Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale
Abstract
1. Introduction
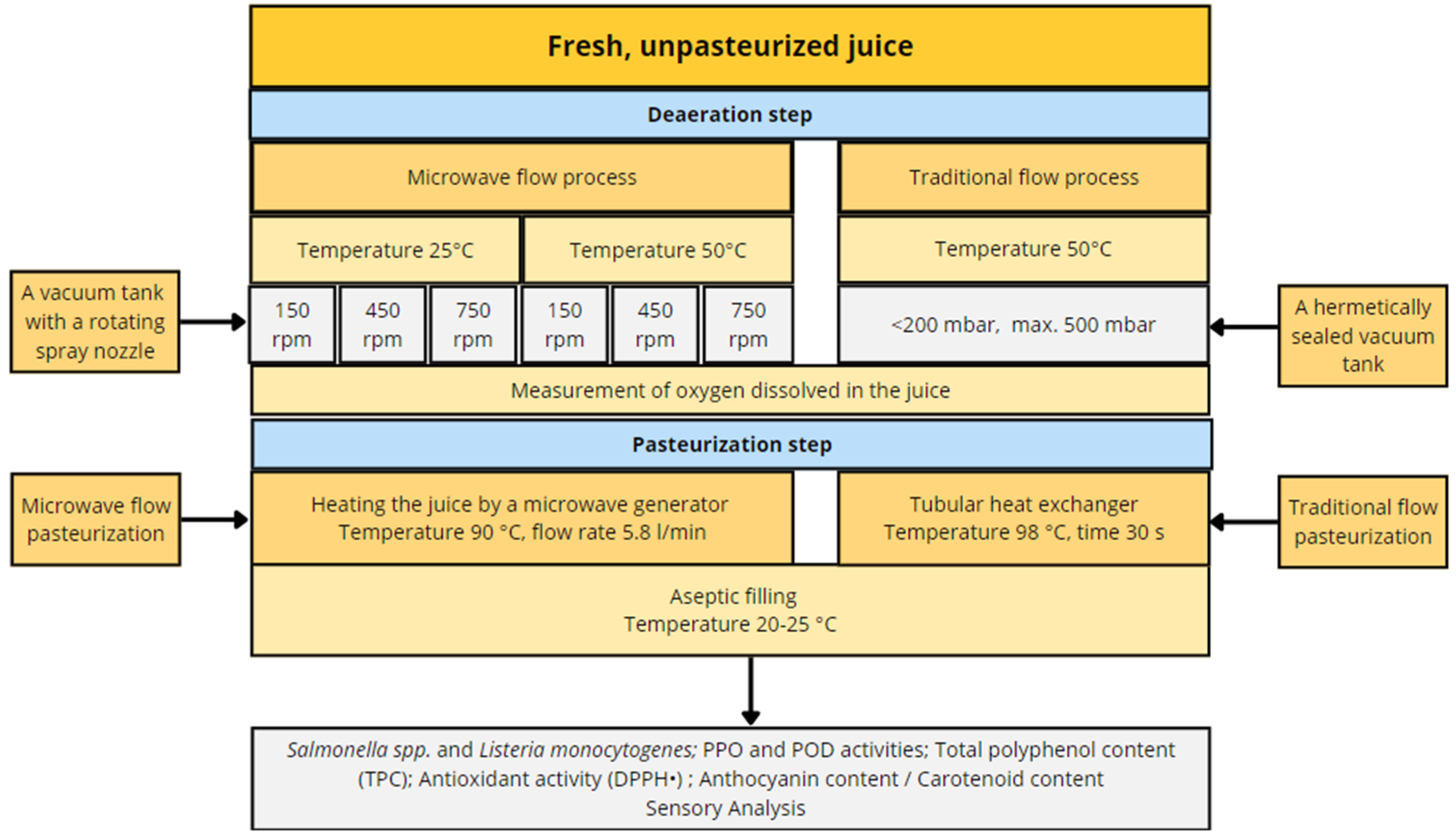
2. Materials and Methods
2.1. Juice Preparation
2.2. Detection of the Pathogenic Bacteria Salmonella spp. and Listeria monocytogenes
2.3. PPO and POD Activities
2.4. Total Polyphenol Content (TPC)
2.5. Anthocyanin Content
2.6. Antioxidant Activity (DPPH•)
2.7. Carotenoid Content
2.8. Sensory Analysis
2.9. Statistical Analysis
3. Results
3.1. Pathogenic Bacteria
3.2. PPO and POD Activities
3.3. Total Phenolic Content (TPC) and Antioxidant Capacity
3.4. Anthocyanin Content
3.5. Carotenoid Content
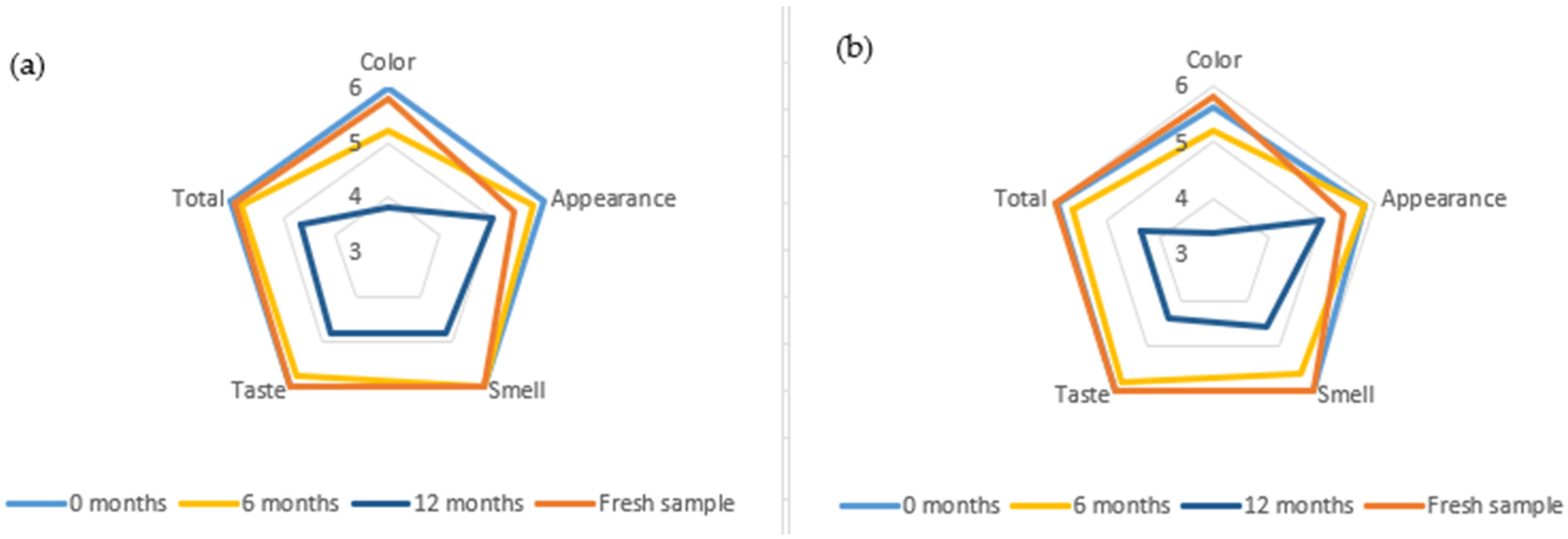
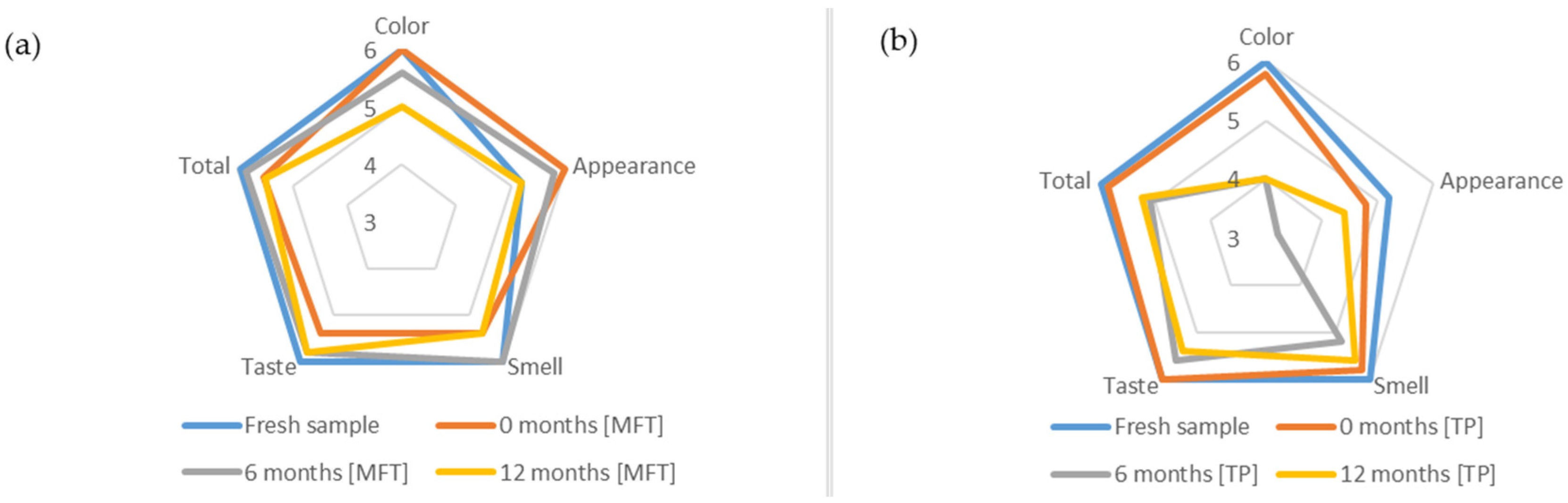
3.6. Sensory Analysis
4. Discussion
4.1. Pathogenic Bacteria Salmonella spp. and Listeria monocytogenes
4.2. PPO and POD Activity Levels
4.3. Total Polyphenol Content (TPC) and Antioxidant Capacity
4.4. Anthocyanin Content in Apple–Chokeberry Juice
4.5. Total Content of Carotenoids
4.6. Sensory Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lepaus, B.M.; Valiati, B.S.; Machado, B.G.; Domingos, M.M.; Silva, M.N.; Faria-Silva, L.; Bernardes, P.C.; Oliveira, D.d.S.; de São José, J.F.B. Impact of ultrasound processing on the nutritional components of fruit and vegetable juices. Trends Food Sci. Technol. 2023, 138, 752–765. [Google Scholar] [CrossRef]
- Nonglait, D.L.; Chukkan, S.M.; Arya, S.S.; Bhat, M.S.; Waghmare, R. Emerging non-thermal technologies for enhanced quality and safety of fruit juices. Int. J. Food Sci. 2022, 57, 6368–6377. [Google Scholar] [CrossRef]
- Altunay, N.; Elik, A.; Tuzen, M.; Lanjwani, M.F.; Mogaddam, M.R.A. Determination and extraction of acrylamide in processed food samples using alkanol-based supramolecular solvent-assisted dispersive liquid-liquid microextraction coupled with spectrophotometer: Optimization using factorial design. J. Food Compos. Anal. 2023, 115, 105023. [Google Scholar] [CrossRef]
- Ravichandran, C.; Jayachandran, L.E.; Kothakota, A.; Pandiselvam, R.; Balasubramaniam, V.M. Influence of high pressure pasteurization on nutritional, functional and rheological characteristics of fruit and vegetable juices and purees-an updated Review. Food Control 2023, 146, 109516. [Google Scholar] [CrossRef]
- Benton, D.; Young, H.A. Role of fruit juice in achieving the 5-a-day recommendation for fruit and vegetable intake. Nutr. Rev. 2019, 77, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Tougeron, K.; Hance, T. Impact of the COVID-19 pandemic on apple orchards in Europe. Agric. Syst. 2021, 190, 103097. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.Š.; Babojelić, M.S. Traditional, indigenous apple varieties, a fruit with potential for beneficial effects: Their quality traits and bioactive polyphenol contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, Q.; Yu, J. Life cycle assessment of concentrated apple juice production in China: Mitigation options to reduce the environmental burden. Sustain. Prod. Consum. 2020, 32, 15–26. [Google Scholar] [CrossRef]
- Kalinowska, M.; Gryko, K.; Wróblewska, A.M.; Jabłońska-Trypuć, A.; Karpowicz, D. Phenolic content, chemical composition and anti-/pro-oxidant activity of Gold Milenium and Papierowka apple peel extracts. Sci. Rep. 2020, 10, 14951. [Google Scholar] [CrossRef] [PubMed]
- Vallée Marcotte, B.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health benefits of apple juice consumption: A review of interventional trials on humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef]
- Cywińska-Antonik, M.; Chen, Z.; Groele, B.; Marszałek, K. Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods 2023, 12, 1181. [Google Scholar] [CrossRef]
- Byrd-Bredbenner, C.; Ferruzzi, M.G.; Fulgoni III, V.L.; Murray, R.; Pivonka, E.; Wallace, T.C. Satisfying America’s fruit gap: Summary of an expert roundtable on the role of 100% fruit juice. J. Food Sci. 2017, 82, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Efimtseva, E.A.; Chelpanova, T.I. Apples as a source of soluble and insoluble dietary fibers: Effect of dietary fibers on appetite. Hum. Physiol. 2020, 46, 224–234. [Google Scholar] [CrossRef]
- Manso, M.C.; Oliveira, F.A.; Oliveira, J.C.; Frías, J.M. Modelling ascorbic acid thermal degradation and browning in orange juice under aerobic conditions. Int. J. Food Sci. 2001, 36, 303–312. [Google Scholar] [CrossRef]
- Feszterová, M.; Mišiaková, M.; Kowalska, M. Bioactive Vitamin C Content from Natural Selected Fruit Juices. Appl. Sci. 2023, 13, 3624. [Google Scholar] [CrossRef]
- Kay, R.M. Dietary fiber. J. Lipid Res. 1982, 23, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Mazón, M.; Iker, D.; Téllez-Medina, L.A.B.; Gutiérrez-López, G.F. Chapter 14 Juice Packaging. In Juice Processing: Quality, Safety and Value-Added Opportunities; CRC Press: Boca Raton, FL, USA, 2014; p. 301. [Google Scholar] [CrossRef]
- Rodrigues, S.S.; Dias, L.G.; Teixeira, A. Emerging Methods for the Evaluation of Sensory Quality of Food: Technology at Service. Curr. Food Sci. Technol. Rep. 2024, 2, 77–90. [Google Scholar] [CrossRef]
- Urango, A.C.M.; Strieder, M.M.; Silva, E.K.; Meireles, M.A.A. Impact of thermosonication processing on food quality and safety: A review. Food Bioprocess Technol. 2022, 15, 1700–1728. [Google Scholar] [CrossRef]
- Tang, J.; Hong, Y.K.; Inanoglu, S.; Liu, F. Microwave pasteurization for ready-to-eat meals. Curr. Opin. Food Sci. 2018, 23, 133–141. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; dos Santos Funcia, E.; Pires, M.N.; Gut, J.A.W. Modeling of time-temperature history and enzymatic inactivation of cloudy apple juice in continuous flow microwave assisted pasteurization. Food Bioprod. Process 2018, 111, 45–53. [Google Scholar] [CrossRef]
- Liang, K.H.; Som, S.; Gupta, K.K.; Lu, C.H. Electrochemical characterization of TiNb2O7 as anode material synthesized using microwave-assisted microemulsion route. J. Am. Ceram. Soc. 2022, 105, 7446–7454. [Google Scholar] [CrossRef]
- Torres-Moya, I.; Harbuzaru, A.; Donoso, B.; Prieto, P.; Ponce Ortiz, R.; Díaz-Ortiz, Á. Microwave Irradiation as a Powerful Tool for the Preparation of n-Type Benzotriazole Semiconductors with Applications in Organic Field-Effect Transistors. Molecules 2022, 27, 4340. [Google Scholar] [CrossRef] [PubMed]
- Strekalova, A.A.; Shesterkina, A.A.; Kustov, A.L.; Kustov, L.M. Recent Studies on the Application of Microwave-Assisted Method for the Preparation of Heterogeneous Catalysts and Catalytic Hydrogenation Processes. Int. J. Mol. Sci. 2023, 24, 8272. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.; Aguayo, E. Continuous microwave pasteurization of a vegetable smoothie improves its physical quality and hinders detrimental enzyme activity. Food Sci. Technol. Int. 2017, 23, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kernou, O.N.; Belbahi, A.; Kaanin-Boudraa, G.; Adel, K.; Madani, P.K. A Review: Ultrasound-Microwave Technologies as Alternative Methods for Inactivation Bacterias in Fruit Juice. Int. J. Anal. Appl. Chem. 2022, 8, 31–40. [Google Scholar] [CrossRef]
- Kosiński, J.; Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Jasińska, U.T.; Woźniak, Ł.; Kaniewska, B.; Marszałek, K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Appl. Sci. 2024, 14, 662. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. Effect of continuous flow microwave and conventional heating on the bioactive compounds, colour, enzymes activity, microbial and sensory quality of strawberry purée. Food Bioprocess Technol. 2015, 8, 1864–1876. [Google Scholar] [CrossRef]
- Seyedabadi, M.M.; Kashaninejad, M.; Jafari, S.M.; Seyedabadi, E.; Khojastehpour, M. Effect of Continuous Flow Microwave Processing System on Quality Attributes of Orange Juice. J. Food Process. Preserv. 2022, 14, 89–104. [Google Scholar] [CrossRef]
- Kernou, O.-N.; Azzouz, Z.; Belbahi, A.; Kerdouche, K.; Kaanin-Boudraa, G.; Amir, A.; Madani, K.; Rijo, P. Inactivation of rescherichia coli in an orange juice beverage by combined ultrasonic and microwave treatment. Foods 2023, 12, 666. [Google Scholar] [CrossRef]
- Jafarpour, D.; Hashemi, S.M.B.; Asadi-Yousefabad, S.H.; Javdan, G. Conventional thermal and microwave processing of guava juice: Process intensification, microbial inactivation and chemical composition. J. Food Meas. Charact. 2023, 17, 3790–3801. [Google Scholar] [CrossRef]
- Marszałek, K. Method of Producing Juices and Fruit Beverages. Patent 238909, 22 July 2021. [Google Scholar]
- Perek, A.; Dolata, W. Zastosowanie mikrofal do obróbki cieplnej żywności. Postęp. Tech. Przetwórstwa Spoż. 2009, 2, 103–108. [Google Scholar]
- Guo, C.; Mujumdar, A.S.; Zhang, M. New development in radio frequency heating for fresh food processing: A review. Food Eng. Rev. 2019, 11, 29–43. [Google Scholar] [CrossRef]
- PN-EN ISO 6579-1:2017-04; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. ISO: Geneva, Switzerland, 2017.
- PN-EN ISO 11290-1:2017-07; Food Microbiology—Horizontal Method Used and Determining the Number of Listeria Monocytogenes and Other Listeria spp.—Part 1: Treatment Method. ISO: Geneva, Switzerland, 2017.
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J. Stabilization and application of anthocyanin chokeberry dye to colouring of beverages. Acta Sci. Pol. Technol. Aliment. 2002, 1, 37–45. [Google Scholar]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food. Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Corte-Real, J.; Meléndez-Martínez, A.J.; Bohn, T. Bioaccessibility of phytoene and phytofluene is superior to other carotenoids from selected fruit and vegetable juices. Food Chem. 2017, 229, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Martinez, A.J.; Stinco, C.M.; Liu, C.; Wang, X.-D. A simple HPLC method for the comprehensive analysis of cis/trans (Z/E) geometrical isomers of carotenoids for nutritional studies. Food Chem. 2013, 138, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- PN-ISO 4121:1998; Sensory Analysis—Methodology—Evaluation of Food Products by Methods Using Scales. ISO: Geneva, Switzerland, 1998.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- Sagong, H.G.; Park, S.H.; Choi, Y.J.; Ryu, S.; Kang, D.H. Inactivation of Escherichia coli O157: H7, Salmonella Typhimurium, and Listeria monocytogenes in orange and tomato juice using ohmic heating. J. Food Prot. 2011, 74, 899–904. [Google Scholar] [CrossRef]
- Schmidt, R.H.; Piotter, H.M. The hygienic/sanitary design of food and beverage processing equipment. In Food Safety Engineering; Springer: Cham, Switzerland, 2020; pp. 267–332. [Google Scholar] [CrossRef]
- Varghese, S.M.; Parisi, S.; Singla, R.K.; Begum, A.A. Food Safety and Quality Control in Food Industry. In Trends in Food Chemistry, Nutrition and Technology in Indian Sub-Continent; Springer: Cham, Switzerland, 2022; pp. 31–44. [Google Scholar] [CrossRef]
- Davanzo, E.F.A.; dos Santos, R.L.; Castro, V.H.d.L.; Palma, J.M.; Pribul, B.R.; Dallago, B.S.L.; Fuga, B.; Medeiros, M.; de Almeida, S.S.T.; da Costa, H.M.B.; et al. Molecular characterization of Salmonella spp. and Listeria monocytogenes strains from biofilms in cattle and poultry slaughterhouses located in the federal District and State of Goiás, Brazil. PLoS ONE 2021, 16, e0259687. [Google Scholar] [CrossRef]
- Qadri, O.S.; Yousuf, B.; Srivastava, A.K. Fresh-cut fruits and vegetables: Critical factors influencing microbiology and novel approaches to prevent microbial risks—A review. Cogent Food Agric. 2015, 1, 1121606. [Google Scholar] [CrossRef]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Mendes-Oliveira, G.; Deering, A.J.; San Martin-Gonzalez, M.F.; Campanella, O.H. Microwave pasteurization of apple juice: Modeling the inactivation of Escherichia coli O157: H7 and Salmonella Typhimurium at 80–90 °C. Food Microbiol. 2020, 87, 103382. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Tinoco, M.; Pina-Pérez, M.C.; Martínez-Navarrete, N.; Rodrigo, D. Listeria monocytogenes inactivation kinetics under microwave and conventional thermal processing in a kiwifruit puree. Innov. Food Sci. Emerg. Technol. 2014, 22, 131–136. [Google Scholar] [CrossRef]
- Cañumir, J.A.; Celis, J.E.; de Bruijn, J.; Vidal, L.V. Pasteurisation of Apple Juice by Using Microwaves. LWT Food Sci. Technol. 2002, 35, 389–392. [Google Scholar] [CrossRef]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf life evaluation during cold storage. J. Food Technol. 2017, 54, 832–841. [Google Scholar] [CrossRef] [PubMed]
- González-Monroy, A.D.; Rodríguez-Hernández, G.; Ozuna, C.; Sosa-Morales, M.E. Microwave-assisted pasteurization of beverages (tamarind and green) and their quality during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2018, 49, 51–57. [Google Scholar] [CrossRef]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Gut, J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave applications in the food industry: An overview of recent developments. Crit. Rev. Food Sci. Nutr. 2022, 62, 7989–8008. [Google Scholar] [CrossRef]
- Baines, B. Comparison of the effects of microwave irradiation and heat treatment of t4 and t7 bacteriophage. J. Exp. Microbiol. Immunol. 2005, 7, 57–61. [Google Scholar]
- Radoiu, M.; Calvo-Carrascal, M.A.; Dodds, C.; Binner, E.R. Flash microwave denaturation of POD and LOX enzymes 624 in whole yellow peas. Chem. Eng. Process. Process. Intensif. 2021, 169, 108601. [Google Scholar] [CrossRef]
- Dhar, R.; Chakraborty, S. Effect of continuous microwave processing on enzymes and quality attributes of bael beverage. Food Chem. 2024, 453, 139621. [Google Scholar] [CrossRef] [PubMed]
- Saxena, J.; Ahmad Makroo, H.; Srivastava, B. E ffect of ohmic heating on Polyphenol Oxidase (PPO) inactivation and color change in sugarcane juice. J. Food Proc. Eng. 2017, 40, e12485. [Google Scholar] [CrossRef]
- Plank, D.W.; Szpylka, J.; Sapirstein, H.; Woollard, D.; Zapf, C.M.; Lee, V.; Chen, C.Y.O.; Liu, R.H.; Tsao, R.; Düsterloh, A.; et al. Determination of antioxidant activity in foods and beverages by reaction with 2,2′-Diphenyl-1-picrylhydrazyl (DPPH): Collaborative study first action 2012.04. J. AOAC Int. 2012, 95, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Wang, J.; Liu, F.; Tang, J.; Bohnet, S. Application of non-enzymatic browning of fructose for heating pattern determination in microwave assisted thermal pasteurization system. J. Food Eng. 2017, 210, 27–34. [Google Scholar] [CrossRef]
- Táborský, J.; Sus, J.; Lachman, J.; Šebková, B.; Adamcová, A.; Šatínský, D. Dynamics of phloridzin and related compounds in four cultivars of apple trees during the vegetation period. Molecules 2021, 26, 3816. [Google Scholar] [CrossRef] [PubMed]
- Stasiak, A.; Ulanowska, A. Aktywnosc przeciwutleniajaca nowych odmian fasoli [Phaseolus vulgaris L.]. Żywn. Nauka Technol. Jakość 2008, 15, 74–82. [Google Scholar]
- Wu, Y.; Xu, L.; Liu, X.; Hasan, K.F.; Li, H.; Zhou, S.; Zhou, Y. Effect of thermosonication treatment on blueberry juice quality: Total phenolics, flavonoids, anthocyanin, and antioxidant activity. LWT 2021, 150, 112021. [Google Scholar] [CrossRef]
- Nayak, P.K.; Basumatary, B.; Chandrasekar, C.M.; Seth, D.; Kesavan, R.K. Impact of thermosonication and pasteurization on total phenolic contents, total flavonoid contents, antioxidant activity, and vitamin C levels of elephant apple (Dillenia indica) juice. J. Food Proc. Eng. 2020, 43, e13447. [Google Scholar] [CrossRef]
- Cavalcante, T.A.B.B.; dos Santos Funcia, E.; Gut, J.A.W. Inactivation of polyphenol oxidase by microwave and conventional heating: Investigation of thermal and non-thermal effects of focused microwaves. Food Chem. 2021, 340, 127911. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Khadka, M.; Mishra, R.; Kohli, D.; Upadhaya, S. Effects of conventional and microwave heating pasteurization on physiochemical properties of pomelo (Citrus maxima) juice. Int. J. Food Process. Technol. 2017, 8, 8–11. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; Purgatto, E.; Hassimotto, N.M.; Gut, J.A. Comparative evaluation of flavour and nutritional quality after conventional and microwave-assisted pasteurization of cloudy apple juice. LWT 2019, 111, 853–860. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Tang, W.; Liu, J.J.; Gong, X.Q.; Kong, L.; Yao, X.M.; Jing, M.; Cai, F.Y.; Li, X.T.; Ju, R.J. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; O Omolola, A.; O Udeh, H.; A Anyasi, T. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Romero-Zúñiga, G.Y.; González-Morones, P.; Sánchez-Valdés, S.; Yáñez-Macías, R.; Sifuentes-Nieves, I.; García-Hernández, Z.; Hernández-Hernández, E. Microwave radiation as alternative to modify natural fibers: Recent trends and opportunities–A review. J. Nat. Fibers 2022, 19, 7594–7610. [Google Scholar] [CrossRef]
- Adam, D. Microwave chemistry: Out of the kitchen. Nature 2003, 421, 571–573. [Google Scholar] [CrossRef]
- Li, A.; Xiao, R.; He, S.; An, X.; He, Y.; Wang, C.; Yin, S.; Wang, B.; Shi, X.; He, J. Research Advances of Purple Sweet Potato Anthocyanins: Extraction, Identification, Stability, Bioactivity, Application, and Biotransformation. Molecules 2019, 24, 3816. [Google Scholar] [CrossRef]
- De la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Stinco, C.M.; Szczepańska, J.; Marszałek, K.; Pinto, C.A.; Inácio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, V.; DerMarderosian, A.; Porter, J.R. Anthocyanins and polyphenol oxidase from dried arils of pomegranate (Punica granatum L.). Food Chem. 2010, 118, 11–16. [Google Scholar] [CrossRef]
- Kim, A.-N.; Lee, K.-Y.; Kim, B.G.; Cha, S.W.; Jeong, E.J.; Kerr, W.L.; Choi, S.-G. Thermal processing under oxygen–free condition of blueberry puree: Effect on anthocyanin, ascorbic acid, antioxidant activity, and enzyme activities. Food Chem. 2021, 342, 128345. [Google Scholar] [CrossRef] [PubMed]
- Vegara, S.; Martí, N.; Mena, P.; Saura, D.; Valero, M. Effect of pasteurization process and storage on color and shelf-life of pomegranate juices. LWT Food Sci. Technol. 2013, 54, 592–596. [Google Scholar] [CrossRef]
- Ziabakhsh Deylami, M.; Abdul Rahman, R.; Tan, C.P.; Bakar, J.; Olusegun, L. Effect of blanching on enzyme activity, color changes, anthocyanin stability and extractability of mangosteen pericarp: A kinetic study. J. Food Eng. 2016, 178, 12–19. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, X.; Lv, S.; Pan, S. Carotenoid profiling of red navel orange “Cara” harvested from five regions in China. Food Chem. 2017, 232, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Peng, Y.; Zhu, C.; Pan, S. Effect of thermal treatment on carotenoids, flavonoids and ascorbic acid in juice of orange cv. Cara. Food Chem. 2018, 265, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, A.; Cinquanta, L.; Panfili, G. Degradation of carotenoids in orange juice during microwave heating. LWT Food Sci. Technol. 2010, 43, 867–871. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N.; Raghavan, V. Plant carotenoids evolution during cultivation, postharvest storage, and food processing: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1561–1604. [Google Scholar] [CrossRef]
- Ngamwonglumlert, L.; Devahastin, S.; Chiewchan, N. Natural colorants: Pigment stability and extraction yield enhancement via utilization of appropriate pretreatment and extraction methods. Crit. Rev. Food Sci. Nutr. 2017, 57, 3243–3259. [Google Scholar] [CrossRef]
- Stolzenbach, S.; Bredie, W.L.P.; Christensen, R.H.B.; Byrne, D.V. Impact of product information and repeated exposure on consumer liking, sensory perception and concept associations of local apple juice. Food Res. Int. 2013, 52, 91–98. [Google Scholar] [CrossRef]
- Carbonell, L.; Izquierdo, L.; Carbonell, I.; Costell, E. Segmentation of food consumers according to their correlations with sensory attributes projected on preference spaces. Food Qual. Prefer. 2008, 19, 71–78. [Google Scholar] [CrossRef]
- Lee, P.Y.; Lusk, K.; Mirosa, M.; Oey, I. Effect of information on Chinese consumers’ acceptance of thermal and non-thermal treated apple juices: A study of young Chinese immigrants in New Zealand. Food Qual. Prefer. 2016, 48, 118–129. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Int. Food Res. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Rodrigo, D.; Van Loey, A.; Hendrickx, M. Combined thermal and high pressure colour degradation of tomato puree and strawberry juice. J. Food Eng. 2007, 79, 553–560. [Google Scholar] [CrossRef]
- Gössinger, M.; Moritz, S.; Hermes, M.; Wendelin, S.; Scherbichler, H.; Halbwirth, H.; Stich, K.; Berghofer, E. Effects of processing parameters on colour stability of strawberry nectar from puree. J. Food Eng. 2009, 90, 171–178. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food. Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef]
| Analysis | Juice | Fresh Sample | Processing Method | Storage Time (Months) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||||
| POD [%] | Appl-peach juice | 100 | TP 1 | <LOQ | 5.9 b ± 0.3 | 9.2 c ± 0.2 | 14.7 f ± 0.6 | 12.1 d ± 0.4 | 14.0 1 e,f ± 0.6 | 13.9 e,f ± 0.5 |
| MFP 2 | <LOQ | 4.2 a ± 0.3 | 6.8 b ± 0.1 | 12.5 d,e ± 0.5 | 17.2 g ± 0.5 | 17.0 g ± 0.4 | 17.1 g ± 0.3 | |||
| Analysis | Juice | Fresh Sample | Processing Method | Storage Time (Months) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||||
| Total phenolic content (mg/100 mL) | Apple–peach juice | 54.15 g ± 0.21 | MFP 2 | 65.07 c ± 1.07 | 60.29 e ± 0.29 | 57.03 f ± 0.55 | 54.45 g ± 0.80 | 46.57 h ± 0.63 | 47.17 h ± 0.63 | 47.02 h ± 0.43 |
| TP 1 | 74.48 a ± 1.07 | 67.35 ab ± 0.70 | 62.49 d ± 0.25 | 61.28 de ± 1.19 | 61.05 de ± 0.46 | 60.75 de ± 1.00 | 59.76 e ± 0.50 | |||
| Apple–chokeberry juice | 127.72 e ± 2.15 | MFP 2 | 152.60 a ± 3.86 | 133.63 de ± 2.82 | 135.61 d ± 2.55 | 131.97 de ± 0.78 | 121.04 f ± 3.07 | 115.13 fg ± 2.00 | 110.12 g ± 3.52 | |
| TP 1 | 165.34 ± 1.29 | 143.49 bc ± 0.61 | 144.25 b ± 3.48 | 137.73 cd ± 1.31 | 134.85 de ± 1.52 | 136.06 d ± 1.74 | 128.63 e ± 3.13 | |||
| DPPH• (µM/100 mL) | Apple–peach juice | 112.76 d ± 0.60 | MFP 2 | 183.57 ab ± 7.87 | 183.59 ab ± 4.22 | 181.80 ab ± 5.21 | 177.63 b ± 2.89 | 146.98 c ± 2.12 | 146.48 c ± 3.51 | 146.20 c ± 4.80 |
| TP 1 | 187.54 ab ± 8.97 | 189.06 a ± 1.88 | 191.29 a ± 5.07 | 188.25 a ± 3.09 | 184.60 ab ± 2.67 | 183.96 ab ± 3.28 | 181.80 ab ± 3.72 | |||
| Apple–chokeberry juice | 512.20 cd ± 10.80 | MFP 2 | 580.16 a ± 10.80 | 532.14 bc ± 4.02 | 543.71 b ± 9.60 | 526.30 bcd ± 3.49 | 480.12 e ± 1.64 | 468.90 e ± 6.74 | 431.91 f ± 1.27 | |
| TP 1 | 543.65 b ± 4.76 | 542.45 b ± 3.67 | 541.52 b ± 11.75 | 507.75 d ± 18.23 | 506.85 d ± 1.99 | 515.54 cd ± 6.17 | 481.35 e ± 6.43 | |||
| Storage Time (Months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Analysis | Juice | Fresh Sample | Processing Method | 0 | 2 | 4 | 6 | 8 | 10 | 12 |
| Total | Apple–chokeberry juice | 33.84 a ± 0.10 | 30.55 b ± 0.05 | 9.16 d ± 0.07 | 6.24 e ± 0.07 | 1.49 g ± 0.06 | 0.57 i ± 0.05 | 0.32 ij ± 0.03 | <LOQ | |
| cyanidin-3-O-galactoside | 23.89 a ± 0.03 | MFP 2 | 21.78 a ± 0.03 | 6.45 c ± 0.05 | 4.41 cd ± 0.05 | 1.06 def ± 0.03 | 0.37 f ± 0.03 | 0.21 f ± 0.02 | <LOQ | |
| cyanidin-3-O-glucoside | 1.43 a ± 0.04 | 0.89 c ± 0.01 | 0.18 d ± 0.01 | 0.11 e ± 0.01 | 0.02 g ± 0.00 | 0.02 g ± 0.00 | <LOQ | <LOQ | ||
| cyanidin-3-O-arabinoside | 8.53 a ± 0.03 | 7.88 b ± 0.01 | 2.53 d ± 0.01 | 1.72 e ± 0.02 | 0.41 h ± 0.03 | 0.18 jkl ± 0.03 | 0.11 klm ± 0.01 | <LOQ | ||
| Total | Apple–chokeberry juice | 33.84 a ± 0.10 | 29.02 c ± 0.11 | 3.71 f ± 0.04 | 1.75 g ± 0.11 | 1.17 h ± 0.03 | 1.06 h ± 0.1 | 0.96 h ± 0.06 | 0.29 ij ± 0.02 | |
| cyanidin-3-O-galactoside | 23.89 a ± 0.03 | TP 1 | 20.69 a ± 0.06 | 2.43 b ± 0.00 | 1.09 def ± 0.06 | 0.82 def ± 0.02 | 0.77 def ± 0.07 | 0.72 def ± 0.04 | 0.18 f ± 0.01 | |
| cyanidin-3-O-glucoside | 1.43 a ± 0.04 | 0.95 b ± 0.01 | 0.05 f ± 0.01 | 0.02 g ± 0.00 | 0.02 g ± 0.00 | 0.01 gh ± 0.00 | 0.01 gh ± 0.00 | <LOQ | ||
| cyanidin-3-O-arabinoside | 8.53 a ± 0.03 | 7.38 c ± 0.04 | 1.23 f ± 0.03 | 0.64 g ± 0.05 | 0.33 hi ± 0.01 | 0.28 ij ± 0.03 | 0.23 ijk ± 0.03 | 0.11 lm ± 0.01 | ||
| Storage Time (Months) | |||||||
|---|---|---|---|---|---|---|---|
| Analysis | Juice | Fresh Sample | Processing Method | 0 | 2 | 4 | 6 |
| Total | Apple–peach juice | 1.10 b ± 0.04 | MFP 2 | 1.77 a ± 0.04 | 0.65 c ± 0.04 | 0.45 d ± 0.03 | <LOQ |
| zeaxanthin | 0.19 a ± 0.01 | 0.11 b ± 0.01 | 0.09 b ± 0.01 | 0.07 b ± 0.01 | <LOQ | ||
| β-cryptoxanthin | 0.56 b ± 0.02 | 0.94 a ± 0.02 | 0.26 cd ± 0.02 | 0.19 d ± 0.01 | <LOQ | ||
| β-carotene | 0.35 b ± 0.01 | 0.72 a ± 0.01 | 0.30 c ± 0.01 | 0.19 de ± 0.01 | <LOQ | ||
| Total | Apple–peach juice | 1.10 b ± 0.04 | TP 1 | 0.69 c ± 0.06 | 0.44 de ± 0.03 | 0.42 e ± 0.04 | <LOQ |
| zeaxanthin | 0.19 a ± 0.01 | 0.12 b ± 0.02 | 0.07 b ± 0.02 | 0.07 b ± 0.02 | <LOQ | ||
| β-cryptoxanthin | 0.56 b ± 0.02 | 0.36 c ± 0.04 | 0.18 d ± 0.01 | 0.17 d ± 0.01 | <LOQ | ||
| β-carotene | 0.35 b ± 0.01 | 0.21 d ± 0.01 | 0.19 de ± 0.01 | 0.18 f ± 0.01 | <LOQ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, M.; Szczepańska-Stolarczyk, J.; Woźniak, Ł.; Jasińska, U.T.; Trych, U.; Cywińska-Antonik, M.; Kosiński, J.; Kaniewska, B.; Marszałek, K. Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale. Appl. Sci. 2024, 14, 6008. https://doi.org/10.3390/app14146008
Wójcik M, Szczepańska-Stolarczyk J, Woźniak Ł, Jasińska UT, Trych U, Cywińska-Antonik M, Kosiński J, Kaniewska B, Marszałek K. Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale. Applied Sciences. 2024; 14(14):6008. https://doi.org/10.3390/app14146008
Chicago/Turabian StyleWójcik, Marta, Justyna Szczepańska-Stolarczyk, Łukasz Woźniak, Urszula Tamara Jasińska, Urszula Trych, Magdalena Cywińska-Antonik, Jakub Kosiński, Beata Kaniewska, and Krystian Marszałek. 2024. "Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale" Applied Sciences 14, no. 14: 6008. https://doi.org/10.3390/app14146008
APA StyleWójcik, M., Szczepańska-Stolarczyk, J., Woźniak, Ł., Jasińska, U. T., Trych, U., Cywińska-Antonik, M., Kosiński, J., Kaniewska, B., & Marszałek, K. (2024). Evaluating the Impact of Microwave vs. Conventional Pasteurization on NFC Apple–Peach and Apple–Chokeberry Juices: A Comparative Analysis at Industrial Scale. Applied Sciences, 14(14), 6008. https://doi.org/10.3390/app14146008








