Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective
Abstract
:1. Introduction
2. Depleted/Depleting Hydrocarbon Reservoirs (DHRs) for H2 Generation
3. Microbial H2–Bio-H2 Generation in DORs
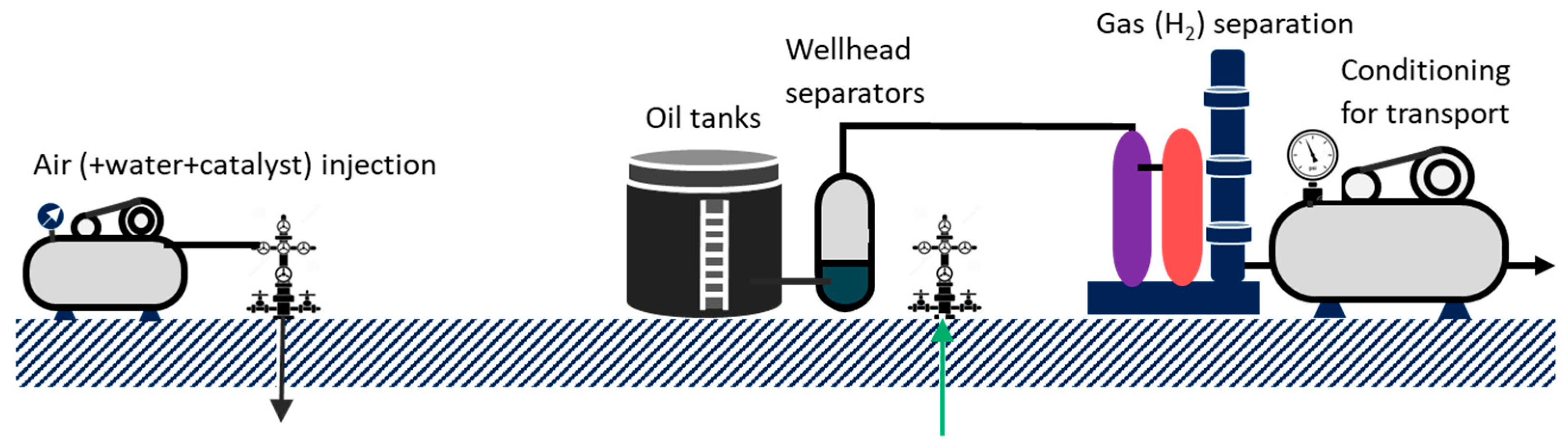
3.1. Concept
| Carbon Source | Experiment | Max H2 | Microorganisms | Ref. |
|---|---|---|---|---|
| Used | Yield 1 | |||
| Glucose | Bottle, atm | 4.08 | Escherichia coli | [43] |
| Glucose | Bioreactor, atm, 35 °C | 1.65 | Clostridium species | [25] |
| Sucrose | Bioreactor, atm, 35 °C | 4.52 | Clostridium species | [25] |
| Sucrose | Bottle, atm | 2.98 | Clostridium butyricum | [28] |
| Glucose | Bioreactor, atm, 32 °C | 3.80 | Clostridium pasteurianum | [31] |
| Glucose | Bottle, atm, 70 °C | 1.10 | Thermotoga strains | [11] |
| Crude oil | Bottle, atm, 70 °C | 0.10 | Thermotoga strains | [11] |
| Glucose + crude oil | Bottle, atm | 1.65 | Thermotoga strains | [11] |
| Glucose | Bottle, atm, 37 °C | 1.12 | Reservoir community 2 | [12] |
| Glucose | Bottle, 16 bar, 37 °C | 0.70 | Reservoir community 2 | [12] |
3.2. Reservoir Engineering Aspects
3.2.1. Mode of Application
3.2.2. Economics
3.2.3. Associated Risks
4. Thermal H2 Generation from Hydrocarbons: Theoretical Basis
4.1. Steam Reforming
4.2. Partial Oxidation
4.3. Autothermal Reforming
4.4. Pyrolysis
5. H2 Generation in DGRs
5.1. Concept
5.2. Reservoir Engineering Aspects
5.2.1. Mode of Application
5.2.2. Economics
5.2.3. Associated Risks
6. H2 Generation in DORs
6.1. Concept
| Application Mode | Reported Mechanisms | H2 1 | AR [Sm3/m3] | Ref. |
|---|---|---|---|---|
| Core tests with reservoir bitumen, T = 280–350 °C | Thermal cracking, WGS, coke gasification | 1 | n.a | [77] |
| Core sandpack, T = ca. 500 °C | Partial combustion, WGS | 2.1 | 168 | [81] |
| Pilot scale DHG unit, with Naphta cut, T = 750 °C | Catalytic steam reforming | 50 | n.a. | [78] |
| Conical combustion cell with Athabaca bitumen | Thermal cracking, LTO/HTO | <5 2 | 400 | [89] |
| Combustion (sandpack) tube, T = 800 °C | Catalytic combustion, WGS, SG | 1.5 | 220 | [80] |
| Core flood tests, hot water-steam, T = 200 °C | Decarboxilation + the reaction with rock | 11 | n.a. | [90] |
| Experimental, 3-D, SADG followed by ISC | Thermal cracking of bitumen | 1.2 | n.a. | [91] |
| Conical sandpack with T = 400–500 °C | Partial combustion, WGS | 6 | n.a. | [57] |
6.2. Reservoir Engineering Aspects
6.2.1. Mode of Application
6.2.2. Economics
6.2.3. Associated Risks
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AR | Air requirement |
| CAPEX | Capital expenditure |
| DF | Dark fermentation |
| DGR | Depleted gas reservoirs |
| DGU | Downhole gasification unit |
| DHR | Depleted hydrocarbon reservoir |
| DOE | Department of Energy (USA) |
| DOR | Depleted oil reservoir |
| EOR | Enhanced oil recovery |
| EU | European Union |
| GCS | Geologic carbon storage |
| H/C | Hydrogen/carbon |
| HC | Hydrocarbon |
| HTO | High-temperature oxidation |
| IEA | International Energy Agency |
| IR | Incremental recovery (oil) |
| ISC | In situ combustion |
| LCOE | Levelized cost of energy |
| LTO | Low-temperature oxidation |
| MC | Methane cracking |
| MEOR | Microbial enhanced oil recovery |
| NG | Natural gas |
| OPEX | Operational expenditure |
| P | Pressure |
| SAGD | Steam-assisted gravity drainage |
| SF | Steam flooding |
| SMR | Steam methane reforming |
| SOR | Steam/oil ratio |
| SRB | Sulfate-reducing bacteria |
| T | Temperature |
| TPOX | Thermal partial oxidation process |
| TRL | Technology readiness level |
| WGS | Water–gas shift |
References
- EU. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions Repowereu Plan; EU: Brussels, Belgium, 2022. [Google Scholar]
- DOE. Hydrogen Shot Summit; Department of Energy: Washington, DC, USA, 2021. [Google Scholar]
- Veziroğlu, T.N. Twenty Years of the Hydrogen Movement: 1974–1994. In Hydrogen Energy System; Yürüm, Y., Ed.; NATO ASI Series (Series E: Applied Sciences); Springer: Dordrecht, The Netherlands, 1995; Volume 295. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. In Energy for a Sustainable World. From the Oil Age to a Sun-Powered Future; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- IEA. Global Hydrogen Review 2023. 2023. Available online: https://iea.blob.core.windows.net/assets/ecdfc3bb-d212-4a4c-9ff7-6ce5b1e19cef/GlobalHydrogenReview2023.pdf (accessed on 1 March 2024).
- Ghiyati, Y.; Bansal, N.; Mohammed Ilyas, M. The Best of Blue Hydrogen; Hydrocabon Engineering: Metairie, LA, USA, 2021; pp. 17–22. [Google Scholar]
- Muradov, N. Low to near-zero CO2 production of hydrogen from fossil fuels: Status and perspectives. Int. J. Hydrogen Energy 2017, 42, 14058–14088. [Google Scholar] [CrossRef]
- Megía, P.J.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Hydrogen production technologies: From fossil fuels toward renewable sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- GCCSI Global CCS Institute. The Global Status of CCS: 2023; GCCSI: Melbourne, Australia, 2023. [Google Scholar]
- Alkan, H.; Burachok, O.; Kowollik, P. Geologic carbon storage: Key components. In Surface Process, Transportation, and Storage; Elsevier BV: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Veshareh, M.J.; Poulsen, M.; Nick, H.M.; Feilberg, K.L.; Eftekhari, A.A.; Dopffel, N. The light in the dark: In-situ biorefinement of crude oil to hydrogen using typical oil reservoir thermotoga strains. Int. J. Hydrogen Energy 2022, 47, 5101–5110. [Google Scholar] [CrossRef]
- Dopffel, N.; Vik, B.F.; Mukherjee, S.; Djurhuus, K. Bio-hydrogen production under pressure by pressure-adapted subsurface microbes. Int. J. Hydrogen Energy 2022, 47, 3690–3698. [Google Scholar] [CrossRef]
- Afanasev, P.; Popov, E.; Cheremisin, A.; Berenblyum, R.; Mikitin, E.; Sorokin, E.; Borisenko, A.; Darishchev, V.; Shchekoldin, K.; Slavkina, O. An Experimental Study of the Possibility of In Situ Hydrogen Generation within Gas Reservoirs. Energies 2021, 14, 5121. [Google Scholar] [CrossRef]
- Afanasev, P.; Popov, E.; Cheremisin, A.; Mikitin, E.; Darishchev, V. In Situ Hydrogen Generation Within Gas Reservoirs. In Proceedings of the SPE-214036-MS, Gas & Oil Technology Showcase and Conference, Dubai, United Arab Emirates, 13–15 March 2023. [Google Scholar] [CrossRef]
- Berenblyum, R.; Surguchev, M. Subsurface Hydrogen Generation: Low Cost and Low Footprint Method of Hydrogen Production, OnePetro. 2022. Available online: https://onepetro.org/SPEBERG/proceedings/22BERG/1-22BERG/D011S002R002/484658 (accessed on 4 December 2022).
- Kapadia, P.R.; Kallos, M.S.; Leskiw, C.; Gates, I.D. Potential for Hydrogen Generation during In Situ Combustion of Bitumen. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar]
- Murthy, B.N.; Sawarkar, A.N.; Deshmukh, N.A.; Mathew, T.; Joshi, J.B. Petroleum coke gasification: A review. Can. J. Chem. Eng. 2013, 92, 441–468. [Google Scholar] [CrossRef]
- Gade, S.K.; Payzant, E.A.; Park, H.J.; Thoen, P.M.; Way, J.D. The effects of fabrication and annealing on the structure and hydrogen permeation of Pd–Au binary alloy membranes. J. Membr. Sci. 2009, 340, 227–233. [Google Scholar] [CrossRef]
- Acquaviva, J. High-Performance, Durable, Palladium Alloy Membrane for Hydrogen Separation and Purification. In Proceedings of the DOE Annual Merit Review Meeting, Arlington, VA, USA, 19 May 2009. [Google Scholar]
- Usman, M.; Kavitha, S.; Kannah, Y.; Yogalakshmi, K.N.; Sivashanmugam, P.; Bhatnagar, A.; Kumar, G. A critical review on limitations and enhancement strategies associated with biohydrogen production. Int. J. Hydrogen Energy 2021, 46, 16565–16590. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Preethi; Karthikeyan, O.P.; Kumar, G.; Dai-Viet, N.V.; Banu, J.R. Techno-economic assessment of various hydrogen production methods—A Review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The future of Hydrogen Energy: Bio-hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Nandi, R.; Sengupta, S. Microbial production of hydrogen: An overview. Crit. Rev. Microbiol. 1998, 24, 61e84. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Yang, M.-H.; Yeh, K.-L.; Liu, C.-H.; Chang, J.-S. Biohydrogen production using sequential two-stage dark and photo fermentation processes. Int. J. Hydrogen Energy 2008, 33, 4755–4762. [Google Scholar] [CrossRef]
- Chen, C.C.; Lin, C.Y. Using Sucrose as a Substrate in an Anaerobic Hydrogen-Producing Reactor, Advances in Environmental Research. Pergamon. 2003. Available online: https://www.sciencedirect.com/science/article/pii/S1093019102000357 (accessed on 20 October 2022).
- Pattra, S.; Sangyoka, S.; Boonmee, M.; Reungsang, A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int. J. Hydrogen Energy 2008, 33, 5256–5265. [Google Scholar] [CrossRef]
- Ntaikou, I.; Gavala, H.N.; Kornaros, M.; Lyberatos, G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int. J. Hydrogen Energy 2008, 33, 1153–1163. [Google Scholar] [CrossRef]
- Ding, J.; Liu, B.-F.; Ren, N.-Q.; Xing, D.-F.; Guo, W.-Q.; Xu, J.-F.; Xie, G.-J. Hydrogen production from glucose by co-culture of Clostridium Butyricum and immobilized Rhodopseudomonas faecalis RLD-53. Int. J. Hydrog. Energy 2009, 34, 3647–3652. [Google Scholar] [CrossRef]
- Ciranna, A.; Ferrari, R.; Santala, V.; Karp, M. Inhibitory effects of substrate and soluble end products on biohydrogen production of the alkalithermophile Caloramator celer: Kinetic, metabolic and transcription analyses. Int. J. Hydrogen Energy 2014, 39, 6391–6401. [Google Scholar] [CrossRef]
- Liu, J.-F.; Mbadinga, S.M.; Ke, W.-J.; Gu, J.-D.; Mu, B.-Z. The diversity of hydrogen-producing microorganisms in a high temperature oil reservoir and its potential role in promoting the in situ bioprocess. Appl. Environ. Biotechnol. 2016, 2, 25–34. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Uddin, N.; Daud, W.M.A.W.; Abbas, H.F. Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables. Renew. Sustain. Energy Rev. 2013, 27, 204–224. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Zumar Bundhoo, M.A.; Mohee, R. Inhibition of Dark Fermentative Bio-Hydrogen Production: A Review. Int. J. Hydrog. Energy 2016, 41, 6713–6733. Available online: https://www.sciencedirect.com/science/article/pii/S0360319915319285 (accessed on 20 October 2022).
- Bundhoo, M.A.Z. Potential of bio-hydrogen production from dark fermentation of crop residues: A review. Int. J. Hydrog. Energy 2019, 44, 17346–17362. [Google Scholar] [CrossRef]
- Stephen, A.J.; Archer, S.A.; Orozco, R.L.; Macaskie, L.E. Advances and bottlenecks in microbial hydrogen production. Microb. Biotechnol. 2017, 10, 1120–1127. [Google Scholar] [CrossRef]
- Magot, M.; Ollivier, B.; Patel, B. Microbiology of petroleum reservoirs. Antonie Van Leeuwenhoek 2000, 77, 103–116. [Google Scholar] [CrossRef]
- Grassia, G.S.; McLean, K.M.; Glénat, P.; Bauld, J.; Sheehy, A.J. A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol. Ecol. 1996, 21, 47–58. [Google Scholar] [CrossRef]
- Magot, M. Similar bacteria in remote oil fields. Nature 1996, 379, 681. [Google Scholar] [CrossRef]
- Donaldson, E.C. Microbial Enhancement of Oil Recovery—Recent Advances; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Alkan, H.; Klueglein, N.; Mahler, E.; Kögler, F.; Beier, K.; Jelinek, W.; Herold, A.; Hatscher, S.; Leonhardt, B. An integrated German MEOR project update: Risk management and Huff’n Puff design. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. SPE-179580-MS. [Google Scholar]
- Alkan, H.; Mukherjee, S.; Kögler, F. Reservoir engineering of in-situ MEOR; impact of microbial community. J. Pet. Sci. Eng. 2020, 195, 107928. [Google Scholar] [CrossRef]
- Keasling, J.D.; Benemann, J.; Pramanik, J.; Carrier, T.A.; Jones, K.L.; Van Dien, S.J. A Toolkit for Metabolic Engineering of Bacteria. In BioHydrogen; Springer: Boston, MA, USA, 1998; pp. 87–97. [Google Scholar] [CrossRef]
- Saajanlehto, M.; Uusi-Kyyny, P.; Alopaeus, V. Hydrogen solubility in heavy oil systems: Experiments and modeling. Fuel 2014, 137, 393–404. [Google Scholar] [CrossRef]
- De Lucia, M.; Pilz, P.; Liebscher, A.; Kühn, M. Measurements of H2 Solubility in Saline Solutions under Reservoir Conditions: Preliminary Results from Project H2STORE. Energy Procedia 2015, 76, 487–494. [Google Scholar] [CrossRef]
- Cai, H.-Y.; Shaw, J.M.; Chung, K.H. Hydrogen solubility measurements in heavy oil and bitumen cuts. Fuel 2001, 80, 1055–1063. [Google Scholar] [CrossRef]
- Liu, B.; Mahmood, B.S.; Mohammadian, E.; Khaksar Manshad, A.; Rosli, N.R.; Ostadhassan, M. Measurement of Solubility of CO2 in NaCl, CaCl2, MgCl2 and MgCl2 + CaCl2 Brines at Temperatures from 298 to 373 K and Pressures up to 20 MPa Using the Potentiometric Titration Method. Energies 2021, 14, 7222. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, Y.; Zheng, Z.; Chen, D. An Accurate Model for Estimating H2 Solubility in Pure Water and Aqueous NaCl Solutions. Energies 2022, 15, 5021. [Google Scholar] [CrossRef]
- Alkan, H.; Mukherjee, S.; Jelinek, W. Front-end engineering practice of in-situ MEOR applications. J. Pet. Sci. Eng. 2022, 216, 110407. [Google Scholar] [CrossRef]
- Alkan, H.; Kögler, F.; Namazova, G.; Hatscher, S.; Jelinek, W.; Amro, M. Assessment of the Biogenic Souring in Oil Reservoirs under Secondary and Tertiary Oil Recovery. Energies 2024, 17, 2681. [Google Scholar] [CrossRef]
- Navarro, R.M.; Peña, M.A.; Fierro, J.L. Hydrogen production reactions from carbon feedstocks: fossil fuels and biomass. Chem. Rev. 2007, 107, 3952–3991. [Google Scholar] [CrossRef]
- Storey, B.M.; Worden, R.H.; McNamara, D.D. The geoscience of in-situ combustion and high-pressure air injection. Geosciences 2022, 12, 340. [Google Scholar] [CrossRef]
- Nodwell, J.; Moore, R.; Ursenbach, M.G.; Laureshen, C.; Mehta, S.K. Economic Considerations for the Design of In-Situ Combustion Projects. J. Can. Pet. Technol. 2000, 34–41. [Google Scholar] [CrossRef]
- Chaar, M.; Venetos, M.; Dargin, J.; Palmer, D. Economics of Steam Generation for Thermal Enhanced Oil Recovery. Oil Gas Facil. 2015, 4, 42–50. [Google Scholar] [CrossRef]
- Coll, R.; Pena, G.; Guitian, J. Surface facilities for a giant thermal EOR project—Cost and HSE implications of alternative fuels for steam generation. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 13–16 November 2017. [Google Scholar] [CrossRef]
- Osma, L.; García, L.; Pérez, R.; Barbosa, C.; Botett, J.; Sandoval, J.; Manrique, E. Benefit–Cost and energy Efficiency index to support the screening of hybrid cyclic steam stimulation methods. Energies 2019, 12, 4631. [Google Scholar] [CrossRef]
- Fassihi, M.; Reza Alamatsaz, A.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G.; Mallory, D.; Pereira Almao, P.; Gupta, S.C.; Chhina, H.S. A Novel Approach in Understanding the Role of Water in Oxidation and Upgrading Reactions during In-Situ Combustion Oil Recovery, Part B: Interpretations. SPE Res. Eval. Eng. 2022, 26, 319–329. [Google Scholar] [CrossRef]
- Araújo, E.A.; Araújo, A.R.; Rodrigues, M.A.F.; Diniz, A.A.R. Economic analysis of oil production by applying in situ combustion. Energy Sources Part B Econ. Plan. Policy 2017, 12, 365–371. [Google Scholar] [CrossRef]
- Deutscher Bundestag, Kosten der Produktion von Grünem Wasserstoff WD 5-3000-029/20. 2022. Available online: https://www.bundestag.de/resource/blob/691748/01a954b2b2d7c70259b19662ae37a575/WD-5-029-20-pdf-data.pdf (accessed on 1 March 2024).
- Wang, A.; Jens, J.; Mavins, D.; Moultak, M. Analysing Future Demand, Supply, and Transport of Hydrogen; European Hydrogen Backbone; Guidehouse: Chicago, IL, USA, 2021. [Google Scholar]
- Abánades, A.; Ruiz, E.; Ferruelo, E.M.; Hernández, F.; Cabanillas, A.; Martínez-Val, J.M.; Rubio, J.A.; López, C.; Gavela, R.; Barrera, G.; et al. Experimental analysis of direct thermal methane cracking. Int. J. Hydrogen Energy 2011, 36, 12877–12886. [Google Scholar] [CrossRef]
- Graham, T. On the Law of the Diffusion of Gase. Lond. Edinb. Philos. Mag. J. Sci. 1833, 2, 175–190, 269–276, 351–358. [Google Scholar] [CrossRef]
- Tsang, C.Y.; Street, W.B. Phase equilibria in the H2/CO2 system at temperatures from 220 to 290 K and pressures to 172 MPA. Chem. Eng. Sci. 1981, 36, 993–1000. [Google Scholar] [CrossRef]
- Panfilov, M.; Gravier, G.; Fillacier, S. Underground storage of H2 and H2-CO2-CH4 mixtures. In Proceedings of the ECMOR 10th European Conference on the Mathematics of Oil Recovery, Amsterdam, The Netherlands, 4–7 September 2006. [Google Scholar] [CrossRef]
- Fandiño, O.; Trusler, J.P.M.; Vega-Maza, D. Phase behavior of (CO2+H2) and (CO2+N2) at temperatures between (218.15 and 303.15) K at pressures up to 15 Mpa. Int. J. Greenh. Gas Control. 2015, 36, 78–92. [Google Scholar] [CrossRef]
- In-Situ Processes to Produce H2 from Underground Hydrocarbon Reservoirs. U.S. Patent WO 2017/136924, 2017.
- Jacobs, T. Canadian Operator Works to Transform an Oil Field into a Hydrogen Factory. JPT 2021. Available online: https://jpt.spe.org/canadian-operator-works-to-transform-an-oil-field-into-a-hydrogen-factory (accessed on 1 April 2024).
- Dwivedy, A. Steam Generation by Solar and Natural Gas Generators for Thermal Enhanced Oil Recovery. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2017. [Google Scholar]
- Sheng, J.J. Enhanced Oil Recovery Field Case Studies; Gulf Professional Publishing: Waltham, MA, USA, 2013. [Google Scholar]
- Droessler, M.; Curkan, B.; Hamilton, K. Technical Considerations of Well Design and Equipment Selection for High Temperature Applications—A Canadian Perspective. In Proceedings of the 38th New Zealand Geothermal Workshop, Auckland, New Zealand, 23–25 November 2016; GRC Transactions. GRC: Boca Raton, FL, USA, 2017; Volume 41. [Google Scholar]
- Shafiei, A.; Dusseault, M.B. Geomechanics of thermal viscous oil production in sandstones. J. Pet. Sci. Eng. 2013, 103, 121–139. [Google Scholar] [CrossRef]
- Chun, Y.N.; Kim, S.C. Production of hydrogen-rich gas from methane by Thermal Plasma Reform. J. Air Waste Manag. Assoc. 2007, 57, 1447–1451. [Google Scholar] [CrossRef]
- Greaves, M.; Xia, T.X. Laboratory Studies of Producing Hydrogen and Incremental Oil from Light Oil Reservoirs Using Downhole Gasification. J. Can. Pet. Technol. 2010, 49, 65–70. [Google Scholar] [CrossRef]
- Cardona, L.; Medina, O.E.; Céspedes, S.; Lopera, S.H.; Cortés, F.B.; Franco, C.A. Effect of Steam Quality on Extra-Heavy Crude Oil Upgrading and Oil Recovery Assisted with PdO and NiO-Functionalized Al2O3 Nanoparticles. Processes 2021, 9, 1009. [Google Scholar] [CrossRef]
- Mahinpey, N.; Ambalae, A.; Asghari, K. In situ combustion in enhanced oil recovery (EOR): A Review. Chem. Eng. Commun. 2007, 194, 995–1021. [Google Scholar] [CrossRef]
- Freitag, N.P. Chemical-Reaction Mechanisms That Govern Oxidation Rates during In-Situ Combustion and High-Pressure Air Injection. SPE Reserv. Eval. Eng. 2016, 19, 645–654. [Google Scholar] [CrossRef]
- Hajdo, L.E.; Hallam, R.J.; Vorndran, L.D.L. Hydrogen Generation During In-Situ Combustion. In Proceedings of the SPE California Regional Meeting, Bakersfield, CA, USA, 27–29 March 1985. [Google Scholar] [CrossRef]
- Greaves, M.; Xia, T.X. Producing hydrogen and incremental oil from light oil reservoirs using downhole gasification. In Proceedings of the Canadian International Petroleum Conference, Calgary, AB, Canada, 17–19 June 2008. [Google Scholar] [CrossRef]
- Yang, X.; Gates, I.D. Combustion Kinetics of Athabasca Bitumen from 1D Combustion Tube Experiments. Nat. Resour. Res. 2009, 18, 193–211. [Google Scholar] [CrossRef]
- Abu, I.I.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G.; Mallory, D.G.; Pereira-Almao, P.; Scott, C.E.; Carbognani Ortega, L. Supported catalyst regeneration and reuse for upgrading of Athabasca bitumen in conjunction with in-situ combustion. J. Can. Pet. Technol. 2015, 54, 372–386. [Google Scholar] [CrossRef]
- Moore, R.G.; Laureshen, C.J.; Mehta, S.A.; Ursenbach, M.G.; Belgrave, J.D.M.; Weissman, J.G.; Kessler, R.V. A Downhole Catalytic Upgrading Process for Heavy Oil Using In Situ Combustion. J. Can. Pet. Technol. 1999, 38. [Google Scholar] [CrossRef]
- Fulford, R.S. Produced fluid changes during a Fireflood. In Proceedings of the SPE Oilfield and Geothermal Chemistry Symposium, Stanford, CA, USA, 28–30 May 1980. [Google Scholar] [CrossRef]
- Fassihi, M.R.; Brigham, W.E.; Ramey, H.J. Reaction Kinetics of In-Situ Combustion: Part 2—Modeling. Soc. Pet. Eng. J. 1984, 24, 408–416. [Google Scholar] [CrossRef]
- Sanmiguel, J.E.; Mehta, S.A.; Moore, R.G. An experimental study of controlled gas-phase combustion in porous media for enhanced recovery of oil and Gas. J. Energy Resour. Technol. 2003, 125, 64–71. [Google Scholar] [CrossRef]
- Cinar, M.; Castanier, L.M.; Kovscek, A.R. Combustion kinetics of heavy oils in porous media. Energy Fuels 2011, 25, 4438–4451. [Google Scholar] [CrossRef]
- Hascakir, B. Introduction to Thermal Enhanced Oil Recovery (EOR) special issue. J. Pet. Sci. Eng. 2017, 154, 438–441. [Google Scholar] [CrossRef]
- Abishev, A.; Tokarev, V.; Sagyndikov, M. Evaluation of in-situ combustion efficiency in Karazhanbas oilfield, western Kazakhstan. In Proceedings of the SPE Annual Caspian Technical Conference and Exhibition, Astana, Kazakhstan, 31 October–2 November 2018. [Google Scholar] [CrossRef]
- Zhao, F.; Xi, C.; Zhang, X.; Shi, X.; Yang, F.; Mu, H.; Guan, W.; Jiang, Y.; Wang, H.; Babadagli, T.; et al. Evaluation of a Field-Wide Post-Steam In-Situ Combustion Performance in a Heavy Oil Reservoir in China. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 26–29 October 2020. [Google Scholar] [CrossRef]
- Alamatsaz, A.; Moore, R.G.; Mehta, S.A.; Ursenbach, M.G. Experimental Investigation of In-Situ Combustion at Low Air Fluxes. J. Can. Pet. Technol. 2011, 50, 48–67. [Google Scholar] [CrossRef]
- Butron, J.; Bryan, J.; Yu, X.; Kantzas, A. Production of Gases during Thermal Displacement Tests, OnePetro. OnePetro. 2015. Available online: https://onepetro.org/SPECHOC/proceedings/15CHOC/All-15CHOC/SPE-174464-MS/181718 (accessed on 1 December 2022).
- Sequera-Dalton, B.M.; Aminfar, E.; Moore, R.G.; Mehta, S.A.R.; Ursenbach, M.G. Compositional Changes in Athabasca Bitumen During Air Injection into Mature SAGD Chambers—Observations from 3-D Large Scale Experiments. In Proceedings of the SPE Canada Heavy Oil Conference, Virtual, 28 September–2 October 2020. [Google Scholar] [CrossRef]
- Surguchev, L.; Berenblyum, R.S.; Dmitrievsky, A.N. Process to Generate Hydrogen from Hydrocarbons in Situ—Hydrogen Production, Carbon Capture, Source of Thermal Energy. In Proceedings of the Third Sustainable Earth Science Conference & Exhibition, Celle, Germany, 13–15 October 2015. [Google Scholar]
- Dusseault, M.B.; Collins, P.M. 24. Geomechanics effects in thermal processes for heavy-oil exploitation. In Heavy Oils: Reservoir Characterization and Production Monitoring; Society of Exploration Geophysicists: Houston, TX, USA, 2010; pp. 287–291. [Google Scholar] [CrossRef]
- Bogdanov, I.I.; Entov, V.M.; Stepanov, V.P. Thermal Decomposition of Carbonate Rocks under In Situ Combustion; Zhurnal, I.F., Translator; Plenum Publishing Corporation: New York, NY, USA, 1990; Volune 58, pp. 828–836. [Google Scholar]
- Turta, A. In Situ Combustion Chapter. In Enhanced Oil Recovery Field Case Studies; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Sur, S. In-Situ combustion: Myths and facts. SPE Reserv. Eval. Eng. 2022, 26, 180–189. [Google Scholar] [CrossRef]
- Ikpeka, P.M.; Ugwu, J.O. In situ hydrogen production from hydrocarbon reservoirs—Modelling study. RSC Adv. 2023, 13, 12100–12113. [Google Scholar] [CrossRef]
- Yan, K.; An, B.; Yuan, Q. Enabling Hydrogen Production from Shale Oil Reservoirs: An Experimental Study Using Microwave-Assisted Catalytic Heating. In Proceedings of the SPE Energy Transition Symposium, Houston, TX, USA, 22–23 August 2023. Paper Number: SPE-215725-MS. [Google Scholar] [CrossRef]
- Edison Group. Hydrogen from Oil Reservoirs—Utilising In-Situ Combustion to Exclusively Produce—Edison Group. 2024. Available online: https://www.edisongroup.com/insight/hydrogen-from-oil-reservoirs-utilising-in-situ-combustion-to-exclusively-produce-hydrogen/ (accessed on 1 April 2024).

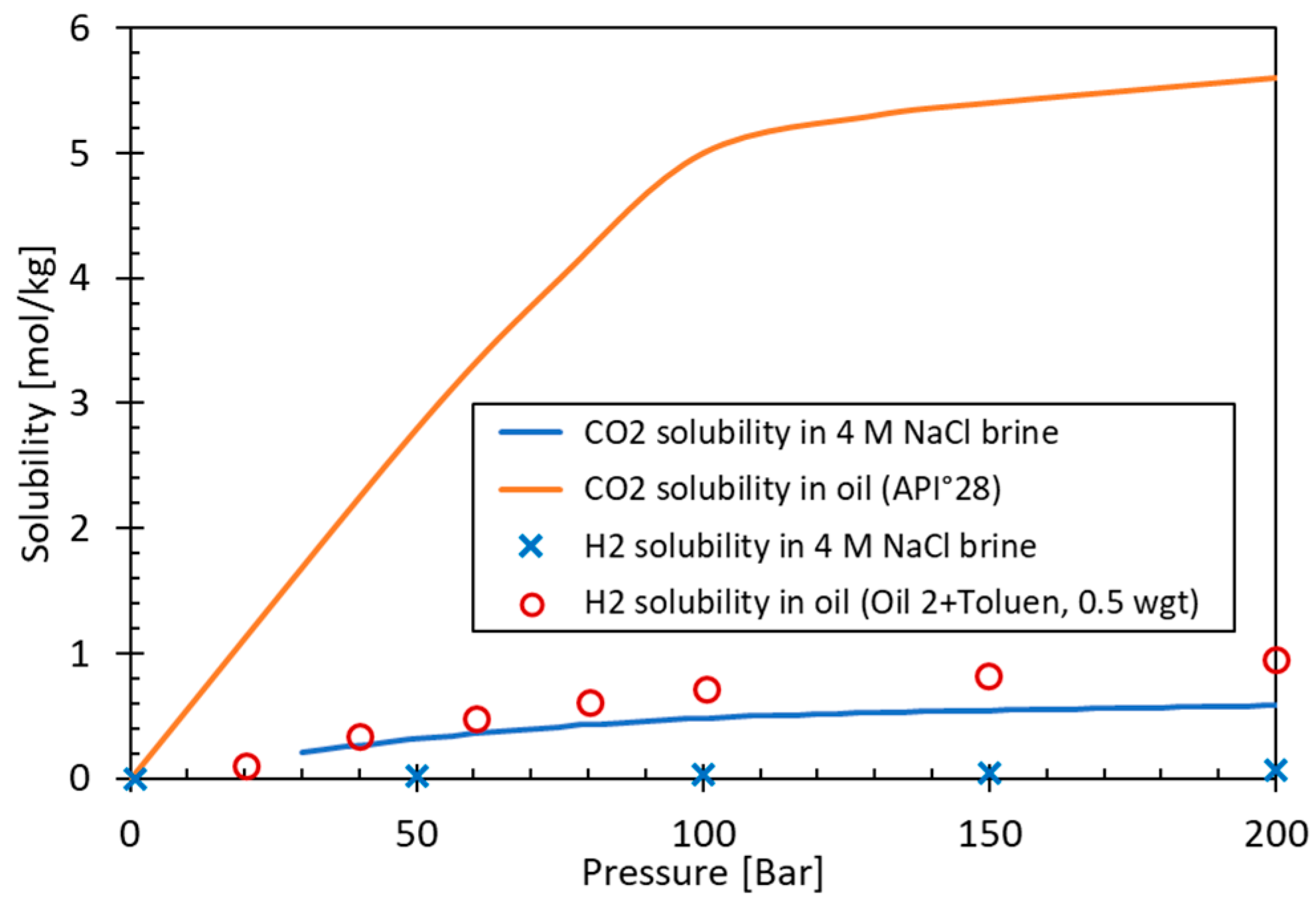
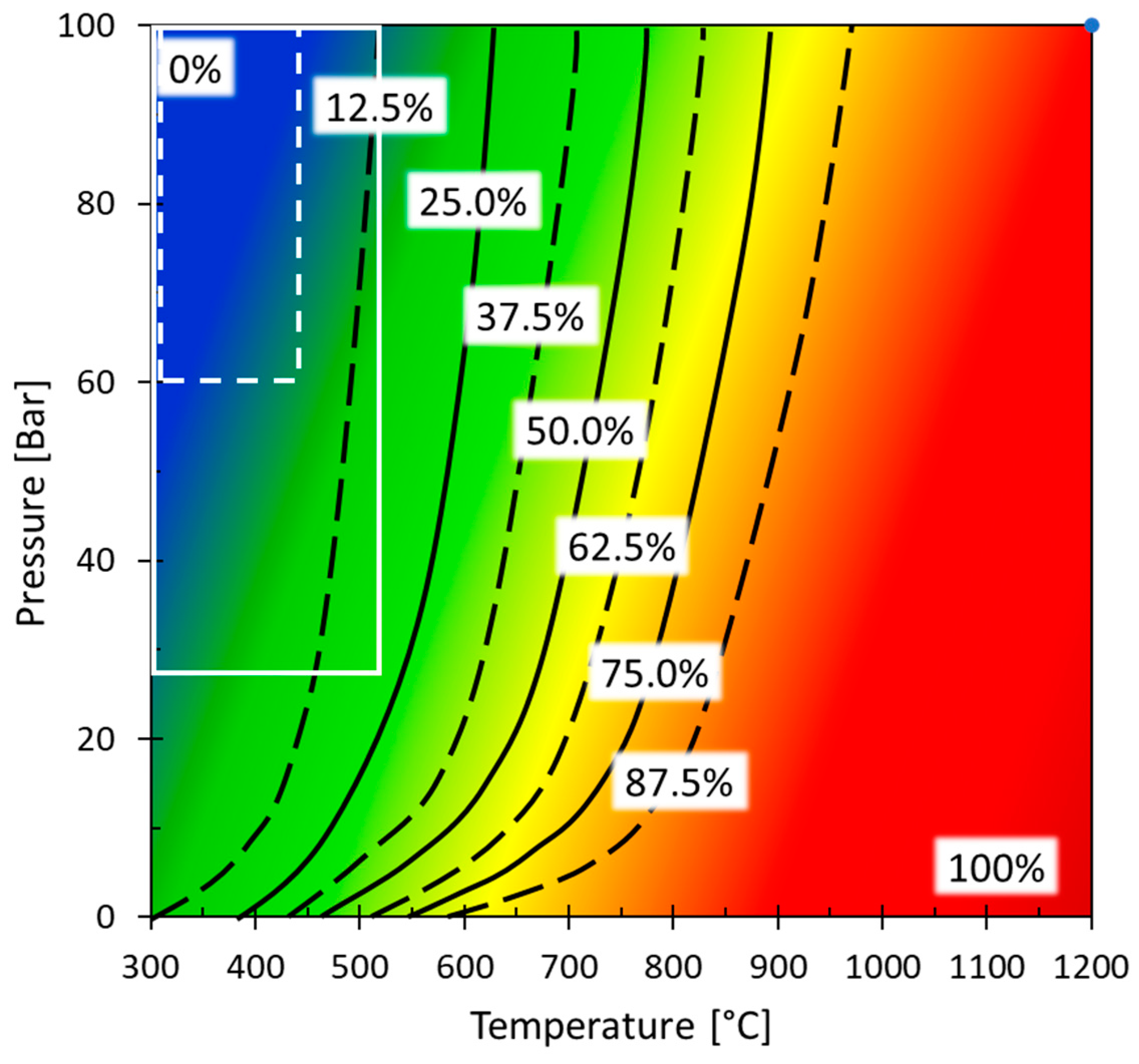
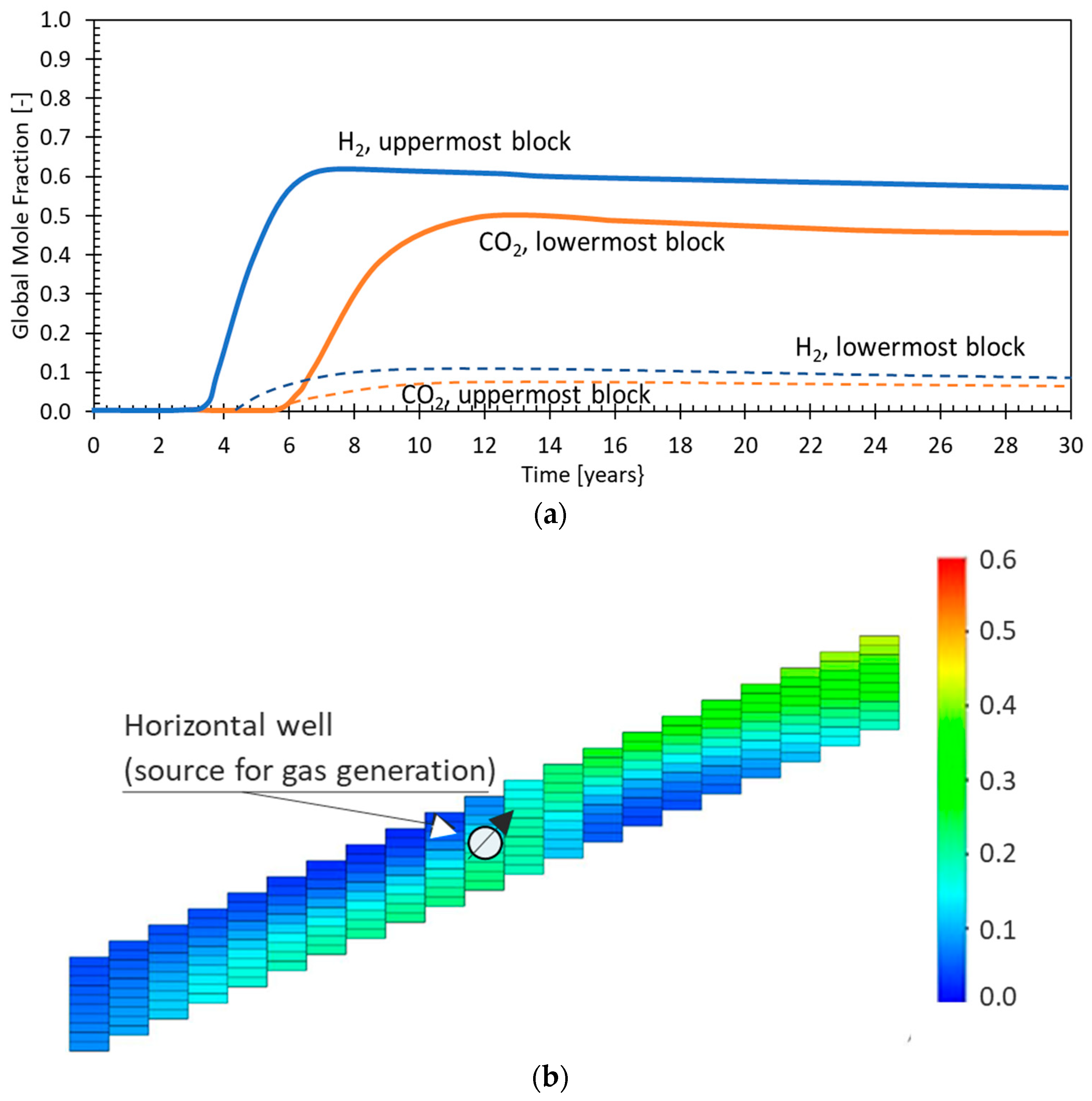
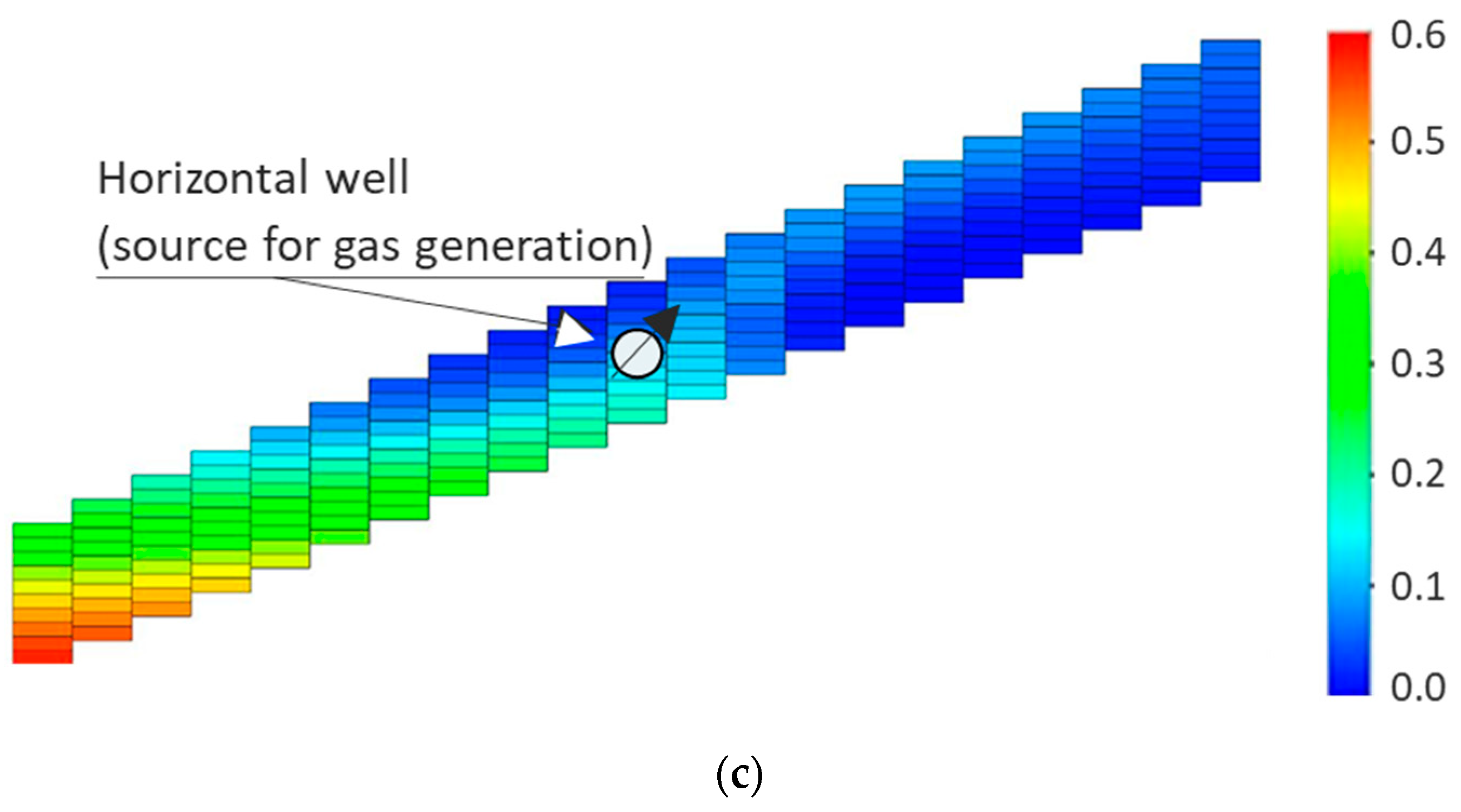

| Method | Process Proposed | Selected References |
|---|---|---|
| Microbial H2 generation (DORs) | Dark fermentation (anaerobic) of sugar. This is a derivative of microbial EOR (MEOR) to improve oil production. | [11,12] |
| Catalytic methane conversion (DGRs) | In situ steam methane reforming (SMR), water–gas shift (WGS) reaction, and methane cracking (MC) requiring heat (350–450 °C with steam flooding); for catalytic MC, Ni-salt-based solutions are used. | [13,14,15] |
| H2 through in situ combustion (DORs) | Converting in situ hydrocarbons of DORs into H2 using reforming techniques. Besides hydrocarbon, the other reactants can involve steam (steam reforming) or oxygen (partial oxidation, with up to 1000 °C using in situ combustion) or both (auto-thermal). | [15,16,17] |
| Thermal EOR | Cost [USD/kg] 1 | Ref. |
| SF field application (USD/IR, Sm3 oil) | 104 | [53] |
| Steam generation (LC of energy 2, USD/t) | 20–30 | [54] |
| Steam generation (OPEX, USD/IR Sm3 oil) | 4–19 | [55] |
| SF, numerical (USD/IR, Sm3 oil) | 108 | [56] |
| ISC air injection (USD/Sm3) | 15 | [57] |
| ISC field application (USD/IR, Sm3 oil) | 78 | [53] |
| ISC, air cost (Sm3/IR Sm3 oil) | 96 | [58] |
| H2 Production | Cost [USD/kg] 3 | |
| Blue H2 with 50 EUR/t CO2 4 | 1.6–2.7 | [5,59,60] |
| Blue H2 with 100 EUR/t CO2 4 | 3.2–7.2 | [5,59,60] |
| Grey H2 with 100 EUR/t CO2 4 | 1.5–5.1 | [5,59,60] |
| Green H2 (Electrolysis with wind/solar energy) | 1.6–12.0 | [5,59,60] |
| Green H2 (Dark fermentation) | 2.0–7.5 | [22,33] |
| Green H2 (Biomass conversion) | 1.6–8.1 | [22,33] |
| Method. | TRL | Cost [USD/kg] | Main Challenges | Perspective |
|---|---|---|---|---|
| Bio-H2 generation (DORs) | 7-8 | >25 | Low H2 yield; H2S co-generation is the main risk. | Very low to no feasibility |
| Catalytic methane conversion (DGRs) | 5-6 | >40 | Low yield; high risks at subsurface. | Very low to no feasibility |
| H2 through ISC (DORs) | 5-6 | >5 | Some higher but inconsistent reported yields; high risks at surface and subsurface. ISC is a challenging technology. | Low feasibility; efficient catalysts and powerful downhole H2 separators can change the situation. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkan, H.; Bauer, J.F.; Burachok, O.; Kowollik, P.; Olbricht, M.; Amro, M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Appl. Sci. 2024, 14, 6217. https://doi.org/10.3390/app14146217
Alkan H, Bauer JF, Burachok O, Kowollik P, Olbricht M, Amro M. Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Applied Sciences. 2024; 14(14):6217. https://doi.org/10.3390/app14146217
Chicago/Turabian StyleAlkan, Hakan, Johannes Fabian Bauer, Oleksandr Burachok, Patrick Kowollik, Michael Olbricht, and Mohd Amro. 2024. "Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective" Applied Sciences 14, no. 14: 6217. https://doi.org/10.3390/app14146217
APA StyleAlkan, H., Bauer, J. F., Burachok, O., Kowollik, P., Olbricht, M., & Amro, M. (2024). Hydrogen from Depleted/Depleting Hydrocarbon Reservoirs: A Reservoir Engineering Perspective. Applied Sciences, 14(14), 6217. https://doi.org/10.3390/app14146217








