IR and Raman Dual Modality Markers Differentiate among Three bis-Phenols: BPA, BPS, and BPF
Abstract
1. Introduction
- Identify spectral wavenumbers in which IR and Raman signals for the same vibrational mode are most different in normalized relative intensity.
- Use these dual-modality data as a marker to determine the most sensitive signal ratio which is specific to BPA, BPS, and BPF.
- Assign these wavenumbers to vibrational modes characteristic of individual compounds.
2. Materials and Methods
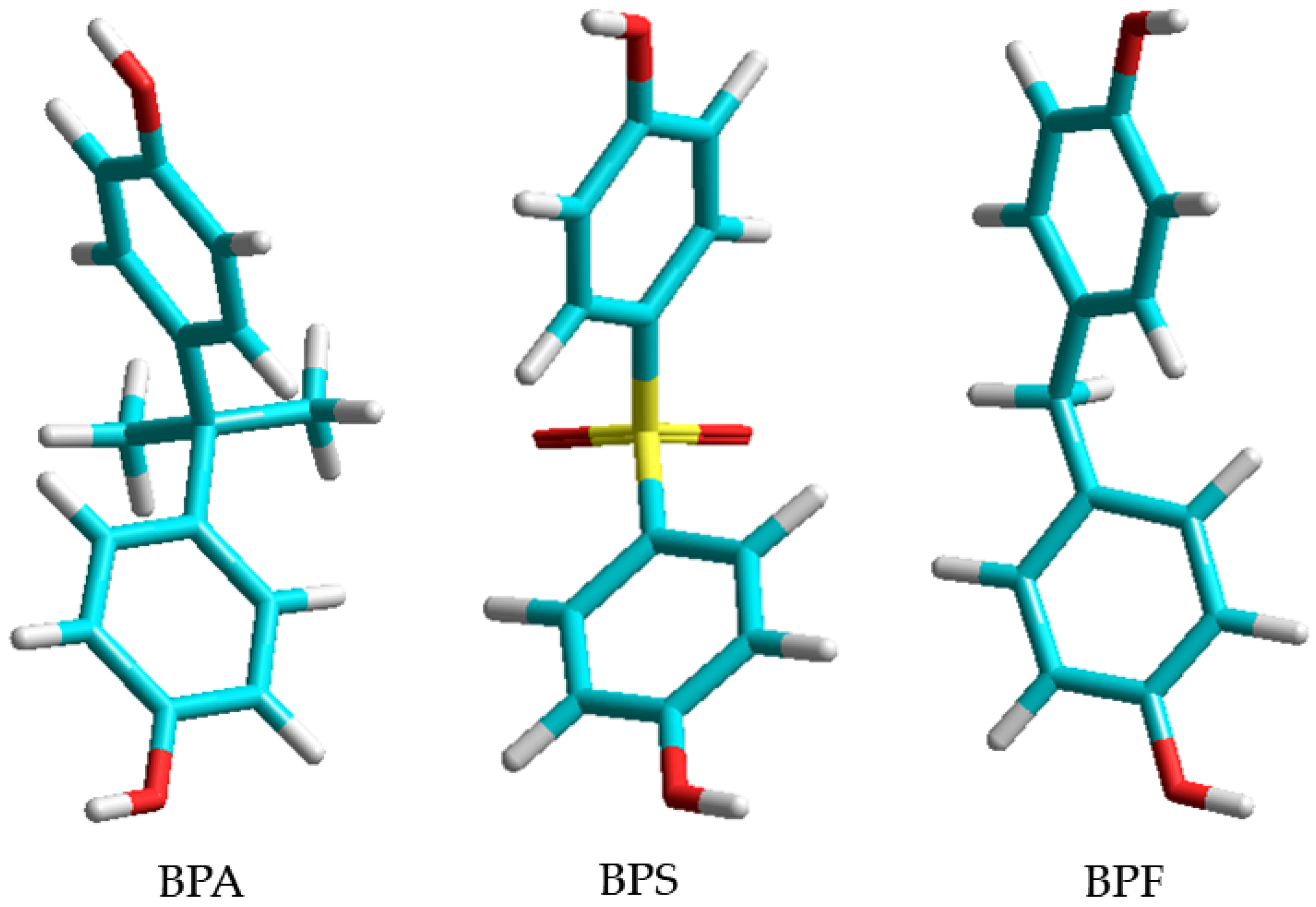
2.1. Plasticizers
2.2. FT-IR Spectroscopy and Spectral Measurement
2.3. Raman Spectroscopy and Spectral Measurement
2.4. Dual-Modality IR and Raman Data Analysis
3. Results
3.1. IR and Raman Band Assignment
3.2. IR and Raman Dual Modality
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, X.L.; Corriveau, J. Migration of bisphenol A from polycarbonate baby and water bottles into water under severe conditions. J. Agric. Food Chem. 2008, 56, 6378–6381. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Carlson, E.M.; Chua, J.P.; Belcher, S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008, 176, 149–156. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Morrissey, R.E.; George, J.D.; Price, C.J.; Tyl, R.W.; Marr, M.C.; Kimmel, C.A. The developmental toxicity of bisphenol A in rats and mice. Fundam. Appl. Toxicol. 1987, 8, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures: I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Hafezi, S.A.; Abdel-Rahman, W.M. The Endocrine Disruptor Bisphenol A (BPA) Exerts a Wide Range of Effects in Carcinogenesis and Response to Therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef]
- Roschi, E.; Gellini, C.; Ricci, M.; Sanchez-Cortes, S.; Focardi, C.; Neri, B.; Otero, J.; López-Tocón, I.; Smulevich, G.; Becucci, M. Surface-enhanced Raman spectroscopy for bisphenols detection: Toward a better understanding of the analyte–nanosystem interactions. Nanomaterials 2021, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, Q.; Zeng, X.; Liu, Q.; Hou, X.; Tian, Y.; Wu, L. Sensitive detection of bisphenol A by coupling solid phase microextraction based on monolayer graphene-coated Ag nanoparticles on Si fibers to surface enhanced Raman spectroscopy. Talanta 2018, 187, 13–18. [Google Scholar] [CrossRef]
- Lin, P.Y.; Hsieh, C.W.; Hsieh, S. Rapid and sensitive SERS detection of bisphenol A using self-assembled graphitic substrates. Sci. Rep. 2017, 7, 16698. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Schmidt, W.F.; Qin, J.; Kim, M.; Chan, D. Evaluation of turmeric powder adulterated with metanil yellow using FT-Raman and FT-IR spectroscopy. Foods 2016, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, S.; Schmidt, W.F.; Kim, M.; Tang, X.; Peng, Y.; Chao, K. Detection of additives and chemical contaminants in turmeric powder using FT-IR spectroscopy. Foods 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Schmutzler, M.; Beganovic, A.; Böhler, G.; Huck, C.W. Methods for detection of pork adulteration in veal product based on FT-NIR spectroscopy for laboratory, industrial and on-site analysis. Food Control 2015, 57, 258–267. [Google Scholar] [CrossRef]
- Almeida, M.R.; Oliveira, K.D.S.; Stephani, R.; de Oliveira, L.F.C. Fourier-transform Raman analysis of milk powder: A potential method for rapid quality screening. J. Raman Spectrosc. 2011, 42, 1548–1552. [Google Scholar] [CrossRef]
- Guelpa, A.; Marini, F.; du Plessis, A.; Slabbert, R.; Manley, M. Verification of authenticity and fraud detection in South African honey using NIR spectroscopy. Food Control 2017, 73, 1388–1396. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y. Application of Fourier transform infrared (FT-IR) spectroscopy combined with chemometrics for authentication of cod-liver oil. Vib. Spectrosc. 2011, 55, 141–145. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y. Application of Fourier Transform Infrared Spectroscopy for Authentication of Functional Food Oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Grace, L.I.; Cohen, R.; Dunn, T.M.; Lubman, D.M.; de Vries, M.S. The R2PI Spectroscopy of Tyrosine: A Vibronic Analysis. J. Mol. Spectrosc. 2002, 215, 204–219. [Google Scholar] [CrossRef]
- Ullah, R.; Wang, X. Raman spectroscopy of Bisphenol ‘S’ and its analogy with Bisphenol ‘A’ uncovered with a dimensionality reduction technique. J. Mol. Struct. 2019, 1175, 927–934. [Google Scholar] [CrossRef]
- Buzgar, N.; Buzatu, A.; Sanislav, I.V. The Raman study on certain sulfates. Ann. Stiint. Univ. 2009, 55, 5–23. [Google Scholar]
- Hernandez, B.; Pflueger, F.; Adenier, A.; Kurglik, S.G.; Ghomi, M. Vibrational analysis of amino acids and short peptides in hydrated media. VIII. Amino acids with aromatic side chains: L-phenylalanine, L-tyrosine and L-tryptphan. J. Phys. Chem. 2010, 114, 15319–15330. [Google Scholar] [CrossRef] [PubMed]




| Wavenumber (cm−1) | IR Assignments | Raman Assignments |
|---|---|---|
| 561 | (C-C-C ip bend) | |
| 636 | C-C-C-OH twist | |
| 728 | C-C-C ip bend | |
| 758 | C-C-C-O-H wag | |
| 824 | C-C-C trigonal bend | |
| 826 | C-C-C trigonal bend | |
| 1013 | C-H oop bend | |
| 1083 | C-H twist | |
| 1106 | CH3 scissoring asym | |
| 1173 | CH2 twisting | |
| 1177 | CH2 twisting | |
| 1216 | CH2 twist + OH bend | |
| 1229 | C-H ip bend | |
| 1250 | C-H bending asym | |
| 1361 | C-H bending sym | |
| 1436 | OH bend asym | |
| 1459 | CH3 deformation asym | |
| 1508 | (C-H ip bend in-phase) | |
| 1592 | C=C stretch in phenol asym | |
| 1598 | C=C stretch in phenol sym | |
| 1611 | C=C stretch in phenol sym | |
| 1612 | C=C stretch in phenol asym | |
| 2965 | C-H str in CH3 asym | |
| 2973 | C-H str in CH3 sym | |
| 3066 | OH op str asym |
| Wavenumber (cm−1) | IR Assignments | Raman Assignments |
|---|---|---|
| 498 | O-S-C bend asym | |
| 544 | O-S-O bend sym | |
| 550 | O-S-O bend asym | |
| 628 | C-C-C-O-H twist asym | |
| 646 | C-C-C-OH twist sym | |
| 688 | S-O stretch sym | |
| 721 | H-C-C-C twist | |
| 813 | C-C-C-H twist | |
| 823 | C-C-C-H twist asym | |
| 826 | C-C-C trigonal bend asym | |
| 835 | C-C-C trigonal bend sym | |
| 1009 | C-S-C trigonal bend sym | |
| 1067 | C-S-C trigonal bend asym | |
| 1071 | S-O stretch sym | |
| 1099 | S-O stretch sym | S-O stretch asym |
| 1135 | C-H oop trigonal bend | C-H oop trigonal bend |
| 1219 | C-O stretch asym | |
| 1221 | C-O stretch sym | |
| 1281 | C-O stretch sym | |
| 1284 | C-O stretch asym | |
| 1441 | C-S-C deformation sym | |
| 1495 | C-S-C deformation asym | |
| 1497 | C-S-C deformation sym | |
| 1580 | C=C stretch in phenol asym | |
| 1582 | C=C stretch in phenol sym | |
| 1596 | C=C stretch in phenol asym | |
| 1601 | C=C stretch in phenol sym | |
| 3070 | OH op stretch |
| Wavenumber (cm−1) | IR Assignments | Raman Assignments |
|---|---|---|
| 489 | C-C-C oop bend | |
| 494 | C-C-C oop bend | |
| 511 | C-C-O-H bend | |
| 562 | C-C-C ip bend | |
| 569 | C-C-C ip bend | |
| 636 | C=C-O torsion | |
| 670 | C-C-C bend + C-H wag | |
| 776 | C-C-C bend + O-H wag | |
| 809 | C-C-C trigonal bending asym | C-C-C bend + O-H wag |
| 815 | a | |
| 845 | b | |
| 909 | Ring breathing | C-C-C trigonal bending asym |
| 1013 | C-H oop bend) sym | C-C-C trigonal bending asym |
| 1096 | ||
| 1100 | H-C-H scissoring sym | |
| 1166 | H-C-H scissoring asym | |
| 1169 | C-H oop bend sym | |
| 1230 | O-H bend + CH2 twist sym | C-H oop bend asym |
| 1250 | ||
| 1382 | C-H in CH2 bending | |
| 1422 | O-H bend + CH2 twist asym | |
| 1450 | H-C-H scissoring sym | |
| 1508 | C-O-H bending asym | |
| 1510 | CH ip bend in phase sym | |
| 1596 | CH ip bend in phase asym | |
| 1599 | C=C stretch in phenol sym | |
| 1608 | C=C stretch in phenol asym | |
| 1612 | C=C stretch in phenol sym | |
| 2911 | C=C stretch in phenol asym | |
| 3021 | Sym CH str on C-CH2-C | |
| 3038 | Asym CH str on C-CH2-C | |
| 3056 | OH ip str | |
| 3062 | OH op str |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, K.; Schmidt, W.; Qin, J.; Kim, M.; Tao, F. IR and Raman Dual Modality Markers Differentiate among Three bis-Phenols: BPA, BPS, and BPF. Appl. Sci. 2024, 14, 6064. https://doi.org/10.3390/app14146064
Chao K, Schmidt W, Qin J, Kim M, Tao F. IR and Raman Dual Modality Markers Differentiate among Three bis-Phenols: BPA, BPS, and BPF. Applied Sciences. 2024; 14(14):6064. https://doi.org/10.3390/app14146064
Chicago/Turabian StyleChao, Kuanglin, Walter Schmidt, Jianwei Qin, Moon Kim, and Feifei Tao. 2024. "IR and Raman Dual Modality Markers Differentiate among Three bis-Phenols: BPA, BPS, and BPF" Applied Sciences 14, no. 14: 6064. https://doi.org/10.3390/app14146064
APA StyleChao, K., Schmidt, W., Qin, J., Kim, M., & Tao, F. (2024). IR and Raman Dual Modality Markers Differentiate among Three bis-Phenols: BPA, BPS, and BPF. Applied Sciences, 14(14), 6064. https://doi.org/10.3390/app14146064







