Doped Epoxy Resins as an Alternative to Luminescent Optical Sensors
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Double-Bond Conversion
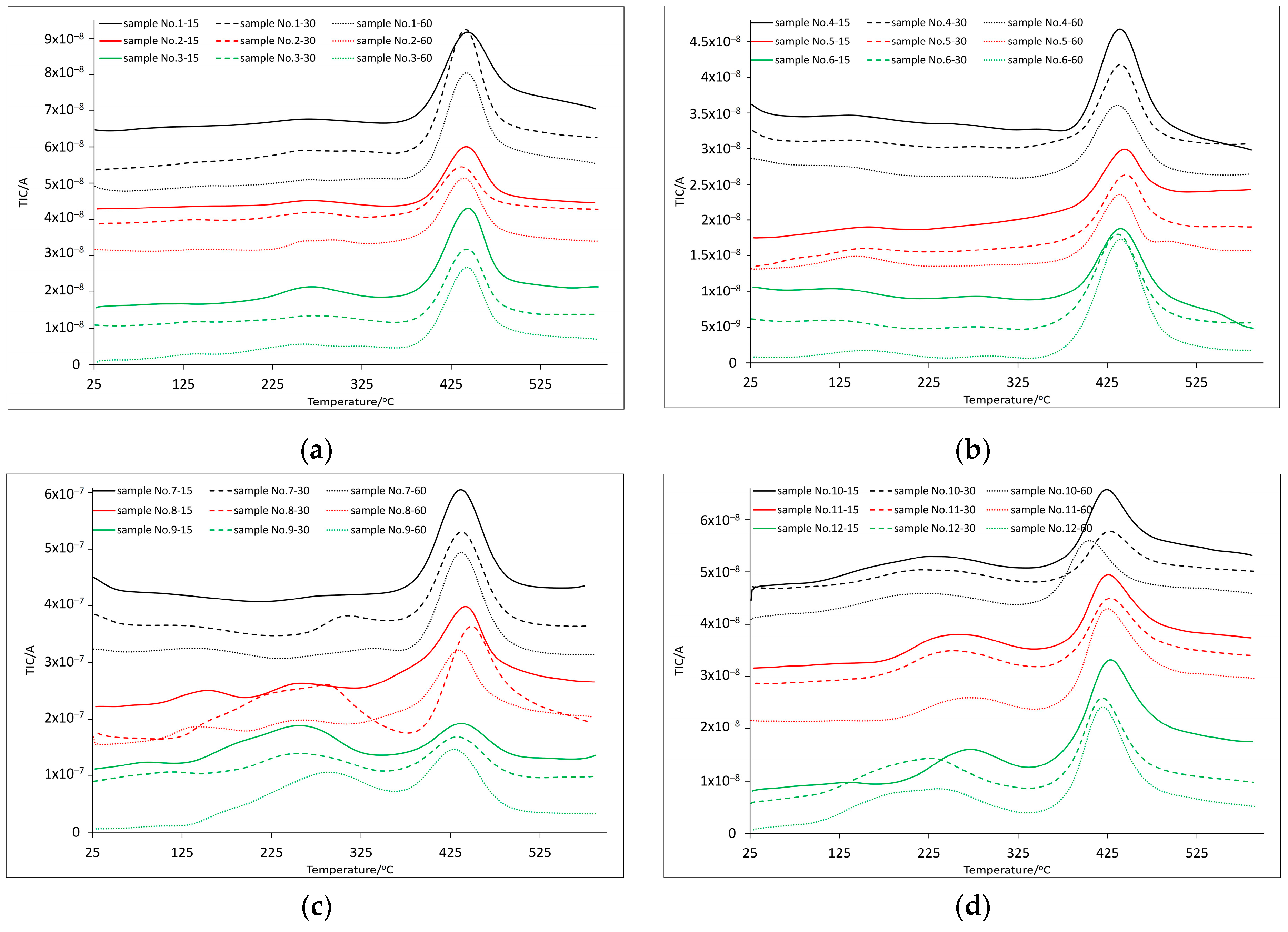
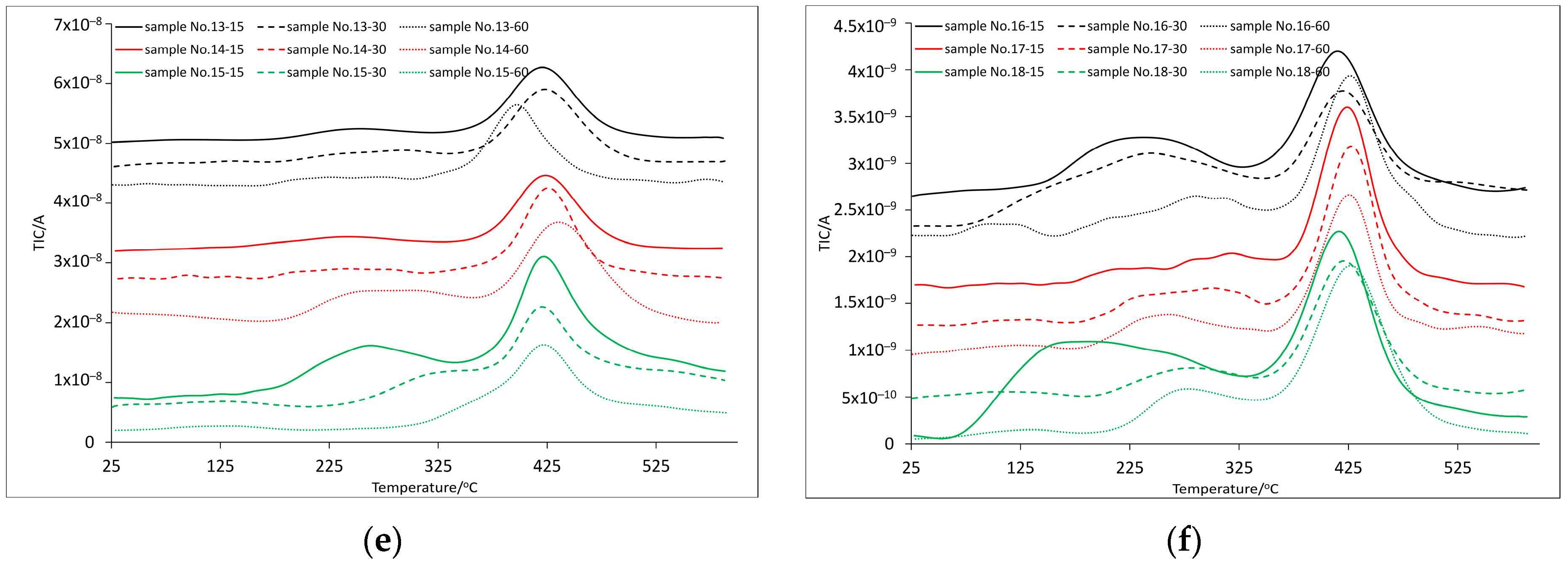
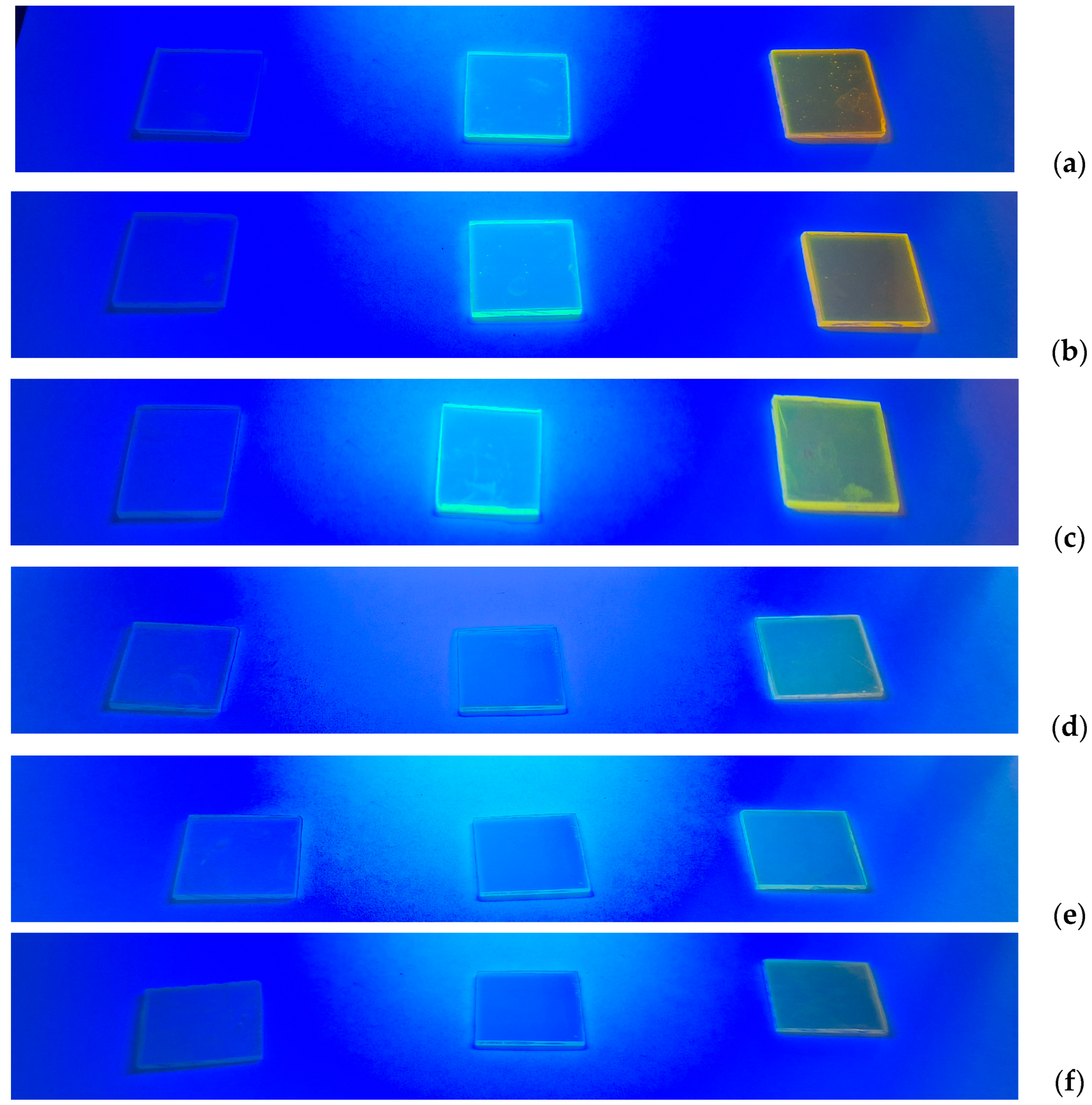
3.2. Thermal and Spectroscopic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Odian, G. Principles of Polymerization, 4th ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- John, Ł.; Ejfler, J. A Brief Review on Selected Applications of Hybrid Materials Based on Functionalized Cage-like Silsesquioxanes. Polymers 2023, 15, 1452. [Google Scholar] [CrossRef] [PubMed]
- Garcia, O.; Garrido, L.; Sastre, R.; Costela, A.; Garcia-Moreno, I. Synthetic Strategies for Hybrid Materials to Improve Properties for Optoelectronic Applications. Adv. Funct. Mater. 2008, 18, 2017–2025. [Google Scholar] [CrossRef]
- Cernadas, T.; Santos, M.; Miquel, S.P.; Correia, I.J.; Alves, P.; Ferreira, P. Photocurable Polymeric Blends for Surgical Application. Materials 2020, 13, 5681. [Google Scholar] [CrossRef] [PubMed]
- Gil-Kowalczyk, M.; Łyszczek, R.; Jusza, A.; Piramidowicz, R. Thermal, Spectroscopy and Luminescent Characterization of Hybrid PMMA/Lanthanide Complex Materials. Materials 2021, 14, 3156. [Google Scholar] [CrossRef] [PubMed]
- Jusza, A.; Lipińska, L.; Baran, M.; Olszyna, A.; Jastrzębska, A.; Gil, M.; Mergo, P.; Piramidowicz, R. Praseodymium doped nanocrystals and nanocomposites for application in white light sources. Opt. Mater. 2019, 95, 109247. [Google Scholar] [CrossRef]
- Piramidowicz, R.; Jusza, A.; Lipińska, L.; Gil, M.; Mergo, P. RE3+:LaALO3 doped luminescent polymer composites. Opt. Mater. 2019, 87, 35–41. [Google Scholar] [CrossRef]
- Łyszczek, R.; Gil, M.; Głuchowska, H.; Podkościelna, B.; Lipke, A.; Mergo, P. Hybrid materials based on PEGDMA matrix and europium(III) carboxylates -thermal and luminescent investigations. Eur. Polym. J. 2018, 106, 318–328. [Google Scholar] [CrossRef]
- Retailleau, M.; Ibrahim, A.; Allonas, X. Dual-cure photochemical/thermal polymerization of acrylates: A photoassisted process at low light intensity. Polym. Chem. 2014, 5, 6503. [Google Scholar] [CrossRef]
- Kapłoń, Ł.; Kochanowski, A.; Molenda, M.; Moskal, P.; Wieczorek, A.; Bednarski, T.; Białas, P.; Czerwiński, E.; Korcyl, G.; Kowal, J.; et al. Plastic scintillators for positron emission tomography obtained by the bulk polymerization method. Bio-Algorithms Med-Syst. 2014, 10, 27–31. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Z.; Hu, P.; Jin, J.; Li, J.; Liu, J.; Lin, X.; Tan, X. Effect of thermal polymerization temperature and time of PQ/PMMA on the holographic data storage. Proc. SPIE 2022, 12231, 1223103. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated Crosslinking polymerization. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Decker, C. Kinetic Analysis and Performance of UV-Curable Coatings. In Radiation Curing. Science and Technology; Pappas, S.P., Ed.; Plenum Press: New York, NY, USA; London, UK, 1992; pp. 135–179. [Google Scholar]
- Jacobine, A.F.; Nakos, S.T. Photopolymerizable Silicone Monomers, Oligomers, and Resins. In Radiation Curing. Science and Technology; Pappas, S.P., Ed.; Plenum Press: New York, NY, USA; London, UK, 1992; pp. 181–240. [Google Scholar]
- Scott, T.F.; Cook, W.D.; Forsythe, J.S. Photo-DSC cure kinetics of vinyl ester resins. I. Influence of temperature. Polymer 2002, 43, 5839–5845. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 606–665. [Google Scholar] [CrossRef]
- Kim, D.S.; Seo, W.H. Ultraviolet-Curing Behavior and Mechanical Properties of a Polyester Acrylate Resin. J. Appl. Polym. Sci. 2004, 92, 3921–3928. [Google Scholar] [CrossRef]
- White, T.J.; Liechty, W.B.; Guymon, C.A. The Influence of N-vinyl Pyrrolidone on Polymerization Kinetics and Thermo-Mechanical Properties of Crosslinked Acrylate Polymers. J. Polym. Sci. A Polym. Chem. 2007, 45, 4062–4073. [Google Scholar] [CrossRef]
- Sysova, O.; Durin, P.; Gablin, C.; Leonard, D.; Teolis, A.; Trombotto, S.; Delair, T.; Berling, D.; Servin, I.; Tiron, R.; et al. Green deep-UV photoresist based on chitosan for microelectronics. J. Appl. Polym. Sci. 2023, 140, e54244. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Rodrigues-Marinho, T.; Vilas, J.L.; Lanceros-Mendez, S. UV curable nanocomposites with tailored dielectric response. Polymer 2020, 196, 122498. [Google Scholar] [CrossRef]
- Lucchetti, L.; Gobbi, L.; Simoni, F. Analysis of the Phase Separation Process in UV Cured Polymer Dispersed Liquid Crystals for Optical Applications. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Crys. Liq. Cryst. 2001, 359, 89–96. [Google Scholar] [CrossRef]
- Carrillo-Betancourt, R.; Lopez-Camero, A.D.; Hernandez-Cordero, J. Luminescent Polymer Composites for Optical Fiber Sensors. Polymers 2023, 15, 505. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, K.; Huang, C.S.; Boesel, L.F.; Hufenus, R.; Heuberger, M. Recent advances in photoluminescent polymer optical fibers. Curr Opin. Solid State Mater. Sci. 2021, 25, 100912. [Google Scholar] [CrossRef]
- Arrospide, E.; IIlarramendi, M.A.; Ayesta, I.; Guarrotxena, N.; Garcia, O.; Zubia, J.; Durana, G. Effects of Fabrication Methods on the Performance of Luminescent Solar Concentrators Based on Doped Polymer Optical Fibers. Polymers 2021, 13, 424. [Google Scholar] [CrossRef]
- Jakubowski, K.; Kerkemeyer, W.; Perret, E.; Heuberger, M.; Hufenus, R. Liquid-core polymer optical fibers for luminescent waveguide applications. Mater. Des. 2020, 196, 109131. [Google Scholar] [CrossRef]
- Xu, S.; Xiang, H.; Wang, Z.; Tang, X.; Zhang, Y.; Zhan, X.; Chen, J. Conjugation of a phenanthrene-imidazole fluorophore with the chondroitin sulfate generated from Escherichia coli K4 polysaccharide. J. Appl. Polym. Sci. 2021, 138, 51538. [Google Scholar] [CrossRef]
- Jankowska, K.; Zdarta, J.; Grzywaczyk, A.; Kijeńska-Gawrońska, E.; Biadosz, A.; Jesionowski, T. Electrospun poly(methyl methacrylate)/polyaniline fibres as a support for laccase immobilisation and use in dye decolourisation. Environ. Res. 2020, 184, 109332. [Google Scholar] [CrossRef] [PubMed]
- Basabe-Desmonts, L.; Reinhoudt, D.N.; Crego-Calama, M. Design of fluorescent materials for chemical sensing. Chem. Soc. Rev. 2007, 36, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Ahumada, G.; Borkowska, M. Fluorescent Polymers Conspectus. Polymers 2022, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the Use of Polymer-Based Composites for the Creation of Optical Sensors: A Review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.C.; Pound-Lana, G.; Oliveira, M.A.; Lanna, E.G.; Fialho, M.C.P.; Brito, A.C.F.; Barboza, A.P.M.; Aguiar-Soares, R.D.O.; Mosqueira, V.C.F. Labeling PLA-PEG nanocarriers with IR780: Physical entrapment versus covalent attachment to polylactide. Drug Deliv. Transl. Res. 2020, 10, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Uttamlal, M.; Sloan, W.D.; Millar, D. Covalent immobilization of fluorescent indicators in photo- and electropolymers for the preparation of fibreoptic chemical sensors. Polym. Int. 2002, 51, 1198–1206. [Google Scholar] [CrossRef]
- Sloan, W.D.; Uttamlal, M. A fibre-optic calcium ion sensor using a calcein derivative. Luminescence 2001, 16, 179–186. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2008−2012). Anal. Chem. 2013, 85, 487–508. [Google Scholar] [CrossRef] [PubMed]
- Isaad, J.; El Achari, A. Azathia crown ether possessing a dansyl fluorophore moiety functionalized silica nanoparticles as hybrid material for mercury detection in aqueous medium. Tetrahedron 2013, 69, 4866–4874. [Google Scholar] [CrossRef]
- Dai, H.J.; Liu, F.; Gao, Q.Q. A highly selective fluorescent sensor for mercury ion (II) based on azathia-crown ether possessing a dansyl moiety. Luminescence 2011, 26, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kaewtong, C.; Niamsa, N.; Wanno, B.; Morakot, N.; Pulpoka, B.; Tuntulani, T. Optical chemosensors for Hg2+ from terthiophene appended rhodamine derivatives: FRET based molecular and in situ hybrid gold nanoparticle sensors. New J. Chem. 2014, 38, 3831–3839. [Google Scholar] [CrossRef]
- Mergo, P.; Martynkien, T.; Urbańczyk, W. Polymer optical microstructured fiber with birefringence induced by stress-applying elements. Opt. Lett. 2014, 39, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Podkościelna, B.; Gawdzik, B. Influence of diluent compositions on the porous structure of methacrylate derivatives of aromatic diols and divinylbenzene. Appl. Surf. Sci. 2010, 256, 2462–2467. [Google Scholar] [CrossRef]
- Fila, K.; Gargol, M.; Goliszek, M.; Podkościelna, B. Synthesis of epoxy resins derivatives of naphthalene-2.7-diol and theircross-linked products. J. Therm. Anal. Calorim. 2019, 138, 4349–4358. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identyfication of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hoyle, C.E. Calorimetric Analysis of Photopolymerization. In Radiation Curing. Science and Technology; Pappas, S.P., Ed.; Plenum Press: New York, NY, USA; London, UK, 1992; pp. 57–133. [Google Scholar]
- Wunderlich, B. Thermal Analysis of Polymeric Materials; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005. [Google Scholar]
- Koga, N. Thermoanalytical Methods: Fundamental Principles and Features. In Thermal Analysis of Polymeric Materials: Methods and Developments, 1st ed.; Kindle, Edition; Pielichowski, K., Pielichowska, K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 1–39. [Google Scholar]
- Toda, A. Modulated Temperature Differential Scanning Calorimetry. In Thermal Analysis of Polymeric Materials: Methods and Developments, 1st ed.; Kindle, Edition; Pielichowski, K., Pielichowska, K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 41–73. [Google Scholar]
- Lin, S.Y.; Chen, L.C.; Cheng, W.T. Use of Different Temperature Control Techniques Coupled with FTIR Spectroscopy to Simultaneously Induce and Identify the Physical Properties, Chemical Reactions, and Thermal Degradation of Polymers. In Thermal Analysis of Polymeric Materials: Methods and Developments, 1st ed.; Kindle, Edition; Pielichowski, K., Pielichowska, K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 169–226. [Google Scholar]
- Johnstone, R.A. Mass Spectrometry for Organic Chemists (Cambridge Texts in Chemistry and Biochemistry); Cambridge University Press: London, UK; New York, NY, USA, 1972. [Google Scholar]
- Danikiewicz, W. Spektrometria Mas, 1st ed.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020. [Google Scholar]
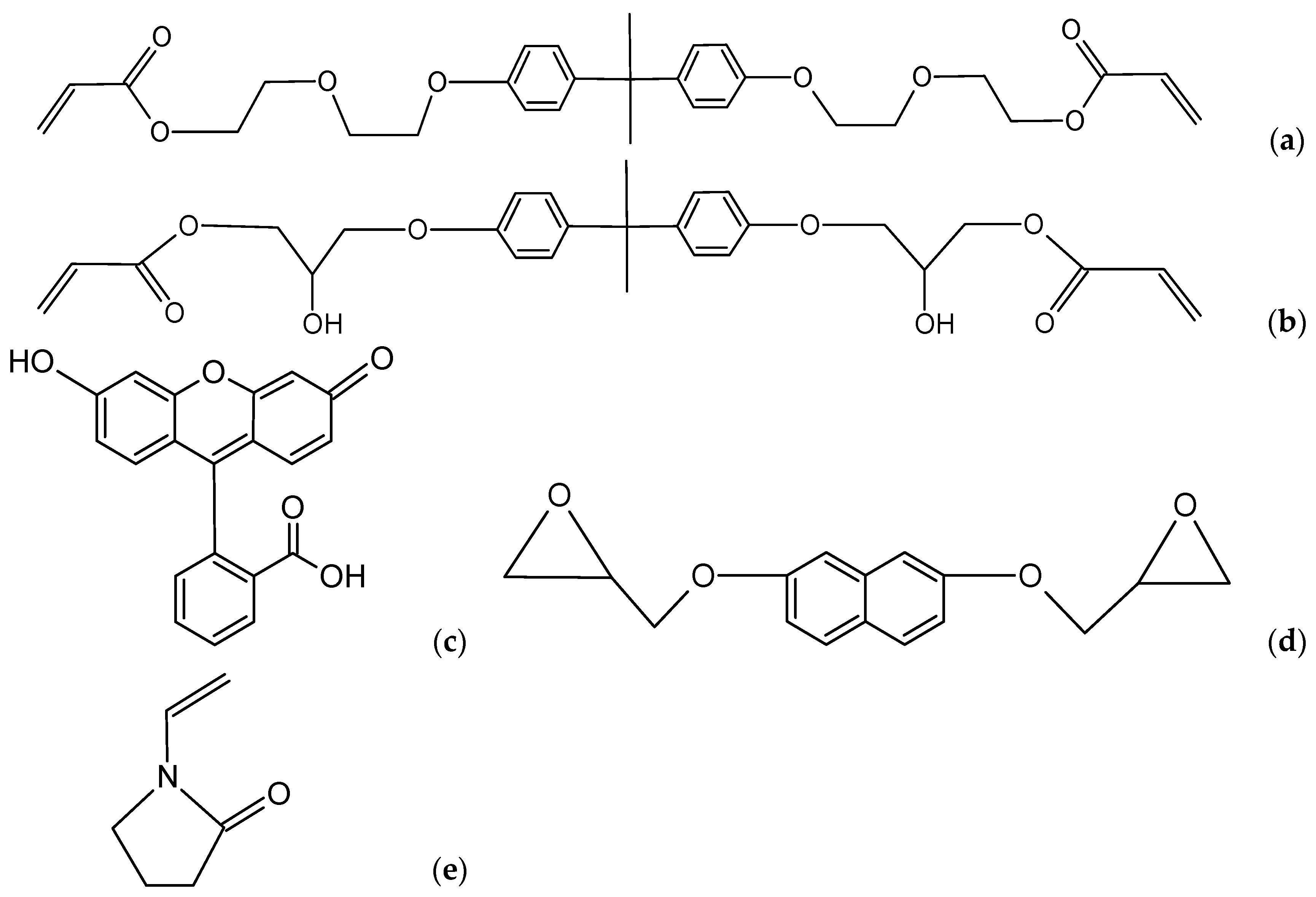
| Sample | EB150/wt.% | NVP/wt.% | Dopant/wt.% | Polymerization Time/s | |
|---|---|---|---|---|---|
| 2.7-NAF.EP | Fluorescein | ||||
| Sample No.1-15 | 75 | 25 | - | - | 15 |
| Sample No.1-30 | 75 | 25 | - | - | 30 |
| Sample No.1-60 | 75 | 25 | - | - | 60 |
| Sample No.2-15 | 75 | 25 | 1 | - | 15 |
| Sample No.2-30 | 75 | 25 | 1 | - | 30 |
| Sample No.2-60 | 75 | 25 | 1 | - | 60 |
| Sample No.3-15 | 75 | 25 | - | 1 | 15 |
| Sample No.3-30 | 75 | 25 | - | 1 | 30 |
| Sample No.3-60 | 75 | 25 | - | 1 | 60 |
| Sample No.4-15 | 67 | 33 | - | - | 15 |
| Sample No.4-30 | 67 | 33 | - | - | 30 |
| Sample No.4-60 | 67 | 33 | - | - | 60 |
| Sample No.5-15 | 67 | 33 | 1 | - | 15 |
| Sample No.5-30 | 67 | 33 | 1 | - | 30 |
| Sample No.5-60 | 67 | 33 | 1 | - | 60 |
| Sample No.6-15 | 67 | 33 | - | 1 | 15 |
| Sample No.6-30 | 67 | 33 | - | 1 | 30 |
| Sample No.6-60 | 67 | 33 | - | 1 | 60 |
| Sample No.7-15 | 50 | 50 | - | - | 15 |
| Sample No.7-30 | 50 | 50 | - | - | 30 |
| Sample No.7-60 | 50 | 50 | - | - | 60 |
| Sample No.8-15 | 50 | 50 | 1 | - | 15 |
| Sample No.8-30 | 50 | 50 | 1 | - | 30 |
| Sample No.8-60 | 50 | 50 | 1 | - | 60 |
| Sample No.9-15 | 50 | 50 | - | 1 | 15 |
| Sample No.9-30 | 50 | 50 | - | 1 | 30 |
| Sample No.9-60 | 50 | 50 | - | 1 | 60 |
| Sample | EB150/wt.% | NVP/wt.% | Dopant/wt.% | Polymerization Time/s | |
|---|---|---|---|---|---|
| 2.7-NAF.EP | Fluorescein | ||||
| Sample No.10-15 | 75 | 25 | - | - | 15 |
| Sample No.10-30 | 75 | 25 | - | - | 30 |
| Sample No.10-60 | 75 | 25 | - | - | 60 |
| Sample No.11-15 | 75 | 25 | 1 | - | 15 |
| Sample No.11-30 | 75 | 25 | 1 | - | 30 |
| Sample No.11-60 | 75 | 25 | 1 | - | 60 |
| Sample No.12-15 | 75 | 25 | - | 1 | 15 |
| Sample No.12-30 | 75 | 25 | - | 1 | 30 |
| Sample No.12-60 | 75 | 25 | - | 1 | 60 |
| Sample No.13-15 | 67 | 33 | - | - | 15 |
| Sample No.13-30 | 67 | 33 | - | - | 30 |
| Sample No.13-60 | 67 | 33 | - | - | 60 |
| Sample No.14-15 | 67 | 33 | 1 | - | 15 |
| Sample No.14-30 | 67 | 33 | 1 | - | 30 |
| Sample No.14-60 | 67 | 33 | 1 | - | 60 |
| Sample No.15-15 | 67 | 33 | - | 1 | 15 |
| Sample No.15-30 | 67 | 33 | - | 1 | 30 |
| Sample No.15-60 | 67 | 33 | - | 1 | 60 |
| Sample No.16-15 | 50 | 50 | - | - | 15 |
| Sample No.16-30 | 50 | 50 | - | - | 30 |
| Sample No.16-60 | 50 | 50 | - | - | 60 |
| Sample No.17-15 | 50 | 50 | 1 | - | 15 |
| Sample No.17-30 | 50 | 50 | 1 | - | 30 |
| Sample No.17-60 | 50 | 50 | 1 | - | 60 |
| Sample No.18-15 | 50 | 50 | - | 1 | 15 |
| Sample No.18-30 | 50 | 50 | - | 1 | 30 |
| Sample No.18-60 | 50 | 50 | - | 1 | 60 |
| Sample | Conversion/% | Sample | Conversion/% |
|---|---|---|---|
| Sample No.1-15 | 64.1 | Sample No.10-15 | 73.9 |
| Sample No.1-30 | 86.5 | Sample No.10-30 | 82.7 |
| Sample No.1-60 | 86.2 | Sample No.10-60 | 92.1 |
| Sample No.2-15 | 76.1 | Sample No.11-15 | 75.4 |
| Sample No.2-30 | 85.9 | Sample No.11-30 | 80.4 |
| Sample No.2-60 | 88.5 | Sample No.11-60 | 84.0 |
| Sample No.3-15 | 38.6 | Sample No.12-15 | 46.9 |
| Sample No.3-30 | 75.2 | Sample No.12-30 | 66.3 |
| Sample No.3-60 | 75.9 | Sample No.12-60 | 70.3 |
| Sample No.4-15 | 75.4 | Sample No.13-15 | 90.6 |
| Sample No.4-30 | 98.4 | Sample No.13-30 | 92.4 |
| Sample No.4-60 | 96.9 | Sample No.13-60 | 95.0 |
| Sample No.5-15 | 54.2 | Sample No.14-15 | 91.7 |
| Sample No.5-30 | 72.4 | Sample No.14-30 | 94.2 |
| Sample No.5-60 | 86.4 | Sample No.14-60 | 90.4 |
| Sample No.6-15 | 79.4 | Sample No.15-15 | 92.6 |
| Sample No.6-30 | 93.5 | Sample No.15-30 | 95.3 |
| Sample No.6-60 | 95.5 | Sample No.15-60 | 93.5 |
| Sample No.7-15 | 31.6 | Sample No.16-15 | 81.5 |
| Sample No.7-30 | 84.3 | Sample No.16-30 | 94.3 |
| Sample No.7-60 | 94.1 | Sample No.16-60 | 96.2 |
| Sample No.8-15 | 23.7 | Sample No.17-15 | 97.5 |
| Sample No.8-30 | 92.9 | Sample No.17-30 | 100.0 |
| Sample No.8-60 | 100.0 | Sample No.17-60 | 100.0 |
| Sample No.9-15 | 23.3 | Sample No.18-15 | 83.3 |
| Sample No.9-30 | 60.7 | Sample No.18-30 | 100.0 |
| Sample No.9-60 | 96.5 | Sample No.18-60 | 100.0 |
| Sample | Temperature at Mass Loss/°C | DTGmax/°C | DSCmax/°C | Residual Mass/wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1/wt.% | 3/wt.% | 5/wt.% | I | II | III | IV | I | II | III | IV | ||
| Sample No.1-15 | 125.5 | 188.0 | 230.0 | 166.0 | 230.8 | 349.0 | 438.5 | 113.4 | 231.4 | - * | 400.9/435.3/ 446.5 | 13.2 |
| Sample No.1-30 | 120.6 | 164.3 | 199.7 | 147.7 | 235.4 | - * | 439.6 | 123.6 | 234.2 | - * | 400.6/434.0/ 442.8 | 10.7 |
| Sample No.1-60 | 112.2 | 156.7 | 198.4 | 148.7 | 237.1 | - * | 434.3 | 124.1 | 232.5 | - * | 406.9/431.6/ 447.2 | 12.3 |
| Sample No.2-15 | 111.6 | 177.6 | 221.8 | 137.6 | 242.3 | - * | 437.4 | 127.0 | 240.0 | - * | 399.3/437.9/ 448.0 | 9.6 |
| Sample No.2-30 | 109.2 | 150.6 | 180.9 | 133.2 | 236.6 | - * | 437.4 | 121.2 | 239.5 | 334.1 | 400.7/432.1/ 441.2 | 9.7 |
| Sample No.2-60 | 127.1 | 172.2 | 207.3 | 138.9 | 238.6 | - * | 438 | 124.8 | 241.0 | 343.1 | 396.9/429.1/ 436.5/444.8 | 10.1 |
| Sample No.3-15 | 147.3 | 192.9 | 222.2 | - * | 233.6 | - * | 439.1 | - * | 232.1 | - * | 394.4/434.2/ 445.4 | 12.1 |
| Sample No.3-30 | 105.0 | 146.6 | 178.9 | 132.5 | 235.5 | - * | 437.6 | 118.9 | 234.0 | - * | 393.9/431.7/ 443.4 | 10.9 |
| Sample No.3-60 | 116.9 | 155.9 | 187.5 | 136.8 | 229.9 | - * | 436.9 | 120.3 | 233.3 | - * | 431.1/441.7 | 13.0 |
| Sample No.4-15 | 102.3 | 139.6 | 168.7 | 134.3 | 188.4 | 242.6 | 431.5 | 129.3/ 166.7 | 240.6 | 322.6/ 351.1 | 429.3 | 8.8 |
| Sample No.4-30 | 96.5 | 122.5 | 170.9 | 143.6 | 187.6 | 252.4 | 432.4 | 131.2/ 170.9 | 250.1 | 325.1/ 352.5 | 428.3 | 10.2 |
| Sample No.4-60 | 92.8 | 117.1 | 170.8 | 137.5 | - | 237.3 | 431.5 | 127.1 | 255.2 | 322.1/ 352.8 | 428.4 | 10.2 |
| Sample No.5-15 | 92.6 | 134.2 | 156.4 | - * | 185.4 | - * | 436.7 | 132.7 | 273.6 | 333.5 | 431.7/444.5 | 9.7 |
| Sample No.5-30 | 98.7 | 137.5 | 159.6 | - * | 172.0 | - * | 436.1 | 130.1 | 279.0 | 345.8 | 414.7/429.7/ 434.8/444.6 | 10.0 |
| Sample No.5-60 | 101.2 | 140.0 | 164.1 | - * | 193.3 | - * | 436.4 | 127.9 | 278.5 | - * | 427.9/436.4 | 8.2 |
| Sample No.6-15 | 95.7 | 134.2 | 166.3 | 140.2 | - * | 235.8 | 432.5 | 130.3/ 160.7 | 243.2 | 323.0/ 352.5 | 430.2 | 9.9 |
| Sample No.6-30 | 85.4 | 135.6 | 163.0 | 138.1 | - * | 230.0 | 432.8 | 122.0/ 174.2 | 239.2 | 322.2/ 350.8 | 430.2 | 10.0 |
| Sample No.6-60 | 100.4 | 145.8 | 175.9 | 141.8 | - * | 231.2 | 433.2 | 128.9/ 162.3 | 235.3 | 321.5/ 351.0 | 431.0 | 9.1 |
| Sample No.7-15 | 80.9 | 130.2 | 160.8 | 162.7 | - * | 276.7 | 433.3 | 131.0/ 163.2 | - * | 284.6/ 320.3/ 350.6 | 431.1 | 10.2 |
| Sample No.7-30 | 80.2 | 130.1 | 160.4 | 165.4 | - * | 277.0 | 432.7 | 131.2/ 155.5 | - * | 282.0/ 323.9/ 352.5 | 428.5 | 10.5 |
| Sample No.7-60 | 82.2 | 129.0 | 159.0 | 153.9 | 192.5 | 239.4 | 433.0 | 147.0 | - * | 325.3/ 353.6 | 427.3 | 11.5 |
| Sample No.8-15 | 89.7 | 118.1 | 133.1 | - * | 199.8 | - * | 435.5 | 129.0 | 199.3 | 332.8 | 429.2/440.3 | 10.2 |
| Sample No.8-30 | 96.1 | 128.2 | 143.6 | - * | 190.2 | - * | 434.4 | 132.2/ 167.8 | 193.4 | 287.6 | 428.6/437.6 | 8.8 |
| Sample No.8-60 | 98.9 | 130.7. | 149.2 | - * | 186.6 | - * | 434.5. | 128.9 | 173.0/ 187.7 | 169.1 | 430.9/439.2 | 12.3 |
| Sample No.9-15 | 85.9 | 118.7 | 137.1 | 157.1 | - * | 222.2 | 434.0 | - * | 159.3/ 223.9 | - * | 430.6/473.4 | 7.3 |
| Sample No.9-30 | 90.2 | 131.8 | 152.5 | 147.1 | - * | 235.9 | 435.2 | - * | 143.2/232.0 | - * | 434.6 | 7.5 |
| Sample No.9-60 | 101.3 | 136.7 | 158.7 | 175.3 | - * | 239.6 | 431.8 | 127.8 | 168.8/235.7 | - * | 429.5/436.8 | 8.8 |
| Sample No.10-15 | 103.0 | 137.2 | 162.8 | 171.8 | - * | 227.2 | 422.8 | 127.4 | 225.3 | 296.0/ 381.0 | 407.0/427.1 | 14.7 |
| Sample No.10-30 | 92.7 | 136.1 | 166.3 | 183.9 | - * | 231.0 | 423.2 | 110.4 | 225.3 | 381.0 | 421.8/428.9 | 16.9 |
| Sample No.10-60 | 113.8 | 152.7 | 180.5 | 190.0 | - * | 230.7 | 421.8 | 129.8 | 224.5 | 298.0/ 377.5 | 404.6/425.7/ 432.3 | 16.1 |
| Sample No.11-15 | 112.7 | 152.1 | 180.0 | 189.1 | - * | - * | 421.8 | 130.2 | 222.4 | 308.3/ 377.8 | 399.9/407.2/ 423.9 | 13.3 |
| Sample No.11-30 | 109.3 | 147.0 | 174.6 | 173.4 | - * | - * | 422.0 | 129.2 | 224.2 | 323.4/ 379.3 | 402.5/417.9/ 427.2 | 9.6 |
| Sample No.11-60 | 102.9 | 149.1 | 183.6 | 188.5 | - * | 236.0 | 420.9 | 128.2 | 223.9 | 374.9 | 397.2/422.3/ 430.6 | 11.5 |
| Sample No.12-15 | 106.0 | 156.5 | 186.4 | 183.0 | - * | 223.5 | 421.3 | 119.3/ 158.3 | 220.7 | 369.5 | 404.5/429.3 | 15.4 |
| Sample No.12-30 | 106.3 | 145.5 | 171.0 | 174.4 | - * | 223.9 | 421.4 | 121.7 | 218.3 | 370.1 | 401.5/415.9/ 428.4 | 15.3 |
| Sample No.12-60 | 112.9 | 146.9 | 173.2 | 162.9 | - * | 231.0 | 421.5 | 125.5 | 222.6 | 372.0 | 402.0/418.7/ 426.5 | 14.8 |
| Sample No.13-15 | 109.8 | 152.4 | 182.4 | 189.7 | - * | 245.7 | 420.3 | 148.1 | 197.9/ 225.7 | 379.8 | 424.4 | 13.0 |
| Sample No.13-30 | 100.9 | 149.4 | 181.4 | 194.2 | - * | 241.6 | 419.7 | 125.7 | 195.3/ 223.8 | 366.6 | 418.6/426.0 | 9.8 |
| Sample No.13-60 | 102.0 | 148.4 | 179.6 | 191.4 | - * | 238.2 | 420.6 | 126.1 | 195.3/ 226.5 | 279.8 | 379.8/402.6/ 417.2/423.5 | 13.9 |
| Sample No.14-15 | 104.8 | 142.0 | 168.1 | - * | 206.8 | 260.6 | 421.8 | - * | 209.6/ 247.1/ 280.4 | 372.6 | 396.4/425.6 | 14.6 |
| Sample No.14-30 | 112.4 | 158.6 | 190.7 | - * | 202.2 | 238.3 | 419.8 | - * | 208.2/ 233.3/ 266.3 | 378.2 | 410.7/421.8 | 13.5 |
| Sample No.14-60 | 99.7 | 141.6 | 170.2 | - * | 200.1 | - * | 421.1 | 123.4 | 208.8/ 246.7/ 278.3 | 381.5 | 406.5/418.6/ 425.7 | 13.4 |
| Sample No.15-15 | 92.7 | 133.4 | 159.1 | 183.7 | - * | 227.3 | 421.9 | 125.3 | 182.7/ 224.7 | 373.1 | 398.5/426.8 | 11.4 |
| Sample No.15-30 | 109.1 | 149.4 | 179.1 | 196.2 | - * | 239.8 | 420.6 | 129.1 | 192.3/223.8 | 375.5 | 395.5/415.1/ 424.3 | 11.8 |
| Sample No.15-60 | 102.1 | 142.3 | 171.9 | 194.4 | - * | 234.7 | 421.7 | 129.6 | 187.9/ 224.2 | 376.6 | 409.7/424.0 | 16.5 |
| Sample No.16-15 | 108.9 | 145.7 | 172.2 | 171.7 | - * | - * | 421.8 | 189.5 | 222.5/ 278.7 | 368.4 | 425.1 | 15.0 |
| Sample No.16-30 | 91.9 | 132.4 | 160.7 | - * | - * | 230.8 | 421.5 | 122.8 | 193.3/ 229.5 | - * | 425.5 | 10.2 |
| Sample No.16-60 | 92.4 | 137.3 | 171.3 | 122.7 | 195.4 | 246.3 | 422.6 | 192.8 | 226.5/ 243.6 | - * | 422.6/432.4 | 12.2 |
| Sample No.17-15 | 94.0 | 135.1 | 160.9 | - * | - * | 200.5 | 419.9 | - * | 206.9 | 379.3 | 409.3/424.5 | 13.6 |
| Sample No.17-30 | 106.8 | 145.9 | 173.1 | 166.2 | - * | 207.5 | 420.8 | 210.8 | - * | 377.4 | 400.9/422.4 | 13.0 |
| Sample No.17-60 | 90.9 | 138.7 | 172.9 | 109.7 | - * | 216.3 | 421.7 | 200.3/ 211.3 | 265.3 | - * | 406.7/421.8/ 435.3 | 12.6 |
| Sample No.18-15 | 92.9 | 129.9 | 155.2 | 188.6 | - * | 235.2 | 421.1 | 125.3 | 188.7/ 226.2 | - * | 420.7/427.4 | 12.2 |
| Sample No.18-30 | 114.9 | 159.4 | 189.8 | 130.3 | - * | 238.0 | 420.6 | 125.3 | 193.7/ 227.1 | - * | 413.8/423.2 | 13.2 |
| Sample No.18-60 | 98.9 | 146.0 | 180.2 | 148.6 | - * | 237.3 | 421.7 | 125.3 | 191.7/234.1 | - * | 407.0/422.2 | 13.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Kowalczyk, M.; Mergo, P. Doped Epoxy Resins as an Alternative to Luminescent Optical Sensors. Appl. Sci. 2024, 14, 6170. https://doi.org/10.3390/app14146170
Gil-Kowalczyk M, Mergo P. Doped Epoxy Resins as an Alternative to Luminescent Optical Sensors. Applied Sciences. 2024; 14(14):6170. https://doi.org/10.3390/app14146170
Chicago/Turabian StyleGil-Kowalczyk, Małgorzata, and Paweł Mergo. 2024. "Doped Epoxy Resins as an Alternative to Luminescent Optical Sensors" Applied Sciences 14, no. 14: 6170. https://doi.org/10.3390/app14146170
APA StyleGil-Kowalczyk, M., & Mergo, P. (2024). Doped Epoxy Resins as an Alternative to Luminescent Optical Sensors. Applied Sciences, 14(14), 6170. https://doi.org/10.3390/app14146170







