Abstract
Gold nanoparticles (AuNPs) radiolabeled with therapeutic and diagnostic radioisotopes have been broadly studied as a promising platform for early diagnosis and treatment of many diseases including cancer. Our main goal for this study was the comparison of the biodistribution profiles of four different concentrations of gold nanoconjugates radiolabeled with Technetium-99m (99mTc). More specifically, AuNPs with an average diameter of 2 nm were functionalized with a tridentate thiol ligand. Four different concentrations were radiolabeled with 99mTc-tricarbonyls with high radiolabeling yields (>85%) and were further purified, leading to radiochemical purity of >95%. In vitro stability of the radiolabeled nanoconstructs was examined in cysteine and histidine solutions as well as in human serum, exhibiting robust radiolabeling up to 24 h post-preparation. Moreover, in vitro cytotoxicity studies were carried out in 4T1 murine mammary cancer cells. In vivo tracking of the radiolabeled nanoconjugates at both concentrations was examined in normal mice in order to examine the effect of AuNPs’ concentration on their in vivo kinetics. Our work demonstrates that varying concentrations of radiolabeled AuNPs lead to notably different biodistribution profiles.
1. Introduction
The multifunctional character of cancer leads to several different types of tumors. This complexity gave rise to the need for more precise and personalized drug development in order to achieve better therapeutic and diagnostic outcomes. For this reason, nanotechnology in combination with nuclear medicine gives us a unique opportunity in order to achieve this goal [1].
Numerous studies have been conducted in order to exploit the unique characteristics of nanoparticles. Many variables such as shape, size, surface coating and charge have been investigated with regard to their effect on the in vivo biokinetics of AuNPs, aiming at their optimization and their effective use in different biomedical applications leading to the design of personalized nanostructures according to every patient’s needs [1]. It is well established that small nanoparticles with a size of 10 nm or less are quickly eliminated from the kidneys [2,3,4]. On the contrary, larger particles are cleared from the bloodstream, accumulating in macrophage-rich organs, namely liver and spleen, limiting their ability to reach the target [5,6,7,8]. Moghimi et al. investigated the size limits for nanospheres in order to avoid spleen filtration and concluded that the maximum size was 150 nm [9]. Sonavane et al. studied the biodistribution of AuNPs of a huge variety of sizes (15, 50, 100 and 200 nm) in mice. The results indicated that the smaller particles (15 and 50 nm) had higher blood concentration 24 h post-administration, while the 15 nm nanoparticles had a higher uptake in organs such as the lung, kidney and brain when compared to the larger-sized nanoparticles. An increasing trend in spleen uptake was again observed with an increase in particle size [10]. Furthermore, Kohane et al. demonstrated that smaller-sized nanoparticles clear more efficiently from the injection site (peritoneum) when compared to larger-sized microparticles in the same time frame [11]. Hence, nanoparticle size has a significant impact on their biodistribution. Nanoparticle size also influences therapeutic delivery aspects such as blood circulation half-life, cellular uptake and tumor penetration ability [4].
Surface charge is also important for the in vivo fate of AuNPs, with several studies revealing the impact in mouse models, since it affects the nanoparticle’s blood circulation time and tumor uptake. Arvizo et al. suggested the substantial role of surface charge on the in vivo behavior of nanoparticles, providing insights for improved therapeutic design. After intravenous injection, neutral and zwitterionic particles exhibit low clearance, while negative particles show rapid clearance due to moderate systemic exposure. After intraperitoneal injection, both positively and negatively charged particles are not sufficiently absorbed, indicating a difficulty in crossing the peritoneal barrier. The ability of surface charge to affect the in vivo behavior is crucial for developing nanotherapeutics for enhanced tumor uptake through both passive and active targeting [7]. Other studies have also shown that particles with positive charge tend to accumulate more in the kidneys, while negatively and uncharged particles showed higher uptake in the liver [8].
Τhe route of administration also has an important impact on the in vivo kinetics of nanoparticles. Wang et al. evaluated the differences between three methods of administration (intratumoral (i.t.), intravenous (i.v.) and intraperitoneal (i.p.)) in order to identify the optimal administration route of a novel nanosystem of hexapeptides and AuNPs (P12) in healthy mice and mice with acute lung injury (ALI). The biodistribution results indicated that even at the lowest concentration of P12, i.t. administrations resulted in higher accumulation in the lung and less in the liver. Intratumoral administration allowed P12 to target lung macrophages while i.v. and i.p. administrations did not. The other two routes of administration (i.v. and i.p.) presented higher accumulation of the injected substance in the liver and lymph nodes [12]. Therefore, it is crucial to select the appropriate administration route for nanostructures in order to achieve the optimum results.
Another factor to consider when designing a nanoparticle is its shape. Black et al. investigated the in vivo behavior of nanostructures radiolabeled with 198Au with different shapes, e.g., nanospheres, nanocages, nanodisks and nanorods. The radiolabeled nanospheres presented enhanced circulation in blood, the lowest clearance by the RES organs and the highest tumor uptake compared to the other three shapes. On the other hand, nanorods and nanocages could reach tumor cores, indicating a distinct advantage for the application of photothermal therapy. Nanostructures with different geometries (e.g., nanorods) can have different enhanced permeability and retention (EPR) effects due to changes in their in vivo properties such as blood circulation, the overcoming of various physiological barriers, and extravasation into the tumor [13].
Over the last decades, gold nanoparticles (AuNPs) have been extensively used for a wide range of applications, such as dual-modality imaging agents and theranostic agents, due to their unique properties, huge variety of sizes and shapes, ability to conjugate with various chemical groups, structural modification and low toxicity and biocompatibility not only in in vitro but also in vivo [14,15,16]. Furthermore, AuNPs can be used for photothermal therapy, a complementary approach for cancer treatment. This procedure is non-invasive, causing minimal functional disturbances [17].
Several studies have been conducted using AuNPs and radiolabeling with the gamma emitter 99mTc, which is the most commonly used radionuclide in diagnostic imaging. In order to achieve stable radiolabeling of AuNPs, sometimes it is necessary to first perform their functionalization with specific chemical groups (e.g., thiol groups) which will also allow conjugation with suitable radioisotopes.
Zhu et al. investigated Au PENs (polyethylenimine-entrapped nanoparticles) radiolabeled with 99mTc after functionalization with APAS. The zeta potential of bare and APAS-modified Au PENs was measured under a range of different pH conditions in order to improve the cellular uptake. The results indicated that under slightly acidic environment, the tumor uptake was increased, leading to a promising contrast agent for dual SPECT/CT imaging [18].
Morales-Avila et al. investigated the functionalization of AuNPs with HYNIC-GGC and c[RGDfK-(C)] through interactions with the thiol groups of cysteine. After intravenous injection of the radiolabeled complex, the biodistribution results indicated high uptake in liver and spleen, which was significantly reduced after intraperitoneal administration. This is normal due to the fact that intravenously injected nanoparticles lead to a substantial uptake by the macrophages present in liver and spleen [19]. On the other hand, it was demonstrated by several studies that intraperitoneal injection results in a minimal uptake of AuNPs by Kupffer cells in liver [20].
Li et al. evaluated AuNPs functionalized with polyamidoamine dendrimers (PAMAM) in a murine HeLa xenograft tumor model which presented high uptake in spleen, lung, liver and kidney compared to tumor [21].
Alric et al. studied AuNPs functionalized with dithiolated DTPA (DTDTPA) and radiolabeled with 99mTc and they investigated their in vivo behavior in healthy rats. After intravenous administration of the radiolabeled nanoparticles in healthy rats, the radioactivity was mostly accumulated in heart and liver (due to the high blood volume presented in these organs), spleen, kidneys and bladder. During the first circulation minutes, the liver and heart uptake decreased, reflecting the slow excretion of the nanoparticles from the bloodstream, while kidney uptake remained mostly stable. The activity in the bladder quickly increased over time, and remained stable after 30 min as well, indicating renal clearance [22]. In our previous study, we investigated the synthesis and functionalization of AuNPs with a thiol ligand and their consequent radiolabeling with 99mTc, via the 99mTc-carbonyl precursor. In order to perform the in vivo tracking of these AuNPs, and after taking all the aforementioned factors into consideration, in this study we focused on another parameter; the concentration of AuNPs. To our knowledge, how the concentration of nanoparticles affects their biodistribution has not been previously investigated in the literature. Therefore, we prepared samples with four different concentrations of AuNPs (312.5, 312.5 × 10−1, 312.5 × 10−2 and 312.5 × 10−3 μg/mL). Biodistribution studies were carried out in CFW mixed-gender mice at different time points (1, 4 and 24 h) after injection via the tail vein. The results showed that at each concentration the accumulation in the organs of interest was different, strongly indicating that concentration is indeed a major factor that affects the in vivo kinetics of AuNPs.
2. Materials and Methods
Technetium-99m (99mTc) is a gamma emitter with a photon energy of 140 keV, which requires radiation protection precautions during handling to reduce the risk of harm. All work associated with radiolabeling procedures was conducted in a licensed radiochemistry facility, where such experiments could be safely conducted.
2.1. Chemical Reagents
All reagents and solvents had a purity >95% and were used without further purification. Acetonitrile (>99.5%) was purchased from Carlo Erba (Val-de-Reuil, France). Dimethylsulfoxide (>99.5%) was purchased from Aldrich Chemical (St. Louis, MO, USA). Human serum was purchased from Sigma Aldrich (St. Louis, MO, USA). Trifluoroacetic acid (>99%) was purchased from Alfa Aesar (Loughborough, UK). 99mTc was eluted as Na [99mTc]TcO4 from a commercial 99Mo/99mTc generator (Mallinckrodt Europe B.V., Hertogenbosch, The Netherlands). The AuNPs were characterized by dynamic light scattering (DLS, Malvern, UK).
Analyses and separation as well as purification processes were performed by High-Performance Liquid Chromatography (HPLC) using a Waters 600 Controller pump, a Waters 996 Photodiode Array detector (set at 220 nm for all experiments) and a γ-RAM radioactivity detector to measure radioactive flow on a Jupiter C4 Column (150 × 4.60 mm, 5 µm, 300 Å, Phenomenex, Torrance, CA, USA). The UV detection wavelength was 220 nm. All HPLC solvents were filtered through 0.22 mm membrane filters (Millipore, Milford, MA, USA) before use. Radioactivity measurements were conducted in a dose calibrator (Capintec, Ramsey, NJ, USA).
The 4T1 cell line was obtained from the cell bank of the Laboratory of Radiobiology, Institute of Nuclear and Radiological Sciences and Technology, Energy, and Safety, NCSR “Demokritos”, Athens, Greece. RPMI culture medium and the reagent [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] for the MTT assay were purchased from Biowest (Nuaillé, France) and Applichem (Darmstadt, Germany), respectively. The LabSystems Multiskan RC Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA) was used for the optical density measurements.
Animals used for the ex vivo biodistribution studies were obtained from the breeding facilities of the Institute of Biosciences and Applications, NCSR “Demokritos”. Our experimental animal facility is registered according to Greek Presidential Decree 56/2013 (Reg. Number: EL 25 BIO 022), in accordance with European Directive 2010/63, which is harmonized with national legislation, on the protection of animals used for scientific purposes. All applicable national guidelines for the care and use of animals were followed. The study protocol was approved by the Department of Agriculture and Veterinary Service of the Prefecture of Athens (Protocol Number 634365/27-7-21). Samples for ex vivo biodistribution studies were measured on a Packard COBRA II Auto-Gamma Counter (Ramsey, MN, USA).
2.2. Functionalization of Gold Nanoparticles with a Thiol Tridentate Ligand
Τhe synthesis and characterization of the AuNPs and the thiol ligand (Figure 1) have been previously described [23]. Both samples were prepared according to the same procedure, during which 500, 500 × 10−1, 500 × 10−2 and 500 × 10−3 μg of AuNPs were each mixed with 0.29 mg of the ligand previously dissolved in DMSO and allowed to stay overnight on a magnetic stirrer. Then, the samples were purified by centrifugation at 4500 rpm for 20 min and the supernatant was carefully removed in order to remove the excess of the ligand. After purification, the nanoparticles were set for radiolabeling, as given below.
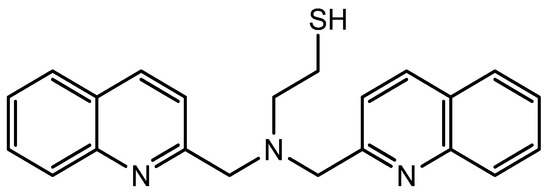
Figure 1.
The thiol tridentate ligand used in this study.
2.3. Characterization of AuNPs by Dynamic Light Scattering (DLS)
Bare and thiol-functionalized AuNPs of both concentrations were analyzed for their average hydrodynamic diameter using the DLS method.
2.4. Cell Viability by MTT Assay
Cytotoxicity studies were performed following a previously reported procedure using the 4T1 cancer cell line [23]. Cell viability of bare AuNPs and ligand-functionalized AuNPs was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. Very briefly, 104 cells/well were seeded in a 96-well plate and incubated overnight at 37 °C and 5% CO2. The following day, increasing concentrations of bare AuNPs (50, 50 × 10−1 and 50 × 10−2 μg/mL) and ligand-functionalized AuNPs (50, 50 × 10−1 and 50 × 10−2 μg/mL) were added to the growing cells in 4 replicates. The control group consisted of cells that were not treated with the AuNPs. The following day, RPMI was replaced with RPMI medium containing MTT (1 mg/mL). Cells were further incubated with the MTT solution for 4 h and, after removing it, 100 µL of isopropanol was added to each well. Finally, absorbance was measured at 560 nm using an ELISA reader. The mean value of the optical density (OD) of the four replicates and the percentage of the OD was calculated with the following equation:
Each assay was repeated three times.
2.5. Radiolabeling with [99mTc][Tc(H2O)3(CO)3]+
The radiolabeling procedure was simple and straightforward. The labeling precursor [99mTc][Tc(H2O)3(CO)3]+ was prepared as described in the literature [23]. After functionalization of AuNPs with the ligand, all samples of AuNPs were incubated with 100 µL of 99mTc-tricarbonyl core—[99mTc][Tc(H2O)3(CO)3]+ (30–80 MBq) at 70 °C for 2 h. Radiochemical yield and purity were determined by HPLC. Purification of the samples was performed by centrifugation at 4500 rpm for 20 min.
2.6. In Vitro Stability Studies
After attaining satisfactory radiochemical purity, the in vitro stability of all concentrations of the radiolabeled nanocomplexes in human serum as well as in cysteine and histidine solutions was tested to predict their in vivo stability, as described in our previous work [23].
2.7. Ex Vivo Biodistribution Studies
The ex vivo behavior of the two radiolabeled complexes was evaluated in mixed-gender Carworth Farms Webster (CFW) mice, 6–8 weeks old, weighing 20–30 g. The animals were housed in individually ventilated cages (IVC) under a 12 h light/dark cycle and allowed free access to food and water. The two radiolabeled nanostructures consisted of different starting concentrations of AuNPs (500, 500 × 10−1, 500 × 10−2 and 500 × 10−3 μg of AuNPs) functionalized with the same concentration of thiol ligand. All samples were reconstituted in 1600 μL water for injection which led to final concentrations of 312.5, 312.5 × 10−1, 312.5 × 10−2 and 312.5 × 10−3 μg/mL, respectively. According to the experimental protocol, a syringe containing 100 μL/~300–700 kBq of diluted radiolabeled AuNPs suspension was counted in a dose calibrator. At 1, 4, and 24 h post-administration, the animals were euthanized by isoflurane inhalation and the major organs and tissues of interest were collected, weighed and measured in an automatic gamma counter. For the calculation of the injected dose in each animal, the radioactivity remaining in the tail was subtracted, and a standard solution was prepared. The syringes containing the radiolabeled samples were measured before and after injection, to determine the precise dose administered to each mouse. All measurements were corrected for background and radioactive decay. All the distribution data were calculated as the percentage of injected dose per gram (%ID/g).
3. Results and Discussion
3.1. Characterization of AuNPs by DLS
The AuNP characterization results are presented in Table 1. The samples were measured after filtration via a syringe filter (pore size 0.45 μm, diameter 13 mm). DLS measurements clearly indicated that there was a noticeable size difference compared to bare nanoparticles, which were found to be 2 nm as measured by TEM, as DLS analysis was not possible due to the small size [23]. The results for size and zeta potentials are summarized in Table 1.

Table 1.
Hydrodynamic diameters of functionalized AuNPs.
3.2. MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide] Assay
The MTT assay was conducted in order to evaluate the toxicity of both bare and functionalized AuNPs against the 4T1 cell line that is used as a model of TNBC (Triple Negative Breast Cancer) [24]. The 4T1 tumor resembles human stage IV breast cancer, with metastatic behavior [25]. Therefore, this cell line provides an interesting tumor model with spontaneous metastases to various sites (e.g., lung, bone, etc.) which can be very useful when evaluating the therapeutic potential of radiolabeled nanoparticles with alpha- or beta-emitting isotopes [26].
The samples were treated with different concentrations of AuNPs for 24 h. The results of the MTT assay are summarized in Figure 2 and indicate that bare AuNPs revealed very low toxicity at the highest examined concentration, with cell viability reducing gradually from 92.41 ± 4.75% at the lowest concentration (0.5 μg/mL) to 79.71 ± 2.68% at the highest concentration (50 μg/mL). In the case of the nanoparticles functionalized with the thiol ligand, cytotoxicity studies also indicated low toxicity at the highest examined concentration, with cell viability reducing from 100.64 ± 2.37% at the lowest concentration (50 × 10−2 μg/mL) to 80.06 ± 5.87% at the highest concentration (50 μg/mL). It is also worth mentioning that the cell viability after incubation with the ligand was ~70% at all tested concentrations, indicating that the presence of AuNPs did not have an important impact on the biological behavior of the ligands on the cells [18].
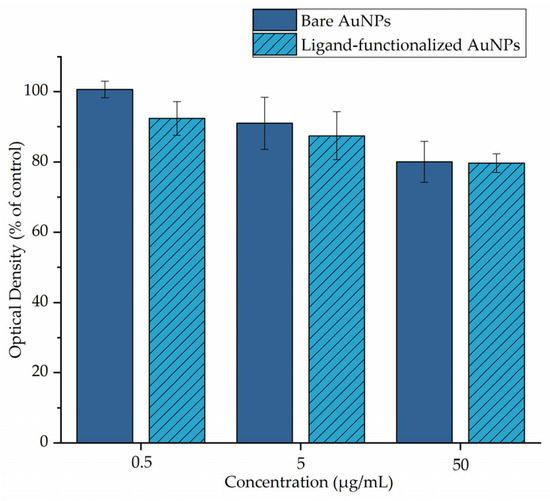
Figure 2.
MTT assay of 4T1 cells treated with different concentrations of bare and ligand-functionalized AuNPs.
3.3. Functionalization and Radiolabeling of AuNPs with [99mTc][Tc(H2O)3(CO)3]+
The functionalization of AuNPs with the ligand and the following radiolabeling procedures are described above. The main purpose of this functionalization was to provide an effective chelator for the successful and robust radiolabeling of AuNPs with 99mTc-carbonyls. After functionalization, strong Au-S bonds were formed between the AuNPs and the thiol group of the ligand. For radiolabeling, functionalized AuNPs of all concentrations were incubated at 70 °C for 2 h with 100 μL of [99mTc][Tc(H2O)3(CO)3]+. The precursor [99mTc][Tc(H2O)3(CO)3]+ carries three labile water molecules which are easily substituted by the three nitrogen atoms of the tridentate thiol ligand to afford the desired 99mTc. The radiolabeling yield was approximately 85%. After purification by centrifugation, radiochemical purity for all samples was >95%, and it remained stable for at least 24 h post-purification, as determined by HPLC analysis (Table 2). Furthermore, the formed radiolabeled nanoparticles present a lipophilic nature, as was demonstrated by our previous work, due to the lipophilic character of the ligand used in the present study.

Table 2.
HPLC results representing the radiochemical purity of [99mTc]Tc-AuNPs.
3.4. Stability Studies
The radiolabeled complexes exhibited very promising in vitro stability not only in cysteine and histidine solutions but also in human serum (>90%), which indicates the satisfactory binding of 99mTc to the AuNPs, thus enhancing their potential to be investigated for in vivo applications (Table 3, Table 4 and Table 5).

Table 3.
HPLC results representing the in vitro stability of [99mTc]Tc-AuNPs in the presence of cysteine.

Table 4.
HPLC results representing the in vitro stability of [99mTc]Tc-AuNPs in the presence of histidine.

Table 5.
HPLC results representing the in vitro stability of [99mTc]Tc-AuNPs in the presence of human serum.
3.5. Biodistribution Studies
Ex vivo biodistribution studies were carried out in CFW mice after intravenous injection for the determination of the in vivo kinetics of the radiolabeled AuNPs at four different concentrations (100 μL per injection, leading to final administered doses of 31.25, 31.25 × 10−1, 31.25 × 10−2 and 31.25 × 10−3 μg per mouse). The results for uptake in the RES organs (lung, liver and spleen) are summarized in Figure 3. Furthermore, the full sets of biodistribution results are provided in Table 6, Table 7 and Table 8.
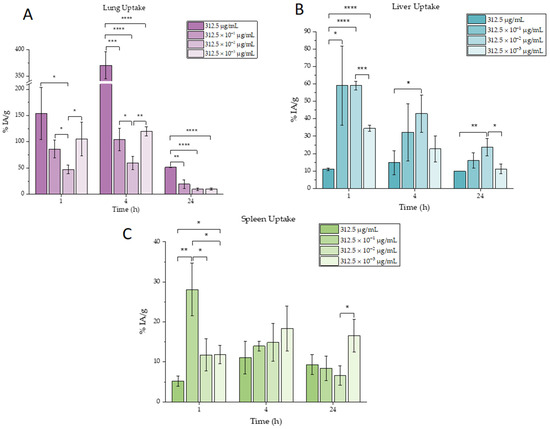
Figure 3.
Ex vivo biodistribution results of [99mTc]Tc-AuNPs for the main organs of interest: (A) lung, (B) liver and (C) spleen. Mean values (n = 3) and SD (bars) are shown (x axis not in scale). Asterisks indicate the statistical significance of the differences between the results (* p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001). The absence of an asterisk denotes a non-significant statistical difference.

Table 6.
Ex vivo biodistribution results for all tested concentrations at 1 h (n = 3 ± SD).

Table 7.
Ex vivo biodistribution results for all tested concentrations at 4 h (n = 3 ± SD).

Table 8.
Ex vivo biodistribution results for all tested concentrations at 24 h (n = 3 ± SD).
As shown in Figure 3, as well as in Table 6, Table 7 and Table 8, the nanoparticles for the three lowest concentrations were accumulated mostly in three organs, liver, spleen and lungs, due to the phenomenon of opsonization which leads to high uptake of the nanoparticles by phagocytic-rich organs (liver, spleen and lungs) [27]. This high accumulation is also normal for nanoparticles of that size since larger nanoparticles are more likely to attach to blood vessels’ walls in the case of flow than smaller ones [28]. On the other hand, it has been observed that the most concentrated sample was almost exclusively distributed in lungs. This can be explained due to the fact that cationic lipophilic compounds accumulate extensively in lung tissues due to trapping mitochondria and lysosomes (as presented in Table 1, the most concentrated sample presents a positive zeta potential, while the other samples present negative zeta potentials). On the contrary, this distribution is minor for anionic and neutral compounds [29]. The positive charge provokes rapid adsorption of plasma proteins after intravenous administration, leading to particle aggregation. These aggregates are trapped in small capillaries, especially in lungs, or taken up by phagocytosis by macrophages [27]. It is very important to highlight that the nanoparticles, once entering the bloodstream, are covered by the so-called protein corona which could alter their physicochemical characteristics. As a result, a nanoparticle’s unique properties could be different during the biodistribution experiment and can therefore lead to diverse biological behavior. The adsorption of the corona protein by the nanoparticle surface depends on its size, charge, shape and lipophilicity, as well as the environment it has moved through. More specifically, regarding their size, it has been demonstrated that the smaller gold nanoparticles tend to form larger protein coronas when compared to larger gold nanoparticles [30]. Furthermore, several studies reveal that increasing the surface charge of the nanoparticle leads to higher protein adsorption. The serum proteins are mostly negatively charged and tend to adsorb on positively charged nanoparticles. The formation of protein corona causes changes in the hydrodynamic diameter and zeta potential of the nanoparticle [31], which in turn affects in vivo biodistribution.
Another point that needs to be highlighted is that the uptake in lungs for the most concentrated sample was very high (>50%) and significantly increased compared to all the other concentrations, as shown in Figure 3.
4. Conclusions
In conclusion, we have investigated the role of AuNPs’ concentration in their in vivo kinetics by evaluating the ex vivo biodistribution in mice after their radiolabeling with 99mTc. The high-concentration sample resulted in pronounced lung, liver and spleen uptake, while all the other less concentrated samples also accumulated in the lungs, liver and spleen, albeit at lower percentages. Our experiments demonstrate that AuNPs’ concentration alters organ distribution, which may be attributed to the difference in zeta potential of the samples, leading to higher protein adsorption for the increased-surface-charge species, and should be taken into account when designing experimental protocols for radiolabeling nanoparticles. Furthermore, the accumulation of nanoparticles for the most concentrated sample remains sufficiently high in lungs (>50%) even at 24 h, making it a very promising therapeutic candidate for future evaluation in lung cancer when radiolabeled with beta-, alpha- or Auger-emitting therapeutic radioisotopes.
Author Contributions
Conceptualization: A.A., P.K. and P.B.; methodology: A.A., E.-A.S., A.C., S.X. and P.K.; validation: I.C.P., P.K. and P.B.; investigation: A.A., E.-A.S. and N.N.P.; resources: I.C.P., P.K. and P.B.; funding acquisition: P.K. and P.B.; writing—original draft preparation: A.A. and P.B.; writing—review and editing, all authors; supervision: P.K. and P.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out as part of the research activities of the Radiochemical Studies Laboratory of the Institute of Nuclear & Radiological Sciences & Technology, Energy & Safety, National Center for Scientific Research “Demokritos”. This work has been supported by the statutory activity of the Institute of Nuclear Chemistry and Technology, Warsaw, Poland.
Institutional Review Board Statement
The animal study protocol was approved by the Department of Agriculture and Veterinary Service of the Prefecture of Athens (protocol code 634365 and date of approval 27 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors. The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors kindly acknowledge P. Petrou for assistance provided during cytotoxicity experiments. The authors would also like to thank F. Kapiris for excellent technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silva, F.; Cabral Campello, M.P.; Paulo, A. Radiolabeled Gold Nanoparticles for Imaging and Therapy of Cancer. Materials 2020, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Zuckerman, J.E.; Choi, C.H.J.; Han, H.; Davis, M.E. Polycation-siRNA Nanoparticles Can Disassemble at the Kidney Glomerular Basement Membrane. Proc. Natl. Acad. Sci. USA 2012, 109, 3137–3142. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in Cellular Drug Delivery. Bioorganic Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Arvizo, R.R.; Miranda, O.R.; Moyano, D.F.; Walden, C.A.; Giri, K.; Bhattacharya, R.; Robertson, J.D.; Rotello, V.M.; Reid, J.M.; Mukherjee, P. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nanoparticles. PLoS ONE 2011, 6, 24374. [Google Scholar] [CrossRef]
- Balogh, L.; Nigavekar, S.S.; Nair, B.M.; Lesniak, W.; Zhang, C.; Sung, L.Y.; Kariapper, M.S.T.; El-Jawahri, A.; Llanes, M.; Bolton, B.; et al. Significant Effect of Size on the in Vivo Biodistribution of Gold Composite Nanodevices in Mouse Tumor Models. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Andresen, T.L. Factors Controlling Nanoparticle Pharmacokinetics: An Integrated Analysis and Perspective. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 481–503. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Kohane, D.S.; Tse, J.Y.; Yeo, Y.; Padera, R.; Shubina, M.; Langer, R. Biodegradable Polymeric Microspheres and Nanospheres for Drug Delivery in the Peritoneum. J. Biomed. Mater. Res. 2006, 77A, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rao, Y.; Liu, X.; Sun, L.; Gong, J.; Zhang, H.; Shen, L.; Bao, A.; Yang, H. Administration Route Governs the Therapeutic Efficacy, Biodistribution and Macrophage Targeting of Anti-Inflammatory Nanoparticles in the Lung. J. Nanobiotechnol. 2021, 19, 56. [Google Scholar] [CrossRef] [PubMed]
- Black, K.C.L.; Wang, Y.; Luehmann, H.P.; Cai, X.; Xing, W.; Pang, B.; Zhao, Y.; Cutler, C.S.; Wang, L.V.; Liu, Y.; et al. Radioactive 198Au-Doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. [Google Scholar] [CrossRef] [PubMed]
- Same, S.; Aghanejad, A.; Akbari Nakhjavani, S.; Barar, J.; Omidi, Y. Radiolabeled Theranostics: Magnetic and Gold Nanoparticles. Bioimpacts 2016, 6, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.M.; Broome, A.-M. Gold Nanoparticles for the Delivery of Cancer Therapeutics. Adv. Cancer Res. 2018, 139, 163–184. [Google Scholar] [PubMed]
- Fan, M.; Han, Y.; Gao, S.; Yan, H.; Cao, L.; Li, Z.; Liang, X.-J.; Zhang, J. Ultrasmall Gold Nanoparticles in Cancer Diagnosis and Therapy. Theranostics 2020, 10, 4944–4957. [Google Scholar] [CrossRef]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and Effective Photodynamic Therapy for Cancer Using Functionalized Nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, L.; Yang, J.; Chen, L.; Shi, J.; Zhao, J.; Shi, X. 99mTc-Labeled Polyethylenimine-Entrapped Gold Nanoparticles with pH-Responsive Charge Conversion Property for Enhanced Dual Mode SPECT/CT Imaging of Cancer Cells. Langmuir 2019, 35, 13405–13412. [Google Scholar] [CrossRef]
- Morales-Avila, E.; Ferro-Flores, G.; Ocampo-García, B.E.; De León-Rodríguez, L.M.; Santos-Cuevas, C.L.; García-Becerra, R.; Medina, L.A.; Gómez-Oliván, L. Multimeric System of 99mTc-Labeled Gold Nanoparticles Conjugated to c[RGDfK(C)] for Molecular Imaging of Tumor α(v)β(3) Expression. Bioconjugate Chem. 2011, 22, 913–922. [Google Scholar] [CrossRef]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin Functionalized Gold Nanoparticles Show in Vitro and in Vivo Cancer Receptor Specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef]
- Li, X.; Xiong, Z.; Xu, X.; Luo, Y.; Peng, C.; Shen, M.; Shi, X. 99mTc-Labeled Multifunctional Low-Generation Dendrimer-Entrapped Gold Nanoparticles for Targeted SPECT/CT Dual-Mode Imaging of Tumors. ACS Appl. Mater. Interfaces 2016, 8, 19883–19891. [Google Scholar] [CrossRef] [PubMed]
- Alric, C.; Miladi, I.; Kryza, D.; Taleb, J.; Lux, F.; Bazzi, R.; Billotey, C.; Janier, M.; Perriat, P.; Roux, S.; et al. The Biodistribution of Gold Nanoparticles Designed for Renal Clearance. Nanoscale 2013, 5, 5930. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, A.; Chiotellis, A.; Salvanou, E.-A.; Makrypidi, K.; Tsoukalas, C.; Kapiris, F.; Paravatou-Petsotas, M.; Papadopoulos, M.; Pirmettis, I.C.; Koźmiński, P.; et al. Synthesis and In Vitro Evaluation of Gold Nanoparticles Functionalized with Thiol Ligands for Robust Radiolabeling with 99mTc. Nanomaterials 2021, 11, 2406. [Google Scholar] [CrossRef]
- Dexter, D.L.; Kowalski, H.M.; Blazar, B.A. Heterogeneity of Tumor Cells from a Single Mouse Mammary Tumor. Cancer Res. 1978, 38, 3174–3181. [Google Scholar] [PubMed]
- Krystofiak, E.S.; Matson, V.Z.; Steeber, D.A.; Oliver, J.A. Elimination of Tumor Cells Using Folate Receptor Targeting by Antibody-Conjugated, Gold-Coated Magnetite Nanoparticles in a Murine Breast Cancer Model. J. Nanomater. 2012, 2012, 431012. [Google Scholar] [CrossRef]
- Heppner, G.H.; Miller, F.R.; Malathy Shekhar, P. Nontransgenic Models of Breast Cancer. Breast Cancer Res. 2000, 2, 331. [Google Scholar] [CrossRef] [PubMed]
- Prokop, A. (Ed.) Intracellular Delivery: Fundamentals and Applications; Fundamental Biomedical Technologies; Springer: Berlin/Heidelberg, Germany, 2011; Volume 5, pp. 73–97. [Google Scholar] [CrossRef]
- Qiao, Q.; Liu, X.; Yang, T.; Cui, K.; Kong, L.; Yang, C.; Zhang, Z. Nanomedicine for Acute Respiratory Distress Syndrome: The Latest Application, Targeting Strategy, and Rational Design. Acta Pharm. Sin. B 2021, 11, 3060–3091. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; Doolette, D. Kinetic Aspects of Drug Disposition in the Lungs. Clin. Exp. Pharma. Physio. 1999, 26, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Brun, E.; Sicard-Roselli, C. Could Nanoparticle Corona Characterization Help for Biological Consequence Prediction? Cancer Nano 2014, 5, 7. [Google Scholar] [CrossRef]
- Forest, V.; Pourchez, J. Preferential Binding of Positive Nanoparticles on Cell Membranes Is Due to Electrostatic Interactions: A Too Simplistic Explanation That Does Not Take into Account the Nanoparticle Protein Corona. Mater. Sci. Eng. C 2017, 70, 889–896. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).