Abstract
Soil microorganisms play an important role on plant development and the homogenization of soil microbiomes is harmful to agri-environments. It is essential that agricultural practices are carried out by taking soil microbiome preservation in consideration. Agroforestry systems are one of the most environmentally friendly agrosystems and its plant diversity directly influences the soil microbiome diversity. In this study, we tested the efficacy of the microbial consortium (MC) obtained from compost and the cyanobacteria Arthrospira platensis (Ap) compared with the application of the vermicompost tea (VT) and bokashi (Bk) in arugula, lettuce, beetroot, and carrot in two seasons in a recently implemented agroforestry system. We aimed to verify if MC and Ap could be new promising sustainable alternatives in vegetables production. The strategy can be broken down into three stages: (1) Green manure management: planting, cutting, griding, and incorporation in the soil, (2) agroforestry system implementation, and (3) treatment application in a completely randomized blocks design. The vegetables yield was measured. Nutritional traits and the plant root system were evaluated for arugula and lettuce. Greater plant yield, nutritional values, and plant root development were observed in the MC-treated plants; Ap and Bk had, in general, similar results. Our data show that both MC and Ap have potential to become a sustainable product for agricultural production.
1. Introduction
Agricultural practices have been intensified over the last few decades, causing harmful impacts on the environment [1]. However, it is known how much conventional agriculture contributes to global warming due to the inadequate management of natural assets such as soil and water, associated with greenhouse gas emissions [2]. With the justification that it is necessary to increase food production due to population growth [3], there has been no concern about the depletion of our natural resources, such as soil and water, which are fundamental for all types of life on the planet.
Agroforestry systems (AFS) are one of the most environmentally friendly agrosystems, where perennial woody plants (trees, shrubs, palm trees) are grown in association with herbaceous plants, agricultural and/or forage crops, and/or with animals in the same area and can be planted all at once or in a time sequence, with a high diversity of species and ecological interactions favouring a more efficient recycling of nutrients and being able to restore soil health [4,5]. The trees, with deep roots, recapture and pump back leached nutrients from the biomass deposited in the soil by falling leaves. Pruning branches and residues from annual crops improves the supply of nutrients and, in addition to the root exudates, favours the action of beneficial microorganisms in the soil [6,7].
Several microorganisms can interact with roots and promote plant growth [8,9]. These microorganisms have been studied for decades and biotechnological products have been developed using plant growth promoting microorganisms (PGPM) in agriculture as inoculants. This biotechnology is very important to Brazilian agriculture [10]. In maize, for example, it is possible to replace 25% of N chemical fertilizers by inoculation with Azospirillum brasilense in the country, saving USD 15 ha−1 and avoiding the emission of 236 kg of CO2e ha−1 [11]. In soybeans, the replacement is 100% using Bradyrhizobium spp. [12]. There is research on inoculation in horticulture, but not so extensive as far as it pertains to crops [12].
Mira et al. (2021) [13] isolated a microbial consortium from compost and inoculated it in arugula, carrots, and radishes, obtaining great results on these three cultures. This microbial consortium differs from the conventional inoculants since it is inoculated in a microbial community and not an isolated strain; it has potential to become a commercial inoculant, but more tests should be done. Another type of PGPM tested in horticulture is cyanobacteria due to their ability to fix atmospheric nitrogen, solubilise phosphorus, and promote plant growth [14,15]. Also, they have potential to be applied as soil conditioners since they can improve soil structure and water retention [16] as well as the availability of nutrients [17,18].
In horticulture, it is common to use organic fertilizers to improve productivity in a sustainable way. Vermicompost tea (worm tea) is a leachate obtained below the vermicomposting heap that has been used as organic fertilizer [19,20]. Bokashi is an organic fertilizer with a high content of organic matter and nutrients, a high porosity, and a high water retention capacity [21]. In addition, it contributes to soil fertility with different types of microorganisms such as fungi and bacteria, increasing life in the soil [22,23].
We tested the efficacy of the microbial consortium presented by Mira et al. (2021) [13] and the cyanobacteria Arthrospira platensis compared with the application of the vermicompost tea and bokashi in arugula, lettuce, beetroot, and carrot in an agroforestry system implemented in an area that was used as a construction debris deposit. Based on the literature and our previous pilot trials, we hypothesized that both the microbial consortium and A. platensis could show good results in vegetable development, creating the potential for a new inoculant development for horticulture.
There are many methodologies applied for soil recovery and a fertility increase, such as technosol [24], reforestation [25], and bioremediation [26], but with the objective being more focused on human being interests rather than ecosystem restoration. A study similar to ours was recently carried out by Theodoro et al. 2021, who adopted the combined use of stonemeal and agroforestry systems technologies. Bicca et al. (2023) [27] studied the addition rates of basaltic breccia and agricultural gypsum on clay stabilization and the nutrient release of a Planosol. However, in addition to applying a different methodology, combining green manure techniques, planting in an agroforestry system, and using inoculants, the objective of our work was the recovery of soil biodiversity and soil physicochemical characteristics. Our focus was to obtain evidence of restoration of biodiversity and functional groups. It is known that the recovery of original biodiversity is almost a utopia, in addition to being an extremely slow process; however, we can help with biological restoration, using strategies that are closer to natural activities such as planting in an agroforestry system and applying inoculants that are a consortium of many microorganisms involved in nutrient cycling.
2. Materials and Methods
2.1. Site Description and Area Preparation
The current study was conducted at the Social Organization Célio Lemos (Obra Social Célio Lemos) in São José dos Campos, State of São Paulo, Brazil. This occurred from April 2021 to August 2022, at the coordinates 23°11′21″ S and 45°52′17″ W, 596 m altitude. The study area presented a history of being used for disposing construction debris; also, one cinnamon and one eucalyptus trees were present there. The soil was classified as Dystrophic Red-Yellow Latosol according to the Brazilian classification system [28]. The climate was classified as Cwa (humid subtropical) according to Köppen–Geiger classification.
Firstly, the area was cleaned by removing the rubble using a truck and cutting the cinnamon and eucalyptus trees. A 00-10 cm soil sample, composed of 15 soil subsamples, was collected on 27 May 2021 and sent to Ribersolo Laboratory in Ribeirão Preto, São Paulo State, Brazil, for determination of soil chemical and granulometric initial properties (Table 1). By using a garden bedder and tilling the soil with a tiller, 10 garden beds spaced by 1 m, with 1 m × 16 m of size each, were built. No chemicals were applied to the soil. Using rakes, some rocks from the rubble remaining in the soil were removed.

Table 1.
Soil chemical properties before starting the experiments.
A mix of seeds from green manure plants was sown by broadcast sowing in the soil that was previously prepared on August, 2021. The mix included: Avena strigosa (7.0 g/m2), Lupinus albus (6.5 g/m2), Cajanus cajan (4.0 g/m2), Crotalaria juncea (3.2 g/m2), Helianthus annus (2.0 g/m2), and Raphanus sativus (1.0 g/m2). The seeds were mixed with garden soil (an amount equivalent to 100 g/m2 of the total area). On October 2021, the plants were cut, crushed, and placed on the garden beds for covering. Additionally, plant material from tree pruning carried out by the city hall was donated for the project and used for soil covering. The weight of the plant material laid in the soil was not measured, but it was sufficient to form a layer of approximately 5 cm of litter. This material was laid on the top of the beds and between them. The study can be divided into three stages: (1) area preparation and cover crop cultivation, (2) first experimental cycle, and (3) second experimental cycle.
2.2. Experimental Design
The experiment was conducted in completely randomized blocks with five treatments: (C) the control; (VT) 0.5 L/m2 of vermicompost tea diluted in a proportion of 1:15; (Ap) 0.5 L/m2 of Artrhospira platensis cultivated in VT; (MC) 0.5 L/m2 of microbial consortium obtained from compost (1.2 × 109 cells/mL) [13]; (Bk) 1.0 kg/m2 of Bokashi. Each block consisted of two rows of garden bed that were 16 m2; each plot had 2 m of length spaced by 1 m length from each other. A space of 1.0 m of length in the ends of the garden beds was considered (Figure 1a). The vegetables were planted at the same time in a consortium, including pepper, a single plant row spaced 1 m apart; lettuce, spaced by 0.3 m × 0.3 m; arugula, six plants spaced by 0.15 m between the pepper and lettuce row; carrot seeds, which were applied in a row with 0.15 m from two rows of lettuce; and beetroot, six plants spaced by 0.15 m between the lettuce row and pepper (Figure 1b). All plants were planted by seedlings, except the carrots, which were planted by seeds. Around 5 days after the emergence, carrots were thinned to 6 plants per row. For the forestry component, two garden beds (one in block 2 and other in block 4) received some plants and trees: Sphagneticola trilobata, Gliricidia sp., Musa paradisiaca, Ricinus communis, Diospyros kaki, Caesalpina pluviosa, Tabebuia roseo-alba, Mangirefa indica, Melia azedarach, Cajanus cajan, and Citrus sp. The experiment was conducted for the duration of two planting and harvesting cycles. The first started on October 2021 and the second on May 2022.
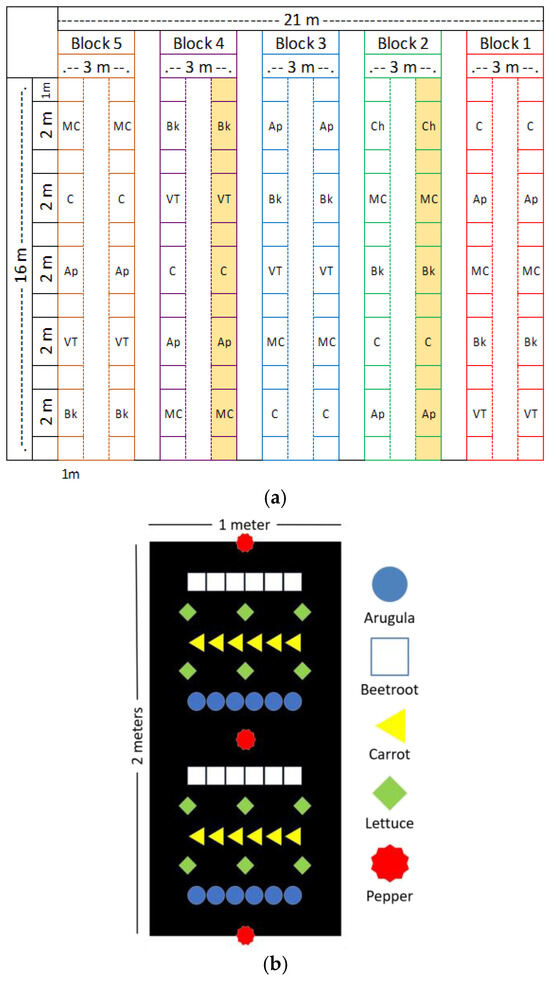
Figure 1.
(a) Experimental design croquis and (b) vegetables arrangement in a plot. C: control; VT: vermicompost tea (1:15 diluted in watrer); AP: Artrhospira platensis; MC: microbial consortium; Bk: bokashi. The highlighted lines in (a) indicate where the trees were planted.
2.3. Inoculum Preparation
The microbial consortium was the same that Mira et al. (2021) obtained from compost. The isolation of the microbial consortium was carried out from ten grams of soil fertilized by organic composting, chosen based on the humidity and colour characteristic of soil with a high content of organic matter as a result of microbial decomposition. The sample was placed in 500 mL of TSB medium (Triptone Soya Broth, Thermo Fisher Scientific, Waltham, MA, USA) for microbial growth for 15 h under agitation at 120 rpm and 30 °C. A 1.0 mL aliquot of this homogenized medium was inoculated into 50 mL tubes containing TSB medium. The tubes were then incubated for 15 h under shaking at 120 rpm at 30 °C. Several aliquots were then cryopreserved in TSB medium with 30% of glycerol.
A cryopreserved sample was reactivated by inoculation in 100 mL of TSB and by maintaining the temperature at 30 °C for 72 h. Then, the reactivated culture was used to produce the inoculum for the experiment. An amount of 1 mL of the reactivated cells was transferred to several flasks containing 300 mL of TSB, which were incubated in static cultivation at 30 °C for 72 h to get the amount necessary for the treatments. The cell concentration in the suspension was adjusted to get 1.2 × 109 cells/mL with the quantitative nephelometry test [29].
The Arthrospira platensis strain BMAKD7 was obtained from the “Aidar & Kutner Microorganism Bank of the Oceanographic Institute of the University of São Paulo”. The cyanobacteria were inoculated in 20 L of 1:5 diluted vermicompost tea in water without sterilisation. An amount of 2.5 g/L of NaNO3 and 16 g/L of NaHCO3 was added to the medium. A photobioreactor was assembled using an organizer box with 29 L of capacity. On the lid of the box, an 18 W LED lamp was attached, which was connected to a timer programmed for 12 h of light time per day. Considering the variations in luminosity in the laboratory and the different depths at which the cells were positioned inside the box, a variation from 1570 to 2500 lux was measured without the presence of culture media and cells, which influence light absorption. The range of luminosity obtained was within good conditions for establishing cell growth [30]. The aeration was controlled using a submersible pump (220 L/h, Elo Imports, Hong Kong, China) and the system worked for 14 days at room temperature for the cyanobacterial growing. For the field application, the cyanobacterial suspension was diluted in water in a proportion of 1:3 to give a 1:15 final dilution for the vermicompost tea and a concentration of 0.83 g/L of A. platensis.
In the place destined to be a vegetable garden at the Obra Social Célio Lemos (OSCL) in São José dos Campos, SP, an artisanal Bokashi was utilised by the local farmer at the experimental site for the experiments. It used 45 kg of garden soil, 10 kg of rice straw, 4 kg of rice bran, 16 kg of manure, 1.4 kg of crushed coal, 800 g of yorin, and 400 g of phosphite. The ingredients were mixed with 10 L of a mixture of 5 L of the efficient microorganisms (E.M.) suspension, 5 kg of sugar cane molasses, and 100 L of water. E.M. were produced from samples of fungi isolated from Atlantic Forest litter in the region of São José dos Campos, using cooked rice as bait. After 7 days, the colonies grown on the cooked rice were selected by their colours: the dark ones were eliminated and the others were used as inoculum in a broth medium based on sugar cane molasses, milk, and rock dust.
2.4. Data Collecting and Analysis
Randomly, taking care to preserve the plants’ roots systems, six plants from each plot for each species were harvested, except for the pepper, from which just the fruits were harvested. The arugula was harvested 28 days after planting (DAP), lettuce 45 DAP, beetroot 90 DAP, carrot 120 DAP, and pepper 120 DAP. The plants were transported to the laboratory, where biometric measures were made. Firstly, the shoots and roots were separated, and then the shoot and root fresh weights for all the plants were measured. For the lettuce and arugula, root volume was measured by immersion and displacement of water in a graduated cylinder. Total root length was assessed in fresh roots by the modified line-intersection method [31]. Root mean diameter (D) was calculated by the formula 2(V/Lπ)0.5 and root superficial area by the formula 2πRL + 2πR2, where V, L, and R represent the root system volume, total root length, and root mean radius (D/2), respectively [32]. The carrots’ root lengths were measured using a ruler. Then, the shoots and roots were dried at 65 °C until they achieved a constant mass. Shoots’ and roots’ dry weights were measured. Due to budget limitations, just 3 repetitions (2, 3, and 4) of the arugula and lettuce in the first experiment were sent to bromatological analysis by the Ribersolo laboratory in Ribeirão Preto, São Paulo State. In the second experiment, a bromatological analysis was not made. Since the pepper did not develop well and some samples were lost, statistical analysis became unfeasible and it was not included in the results.
2.5. Statistical Analysis
All data obtained were tested for the normality of variables and variance homogeneity, followed by an analysis of variance (ANOVA) at p < 0.05; means were compared by the Duncan test at p < 0.05. In cases where the variables did not show normality and/or variance homogeneity, data were transformed to √(x + 1). These tests were performed using the ExpDes package of the R software (version 4.2.2, 2022). Data from biometric analysis were transformed into log(x + 1) for clustering; the number of clusters were determined by the function “fviz_nbclust” and then the cluster representations were generated with the “fviz_cluster” function, both from the “factoextra” package in R.
3. Results
In general, the first experiment showed more differences between the treatments than the second. In most cases, the vegetables were benefited by the AP, MC, and Bk treatments, with a highlight to MC. In arugula, these treatments resulted in a greater shoot dry weight in the first and second experiment (Table 2). The shoot dry weight of the MC-treated plants in the second experiment is the only one of these three treatments mentioned that differs from VT. Furthermore, MC has more root dry weight and volume than the others in the first experiment. In the second experiment, AP, MC, and Bk did not differ from each other in relation to root dry weight and volume, but differ from the control group. The MC treatment also resulted in longer roots and in more root surface area in the first experiment. The treatments did not differ from each other in the root mean diameter. The Bk-treated arugulas showed the lowest root specific length and the major root density values in the first experiment. The clustering plots based on the biometric analysis (Figure 2) showed two groups of treatments, the first comprising Ap, MC, and Bk, and the second comprising C and VT.

Table 2.
Means of the variables analysed in the productivity of arugula with five repetitions in different treatments in the agroforestry system.
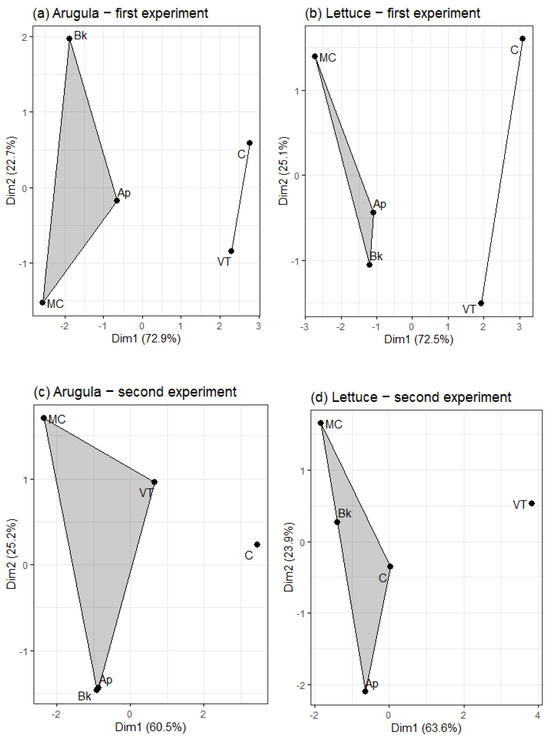
In lettuce, the treatments only showed differences between each other in the first experiment (Table 3). MC-treated lettuce had more shoot and root dry weight and root volume than the others. Ap, MC, and Bk did not differ from each other in root system length, but were longer than C and VT. There was no difference in root mean diameter between all the treatments. MC had the major root surface area, followed by Bk and Ap, then VT and C. Only Ap and Bk differ from the control group in the root specific length. There were no differences between the treatments in root density. As it was for arugula, the treatments Ap, MC, and Bk in lettuce were similar to each other, being clustered together and separated from C and VT (Figure 2).

Table 3.
Means of the variables analysed in the productivity of lettuce with five repetitions in different treatments in the agroforestry system.
The bromatology analysis, performed in the first experiment, showed that, except for the Zn total content in the Ap treatment, Ap and Bk had always higher nutrient and protein content than C and VT. In relation to MC, this treatment had the best results with the highest level of statistical significance (Table 4). As it occurred on the arugulas, the bromatology analysis performed in the first experiment showed that, except for the Zn total content in the Ap treatment, Ap and Bk had always higher nutrient and protein content than C and VT. The MC treatment had, in general, higher nutrient content than the others, being similar to Ap and Bk in some of those (Table 4).

Table 4.
Total content of macronutrients, micronutrients, and proteins in arugula with different agroforestry system treatments.
In the first experiment, beetroot treated with Ap, MC, and Bk resulted in a greater shoot fresh weight and root dry weight compared to the control group but did not differ from the VT group (Table 5). In the second experiment, there were no differences between the treatments in beetroots. The treatments in carrots did not differ from each other in the first experiments but in the second, VT-treated carrots had more dry weight (Table 5).

Table 5.
Means of the variables analysed in the productivity of beetroot and carrot with five repetitions in different treatments in the agroforestry system.
4. Discussion
Maintaining soil quality depends on environmental conditions, physical-chemical characteristics of the soil, such as permeability and capacity to store water, and soil microbial communities. Building a microbial functional diversity and redundancy is a key factor for a stable plant–soil system. This can be reached by adopting several sustainable practices such as no tilling, soil covering, no or low inputs of chemicals, culture rotation, and microorganism application [33,34,35]. In the present study, microbial treatments were presented for use in agriculture with the aim of increasing soil biodiversity, promoting plant growth with greater efficiency, enhancing nutrient cycling, and making them available to the roots. The microbial consortium has a biodiversity that came from organic compounds; many bacteria present in the symbiotic consortium are already known in the literature as bacteria that promote plant growth, such as Clostridium spp. and Ruminococcus, which also have cellulolytic activity [36,37]. Bokashi has a microbial diversity which came from production processes and from EM, and vermicompost tea also has microorganism diversity [38]. Although A. platensis is a single organism, it is known that cyanobacteria can stimulate soil microbiota diversity and represent a rich source of macro and micro nutrients and phytohormones [39]. Moreover, non-sterilized vermicompost tea was used for the production; this tea is supposed to carry other beneficial microorganisms [38,40]. The initial hypothesis was that these microorganisms could improve the plants’ development and soil quality.
Plant growth-promoting bacteria can induce improvements in morphological root traits by producing plant growth regulators and signaling molecules in the rhizosphere [41,42,43]. With a longer root system and more root surface area, the plant can scavenge more efficiently the soil nutrients, especially those with low motility in the soil [44,45]. Root tissue density is related with how the plant interact with the soil, even having impact in soil carbon dynamic. Specific root exudation rate (amount of root exudate per gram of dry root over time) is decreased with high root tissue density [46]. Root exudates represent a chemical interface for the communication between the plant and microorganisms, which will use it as energy source [47]. Also, high root tissue density is negatively correlated with root life span, it means a faster degradation of roots with lower tissue density in soil [48]. Regarding the plant physiology, low root tissue density may indicate less metabolic expenditure with the respiratory rate of the roots and faster shoot growth rate [49]. On other hand, plant can reduce root tissue density by the formation of air spaces in the root cortex when it has low nitrogen or phosphorus availability [2,50]. C and VT treatments had conditions that led to low general development comparing with the other treatments in the first experiment with arugula, one hypothesis is that these plants were in an environment with less plant growth promoting bacteria, reducing, for example, the N and P uptake. In the MC treatment, the low root tissue density was accompanied with a good plant development compared with the other treatments. In this case, root tissue density reflected not a worse plant development but a reduction of the necessity of high respiration rates by the roots. Although the treatments Ap, MC, and Bk did not show thinner roots than the control group on arugula and lettuce, in general, they had more root length, more root surface area, and less root specific length. These results may be attributed to the inoculated microorganisms and it leaded to a greater plant development and nutrient uptake when comparing to the control group.
Our results show that the A. platensis suspension in general improved the plants development and can also be used for agricultural systems improvements. There are corroborations in the literature with related benefits due to the inoculation of A. platensis in arugula, eggplant, lettuce, maize, and onion [51,52,53,54]. Micronutrient concentration increasing in plant parts has also been reported [55,56]. In the present work, A. platensis increased macro and micronutrients in arugula and lettuce, when compared with the control and VT groups, excepting for Zn in arugula, these results can be explained due to the roots development improving, which aids the plants to explore more efficiently the soil. The same rational thinking can be applied to the MC and Bk treatments.
The first trial with the microbial consortium used in this study was carried out by Mira et al. (2021) [13]. The authors isolated the microbial consortium from compost and applied in arugula, carrots, and radish. The MC significantly improved the fresh mass of the arugula, carrots, and radish in 2.5, 1.3, and 16 times, respectively. The increase in the arugula shoots dry weight in 2.98 and 1.94 times in the first and second experiment of the present study corroborates with it. On the other hand, while Mira et al. (2021) [13] related an increase in carrots biomass, the present results do not show that. Although the authors made just one application of the MC, it can be worthy to do another application in the middle of the growing period, since carrots takes a longer time to be harvested than arugula and lettuce. This approach of applying the microorganisms more than one time could also be adopted for the Ap treatment, Dias et al. (2016) [51] for example did four applications of A. platensis in an experiment with eggplant.
Bokashi, as an organic fertilizer, has a high content of organic matter and nutrients, in addition to the microorganisms [21]. The results with bokashi application show a general improvement on plan development when compared to the control group (Table 1, Table 2 and Table 3). Controversially, Mayer et al. (2010) [57] did not observe yield increase in a four-year experiment with potato, winter barley, alfalfa, and winter wheat, each one in a year as a rotation in temperate climate. The authors related some effects on soil traits but attributed it to the nutrient content in bokashi rather than the microorganisms, since the autoclaved bokashi treated group had similar results.
The use of bokashi through badly executed management can harm a crop. When the material is added to a substrate for seed germination or even when applied to the soil, the decomposition of the material continues and the negative impacts are caused by the decline in oxygen availability, N immobilization [58] or by the phytotoxicity generated by the presence of phytotoxic compounds [21]. Organic acids and ammonium are examples of these compounds and are generated when the raw material has a low C/N ratio [59]. Thus, it is recommended that biological tests be performed as quality control to assess the phytotoxicity of the product [21].
5. Conclusions
Therefore, adopting the inoculation of MC or Ap instead of Bk can be better for crop safety and yield, since the results are better in terms of MC and Ap treatment compared to Bokashi. However, for using MC in agriculture as a product, more research on formulation and scaling bioprocess is needed, since using the TSB medium for growing the consortium is not economically worthwhile. Future research includes testing different culture media formulation for MC as well as Ap and their efficacy for other plant species.
Author Contributions
Conceived and designed the experiments: T.F.R., M.P.M.I., V.M., C.O.V. and E.E. Performed the experiments: T.F.R., M.P.M.I., G.B., V.A.R.M. and E.E. Analyzed the data: T.F.R., M.P.M.I. and E.E. Contributed reagents/materials/analysis tools: C.O.V. and E.E. Wrote the paper: T.F.R. and E.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cargill, Sustainability Notice 2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
T. F. Rodrigues acknowledges a PhD fellowship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil—Finance Code 001) and M.P.M. Itkes Msc fellowship from Cargill.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Muller, A.; Schader, C.; El-Hage Scialabba, N.; Brüggemann, J.; Isensee, A.; Erb, K.-H.; Smith, P.; Klocke, P.; Leiber, F.; Stolze, M.; et al. Strategies for Feeding the World More Sustainably with Organic Agriculture. Nat. Commun. 2017, 8, 1290. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Ho, M.D.; Phosphorus, L. Rhizoeconomics: Carbon Costs of Phosphorus Acquisition. Plant Soil 2005, 269, 45–56. [Google Scholar] [CrossRef]
- Beltran-Peña, A.; Rosa, L.; D’Odorico, P. Global Food Self-Sufficiency in the 21st Century under Sustainable Intensification of Agriculture. Environ. Res. Lett. 2020, 15, 095004. [Google Scholar] [CrossRef]
- Miccolis, A.; Peneireiro, F.M.; Vieira, D.L.M.; Marques, H.R.; Hoffmann, M.R.M. Restoration through Agroforestry: Options for Reconciling Livelihoods with Conservation in the Cerrado and Caatinga Biomes in Brazil. Exp. Agric. 2019, 55, 208–225. [Google Scholar] [CrossRef]
- Matos, P.S.; Cherubin, M.R.; Damian, J.M.; Rocha, F.I.; Pereira, M.G.; Zonta, E. Short-Term Effects of Agroforestry Systems on Soil Health in Southeastern Brazil. Agrofor. Syst. 2022, 96, 897–908. [Google Scholar] [CrossRef]
- Beule, L.; Vaupel, A.; Moran-Rodas, V.E. Abundance, Diversity, and Function of Soil Microorganisms in Temperate Alley-Cropping Agroforestry Systems: A Review. Microorganisms 2022, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Chavan, S.B.; Chichaghare, A.R.; Uthappa, A.R.; Kumar, M.; Kakade, V.; Pradhan, A.; Jinger, D.; Rawale, G.; Yadav, D.K.; et al. Agroforestry Systems for Soil Health Improvement and Maintenance. Sustainability 2022, 14, 14877. [Google Scholar] [CrossRef]
- de Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Lopes, M.J.d.S.; Dias-Filho, M.B.; Gurgel, E.S.C. Successful Plant Growth-Promoting Microbes: Inoculation Methods and Abiotic Factors. Front. Sustain. Food Syst. 2021, 5, 606454. [Google Scholar] [CrossRef]
- Bomfim, C.A.; Coelho, L.G.F.; do Vale, H.M.M.; de Carvalho Mendes, I.; Megías, M.; Ollero, F.J.; dos Reis Junior, F.B. Brief History of Biofertilizers in Brazil: From Conventional Approaches to New Biotechnological Solutions. Braz. J. Microbiol. 2021, 52, 2215–2232. [Google Scholar] [CrossRef]
- Hungria, M.; Barbosa, J.Z.; Rondina, A.B.L.; Nogueira, M.A. Improving Maize Sustainability with Partial Replacement of N Fertilizers by Inoculation with Azospirillum Brasilense. Agron. J. 2022, 114, 2969–2980. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial Inoculants: Reviewing the Past, Discussing the Present and Previewing an Outstanding Future for the Use of Beneficial Bacteria in Agriculture. AMB Express 2019, 9, 205. [Google Scholar] [CrossRef]
- Mira, W.V.W.; Esposito, E.; Vilarraga, C.O.; Anatriello, E. APLICAÇÃO DE BIOINOCULANTES DE SOLO COMPOSTADO PARA PROMOÇÃO DO CRESCIMENTO DE RÚCULA (Eruca sativa), CENOURA (Daucus carota sativus) e RABANETE (Raphanus sativus). Rev. Bras. Agroecol. 2021, 16, 117–122. [Google Scholar] [CrossRef]
- Kuraganti, G.; Edla, S.; Pallaval, V.B. Cyanobacteria as Biofertilizers: Current Research, Commercial Aspects, and Future Challenges. In Advances in Plant Microbiome and Sustainable Agriculture; Yadav, A., Rastegari, A., Yadav, N., Kour, D., Eds.; Springer: Singapore, 2020; pp. 259–278. [Google Scholar]
- Zapata, D.; Arroyave, C.; Cardona, L.; Aristizábal, A.; Poschenrieder, C.; Llugany, M. Phytohormone Production and Morphology of Spirulina Platensis Grown in Dairy Wastewaters. Algal Res. 2021, 59, 102469. [Google Scholar] [CrossRef]
- Rossi, F.; Li, H.; Liu, Y.; De Philippis, R. Cyanobacterial Inoculation (Cyanobacterisation): Perspectives for the Development of a Standardized Multifunctional Technology for Soil Fertilization and Desertification Reversal. Earth Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Alobwede, E.; Leake, J.R.; Pandhal, J. Circular Economy Fertilization: Testing Micro and Macro Algal Species as Soil Improvers and Nutrient Sources for Crop Production in Greenhouse and Field Conditions. Geoderma 2019, 334, 113–123. [Google Scholar] [CrossRef]
- Manjunath, M.; Kanchan, A.; Ranjan, K.; Venkatachalam, S.; Prasanna, R.; Ramakrishnan, B.; Hossain, F.; Nain, L.; Shivay, Y.S.; Rai, A.B.; et al. Beneficial Cyanobacteria and Eubacteria Synergistically Enhance Bioavailability of Soil Nutrients and Yield of Okra. Heliyon 2016, 2, e00066. [Google Scholar] [CrossRef]
- Churilova, E.; Midmore, D. Vermiliquer (Vermicompost Leachate) as a Complete Liquid Fertilizer for Hydroponically-Grown Pak Choi (Brassica chinensis L.) in the Tropics. Horticulturae 2019, 5, 26. [Google Scholar] [CrossRef]
- Helaly, A.; El-Dakak, R. Effect of Organic Liquid Vermicompost as a Substitute for Chemical Fertilizer on Morphological and Biochemical Characteristics in Lettuce. Assiut J. Agric. Sci. 2021, 52, 69–81. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as an Amendment and Source of Nitrogen in Sustainable Agricultural Systems: A Review. J. Soil. Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Restrepo, J.; Hensel, J. El ABC de La Agricultura Orgánica, Fosfitos y Panes de Piedra; Impressora Feriva S.A.: Santiago de Cali, Colombia, 2013; 396p. [Google Scholar]
- Wilmer, J.; Rodriguez, W.; Rosas, G. Caracterización Física y Química de Bokashi y Lombricompost y su Evaluación Agronómica en Plantas de Maíz. Ing. Amazon. 2014, 7, 5–16. [Google Scholar]
- Theodoro, S.H.; de Paula Medeiros, F.; Ianniruberto, M.; Baiocchi Jacobson, T.K. Soil Remineralization and Recovery of Degraded Areas: An Experience in the Tropical Region. J. South. Am. Earth Sci. 2021, 107, 103014. [Google Scholar] [CrossRef]
- Gris, D.; Temponi, L.G.; Marcon, T.R. Native Species Indicated for Degraded Area Recovery in Western Paraná, Brazil. Rev. Árvore 2012, 36, 113–125. [Google Scholar] [CrossRef][Green Version]
- Alori, E.T.; Gabasawa, A.I.; Elenwo, C.E.; Agbeyegbe, O.O. Bioremediation Techniques as Affected by Limiting Factors in Soil Environment. Front. Soil Sci. 2022, 2, 937186. [Google Scholar] [CrossRef]
- Bicca, J.M.; Nunes Arduin, R.L.; Spinelli Pinto, L.F.; Bamberg, A.L.; Miguel, P.; Stumpf, L. Clay Stabilization and Recovery of Soil Functions of a Degraded Solodic Planosol through Incorporation of Agrominerals: A Case Study in Southern Brazil. J. S. Am. Earth Sci. 2023, 122, 104177. [Google Scholar] [CrossRef]
- Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.; Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; Almeida, J.A.; Araujo-Filho, J.C.; Oliveira, J.B.; Cunha, T.J.F. Brazilian Soil Classification System, 5th ed.; Embrapa: Brasília, Brazil, 2018. [Google Scholar]
- Morais, I.P.A.; Tóth, I.V.; Rangel, A.O.S.S. Turbidimetric and Nephelometric Flow Analysis: Concepts and Applications. Spectrosc. Lett. 2006, 39, 547–579. [Google Scholar] [CrossRef]
- Muliterno, A.; Mosele, P.C.; Costa, J.A.V.; Hemkemeier, M.; Bertolin, T.E.; Colla, L.M. Cultivo Mixotrófico Da Microalga Spirulina Platensis Em Batelada Alimentada. Ciência Agrotecnologia 2005, 29, 1132–1138. [Google Scholar] [CrossRef][Green Version]
- Tennant, D. A Test of a Modified Line Intersect Method of Estimating Root Length. J. Ecol. 1975, 63, 995. [Google Scholar] [CrossRef]
- Zangaro, W.; Rostirola, L.V.; de Souza, P.B.; de Almeida Alves, R.; Lescano, L.E.A.M.; Rondina, A.B.L.; Nogueira, M.A.; Carrenho, R. Root Colonization and Spore Abundance of Arbuscular Mycorrhizal Fungi in Distinct Successional Stages from an Atlantic Rainforest Biome in Southern Brazil. Mycorrhiza 2013, 23, 221–233. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of Soil Microbial Diversity through Sustainable Agricultural Practices and Its Evaluation by -Omics Approaches: A Perspective for the Environment, Food Quality and Human Safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a Key Player in Sustainable Agriculture and Human Health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Polyanskaya, L.M.; Vedina, O.T.; Lysak, L.V.; Zvyagintsev, D.G. The Growth-Promoting Effect of Beijerinckia and Clostridium Sp. Cultures on Some Agricultural Crops. Microbiology 2002, 71, 109–115. [Google Scholar] [CrossRef]
- Nwachukwu, B.C.; Ayangbenro, A.S.; Babalola, O.O. Elucidating the Rhizosphere Associated Bacteria for Environmental Sustainability. Agriculture 2021, 11, 75. [Google Scholar] [CrossRef]
- Fritz, J.I.; Franke-Whittle, I.H.; Haindl, S.; Insam, H.; Braun, R. Microbiological Community Analysis of Vermicompost Tea and Its Influence on the Growth of Vegetables and Cereals. Can. J. Microbiol. 2012, 58, 836–847. [Google Scholar] [CrossRef]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential Applications of Cyanobacteria: Spirulina Platensis Filtrates and Homogenates in Agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef]
- Prasanna, R.; Kanchan, A.; Ramakrishnan, B.; Ranjan, K.; Venkatachalam, S.; Hossain, F.; Shivay, Y.S.; Krishnan, P.; Nain, L. Cyanobacteria-Based Bioinoculants Influence Growth and Yields by Modulating the Microbial Communities Favourably in the Rhizospheres of Maize Hybrids. Eur. J. Soil Biol. 2016, 75, 15–23. [Google Scholar] [CrossRef]
- Molina-Favero, C.; Creus, C.M.; Simontacchi, M.; Puntarulo, S.; Lamattina, L. Aerobic Nitric Oxide Production by Azospirillum Brasilense Sp245 and Its Influence on Root Architecture in Tomato. Mol. Plant-Microbe Interact. 2008, 21, 1001–1009. [Google Scholar] [CrossRef]
- Cassán, F.; Vanderleyden, J.; Spaepen, S. Physiological and Agronomical Aspects of Phytohormone Production by Model Plant-Growth-Promoting Rhizobacteria (PGPR) Belonging to the Genus Azospirillum. J. Plant Growth Regul. 2014, 33, 440–459. [Google Scholar] [CrossRef]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits That Go Far beyond Biological Nitrogen Fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerling, D.J. Evolutionary Patterns and Biogeochemical Significance of Angiosperm Root Traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in Root Morphological Traits in Soybean Co-Inoculated with Bradyrhizobium Spp. and Azospirillum Brasilense or Treated with A. Brasilense Exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Williams, A.; Langridge, H.; Straathof, A.L.; Muhamadali, H.; Hollywood, K.A.; Goodacre, R.; de Vries, F.T. Root Functional Traits Explain Root Exudation Rate and Composition across a Range of Grassland Species. J. Ecol. 2022, 110, 21–33. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Ryser, P. The Importance of Tissue Density for Growth and Life Span of Leaves and Roots: A Comparison of Five Ecologically Contrasting Grasses. Funct. Ecol. 1996, 10, 717. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root Traits Are Multidimensional: Specific Root Length Is Independent from Root Tissue Density and the Plant Economic Spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- McPherson, D.C. Cortical Air Spaces in the Roots of Zea Mays L. New Phytol. 1939, 38, 190–202. [Google Scholar] [CrossRef]
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; Lima, J.F.; Guedes, W.A. Growth, Yield, and Postharvest Quality in Eggplant Produced under Different Foliar Fertilizer (Spirulina platensis) Treatments. Semin. Cienc. Agrar. 2016, 37, 3893. [Google Scholar] [CrossRef]
- Dineshkumar, R.; Subramanian, J.; Arumugam, A.; Ahamed Rasheeq, A.; Sampathkumar, P. Exploring the Microalgae Biofertilizer Effect on Onion Cultivation by Field Experiment. Waste Biomass Valorization 2020, 11, 77–87. [Google Scholar] [CrossRef]
- Siringi, J.O.; Turoop, L.; Njonge, F. Biostimulant Effect of Spirulina (Arthrospira platensis) on Lettuce (Lactuca sativa) Cultivated under Aquaponic System. SCIREA J. Biol. 2022, 7, 23–40. [Google Scholar] [CrossRef]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina Biomass Produced from Treatment of Aquaculture Wastewater as Agricultural Fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial Inoculation Elicits Plant Defense Response and Enhanced Zn Mobilization in Maize Hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of Wheat through Inoculation of Plant Growth Promoting Rhizobacteria and Cyanobacteria. Eur. J. Soil. Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließbach, A.; Oberholzer, H.-R. How Effective Are ‘Effective Microorganisms® (EM)’? Results from a Field Study in Temperate Climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Zucconi, F.; Monaco, A.; Forte, M. Phytotoxins during the Stabilization of Organic Matter; Elsevier: Amsterdam, The Netherlands, 1985. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).