Impact of Shaking Exercise on Functional Recovery in Patients with Chronic Post-Stroke Upper Limb Impairment: A Multicenter, Open-Label, Quasi-Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Participants
2.3. Addressing Potential Biases
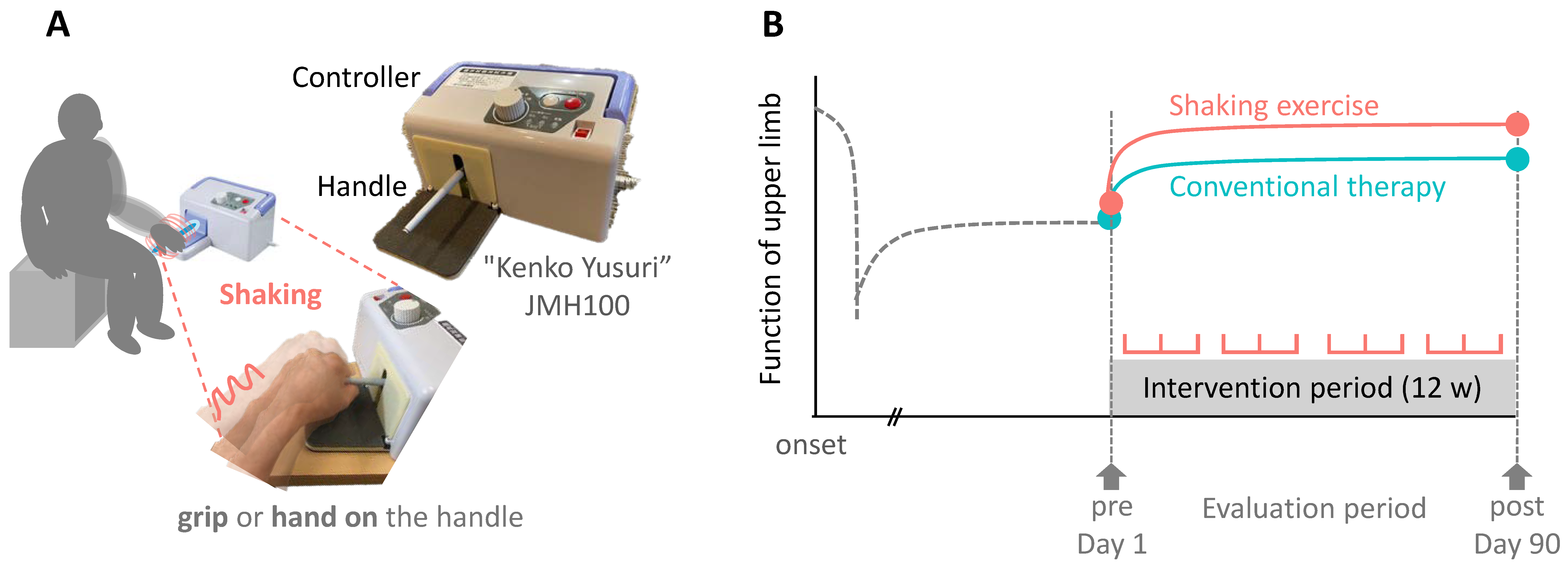
2.4. Self-Training Using the Shaking Device
2.5. Adjunctive Therapy
2.6. Outcome Measures
2.7. Statistical Analysis
| FMA-UE total = β0 + β1 × periods + β2 × group + β3 × (periods × group) + β4 × age + β5 |
| × sex + β6 × diagnosis + β7 × affected side + β8 × BoNT-A dosage + ui + ϵ |
3. Results
3.1. Participants and Baseline Characteristics
3.2. Results from GLMM for FMA-UE Score
3.3. Changes in FMA-UE Scores Pre- and Post-Intervention
3.4. Baseline Characteristics According to Severity
3.5. Stratified Analysis of FMA-UE Score by Severity Level
3.6. Analysis of Secondary Outcomes and Data Imputation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Appendix A
| Intervention Group (n = 39) | Control Group (n = 39) | Between-Group | Mean Change | ||||
|---|---|---|---|---|---|---|---|
| Secondary Endpoints (n = 78) | Pre-Test | Post-Test | Pre-Test | Post-Test | p-Value | Intervention Group | Control Group |
| (95% CI) | |||||||
| MAS (shoulder flexion) | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 1 [1, 2] | 1 [1, 1] ‡ | 0.574 | 0 [1, 0] | 0 [0, 0] |
| MAS (shoulder extension) | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 0.59 | 0 [0, 0] | 0 [0, 0] |
| MAS (Elbow flexion) | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 0.215 | 0 [−1, 0] | 0 [0, 0] |
| MAS (Elbow extension) | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 1 [1, 1] ‡ | 1 [0, 1] ‡ | 0.297 | 0 [−1, 0] | 0 [, 1, 0] |
| MAS (Wrist flexion) | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 1 [1, 2] | 1 [1, 1] ‡ | 0.367 | 0 [−1, 0] | 0 [0, 0] |
| MAS (Wrist extension) | 1 [1, 2] ‡ | 1 [1, 1] ‡ | 1 [1, 2] | 1 [1, 1] ‡ | 0.57 | 0 [−1, 0] | 0 [0, 0] |
| MAS (Finger flexion) | 1 [0, 2] ‡ | 1 [0, 2] | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 0.64 | 0 [−1, 0] | 0 [0, 0] |
| MAS (Finger extension) | 0 [0, 1] ‡ | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 1 [0, 1] ‡ | 0.461 | 0 [0, 0] | 0 [0, 0] |
| Sholder flexion active ROM | 102.50 ± 32.39 † | 102.78 ± 35.65 | 80.00 ± 46.73 | 81.03 ± 47.69 | 0.808 (−5.33, 6.83) | 0.28 ± 10.07 | 1.03 ± 15.06 |
| Sholder flexion passive ROM | 138.47 ± 23.11 | 140.28 ± 22.86 | 131.76 ± 29.56 | 134.12 ± 29.04 | 0.796 (−3.71, 4.81) | 1.81 ± 10.36 | 2.35 ± 7.10 |
| Sholder extension active ROM | 32.08 ± 15.92 | 37.50 ± 13.91 | 30.88 ± 15.54 | 32.21 ± 25.14 | 0.019 (−12.39, −1.09) † | 5.42 ± 14.31 | −1.32 ± 8.47 |
| Sholder extension passive ROM | 49.58 ± 11.97 | 51.06 ± 11.54 | 45.15 ± 11.38 | 43.82 ± 11.68 | 0.162 (−6.78, 1.19) | 1.47 ± 9.67 | −1.32 ± 6.66 |
| Sholder abduction active ROM | 88.19 ± 38.29 † | 88.89 ± 36.80 | 65.29 ± 33.93 | 65.15 ± 35.52 | 0.819 (−8.19, 6.51) | 0.69 ± 16.13 | −0.15 ± 14.59 |
| Sholder abduction passive ROM | 122.50 ± 35.73 | 124.86 ± 35.89 | 113.68 ± 37.42 | 112.06 ± 40.99 | 0.381 (−12.86, 4.90) | 2.36 ± 14.42 | −1.62 ± 22.22 |
| Sholder adduction active ROM | 7.36 ± 20.86 | 7.50 ± 20.79 | 3.09 ± 12.67 | 3.09 ± 12.43 | 0.937 (−3.65, 3.37) | 0.14 ± 8.58 | 0.00 ± 5.77 |
| Sholder adduction passive ROM | 10.56 ± 20.24 | 10.28 ± 19.82 | 7.24 ± 12.06 | 7.21 ± 13.15 | 0.885 (−3.20, 3.70) | −0.28 ± 8.10 | −0.03 ± 6.16 |
| Elbow flexion active ROM | 111.33 ± 21.52 | 109.03 ± 29.71 | 102.50 ± 23.07 | 104.56 ± 21.30 | 0.366 (−5.37, 14.09) | −2.31 ± 26.06 | 2.06 ± 11.69 |
| Elbow flexion passive ROM | 135.14 ± 8.49 | 136.25 ± 8.14 | 132.21 ± 16.34 | 131.47 ± 15.45 | 0.197 (−4.66, 0.97) | 1.11 ± 6.29 | −0.74 ± 6.29 |
| Elbow extension active ROM | −22.78 ± 22.57 | −20.69 ± 21.22 | −24.71 ± 25.88 | −25.29 ± 27.33 | 0.281 (−7.60, 2.26) | 2.08 ± 11.17 | −0.59 ± 9.36 |
| Elbow extension passive ROM | −5.69 ± 12.14 | −5.28 ± 12.07 | −5.15 ± 12.94 | −5.29 ± 13.14 | 0.647 (−3.05, 1.92) | 0.42 ± 6.48 | −0.15 ± 3.37 |
| Wrist flexion active ROM | 23.33 ± 28.23 | 16.67 ± 27.95 | 16.18 ± 30.00 | 17.65 ± 29.47 | 0.026 (0.94, 15.34) † | −6.67 ± 17.81 | 1.47 ± 11.52 |
| Wrist flexion passive ROM | 68.47 ± 13.46 | 66.94 ± 14.80 | 70.44 ± 13.62 | 72.06 ± 12.86 | 0.106 (−0.70, 7.00) | −1.53 ± 8.69 | 1.62 ± 7.36 |
| Wrist extension active ROM | 13.47 ± 38.24 | 23.33 ± 28.88 | 7.65 ± 30.83 | 9.41 ± 28.81 | 0.14 (−19.05, 2.86) | 9.86 ± 28.55 | 1.76 ± 14.87 |
| Wrist extension passive ROM | 58.33 ± 21.18 | 60.14 ± 23.25 | 60.15 ± 18.47 | 63.53 ± 15.15 | 0.522 (−3.33, 6.49) | 1.81 ± 10.77 | 3.38 ± 9.75 |
| Pain VAS | 3.17 ± 3.04 | 2.56 ± 2.50 * | 2.65 ± 2.84 | 2.79 ± 3.05 | 0.03 (−1.44, 0.07) † | −0.61 ± 1.59 | 0.15 ± 1.26 |
References
- Director-General For Statistics IPAIR, Ministry Of Health, Labour And Welfare. Patient Survey 2020 (Classification of Diseases). Ministry of Health, Labour and Welfare. 2020. Available online: https://www.mhlw.go.jp/toukei/saikin/hw/kanja/10syoubyo/dl/r02syobyo.pdf (accessed on 6 May 2024).
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.; Winstein, C.J.; Miller, J.P.; Taub, E.; Uswatte, G.; Morris, D.; Giuliani, C.; Light, K.E.; Nichols-Larsen, D.; Investigators, E. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: The EXCITE randomized clinical trial. JAMA 2006, 296, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Veerbeek, J.M.; van Wegen, E.E.; Wolf, S.L. Constraint-induced movement therapy after stroke. Lancet Neurol. 2015, 14, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Jorgensen, H.S.; Raaschou, H.O.; Olsen, T.S. Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1994, 75, 394–398. [Google Scholar] [CrossRef]
- Palstam, A.; Sjodin, A.; Sunnerhagen, K.S. Participation and autonomy five years after stroke: A longitudinal observational study. PLoS ONE 2019, 14, e0219513. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Vive-Larsen, J.; Stoier, M.; Olsen, T.S. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1995, 76, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Francisco, G.E.; Bandari, D.S.; Bavikatte, G.; Jost, W.H.; McCusker, E.; Largent, J.; Zuzek, A.; Esquenazi, A. High clinician- and patient-reported satisfaction with individualized onabotulinumtoxinA treatment for spasticity across several etiologies from the ASPIRE study. Toxicon X 2020, 7, 100040. [Google Scholar] [CrossRef] [PubMed]
- Francisco, G.E.; Jost, W.H.; Bavikatte, G.; Bandari, D.S.; Tang, S.F.T.; Munin, M.C.; Largent, J.; Adams, A.M.; Zuzek, A.; Esquenazi, A. Individualized OnabotulinumtoxinA Treatment for Upper Limb Spasticity Resulted in High Clinician- and Patient-Reported Satisfaction: Long-Term Observational Results from the ASPIRE Study. PM R 2020, 12, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Niimi, M.; Yamada, N.; Shimamoto, Y.; Masuda, G.; Hara, H.; Abo, M. Prognosis prediction of the effect of botulinum toxin therapy and intensive rehabilitation on the upper arm function in post-stroke patients using hierarchical cluster analysis. Disabil. Rehabil. 2022, 44, 6815–6823. [Google Scholar] [CrossRef]
- Sunnerhagen, K.S.; Francisco, G.E. Enhancing patient-provider communication for long-term post-stroke spasticity management. Acta Neurol. Scand. 2013, 128, 305–310. [Google Scholar] [CrossRef]
- Palmcrantz, S.; Holmqvist, L.W.; Sommerfeld, D.K. Long-term health states relevant to young persons with stroke living in the community in southern Stockholm—A study of self-rated disability and predicting factors. Disabil. Rehabil. 2012, 34, 817–823. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abo, M.; Kakuda, W.; Momosaki, R.; Harashima, H.; Kojima, M.; Watanabe, S.; Sato, T.; Yokoi, A.; Umemori, T.; Sasanuma, J. Randomized, multicenter, comparative study of NEURO versus CIMT in poststroke patients with upper limb hemiparesis: The NEURO-VERIFY Study. Int. J. Stroke 2014, 9, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Kakuda, W.; Abo, M.; Sasanuma, J.; Shimizu, M.; Okamoto, T.; Kimura, C.; Kakita, K.; Hara, H. Combination Protocol of Low-Frequency rTMS and Intensive Occupational Therapy for Post-stroke Upper Limb Hemiparesis: A 6-year Experience of More Than 1700 Japanese Patients. Transl. Stroke Res. 2016, 7, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Sasaki, N.; Kimura, C.; Hara, T.; Yamada, N.; Abo, M. Sleep during low-frequency repetitive transcranial magnetic stimulation is associated with functional improvement in upper limb hemiparesis after stroke. Acta Neurol. Belg. 2019, 119, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, D.; Hamaguchi, T.; Murata, K.; Ito, H.; Nakayama, Y.; Abo, M. Upper Limb Function Recovery by Combined Repetitive Transcranial Magnetic Stimulation and Occupational Therapy in Patients with Chronic Stroke According to Paralysis Severity. Brain Sci. 2023, 13, 284. [Google Scholar] [CrossRef]
- Sanchez-Cuesta, F.J.; Gonzalez-Zamorano, Y.; Arroyo-Ferrer, A.; Moreno-Verdu, M.; Romero-Munoz, J.P. Repetitive transcranial magnetic stimulation of primary motor cortex for stroke upper limb motor sequelae rehabilitation: A systematic review. NeuroRehabilitation 2023, 52, 329–348. [Google Scholar] [CrossRef] [PubMed]
- Almhdawi, K.A.; Mathiowetz, V.G.; White, M.; delMas, R.C. Efficacy of Occupational Therapy Task-oriented Approach in Upper Extremity Post-stroke Rehabilitation. Occup. Ther. Int. 2016, 23, 444–456. [Google Scholar] [CrossRef]
- Rand, D.; Weingarden, H.; Weiss, R.; Yacoby, A.; Reif, S.; Malka, R.; Shiller, D.A.; Zeilig, G. Self-training to improve UE function at the chronic stage post-stroke: A pilot randomized controlled trial. Disabil. Rehabil. 2017, 39, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Nudo, R.J. Recovery after brain injury: Mechanisms and principles. Front. Hum. Neurosci. 2013, 7, 887. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil Neural Repair. 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Propst, M.; Nelson, S.G. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys. Ther. 1983, 63, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.D.; Gregoric, M.; Barnes, M.P.; Wood, D.; Van Wijck, F.; Burridge, J.; Hermens, H.; Johnson, G.R. Spasticity: Clinical perceptions, neurological realities and meaningful measurement. Disabil. Rehabil. 2005, 27, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Noma, T.; Matsumoto, S.; Shimodozono, M.; Etoh, S.; Kawahira, K. Anti-spastic effects of the direct application of vibratory stimuli to the spastic muscles of hemiplegic limbs in post-stroke patients: A proof-of-principle study. J. Rehabil. Med. 2012, 44, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.N.; Lo, P.C.; Yu, Y.S.; Cheuk, C.K.; Tsang, T.H.; Po, A.S.; Chan, C.C. Effects of sensory cueing on voluntary arm use for patients with chronic stroke: A preliminary study. Arch. Phys. Med. Rehabil. 2011, 92, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, Y.; Fukushima, K.; Koyama, T.; Ohashi, Y.; Uchiyama, K.; Takahira, N.; Takaso, M. Impact of Jiggling Exercise as Conservative Treatment for Hip Osteoarthritis: A Report of Two Cases. Case Rep. Orthop. 2020, 2020, 2804193. [Google Scholar] [CrossRef] [PubMed]
- Yoshizuka, H.; Sato, T.; Murakami, J.; Mitsutake, T.; Hiromatsu, M. Short-term changes in radiographic joint space width after jiggling exercise as conservative treatment for hip osteoarthritis: A retrospective case series of nine patients. PLoS ONE 2021, 16, e0253643. [Google Scholar] [CrossRef] [PubMed]
- Hijikata, N.; Kawakami, M.; Ishii, R.; Tsuzuki, K.; Nakamura, T.; Okuyama, K.; Liu, M. Item Difficulty of Fugl-Meyer Assessment for Upper Extremity in Persons With Chronic Stroke With Moderate-to-Severe Upper Limb Impairment. Front. Neurol. 2020, 11, 577855. [Google Scholar] [CrossRef] [PubMed]
- Tauchi, Y.; Kyougoku, M.; Takahashi, K.; Okita, Y.; Takebayashi, T. Dimensionality and item-difficulty hierarchy of the Fugl-Meyer assessment of the upper extremity among Japanese patients who have experienced stroke. Top. Stroke Rehabil. 2022, 29, 579–587. [Google Scholar] [CrossRef]
- Woytowicz, E.J.; Rietschel, J.C.; Goodman, R.N.; Conroy, S.S.; Sorkin, J.D.; Whitall, J.; McCombe Waller, S. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. Arch. Phys. Med. Rehabil. 2017, 98, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Joyce, C.R.; Zutshi, D.W.; Hrubes, V.; Mason, R.M. Comparison of fixed interval and visual analogue scales for rating chronic pain. Eur. J. Clin. Pharmacol. 1975, 8, 415–420. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.B.; Daldegan-Bueno, D.; Menezes Oliveira, M.G.; de Souza, A.L. Beyond ANOVA and MANOVA for repeated measures: Advantages of generalized estimated equations and generalized linear mixed models and its use in neuroscience research. Eur. J. Neurosci. 2022, 56, 6089–6098. [Google Scholar] [CrossRef] [PubMed]
- Hayward, K.S.; Ferris, J.K.; Lohse, K.R.; Borich, M.R.; Borstad, A.; Cassidy, J.M.; Cramer, S.C.; Dukelow, S.P.; Findlater, S.E.; Hawe, R.L.; et al. Observational Study of Neuroimaging Biomarkers of Severe Upper Limb Impairment After Stroke. Neurology 2022, 99, e402–e413. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Cohen, L.G. Mechanisms underlying recovery of motor function after stroke. Arch. Neurol. 2004, 61, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, C.; Chen, H.; Qin, W.; He, Y.; Fan, F.; Zhang, Y.; Wang, M.; Li, K.; Zang, Y.; et al. Dynamic functional reorganization of the motor execution network after stroke. Brain 2010, 133, 1224–1238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, J.; Wu, X. Effects of whole-body vibration training on lower limb motor function and neural plasticity in patients with stroke: Protocol for a randomised controlled clinical trial. BMJ Open 2022, 12, e060796. [Google Scholar] [CrossRef] [PubMed]
- Teasell, R.; Salbach, N.M.; Foley, N.; Mountain, A.; Cameron, J.I.; Jong, A.; Acerra, N.E.; Bastasi, D.; Carter, S.L.; Fung, J.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part One: Rehabilitation and Recovery Following Stroke; 6th Edition Update 2019. Int. J. Stroke 2020, 15, 763–788. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Rothwell, J.C. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J. Physiol. 2003, 551, 649–660. [Google Scholar] [CrossRef]
- Rosenkranz, K.; Butler, K.; Williamon, A.; Cordivari, C.; Lees, A.J.; Rothwell, J.C. Sensorimotor reorganization by proprioceptive training in musician’s dystonia and writer’s cramp. Neurology 2008, 70, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Takebayashi, T.; Hanioka, D.; Okita, Y.; Shimada, S. Comparison of tendon and muscle belly vibratory stimulation in the treatment of post-stroke upper extremity spasticity: A retrospective observational pilot study. Sci. Rep. 2024, 14, 4151. [Google Scholar] [CrossRef]
- Afzal, T.; Chardon, M.K.; Rymer, W.Z.; Suresh, N.L. Stretch reflex excitability in contralateral limbs of stroke survivors is higher than in matched controls. J. Neuroeng. Rehabil. 2019, 16, 154. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, M.; Murata, K.; Taguchi, K.; Tanaka, T.; Sasaki, N.; Abo, M. The effect of 6 treatment with botulinum neurotoxin type A for patients with upper limb hemiparesis after stroke. Tokyo Jikei Med. J. 2017, 132, 162–167. [Google Scholar]
- Hamaguhi, T.; Abo, M. Recovery of Patients With Upper Limb Paralysis Due to Stroke Who Underwent Intervention Using Low-Frequency Repetitive Transcranial Magnetic Stimulation Combined With Occupational Therapy: A Retrospective Cohort Study. Neuromodulation 2023, 26, 861–877. [Google Scholar] [CrossRef]
- Godlove, J.; Anantha, V.; Advani, M.; Des Roches, C.; Kiran, S. Comparison of Therapy Practice at Home and in the Clinic: A Retrospective Analysis of the Constant Therapy Platform Data Set. Front. Neurol. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- National Clinical Guideline for Stroke for the UK and Ireland. 2023. Available online: https://www.strokeguideline.org/ (accessed on 2 March 2024).
- Lee, H.H.; Kim, D.Y.; Sohn, M.K.; Shin, Y.I.; Oh, G.J.; Lee, Y.S.; Joo, M.C.; Lee, S.Y.; Han, J.; Ahn, J.; et al. Revisiting the Proportional Recovery Model in View of the Ceiling Effect of Fugl-Meyer Assessment. Stroke 2021, 52, 3167–3175. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Senoo, A.; Abo, M. Changes in Structural Neural Networks in the Recovery Process of Motor Paralysis after Stroke. Brain Sci. 2024, 14, 197. [Google Scholar] [CrossRef]
- Page, S.J.; Fulk, G.D.; Boyne, P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys. Ther. 2012, 92, 791–798. [Google Scholar] [CrossRef]


| Variables | All | Shaking | Control | Statistics | ||
|---|---|---|---|---|---|---|
| (n = 93) | (n = 47) | (n = 46) | χ2 or U | p-Value | r or V | |
| Sex (Female/Male) | 50/43 | 26/22 | 24/21 | 0.00 | 1.00 | 0.00 |
| Age (median, [Q1, Q3]) | 59 [53, 68] | 59 [53, 67] | 59 [53, 68] | 1065 | 0.91 | 0.49 |
| Diagnosis (CI/ICH/other) | 33/55/5 | 16/30/2 | 17/25/3 | 0.59 | 0.75 | 0.08 |
| Affected side (L/R) | 46/47 | 27/21 | 19/26 | 1.31 | 0.25 | 0.12 |
| Month from onset | 131 [92, 175] | 136 [98, 177] | 126 [81, 175] | 1487 | 0.36 | 0.56 |
| FMA-UE | 24 [18, 30] | 27 [23, 31] * | 20 [17, 23] | 1343 | 0.04 | 0.62 |
| BoNT-A treatment (times) | 14 [10, 18] | 14 [11, 19] | 13 [9, 17] | 1124 | 0.81 | 0.49 |
| BoNT-A dose (Unit) | 250 [200, 350] | 250 [200, 400] | 250 [200, 300] | 1208 | 0.32 | 0.56 |
| Variable | β | SE | z-Value | p-Value | 95% CI | ||
|---|---|---|---|---|---|---|---|
| Intercept | 0.467 | 0.550 | 0.849 | 0.396 | 0.611 | 1.545 | |
| Periods | 0.467 | 0.298 | 1.565 | 0.118 | −0.118 | 1.051 | |
| Group | 1.363 | 0.447 | 3.048 | 0.002 | * | 0.486 | 2.239 |
| Periods × Group | 1.359 | 0.419 | 3.242 | 0.001 | * | 0.538 | 2.181 |
| Group Variance | 25.368 | 4.721 | 5.373 | 0.000 | * | 16.115 | 34.621 |
| Periods Variance | 25.057 | 4.150 | 6.038 | 0.000 | * | 16.923 | 33.191 |
| Group × periods Covariance | 23.963 | 4.300 | 5.572 | 0.000 | * | 15.534 | 32.391 |
| FMA-UE | Shaking (n = 47) | Control (n = 46) | U | p | r | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Delta | Pre | Post | Delta | |||||
| Total | 26.8 [23.0, 31.2] | 28.4 [24.4, 33.0] | 1.8 [0.8, 2.8] | 20.0 [17.1, 23.3] | 20.3 [17.4, 23.7] | 0.4 [−0.1, 1.0] | 823 | 0.04 | * | 0.24 |
| part A | 21.4 [19.4, 23.5] | 22.6 [20.6, 24.7] | 1.2 [0.6, 1.8] | 17.5 [15.5, 19.6] | 17.9 [15.8, 20.0] | 0.3 [−0.2, 0.9] | 770 | 0.01 | * | 0.29 |
| part B | 2.9 [2.1, 3.7] | 3.1 [2.3, 3.9] | 0.2 [−0.2, 0.6] | 1.7 [0.9, 2.4] | 1.9 [1.1, 2.7] | 0.2 [−0.1, 0.6] | 1015 | 0.56 | 0.06 | |
| part C | 4.1 [2.9, 5.2] | 4.2 [3.1, 5.4] | 0.2 [−0.3, 0.6] | 3.5 [2.4, 4.7] | 3.3 [2.2, 4.5] | −0.2 [−0.5, 0.1] | 977 | 0.37 | 0.10 | |
| part D | 1.3 [0.8, 1.7] | 1.4 [1.0, 1.9] | 0.2 [0.0, 0.4] | 0.8 [0.3, 1.2] | 0.7 [0.3, 1.2] | 0.0 [−0.2, 0.1] | 964 | 0.16 | 0.11 | |
| Variable | Severe (n = 34) | Moderate (n = 50) | Mild (n = 9) | |||
|---|---|---|---|---|---|---|
| Shaking | Control | Shaking | Control | Shaking | Control | |
| Sex (Female/Male) | 8/5 | 13/8 | 16/14 | 13/7 | 3/2 | 4/0 |
| Age | 66 [52, 74] | 59 [54, 70] | 56.5 [53, 66.5] | 56.5 [52.75, 64] | 58 [57, 61] | 69 [58, 73] |
| Diagnosis (CI:ICH; Other) | 7:6:0 | 7:13:1 | 8:3:2 | 8:11:1 | 1:4:0 | 2:1:1 |
| Affected Side (L/R) | 9/4 | 11/10 | 15/15 | 7/13 | 3/2 | 1/3 |
| Month of Onset | 167 [94, 248] | 138 [92, 176] | 134 [103, 174] | 116 [80, 169] | 122 [110, 137] | 88 [79, 136] |
| FMA-UE Total | 15 [11, 18] | 11 [10, 18] | 30.5 [24.25, 34.75] | 26 [23, 37.5] | 49 [49, 52] | 50.5 [49, 52] |
| BoNT-A treatment (times) | 14 [11, 23] | 13 [10, 17] | 15 [10, 21] | 10 [6, 15] | 18 [14, 27] | 14 [6, 22] |
| BoNT-A (unit) | 275 [200, 300] | 250 [250, 400] | 250 [200, 400] | 200 [197.5, 250] | 250 [100, 250] | 175 [138, 250] |
| Severity | Group | Pre | Post | Delta | U | p | r | |
|---|---|---|---|---|---|---|---|---|
| Severe (n = 34) | Shaking | 15.0 [11.0, 18.0] | 16.0 [12.5, 18.3] | 1.0 [−0.25, 1.0] | 16.00 | 0.22 | 0.43 | |
| Control | 11.0 [10.0, 18.0] | 13.0 [9.75, 17.0] | 0.0 [0.0, 1.25] | 21.50 | 0.09 | 0.00 | ||
| Moderate (n = 50) | Shaking | 30.5 [24.3, 34.8] | 33.5 [29.0, 37.5] | 1.0 [0.0, 4.0] | 59.50 | 0.01 | * | 0.25 |
| Control | 26.0 [23.0, 37.5] | 27.0 [23.0, 37.3] | 0.0 [0.0, 1.0] | 25.50 | 0.50 | 0.00 | ||
| Mild (n = 9) | Shaking | 49.0 [49.0, 52.0] | 51.5 [47.75, 57.0] | 2.0 [0.5, 3.5] | 1.50 | 0.25 | 0.77 | |
| Control | 50.5 [49.0, 52.0] | 51.0 [51.0, 52.0] | 1.5 [−0.5, 2.75] | 3.50 | 0.63 | 0.36 |
| Variables | Intervention Group | Control Group | Δ Control vs. Δ Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Delta | Pre | Post | Delta | U | p | r | |||
| modified Ashworth scale | shoulder | flex | 1 [1, 2] | 1 [1, 2] | 0 [0, 1] | 1 [1, 2] | 1 [0, 2] | 0 [0, 0] | 908 | 0.14 | −0.16 |
| ext | 1 [0, 2] | 1 [0, 2] | 0 [0, 0] | 1 [0, 2] | 1 [0, 2] | 0 [0, 0] | 1028 | 0.62 | −0.05 | ||
| elbow | flex | 1 [1, 2] | 1 [1, 2] | 0 [0, 1] | 1 [1, 2] | 1 [1, 2] | 0 [0, 0] | 1202 | 0.31 | 0.11 | |
| ext | 2 [1, 2] | 2 [1, 2] | 0 [0, 1] | 2 [1, 2] * | 1 [0, 2] | 0 [0, 1] | 930 | 0.20 | −0.14 | ||
| wrist | flex | 1 [0, 2] | 1 [0, 2] | 0 [0, 1] | 1 [1, 2] | 1 [1, 2] | 0 [0, 0] | 1082 | 1.00 | 0.00 | |
| ext | 2 [1, 2] | 2 [1, 2] | 0 [0, 1] | 1 [0, 2] | 1 [1, 2] | 0 [0, 0] | 1087 | 0.97 | 0.01 | ||
| finger | flex | 2 [0, 2] | 1 [0, 2] | 0 [0, 1] | 1 [0, 2] | 1 [0, 2] | 0 [0, 0] | 1091 | 0.93 | 0.01 | |
| ext | 0 [0, 2] | 1 [0, 2] | 0 [0, 0] | 1 [0, 2] | 1 [0, 2] | 0 [0, 0] | 1037 | 0.68 | −0.04 | ||
| Active Range of Motion | shoulder | flex | 110 [125, 80] | 110 [125, 88] | 0 [−5, 10] | 83 [110, 43] | 83 [120, 50] | 0 [−5, 9] | 1094 | 0.92 | 0.01 |
| ext | 40 [40, 20] | 40 [50, 30] | 0 [−5, 5] | 35 [40, 25] | 35 [40, 20] | 0 [−5, 5] | 1311 | 0.06 | 0.21 | ||
| abd | 75 [120, 60] | 85 [113, 60] | 5 [0, 10] | 60 [85, 50] | 60 [80, 41] | 0 [−5, 5] | 1187 | 0.41 | 0.10 | ||
| add | 0 [10, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 999 | 0.46 | −0.08 | ||
| elbow | flex | 115 [130, 103] | 115 [130, 100] | 5 [−10, 0] | 100 [114, 76] | 100 [120, 83] | 0 [−5, 5] | 1036 | 0.73 | −0.04 | |
| ext | −15 [−38, 0] | −10 [0, −30] | 5 [0, 10] | −18 [−49, 0] | −15 [−39, −6] | 5 [0, 10] | 1214 | 0.30 | 0.12 | ||
| wrist | flex | 15 [40, 0] | 15 [40, −5] | 0 [−5, 5] | 13 [0, 30] | 18 [−10, 30] | 0 [−5, 5] | 941 | 0.27 | −0.13 | |
| ext | 30 [40, 0] | 30 [45, 5] | 0 [0, 0] | 5 [−28, 35] | 8 [−20, 34] | 0 [−10, 0] | 1141 | 0.64 | 0.06 | ||
| Passive Range of Motion | shoulder | flex | 140 [125, 155] | 140 [120, 160] | 0 [−5, 10] | 138 [120, 160] | 135 [120, 154] | 0 [−5, 9] | 1048 | 0.80 | 0.03 |
| ext | 50 [45, 60] | 50 [48, 58] | 0 [−5, 5] | 50 [41, 50] | 50 [40, 50] | 0 [−5, 0] | 1006 | 0.55 | 0.07 | ||
| abd | 135 [130, 140] | 140 [130, 143] | 0 [0, 5] | 140 [130, 145] | 133 [125, 145] | 0 [−5, 0] | 892 | 0.12 | 0.18 | ||
| add | 0 [−10, 0] | 0 [−5, 0] | 0 [0, 0] | 0 [−9, 0] | 0 [−5, 0] | 0 [0, 0] | 1054 | 0.81 | 0.03 | ||
| elbow | flex | 120 [95, 150] | 120 [98, 155] | 5 [−10, 10] | 118 [90, 145] | 110 [90, 144] | 0 [−14, 5] | 898 | 0.16 | 0.17 | |
| ext | 0 [0, 13] | 0 [0, 0] | 0 [0, 0] | 0 [0, 12] | 0 [0, 0] | 0 [0, 0] | 1187 | 0.27 | −0.10 | ||
| wrist | flex | 70 [60, 80] | 70 [60, 80) | 0 [−8, 5) | 70 [65, 80] | 70 [60, 80] | 0 [0, 5] | 1144 | 0.62 | −0.06 | |
| ext | 60 [45, 70] | 70 [48, 80) | 0 [0, 10) | 68 [50, 75] | 68 [55, 74] | 0 [0, 9] | 1029 | 0.68 | 005 | ||
| Pain VAS | 2 [0, 5] | 2 [0, 6] | 0 [0, 0] | 3 [0, 6] | 2 [0, 5] * | 0 [−1, 0] | 914 | 0.10 | 0.10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hada, T.; Hamaguchi, T.; Abo, M. Impact of Shaking Exercise on Functional Recovery in Patients with Chronic Post-Stroke Upper Limb Impairment: A Multicenter, Open-Label, Quasi-Randomized Controlled Trial. Appl. Sci. 2024, 14, 6295. https://doi.org/10.3390/app14146295
Hada T, Hamaguchi T, Abo M. Impact of Shaking Exercise on Functional Recovery in Patients with Chronic Post-Stroke Upper Limb Impairment: A Multicenter, Open-Label, Quasi-Randomized Controlled Trial. Applied Sciences. 2024; 14(14):6295. https://doi.org/10.3390/app14146295
Chicago/Turabian StyleHada, Takuya, Toyohiro Hamaguchi, and Masahiro Abo. 2024. "Impact of Shaking Exercise on Functional Recovery in Patients with Chronic Post-Stroke Upper Limb Impairment: A Multicenter, Open-Label, Quasi-Randomized Controlled Trial" Applied Sciences 14, no. 14: 6295. https://doi.org/10.3390/app14146295
APA StyleHada, T., Hamaguchi, T., & Abo, M. (2024). Impact of Shaking Exercise on Functional Recovery in Patients with Chronic Post-Stroke Upper Limb Impairment: A Multicenter, Open-Label, Quasi-Randomized Controlled Trial. Applied Sciences, 14(14), 6295. https://doi.org/10.3390/app14146295







