The Effect of Cold Plasma on Selected Parameters of Bovine Colostrum
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microbiological Analysis
2.3. Measurement of pH
2.4. Colour Analysis
2.5. Sensory Evaluation
2.6. Cold Plasma Treatment
2.7. Protein Extract Preparation
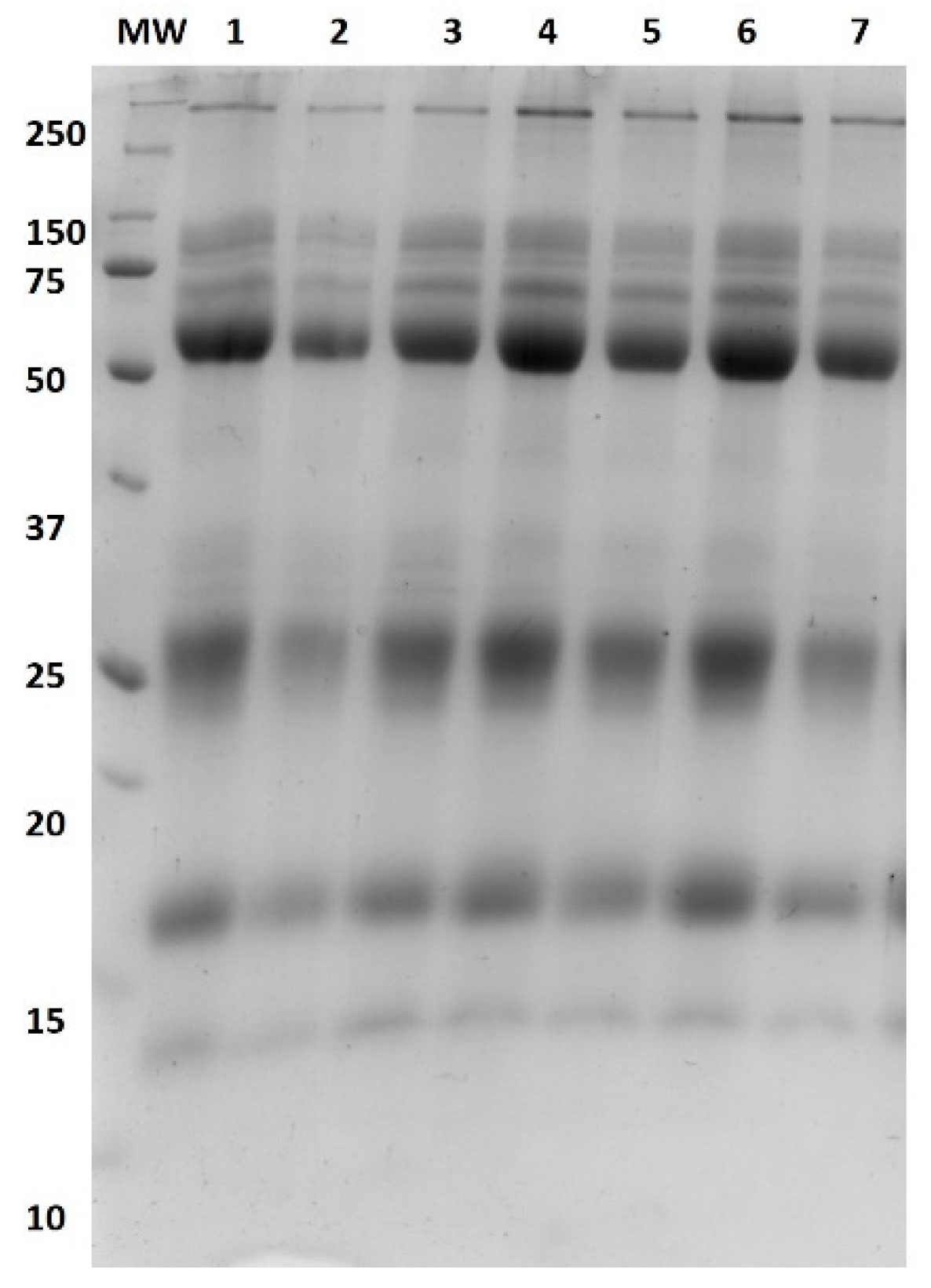
2.8. Protein Profiling Using SDS-PAGE (1-DE)
2.9. Two-Dimensional Electrophoresis (2-DE)
2.10. MALDI—TOF (Matrix Assisted Laser Desorption/Ionisation-Time of Flight) Mass Spectrometry
2.11. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brian, A.M.; Patric, F.F.; Paul, S.; Alan, K. Composition and properties of bovine colostrum: A review. Dairy Sci. Technol. 2016, 96, 133–158. [Google Scholar]
- Pakkanen, R.; Aalto, J. Growth factors and antimicrobial factors of bovine colostrum. Int. Dairy J. 1997, 7, 258–297. [Google Scholar] [CrossRef]
- Shen, R.L.; Thymann, T.; Østergaard, M.V.; Støy, A.C.; Krych, Ł.; Nielsen, D.S.; Lauridsen, C.; Hartmann, B.; Holst, J.J.; Burrin, D.G.; et al. Early gradual feeding with bovine colostrum improves gut function and NEC resistance relative to infant formula in preterm pigs. Am. J. Physiol. Gastrointest. Liver. Physiol 2015, 309, 310–320. [Google Scholar] [CrossRef]
- Playford, R.J.; Weiser, M.J. Bovine Colostrum: Its Constituents and Uses. Nutrients 2021, 13, 265. [Google Scholar] [CrossRef]
- Płusa, T. Immunomodulatory proteins in colostrum. Pol. Merkur. Lekarski. 2009, 26, 8–10. [Google Scholar]
- Chen, D.; Peng, P.; Zhou, N.; Cheng, Y.; Min, M.; Ma, Y.; Mao, Q.; Chen, P.; Chen, C.; Ruan, R. Evaluation of Cronobacter sakazakii inactivation and physicochemical property changes of non-fat dry milk powder by cold atmospheric plasma. Food Chem. 2019, 290, 270–276. [Google Scholar] [CrossRef]
- Chiang, S.H.; Chang, C.Y. Antioxidant properties of caseins and whey proteins from colostrums. J. Food Drug. Anal. 2005, 13, 57–63. [Google Scholar] [CrossRef]
- Wieczorek—Dąbrowska, M.; Wójcik, P.; Malinowski, E. Importance of cow colostrum and factors determining its quality. Balice Przegląd Hod. 2013, 81, 9–10. [Google Scholar]
- Kim, J.S.; Lee, E.J.; Choi, E.H.; Kim, Y.J. Inactivation of Staphylococcus aureus on the beef jerky by radio-frequency atmospheric pressure plasma discharge treatment. Innov. Food Sci. Emerg. Technol. 2014, 22, 124–130. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef]
- Fecteau, G.; Baillargeon, P.; Higgins, R.; Paré, J.; Fortin, M. Bacterial contamination of colostrum fed to newborn calves in Québec dairy herds. Can. Vet. J. 2002, 43, 523–527. [Google Scholar]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Yepez, X.V.; Misra, N.N.; Keener, K.M. Nonthermal Plasma Technology. In Food Safety Engineering. Food Engineering Series; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer: New York, NY, USA, 2020; pp. 607–628. [Google Scholar]
- Reineke, K.; Langer, K.; Hertwig, C.; Ehlbeck, J.; Schlüter, O. The impact of different process gas compositions on the inactivation effect of an atmospheric pressure plasma jet on Bacillus spores. Innov. Food Sci. Emerg. Technol. 2015, 30, 112–118. [Google Scholar] [CrossRef]
- Kim, H.-J.; Yong, H.I.; Park, S.; Choe, W.; Jo, C. Effects of dielectric barrier discharge plasma on pathogen inactivation and the physicochemical and sensory characteristics of pork loin. Curr. Appl. Phys. 2013, 13, 1420–1425. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, S.; Jung, H.; Park, S.; Choe, W.; Ham, J.S.; Jo, C. Evalution of dielectric barier discharge plasma system for inactivatating pathogens on cheese slices. J. Anim. Sci. Technol. 2012, 54, 191–198. [Google Scholar] [CrossRef]
- Moreau, M.; Orange, N.; Feuilloley, M.G.J. Non-thermal plasma technologies: New tools for bio-decontamination. Biotechnol. Adv. 2008, 26, 610–617. [Google Scholar] [CrossRef]
- PN—93/A-86034/02; Milk and Dairy Products. Microbiological Testing. General Principles of Testing. Polish Committee for Standardization: Warsaw, Poland, 2018.
- ISO 6887-5:2020-10; Microbiology of the Food Chain—Preparation of Samples, Stock Suspension and Decimal Dilutions for Microbiological Testing—Part 5: Specific Rules for the Preparation of Milk and Milk Products. Polish Committee for Standardization: Warsaw, Poland, 2020.
- ISO 4833-2:2013-12; Microbiology of the Food Chain—Horizontal Method for the Determination of Microbial Counts—Part 2: Determination of Counts by Surface Culture at 30 Degrees C. Polish Committee for Standardization: Warsaw, Poland, 2013.
- ISO 21527-1:2009; Microbiology of Food and Feed—Horizontal Method for the Determination of Yeast and Mold Counts—Part 1: Method for Counting Colonies in Products with Water Activity Higher than 0.95. Polish Committee for Standardization: Warsaw, Poland, 2009.
- ISO 21528-2:2017-08; Food Chain Microbiology—Horizontal Method for Detection and Enumeration of Enterobacteriaceae—Part 2: Colony Counting Method. Polish Committee for Standardization: Warsaw, Poland, 2017.
- ISO 6888-2:2001+A1:200; Microbiology of Food and Feed—Horizontal Method for the Determination of Coagulase-Positive Staphylococci (Staphylococcus Aureus and Other Species)—Part 2: Method Using Rabbit Plasma Agar Medium and Fibrynogen. Polish Committee for Standardization: Warsaw, Poland, 2004.
- ISO 7932:2005/A1:2020-09; Microbiology of Food and Feed—Horizontal Method for Determining the Number of Putative Bacillus Cereus—Method of Counting Colonies at 30 Degrees C. Polish Committee for Standardization: Warsaw, Poland, 2020.
- PN-EN 15788:2009; Detection and Enumeration of Enterococcus (E. faecium) spp. Polish Committee for Standardization: Warsaw, Poland, 2009.
- ISO 15214:2002; Microbiology of Food and Feed—Horizontal Method for the Determination of the Number of Mesophilic Lactic Fermentation Bacteria—Plate Method at 30 Degrees C. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Dmytrów, I.; Szymczak, M.; Szkolnicka, K.; Kamiński, P. Development of Functional Acid Curd Cheese (Tvarog) with Antioxidant Activity Containing Astaxanthin from Shrimp Shells Preliminary Experiment. Foods 2021, 10, 895. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Physicochemical characterization of plasma-treated sodium caseinate film. Food Res. Int. 2014, 66, 438–444. [Google Scholar] [CrossRef]
- ISO 22935-2:2013-07; Milk and Dairy Products—Sensory Analysis—Part 2: Recommended Methods for Sensory Evaluation. Polish Committee for Standardization: Warsaw, Poland, 2013.
- ISO 22935-3:2013-07; Milk and Dairy Products—Sensory Analysis—Part 3: Guidelines for Evaluating the Conformity of the Properties of Sensory Attributes with Product Specifications Using the Scoring Method. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Drake, M.A.; Karagul-Yuceer, Y.; Cadwallader, K.R.; Civtlle, G.V.; Tong, P.S. Determination of the sensory attributes of dried milk powders and dairy ingredients. J. Sens. Stud. 2003, 18, 199–2016. [Google Scholar] [CrossRef]
- Abdalla, A.K.; Smith, J.K.; Lucey, K. Physical Properties of Nonfat Dry Milk and Skim Milk Powder. Int. J. Dairy Sci. 2017, 12, 149–154. [Google Scholar] [CrossRef]
- Lepczyński, A.; Ożgo, M.; Dratwa-Chałupnik, A.; Robak, P.; Pyć, A.; Zaborski, D.; Herosimczyk, A. An update on medium- and low-abundant blood plasma proteome of horse. Animal 2018, 12, 76–87. [Google Scholar] [CrossRef]
- Lepczyński, A.; Ożgo, M.; Michałek, K.; Dratwa-Chałupnik, A.; Grabowska, M.; Herosimczyk, A.; Liput, K.P.; Poławska, E.; Kram, A.; Pierzchała, M. Effects of three-month feeding high fat diets with different fatty acid composition on myocardial proteome in mice. Nutrients 2021, 13, 330. [Google Scholar] [CrossRef]
- Ozgo, M.; Lepczynski, A.; Herosimczyk, A. Two-dimensional gel-based serum protein profile of growing piglets. Turk. J. Biol. 2015, 39, 320–327. [Google Scholar] [CrossRef]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum Management for Dairy Calves. Vet. Clin. North. Am. Food. Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef]
- Lorenz, I.; Mee, J.F.; Earley, B.; More, S.J. Calf health from birth to weaning. General aspects of disease prevention. Ir. Vet. J. 2011, 16, 3–8. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold atmospheric plasma: Charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Fröhling, A.; Baier, M.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Atmospheric pressure plasma treatment of Listeria innocua and Escherichia coli at polysaccharide surfaces: Inactivation kinetics and flow cytometric characterization. Innov. Food Sci. Emerg. Technol. 2012, 13, 142–150. [Google Scholar] [CrossRef]
- Hertwig, C.; Reineke, K.; Ehlbeck, J.; Erdoğdu, B.; Rauh, C.; Schlüter, O. Impact of remote plasma treatment on natural microbial load and quality parameters of selected herbs and spices. J. Food Eng. 2015, 167, 12–17. [Google Scholar] [CrossRef]
- Cummins, C.; Lorenz, I.; Kennedy, E. Short communication: The effect of storage conditions over time on bovine colostral immunoglobulin G concentration, bacteria, and pH. J. Dairy Sci. 2016, 99, 4857–4863. [Google Scholar] [CrossRef] [PubMed]
- Fasse, S.; Jarmo, A.; Björn, F.; Gun, W. Bovine Colostrum for Human Consumption—Improving Microbial Quality and Maintaining Bioactive Characteristics through Processing. Dairy 2021, 2, 556–575. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Moreau, S.; Pelletier, J.; Tabrizian, M.; Yahia, L.H. Low-temperature sterilization using gas plasmas: A review of the experiments and an analysis of the inactivation mechanisms. Int. J. Pharm. 2001, 226, 1–21. [Google Scholar] [CrossRef]
- Schlüter, O.; Fröhling, A. Non-Thermal Processing Cold Plasma for Bioefficient Food Processing, Reference Module in Food Science Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: New York, NY, USA, 2014; pp. 948–953. [Google Scholar]
- Li, J.; Sakai, N.; Watanabe, M.; Hotta, E.; Wachi, M. Study on plasma agent effect of a direct-current atmospheric pressure oxygen-plasma jet on inactivation of E. coli using bacterial mutants. IEEE Trans. Plasma Sci. 2013, 41, 935–941. [Google Scholar] [CrossRef]
- Joshi, S.G.; Cooper, M.; Yost, A.; Paff, M.; Ercan, U.K.; Fridman, G.; Friedman, G.; Fridman, A.; Brooks, A.D. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob. Agents. Chemother. 2011, 55, 1053–1062. [Google Scholar] [CrossRef]
- Hertwig, C.; Meneses, N.; Mathys, A. Cold atmospheric pressure plasma and low energy electron beam as alternative nonthermal decontamination technologies for dry food surfaces: A review. Trends Food Sci. Technol. 2018, 77, 131–142. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical mechanisms of inactivation of Bacillus subtilis spores using cold atmospheric plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Tseng, S.; Abramzon, N.; Jackson, J.O.; Lin, W.J. Gas discharge plasmas are effective in inactivating Bacillus and Clostridium spores. Appl. Microbiol. Biotechnol. 2012, 93, 2563–2570. [Google Scholar] [CrossRef]
- Wang, S.; Doona, C.J.; Setlow, P.; Li, Y.Q. Use of Raman Spectroscopy and Phase-Contrast Microscopy to Characterize Cold Atmospheric Plasma Inactivation of Individual Bacterial Spores. Appl. Environ. Microbiol. 2016, 82, 5775–5784. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Effect of atmospheric pressure cold plasma (ACP) on activity and structure of alkaline phosphatase. Food Bioprod. Process. 2016, 98, 181–188. [Google Scholar] [CrossRef]
- Manoharan, D.; Stephen, J.; Radhakrishnan, M. Study on low-pressure plasma system for continuous decontamination of milk and its quality evaluation. J. Food Process. Preserv. 2020, 45, 234–245. [Google Scholar] [CrossRef]
- Wan, Z.; Misra, N.N.; Li, G.; Keener, K.M. High voltage atmospheric cold plasma treatment of Listeria innocua and Escherichia coli K-12 on Queso Fresco (fresh cheese). LWT 2021, 146, 111406. [Google Scholar] [CrossRef]
- Oehmigen, K.; Hahnel, M.; Brandenburg, R.; Wilke, C.; Weltmann, K.D.; von Woedtke, T. The Role of Acidification for Antimicrobial Activity of Atmospheric Pressure Plasma in Liquids. Plasma Process. Polym. 2010, 7, 250–257. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. Appl. Phys. 2011, 44, 134–145. [Google Scholar] [CrossRef]
- Gross, J.J.; Kessler, E.C.; Bruckmaier, R.M. Colour measurement of colostrum for estimation of colostral IgG and colostrum composition in dairy cows. J. Dairy. Res. 2014, 81, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Gurol, C.; Ekinci, F.Y.; Aslan, N.; Korachi, M. Low Temperature Plasma for decontamination of E. coli in milk. Int. J. Food. Microbiol. 2012, 157, 1–5. [Google Scholar] [CrossRef]
- Yong, H.I.; Kim, H.-J.; Park, S.; Kim, K.; Choe, W.; Yoo, S.J.; Jo, C. Pathogen inactivation and quality changes in sliced cheddar cheese treated using flexible thin-layer dielectric barrier discharge plasma. Food Res. Int. 2015, 69, 57–63. [Google Scholar] [CrossRef]
- Nikmaram, N.; Keener, K.M. The effects of cold plasma technology on physical, nutritional, and sensory properties of milk and milk products. LWT 2022, 154, 112729. [Google Scholar] [CrossRef]
- Segat, A.; Misra, N.N.; Cullen, P.J.; Innocente, N. Atmospheric pressure cold plasma (ACP) treatment of whey protein isolate model solution. Innov. Food Sci. Emerg. Technol. 2015, 29, 247–254. [Google Scholar] [CrossRef]
- Wu, X.; Luo, Y.; Zhao, F.; Murad, M.S.; Mu, G. Influence of dielectric barrier discharge cold plasma on physicochemical property of milk for sterilization. Plasma Process. Polym. 2021, 18, 245–256. [Google Scholar] [CrossRef]
- Singh, P.K.; Huppertz, T. Chapter 8—Effect of Nonthermal Processing on Milk Protein Interactions and Functionality. In Milk Proteins; Boland, M., Singh, H., Eds.; Academic Press: Toronto, ON, Canada, 2020; pp. 293–324. [Google Scholar]
- Ng, S.W.; Lu, P.; Rulikowska, A.; Boehm, D.; O’Neill, G.; Bourke, P. The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chem. 2021, 342, 128283. [Google Scholar] [CrossRef] [PubMed]
- Ramazzina, I.; Tappi, S.; Rocculi, P.; Sacchetti, G.; Berardinelli, A.; Marseglia, A.; Rizzi, F. Effect of Cold Plasma Treatment on the Functional Properties of Fresh-Cut Apples. J. Agric. Food. Chem. 2016, 64, 8010–8018. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zhuang, J.; Zong, Z.; Zhang, X.; Liu, D.; Bazaka, K.; Ostrikov, K. Interaction of Atmospheric-Pressure Air Microplasmas with Amino Acids as Fundamental Processes in Aqueous Solution. PLoS ONE 2016, 11, 134–148. [Google Scholar] [CrossRef]
- Held, S.; Tyl, C.E.; Annor, G.A. Effect of radio frequency cold plasma treatment on intermediate wheatgrass (Thinopyrum intermedium) flour and dough properties in comparison to hard and soft wheat (Triticum aestivum L.). J. Food. Qual. 2019, 34, 156–176. [Google Scholar] [CrossRef]
- Ji, H.; Dong, S.; Han, F.; Li, Y.; Chen, G.; Li, L.; Chen, Y. Effects of Dielectric Barrier Discharge (DBD) Cold Plasma Treatment on Physicochemical and Functional Properties of Peanut Protein. Food Bioprocess Technol. 2018, 11, 344–354. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102–112. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food. Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]

| Sample | Gas Flow | Time to Obtain Vacuum | Temperature | Power | Process Time | Pressure in the Chamber |
|---|---|---|---|---|---|---|
| [cm3/min] | [min] | [°C] | [W] | [min] | [mbar] | |
| Air | 20–50 | 60 | 27–30 | 300 | 60 | 0.6 |
| Nitrogen | 20–48 | 60 | 28–32 | |||
| Oxygen | 20–50 | 70 | 26–30 |
| Bacteria | Average | LL-UL | SEM | p-Value |
|---|---|---|---|---|
| Normal inhabitants of bovine skin and mucosa (NI) | log cfu/g | |||
| Mesophilic bacteria (THMC) | 4.70 * | 3.21–4.86 | 0.082 | 0.001 |
| Staphylococcus spp. (TSt) | 1.89 * | 0.5–3.29 | 0.148 | 0.031 |
| Streptococcus spp. (TStr) | 1.87 * | 1.00–2.30 | 0.135 | 0.049 |
| Yeast (TYMC) | 2.95 * | 1.61–3.25 | 0.019 | 0.046 |
| LAB | 2.94 * | 1.29–3.72 | 0.006 | 0.001 |
| Environmental contaminants (EC) | ||||
| Psychrotrophic bacteria (THPC) | 5.24 | 4.56–5.78 | 0.366 | 0.897 |
| Bacillus spp. (TCBac) | 2.96 | 2.48–3.02 | 0.313 | 0.604 |
| Moulds (TMMC) | 1.97 | 1.74–2.24 | 0.185 | 0.091 |
| Faecal contaminants (FC) | ||||
| Enterobacteriacea (TEb) | 2.43 * | 1.18–2.98 | 0.267 | 0.049 |
| E. coli (Ec) | 1.38 * | 0.00–2.00 | 0.348 | 0.048 |
| Enterococcus spp. (TEcc) | 2.37 | 1.69–2.59 | 0.134 | 0.167 |
| Normal Inhabitants of Bovine Skin and Mucosa | Environmental Contaminants | Faecal Contaminants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | THMC | TSt | TStr | TYMC | LAB | THPC | TCBac | TMMC | TEb | Ec | TEcc |
| log cfu/g | |||||||||||
| Air | 0.72 | 0.61 | 0.54 | 0.91 | 0.16 a | 0.73 | 0.76 | 0.45 | 0.68 a | 0.64 | 0.79 |
| Nitrogen | 1.19 | 0.78 | 0.65 | 0.62 | 0.56 | 1.09 | 0.53 | 0.49 | 1.14 | 0.88 | 0.75 |
| Oxygen | 1.02 | 0.73 | 0.75 | 0.92 | 0.88 b | 1.24 | 0.81 | 0.45 | 1.16 b | 0.96 | 0.74 |
| SEM | 0.127 | 0.149 | 0.158 | 0.174 | 0.084 | 0.124 | 0.369 | 0.681 | 0.079 | 0.079 | 0.019 |
| p-value | 0.101 | 0.122 | 0.326 | 0.094 | 0.032 | 0.701 | 0.112 | 0.843 | 0.043 | 0.133 | 0.052 |
| Samples | pH | a*(-) | b* | L* | h (-) | C | ΔE |
|---|---|---|---|---|---|---|---|
| Control | 6.656 | 1.94 | 18.29 | 82.84 a | 83.92 | 18.40 a | - |
| Air | 6.663 | 2.31 | 18.43 | 82.11 | 82.87 | 18.57 | 0.82 A |
| Nitrogen | 6.654 | 2.04 | 19.64 | 81.49 b | 84.18 | 19.74 b | 1.90 B,C |
| Oxygen | 6.667 | 1.87 | 19.27 | 82.49 | 84.46 | 19.36 | 1.04 D |
| SEM | 0.033 | 0.039 | 0.554 | 0.166 | 0.855 | 0.338 | 0.159 |
| p-value | 0.992 | 0.056 | 0.082 | 0.012 | 0.055 | 0.000 | 0.000 |
| Sample | Smell | Taste | Structure | Overall Sensory Quality |
|---|---|---|---|---|
| Control | 3.954 | 3.944 | 3.978 | 3.972 |
| Air | 3.833 | 3.899 | 3.944 | 3.745 |
| Nitrogen | 4.012 | 4.051 | 3.816 | 4.034 |
| Oxygen | 3.675 | 3.749 | 3.801 | 3.933 |
| SEM | 0.450 | 0.801 | 0.394 | 0.654 |
| p-value | 0.178 | 0.450 | 0.765 | 0.125 |
| No. | Protein Name | Gene Name | Accession Number | C | N2 | N2/C | A | A/C | O2 | O2/C | Pep. Mach | Seq.% /Score | pI/Mr pH/kDa | Sp. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Immunoglobulin gamma | IGHG | AQT27060 | 251.6 | 25.8 | 0.1 | 14.9 | 0.06 | 51 | 0.2 | 28 | 65/82 | 6.21/51.09 | B |
| 2 | heavy chain | 144.1 | 9.6 | 0.07 | 7.3 | 0.05 | 31.7 | 0.22 | 18 | 42/87 | ||||
| 3 | 174.4 | 146 | 0.84 | 118.9 | 0.68 | 50.4 | 0.29 | 18 | 42/100 | |||||
| 4 | 173.6 | 82.6 | 0.48 | 31.2 | 0.18 | 64.7 | 0.37 | 16 | 42/82 | |||||
| 5 | 152.1 | 121.4 | 0.8 | 257.9 | 1.7 | 52.3 | 0.34 | 15 | 41/84 | |||||
| 6 | 57.9 | 125.4 | 2.17 | 203.3 | 3.51 | 89.8 | 1.55 | 14 | 41/84 | |||||
| 7 | 43.8 | 7.8 | 0.18 | 133.6 | 3.05 | 34.5 | 0.79 | 14 | 41/86 | |||||
| 8 | l-lactate dehydrogenase A chain | LDHA | P00339 | 58.6 | 22.8 | 0.39 | 38.2 | 0.65 | 23.4 | 0.4 | 13 | 58/64 | S | |
| 9 | Sclerostin domain-containing protein 1 | SOSTDC1 | Q9CQN4 | 28 | 16.9 | 0.61 | 41.3 | 1.48 | 16.5 | 0.59 | 7 | 33/63 | 9.87/23.73 | M |
| 10 | Serotransferrin | TRF | Q299443 | 15.9 | 4.4 | 0.28 | 11.4 | 0.72 | 0.3 | 0.02 | 39 | 63/78 | 6.75/79.87 | B |
| 11 | 24.7 | 24.3 | 0.98 | 50.5 | 2.04 | 19.8 | 0.8 | 40 | 62/63 | |||||
| 12 | 42 | 25.2 | 0.6 | 85.7 | 2.04 | 116.9 | 2.78 | 41 | 65/66 | |||||
| 13 | 31.2 | 40.7 | 1.3 | 62.5 | 2 | 57.2 | 1.83 | 41 | 66/99 | |||||
| 14 | 38 | 22.1 | 0.58 | 28.8 | 0.76 | 2.5 | 0.06 | 17 | 33/61 | |||||
| 15 | 23.7 | 24.2 | 1.02 | 12.3 | 0.52 | 15.4 | 0.65 | 45 | 68/81 | |||||
| 16 | 10.2 | 18.1 | 1.78 | 23.1 | 2.27 | 13.6 | 1.34 | 43 | 68/120 | |||||
| 17 | 16 | - | - | - | - | - | - | 36 | 62/82 | |||||
| 18 | Secreted immunoglobulin mu 2 heavy chain constant region, partial | IGMG | ANN46371 | 87.4 | 17.9 | 0.2 | 73.1 | 0.84 | 23.4 | 0.27 | 23 | 74/93 | 5.48/50.30 | B |
| 19 | Immunoglobulin mu heavy chain constant region, partial | IGMG | AAO37096 | 99.6 | 63.2 | 0.63 | 114.5 | 1.15 | 77 | 0.77 | 22 | 69/88 | 5.49/49.85 | B |
| 20 | Secreted immunoglobulin mu 2 heavy chain constant region, partial | IGMG | ANN46371 | 49.7 | 20.8 | 0.42 | 0.3 | 0.01 | 14.1 | 0.28 | 19 | 66/71 | 5.48/50.30 | B |
| 21 | Serum albumin precursor | ALB | NP851335 | 30.1 | 4.2 | 0.14 | 14.4 | 0.48 | 2.6 | 0.09 | 24 | 43/98 | 5.82/71.27 | B |
| 22 | Serum albumin | ALB | P02769 | 379.9 | 62 | 0.16 | 54.5 | 0.14 | 71.7 | 0.19 | 17 | 34/75 | 5.82/71.24 | B |
| 23 | 465.7 | 248.2 | 0.53 | 334.9 | 0.72 | 206.5 | 0.44 | 17 | 31/75 | |||||
| 24 | 351.2 | 264.6 | 0.75 | 738.1 | 2.1 | 328.3 | 0.93 | 21 | 41/87 | |||||
| 25 | 604.4 | 69.7 | 0.12 | 99.9 | 0.17 | 162.6 | 0.27 | 18 | 34/76 | |||||
| 26 | Immunoglobulin gamma | IGHG | AQT27060 | 596.1 | 363.9 | 0.61 | 475.3 | 0.8 | 111.5 | 0.19 | 16 | 48/22 | 6.21/51.09 | B |
| 27 | heavy chain | 866.9 | 105.1 | 0.12 | 390.6 | 0.45 | 1891 | 2.18 | 24 | 53/80 | ||||
| 28 | Secreted immunoglobulin gamma1 heavy chain constant region, partial | IGHG | ANN46377 | 2237 | 2301 | 1.03 | 2428 | 1.09 | 287.2 | 0.13 | 14 | 60/79 | 6.07/36.32 | B |
| 29 | Immunoglobulin gamma | IGHG | AQT27060 | 2275 | 1017 | 0.45 | 1618 | 0.71 | 2225 | 0.98 | 26 | 56/89 | 6.21/50.09 | B |
| 30 | heavy chain | 5708 | 4441 | 0.78 | 4624 | 0.81 | 3248 | 0.57 | 15 | 48/82 | 6.21/50.09 | B | ||
| 31 | 69.1 | 759.5 | 11 | 160.6 | 2.33 | 1212 | 17.6 | 23 | 53/91 | |||||
| 32 | 1777 | 657.4 | 0.37 | 5782 | 3.25 | 627 | 0.35 | 16 | 49/109 | |||||
| 33 | 94 | 759.5 | 8.08 | 160.6 | 1.71 | 1212 | 12.9 | 17 | 50/107 | |||||
| 34 | 698.5 | 990.2 | 1.42 | 1057 | 1.51 | 779.5 | 1.12 | 16 | 47/87 | |||||
| 35 | 1087 | 566.7 | 0.52 | 1282 | 1.18 | 1709 | 1.57 | 15 | 41/81 | |||||
| 36 | 952.9 | 2962 | 3.11 | 413.2 | 0.43 | 1967 | 2.06 | 15 | 48/80 | |||||
| 37 | 1122 | 100.3 | 0.09 | 219.6 | 0.2 | 494.7 | 0.44 | 16 | 53/90 | |||||
| 38 | t-RNA specific adenosine | ADAT2 | Q5E9J7 | 55.8 | 24.9 | 0.45 | 49.7 | 0.89 | 41.3 | 0.74 | 14 | 61/61 | 6.33/21.57 | B |
| 39 | deaminase 2 | 29.4 | 11.5 | 0.39 | 14.9 | 0.51 | 17.9 | 0.61 | 14 | 61/58 | ||||
| 40 | Immunoglobulin gamma | IGHG | AQT27060 | 43.2 | 18.5 | 0.43 | 35.8 | 0.83 | 12.3 | 0.28 | 26 | 58/83 | 6.21/50.09 | B |
| 41 | heavy chain | 51.4 | 21.8 | 0.42 | 30.3 | 0.59 | 10.8 | 0.21 | 15 | 43/91 | ||||
| 42 | IgG heavy chain precursor (B/MT.4A.17.H5.A5) | IGHG | CAA44699 | 25.7 | 18.7 | 0.73 | 21.5 | 0.83 | 5.6 | 0.22 | 22 | 58/91 | 6.10/51.39 | B |
| 43 | Alpha-S1-casein | CSN1S1 | P02662 | 283.9 | 58.1 | 0.2 | 45.7 | 0.16 | 34.6 | 0.12 | 6 | 35/59 | 4.98/24.57 | B |
| 44 | T-complex protein 1 subunit epsilon isoform d | TCP1 | NP_001293084 | 668.8 | 643.3 | 0.96 | 2000 | 2.99 | 863.8 | 1.29 | 29 | 72/81 | 5.86/49.95 | H |
| 45 | Unnamed protein product, partial | CAF90119 | CAF90119 | 1558 | 1783 | 1.14 | 1941 | 1.25 | 710 | 0.46 | 12 | 64/95 | 9.78/13.15 | T |
| 46 | PREDICTED: nck-associated protein 5 isoform X5 | XP_012901706 | 4206 | 1057 | 0.25 | 1757 | 0.42 | 1033 | 0.25 | 95 | 45/83 | 8.25/22.35 | MP | |
| 47 | hypothetical protein EI555_020003, partial | TKC51465 | TKC51465 | 1185 | 191.5 | 0.16 | 489.4 | 0.41 | 840.7 | 0.71 | 21 | 61/82 | 10.5/46.66 | MM |
| 48 | Kv channel-interacting protein 1 | KCIP1 | Q9NZI2 | 1325 | 50 | 0.04 | 253.6 | 0.19 | 433.9 | 0.33 | 26 | 81/66 | 5.10/26.97 | H |
| 49 | Coiled-coil domain- | CCDC183 | XP_030896 | 3331 | 1156 | 0.35 | 1247 | 0.37 | 3818 | 1.15 | 38 | 67/85 | 9.90/32.80 | L |
| 50 | containing protein 183 | 878 | 2019 | 749.5 | 0.37 | 1594 | 0.79 | 2021 | 1 | 37 | 68/86 | |||
| 51 | isoform X2 | 4315 | 2094 | 0.49 | 144.7 | 0.03 | 2743 | 0.64 | 41 | 71/89 | ||||
| 52 | 44.6 | 181.5 | 4.07 | 187.3 | 4.2 | 928.3 | 20.8 | 35 | 76/82 | |||||
| 53 | Beta-lactoglobulin | LGB | P02754 | 155.1 | 22.3 | 0.14 | 21.7 | 0.14 | 6.9 | 0.04 | 23 | 96/62 | 4.93/20.27 | B |
| 54 | Prepronociceptin | PNOC | O62647 | 31 | 4.2 | 0.14 | 10.9 | 0.35 | 0.2 | 0.01 | 5 | 46/56 | 9.67/20.64 | B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusławska-Wąs, E.; Dłubała, A.; Sawicki, W.; Ożgo, M.; Lepczyński, A. The Effect of Cold Plasma on Selected Parameters of Bovine Colostrum. Appl. Sci. 2023, 13, 5490. https://doi.org/10.3390/app13095490
Bogusławska-Wąs E, Dłubała A, Sawicki W, Ożgo M, Lepczyński A. The Effect of Cold Plasma on Selected Parameters of Bovine Colostrum. Applied Sciences. 2023; 13(9):5490. https://doi.org/10.3390/app13095490
Chicago/Turabian StyleBogusławska-Wąs, Elżbieta, Alicja Dłubała, Wojciech Sawicki, Małgorzata Ożgo, and Adam Lepczyński. 2023. "The Effect of Cold Plasma on Selected Parameters of Bovine Colostrum" Applied Sciences 13, no. 9: 5490. https://doi.org/10.3390/app13095490
APA StyleBogusławska-Wąs, E., Dłubała, A., Sawicki, W., Ożgo, M., & Lepczyński, A. (2023). The Effect of Cold Plasma on Selected Parameters of Bovine Colostrum. Applied Sciences, 13(9), 5490. https://doi.org/10.3390/app13095490






