Improvement of Buildings’ Air Quality and Energy Consumption Using Air Purifying Paints
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of VISIONS Photo-Powder
2.2.2. Preparation of Photo-Paint
2.3. Characterization
2.4. Photocatalytic Evaluation in Terms of IAQ Improvement_Lab-Scale
2.5. Photocatalytic Evaluation in Terms of IAQ Improvement_Real-Scale
2.6. Photocatalytic Evaluation in Terms of Energy Saving_Real-Scale
3. Results
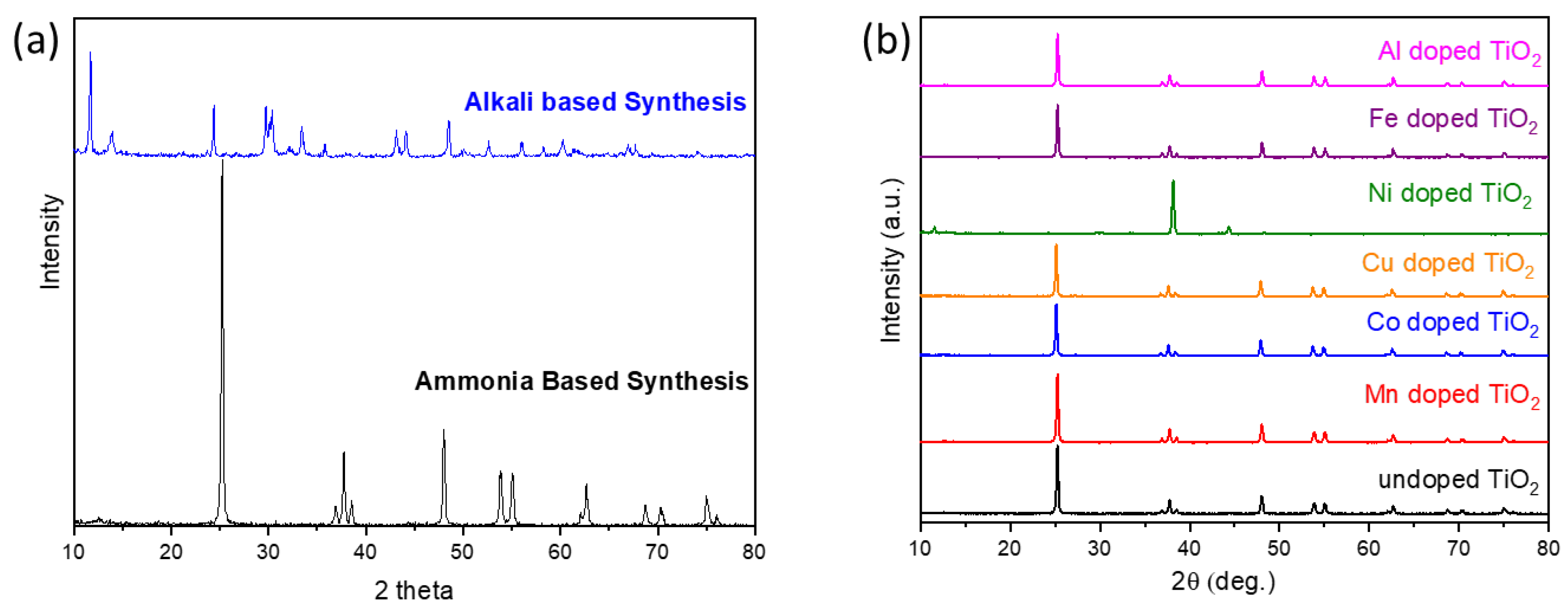
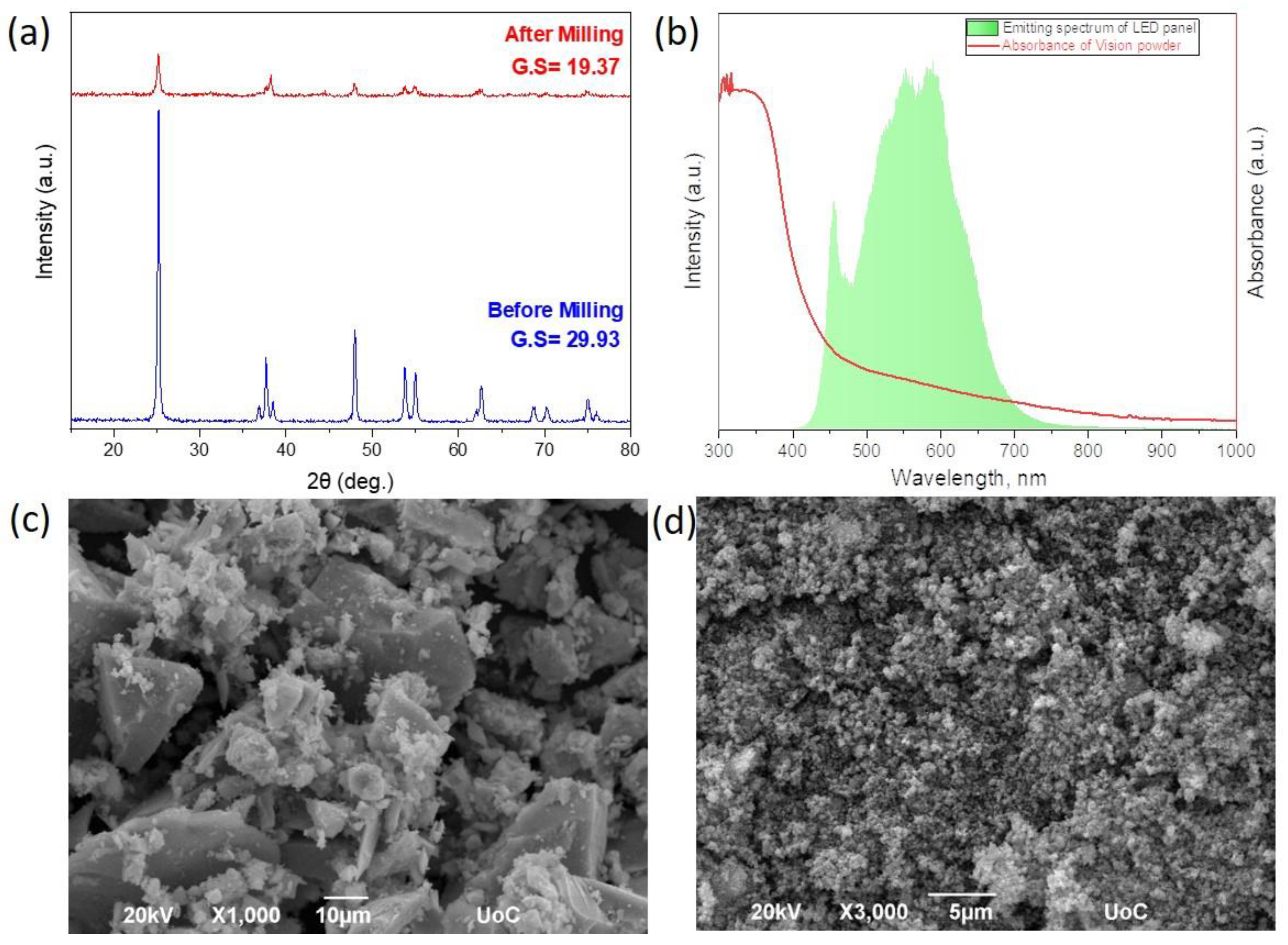
3.1. Physical and Chemical Properties
3.2. Laboratory Photocatalytic Performance of the Photo-Powder
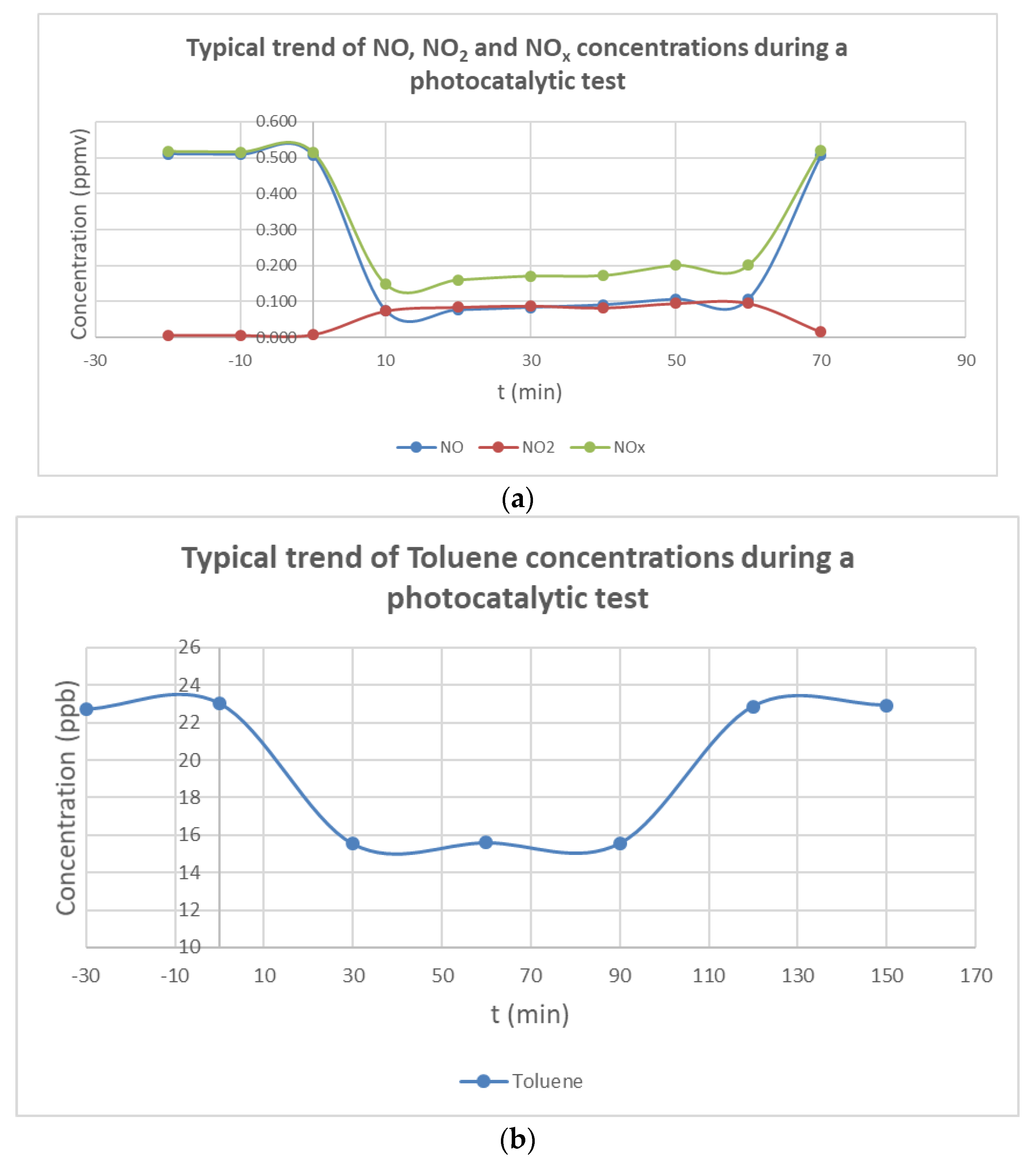
3.3. Real-Scale Photocatalytic Performance of the Photo-Paints in Air Pollutant Degradation
3.4. Real-Scale Photocatalytic Performance of the Photo-Paints in Energy Saving
4. Discussion
5. Conclusions
- Fe-doped TiO2 was prepared successfully using an easy and cost-effective co-precipitation process. In the case of Fe, doping shifted the optical absorption edge to the visible region significantly.
- The photocatalytic paint formulation has the same physicochemical and application characteristics as the commodity paints while presenting significant photocatalytic properties in terms of air pollutant elimination under real-scale application.
- The real-scale application of the photo-paint is shown with lower photocatalytic performance than the corresponding photo-powder, which was tested under a laboratory scale. The reason for the lower activity of the paint compared with powder is the polyparametric conditions (such as humidity, temperature, initial concentration, etc.) in the real-scale application.
- The ability of both organic and inorganic paints to photocatalytically remove NO was higher than toluene. The chemical structure of the pollutant plays a significant role during the oxidation reaction with the photocatalyst. Although the removal rate of toluene was lower than NO, it was very promising for IAQ applications.
- As a result of IAQ improvement, which was obtained from the application of the photocatalytic paint, the ventilation rate was reduced. Accordingly, the energy consumption in the “green” room was reduced by more than 7% compared to the “conventional” room.
- Although a real-scale application was conducted in the frame of the current work (Demo Houses), the latter was performed under controlled conditions (initial pollutant concentration, light intensity, access restrictions, etc.). To that end, there is a need for real-world building applications in order to prove the efficiency of photocatalytic paints to improve IAQ on-site. The latter is ongoing in the Hellenic Naval Academy and the results will be presented in a future manuscript.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic Oxidation Technology for Indoor Environment Air Purification: The State-of-the-Art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic Air Cleaners and Materials Technologies—Abilities and Limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F.; Blondeau, P.; Kozinski, J. Modeling and Physical Interpretation of Photocatalytic Oxidation Efficiency in Indoor Air Applications. Build. Environ. 2010, 45, 2689–2697. [Google Scholar] [CrossRef]
- Salvadores, F.; Reli, M.; Alfano, O.M.; Kočí, K.; Ballari, M.D.L.M. Efficiencies Evaluation of Photocatalytic Paints Under Indoor and Outdoor Air Conditions. Front. Chem. 2020, 8, 551710. [Google Scholar] [CrossRef] [PubMed]
- 2021 Global Status Report for Buildings and Construction Towards a Zero-Emission Efficient and Resilient Buildings and Construction Sector.Pdf. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://globalabc.org/sites/default/files/2021-10/GABC_Buildings-GSR-2021_BOOK.pdf&ved=2ahUKEwiExM6T8Y6HAxVus1YBHc3oBKUQFnoECBEQAQ&usg=AOvVaw1pfV3gOxQ4C3_NXO-s0UPr (accessed on 14 March 2024).
- BPIE. Indoor Air Quality, Thermal Comfort and Daylight. Analysis of Residential Building Regulations in Eight EU Member States.Pdf. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.bpie.eu/wp-content/uploads/2015/10/BPIE_executive_briefing_IAQ2015.pdf&ved=2ahUKEwj3sNnA8Y6HAxVKqFYBHQ2KDGkQFnoECA4QAQ&usg=AOvVaw35l0s9gEh-wXa5nNhmJLh5 (accessed on 14 March 2024).
- Shayegan, Z.; Bahri, M.; Haghighat, F. A Review on an Emerging Solution to Improve Indoor Air Quality: Application of Passive Removal Materials. Build. Environ. 2022, 219, 109228. [Google Scholar] [CrossRef]
- Directive 2010/31/EU, Off. J. Eur. Union, Vol. L153/13, No. 18.6.2010.Pdf. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://eur-lex.europa.eu/LexUriServ/LexUriServ.do%3Furi%3DOJ:L:2010:153:0013:0035:EN:PDF&ved=2ahUKEwiLsq7d8Y6HAxU7slYBHS6vCTMQFnoECBEQAQ&usg=AOvVaw0pOZGXaqo5VZyusosxfeop (accessed on 14 March 2024).
- Regulation EU No 305/2011 of the European Parliament and of the Council of 9 March 2011 Laying down Harmonised Conditions for the Marketing of Construction Products and Repealing Council Directive 89/106/EEC Text with EE.Pdf. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://eur-lex.europa.eu/LexUriServ/LexUriServ.do%3Furi%3DOJ:L:2011:088:0005:0043:EN:PDF&ved=2ahUKEwi8rbD38Y6HAxXSslYBHeg0C5IQFnoECBwQAQ&usg=AOvVaw1LrzHJNHg4WJujhH3i-TCe (accessed on 14 March 2024).
- Águia, C.; Ângelo, J.; Madeira, L.M.; Mendes, A. Influence of Photocatalytic Paint Components on the Photoactivity of P25 towards NO Abatement. Catal. Today 2010, 151, 77–83. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Indoor Air Purification by Photocatalyst TiO2 Immobilized on an Activated Carbon Filter Installed in an Air Cleaner. Chem. Eng. Sci. 2005, 60, 103–109. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Design of Graphene-Based TiO2 Photocatalysts—A Review. Environ. Sci. Pollut. Res. 2012, 19, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite–TiO2 Hybrid Composites for Pollutant Degradation in Gas Phase. Appl. Catal. B Environ. 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, S.; Zhao, S.; Li, C.; Wang, R.; Cao, D.; Liu, G. Construction of Bi-Assisted Modified CdS/TiO2 Nanotube Arrays with Ternary S-Scheme Heterojunction for Photocatalytic Wastewater Treatment and Hydrogen Production. Fuel 2022, 322, 124163. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, S.; Zhao, Y.; Deng, Y.; Yang, W.; Ye, Y.; Wang, K. Construction of Z-Scheme Bi2O3/CeO2 Heterojunction for Enhanced Photocatalytic Capacity of TiO2 NTs. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123405. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, P.; Wang, Q.; Wu, Y.; Cao, D.; Qiao, Q.-A. Construction of Bi2S3-BiOBr Nanosheets on TiO2 NTA as the Effective Photocatalysts: Pollutant Removal, Photoelectric Conversion and Hydrogen Generation. J. Colloid Interface Sci. 2021, 585, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhao, Y.; Zhang, Z.; Liao, S.; Deng, Y.; Wang, X.; Ye, Q.; Wang, K. Hydrothermal Preparation of Sn3O4/TiO2 Nanotube Arrays as Effective Photocatalysts for Boosting Photocatalytic Dye Degradation and Hydrogen Production. Ceram. Int. 2023, 49, 5977–5985. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Yu, X.; Jia, Y.; Chang, Y.; Gao, S. Morphology Regulated Bi2WO6 Nanoparticles on TiO2 Nanotubes by Solvothermal Sb3+ Doping as Effective Photocatalysts for Wastewater Treatment. Electrochim. Acta 2020, 330, 135167. [Google Scholar] [CrossRef]
- Giosuè, C.; Belli, A.; Mobili, A.; Citterio, B.; Biavasco, F.; Ruello, M.; Tittarelli, F. Improving the Impact of Commercial Paint on Indoor Air Quality by Using Highly Porous Fillers. Buildings 2017, 7, 110. [Google Scholar] [CrossRef]
- Morin, J.; Gandolfo, A.; Temime-Roussel, B.; Strekowski, R.; Brochard, G.; Bergé, V.; Gligorovski, S.; Wortham, H. Application of a Mineral Binder to Reduce VOC Emissions from Indoor Photocatalytic Paints. Build. Environ. 2019, 156, 225–232. [Google Scholar] [CrossRef]
- Gandolfo, A.; Marque, S.; Temime-Roussel, B.; Gemayel, R.; Wortham, H.; Truffier-Boutry, D.; Bartolomei, V.; Gligorovski, S. Unexpectedly High Levels of Organic Compounds Released by Indoor Photocatalytic Paints. Environ. Sci. Technol. 2018, 52, 11328–11337. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, J.; Wirtanen, L. The Influence of Photocatalytic Interior Paints on Indoor Air Quality. Atmos. Environ. 2008, 42, 4101–4112. [Google Scholar] [CrossRef]
- Salthammer, T.; Fuhrmann, F. Photocatalytic Surface Reactions on Indoor Wall Paint. Environ. Sci. Technol. 2007, 41, 6573–6578. [Google Scholar] [CrossRef]
- Guo, M.-Z. TiO2-Based Self-Compacting Glass Mortar: Comparison of Photocatalytic Nitrogen Oxide Removal and Bacteria Inactivation. Build. Environ. 2012, 53, 1–6. [Google Scholar] [CrossRef]
- Mills, A.; Burns, L.; O’Rourke, C.; Elouali, S. Kinetics of the Photocatalysed Oxidation of NO in the ISO 22197 Reactor. J. Photochem. Photobiol. A Chem. 2016, 321, 137–142. [Google Scholar] [CrossRef]
- De Melo, J.V.S.; Trichês, G. Evaluation of the Influence of Environmental Conditions on the Efficiency of Photocatalytic Coatings in the Degradation of Nitrogen Oxides (NOx). Build. Environ. 2012, 49, 117–123. [Google Scholar] [CrossRef]
- Pirola, C.; Boffito, D.C.; Vitali, S.; Bianchi, C.L. Photocatalytic Coatings for Building Industry: Study of 1 Year of Activity in the NO x Degradation. J. Coat. Technol. Res. 2012, 9, 453–458. [Google Scholar] [CrossRef]
- Cárdenas, C.; Tobón, J.I.; García, C.; Vila, J. Functionalized Building Materials: Photocatalytic Abatement of NOx by Cement Pastes Blended with TiO2 Nanoparticles. Constr. Build. Mater. 2012, 36, 820–825. [Google Scholar] [CrossRef]
- Suárez, S.; Portela, R.; Hernández-Alonso, M.D.; Sánchez, B. Development of a Versatile Experimental Setup for the Evaluation of the Photocatalytic Properties of Construction Materials under Realistic Outdoor Conditions. Environ. Sci. Pollut. Res. 2014, 21, 11208–11217. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.A.R. Are TiO2-Based Exterior Paints Useful Catalysts for Gas-Phase Photooxidation Processes? A Case Study on n-Decane Abatement for Air Detoxification. Appl. Catal. B Environ. 2014, 147, 988–999. [Google Scholar] [CrossRef]
- Le Bechec, M.; Kinadjian, N.; Ollis, D.; Backov, R.; Lacombe, S. Comparison of Kinetics of Acetone, Heptane and Toluene Photocatalytic Mineralization over TiO2 Microfibers and Quartzel® Mats. Appl. Catal. B Environ. 2015, 179, 78–87. [Google Scholar] [CrossRef]
- Huang, H. Efficient Degradation of Gaseous Benzene by VUV Photolysis Combined with Ozone-Assisted Catalytic Oxidation: Performance and Mechanism. Appl. Catal. B Environ. 2016, 186, 62–68. [Google Scholar] [CrossRef]
- Antonello, A.; Soliveri, G.; Meroni, D.; Cappelletti, G.; Ardizzone, S. Photocatalytic Remediation of Indoor Pollution by Transparent TiO2 Films. Catal. Today 2014, 230, 35–40. [Google Scholar] [CrossRef]
- Pham, T.-D.; Lee, B.-K.; Lee, C.-H. The Advanced Removal of Benzene from Aerosols by Photocatalytic Oxidation and Adsorption of Cu–TiO2/PU under Visible Light Irradiation. Appl. Catal. B Environ. 2016, 182, 172–183. [Google Scholar] [CrossRef]
- Dhada, I.; Nagar, P.K.; Sharma, M. Photo-Catalytic Oxidation of Individual and Mixture of Benzene, Toluene and p-Xylene. Int. J. Environ. Sci. Technol. 2016, 13, 39–46. [Google Scholar] [CrossRef]
- Dong, F.; Ni, Z.; Li, P.; Wu, Z. A General Method for Type I and Type II G-C3N4/g-C3N4 Metal-Free Isotype Heterostructures with Enhanced Visible Light Photocatalysis. New J. Chem. 2015, 39, 4737–4744. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-Light-Driven N−F−Codoped TiO2 Photocatalysts. 2. Optical Characterization, Photocatalysis, and Potential Application to Air Purification. Chem. Mater. 2005, 17, 2596–2602. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Y.; Xu, L.; Cao, J.; Ho, W.; Lee, S.C. Visible-Light-Active Plasmonic Ag–SrTiO3 Nanocomposites for the Degradation of NO in Air with High Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 4165–4174. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Colón, G.; Fernández-García, M. Cationic (V, Mo, Nb, W) Doping of TiO2–Anatase: A Real Alternative for Visible Light-Driven Photocatalysts. Catal. Today 2009, 143, 286–292. [Google Scholar] [CrossRef]
- Zhong, L.; Brancho, J.J.; Batterman, S.; Bartlett, B.M.; Godwin, C. Experimental and Modeling Study of Visible Light Responsive Photocatalytic Oxidation (PCO) Materials for Toluene Degradation. Appl. Catal. B Environ. 2017, 216, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Maggos, T.; Binas, V.; Siaperas, V.; Terzopoulos, A.; Panagopoulos, P.; Kiriakidis, G. A Promising Technological Approach to Improve Indoor Air Quality. Appl. Sci. 2019, 9, 4837. [Google Scholar] [CrossRef]
- EN16980-1:2021; Photocatalysis—Continuous Flow Test Methods—Part 1: Determination of the Degradation of Nitric Oxide (NO) in the Air by Photocatalytic Materials. Available online: https://standards.iteh.ai/catalog/standards/cen/e553c4c6-a577-4530-b32b-c9165aba25c3/en-16980-1-2021 (accessed on 4 July 2024).
- Binas, V.D.; Sambani, K.; Maggos, T.; Katsanaki, A.; Kiriakidis, G. Synthesis and Photocatalytic Activity of Mn-Doped TiO2 Nanostructured Powders under UV and Visible Light. Appl. Catal. B Environ. 2012, 113–114, 79–86. [Google Scholar] [CrossRef]
- Giama, E.; Chantzis, G.; Kontos, S.; Keppas, S.; Poupkou, A.; Liora, N.; Melas, D. Building Energy Simulations Based on Weather Forecast Meteorological Model: The Case of an Institutional Building in Greece. Energies 2022, 16, 191. [Google Scholar] [CrossRef]
- Giama, E. Review on Ventilation Systems for Building Applications in Terms of Energy Efficiency and Environmental Impact Assessment. Energies 2021, 15, 98. [Google Scholar] [CrossRef]
- ASTM D 562; Standard Test Method for Consistency of Paints Measuring Krebs Unit (KU) Viscosity Using a Stormer-Type Viscometer. ASTM International: West Conshohocken, PA, USA, 2018.
- ISO 2811; Paints and Varnishes—Determination of Density—Part 1: Pycnometer Method. ISO: Geneva, Switzerland, 2023.
- ASTM D1210; Standard Test Method for Fineness of Dispersion of Pigment-Vehicle Systems by Hegman-Type Gage. ASTM International: West Conshohocken, PA, USA, 2022.
- ISO 787-9; General Methods of Test for Pigments and extenders—Part 9: Determination of pH Value of an Aqueous Suspension. ISO: Geneva, Switzerland, 2019.
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated Kinetic and Photochemical Data for Atmospheric Chemistry: Volume I—Gas Phase Reactions of OX, HOX, NOX and SOX Species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere; Academic Press: Cambridge, MA, USA, 1999; ISBN 978-0-12-257060-5. [Google Scholar]
- Boonen, E.; Beeldens, A. Photocatalytic Roads: From Lab Tests to Real Scale Applications. Eur. Transp. Res. Rev. 2013, 5, 79–89. [Google Scholar] [CrossRef]
- Hunger, M.; Hüsken, G.; Brouwers, H.J.H. Photocatalytic Degradation of Air Pollutants—From Modeling to Large Scale Application. Cem. Concr. Res. 2010, 40, 313–320. [Google Scholar] [CrossRef]







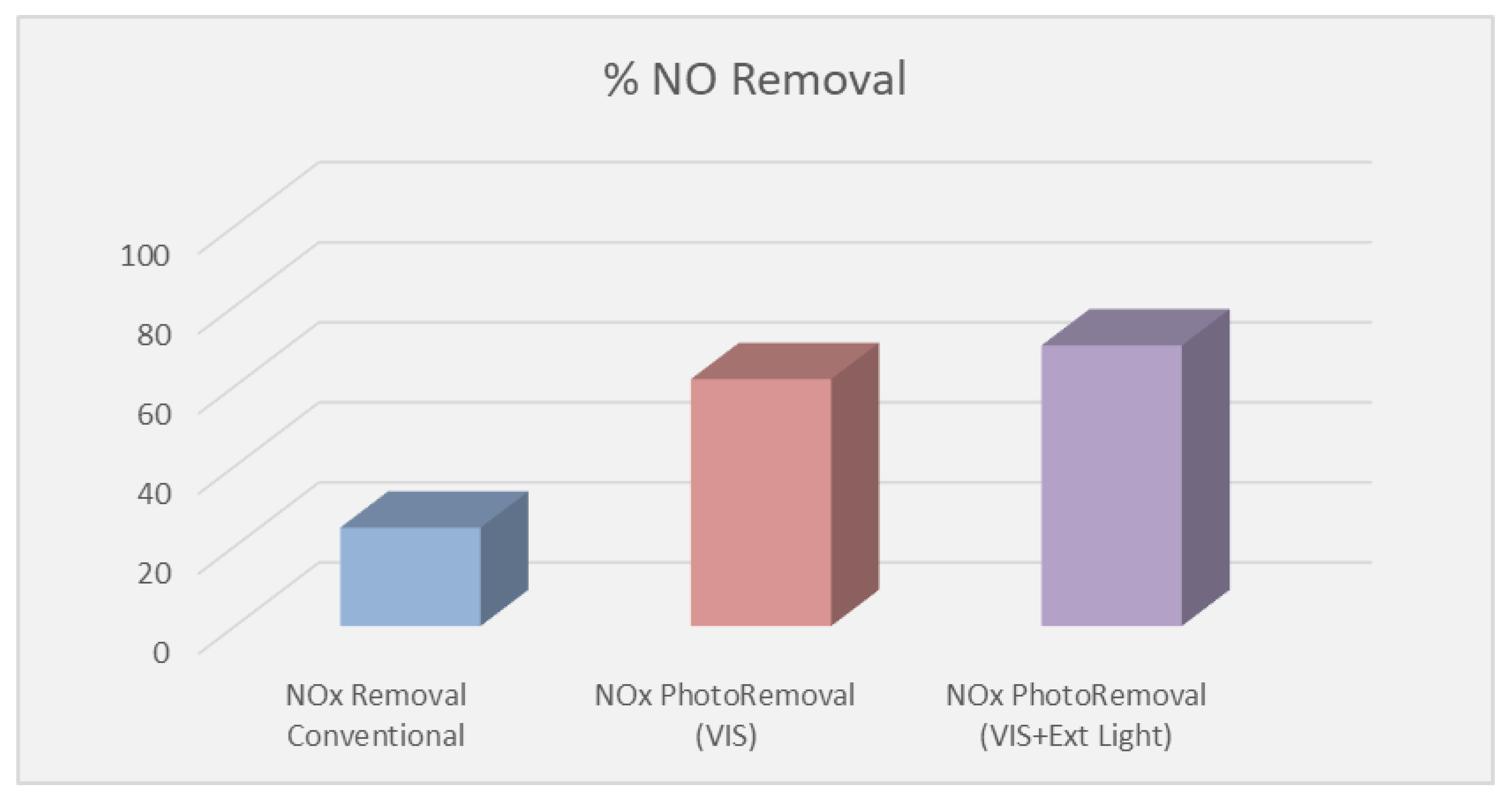
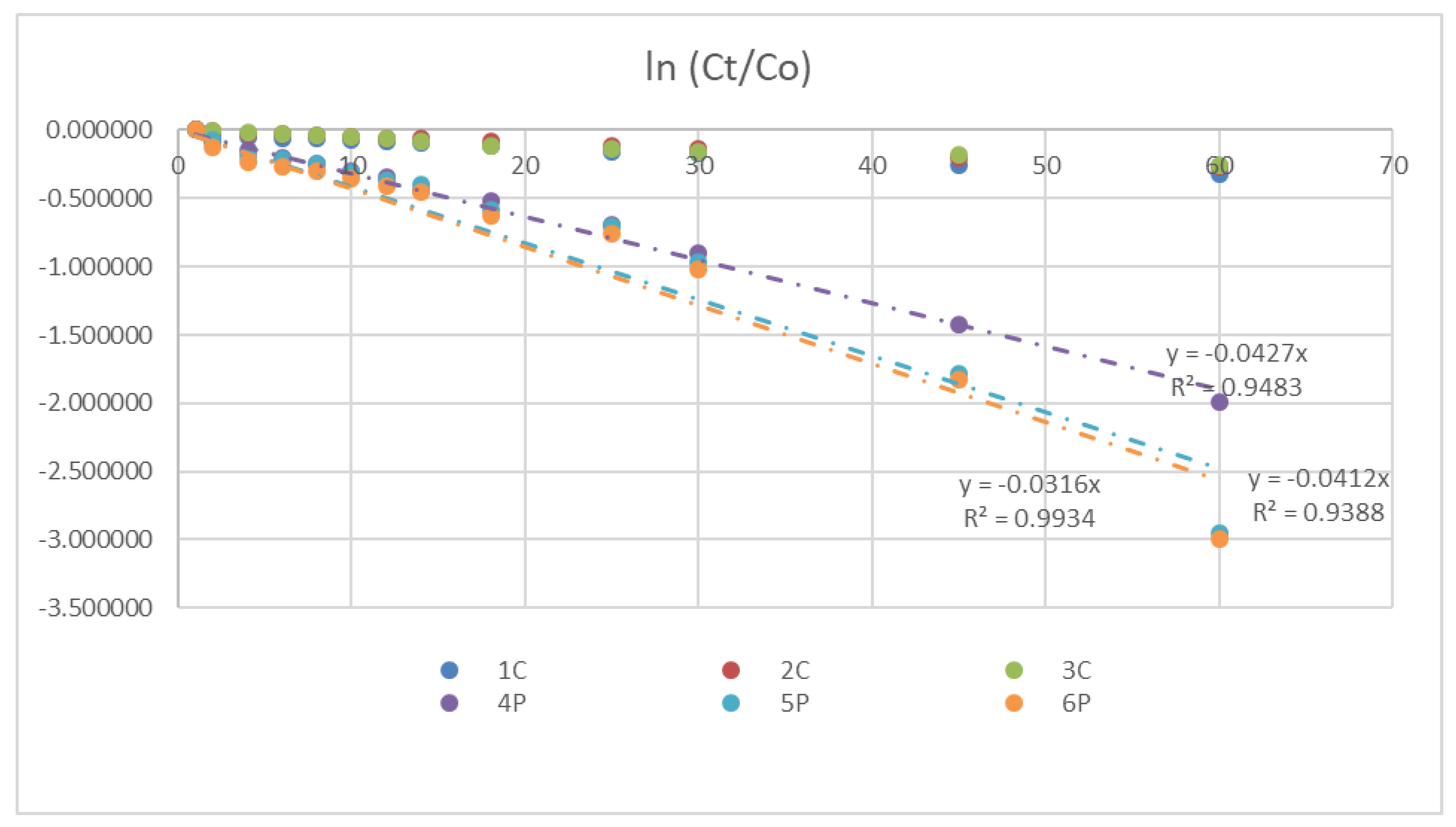
| Property | Value | Test Method |
|---|---|---|
| Viscosity at 25 °C (KU) | 95 | ASTM D 562 [46] |
| Density at 25 °C (kg/L) | 1.464 | ISO 2811 [47] |
| Fineness and dispersion | <40 μm | ASTM D1210 [48] |
| pH at 25 °C | 7.85 | ISO 787-9 [49] |
| PVC (%) | 78 | Calculated |
| Usage rate for a 50 μm dry film thickness (m2/kg) | 6.52 | Calculated |
| Parameter | Lab Scale—Photo-Powder under Vis Light | |
|---|---|---|
| NO | Toluene | |
| % η | 85.4 | 32.4 |
| r photo (μg/m2s) | 15.8 | 0.01 |
| Vd (m/s) | 0.03 | 0.0001 |
| Parameter | Real Scale—Organic Photo-Paint under Vis Light | Real Scale—Inorganic Photo-Paint under Vis Light | ||
|---|---|---|---|---|
| NO | Toluene | NO | Toluene | |
| % η | 61.7 | 5.8 | 36.8 | 2.3 |
| rphoto (μg/m2s) | 0.10 | 0.01 | 0.06 | 0.004 |
| Vd (m/s) | 0.03 | 0.001 | 0.01 | 0.0003 |
| Winter Period—One-Week Simulation Output (Energy Data in kWh) | Summer Period—One-Week Simulation Output (Energy Data in kWh) | |
|---|---|---|
| Heating | 43.23 | 0 |
| Cooling | 0 | 78.74 |
| Mech. ventilation | 34.04 | 34.04 |
| Total | 82.91 | 117.25 |
| CO2 (kg) emissions | 301.45 | 426.32 |
| Mech. ventilation (ac/h) | 1.16 | 1.16 |
| Winter Period—One-Week Simulation Output (kWh) | Summer Period—One-Week Simulation Output (kWh) | |
|---|---|---|
| Heating | 40.12 | 0 |
| Cooling | 0 | 74.44 |
| Mech. ventilation | 33.41 | 33.41 |
| Total | 79.2 | 112.32 |
| CO2 emissions | 287.98 | 408.38 |
| Mech. ventilation (ac/h) | 0.88 | 0.88 |
| Winter Period—One-Week Simulation Output (kWh) | Summer Period—One-Week Simulation Output (kWh) | |
|---|---|---|
| Heating | 38.18 | 0 |
| Cooling | 0 | 71.83 |
| Mech. ventilation | 33.16 | 33.16 |
| Total | 77.05 | 109.46 |
| CO2 emissions | 280.17 | 397.98 |
| Mech. ventilation (ac/h) | 0.72 | 0.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maggos, T.; Binas, V.; Panagopoulos, P.; Skliri, E.; Theodorou, K.; Nikolakopoulos, A.; Kiriakidis, G.; Giama, E.; Chantzis, G.; Papadopoulos, A. Improvement of Buildings’ Air Quality and Energy Consumption Using Air Purifying Paints. Appl. Sci. 2024, 14, 5997. https://doi.org/10.3390/app14145997
Maggos T, Binas V, Panagopoulos P, Skliri E, Theodorou K, Nikolakopoulos A, Kiriakidis G, Giama E, Chantzis G, Papadopoulos A. Improvement of Buildings’ Air Quality and Energy Consumption Using Air Purifying Paints. Applied Sciences. 2024; 14(14):5997. https://doi.org/10.3390/app14145997
Chicago/Turabian StyleMaggos, Thomas, Vassiliοs Binas, Panagiotis Panagopoulos, Evangelia Skliri, Konstantinos Theodorou, Aristotelis Nikolakopoulos, George Kiriakidis, Effrosyni Giama, Georgios Chantzis, and Agis Papadopoulos. 2024. "Improvement of Buildings’ Air Quality and Energy Consumption Using Air Purifying Paints" Applied Sciences 14, no. 14: 5997. https://doi.org/10.3390/app14145997
APA StyleMaggos, T., Binas, V., Panagopoulos, P., Skliri, E., Theodorou, K., Nikolakopoulos, A., Kiriakidis, G., Giama, E., Chantzis, G., & Papadopoulos, A. (2024). Improvement of Buildings’ Air Quality and Energy Consumption Using Air Purifying Paints. Applied Sciences, 14(14), 5997. https://doi.org/10.3390/app14145997









