Clinical Tolerability and Efficacy Establishment of a New Cosmetic Treatment Regimen Intended for Sensitive Skin
Abstract
Featured Application
Abstract
1. Introduction
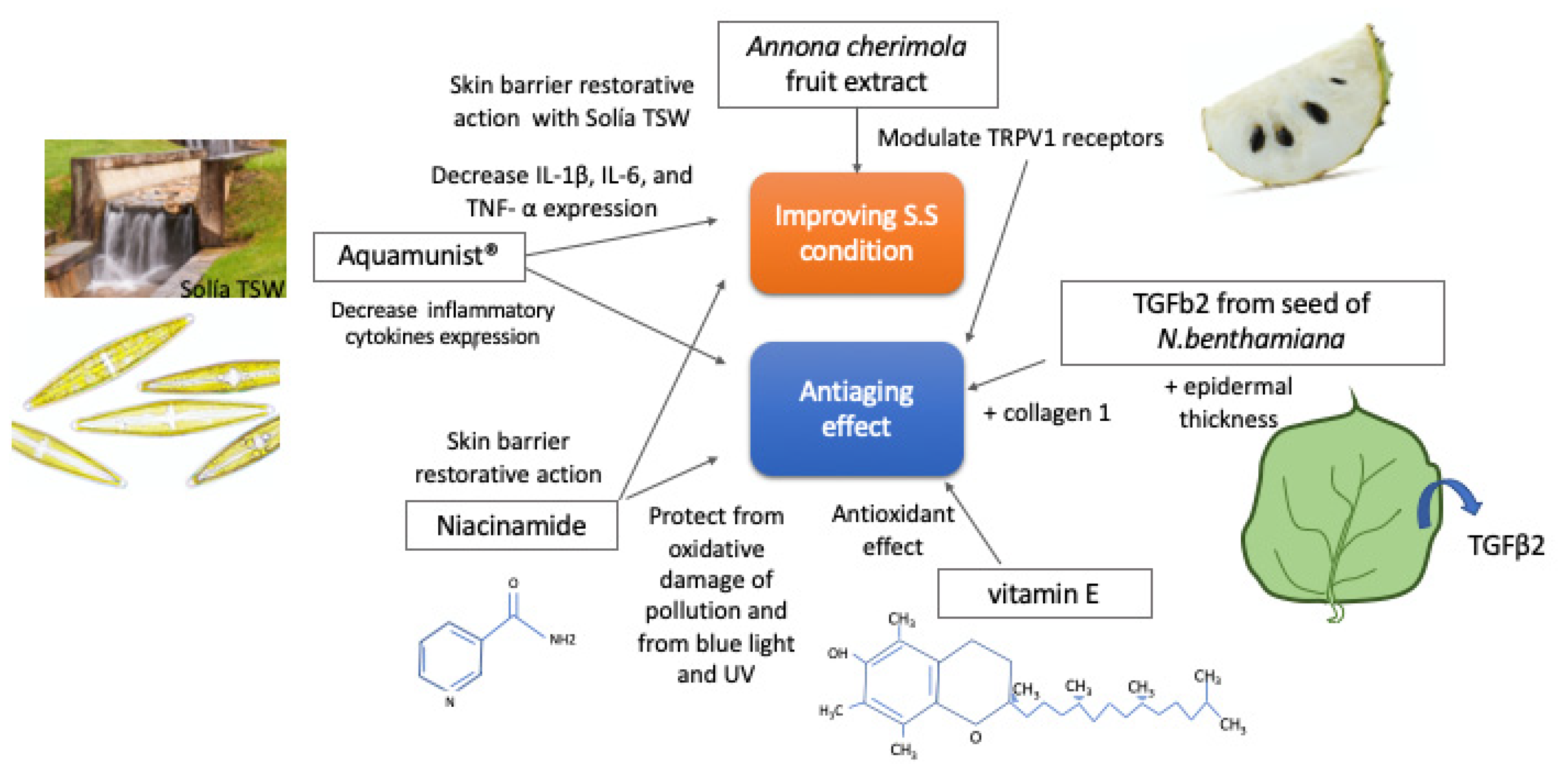
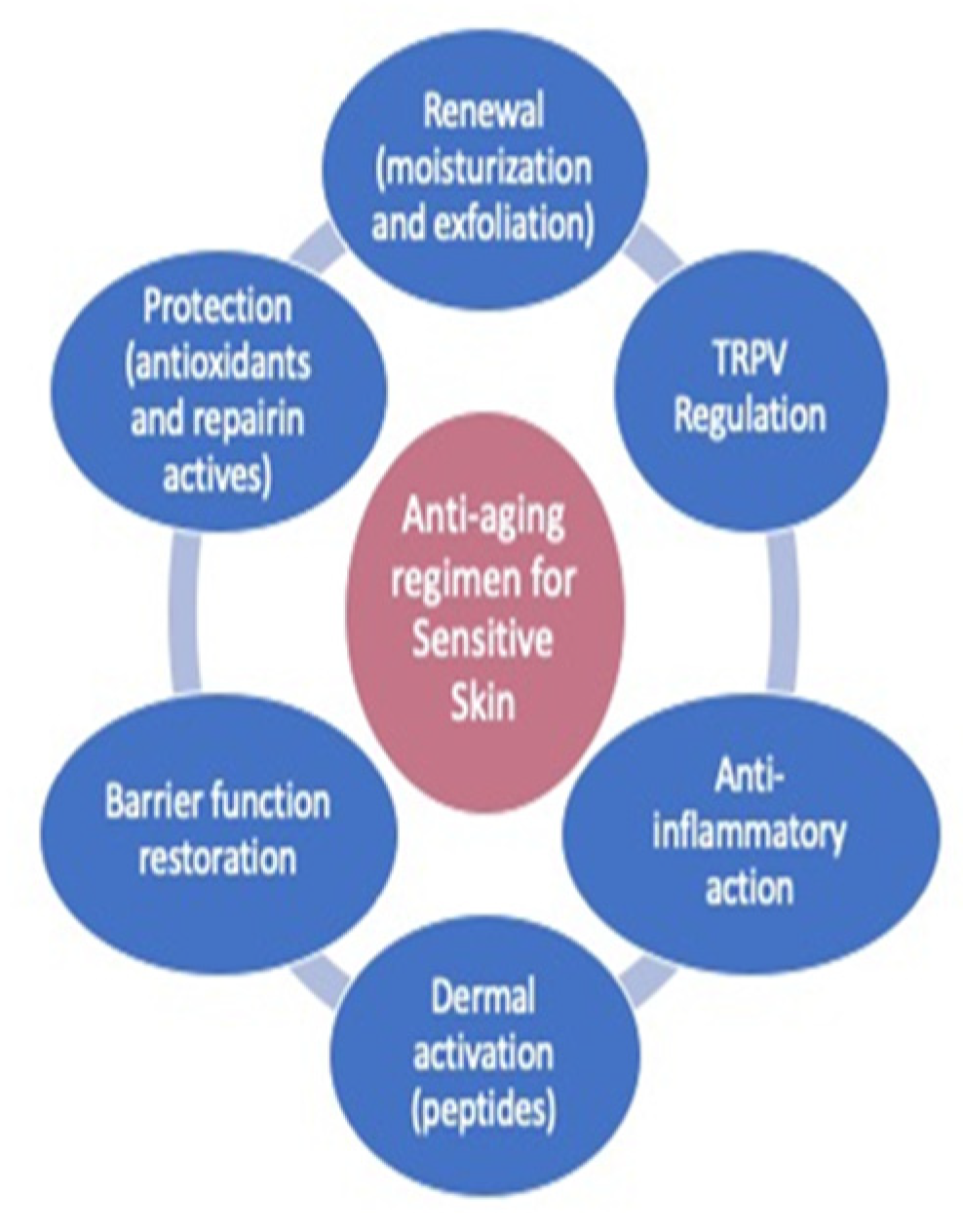
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
2.3. Composition of the Tested Products
2.4. Study Protocol
2.5. Evaluation Methods
- -
- Skin moisturization Corneometer® CM 825 Courage + Khazaka, electronic at T0, T14, T28, T42.
- -
- Transepidermal water loss (TEWL) to assess skin barrier function with Tewameter® TM 300—Courage + Khazaka Electronic at T0-T14-T28-T42.
- -
- Skin erythema with Mexameter 18® Mexameter® MX 18—Courage + Khazaka Electronic at T0-T14-T28-T42.
- -
- Skin firmness (R0) and elasticity (R2) with Cutometer® MPA 580, Courage + Khazaka, electronic at T0-T14-T28-T42
- -
- Skin profilometry (wrinkle depth) with Primos 3D (GFMesstechnik GmbH, Brandenburg, Germany) at T0-T28-T42.
- -
- Skin wrinkle according to RAO Goldman scale (1–5 score) at all visits and improvement of skin appearance (1–4 score) at T14, T28 and T42.
- -
- Dermatological evaluation and product tolerability: with collaboration of the enrolled subject, the dermatologist assessed both physical signs (skin erythema, skin edema, skin dryness, skin desquamation) and functional signs (stinging/itching sensation, burning sensation, tight feeling), scoring from 0 (no one) to 4 (severe).
- -
- A self-assessment questionnaire was filled in by the enrolled subjects at the end of the study (T42), ranging from completely disagree, disagree, agree or completely agree (Table 2).
- -
- Digital pictures of faces were acquired by means of Visia®-CR (Canfield Scientific, Parsippany, NJ, USA). This instrument ensures a reproducible subject positioning between timepoints and acquires pictures using different light modalities in order to enhance the visualization of the skin features to be analyzed.
| Regarding the products | |
| 1 | Do you find the look and smell of the products pleasant? |
| 2 | Do you find the texture of the products pleasant? |
| 3 | Is the absorption of the products into the skin rapid after application? |
| 4 | Do you feel freshness effect after application? |
| 5 | Do you notice an oily sensation during application? |
| 6 | Do you notice the product leave residue on the skin, or feeling stickiness? |
| 7 | Do you notice your skin is softer? |
| Regarding the scheme of use | |
| 8 | Is it easy for you to follow the protocol using the products in your daily routine? |
| Regarding subjective efficacy | |
| 9 | You notice your skin more hydrated, immediately after application. |
| 10 | You notice your skin is more hydrated at the end of treatment. |
| 11 | You notice your skin tone is more even |
| 12 | The product relieves tightness immediately after application. |
| 13 | Does the product relieve skin tightness? |
| 14 | The treatment reduces symptoms associated with sensitive/reactive skin |
| 15 | Reduce the sensation of heat on the skin |
| 16 | Reduce the dryness of the skin. |
| 17 | Reduce the redness of the skin. |
| 18 | Instantly calms the feeling of discomfort |
| 19 | The treatment helps control sensations of irritation and tingling. |
| 20 | Notice more renewed and repaired skin |
| 21 | You notice fewer reactions and less sensitivity in your skin since using the products |
| Regarding the purchase intention | |
| 22 | Would you recommend this protocol? |
| 23 | Did product protocol meet your expectations? |
2.6. Statistical Analysis
3. Results
3.1. Quantitative Variables
3.1.1. Skin Moisturization
3.1.2. TEWL
3.1.3. Skin Erythema
3.1.4. Skin Firmness (R0)
3.1.5. Skin Elasticity (R2)
3.1.6. Skin Profilometry
3.2. Qualitative Variables
3.2.1. Clinical Evaluation
3.2.2. Dermatological Evaluation of Tolerance
3.2.3. Self-Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Misery, L.; Weisshaar, E.; Brenaut, E.; Evers, A.W.M.; Huet, F.; Ständer, S.; Reich, A.; Berardesca, E.; Serra-Baldrich, E.; Wallengren, J.; et al. Pathophysiology and management of sensitive skin: Position paper from the special interest group on sensitive skin of the International Forum for the Study of Itch (IFSI). J. Eur. Acad. Dermatol. Venereol. 2020, 34, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Ständer, S.; Szepietowski, J.C.; Reich, A.; Wallengren, J.; Evers, A.W.; Takamori, K.; Brenaut, E.; Le Gall-Ianotto, C.; Fluhr, J.; et al. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. Revisiting the Skin Health and Beauty Pyramid: A Clinically Based Guide to Selecting Topical Skincare Products. J. Drugs Dermatol. 2021, 20, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Sousa Lobo, J.M.; Almeida, I.F. Sensitive Skin: Active Ingredients on the Spotlight. Int. J. Cosmet. Sci. 2021, 44, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Marson, J.; Bhatia, N.; Graber, E.; Harper, J.; Lio, P.; Tlougan, B.; Nussbaum, D.; Baldwin, H. Supplement Article: The Role of Epidermal Barrier Dysfunction and Cutaneous Microbiome Dysbiosis in the Pathogenesis and Management of Acne Vulgaris and Rosacea. J. Drugs Dermatol. 2022, 21, SF3502915–SF35029114. [Google Scholar] [PubMed]
- Do, L.H.D.; Azizi, N.; Maibach, H. Sensitive Skin Syndrome: An Update. Am. J. Clin. Dermatol. 2020, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Buhé, V.; Vié, K.; Guéré, C.; Natalizio, A.; Lhéritier, C.; Le Gall-Ianotto, C.; Huet, F.; Talagas, M.; Lebonvallet, N.; Marcorelles, P.; et al. Pathophysiological Study of Sensitive Skin. Acta Derm. Venereol. 2016, 96, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Escalas-Taberner, J.; González-Guerra, E.; Guerra-Tapia, A. La Piel Sensible: Un Síndrome Complejo. Actas Dermo-Sifiliogr. 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Misery, L.; Loser, K.; Ständer, S. Sensitive skin. J. Eur. Acad. Dermatol. Venereol. 2016, 30 (Suppl. 1), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.I.; Olah, A.; Szollosi, A.G.; Biro, T. TRP channels in the skin. Br. J. Dermatol. 2014, 171, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ye, L.; Mauro, T.M.; Dang, E.; Wang, G.; Hu, L.Z.; Yu, C.; Jeong, S.; Feingold, K.; Elias, P.M.; Lv, C.Z.; et al. Topical applications of an emollient reduce circulating pro-inflammatory cytokine levels in chronically aged humans: A pilot clinical study. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2197–2201. [Google Scholar] [CrossRef] [PubMed]
- Mourelle, M.L.; Segura de Yebra, J.; Ayats, J.; Vitale, M.; López Sánchez, A. Potential Benefits of a Cosmetic Ingredient Combining Thermal Spring Water and Diatom Algae Extract. Cosmetics 2024, 11, 62. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Heiland, J.; Doppler, S.; Rawlings, A.V. Efficient, high skin barrier improvement by a daily use cosmetic moisturizer: Results from two clinical studies. Int. J. Cosmet. Sci. 2007, 29, 485–492. [Google Scholar] [CrossRef]
- Lee, H.P.; Choi, Y.J.; Cho, K.A.; Woo, S.Y.; Yun, S.T.; Lee, J.T.; Kim, H.J.; Lee, K.H.; Kim, J.W. Effect of Spa Spring Water on Cytokine Expression in Human Keratinocyte HaCaT Cells and on Differentiation of CD4(+) T Cells. Ann. Dermatol. 2012, 24, 324–336. [Google Scholar] [CrossRef]
- Cacciapuoti, S.; Luciano, M.A.; Megna, M.; Annunziata, M.C.; Napolitano, M.; Patruno, C.; Scala, E.; Colicchio, R.; Pagliuca, C.; Salvatore, P.; et al. The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature. J. Clin. Med. 2020, 9, 3047. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Dermothermal Cosmetics: Added Value for Thermal Centers. In Proceedings of the 1st International Congress on Water Healing Spa and Quality of Life, Ourense, Spain, 23–24 September 2015; pp. 1–7. [Google Scholar]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Hydrobiome of Thermal Waters: Potential Use in Dermocosmetics. Cosmetics 2023, 10, 94. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Rodrigues, M.; Mourelle, M.L.; Araujo, A.R.T.S. Thermal Spring Waters as an Active Ingredient in Cosmetic Formulations. Cosmetics 2023, 10, 27. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Awwad, F.; Diamond, A.; Desgagné-Penix, I. Diatoms’ Breakthroughs in Biotechnology: Phaeodactylum tricornutum as a Model for Producing High-Added Value Molecules. Am. J. Plant Sci. 2020, 11, 1632–1670. [Google Scholar] [CrossRef]
- Mosxou, D.; Letsiou, S. Exploring the Protective Effects of Phaeodactylum tricornutum Extract on LPS-Treated Fibroblasts. Cosmetics 2021, 8, 76. [Google Scholar] [CrossRef]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosmet. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Madaan, P.; Sikka, P.; Malik, D.S. Cosmeceutical Aptitudes of Niacinamide: A Review. Recent Adv. Anti-Infect. Drug Discov. 2021, 16, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef]
- Burgess, C. Topical vitamins. J. Drugs Dermatol. 2008, 7 (Suppl. 7), s2–s6. [Google Scholar] [PubMed]
- Rojas-García, A.; Rodríguez, L.; Cádiz-Gurrea, M.d.l.L.; García-Villegas, A.; Fuentes, E.; Villegas-Aguilar, M.d.C.; Palomo, I.; Arráez-Román, D.; Segura-Carretero, A. Determination of the Bioactive Effect of Custard Apple By-Products by In Vitro Assays. Int. J. Mol. Sci. 2022, 23, 9238. [Google Scholar] [CrossRef]
- Ferreira, E.; Pastor, S.; Grau-Campistany, A.; Mateu, M. Una alternativa de origen vegetal para aplicaciones similares al retinol y al ácido retinoico conpropiedades antienvejecimiento de amplio espectro, según la a-beauty. Cosmetica 2022, 111, 71–89. [Google Scholar]
- Jutley, J.K.; Wood, E.J.; Cunliffe, W.J. Influence of retinoic acid and TGF-beta on dermal fibroblast proliferation and collagen production in monolayer cultures and dermal equivalents. Matrix 1993, 13, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Pendaries, V.; Verrecchia, F.; Michel, S.; Mauviel, A. Retinoic acid receptors interfere with the TGF-beta/Smad signaling pathway in a ligand-specific manner. Oncogene 2003, 22, 8212–8220. [Google Scholar] [CrossRef] [PubMed]
- Macias, M.J.; Martin-Malpartida, P.; Massagué, J. Structural determinants of Smad function in TGF-β signaling. Trends Biochem. Sci. 2015, 40, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Hoover, L.L.; Burton, E.G.; O’Neill, M.L.; Brooks, B.A.; Sreedharan, S.; Dawson, N.A.; Kubalak, S.W. Retinoids regulate TGFbeta signaling at the level of Smad2 phosphorylation and nuclear accumulation. Biochim. Biophys. Acta 2008, 1783, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.; Costa, P.C.; Bahia, M.F. Effect of São Pedro Do Sul Thermal Water on Skin Irritation. Int. J. Cosmet. Sci. 2010, 32, 205–210. [Google Scholar] [CrossRef]
- Petraccia, L.; Liberati, G.; Masciullo, S.G.; Grassi, M.; Fraioli, A. Water, mineral waters and health. Clin. Nutr. 2006, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Sun, M.; Zhao, C.; Kang, J. TRPV1: A promising therapeutic target for skin aging and inflammatory skin diseases. Front. Pharmacol. 2023, 14, 1037925. [Google Scholar] [CrossRef]
- Voegeli, R.; Rawlings, A.V.; Serup, J.; Loden, M. The Effect of a Bi-functional Moisturizer Containing Niacinamide on Facial Skin Quality: A Randomized, Double-blind, Vehicle-controlled Study. Int. J. Cosmet. Sci. 2016, 38, 237–245. [Google Scholar] [CrossRef]
- Lodén, M. The Clinical Benefit of Moisturizers. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 959–965. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialization. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Kandasamy, D.; Nithyanand, P. A review on Antioxidant activity of marine organisms. Int. J. ChemTech Res. 2014, 6, 3431–3436. [Google Scholar]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for Marine Resources in Cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Uppala, L. A Review on Active Ingredients from Marine Sources used in Cosmetics. SOJ Pharm. Pharm. Sci. 2015, 2, 1–3. [Google Scholar] [CrossRef][Green Version]
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Mar. Drugs 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Bueno Ariede, M.; Marcílio Candido, T.; Morocho Jacome, A.L.; Robles Velasco, M.V.; de Carvalho, J.C.M.; Rolim Baby, A. Cosmetic attributes of algae—A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Verdy, C.; Branka, J.E.; Lefeuvre, L. Modulation of Sodium-Dependent Transporters Expression in Normal Human Keratinocytes by a Sodium Rich Isotonic Thermal Water. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 254–262. [Google Scholar] [CrossRef]
- Messaraa, C.; Drevet, J.; Jameson, D.; Zuanazzi, G.; De Ponti, I. Can Performance and Gentleness Be Reconciled? A Skin Care Approach for Sensitive Skin. Cosmetics 2022, 9, 34. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Gunt, H.; Zeichner, J.; Levy, S. Clinical Evaluation of a Nature-Based Bakuchiol Anti-Aging Moisturizer for Sensitive Skin. J. Drugs Dermatol. 2020, 19, 1181–1183. [Google Scholar] [CrossRef] [PubMed]






| Evaluation Time | Qualitative and Quantitative Evaluations Performed at Each Visit |
|---|---|
| T0 |
|
| T14 |
|
| T28 |
|
| T42 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, M.; Truchuelo, M.T.; Nobile, V.; Gómez-Sánchez, M.J. Clinical Tolerability and Efficacy Establishment of a New Cosmetic Treatment Regimen Intended for Sensitive Skin. Appl. Sci. 2024, 14, 6252. https://doi.org/10.3390/app14146252
Vitale M, Truchuelo MT, Nobile V, Gómez-Sánchez MJ. Clinical Tolerability and Efficacy Establishment of a New Cosmetic Treatment Regimen Intended for Sensitive Skin. Applied Sciences. 2024; 14(14):6252. https://doi.org/10.3390/app14146252
Chicago/Turabian StyleVitale, María, Maria Teresa Truchuelo, Vincenzo Nobile, and María José Gómez-Sánchez. 2024. "Clinical Tolerability and Efficacy Establishment of a New Cosmetic Treatment Regimen Intended for Sensitive Skin" Applied Sciences 14, no. 14: 6252. https://doi.org/10.3390/app14146252
APA StyleVitale, M., Truchuelo, M. T., Nobile, V., & Gómez-Sánchez, M. J. (2024). Clinical Tolerability and Efficacy Establishment of a New Cosmetic Treatment Regimen Intended for Sensitive Skin. Applied Sciences, 14(14), 6252. https://doi.org/10.3390/app14146252






