Lee, A. J., Ojediran, I. G., Sharma, R., Chahine, N. O., Ateshian, G. A., Shah, R. P., & Hung, C. T. (2024). Effect of Blood on Synovial Joint Tissues: Potential Role of Ferroptosis. Applied Sciences, 14(14), 6292. https://doi.org/10.3390/app14146292






