Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
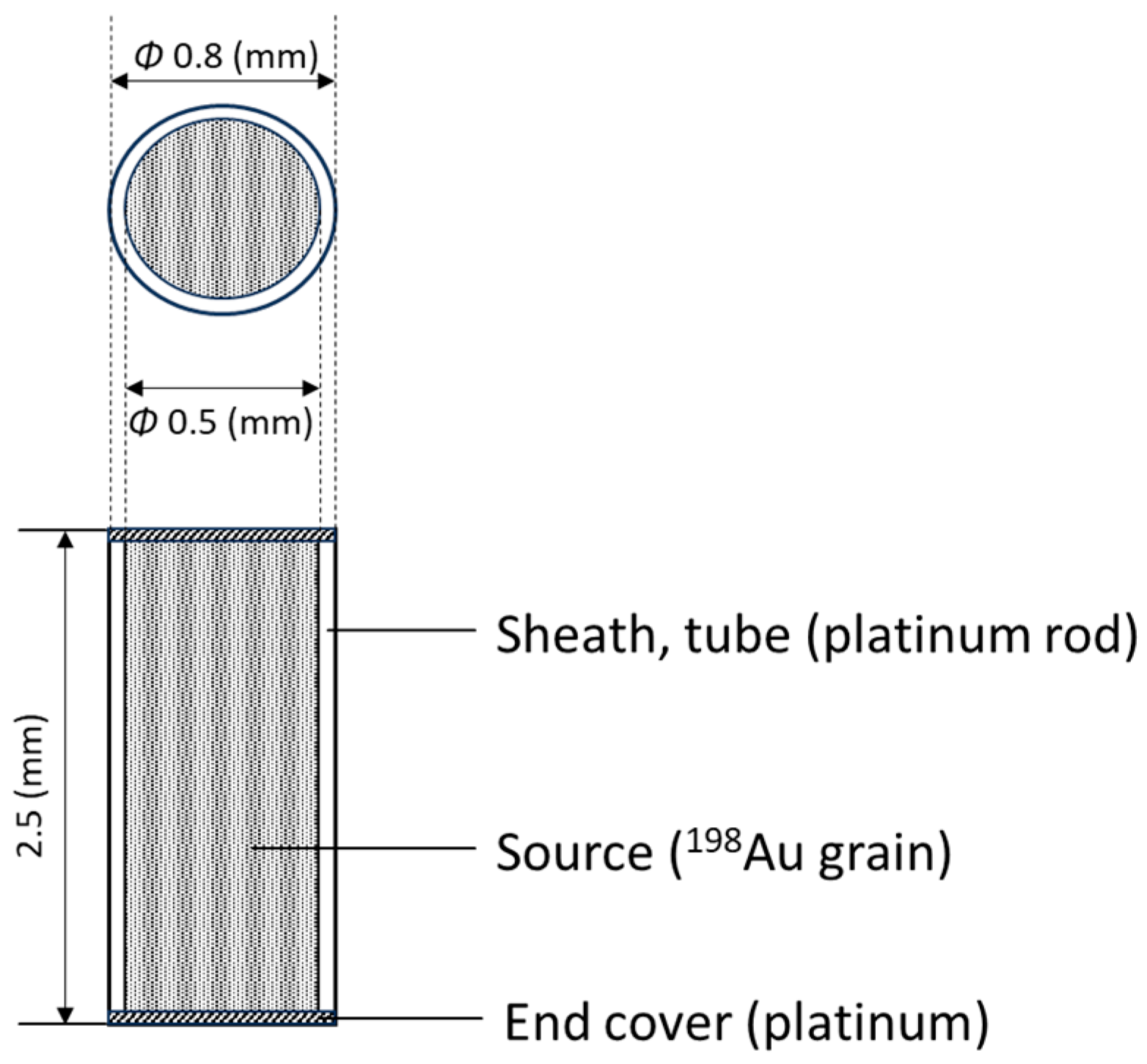
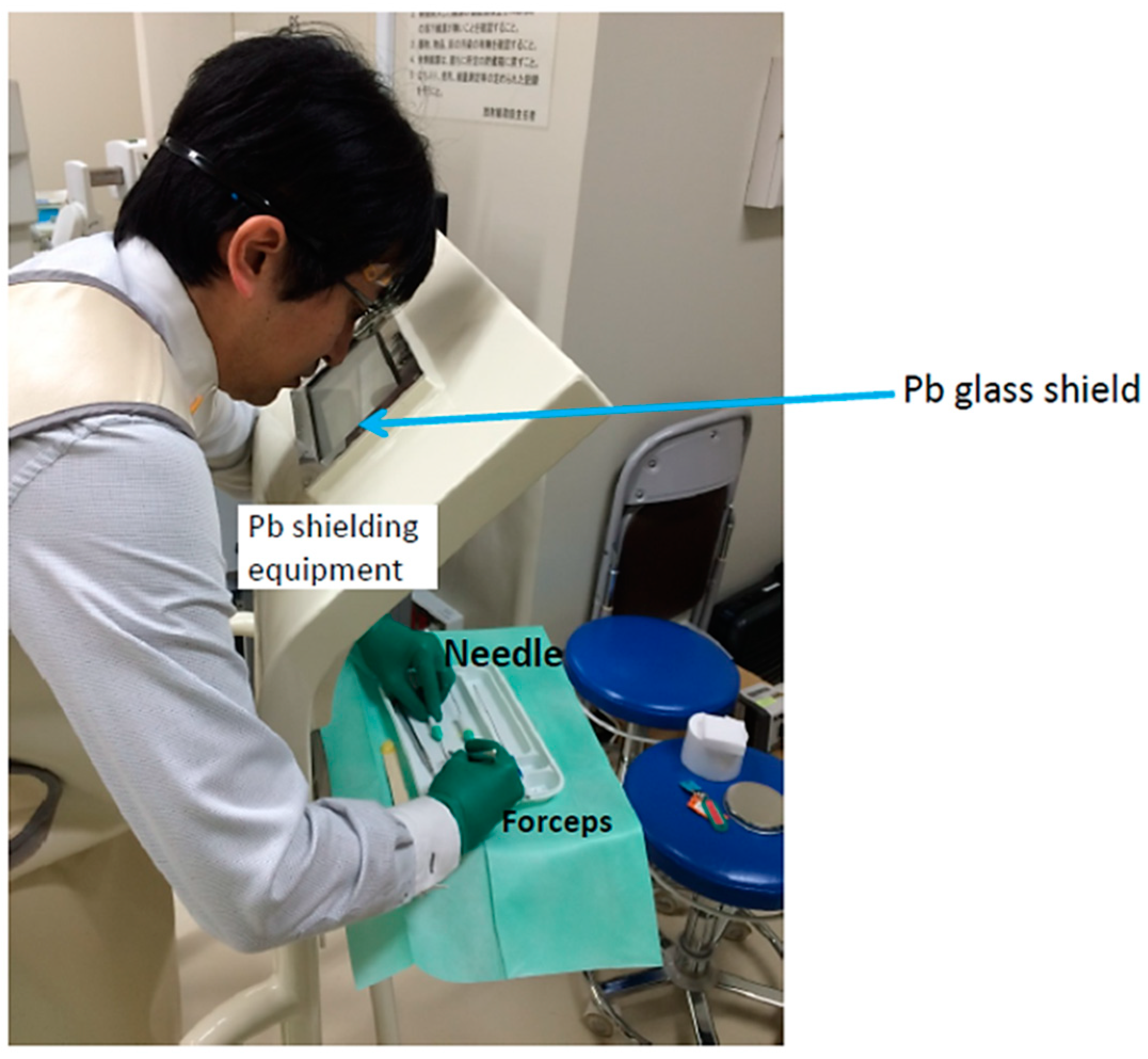
2.1. 198Au Brachytherapy
2.2. Subjects
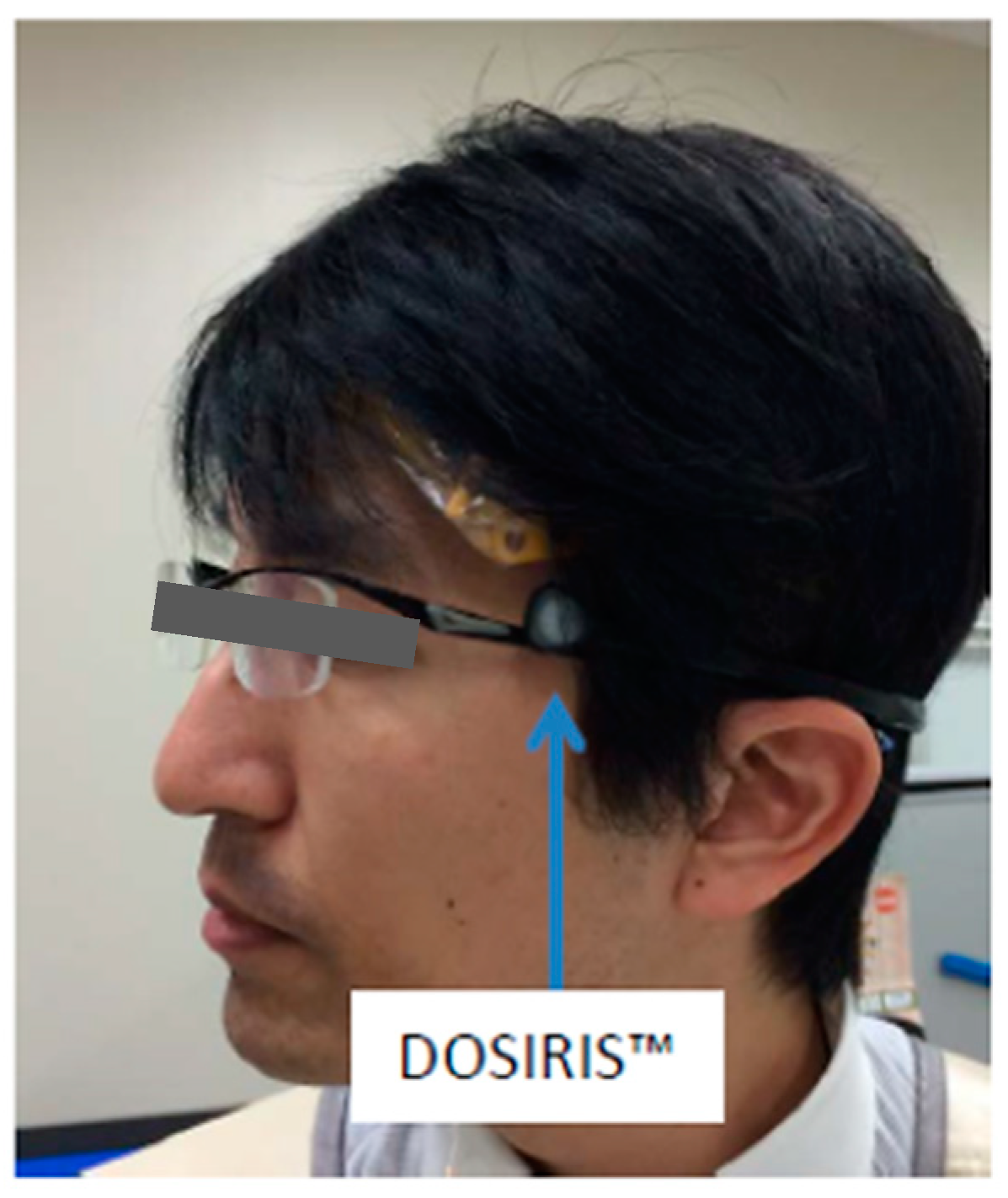
2.3. Dosimetry
2.4. Statistical Analyses
3. Results
3.1. Physician’s Eye/Neck Doses
3.2. Physician’s Hand Doses
3.3. Correlations among the Dose Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Parsa, R.; Chauhan, K.; Lukovic, J.; Han, K.; Taggar, A.; Raman, S. Review of brachytherapy clinical trials: A cross-sectional analysis of ClinicalTrials.gov. Radiat. Oncol. 2024, 19, 22. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, N.; Yang, J.; He, J.; Zhu, F.; Liao, W.; Xiong, M.; Li, Y. Advancements of radiotherapy for recurrent head and neck cancer in modern era. Radiat. Oncol. 2023, 18, 166. [Google Scholar] [CrossRef]
- Neugebauer, J.; Blum, P.; Keiler, A.; Süß, M.; Neubauer, M.; Moser, L.; Dammerer, D. Brachytherapy in the Treatment of Soft-Tissue Sarcomas of the Extremities-A Current Concept and Systematic Review of the Literature. Cancers 2023, 15, 1133. [Google Scholar] [CrossRef]
- Poder, J.; Rivard, M.J.; Howie, A.; Carlsson Tedgren, Å.; Haworth, A. Risk and Quality in Brachytherapy From a Technical Perspective. Clin. Oncol. (R. Coll. Radiol.) 2023, 35, 541–547. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Prevention of High-dose-rate Brachytherapy Accidents. ICRP Publication 97. Ann. ICRP 2005, 35, 5–75. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Radiation Safety Aspects of Brachytherapy for Prostate Cancer using Permanently—Implanted Sources. ICRP Publication 98. Ann. ICRP 2005, 35, 3–50. [Google Scholar]
- Fujita, M.; Hirokawa, Y.; Kashiwado, K.; Akagi, Y.; Kashimoto, K.; Kiriu, H.; Ohtani, K.; Wada, T. An analysis of mandibular bone complications in radiotherapy for T1 and T2 carcinoma of the oral tongue. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 333–339. [Google Scholar] [CrossRef]
- Chargari, C.; Deutsch, E.; Blanchard, P.; Gouy, S.; Martelli, H.; Guérin, F.; Dumas, I.; Bossi, A.; Morice, P.; Viswanathan, A.N.; et al. Brachytherapy: An overview for clinicians. CA Cancer J. Clin. 2019, 69, 386–401. [Google Scholar] [CrossRef]
- Skowronek, J. Current status of brachytherapy in cancer treatment—Short overview. J. Contemp. Brachyther. 2017, 9, 581–589. [Google Scholar] [CrossRef]
- Kovács, G.; Martinez-Monge, R.; Budrukkar, A.; Guinot, J.L.; Johansson, B.; Strnad, V.; Skowronek, J.; Rovirosa, A.; Siebert, F.A. GEC-ESTRO Head & Neck Working Group. GEC-ESTRO ACROP recommendations for head & neck brachytherapy in squamous cell carcinomas: 1st update—Improvement by cross sectional imaging based treatment planning and stepping source technology. Radiother. Oncol. 2017, 122, 248–254. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, X.; Liao, X.; Zheng, W.; Xu, H.; Yang, L.; Wei, Q. Nonsurgical salvage options for locally recurrent prostate cancer after primary definitive radiotherapy: A systematic review and meta-analysis. Int. J. Surg. 2024, 15, 81–88. [Google Scholar] [CrossRef]
- Gordon, K.; Smyk, D.; Gulidov, I.; Golubev, K.; Fatkhudinov, T. An Overview of Head and Neck Tumor Reirradiation: What Has Been Achieved So Far? Cancers 2023, 15, 4409. [Google Scholar] [CrossRef]
- Slevin, F.; Zattoni, F.; Checcucci, E.; Cumberbatch, M.G.K.; Nacchia, A.; Cornford, P.; Briers, E.; De Meerleer, G.; De Santis, M.; Eberli, D.; et al. A Systematic Review of the Efficacy and Toxicity of Brachytherapy Boost Combined with External Beam Radiotherapy for Nonmetastatic Prostate Cancer. Eur. Urol. Oncol. 2023. [Google Scholar] [CrossRef]
- Schaulin, M.S.; Delouya, G.; Zwahlen, D.; Taussky, D. Tracing the Evolution of Prostate Brachytherapy in the 20th Century. Oncology 2024, 102, 283–290. [Google Scholar] [CrossRef]
- Liang, Z.; Yuliang, C.; Zhu, M.; Zhou, Y.; Wu, X.; Li, H.; Fan, B.; Zhou, Z.; Yan, W. The direct prognosis comparison of. Eur. J. Med. Res. 2023, 28, 181. [Google Scholar] [CrossRef]
- Fionda, B.; Bussu, F.; Placidi, E.; Rosa, E.; Lancellotta, V.; Parrilla, C.; Zinicola, T.; De Angeli, M.; Greco, F.; Rigante, M.; et al. Interventional Radiotherapy (Brachytherapy) for Nasal Vestibule: Novel Strategies to Prevent Side Effects. J. Clin. Med. 2023, 12, 6154. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, L.; Xiao, Y.; Guo, X.; Hu, Y. Iodine-125 seed brachytherapy combined with pembrolizumab for advanced non-small-cell lung cancer after failure of first-line chemotherapy: A report of two cases and literature review. J. Contemp. Brachyther. 2023, 15, 81–88. [Google Scholar] [CrossRef]
- Yamazaki, H.; Yoshida, K.; Yoshioka, Y.; Shimizutani, K.; Furukawa, S.; Koizumi, M.; Ogawa, K. High dose rate brachytherapy for oral cancer. J. Radiat. Res. 2013, 54, 1–17. [Google Scholar] [CrossRef]
- Harada, H.; Ishikawa, Y.; Tanaka, S.; Kishida, K.; Umezawa, R.; Yamamoto, T.; Takahashi, N.; Takeda, K.; Suzuki, Y.; Jingu, K. Brachytherapy for primary nasal vestibule cancer using Au-198 grains. Int. Cancer Conf. J. 2022, 11, 184–187. [Google Scholar] [CrossRef]
- Tuček, L.; Vošmik, M.; Petera, J. Is There Still a Place for Brachytherapy in the Modern Treatment of Early-Stage Oral Cancer? Cancers 2022, 14, 222. [Google Scholar] [CrossRef]
- Arboleda, L.P.A.; de Carvalho, G.B.; Santos-Silva, A.R.; Fernandes, G.A.; Vartanian, J.G.; Conway, D.I.; Virani, S.; Brennan, P.; Kowalski, L.P.; Curado, M.P. Squamous Cell Carcinoma of the Oral Cavity, Oropharynx, and Larynx: A Scoping Review of Treatment Guidelines Worldwide. Cancers 2023, 15, 4405. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Komatsubara, H.; Ojima, Y.; Minamikawa, T.; Shibuya, Y.; Yokoo, S.; Ishii, J.; Komori, T. A comparison of brachytherapy and surgery for the treatment of stage I-II squamous cell carcinoma of the tongue. Int. J. Oral Maxillofac. Surg. 2005, 34, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Shibuya, H.; Miura, M.; Watanabe, H.; Ayukawa, F.; Hayashi, K.; Toda, K. Quality of life of oral cancer patients after low-dose-rate interstitial brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 772–778. [Google Scholar] [CrossRef]
- Yamazaki, H.; Inoue, T.; Yoshida, K.; Yoshioka, Y.; Furukawa, S.; Kakimoto, N.; Shimizutani, K. Brachytherapy for early oral tongue cancer: Low dose rate to high dose rate. J. Radiat. Res. 2003, 44, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, N.; Inoue, T.; Murakami, S.; Furukawa, S.; Yoshida, K.; Yoshioka, Y.; Yamazaki, H.; Tanaka, E.; Shimizutani, K. Results of low- and high-dose-rate interstitial brachytherapy for T3 mobile tongue cancer. Radiother. Oncol. 2003, 68, 123–128. [Google Scholar] [CrossRef]
- Khalilur, R.; Hayashi, K.; Shibuya, H. Brachytherapy for tongue cancer in the very elderly is an alternative to external beam radiation. Br. J. Radiol. 2011, 84, 747–749. [Google Scholar] [CrossRef]
- Okazawa, K.; Yuasa-Nakagawa, K.; Yoshimura, R.; Shibuya, H. Permanent interstitial re-irradiation with Au-198 seeds in patients with post-radiation locally recurrent uterine carcinoma. J. Radiat. Res. 2013, 54, 299–306. [Google Scholar] [CrossRef]
- Konishi, M.; Fujita, M.; Takeuchi, Y.; Kubo, K.; Imano, N.; Nishibuchi, I.; Murakami, Y.; Shimabukuro, K.; Wongratwanich, P.; Verdonschot, R.G.; et al. Treatment outcomes of real-time intraoral sonography-guided implantation technique of 198Au grain brachytherapy for T1 and T2 tongue cancer. J. Radiat. Res. 2021, 62, 871–876. [Google Scholar] [CrossRef]
- Konishi, M.; Takeuchi, Y.; Imano, N.; Kubo, K.; Nishibuchi, I.; Murakami, Y.; Shimabukuro, K.; Wongratwanich, P.; Kakimoto, N.; Nagata, Y. Brachytherapy with 198Au grains for cancer of the floor of the mouth: Relationships between radiation dose and complications. Oral. Radiol. 2022, 38, 105–113. [Google Scholar] [CrossRef]
- Horiuchi, J.; Takeda, M.; Shibuya, H.; Matsumoto, S.; Hoshina, M.; Suzuki, S. Usefulness of 198Au grain implants in the treatment of oral and oropharyngeal cancer. Radiother. Oncol. 1991, 21, 29–38. [Google Scholar] [CrossRef]
- Konishi, M.; Shimabukuro, K.; Hirokawa, J.; Sadatoki, T.; Katsuta, T.; Imano, N.; Nishibuchi, I.; Murakami, Y.; Kakimoto, N. Radiation doses of medical radiation workers performing low-dose-rate brachytherapy with 198Au grains and 192Ir pins for patients with oral cancers. Oral Radiol. 2023, 40, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.; Shibuya, H.; Hayashi, K. 198Au grain implantation for early tongue cancer in patients of advanced age or poor performance status. J. Radiat. Res. 2013, 54, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Penfold, S.N.; Marcu, L.; Lawson, J.M.; Asp, J. Evaluation of physician eye lens doses during permanent seed implant brachytherapy for prostate cancer. J. Radiol. Prot. 2012, 32, 339–347. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). Statement on Tissue Reactions. Available online: https://www.icrp.org/page.asp?id=123 (accessed on 10 March 2024).
- International Atomic Energy Agency. Radiation Protection and Safety of Radiation Sources: International Basic Safety Standards; General Safety Requirements Part 3; IAEA: Vienna, Austria, 2014. [Google Scholar]
- Chida, K. What are useful methods to reduce occupational radiation exposure among radiological medical workers, especially for interventional radiology personnel? Radiol. Phys. Technol. 2022, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Moritake, T.; Morota, K.; Nagamoto, K.; Nakagami, K.; Kuriyama, T.; Kunugita, N. Development and assessment of an educational application for the proper use of ceiling-suspended radiation shielding screens in angiography rooms using augmented reality technology. Eur. J. Radiol. 2021, 143, 109925. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Eguchi, Y.; Yamazaki, C.; Hino, T.; Saida, T.; Chida, K. Development of a New Radiation Shield for the Face and Neck of IVR Physicians. Bioengineering 2022, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Takahashi, T.; Ito, D.; Shimura, H.; Takeda, K.; Zuguchi, M. Clarifying and visualizing sources of staff-received scattered radiation in interventional procedures. AJR Am. J. Roentgenol. 2011, 197, W900–W903. [Google Scholar] [CrossRef] [PubMed]
- Zuguchi, M.; Chida, K.; Taura, M.; Inaba, Y.; Ebata, A.; Yamada, S. Usefulness of non-lead aprons in radiation protection for physicians performing interventional procedures. Radiat. Prot. Dosim. 2008, 131, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Fujibuchi, T. Radiation protection education using virtual reality for the visualisation of scattered distributions during radiological examinations. J. Radiol. Prot. 2021, 41, S317–S328. [Google Scholar] [CrossRef]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Radiation Eye Dose for Physicians in CT Fluoroscopy-Guided Biopsy. Tomography 2022, 8, 438–446. [Google Scholar] [CrossRef]
- Osanai, M.; Sato, H.; Sato, K.; Kudo, K.; Hosoda, M.; Hosokawa, S.; Kitajima, M.; Tsushima, M.; Fujita, A.; Hosokawa, Y.; et al. Occupational Radiation Dose, Especially for Eye Lens: Hp(3), in Medical Staff Members Involved in Computed Tomography Examinations. Appl. Sci. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Imai, S.; Akahane, M.; Ogata, Y.; Tanki, N.; Sato, H.; Tameike, K. Occupational eye lens dose in endoscopic retrograde cholangiopancreatography using a dedicated eye lens dosimeter. J. Radiol. Prot. 2021, 41, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N. Ionizing radiation sensitivity of the ocular lens and its dose rate dependence. Int. J. Radiat. Biol. 2017, 93, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Azizova, T.V.; Little, M.P. An update on effects of ionizing radiation exposure on the eye. Br. J. Radiol. 2020, 93, 20190829. [Google Scholar] [CrossRef] [PubMed]
- Haskal, Z.J. Interventional radiology carries occupational risk for cataracts. RSNA News 2004, 14, 5–6. [Google Scholar]
- Vañó, E.; Gonzalez, L.; Fernández, J.M.; Haskal, Z.J. Eye lens exposure to radiation in interventional suites: Caution is warranted. Radiology 2008, 248, 945–953. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). Avoidance of Radiation Injuries from Medical Interventional Procedures; ICRP Publication 85; Pergamon: Oxford, UK, 2000; Volume 30. [Google Scholar]
- IAEA. Implications for Occupational Radiation Protection of the New Dose Limit for the Lens of the Eye; IAEA: Vienna, Austria, 2013; Volume 1731, pp. 1–34. [Google Scholar]
- Kato, M.; Chida, K.; Munehisa, M.; Sato, T.; Inaba, Y.; Suzuki, M.; Zuguchi, M. Non-Lead Protective Aprons for the Protection of Interventional Radiology Physicians from Radiation Exposure in Clinical Settings: An Initial Study. Diagnostics 2021, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Haga, Y.; Sota, M.; Tanaka, A.; Otomo, K.; Murabayashi, Y.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; et al. Evaluation of novel X-ray protective eyewear in reducing the eye dose to interventional radiology physicians. J. Radiat. Res. 2021, 62, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Takei, Y.; Mori, H.; Kobayashi, I.; Noto, K.; Igarashi, T.; Suzuki, S.; Akahane, K. A multicenter study of radiation doses to the eye lenses of medical staff performing non-vascular imaging and interventional radiology procedures in Japan. Phys. Medica 2020, 74, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Hitachi, S.; Watanuki, M.; Chida, K. Occupational Radiation Dose to Eye Lenses in CT-Guided Interventions Using MDCT-Fluoroscopy. Diagnostics 2021, 11, 646. [Google Scholar] [CrossRef]
- Shindo, R.; Ohno, S.; Yamamoto, K.; Konta, S.; Inaba, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Comparison of shielding effects of over-glasses-type and regular eyewear in terms of occupational eye dose reduction. J. Radiol. Prot. 2024, 44, 023501. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Suzuki, S.; Toyama, H.; Arakawa, S.; Inoue, S.; Kinomura, Y.; Kobayashi, I. Evaluation of eye lens dose of interventional cardiologists. Radiat. Prot. Dosim. 2017, 173, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Maas, J.; Viniol, S.; Etzel, R.; Fiebich, M.; Thomas, R.; Mahnken, A. Scatter radiation reduction with a radiation-absorbing pad in interventional radiology examinations. Eur. J. Radiol. 2020, 132, 109245. [Google Scholar] [CrossRef] [PubMed]
- Haga, Y.; Chida, K.; Kimura, Y.; Yamanda, S.; Sota, M.; Abe, M.; Kaga, Y.; Meguro, T.; Zuguchi, M. Radiation eye dose to medical staff during respiratory endoscopy under X-ray fluoroscopy. J. Radiat. Res 2020, 61, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, F.H.; Razakamanantsoa, L.; Ammar, M.B.; Lehrer, R.; Haffaf, I.; El-Mouhadi, S.; Gardavaud, F.; Najdawi, M.; Barral, M. Ergonomics in interventional radiology: Awareness is mandatory. Medicina 2021, 57, 500. [Google Scholar] [CrossRef] [PubMed]
- Ikezawa, K.; Hayashi, S.; Takenaka, M.; Yakushijin, T.; Nagaike, K.; Takada, R.; Yamai, T.; Matsumoto, K.; Yamamoto, M.; Omoto, S.; et al. Occupational radiation exposure to the lens of the eyes and its protection during endoscopic retrograde cholangiopancreatography. Sci. Rep. 2023, 13, 7824. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Chida, K.; Ishida, T.; Toyoshima, H.; Yoshida, Y.; Yoshioka, S.; Moroi, J.; Kinoshita, T. Occupational Radiation Exposure of the Eye in Neurovascular Interventional Physician. Radiat. Prot. Dosim. 2019, 185, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Haga, Y.; Chida, K.; Kaga, Y.; Sota, M.; Meguro, T.; Zuguchi, M. Occupational eye dose in interventional cardiology procedures. Sci. Rep. 2017, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Education and Training in Radiological Protection for Diagnostic and Interventional Procedures. ICRP Publication 113. Ann. ICRP 2009, 39, 7–68. [Google Scholar]
- International Commission on Radiological Protection (ICRP). Occupational radiological protection in brachytherapy. ICRP Publication 149. Ann. ICRP 2021, 50, 5–75. [Google Scholar] [CrossRef]
- International Commission on Radiological Protection (ICRP). Occupational radiological protection in interventional procedures. ICRP Publication 139. Ann. ICRP 2018, 47, 1–118. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Radiological Protection (ICRP). Radiological Protection in Fluoroscopically Guided Procedures outside the Imaging Department. ICRP Publication 117. Ann. ICRP 2010, 40, 1–102. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Haga, Y.; Sota, M.; Abe, M.; Kaga, Y.; Inaba, Y.; Suzuki, M.; Meguro, T.; Hosoi, Y.; Chida, K. Evaluation of Lens Doses among Medical Staff Involved in Nuclear Medicine: Current Eye Radiation Exposure among Nuclear-Medicine Staff. Appl. Sci. 2023, 13, 9182. [Google Scholar] [CrossRef]
- Ishii, H.; Chida, K.; Satsurai, K.; Haga, Y.; Kaga, Y.; Abe, M.; Inaba, Y.; Zuguchi, M. Occupational eye dose correlation with neck dose and patient-related quantities in interventional cardiology procedures. Radiol. Phys. Technol. 2022, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chida, K.; Kaga, Y.; Haga, Y.; Kataoka, N.; Kumasaka, E.; Meguro, T.; Zuguchi, M. Occupational dose in interventional radiology procedures. AJR Am. J. Roentgenol. 2013, 200, 138–141. [Google Scholar] [CrossRef]
- Martin, C.J. Personal dosimetry for interventional operators: When and how should monitoring be done? Br. J. Radiol. 2011, 84, 639–648. [Google Scholar] [CrossRef]
- Fujii, K.; Ko, S.; Nako, Y.; Tonari, A.; Nishizawa, K.; Akahane, K.; Takayama, M. Dose measurement for medical staff with glass dosemeters and thermoluminescence dosemeters during 125I brachytherapy for prostate cancer. Radiat. Prot. Dosim. 2011, 144, 459–463. [Google Scholar] [CrossRef]
- Aronowitz, J.N.; Connock, G.; Haq, R.; Morin, M.J. Radiation exposure from permanent prostate brachytherapy without fluoroscopy. Nowotwory 2009, 59, 184–187. [Google Scholar]














| Patient | Type of Cancer | Number of Au Grains | Total Radiation Activity (MBq) | DOSIRIS (mSv) | Personal Badge (mSv) | RPLD (mSv) | |||
|---|---|---|---|---|---|---|---|---|---|
| Left Eye | Right Eye | Left Neck | Right Neck | Left Hand | Right Hand | ||||
| 1 | Tongue | 11 | 2035 | 0.08 | - | - | - | 0.380 | 0.576 |
| 2 | Tongue | 12 | 2115 | 0.07 | - | - | - | 0.241 | 0.599 |
| 3 | Tongue | 8 | 1426 | 0.04 | 0.03 | 0.01 | 0.02 | 0.340 | 0.609 |
| 4 | Tongue | 10 | 1735 | 0.05 | 0.05 | 0.02 | 0.03 | 0.310 | 0.221 |
| 5 | Tongue | 8 | 1488 | 0.03 | 0.03 | 0.02 | 0.02 | 0.074 | 0.150 |
| 6 | Tongue | 17 | 1947 | 0.06 | 0.06 | 0.05 | 0.06 | - | 0.527 |
| 7 | Tongue | 12 | 2150 | 0.07 | 0.06 | 0.03 | 0.04 | 0.508 | 0.763 |
| 8 | Oral | 15 | 2659 | 0.01 | 0 | 0 | 0 | 0.461 | 0.980 |
| 9 | Nose | 9 | 1630 | 0.09 | 0.10 | 0.06 | 0.10 | 0.142 | 0.243 |
| 10 | Tongue | 9 | 1322 | 0.05 | 0.06 | 0 | 0 | 0.143 | 0.221 |
| 11 | Tongue | 15 | 2453 | 0.06 | 0.04 | 0.04 | 0.04 | 0.502 | 0.809 |
| Average | 11.5 | 1905.5 | 0.06 | 0.05 | 0.03 | 0.03 | 0.310 | 0.518 | |
| Standard deviation | 3.1 | 428.9 | 0.02 | 0.03 | 0.02 | 0.03 | 0.157 | 0.276 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inaba, Y.; Jingu, K.; Fujisawa, M.; Otomo, K.; Ishii, H.; Kato, T.; Murabayashi, Y.; Suzuki, M.; Zuguchi, M.; Chida, K. Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer. Appl. Sci. 2024, 14, 6010. https://doi.org/10.3390/app14146010
Inaba Y, Jingu K, Fujisawa M, Otomo K, Ishii H, Kato T, Murabayashi Y, Suzuki M, Zuguchi M, Chida K. Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer. Applied Sciences. 2024; 14(14):6010. https://doi.org/10.3390/app14146010
Chicago/Turabian StyleInaba, Yohei, Keiichi Jingu, Masaki Fujisawa, Kazuki Otomo, Hiroki Ishii, Toshiki Kato, Yuuki Murabayashi, Masatoshi Suzuki, Masayuki Zuguchi, and Koichi Chida. 2024. "Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer" Applied Sciences 14, no. 14: 6010. https://doi.org/10.3390/app14146010
APA StyleInaba, Y., Jingu, K., Fujisawa, M., Otomo, K., Ishii, H., Kato, T., Murabayashi, Y., Suzuki, M., Zuguchi, M., & Chida, K. (2024). Evaluation of Radiation Doses Received by Physicians during Permanent 198Au Grain Implant Brachytherapy for Oral Cancer. Applied Sciences, 14(14), 6010. https://doi.org/10.3390/app14146010






