Featured Application
In this work, cheese whey, which is a by-product of the cheese industry, was used to obtain lactic acid by biotechnological processes (using strains isolated from the same cheese whey). Lactic acid has several uses in the cosmetic, food, medical, textile, and manufacturing industries. Therefore, while lactic acid can have multiple uses, we want to use it as a raw material for biopolymers for the manufacturing of bioplastics in future research.
Abstract
Cheese whey is a byproduct of the cheese industry that causes high levels of pollution in the environment, but its high lactose content means that it can be used as a source to obtain lactic acid. In this study, two strains, one belonging to a yeast and the other one to a bacteria (Kluyveromyces lactis and Enterococcus faecalis), were isolated from cheese whey and molecularly characterized, and the optimal growth conditions were determined. Then, using proteinized and deproteinized cheese whey, batch fermentation was carried out with the strains arranged in suspension and immobilized. The consumption of lactose and the production of lactic acid were measured through Brix degrees and acidity analysis. Afterwards, the lactic acid was purified, and its yield and physical and chemical characteristics were determined. It was proven that there were differences between each of the strains; arranged in free or encapsulated cells, the proteinized and deproteinized cheese wheys, under the same purification conditions, achieved different yields, colors, and densities of lactic acid. Immobilized Enterococcus faecalis had the highest yield (50.61 ± 34.94 g/L) using the deproteinized cheese whey compared to the immobilized Kluyveromyces lactis (35.70 ± 0.15 g/L) using the proteinized cheese whey.
1. Introduction
In the production of cheese, a by-product is generated, which is a yellowish liquid called cheese whey that is composed of proteins, lactose, lipids, and mineral salts [1]. In Latin America, cheese whey is usually used as food for animals or discarded without any treatment, and due to its composition, it generates a huge amount of contamination in the environment from processes such as water eutrophication, soil contamination, etc. [2]. Furthermore, considering that from 9 to 10 L of cheese whey is generated from 1 kg worth of cheese production and that it is typically discarded without any treatment [1], it is important to see what other uses it can be given. Thus, in some studies, cheese whey has been used for conversion into biofuels and as a fermentation substrate for the biotechnological production of fermented beverages, foods, etc., being recognized as a source of bioactive and functional compounds such as proteins and peptides [1]. It is essential to mention that cheese whey, due to its high lactose composition (44–46 g L−1) [1], can be used for other applications, such as the biotechnological production of lactic acid.
Lactic acid is a product of high importance due to its wide applications, mainly in the pharmaceutical, cosmetic, chemical, and food industries [3]; in addition, there is currently a high demand to produce polylactic acid (PLA), which is a biopolymer that is classified as environmentally friendly. So, lactic acid has increased in demand as a raw material to produce polylactic acid (PLA) [4]. Lactic acid is an organic acid that can be acquired naturally by extraction from milk sugar or synthetically by extraction from cane, grape, or starch sugar [5]. Its price in the market varies according to its application and is estimated to increase from 1220 kilotons in 2016 to 1960.1 kilotons by 2025; it is used most widely in medicine and cosmetics in Latin America and Asia [6].
Lactic acid production can be synthetically obtained either by fermentation or chemically. The fermentation production process has gained more interest because it has the advantage of obtaining lactic acid with pure isomers of L (+) or D (−); additionally, it is cheaper because of the low cost of renewable raw materials, its low energy consumption, and its easy operating conditions [4]. For the synthesis of lactic acid through fermentation, lactic acid bacteria (LAB) are used, which include Lactobacillus, Enterococcus, and Streptococcus, which are capable of consuming cheese whey to produce lactic acid as a result of lactose fermentation. Among the Lactobacillus strains are Lactobacillus helveticus, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus casei, and Lactobacillus acidophilus, among others, and within of the genus Enterococcus there are Enterococcus faecalis, Enterococcus lactis, and Enterococcus camelliae, among others. Depending on the species of microorganisms used, a D (−), L (+), or DL lactic acid configuration can be generated. However, some species of yeast that ferment lactose have also been seen, including E. coli, K. lactis, and S. cerevisiase, as well as fungi such as Rhizopus oryzae [6]. Regarding their environmental conditions, it has been seen that the majority of microorganisms grow at low fermentation temperatures (less than 45 °C) that favor contamination by other species. In addition, bacteria, yeasts, and fungi have distinct advantages and disadvantages when used in the production of lactic acid [6].
Lactic acid bacteria (LAB) are Gram-positive bacteria, with cocci and/or coccobacilli, and they are catalase-negative and usually aerotolerant; that is, they can be strict anaerobes or microaerobes, acid-tolerant, and non-spore-forming [7,8]. From them, Lactobacillus, Streptococcus, and Enterococcus are the most studied for lactic acid production, with the most abundant strain being the genus Lactobacillus. However, Enterococcus are Gram-positive bacteria, cocci, and facultative anaerobes [9] that are clustered in short chains, pairs, or individual cocci; they are considered some of the producers of lactic acid and are homofermentative, and they have been isolated from different human, animal, and fermented food sources [10]. Additionally, they are also considered among the alkaliphilic LAB that produce LA at high pH values between 7 and 11.5 [6]. Enterococcus even generate bacteriocins or antimicrobial peptides, and it has been seen that they also have immunomodulatory properties [10]. However, they are also associated with opportunistic infections such as those in the central nervous system, urinary tract, endocarditis, and abdomen, but since they do not have strong virulent factors or toxins, their limited use is sometimes tolerated in the food industry [7,10]. Regarding the production of lactic acid, Enterococcus faecalis AG5 has been shown to be used for the production of lactic acid [10] in addition to Enterococcus lactis, Enterococcus camelliae, and Weissella paramesenteroides [11].
Regarding yeasts, they have an advantage over LAB in that at low pH, they are very stable; accordingly, with yeasts, we can avoid using neutralizing agents [6]. Among yeasts, there is Kluyveromyces lactis, a yeast that can carry out fermentation under anoxic and aerobic conditions; additionally, it is capable of the consumption of lactose [12] and it is usually employed to produce proteins [13], organic acids, and ethanol [12]. Kluyveromyces lactis has been seen to generate high-cell-density lactic acid by using low-cost carbon sources such as lactose and cheese whey and, due to its ease of genetic manipulation, it has also been modified to produce biopolymers such as hyaluronic acid [14] and to increase its production of lactic acid [6]. In the fermentation process, immobilized or suspended cells can be used. The immobilization process is known to allow for increasing cell retention and cell density in bioreactors, to diminish the microbial latency phase, to improve its tolerance at high sugar concentrations, to have a better pH control, and to generate greater productivity because the purification process is easier than fermentation with suspended cells. The fermentation can be carried out with immobilized or suspended cells. The immobilization of microorganisms has the following advantages: it allows for increasing cell retention and density in bioreactors, the microbial latency phase is diminished, it has a high tolerance at sugar concentrations, the pH control is better, and it has more productivity due to the purification processes being easier [9]. To immobilize LAB strains, sodium alginate, calcium chloride, polyethylene amine, and plastics have been used, and they acted as supports for the production of lactic acid that was used in batch fermentations for more than 126 days with Enterococcus faecalis and Enterococcus hirae [9]. Additionally, this advantage has also been observed when immobilized strains of Kluyveromyces lactis generated an increase in lactose removal efficiency that was 3.4 times greater than when they were used as suspended cells [15].
Therefore, in this research, it was proposed to obtain lactic acid through the batch fermentation of cheese whey with its native strains (Kluyveromyces lactis and Enterococcus faecalis) and to determine its influence on the production and characteristics of lactic acid. Likewise, we not only sought to obtain lactic acid but also to evaluate the influence of the use of native strains that were encapsulated in alginate beads and suspended on obtaining lactic acid and on its yield and physicochemical characteristics. So, this research aims to give added value to cheese whey in obtaining lactic acid, and at the same time, the lactic acid could be a source for obtaining polylactic acid (PLA), which is used as a raw material for manufacturing biodegradable plastics, considering that plastics currently generate serious pollution due to their long delay in degradation, which can be up to more than 100 years.
Thus, it was shown in this research that there is a difference between obtaining lactic acid using LAB such as Enterococcus faecalis and the yeast Kluyveromyces lactis. However, in both cases, their immobilization favored obtaining a greater performance than when they were arranged freely. The greatest generation of lactic acid was achieved with immobilized strains of Enterococcus faecalis at 5% CaCl2 and 3% sodium alginate for 1 h of stabilization, reaching up to 50.61 ± 34.94 g/L of lactic acid with a fermentation pH that varied between 3.5 and 5 using deproteinized cheese whey as a culture medium without nutritional supplements. Meanwhile, with Kluyveromyces lactis, it was determined that the lactic acid production was favorable at an acidic fermentation pH with deproteinized cheese whey, reaching up to 35.70 ± 0.15 g/L for 168 h. In addition, differences were observed in their physical properties, such as their density and color, as well as in the lactic acid purification performance and in the FTIR spectra, which can provide different properties and, therefore, uses.
2. Materials and Methods
2.1. Isolation and Biochemical and Molecular Identification
LAB and yeast strains were isolated from cheese whey from the cheese industry located in the city of Arequipa (Peru). An agar MRS medium (Man, Rogosa, and Sharpe medium), acquired from the Merck company, was prepared, autoclaved, and plated on plates, and a cheese whey sample was spread by exhaustion. The growth conditions were micro anaerobiosis (in an anaerobic chamber) at 32 °C for 48 h in a Witeg incubator, model WIG-50. The colonies of interest were replicated until isolated, and Gram staining was carried out. A catalase essay (3% H2O2) was carried out, and the isolated strains were molecularly identified by PCR in the Uchumayo DNA Laboratory (Peru), where genomic DNA was extracted from the samples. The taxonomy marker genes were amplified using PCR technology (RNAr16S for bacteria and the ITS gene for yeast), and the amplicons were sequenced by the Sanger method. These sequences were compared with the NCBI database using the “BLAST ®” blast suite 2.13.0+ software.
2.2. Determination of Optimal Microorganism Growth Conditions
It was important to know the optimal growth conditions of both strains, so the growth temperature was tested at 4, 8, 20, 25, 29, 32, 34, 35, 36, 37, and 45 °C, with the pH at 4, 7, and 9, and with growth inhibition by increasing the saline concentration (NaCl) at 1, 2, 4, 6, 8, 10, 12, and 13%.
2.3. Batch Fermentation
2.3.1. Batch Fermentation with Strains in Suspension Using Enterococcus faecalis and Kluyveromyces lactis
Batch fermentation was carried out using inactivated strains (due to the fact that using activated strains in the fermentation process did not result in a higher LA production in both samples) and untreated (proteinized) and treated (deproteinized) cheese. In order to deproteinize the cheese whey (to eliminate not only the proteins but also the fats), it was put into an autoclave at 121 °C and 1.03 bar pressure for 15 min. After it was passed through a 60 sieve (0.050 nm) to eliminate the majority of the proteins, it was centrifuged at 4500 RPM for 10 min to finally go through vacuum filtration.
The fermentation culture medium was composed of 0.5% p/v yeast extract, 0.9676% p/v ammonium phosphate, a neutralizer (5 g/L calcium carbonate), and 200 mL of deproteinized and proteinized cheese whey, which was mixed and placed in a water bath at 65 °C for 10 min. It was cooled and inoculated with a separately native strain (3 spades). The fermentation parameters were as follows: 32 °C and 120 RPM for 7 days.
The proteinized cheese whey used in the fermentation process had the following physicochemical characteristics: 0.45% acidity, 0.50% ash, 3.41% lactose, 0.81% proteins, 0.85% fats, and a pH of 5. Meanwhile, the deproteinized cheese whey had the following characteristics: 0.38% acidity, 0.52% ashes, 3.57% lactose, 0.04% proteins, 0.38% fats, and a pH of 4.84.
2.3.2. Batch Fermentation with Strains Immobilized Using Enterococcus faecalis and Kluyveromyces lactis
Batch fermentation using strains immobilized in alginate beads was carried out in deproteinized and proteinized cheese whey. The culture medium was composed only of the deproteinized and proteinized cheese whey without adding other additives. This was due to the fact that in preliminary tests, it was observed that the alginate beads lost their stability, so it was decided to use the cheese whey without any additives.
To produce the alginate beads, each strain was cultivated individually in MRS broth at 32 °C and 120 RPM for 24 h. Then, to concentrate the bacteria in a pellet, a volume of this broth was taken and centrifuged at 4500 RPM for 20 min. Then, this pellet was washed three times with a saline solution that was discarded, and 1 mL of saline solution was added to the biomass pellet to dissolve it. After the biomass solution was mixed in a 2.5% sodium alginate solution (1 mL of biomass in 25 mL of sodium alginate) and then drip-added to a 4% CaCl2 solution for 1 h for its stabilization, and these conditions were for Kluyveromyces lactis. This was carried out with 5% CaCl2 and 3% sodium alginate solution for 1 h of stabilization for Enterococcus faecalis.
The calcium chloride solution was prepared and placed in a beaker on a magnetic stirrer at 120 RPM. Then, the biomass solution was added to a burette and dropped into the beaker with the calcium chloride solution, and when all the mixture passed, it was left in contact for one hour h for its stabilization. Finally, the beads were washed with sterile distilled water to remove excess calcium ions before use, for which the calcium chloride was recovered. Table 1 shows the fermentation systems using batch fermentation with suspended cells and immobilized cells.

Table 1.
Coding of the proposed fermentation systems to obtain lactic acid.
2.4. Fermentation Monitoring
Lactic acid was monitored through acidity analysis, via the pH variation in an OAKTON ion 2700 pH meter, and via the lactose concentration.
2.4.1. Acidity Analysis
A known volume from the fermentation broth was titrated with a 0.1 N NaOH solution and 2 drops of phenolphthalein (an indicator solution). This is a way to determine lactic acid production.
2.4.2. Lactose Consumption
The lactose consumption was measured in Brix degrees, which is equivalent to 1 g of solute (lactose) in 100 g of solution. For this, 1 mL of the fermentation medium was taken and centrifuged at 4500 RPM for 10 min in order to eliminate the solids and make the strains precipitate. The suspended solution was recovered, and a few drops of the solution were placed in the refractometer for measurement.
2.5. Lactic Acid Purification and Characterization
Lactic acid purification was carried out on the samples that had the highest LA yield, i.e., ES1, KS2, EI1, and KI1, but each of them went through two different lactic acid purification processes (P1: purification process number 1 and P2: purification process number 2), as shown in Table 2.

Table 2.
Coding of the proposed fermentation systems and purification conditions for obtaining lactic acid.
After the fermentation process was finished, 15 mL was taken into falcon tubes to be centrifugated at 4500 RPM for 20 min. Later, the supernatant was vacuum-filtered using a Whatman 42 filter paper and passed through a vacuum microfiltration process using a 47 mm membrane filter. The filtered sample was taken to a rotary evaporator at 95 RPM with the temperature and time shown in Table 2 with the aim of concentrating the lactic acid. Then, to extract the lactic acid, a known volume of sample and solvent (diethyl ether) in a 1:1 ratio were added into a 500 mL separating funnel. Later, it was shaken vigorously and left to rest until the two phases differentiated. These two phases were separated; the clearest and most transparent one was taken to a rotary evaporator at atmospheric pressure and at 40 °C to evaporate the diethyl ether and thus concentrate and purify the lactic acid. Table 2 depicts the coding of the samples according to the fermentation and purification conditions to obtain lactic acid.
These samples were analyzed in the Chemistry Laboratory of the National University of San Agustin (Arequipa, Peru) by infrared spectroscopy (FTIR) analysis according to the ASTM E1252-98 [16] “Standard Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis”, with the wave number range from 650 cm−1 to 4000 cm−1.
3. Results and Discussion
3.1. Characteristics of the Strains Isolated
There were two isolated strains that belonged to a LAB, one that had a 100% similarity with the rRNA16S of Enterococcus faecalis, and its phylogenetic analysis indicated that it was Enterococcus faecalis. The second strain was a yeast that presented a 100% similarity with the ITS gene of Kluyveromyces lactis, and its phylogenetic analysis indicated that it was Kluyveromyces lactis. Their characteristics are depicted in Table 3.

Table 3.
Characteristics of strains isolated from cheese whey (Enterococcus faecalis and Kluyveromyces lactis).
3.2. Determination of Optimal Bacterial Growth Conditions
The optimal growth conditions at several temperatures (4, 8, 20, 25, 29.32, 34, 35, 36, 37, and 45 °C), the pH (4, 7, and 9), and the growth inhibition caused by increasing the saline concentration (NaCl) at 1, 2, 4, 6, 8, 10, 12, and 13% for both strains are shown in Table 4.

Table 4.
Optimal bacterial growth conditions of the strains isolated from cheese whey (Enterococcus faecalis and Kluyveromyces lactis).
The optimal growth temperatures were determined to be 32 °C for Enterococcus faecalis and Kluyveromyces lactis, and they were also neutrophilic and alkalophilic. It is important to note that Kluyveromyces lactis has a greater tolerance to acidic pH, which has been detailed as an advantage of yeasts over LAB [6]. Enterococcus spp. are considered along with other bacteria as alkaliphilic LAB, that is, they can grow at 7.0–11.5 pH [6]. This high tolerance of Kluyveromyces lactis was also observed against salinity, reaching up to 12% compared to 4% for Enterococcus faecalis, which corroborates the greater resistance to extreme conditions of yeasts compared to bacteria.
3.3. Batch Fermentation with Strains in Suspension and Immobilized
3.3.1. Batch Fermentation with Strains in Suspension Using Enterococcus faecalis and Kluyveromyces lactis
Figure 1 shows the fermentation with Enterococcus faecalis in suspension using the deproteinized cheese whey (ES1) and cheese whey without deproteinized (ES2). It was observed that there were differences in the lactic acid yield using both cheese whey samples and, in both cases, when the pH decreased, there was a greater generation of lactic acid. With the deproteinized cheese whey, the generation of lactic acid was low given that its pH tends toward neutrality, and the presence of fats, proteins, and other components of the cheese whey probably influenced the behavior of the strains and therefore the production of lactic acid. This behavior was clearly observed, since the maximum value of lactic acid obtained was 13.09 ± 1.54 g/L, which occurred after 48 h of fermentation with the lowest pH value (4.90 ± 0.16) with the deproteinized cheese whey. However, raising the pH inhibited the generation of lactic acid. Using Enterococcus isolated from cheese whey to obtain lactic acid has been demonstrated in another study that achieved up to 15.93 ± 0.13 g/L and 15.43 ± 0.03 g/L levels of lactic acid using proteinized cheese whey and nutritional additives, respectively [9].
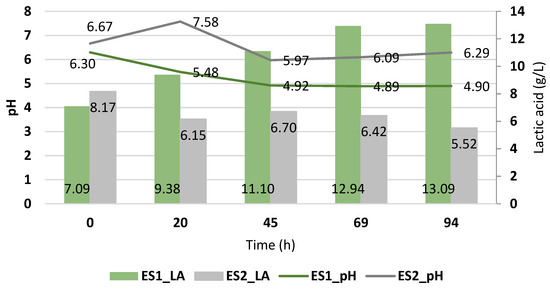
Figure 1.
Lactic acid yield (g/L) of Enterococcus faecalis and its pH variation during the cheese whey fermentation.
Figure 2 shows the lactose consumption against the production of lactic acid. It was observed that when using the deproteinized cheese whey (ES1), there was a greater reduction in the lactose concentration, reaching up to 2.80 ± 0.0 °Brix with an acidity of 13.09 ± 1.54 g/L of lactic acid, starting from 8.10 ± 0.0 °Brix. Meanwhile, with the proteinized cheese whey (ES2), although there was a decrease in lactose, there was no generation of lactic acid. This was due to the pH medium, which was in the neutrophilic range, which has been seen to disfavor the production of lactic acid. In addition, it contained other components that may have affected the assimilation of lactose, such as proteins and fats. This behavior of Enterococcus faecalis is similar to other works, since it has been shown that with a pH of between 4 and 5.79, good yields of lactic acid were obtained [11]. In another study at pH 5, a high yield of lactic acid was also obtained using Enterococcus faecalis and Enterococcus hirae from cheese whey [9]. However, there is another study whose highest production of lactic acid (4.5 mg/mL) was determined at pH 3.5 and 37 °C in MRS broth using Enterococcus isolated from the rectal area of rats [10]. Additionally, a strain of Enterococcus faecalis isolated from food remains produced 19.5 g/L of lactic acid at 37 °C and at a pH of between 6 and 8, achieving the highest production at pH 7 [17]. These results show that Enterococcus can produce lactic acid but under certain conditions, depending on the source of isolation. Therefore, for Enterococcus faecalis isolated from cheese whey, using deproteinized cheese whey provides more favorable conditions for the production of lactic acid; that is, the composition of cheese whey and the pH conditions affect the metabolism of Enterococcus faecalis.
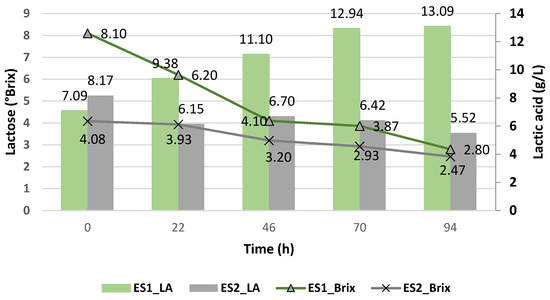
Figure 2.
Lactic acid yield (g/L) of Enterococcus faecalis and its lactose consumption during the cheese whey fermentation.
Figure 3 shows the production of lactic acid with the yeast Kluyveromyces lactis using the deproteinized cheese whey (KS1) and non-deproteinized or proteinized cheese whey (KS2). For this case, it was observed that the highest production of lactic acid was generated with the proteinized cheese whey (KS2), reaching up to 12.45 ± 0.91 g/L at 47 h and at pH 5. Meanwhile, with the deproteinized cheese whey (KS1), the production of lactic acid was restricted by the pH value, which in this case tended to neutrality, a range that has been seen to influence the production of lactic acid. Therefore, for Kluyveromyces lactis, an acidic pH favors the production of lactic acid, which is consistent with other works, where Kluyveromyces lactis produced 4.70 g/L of lactic acid at 30 °C for 36 h of fermentation [18]. So, it has been shown that at the lowest pH, the greatest generation of lactic acid occurred, and when it rose, the production of lactic acid tended to decrease, a behavior also observed by Enterococcus faecalis.
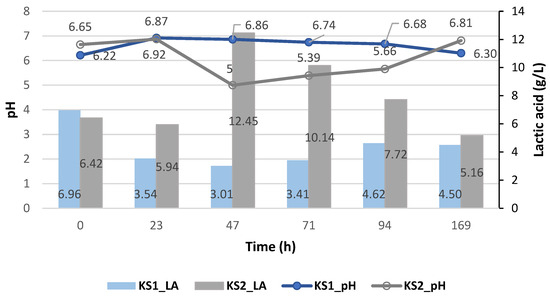
Figure 3.
Lactic acid yield (g/L) of Kluyveromyces lactis and its pH variation during the cheese whey fermentation.
Figure 4 shows the lactose consumption against the production of lactic acid with Kluyveromyces lactis, and it was observed that although the deproteinized cheese whey (KS1) had a higher concentration of lactose, the least amount of lactic acid was generated, while with the proteinized cheese whey (KS2), it had a lower amount of lactose but generated a greater amount of lactic acid, which was due to the effect of the pH.
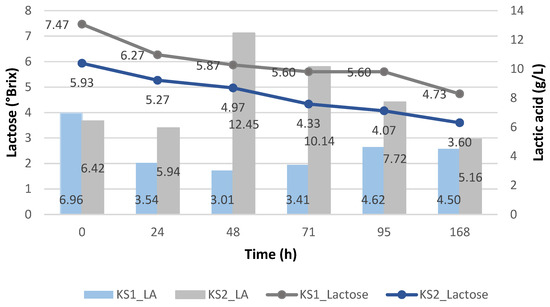
Figure 4.
Lactic acid yield (g/L) of Kluyveromyces lactis and its lactose consumption during the cheese whey fermentation.
In both strains, when the lactose was most consumed, the highest yield of lactic acid was obtained. Therefore, the use of proteinized and deproteinized cheese whey and the pH medium influenced the lactose metabolism of the yeast Kluyveromyces lactis as well as that of Enterococcus faecalis and, hence, the production of lactic acid.
3.3.2. Batch Fermentation with Strains Immobilized Using Enterococcus faecalis and Kluyveromyces lactis
The batch fermentation results using immobilized Enterococcus faecalis and using the deproteinized cheese whey (EI1) and proteinized cheese whey (EI2) are shown in Figure 5.
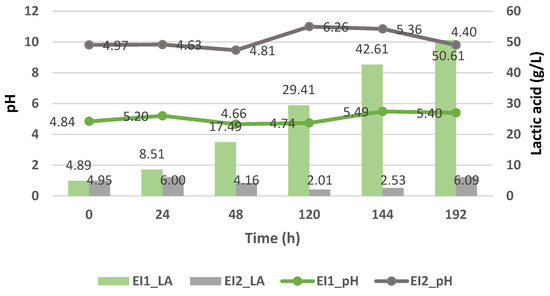
Figure 5.
Lactic acid yield (g/L) of Enterococcus faecalis and its pH variation during the cheese whey fermentation.
As observed in the previous case of the suspended strains, the immobilized strains did not generate a high production of lactic acid because of their pH, which tended toward neutrality. As in the case of the proteinized cheese whey (EI2) with Enterococcus faecalis, it reached a value of 6.09 g/L of lactic acid at pH 4.40, with a drastic drop in lactic acid (2.01 g/L) at pH 6.26. Meanwhile, with the deproteinized cheese whey (EI1), a higher production of lactic acid was generated, reaching values of up to 50.61 ± 34.94 g/L at a pH ranging from 4.84 to 3.61.
Therefore, the optimal pH for growth would be between 3.5 and 5 for Enterococcus faecalis, since at that pH range, a greater yield of lactic acid was obtained, unlike when the proteinized cheese whey was used.
Hence, the influence of the pH on the generation of lactic acid was confirmed, since it affected two aspects of microbial metabolism, namely, the metabolism of enzymes and the transport of nutrients within cells [19].
It should be noted, as shown in Figure 6, that regarding the lactose consumption with the immobilized Enterococcus faecalis, if the data on the lactose consumption in both cases are taken, there was a greater level of consumption when using the proteinized cheese whey (from 6 to 2.67 °Bx) than with the deproteinized cheese whey (from 5.07 to 2.47 °Bx). This was probably due to the pH medium, which was not favorable for an optimal environment for further lactose metabolism to occur.
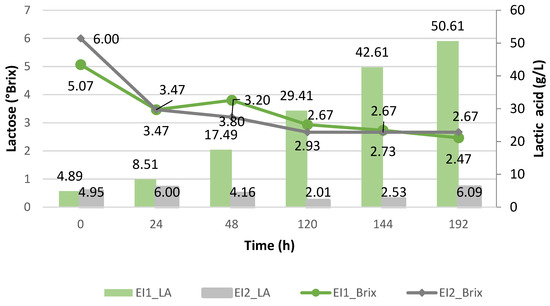
Figure 6.
Lactic acid yield (g/L) of Enterococcus faecalis and its lactose consumption during the cheese whey fermentation.
If we examine the amount of lactic acid obtained with the immobilized Enterococcus faecalis and only with the deproteinized cheese whey as a substrate, it had the highest lactic acid yield compared to the suspended Enterococcus faecalis in both the proteinized and deproteinized cheese whey. This behavior was also observed with the Enterococcus strains isolated from cheese whey, which obtained a level of 41 ± 0.4 g/L at 96 h of incubation when using the encapsulated Enterococcus compared to using them without encapsulation, where they obtained 16.27 ± 0.09 g/L [9]. Therefore, it can be inferred that the isolated strain of Enterococcus faecalis generated a greater production of lactic acid using the immobilized strains and deproteinized cheese whey.
Figure 7 shows the production of lactic acid using the immobilized Kluyveromyces lactis and using the deproteinized cheese whey (KI1) and proteinized cheese whey (KI2). Like the Enterococcus faecalis strain, when using the immobilized Kluyveromyces lactis, the highest generation of lactic acid (35.70 ± 0.15 g/L) was obtained when the pH decreased and when the deproteinized cheese whey was used, while when the pH increased and the proteinized cheese whey was used, the lactic acid generated was quite limited.
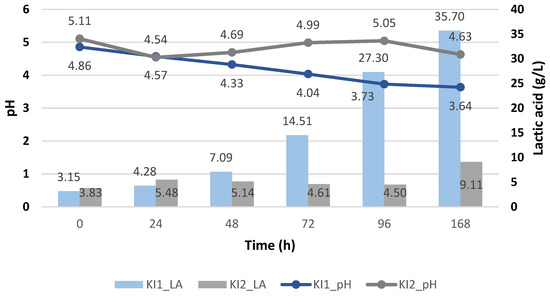
Figure 7.
Lactic acid yield (g/L) of Kluyveromyces lactis and its pH variation during the cheese whey fermentation.
Therefore, it is corroborated that an acidic pH favored the metabolism of lactose and the production of lactic acid using the Kluyveromyces lactis, since at pH 3.64, it obtained the highest value of lactic acid, unlike Enterococcus faecalis, where the pH tended to become more acidic and the production of lactic acid was interrupted. This advantage is not only observed over Enterococcus but also over other strains like Lactobacillus casei, Enterococcus lactis, Enterococcus camelliae, and Weissella paramesenteroi, where at pH 4, the least generation of lactic acid occurred, at pH 5, it increased and rose to its maximum at pH 6 and 7, but at pH 8, it decreased again when using the deproteinized cheese whey, with Lactobacillus casei being the one that generated the highest lactic acid content, generating 44.25 g/L after 10 h of fermentation [11].
Figure 8 shows the lactose consumption of the immobilized Kluyveromyces lactis, demonstrating higher amounts for the deproteinized cheese whey (KI1), achieving up to 2.07 °Brix, compared to using the proteinized cheese whey (KI2). Hence, when we used the proteinized cheese whey without any supplements (KI2), the lactose metabolism and its uptake were affected, despite the fact that the pH values were lower than 5.
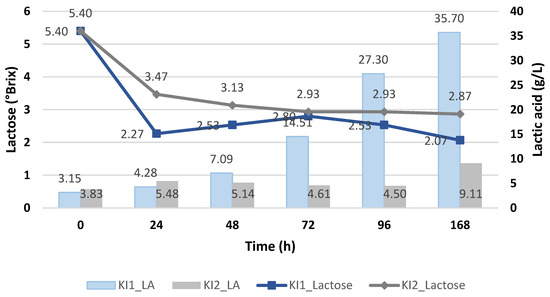
Figure 8.
Lactic acid yield (g/L) of Kluyveromyces lactis and its lactose consumption during the cheese whey fermentation.
These results allow us to conclude that if we use suspended Kluyveromyces lactis, we have to use proteinized cheese whey with nutritional additives, but for deproteinized cheese whey, no other additives are required, and the Kluyveromyces lactis strain must be immobilized, with the latter being more favorable since it would reduce costs.
Therefore, the stability of immobilized Kluyveromyces lactis and Enterococcus faecalis when generating lactic acid in acidic conditions is favored, since one of the advantages of immobilization is tolerance to extreme conditions, high sugar concentrations, improved pH control, greater productivity, and the facilitation of purification processes [9].
Therefore, the immobilization of Kluyveromyces lactis and Enterococcus faecalis significantly favored the production of lactic acid, and this encourages the use of Kluyveromyces lactis and Enterococcus faecalis, since they are used to produce recombinant proteins in large-scale industry [13]. Therefore, the use of suspended and immobilized strains in a culture medium with treated (deproteinized) and untreated (proteinized) cheese whey and with the addition of nutritional supplements affects lactose metabolism and, hence, lactic acid production.
3.4. Lactic Acid Purification and Characterization
An analysis of the purification conditions was carried out, since the use of temperatures in the purification process under normal conditions and without pressure is inefficient when recovering lactic acid from a fermentation broth; therefore, the optimal temperature and time were determined in order to concentrate the lactic acid.
Table 5 shows how the use of these temperatures influenced the purification performance of the lactic acid as well as the characteristics of the lactic acid obtained (color and density). Among the samples that were purified, the one with the highest yield (16.92%) belonged to ES1_P2 (Enterococcus faecalis in suspension and with the lactic acid concentrated at 40 °C for 1 h in a rotary evaporator). This was followed by KI1_P1 (immobilized Kluyveromyces lactis and with the lactic acid concentrated at 40 °C for 5 h in a rotary evaporator) with a yield of 8.44%. The third place corresponded to EI1_P2 (immobilized Enterococcus faecalis treated at 45 °C for 5 h to concentrate the lactic acid) with an 8.33% yield. Likewise, a difference in color was observed, obtaining whitish and yellowish colors, and this also occurred in lactic acid obtained by homofermentative strains and purified by a microfiltration process [20]. However, it has been mentioned that when using nanofiltration, a lighter color is obtained, and when using reverse osmosis, a transparent color is obtained. In addition to this, a higher concentration of lactic acid is obtained [1]. Likewise, using Lactobacillus casei at 40.2 °C without any nutritional additives and after 3 days of fermentation, it produced a yellowish lactic acid with a density of 1.10 g/mL [5].

Table 5.
Lactic acid characteristics obtained from Enterococcus faecalis and Kluyveromyces lactis.
Therefore, it is confirmed how the fermentation conditions, strains used in the fermentation, and mode of use (suspended and encapsulated strains) generate variation in the yield of the lactic acid obtained as well as in its color and density.
Likewise, these differences were also observed in the chemical characteristics of the FTIR spectra that were obtained for the purified lactic acid samples that are shown in Figure 9 and Figure 10 and belong to those obtained using Enterococcus faecalis and Kluyveromyces lactis, respectively.
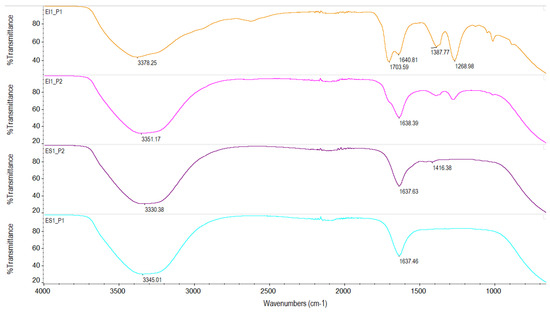
Figure 9.
FTIR spectra of lactic acid obtained by Enterococcus faecalis in suspension (ES) and immobilized (EI). ES1_P1: deproteinized cheese whey, concentrated at 60 °C for 45 min; ES1_P2: deproteinized cheese whey, concentrated at 40 °C for 1 h; EI1_P1: deproteinized cheese whey, concentrated at 40 °C for 5 h; and EI1_P2: deproteinized cheese whey, concentrated at 45 °C for 5 h.
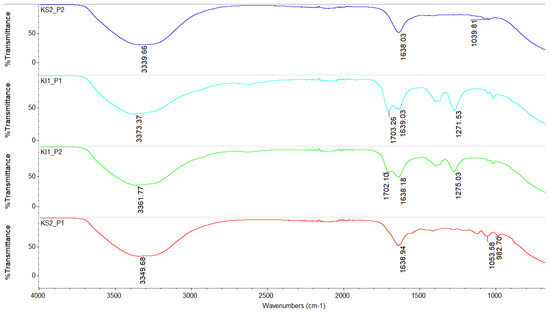
Figure 10.
FTIR spectra of lactic acid obtained by Kluyveromyces lactis in suspension (KS) and immobilized (KI). KS2_P1: proteinized cheese whey, concentrated at 40 °C for 5 h; KS2_P2: proteinized cheese whey, concentrated at 45 °C for 5 h; KI1_P1: deproteinized cheese whey, concentrated at 40 °C for 5 h; and KI1_P2: deproteinized cheese whey, concentrated at 45 °C for 5 h.
Figure 9 shows the results for the ES1_P1 and ES1_P2 samples using the deproteinized cheese whey with Enterococcus faecalis in suspension at the purification conditions depicted in Table 5. It is observed that the thick band in both spectral regions between 3000 and 3500 cm−1 corresponded to OH, while the sharp peak at 1637.63 cm−1 indicated the presence of C=O, characteristic functional groups of lactic acid. Furthermore, by varying the purification conditions, the signal was accentuated, and a peak corresponding to the C-H group (1416.38 cm−1) appeared slightly. This variation in and value of the carboxyl group allowed us to presume that both corresponded to L-lactic acid, and in this research, there was also the presence of a peak corresponding to C=O at around 1651.07 cm−1 [21], but this variation in the peaks, unlike with pure lactic acid, indicates that there were probably some impurities.
Also, Figure 9 shows the spectra of the EI1_P1 and EI1_P2 samples, which corresponded to the lactic acid obtained from the deproteinized cheese whey with immobilized Enterococcus faecalis. A characteristic OH peak was also obtained at 3351.17 cm−1 (EI1_P2) and at 3378.25 cm−1 (EI1_P1), and that of the C=O group was also obtained at 1703.59 cm−1 and 1640.81 cm−1 (EI1_P1) and at 1638.39 cm−1 (EI1_P2). The EI1_P1 sample had a peak at 1268.98 cm−1 that corresponded to the C-O-C group, and this same group was present in EI1_P2 sample but at a lower intensity (1277.22 cm−1). Neither of the peaks appeared in the lactic acid obtained with the strains in suspension. Between these two samples, the EI1_P1 sample was selected, which had a peak at 1703.59 cm−1, since this peak is characteristic of the C=O group of lactic acid, so it probably had a higher purity of 85% lactic acid. This was due to the characteristic peaks at 1723 cm−1 (C=O) and 1120 cm−1 of the C-O group [22].
Figure 10 shows the results of the lactic acid using Kluyceromyces lactis, and, as with the previous strain, there was a difference when fermenting with the suspended and immobilized strains [23], with more peaks appearing when using the immobilized strains. In all cases, the presence of the characteristic peak of the OH group was observed in the range from 3000 to 3500 cm−1 and at 1702.10 cm−1 and 1703.26 cm−1, which corresponded to C=O for KI1_P2 and KI1_P1, respectively. However, for both cases, a peak was observed at around 1638 cm−1 that corresponded to C=O, which indicates that there were impurities in the lactic acid. In terms of the C=O peaks, these last peaks probably corresponded to the DL-lactic acid monomer, since the peaks are like those indicated by Nikolic et al. [24]. Among both samples, the KI1_P2 sample could be considered the one with the highest purity, since it tended to have a sharper peak at 1702.10 cm−1 and had a higher yield. Regarding the KS2_P1 and KS2_P1 samples, the peaks that belonged to OH were 3349.68 (KS2_P1) and 3339.66 (KS2_P2). There was a difference between them in the range from 1700 to 650 cm−1, where there were peaks for the KS2_P1 sample that belonged to C=O at 1638.94 cm−1, C-O at 1053.58, and 982.70 cm−1, while for the KS2_P2 sample, there were only two peaks at 1638.03 cm−1 (C=O group) and at 1039.81 cm−1 (C-O group).
It is important to mention that in the present work, what was indicated by Orozco Olivarez [20] was also observed, who stated that the purification step of lactic acid in proteinized cheese whey samples is more difficult due to the lack of deproteinization and prior filtration, which was also observed in the purification stage. Additionally, not using encapsulated strains makes the filtration stage slower than when using immobilized strains. Therefore, the use of deproteinized or non-deproteinized cheese whey, as well as using suspended or immobilized strains, favors the case in which the purification of lactic acid is delayed and lactic acid is generated with different physical and chemical characteristics.
4. Conclusions
Two strains were isolated from cheese whey, one that corresponded to the LAB Enterococcus faecalis, which has an optimal growth temperature of 32 °C, is neutrophilic and alkalophilic, and has a tolerance to salinity up to 4% NaCl. The second strain belonged to the yeast Kluyveromyces lactis, which has an optimal growth temperature of 32 °C, tolerance to acidic pH and mainly to neutral and alkaline pH, and a high resistance to salinity, reaching a tolerance of up to 12%, which gives it an advantage against extreme environments.
The Enterococcus faecalis had a greater generation of lactic acid in the deproteinized cheese whey, both in suspension and immobilized. The highest generation of lactic acid was achieved with the immobilized strains, achieving up to 50.61 ± 34.94 g/L of lactic acid with a fermentation pH that varied between 3.5 and 5 using the deproteinized cheese whey as culture medium without any nutritional supplements. The Enterococcus faecalis suspension strain produced 13.09 ± 1.54 g/L of lactic acid at pH 4.90, while when the pH increased, a decline in the generation of lactic acid was observed.
The immobilized Kluyveromyces lactis showed a greater tolerance to acidic pH and the highest production of lactic acid (35.70 ± 0.15 g/L) at 168 h and pH 3.64 using the proteinized cheese whey. The lactic acid production when the strain was suspended was favorable at an acidic pH and when using the proteinized cheese whey, reaching a value of up to 12.45 ± 0.91 g/L at 47 h.
The optimal purification conditions were determined, and the highest yield of lactic acid after the purification process was 16.92%, which was obtained with Enterococcus faecalis in suspension using the deproteinized cheese whey (purified at 40 °C for 1 h). Meanwhile, for Kluyveromyces lactis, the highest yield was 8.44% using the deproteinized cheese whey and the immobilized strains and when it was concentrated at 40 °C for 5 h. It was verified through FTIR analysis that all the samples obtained corresponded to lactic acid, with some variation in the peaks being observed at the different fermentation and purification conditions.
In conclusion, although Kluyveromyces lactis showed a greater resistance to acidic pH, Enterococcus faecalis had the highest performance in obtaining and purifying lactic acid. It was determined there is a difference when using deproteinized and proteinized cheese whey, in the strain used (Enterococcus faecalis and Kluyveromyces lactis), in the disposition of the strain (in suspension and immobilized), in the yield, and in the physicochemical characteristics of the lactic acid, which could probably affect their probable uses.
Author Contributions
Conceptualization, J.A. and C.G.-A.; methodology, J.E.B.-D.-C. and M.V.; validation, D.T.-Q. and F.R.; formal analysis, J.A.; investigation, M.V. and J.A.; resources, J.E.B.-D.-C. and C.G.-A.; data curation, F.R.; writing—original draft preparation, M.V.; writing—review and editing, F.R. and J.A.; visualization, C.G.-A.; supervision, C.G.-A.; project administration, D.T.-Q.; funding acquisition, C.G.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Catholic University of Saint Mary, grant number 26617-R-2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and we have published a previous article [23]: “https://avestia.com/NewTech2023_Proceedings/files/paper/ICBB/ICBB_108.pdf (accessed on 1 August 2023).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Treu, L.; Tsapekos, P.; Peprah, M.; Campanaro, S.; Giacomini, A.; Corich, V.; Kougias, P.G.; Angelidaki, I. Microbial profiling during anaerobic digestion of cheese whey in reactors operated at different conditions. Bioresour. Technol. 2019, 275, 375–385. [Google Scholar] [CrossRef]
- Eş, I.; Khaneghah, A.M.; Barba, F.J.; Saraiva, J.A.; Sant’Ana, A.S.; Hashemi, S.M.B. Recent advancements in lactic acid production—A review. Food Res. Int. 2018, 107, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Komesu, A.; de Oliveira, J.A.R.; da Silva Martins, L.H.; Maciel, M.R.W.; Filho, R.M. Lactic Acid Production to Purification: A Review. Bioresources 2017, 12, 4364–4383. [Google Scholar] [CrossRef]
- Tixicuro, J.M.F.; Chanfrau, J.M.P.; de Céspedes, I.S.S.; Fiallos, M.V.L.; Pérez, J.N. Optimización estadística de un bioproceso de ácido láctico a partir de lactosuero. Cienc. Lat. Rev. Científica Multidiscip. 2021, 5, 3259–3274. [Google Scholar] [CrossRef]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production–producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Lactic acid bacteria as starter cultures: An update in their metabolism and genetics. AIMS Microbiol. 2018, 4, 665–684. [Google Scholar] [CrossRef]
- Veerapagu, M.; Jeya, K.R. Evaluation of probiotic characteristics of bacteria isolated from fermented foods. Pharma Innov. J. 2017, 6, 322–325. [Google Scholar]
- Dosuky, A.S.; Elsayed, T.R.; Yousef, E.T.; Barakat, O.S.; Nasr, N.F. Isolation, identification, and application of lactic acid-producing bacteria using salted cheese whey substrate and immobilized cells technology. J. Genet. Eng. Biotechnol. 2022, 20, 26. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ghosh, A.R. Characterization of Functional, Safety, and Probiotic Properties of Enterococcus faecalis AG5 Isolated From Wistar Rat, Demonstrating Adherence to HCT 116 Cells and Gastrointestinal Survivability. Probiotics Antimicrob. Proteins 2018, 10, 435–445. [Google Scholar] [CrossRef]
- Sayed, W.F.; Salem, W.M.; Sayed, Z.A.; Abdalla, A.K. Production of lactic acid from whey permeates using lactic acid bacteria isolated from cheese. SVU-Int. J. Vet. Sci. 2020, 3, 78–95. [Google Scholar] [CrossRef]
- Marcus, J.F.; Demarsh, T.A.; Alcaine, S.D. Upcycling of whey permeate through yeast-and mold-driven fermentations under anoxic and oxic conditions. Fermentation 2021, 7, 16. [Google Scholar] [CrossRef]
- Spohner, S.C.; Schaum, V.; Quitmann, H.; Czermak, P. Kluyveromyces lactis: An emerging tool in biotechnology. J. Biotechnol. 2016, 222, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.M.V.; Netto, J.H.C.M.; Carvalho, L.S.; Parachin, N.S. Heterologous Hyaluronic Acid Production in Kluyveromyces lactis. Microorganisms 2019, 7, 294. [Google Scholar] [CrossRef]
- Yeo, I.S.; Yoon, Y.J.; Seo, N.; An, H.J.; Kim, J.H. Biopurification of oligosaccharides by immobilized Kluyveromyces lactis. Appl. Sci. 2019, 9, 2845. [Google Scholar] [CrossRef]
- ASTM E1252-98; Standard Practice for General Techniques for Obtaining Infra-red Spectra for Qualitative Analysis. ASTM: West Conshohocken, PA, USA, 2021.
- Yuan, S.F.; Hsu, T.C.; Wang, C.A.; Jang, M.F.; Kuo, Y.C.; Alper, H.S.; Guo, G.L.; Hwang, W.S. Production of optically pure l(+)-lactic acid from waste plywood chips using an isolated thermotolerant Enterococcus faecalis SI at a pilot scale. J. Ind. Microbiol. Biotechnol. 2018, 45, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Hun, C.H.; Sueb, M.; Abd Malek, R.; Othman, Z.; Elsayed, E.A.; Ramili, S.; Elmarzugi, N.A.; Sarmidi, M.R.; Aziz, R.; El Enshasy, H.A. Bioprocess Development for High Cell Mass Production of the Probiotic Yeast-Kluyveromyces lactis. IOSR J. Pharm. Biol. Sci. 2013, 8, 49–59. [Google Scholar] [CrossRef]
- Bahry, H.; Abdalla, R.; Pons, A.; Taha, S.; Vial, C. Optimization of lactic acid production using immobilized Lactobacillus Rhamnosus and carob pod waste from the Lebanese food industry. J. Biotechnol. 2019, 306, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Orozco, F. Producción de Ácido Láctico por Medio de Fermentación Anaerobia y su Polimerización a Partir de Reacciones de Apertura de Anillo. Master’s Thesis, Centro de Investigación Científica de Yucata, Mérida, Mexico, 2011. Available online: https://cicy.repositorioinstitucional.mx/jspui/bitstream/1003/1333/1/PMP_M_Tesis_2011_Fatima_Orozco_Olivarez.pdf (accessed on 22 April 2024).
- Karande, R.D.; Abitha, V.; Rane, A.V.; Mishra, R.K. Preparation of Polylactide from Synthesized Lactic Acid and Effect of reaction Parameters on Conversion. J. Mater. Sci. Eng. Adv. Technol. 2015, 12, 1–37. [Google Scholar] [CrossRef]
- Mazo, P.; Rios, L.A.; Restrepo, G. Síntesis de poli ácido láctico y poli ricinoleato empleando calentamiento por microondas y su utilización en la producción de termoplasticos de poliuretano. Polimeros 2011, 21, 83–89. [Google Scholar] [CrossRef][Green Version]
- Vargas, M.; Gordillo-Andia, C.; Tupayachy-Quispe, D.; Almirón, J.; Roudet, F. Influence of Kluyveromyces lactis Arranged in Suspension and Immobilized on Obtaining Lactic Acid by Cheese Whey Fermentation. In Proceedings of the 9th World Congress on New Technologies, London, UK, 9–11 August 2023. [Google Scholar]
- Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M. Novel microwave-assisted synthesis of poly(D,L-lactide): The influence of monomer/initiator molar ratio on the product properties. Sensors 2010, 10, 5063. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).