The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anode Sysnthesis
2.3. Cathode Synthesis
2.4. Characterization
2.5. Electrochemical Performance
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SEM | Scanning electron microscope |
| EDX | Energy dispersive X-ray spectroscopy |
| CV | Cyclic voltammetry |
| ORR | Oxygen reduction reaction |
| EDTA | Ethylenediaminetetraacetic acid |
| PVDF | polyvinylidene fluoride |
| NMP | N-methyl-2-pyrrolidone |
| OCP | Open circuit potential |
References
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Mohamad, A. Electrochemical properties of aluminum anodes in gel electrolyte-based aluminum-air batteries. Corros. Sci. 2008, 50, 3475–3479. [Google Scholar] [CrossRef]
- Ryu, J.; Park, M.; Cho, J. Advanced technologies for high-energy aluminum–air batteries. Adv. Mater. 2019, 31, 1804784. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Rahman, A.; Wang, X.; Wen, C. High energy density metal-air batteries: A review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Ren, J.; Zhang, Y.; Peng, H. An all-solid-state fiber-shaped aluminum–air battery with flexibility, stretchability, and high electrochemical performance. Angew. Chem. 2016, 128, 8111–8114. [Google Scholar] [CrossRef]
- Ma, J.; Li, W.; Wang, G.; Li, Y.; Ren, F.; Xiong, Y. Influences of L-Cysteine/Zinc Oxide Additive on the Electrochemical Behavior of Pure Aluminum in Alkaline Solution. J. Electrochem. Soc. 2018, 165, A266. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wang, X.; Yang, S.; Xiong, C. Progress and Applications of Seawater-Activated Batteries. Sustainability 2023, 15, 1635. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, C.X.; Liu, J.N.; Li, B.Q.; Tang, C.; Zhang, Q. Seawater-based electrolyte for Zinc–air batteries. Green Chem. Eng. 2020, 1, 117–123. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.Q.; Zhao, C.X.; Zhang, Q. Seawater electrolyte-based metal–air batteries: From strategies to applications. Energy Environ. Sci. 2020, 13, 3253–3268. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, H. Performance of Al-0.5In as Anode for Al–Air Battery in Inhibited Alkaline Solutions. J. Electrochem. Soc. 2015, 162, A1617. [Google Scholar] [CrossRef]
- Lv, C.; Zhang, Y.; Ma, J.; Zhu, Y.; Huang, D.; Li, Y.; Wang, H.; Tang, Y. Regulating Solvation and Interface Chemistry to Inhibit Corrosion of the Aluminum Anode in Aluminum–air Batteries. J. Mater. Chem. A 2022, 10, 9506–9514. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.; Lei, H.; Yu, Z.; Chen, L.; Wang, M.; Jiao, S. Nonaqueous rechargeable aluminum batteries: Progresses, challenges, and perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Choi, S.R.; Kim, J.G. Effect of Agar as Electrolyte Additive on the Aluminum-Air Batteries. J. Electrochem. Soc. 2020, 167, 110503. [Google Scholar] [CrossRef]
- Taeri, O.; Hassanzadeh, A.; Ravari, F. Synergistic Inhibitory Effect of Potassium Sodium Tartrate Tetrahydrate and Sodium Stannate Trihydrate on Self-Corrosion of Aluminum in Alkaline Aluminum-Air Batteries. Chemelectrochem 2020, 7, 2123–2135. [Google Scholar] [CrossRef]
- Cho, Y.J.; Park, I.J.; Lee, H.J.; Kim, J.G. Aluminum anode for aluminum–air battery–Part I: Influence of aluminum purity. J. Power Sources 2015, 277, 370–378. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, L.; Jiang, H.; Li, X.; Mu, X. Atmospheric Corrosion Comparison of Antirust Aluminum Exposed to Industrial and Coastal Atmospheres. Mater. Corros. 2018, 69, 1516–1525. [Google Scholar] [CrossRef]
- Meroueh, L.; Eagar, T.W.; Hart, D.P. Effects of Mg and Si Doping on Hydrogen Generation via Reduction of Aluminum Alloys in Water. Acs Appl. Energy Mater. 2020, 3, 1860–1868. [Google Scholar] [CrossRef]
- Sun, S.; Li, C.L.; Zheng, Q.; Wang, X.M.; Hu, S. The Effect of Environmental Factors on Long-term Atmospheric Corrosion Behavior of Commercially Pure Aluminum AA1035. Mater. Corros. 2017, 68, 450–457. [Google Scholar] [CrossRef]
- Desai, P.S.; Vashi, R.T. Inhibitive Efficiency of Sulphathiazole for Aluminum Corrosion in Trichloroacetic Acid. Anti-Corros. Methods Mater. 2011, 58, 70–75. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Z.; Zexu, D. Electrochemical performance and interface analysis of Al-MgO composite anodes for Al-air batteries. J. Electrochem. Soc. 2020, 167, 140514. [Google Scholar] [CrossRef]
- Macdonald, D.; Lee, K.; Moccari, A.; Harrington, D. Evaluation of alloy anodes for aluminum-air batteries: Corrosion studies. Corrosion 1988, 44, 652–657. [Google Scholar] [CrossRef]
- Park, I.J.; Choi, S.R.; Kim, J.G. Aluminum anode for aluminum-air battery–Part II: Influence of In addition on the electrochemical characteristics of Al-Zn alloy in alkaline solution. J. Power Sources 2017, 357, 47–55. [Google Scholar] [CrossRef]
- Ma, J.; Wen, J.; Gao, J.; Li, Q. Performance of Al- 1Mg- 1Zn- 0.1 Ga- 0.1 Sn as anode for Al-air battery. Electrochim. Acta 2014, 129, 69–75. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Qin, K.; Zou, J.; Qin, K.; Ban, C.; Cui, J.; Nagaumi, H. The role of gallium and indium in improving the electrochemical characteristics of Al–Mg–Sn-based alloy for Al–air battery anodes in 2 M NaCl solution. J. Mater. Sci. 2020, 55, 11545–11560. [Google Scholar] [CrossRef]
- Radošević, J.; Kliškić, M.; Dabić, P.; Stevanović, R.; Despić, A. Processes on aluminium on the negative side of the open-circuit potential. J. Electroanal. Chem. Interfacial Electrochem. 1990, 277, 105–119. [Google Scholar] [CrossRef]
- Deepa, P.; Padmalatha, R. Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 2017, 10, S2234–S2244. [Google Scholar] [CrossRef]
- Du, X.; Chen, Y. Corrosion inhibition by a superhydrophobic surface on aluminum that was prepared with a facile electrochemical route. Mater. Res. Express 2020, 7, 056405. [Google Scholar]
- Prakashaiah, B.; Kumara, D.V.; Pandith, A.A.; Shetty, A.N.; Rani, B.A. Corrosion inhibition of 2024-T3 aluminum alloy in 3.5% NaCl by thiosemicarbazone derivatives. Corros. Sci. 2018, 136, 326–338. [Google Scholar] [CrossRef]
- Albasre, N.M.M.; Zakaria, A.; Hussin, M.H. The Study of Electroless Ni-Cu-P Plating on Corrosion Resistance of Aluminium in 3.5% NaCl. Int. Electroact. Mater. 2021, 9, 12–26. [Google Scholar]
- Charitha, B.; Rao, P. Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: Electrochemical and surface studies. Int. J. Biol. Macromol. 2018, 112, 461–472. [Google Scholar]
- Mutlu, R.N.; Yazıcı, B. Copper-deposited aluminum anode for aluminum-air battery. J. Solid State Electrochem. 2019, 23, 529–541. [Google Scholar] [CrossRef]
- Zhang, K.; Yan, Z.; Chen, J. Electrodeposition accelerates metal-based batteries. Joule 2020, 4, 10–11. [Google Scholar] [CrossRef]
- Liu, F.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of metals and alloys from ionic liquids. J. Alloys Compd. 2016, 654, 163–170. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, L.; Roesky, H.W.; Wang, L.; Huang, B. The effect of zinc on the aluminum anode of the aluminum–air battery. J. Power Sources 2004, 138, 313–318. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, J.; Zhu, M. An electrochemical investigation of the EDTA influence on the cobalt-manganese electrodeposition in aqueous chloride electrolytes. Int. J. Electrochem. Sci. 2017, 12, 8368–8380. [Google Scholar] [CrossRef]
- Fashu, S.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Wang, X.L.; Tu, J.P. Effect of EDTA and NH4Cl additives on electrodeposition of Zn–Ni films from choline chloride-based ionic liquid. Trans. Nonferrous Met. Soc. China 2015, 25, 2054–2064. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, Y.; Wang, X.; Xiao, P.; Tian, E.; Wang, X.; Li, J. An initial study of EDTA complex based draw solutes in forward osmosis process. Desalination 2016, 378, 28–36. [Google Scholar] [CrossRef]
- Guidelli, R.; Compton, R.G.; Feliu, J.M.; Gileadi, E.; Lipkowski, J.; Schmickler, W.; Trasatti, S. Defining the transfer coefficient in electrochemistry: An assessment (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 245–258. [Google Scholar] [CrossRef]
- Tian, H.; Li, Z.; Feng, G.; Yang, Z.; Fox, D.; Wang, M.; Zhou, H.; Zhai, L.; Kushima, A.; Du, Y.; et al. Stable, high-performance, dendrite-free, seawater-based aqueous batteries. Nat. Commun. 2021, 12, 237. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-air batteries—A review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Tsurtsumia, G.; Gogoli, D.; Koiava, N.; Kakhniashvili, I.; Jokhadze, N.; Lezhava, T.; Nioradze, N.; Tatishvili, D. Electrodeposition and characterization of Mn–Cu–Zn alloys for corrosion protection coating electrodeposition and characterization of Mn–Cu–Zn alloys for corrosion PROTECTION coating. World Multidiscip. Earth Sci. Symp. Ser. Earth Environ. Sci. 2017, 95, 042035. [Google Scholar]
- Nur’aini, S.; Susanto, S.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. Unravelling the deposition of Zn and Sn as corrosion inhibitor on aluminum (Al) surface for Al-air battery application with sodium chloride electrolytes. Mater. Today Proc. 2024. [Google Scholar] [CrossRef]
- Montiel Guerrero, S.S.; Durmus, Y.E.; Dzieciol, K.; Basak, S.; Tempel, H.; van Waasen, S.; Kungl, H.; Eichel, R.A. Improved Electrochemical Performance of Zinc Anodes by EDTA in Near-Neutral Zinc-Air Batteries. Batter. Supercaps 2021, 4, 1830–1842. [Google Scholar] [CrossRef]
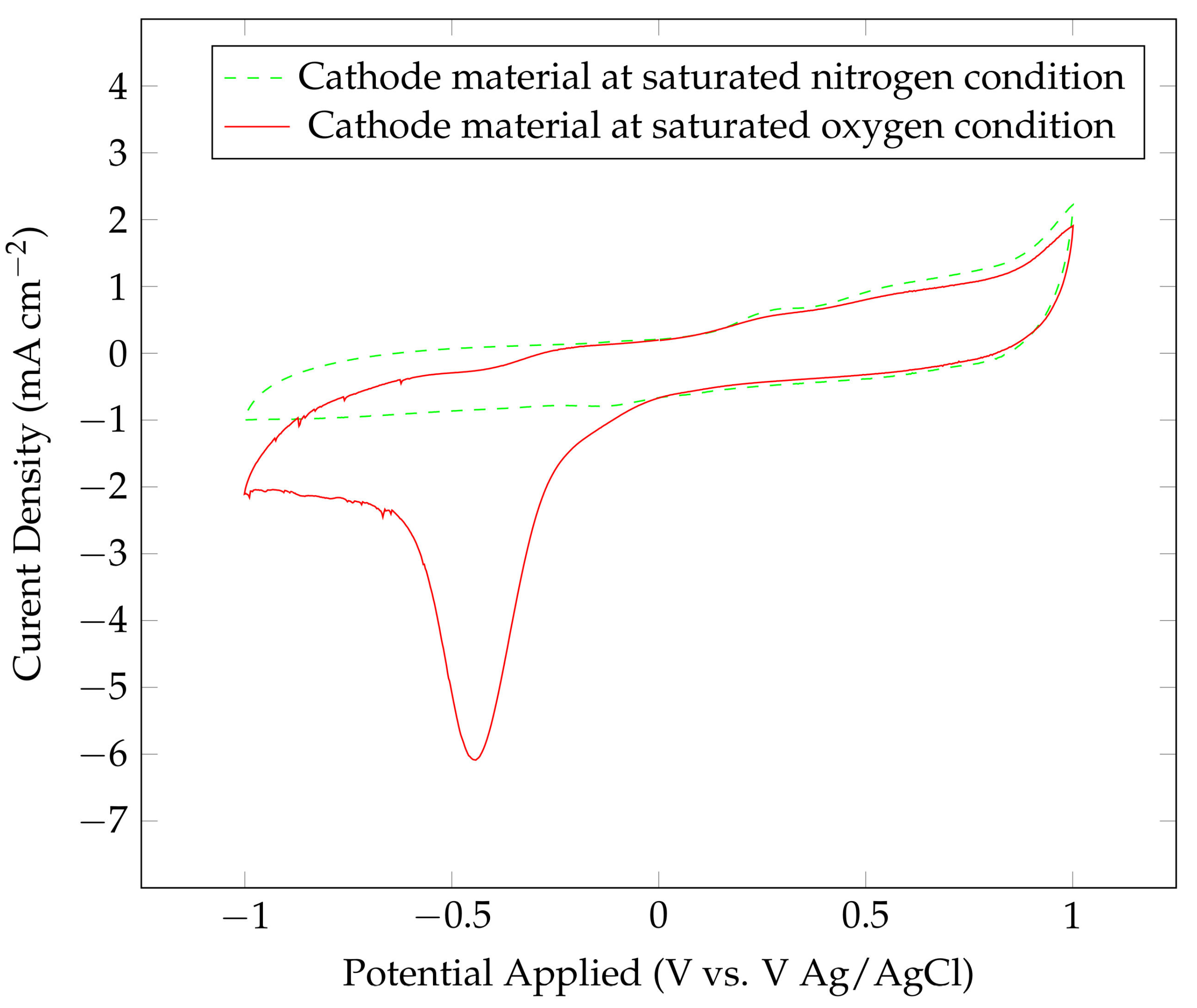
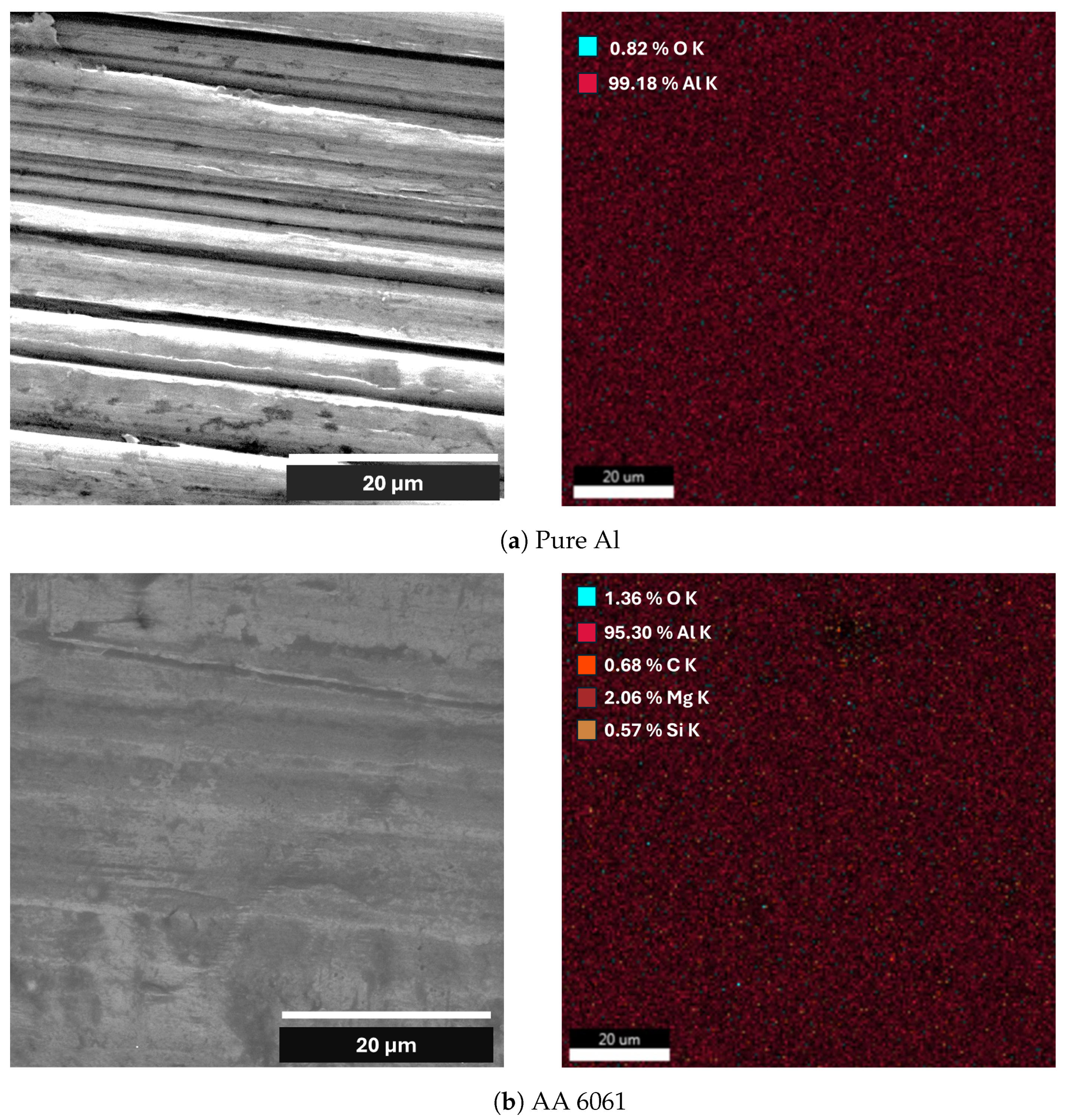
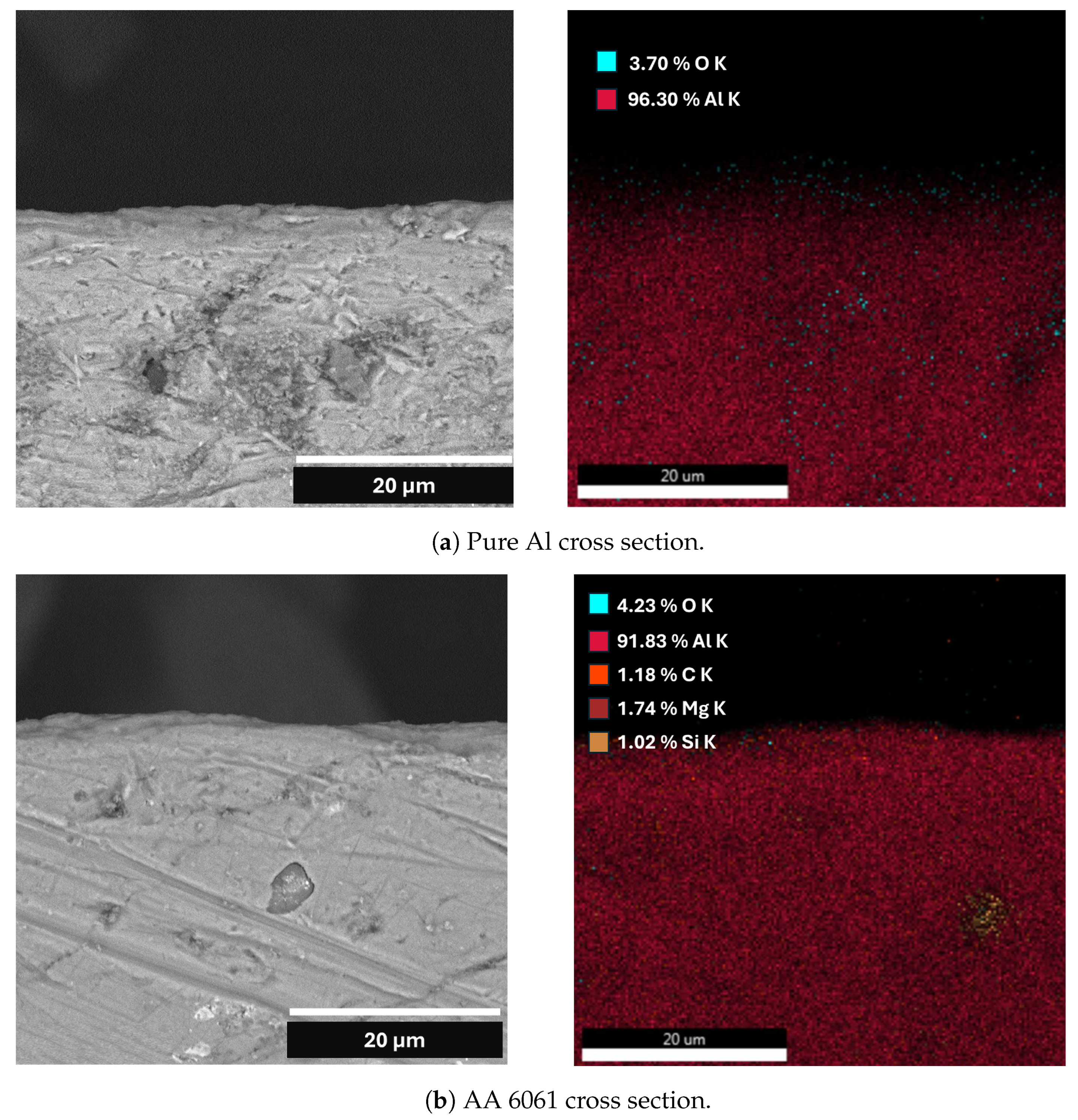
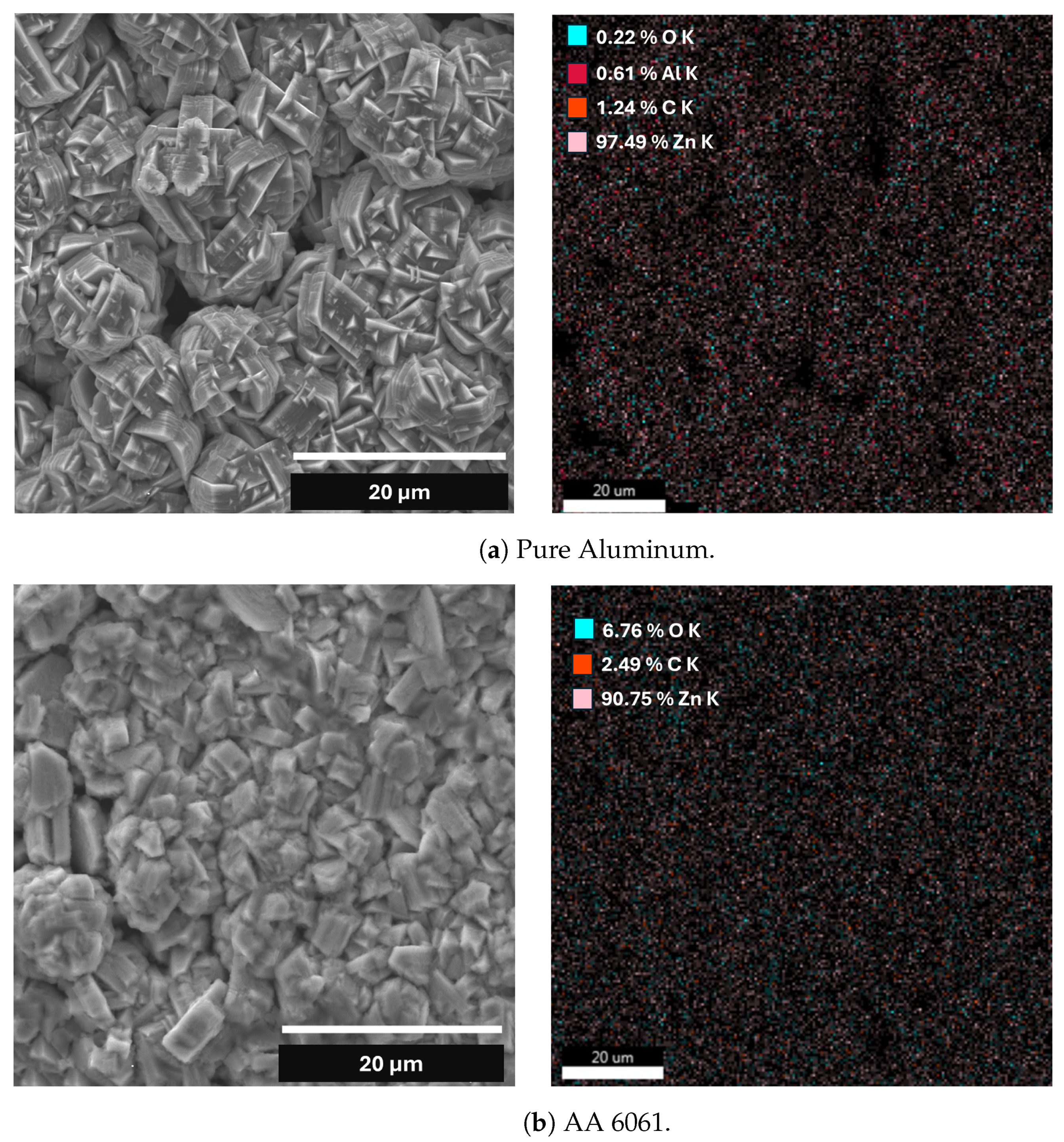
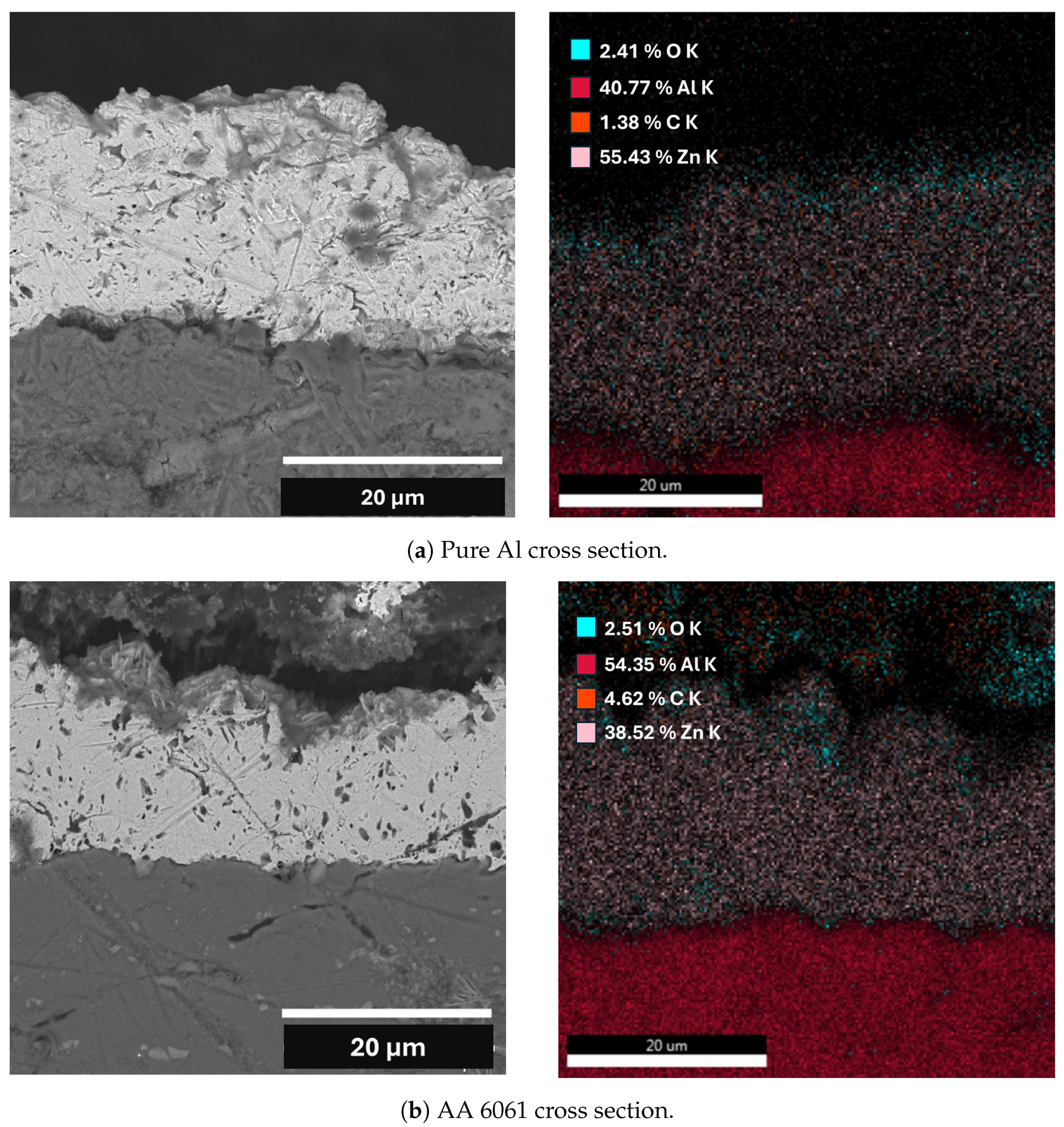
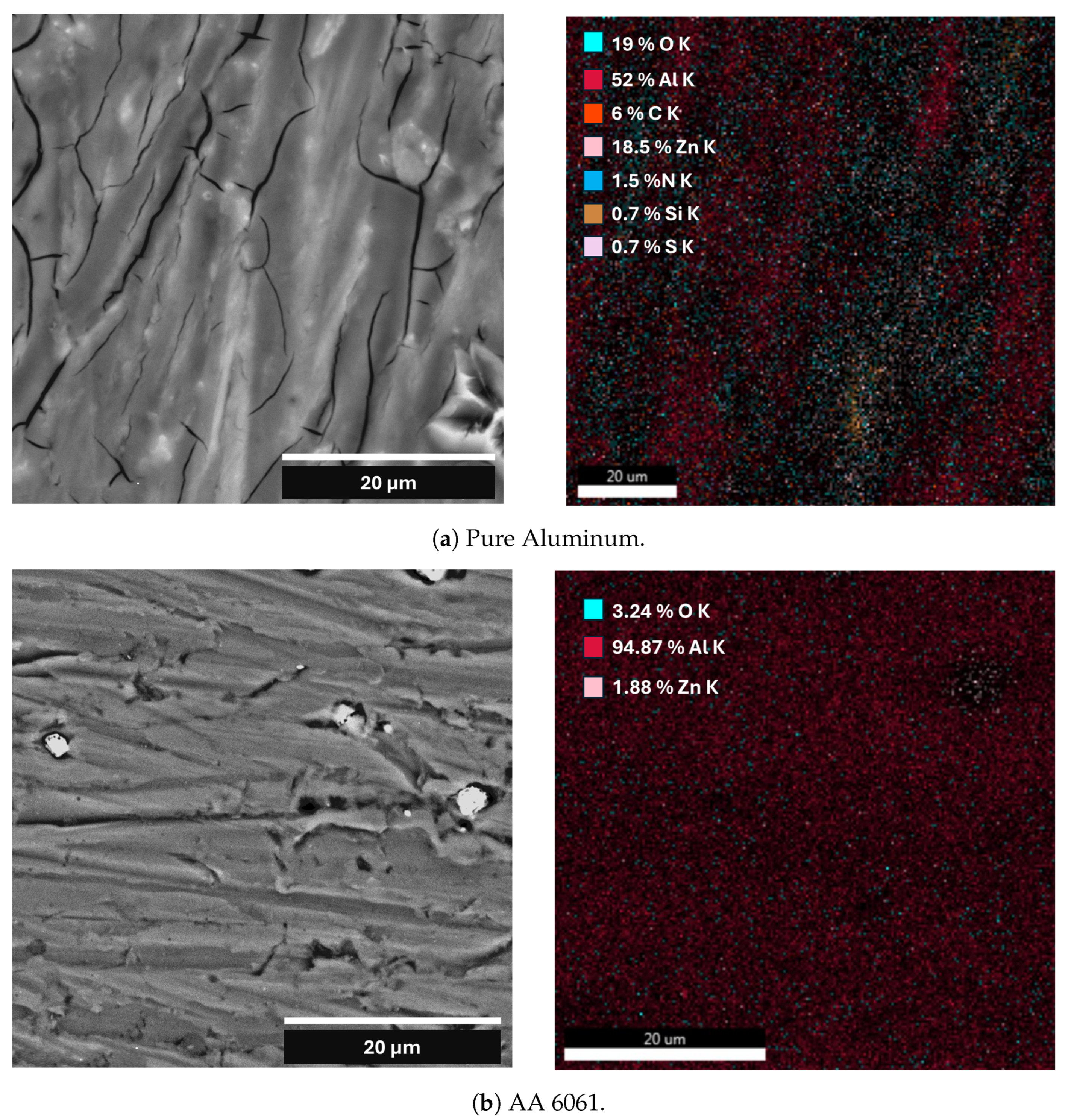
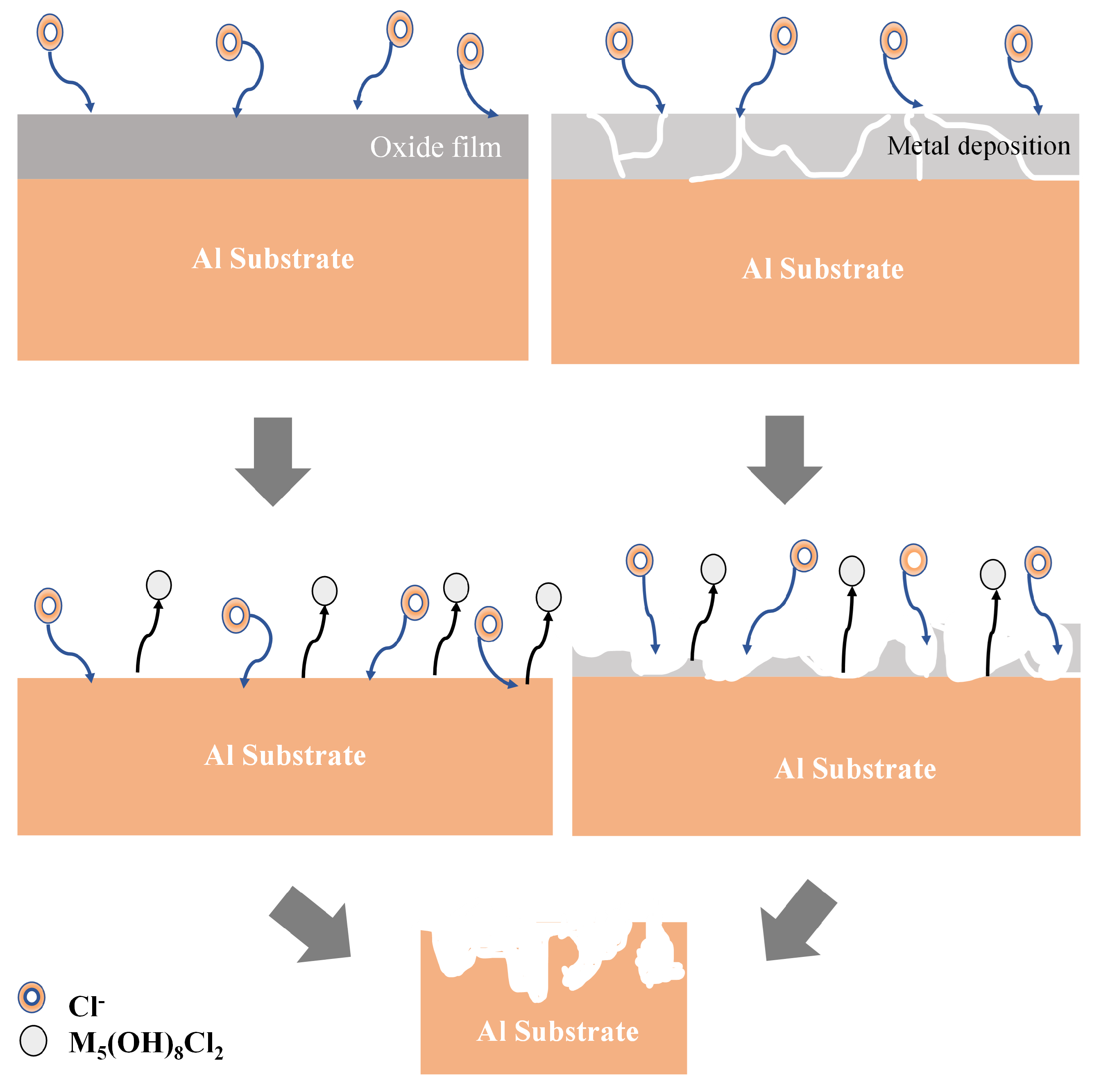
| Element | Pure Al | Al Alloy AA6061 |
|---|---|---|
| % wt. | % wt. | |
| Al (Aluminum) | ~100 | 97 |
| Si (Silicon) | ≤ | 0.73 |
| Mn (Manganese) | ≤ | 0.08 |
| Zn (Zinc) | ≤ | 0.01 |
| Ni (Nickel) | - | 0.015 |
| Ti (Titania) | - | 0.03 |
| Cu (Copper) | ≤ | 0.31 |
| Mg (Magnesium) | - | 1.51 |
| Fe (Iron) | ≤ | 0.18 |
| Cr (Chrome) | - | 0.08 |
| N (Nitrogen total) | ≤ | - |
| As (Arsenic) | ≤ | - |
| Bath Composition | ZnSO4 mol L−1 |
C6H5Na5O7 mol L−1 |
C10H16N2O8 mol L−1 |
|---|---|---|---|
| First route | 0.2 | 0.2 | - |
| Second route | 0.2 | 0.2 | 0.6 |
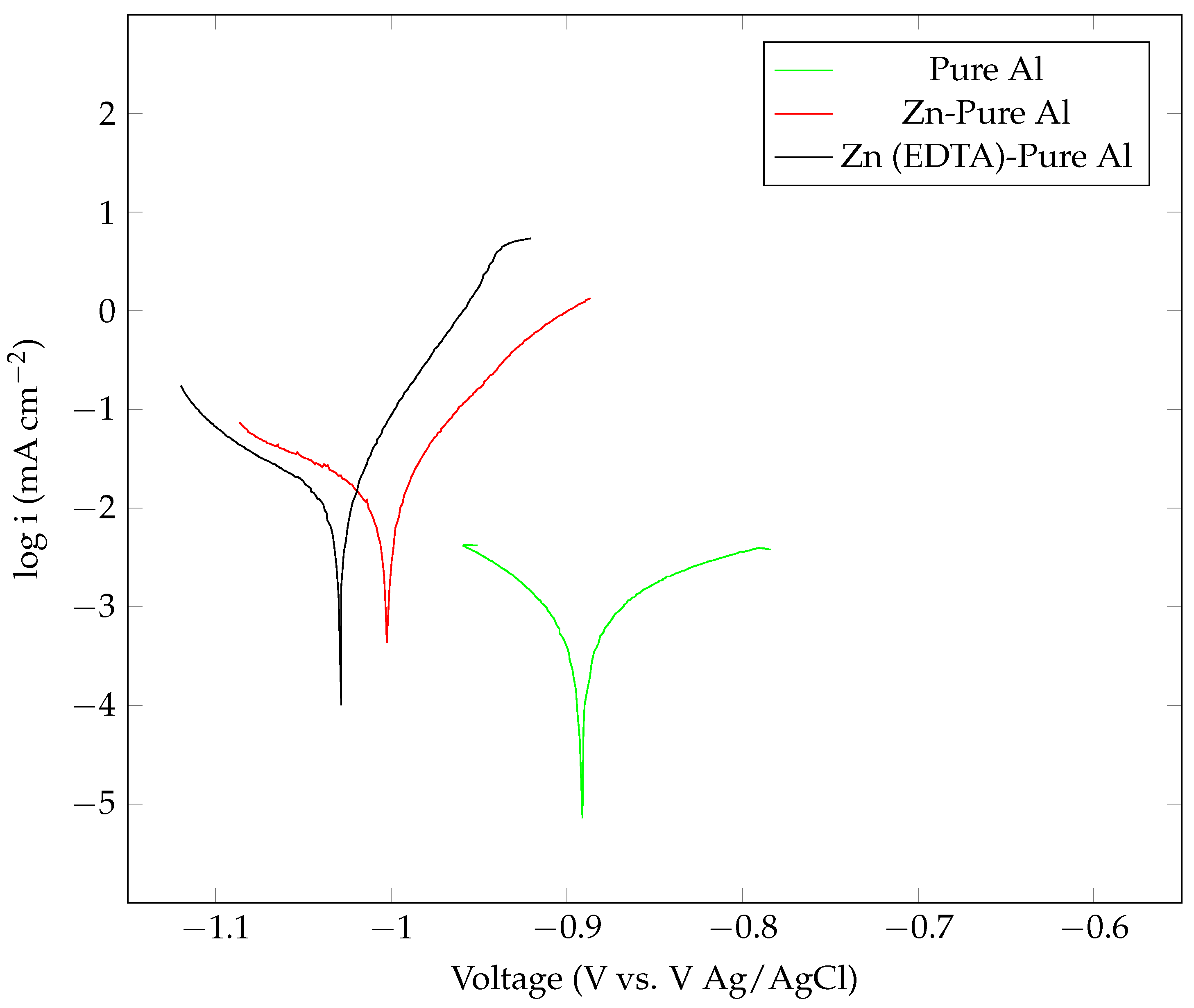
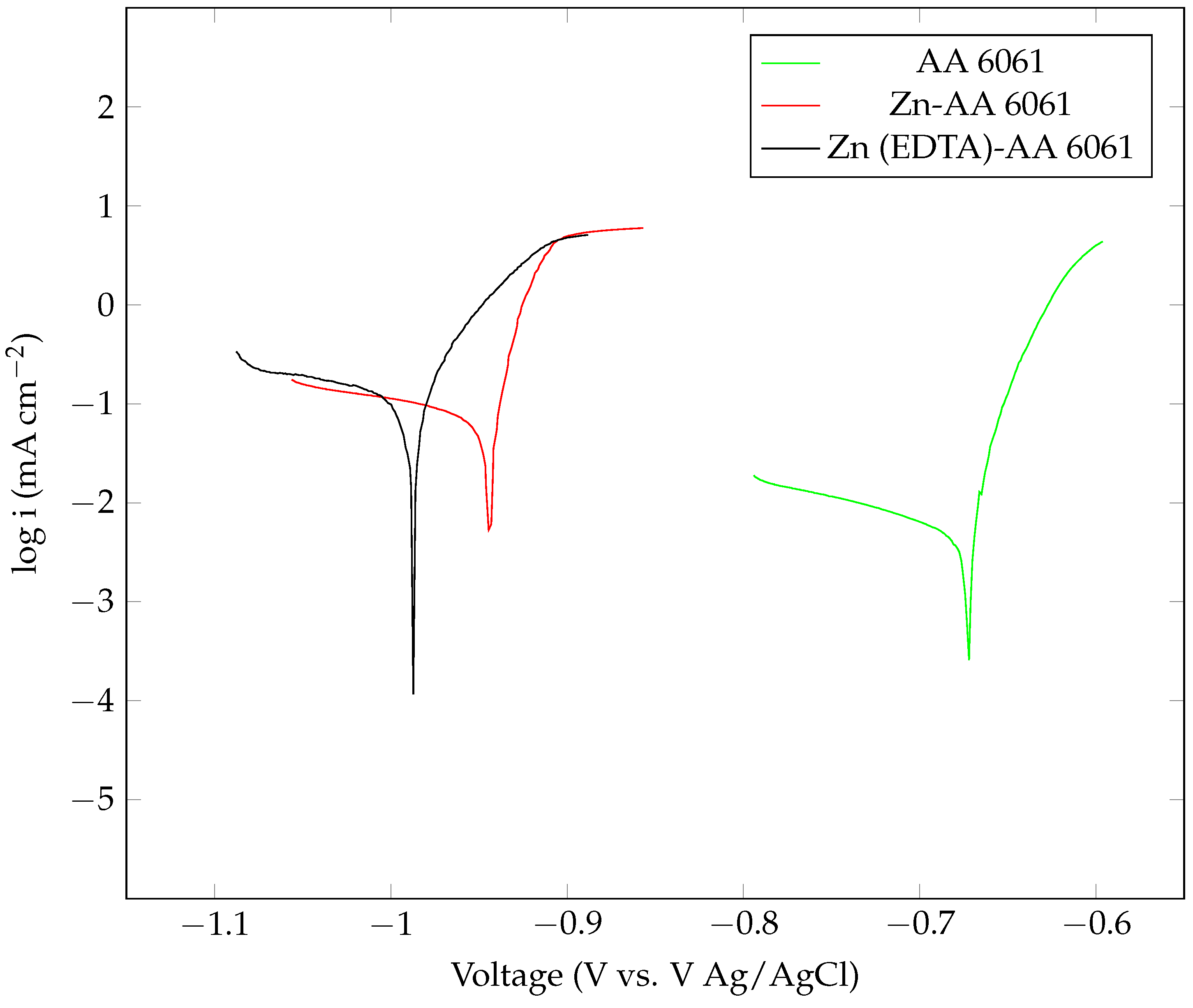
| Anode Sample | Icorr () |
Ecorr (V, Ag/AgCl) |
Corrosion Rate (mm/year) | |
|---|---|---|---|---|
| Pure Al | 2.18 | −0.912 | 0.323 | 0.025361 |
| Zn-pure Al | 16.16 | −0.983 | 0.332 | 0.13666 |
| Zn (EDTA) pure Al | 18.41 | −1.032 | 0.436 | 0.21395 |
| AA 6061 | 29.72 | −0.696 | 0.192 | 0.3454 |
| Zn-AA 6061 | 72.56 | −0.9466 | 0.252 | 0.84322 |
| Zn (EDTA)-AA 6061 | 110.59 | −0.9949 | 0.306 | 1.285 |
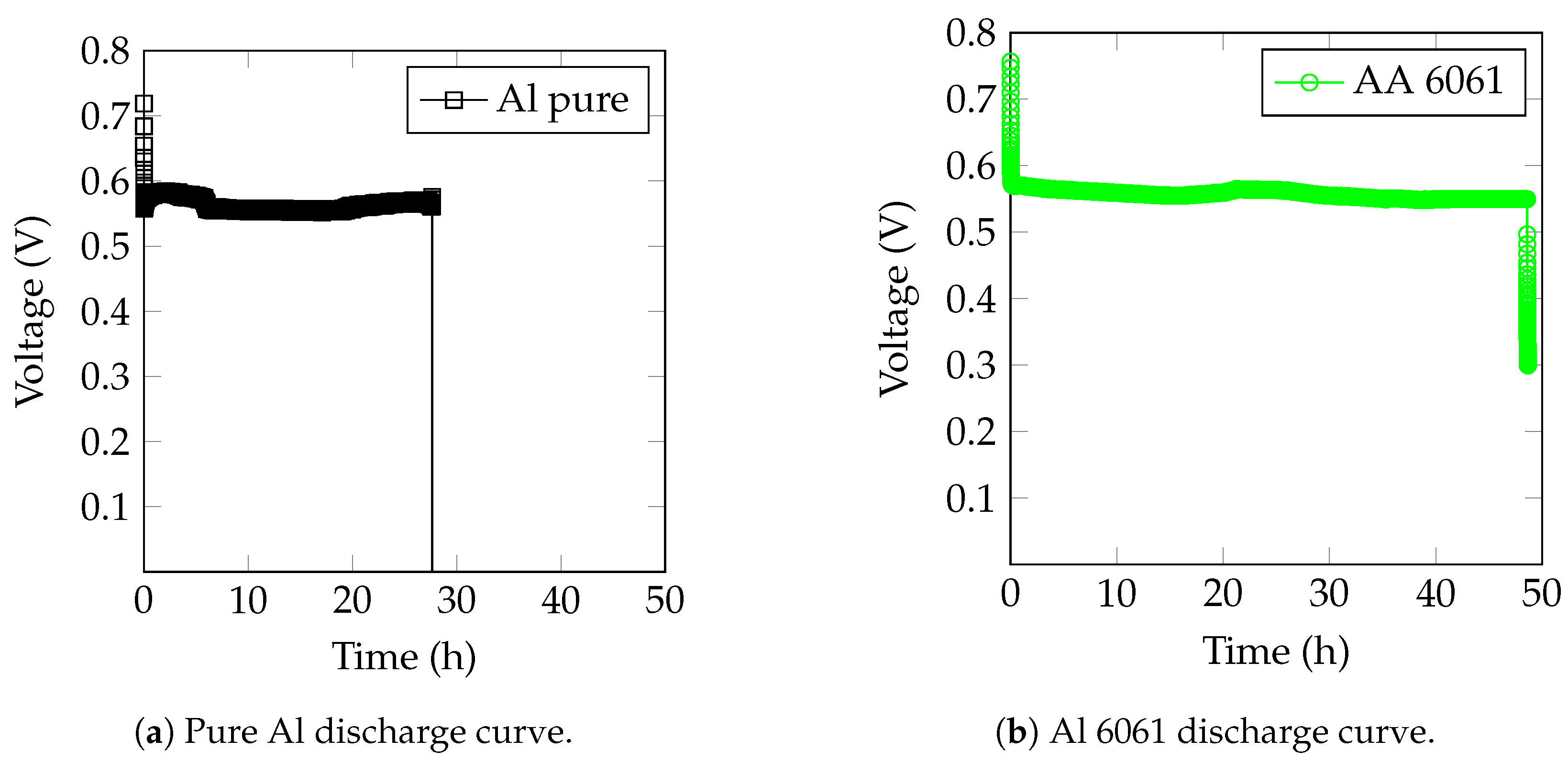
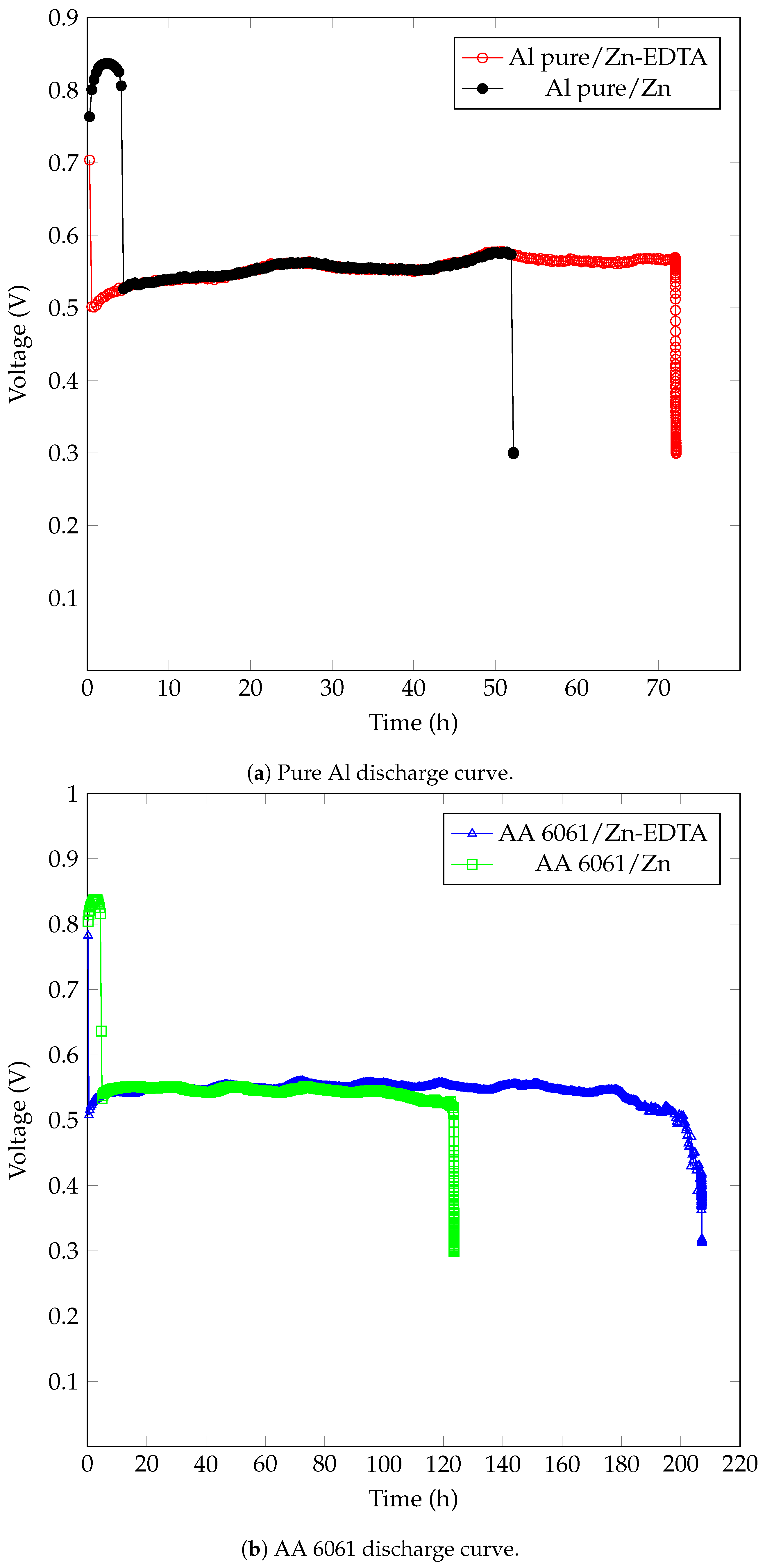
| Anode Sample |
Discharge
Capacity (mAh.) |
Discharge
Energy (mWh.) |
|---|---|---|
| Pure Al | 0.267 | 0.11 |
| Zn-pure Al | 104.55 | 60.017 |
| Zn (EDTA)-pure Al | 144.501 | 80.143 |
| AA 6061 | 94.892 | 54.466 |
| Zn-AA 6061 | 247.039 | 137.109 |
| Zn (EDTA)-AA 6061 | 414.561 | 255.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitanggang, R.B.; Nur’aini, S.; Susanto, S.; Widiyastuti, W.; Setyawan, H. The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Appl. Sci. 2024, 14, 6263. https://doi.org/10.3390/app14146263
Sitanggang RB, Nur’aini S, Susanto S, Widiyastuti W, Setyawan H. The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Applied Sciences. 2024; 14(14):6263. https://doi.org/10.3390/app14146263
Chicago/Turabian StyleSitanggang, Ruly Bayu, Syarifa Nur’aini, Susanto Susanto, Widiyastuti Widiyastuti, and Heru Setyawan. 2024. "The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution" Applied Sciences 14, no. 14: 6263. https://doi.org/10.3390/app14146263
APA StyleSitanggang, R. B., Nur’aini, S., Susanto, S., Widiyastuti, W., & Setyawan, H. (2024). The Enhancement Discharge Performance by Zinc-Coated Aluminum Anode for Aluminum–Air Battery in Sodium Chloride Solution. Applied Sciences, 14(14), 6263. https://doi.org/10.3390/app14146263





