Abstract
Fabricating and developing superhydrophobic anti-icing surfaces have been a research hotspot for eliminating undesired icing issues. Among various fabricating strategies, ultrafast laser micro-nano fabrication is regarded as a greatly promising technique owing to its advantages of high geometric accuracy, highly flexible microstructure or dimension availability, no contact, and no material limitation. A number of diverse micro-nanostructured superhydrophobic surfaces have been developed by ultrafast lasers and demonstrated extraordinary anti-icing properties. They are collectively known as ultrafast laser-fabricated superhydrophobic anti-icing surfaces (ULSASs). In this article, we reviewed the recent advances in ULSASs from micro-nano structure fabricating to anti-icing performances and to potential applications. The surface wettability and mechanisms of ultrafast laser micro-nano fabrication are first introduced, showing the strong ability of ultrafast laser for fabricating superhydrophobic surfaces. Then the deepened understanding of the relationship between superhydrophobicity and icephobicity is discussed in detail, including Cassie–Baxter stability, surface durability and environmental adaptability. Eventually, the passive anti-icing technique, the passive/active combined anti-icing technique and their practical applications are presented together with current challenges and future prospects.
1. Introduction
Icing phenomena have brought tremendous troubles in many fields such as telecommunication, transportation, energy, etc. [1,2,3,4]. Especially in aviation applications, the formed and accumulated ice on airplanes greatly damages aerodynamic properties [5,6,7]. It has been shown that mm-thick ice on airfoils will result in about 30% loss of lift force per unit area, eventually causing catastrophic accidents [8,9]. In recent years, many crashes related to icing have taken place. To eliminate ice formation and build-up, some conventional anti/deicing technologies such as electrothermal, pneumatic, and ultrasonic deicing have been developed and widely used [10,11,12]. This notwithstanding, such active deicing strategies tend to be extremely wasteful of energy, are of low-efficiency, and not environmentally friendly [13]. These drawbacks overwhelmingly restrict their applications in situations such as for diminutive airplanes from drones to warcraft and some industrial applications from pipelines to power lines [14,15]. Therefore, novel anti/deicing technologies are in urgent need of researching and developing to meet the application demands.
Inspired by the water repellency of the lotus in nature, superhydrophobic surfaces (SHSs) have been one of the most promising candidates for achieving passive anti/deicing [16,17]. With extremely high contact angles of >150° and low sliding angles of <10°, SHSs can significantly reduce the solid-liquid contact area to repel external water droplets in time and thus keep surfaces dry [18,19,20]. The unique properties have attracted a flurry of researchers and engineers to pay great attention to moving SHSs toward anti-icing applications [21,22,23,24]. Figure 1 shows the increasing number of published articles on the topic of “anti-icing” and “superhydrophobic anti-icing” in recent years. Among these, papers about superhydrophobic anti-icing account for about one-quarter of all publications, showing their dominant role. However, with the deepening of research, it is found that superhydrophobic surfaces cannot always eliminate icing issues and have greater ice adhesion strengths than smooth pristine surfaces in some cases [25,26]. With regard to mechanisms, superhydrophobicity is mainly attributed to a large number of trapped air pockets among micro-nanostructures, where a triple-phase solid-liquid-gas interface will be constructed. However, the air pockets are very fragile. Once they are broken, greater ice adhesion will be generated owing to the mechanical locking between ice and micro-nanostructures. In this case, the micro-nanostructures also provide more ice nucleation sites to promote the icing process [27,28,29]. These negative results have given rise to a number of debates on whether SHSs are advantageous for anti-icing applications. Kreder et al. compared different types of anti-icing surfaces, including superhydrophobic surfaces, slippery surfaces, and hydrated surfaces. They emphasized the decisive role of surface structures on anti-icing properties [30]. To further understand the relationship between superhydrophobicity and icephobicity and explore the optimized superhydrophobic anti-icing surfaces, fabricating and easily controlling micro-nanostructures is of great significance.
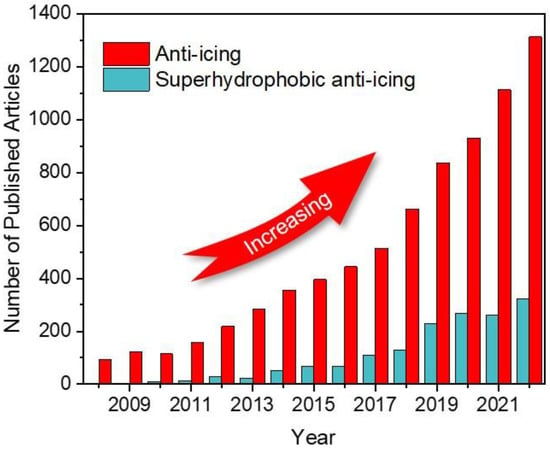
Figure 1.
Number of published articles per year indexed by Web of Science. The keywords for searching are “anti-icing” and “superhydrophobic anti-icing”.
To date, many state-of-the-art strategies have been developed to fabricate micro-nanostructures, including chemical etching, machining, 3D printing, sol–gel methods, laser processing, and others [31,32,33,34,35]. Among these, ultrafast laser micro-nano fabricating, as a novel top-down processing strategy, shows the superior advantages of nm-accuracy, contact-free, and highly programmable processing without material dependence. This method is not only easy for fabricating surface micro-nanostructures but also greatly promising for practical applications [36,37,38,39] (Table 1). Meanwhile, compared to other conventional lasers with continuous wave and short pulses, ultrafast lasers have an ultrashort pulse width from several tens of femtoseconds to tens of picoseconds, which is equivalent to or shorter than the heat conduction time of an electron lattice. Hence, a smaller heat-affected zone and higher processing quality could be realized [40,41]. Generally, the surface structures formed by ultrafast laser irradiation can be divided into two types: laser-ablated structures (arrayed microstructures) and laser-induced structures (random and/or periodic nanostructures) [42,43]. The former’s geometry is mainly determined by the ablation process with intense laser energy interaction with materials and can be closely controlled by user-defined structure shapes. The most typical representative of the latter, the laser-induced periodic surface structure (LIPPS), is formed by linearly polarized laser radiation with fluences slightly above the ablation threshold [44]. By varying laser processing conditions such as wavelength, pulse duration, power density, incidence angle, scanning speed, and processing environments, the morphologies of LIPPS could also be closely controlled. Adopting the above two structures, microscale and nanoscale structures on surfaces could be easily and tunably fabricated with ultrafast lasers. In the meantime, during laser ablation, the plasma plume contains abundant energetic species, which reunite and rapidly deposit on microstructures to form nanostructures, thus constituting the hierarchical micro-nanostructures [45,46,47]. The fabrication of various micro/nanostructures enabled by ultrafast lasers has paved a solid pathway to preparing SHSs. Although most materials such as metals and ceramics are intrinsically hydrophilic so that the superhydrophilic interfaces are produced after laser processing, the superhydrophobicity can be easily achieved by surface chemical modification, including vapor deposition, solution immersing, spinning coating, and so on [48,49,50]. Many reports and advances have been produced in fabricating SHSs and controlling their superhydrophobic states by ultrafast lasers [51,52]. Compared to the SHSs fabricated by other methods, laser-fabricated superhydrophobic surfaces have shown tremendous advantages in durability, stability, and repeatability as well as high tunability [53,54,55,56]. Many reviews have summarized laser-fabricated superhydrophobic surfaces in various application scenarios [40,42,57]. Specifically, research articles about ultrafast laser-fabricated superhydrophobic anti-icing surfaces (ULSASs) have sprung up in recent years [58,59,60,61], as shown in Figure 2. However, to our best knowledge, related reviews and perspectives are rarely reported. In response to the special issue “Superhydrophobic and Icephobic Coatings”, it is of significance and value to summarize recent progress in ULSASs and to propose current challenges and future prospects.

Table 1.
Comparison of common micro-nanofabrication techniques in terms of fabrication rate, accuracy, structural tunability, applicable materials, and large-scale fabricating.
In this review, we focus on ultrafast laser enabled superhydrophobic anti-icing performances and draw examples from surface fabrication, anti-icing mechanisms, and advances and applications, as shown in Figure 3. The fundamental wettability and ultrafast laser micro-nano fabricating examples are discussed first, elucidating the structural diversity and tunability of ultrafast laser fabricating. Adopting ultrafast laser-fabricated SHSs, some novel and interesting phenomena involving icing and melting processes are discussed with a deepened understanding of the relationship between superhydrophobicity and icephobicity. We clarify that superhydrophobicity is one necessary but insufficient condition of icephobicity and present three common failure factors of superhydrophobic anti-icing surfaces, including Cassie–Baxter stability, surface durability, and environmental adaptivity. The next section introduces the recent progress of ULSASs in the three aspects of passive anti-icing, passive/active combined anti-icing, and their practical outdoor applications in detail. Combining the storylines from fabrication to mechanisms and then to applications, we present current challenges in moving ULSASs toward practical applications. Finally, we give a conclusion and provide prospects for the future development of ULSASs.
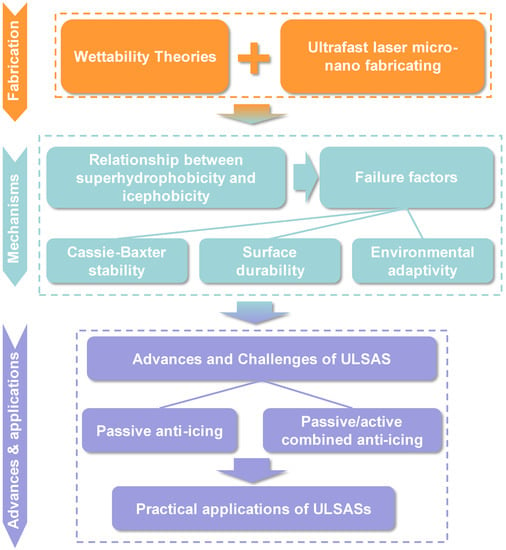
Figure 3.
Flow chart describing the arrangement and order of each part in this review.
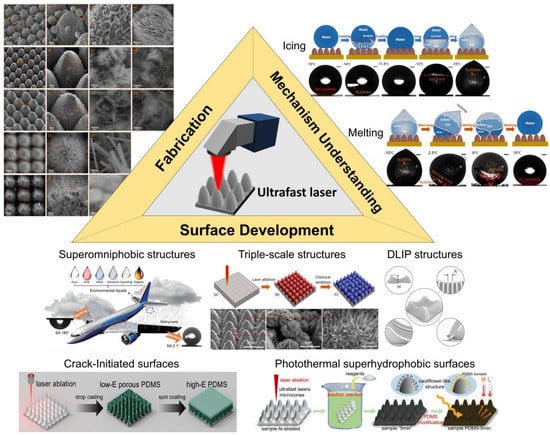
Figure 2.
Ultrafast laser-fabricated superhydrophobic anti-icing surfaces: fabrication, mechanism understanding and surface development. Reproduced with permission from [78], copyright (2022) Springer; reproduced with permission from [79], copyright (2022) Nature Publishing Group; reproduced with permission from [80], copyright (2020) American Chemical Society; reproduced with permission from [81], copyright (2023) American Chemical Society; reproduced with permission from [6], copyright (2023) American Chemical Society; reproduced with permission from [82], copyright (2022) Elsevier.
2. Ultrafast Laser-Fabricating Micro-Nanostructured Superhydrophobic Surfaces
2.1. Wettability and Superhydrophobicity
Surface wettability represents the ability of liquids to spread on surfaces. When the spreading reaches the equilibrium, droplets statically rest on surfaces and form an angle, namely, contact angle (CA). From thermodynamics, CA is the characterization of three-phase interfacial free energies. Back in 1805, the relationship between them has been well described by Young’s equation [83], as shown in Figure 4a:
where , and denote the interface tensions of solid-vapor, liquid-solid, and liquid-vapor, respectively. Although Young’s equation establishes the qualitative relationship between interfacial energies and wettability, it is not appropriate for describing rough surfaces in the real world. Wenzel et al. introduced the roughness factor r and took the apparent contact angle to characterize surface wettability [84], as shown in the following equation:
where the roughness factor r is the ratio of the actual solid-liquid contact area to the nominal contact area. However, the model is limited to the occasion that liquids completely penetrate micro-nano valleys and cannot explain some common wetting phenomena in nature such as the rolling droplets on lotuses. Cassie and Baxter further introduced the contact fractions of liquid-solid and liquid-vapor to describe the mixed triple-phase interfaces [85]. Considering , the Cassie–Baxter equation is expressed by:
Generally, droplets on superhydrophobic surfaces show a large CA and low adhesion strength. Contact angle hysteresis is one of the most common evaluation standards of droplet adhesion. The maximum and minimum static CAs are defined as the advancing angle and receding angle , respectively. The difference between and is the contact angle hysteresis (CAH), as shown in Figure 4a. Smaller CAHs mean smaller adhesion forces exist between interfaces so that droplets can move on surfaces more easily. In this case, surfaces have better liquid-repellency [86,87]. In nature, superhydrophobic phenomena exist widely. For example, luxuriant micropapillae covered by low-surface-energy epicuticular wax endow lotuses with excellent superhydrophobic and anti-fouling properties [88] (Figure 4(d1,d2)), and needle-shaped micro-nano setae combined with wax make water striders walk on rivers freely [89] (Figure 4(e1,e2)). Meanwhile, diverse micro-nanostructure arrangements also bring about many unique superhydrophobic properties, such as the high droplet adhesion on rose petals enabled by single-tier microstructures (Figure 4(f1,f2)) [90], directional adhesion on rice leaves [88] and butterflies enabled by directional microgrooves [91] (Figure 4(g1,g2,h1,h2)), and the anti-fogging properties on mosquito eyes enabled by elaborate nanonipples [92] (Figure 4(i1,i2)).
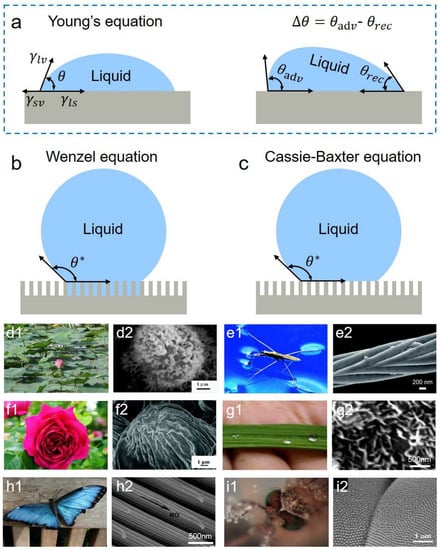
Figure 4.
Three classical wetting states and the superhydrophobic phenomena in nature. (a–c) Schematics of the three states: (a) Young’s equation; (b) Wenzel equation; (c) Cassie–Baxter equation. (d–i) Creatures with superhydrophobicity: (d1,d2) lotus leaves (reproduced with permission from [88]. Copyright (2007) Elsevier); (e1,e2) water striders (reproduced with permission from [89]. Copyright (2007) American Chemical Society); (f1,f2) rose petals (reproduced with permission from [90]. Copyright (2008) American Chemical Society); (g1,g2) rice leaves (reproduced with permission from [88]. Copyright (2007) Elsevier); (h1,h2) butterflies (reproduced with permission from [91]. Copyright (2018) American Chemical Society); (i1,i2) mosquito eyes (reproduced with permission from [92]. Copyright (2007) John Wiley and Sons).
2.2. Ultrafast Laser Micro-Nano Fabricating
As discussed above, ultrafast lasers have many incomparable advantages in micro-nanofabrication. These advantages are not only ascribed to the convenience and programmability of processing methods but also attributed to the complicated light-matter interactions [39,93]. Generally, ultrafast lasers are defined as lasers with ultrashort pulse duration from several tens of femtoseconds to tens of picoseconds [94]. Figure 5a shows the timescales and intensity of the main phenomena during and after irradiating a solid with a femtosecond laser. The light-matter interacting processes could be divided mainly into three types: electronic excitation, nonequilibrium phase transformation, and morphology formation [95], as illustrated in Figure 5a. Overall, in most cases, electronic excitation occurs during the laser pulse (fs—ps), and nonequilibrium phase transformations take place on the picosecond timescale (about ps—100 ps). The timescale of morphology formation reaches up to nanoseconds (about ns—100 ns). There also exist some differences when processing different materials. For semiconductors or dielectrics, the irradiated laser on the one hand can prompt the generation of high-concentration free electrons across the bandgap, which might further induce non-thermal melting. Meanwhile, on the other hand, the laser also provides the excited electrons with more energy, triggering the switching to the plasma state or producing a coulomb explosion. When reaching the electron lattice equilibration, rapid heating at a rate of >1014 K/s occurs to further lead to homogeneous melting or the formation of a supercritical fluid [96]. Many new surface morphologies or metastable phases are produced in extreme environments. After the temperature decreases below material melting points, the melts start to solidify or resolidify to form surface micro-nanostructures. For metals, laser-induced electron heating dominates, resulting in localized high-temperature melting. However, the high thermal conductivities of metals can additionally result in a high supercooling state, where some unexpected structures or defects will be constructed, such as some amorphous structures or nanocrystals [97,98].
Following the interaction mechanisms between ultrafast lasers and matter, numerous diverse micro-nanostructures can be easily fabricated and can be further tuned by varying laser processing parameters or adjusting processing environments. Some common laser parameters include laser wavelengths, pulse duration, pulse repetition frequency, pulse input number, laser fluence, and so on [39,99]. Here, we take two important parameters (laser pulse duration and pulse input number) as examples to introduce their effects on micro-nanostructures. Generally, different laser pulse durations result in different heat affected zones, eventually affecting the processing quality. As shown in Figure 5b, with the shortening of pulse duration from −10−9 to 10−15, the processing quality gradually improves as a result of the smaller heat-affected zone [94]. This phenomenon is mainly attributed to the pulse durations of ultrafast laser (picosecond and femtosecond laser) being shorter than the electron-lattice relaxation time (~10−12 s). Hence, less heat is transferred, causing a smaller thermal effect. For the pulse input number, the micro-nanostructure sizes and morphologies are directly influenced. Figure 5c exhibits the evolution of micro-nanostructures with the pulse input number. It is found that the particles can be flexibly controlled not only in the wide range of nanoscale, sub-microscale, fine-microscale, microscale, and coarse-microscale but also with various structural sizes, including heights and intervals [100].
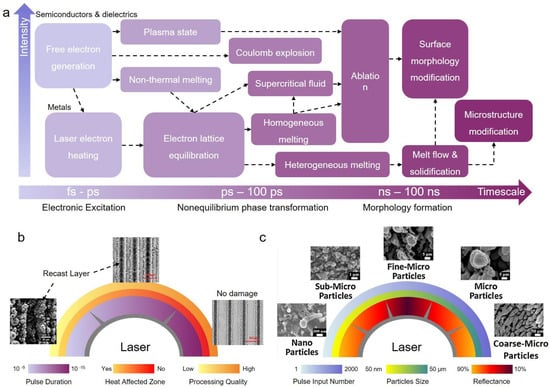
Figure 5.
Mechanisms and morphologies of ultrafast laser micro-nano fabrication. (a) Timescales and intensity of main phenomena during and after irradiating a solid with a femtosecond laser. (b) Evolution of surface morphologies and processing quality with the laser wavelength (reproduced with permission from [101] (copyright (2021) Elsevier) and [102] (copyright (2019) Elsevier). (c) Evolution of micro-nanostructure sizes with the laser pulse input number (reproduced with permission from [100]. Copyright (2015) Elsevier).
Besides the fundamental laser parameters, processing environments also play a significant role in structural morphologies and chemical elements. For example, we once adopted a femtosecond laser to process Ag samples in air and Argon atmosphere [103]. It was found that more nanoparticle clusters and less oxide were generated in Argon. In the meantime, by ablating NiFe in the NH3 atmosphere, we also fabricated more abundant nanoclusters than in the air [104]. These discoveries confirm that the processing atmosphere has a great influence on micro-nanostructure morphologies.
Based on diverse processing parameters and environments, ultrafast laser-fabricated micro-nanostructures could be easily tuned to further realize the fine control for superhydrophobic states. Long et al. achieved the superhydrophobic state tunability from the Wenzel state to the CB state by simply changing the laser scanning speeds on the copper material [51]. The superhydrophobic state evolution was ascribed to the changes in microstructure sizes and nanostructure amounts, where lower scanning speeds could induce deeper microstructures and more abundant nanostructures to contribute to the CB state. Yin et al. discovered the controllable wettability on Polyimide film via femtosecond laser thermal accumulation engineering [105]. By regulating local thermal accumulation effects, different micro-nanostructures were induced, realizing the wettability changes from superhydrophobicity (~3.6°) to superhydrophobicity (~151.6°). Zheng et al. adopted a femtosecond laser to fabricate a temperature-response ceramic surface with switchable wettability [106]. Superhydrophobic and superhydrophilic interfaces were reversibly and repeatedly transited after alternate heating treatments. It was confirmed that laser-induced micro-nanostructures contributed to the removal and re-absorption of organic compounds, thus resulting in the wettability transition.
3. Anti-Icing Mechanisms of SHSs
Adopting diverse and tunable micro-nanostructures fabricated by ultrafast lasers, a large number of applications have been explored [48,107,108]. Among that, ULSASs have been extensively researched in recent years. Many new phenomena have been discovered to deepen the understanding of superhydrophobic anti-icing properties. In this section, we combine recent reports and our understanding to explain the relationship between superhydrophobicity and icephobicity and give the analysis of common failure factors of superhydrophobic anti-icing surfaces.
3.1. Relationship between Superhydrophobicity and Icephobicity
In terms of the interfacial states between droplets and micro-nanostructures, droplets on superhydrophobic surfaces mainly exhibit two states: Cassie-Baxter (CB) and Wenzel. Although droplets in both states have CAs exceeding 150°, their icephobicity performs differently [5]. Icephobicity works only when droplets are supported by air pockets to retain the CB state. However, once air pockets are broken, droplets will rapidly penetrate into the valleys and be stuck by surrounding micro-nanostructures. In this case, the SHSs not only lose their icephobicity but also bring about more icing danger such as easy-icing and ice difficult-removal [109]. Chen et al. reported that the Wenzel ice on SHSs even showed over four times higher ice adhesion strengths than on hydrophilic surfaces [25]. Hence, superhydrophobicity is not equal to icephobicity but is one necessary but not insufficient condition.
To ensure the icephobicity of superhydrophobic surfaces, droplets should retain the CB state but not the Wenzel state. Nonetheless, much research indicates droplets tend to passively transit from the CB state to the Wenzel state under external disturbances [79,110,111], such as freezing, vibration, external pressure, and so on. Affected by the energy barrier between the Wenzel state and the CB state, the spontaneously reversible transition was once regarded as impossible. Many experts have systematically investigated the icing and melting processes on different micro-nanostructured superhydrophobic surfaces [79]. Although some found ultralow ice adhesion strengths for mL-scale ice columns, the transition from the CB state to the Wenzel state still inevitably occurred when the droplet size diminished to the μL-scale. Figure 6 shows the icing and melting processes of droplets on laser-fabricated superhydrophobic surfaces. Compared to other SHSs, it was discovered that droplets on laser-fabricated SHSs possessed a significantly delayed icing ability, which contributed to more bubbles dissolving in supercooled droplets and further dissolving out in ice droplets. During melting processes, these bubbles could spontaneously and rapidly impact downwards under the Marangoni effect, leading to the recovery of the CB state. The dewetting transitions were closely related to surface structures, which further affected the superhydrophobicity, the delayed icing time, and the liquid retraction resistance forces. Although it was confirmed that laser-induced micro-nanostructures could well ensure the robustness of dewetting transitions, the room-temperature superhydrophobicity was still overwhelmingly different from the low-temperature icephobicity owing to complex interfacial interactions at low temperatures [112]. To ensure the anti-icing properties of SHSs, extraordinary superhydrophobicity is undoubtedly one of the most significant conditions, but there are still many other factors that need to be guaranteed. As follows, three main failure factors of superhydrophobic anti-icing surfaces are discussed.
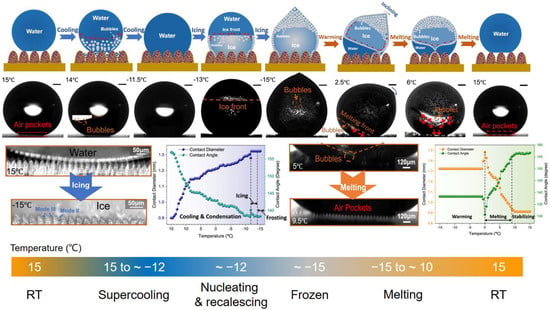
Figure 6.
Icing and melting processes on the superhydrophobic surfaces fabricated by ultrafast laser (reproduced with permission from [79], copyright (2022) Nature Publishing Group).
3.2. Failure Factors of Superhydrophobic Anti-Icing Surfaces
3.2.1. Cassie–Baxter Stability
The CB stability represents the ability of SHSs to withstand the impalement of air pockets by droplets [113,114]. In room-temperature environments, the CB stability is evaluated by the Laplace pressure of droplets, namely, , where is the liquid surface tension and is the radius of droplets. Higher CB stability means that SHSs can withstand higher Laplace pressure to retain the CB state of droplets. The critical Laplace pressure is generally determined by two criteria [80,115]: (i) the critical Laplace pressure for the CA at the moment of droplet transiting from the superhydrophobic state to the hydrophobic state (); and (ii) the critical Laplace pressure for the contact diameter at the moment of the triple-phase line stops retracting (). In terms of the two criteria, is expressed by:
Many researches have confirmed that some finely designed ultrafast laser-fabricated SHSs show superior CB stability at room temperature owing to highly controllable and robust micro-nanostructures [115,116,117]. It is widely accepted that SHSs with higher could show better anti-icing properties. Pan et al. combined ultrafast laser ablation and chemical oxidation to fabricate two types of three-tier micro-nanostructures: the microcone-microflower-nanosheet (MNSF) and the microcone-microflower-nanograss (MNGF) [80]. The two surfaces showed ultrahigh room-temperature CB stability with the highest critical Laplace pressure of ~1450 Pa among all the published reports (Figure 7a,b). However, although the two surfaces showed similar CB stability at room temperature, there still existed significant differences in their anti-icing properties. For example, the ice adhesion strength on MNGF was only 1.7 kPa while that on the MNSF reached 16.9 kPa (Figure 7c). The results indicated that room-temperature was not appropriate for evaluating the low-temperature Cassie–Baxter stability. Focusing on this problem, related calculation models based on icing phenomena were recently established to evaluate the low-temperature CB stability of SHSs with air pocket pressure (Figure 8a,b) [118]. It was found that both microstructure morphologies and temperatures had a great influence on the air pocket pressure. Among these, the open-cell microstructures had higher air pocket pressure at low temperatures than the semi-open or closed microstructures, thus showing higher CB stability. As demonstrated in Figure 8c,d, the anti-icing properties corresponded well to the air pocket stability induced by microstructures, where the SHSs with more open-cell structures showed better and robust anti-icing properties. Hence, to avoid the failure of superhydrophobic anti-icing surfaces, open-cell structures should be adopted to maintain the low-temperature CB stability.
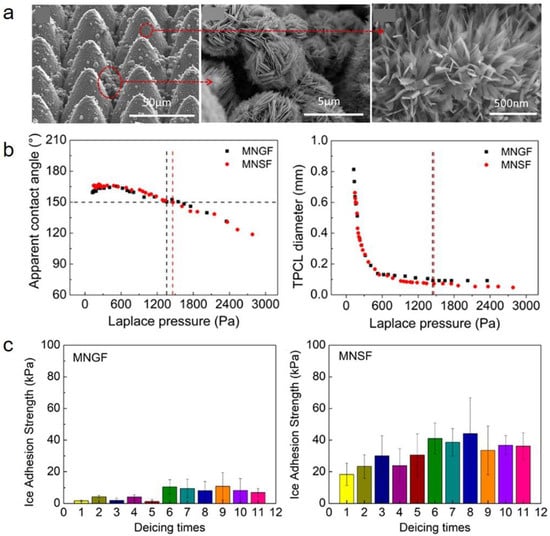
Figure 7.
Morphologies (a), CB stability (b) and ice adhesion strengths (c) of laser-fabricated superhydrophobic surfaces. MNGF denotes the surface with microcone-microflower-nanosheet structures while MNSF denotes the surface with microcone-microflower-nanograss structures. The SEM images in (a) are from the MNGF surfaces. (Reproduced with permission from [80]. Copyright (2020) American Chemical Society.)
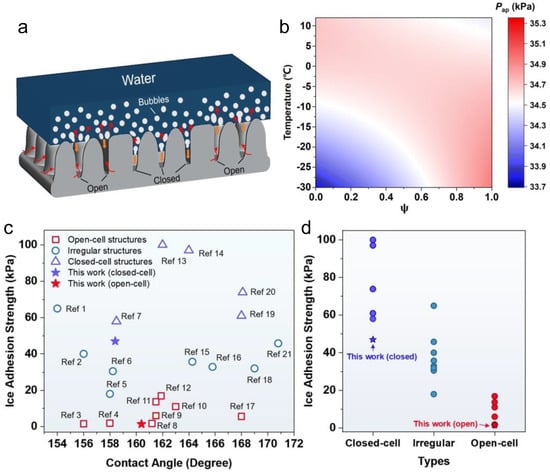
Figure 8.
Effect of air pocket stability at low temperatures on anti-icing performances. (a) Schematics displaying the triple-phase interface changes during icing processes. (b) Phase diagram of air pocket pressure at different temperatures and on the surfaces with different closed/open area ratios. (c) Comparison of ice adhesion strengths on different surfaces with different structural types and contact angles. (d) Comparison of ice adhesion strengths on the surfaces with different structural types. (Reproduced with permission from [118]. Copyright (2023) Royal Society of Chemistry.)
3.2.2. Surface Durability
Surface durability plays a key role in long-term practical applications. Among these, micro-nanostructures and low-surface-energy coatings are two crucial factors. For micro-nanostructures, it is well-known that the structures on SHSs are generally fragile and can be abrased easily when subjected to external shear forces [119]. To improve structural durability, much research has conducted [120,121]. In the best-known study, Wang et al. used armor-like microstructures combined with nanoparticles to realize ultrahigh superhydrophobic durability [87]. However, based on the above analysis, the introduction of armor-like microstructures would result in a decrease in air pocket pressure at low temperatures, eventually causing the failure of anti-icing properties. Hence, the closed-cell structures are not icephobic-durable (Figure 9a). Both room-temperature durability and icephobic durability need to be met for long-term anti-icing applications. Designing the micro-nanostructures to make this comprise is of significance.
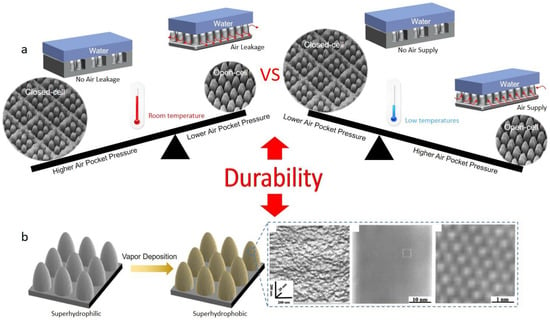
Figure 9.
Two key factors influencing surface durability. (a) Surface structure types (Reproduced with permission from [118]. Copyright (2023) Royal Society of Chemistry.) (b) Low-surface-energy coatings (Reproduced with permission from [122]. Copyright (1999) American Chemical Society.)
For low-surface-energy coatings, durability is related only to intrinsically hydrophilic materials, such as metals, ceramics, etc. The most common coating substance is fluorosilanes, which can be facilely coated on micro-nanostructures by the methods of vapor deposition or solution immersing [123]. Although excellent superhydrophobicity could be achieved with the methods, the durability of such coatings is usually poor owing to the single molecular layer thickness of the coated fluorosilanes by bonding with hydroxyl groups [82]. Nishino et al. measured the surface roughness of the coating on glass [122], where n-Perfluroeicosane (C20F42, one of the low-surface-energy substances) was vapor deposited. They found that the vapor-deposited layer was single crystal-like with a maximum roughness of only 8 Å (Figure 9b). Under external abrasion, the coatings can be easily peeled off to expose the inner hydrophilic sites, eventually leading to the failure of anti-icing properties. Therefore, research on chemical synthesis and coating materials still needs to be made in developing novel durable low-surface-energy coatings.
3.2.3. Environmental Adaptivity
Numerous complex and unexpected disturbances exist in practical application environments, including high-speed droplet impact, dust accretion, strong wind, etc. [124]. In this case, superhydrophobic anti-icing surfaces fail more easily compared to static laboratory environments. We take the common dynamic impact as an example to elucidate the influences of environments on anti-icing properties. Figure 10a,b show the comparison of four typical dynamic tests: water jet impacting, solid particle impacting, supercooled droplet impinging, and icing wind tunnel tests [81]. It can be observed that there are tremendous differences for different test conditions. For icing wind tunnel tests (a widely adopted method to simulate real aviation environments), the droplet sizes and impacting velocities are generally in the range of ~20–100 μm and ~10–100 m/s. The dynamic pressures in icing wind tunnel tests reach up to over 1 Mpa, which is more than an order of magnitude higher than those in other tests. Under continuous, high-speed, high-pressure and tiny droplet impact, superhydrophobic anti-icing surfaces tend to fail extremely easily. Figure 10c shows the icing processes of the superhydrophobic anti-icing surfaces fabricated by the DLIP. The surfaces, which could easily repel supercooled droplets (−5 °C; diameter: ~2 mm; impact speed: 3.4 m/s) at the substate temperature of −25 °C, not only failed in 0.5 min in the icing wind tunnel tests but also even resulted in more ice accretion than pristine smooth surfaces. We ascribed the phenomenon to the preferentially bottom freezing when supercooled tiny droplets contacted surfaces. Within several milliseconds, the ice embryo nucleated and generated a strong adhesion to the substrate instead of rebounding (Figure 10d). Meanwhile, subsequent high-pressure droplets continuously impacted the surfaces to further increase the ice adhesion and lead to ice accretion.
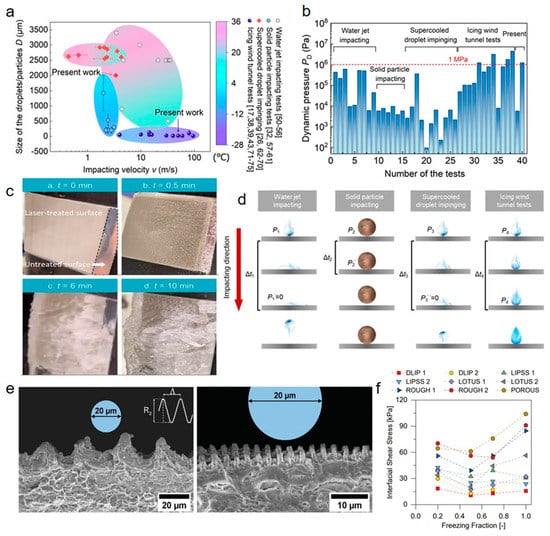
Figure 10.
Influence of environmental disturbances on anti-icing performances. (a) Summarization of the relationships between the size of the droplets/particles and the impacting velocity from the four dynamic experiments: water-jet impacting, solid-particle impacting, supercooled-droplet impinging, and icing wind tunnel tests. (b) Dynamic impact pressure when the droplets/particles contact the target surfaces. (c) Image sequences of the icing processes on SHSs in icing wind tunnel tests. (d) Schematic diagrams showing the physical processes at dynamic impacting (Reproduced with permission from [81]. Copyright (2023) American Chemical Society.) (e) Comparison of structural sizes with droplet sizes. (f) Interfacial shear stress versus freezing fraction (Reproduced with permission from [125]. Copyright (2020) John Wiley and Sons.)
To ensure that superhydrophobic anti-icing surfaces have qualified environmental adaptivity, Vercillo et al. explored the design rules of ULSASs by testing various micro-nanostructures in icing wind tunnel conditions [125]. Two dominant but opposite factors were summarized: stress concentrations and surface contact area. They found that although micro-nanostructures with small sizes had a smaller solid-liquid contact area, the structures also induced lower stress concentration. For those with larger sizes, higher stress concentration but a larger contact area was produced. It was concluded that the laser-fabricated structures should be with a spatial period of one order of magnitude smaller than the mean volume diameter (MVD) and with abundant nanostructures (Figure 10e). They also confirmed that the ice types, i.e., freezing fraction, had a strong influence on anti-icing properties, where droplets could freeze faster at higher freezing fractions and were also more difficult to remove (Figure 10f).
4. Advances and Challenges of ULSASs
Based on the above understanding, we summarize recent advances of ULSASs and proposed corresponding challenges in this section. Two typical types of SHS anti-icing technologies are discussed, including passive superhydrophobic anti-icing surfaces and passive/active combined superhydrophobic anti-icing surfaces. For ease of understanding, the challenges are presented by following their respective progress. Finally, current attempts and explores of ULSASs in their practical applications are introduced and discussed.
4.1. Passive Superhydrophobic Anti-Icing Surfaces
4.1.1. Delayed Icing/Frosting
Five stages are divided into the whole icing processes: supercooling, nucleating, recalescing, freezing, and solid cooling [79,126]. The delayed icing time generally denotes the duration time of the first three stages, which start in the original cooling from room temperatures and end in the occurrence of recalescence. Among these stages, avoiding ice embryo nucleation is the key to delaying icing [127].
Nucleation processes are divided into two types: homogeneous nucleation and heterogeneous nucleation [128]. The former generally takes place in environments without any disturbance and is completed by structure fluctuation and energy fluctuation. However, in practical environments, heterogeneous nucleation generally takes the dominant [129]. The nucleation rate of liquids can be expressed by:
where are the nucleation rates of the total, the bulk, the liquid-vapor interface, and the liquid-solid interface, respectively; and are the contact areas of the liquid-vapor interface and the liquid-solid interface, respectively; is the liquid volume, and is the surface temperature. Compared to , the value of is very small. Hence the total nucleation rate is mainly dependent of and [5]. Among them, is determined by the heterogeneous nucleation energy barrier, which is expressed by:
where is the energy barrier of heterogeneous nucleation; is the liquid-vapor interface energy; is the Gibbs free energy per unit volume; and is the contact angle of the ice embryo on a surface. The value of is proportional to the contact angles, where higher contact angles could induce a higher heterogeneous nucleation energy barrier, further slowing down the nucleation rate. However, as discussed above, superhydrophobic delayed icing properties work only when the CB state is retained. Once droplets transit to the Wenzel state, the significantly increased solid-liquid contact area will greatly accelerate the icing proceedings. Hence, maintaining the CB stability at low temperatures is of great importance. Many researches reveal that it is mainly affected by surface wettability and micro-nanostructure morphologies [11,30]. Undoubtedly, wettability is the key factor that influences delayed icing properties. However, the above analysis confirms that the micro-nanostructure topologies play a more important role in low-temperature air pocket stability [118]. To explore the optimal ULSASs, numerous state-of-the-art micro-nanostructures have been developed. Pan et al. combined femtosecond laser ablation and chemical etching to fabricate the triple-scale micro-nanostructures on copper and aluminum alloy materials [130], where the open microcone arrays were covered by densely distributed submicroflowers and nanograss (Figure 11a). It was found that the triple-scale micro-nanostructures showed a significantly longer delayed icing time of 52 min 39 s, longer than on smooth surfaces (8 min 15 s) and double-scale structured SHSs (34 min 38 s). Ge et al. fabricated a hybrid micro-nanostructure with square pillars integrated Siberian–Cocklebur-like microstructures on PTFE by controlling femtosecond laser parameters [131], as shown in Figure 11b. They found that the surfaces not only had excellent superhydrophobicity but also exhibited significantly enhanced delayed icing time. Xing et al. adopted a picosecond laser to fabricate the cauliflower-like protrusions with nanostructures on the open micro-gratings [132], as shown in Figure 11c. They revealed that the laser-processed multi-tier micro-nanostructures moderated the heat loss to delay the icing process. Summarizing recently reported data on ULSASs [61,129,131,133,134,135,136], Figure 12 shows the delayed icing times versus different droplet volumes and materials. Compared to organic and inorganic ULSASs, most metallic surfaces have better delayed icing properties. This is attributed to the differences of the formed micro-nanostructures on different materials owing to different light-matter interaction mechanisms (discussed in Section 2.2). Some chemical component changes during ablating also account for the differences.
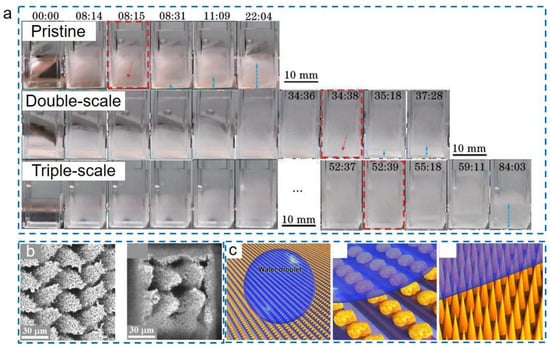
Figure 11.
Delayed icing properties of ULSASs. (a) Image sequence of icing processes on the pristine copper surface, the double-scale structured ULSAS, and triple-scale structured ULSASs (reproduced with permission from [130]. Copyright (2021) Chinese Laser Press). (b) SEM images of the hybrid micro-nanostructure with square pillars integrated Siberian–Cocklebur-like microstructures (reproduced with permission from [131]. Copyright (2020) Elsevier). (c) Schematics showing the cauliflower-like protrusions with nanostructures on the micro-gratings (reproduced with permission from [132]. Copyright (2020) Elsevier).
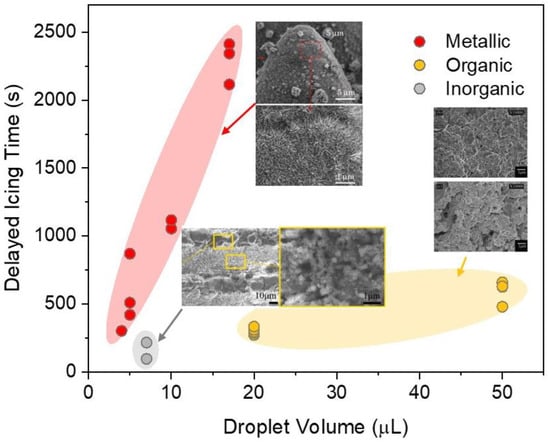
Figure 12.
Delayed icing times on ULSASs versus different droplet volumes and materials. The test temperature of all data was −10 °C. Data were from the references [61,129,131,133,134,135,136].
In contrast to droplet freezing, frosting is a more complicated process, involving more phase transformations [137,138,139]. Generally, two types of frosting modes are included: desublimation frosting and condensation frosting [140]. Desublimation frosting involves the gas-solid phase change and occurs mostly when the surface temperature is lower than the triple point of water. In condensation frosting, vapor first condenses onto surfaces, and then condensates freeze to form frosts when surface temperatures decrease below the icing point [4]. On hydrophobic surfaces, the required supersaturation degree for desublimation frosting is an order of magnitude larger than on hydrophilic surfaces [137]. Therefore, in practical applications, the delayed frosting on SHSs generally represents the delayed condensation frosting. Numerous studies have been made on understanding the mechanisms of condensation frosting on SHSs and developing related anti-frosting surfaces [24,68,141,142]. The most remarkable is the discovery of droplet jumping on SHSs induced by the decreased Laplace pressure and droplet coalescence [21,24]. Taking advantage of the spontaneous droplet jumping, condensates could be swept off the surfaces in time before freezing. However, some remaining droplets can still freeze to inevitably form frosts. To diminish the frosting ratio, two strategies have been mainly developed: (i) designing and optimizing micro-nanostructures to inhibit the growth of frost layers; (ii) designing the chemical components of surfaces to enhance droplet jumping. For ULSASs, the first strategy is adopted more frequently. By adjusting the microstructure sizes and morphologies, the frost growing extent and spreading directions could be well controlled. Many experts have investigated frosting behaviors on ULSASs. He et al. adjusted laser fluence and pulse repetition frequency to fabricate different micro-nanostructures and investigated the relationship between surface morphologies and anti-frosting properties [143]. They found the smaller tip area and the larger nanostructures were preferred for delaying frosting. Long et al. fabricated 3D conical microtextures covered with nanowires on copper materials [144]. In condensation environments, the fine structures could contribute to the departure of condensate droplets, thus realizing the dropwise condensation and delaying frosting. Although much effort has been expended on developing diverse laser-induced micro-nanostructures for anti-frosting, which type of structures performed better was still unclear. In 2023, we classified all micro-nanostructures into two types: open-cell and closed-cell structures [118]. By comparing the condensation frosting processes on the two structures, it was found that more small condensate droplets emerged on the closed-cell structures since the microframes provided more nucleation sites (Figure 13). The delayed frosting time decreased as the area ratio of closed structures increased, indicating open structures performed better than closed structures. This conclusion could greatly guide the structural design of ULSASs for better anti-frosting properties.
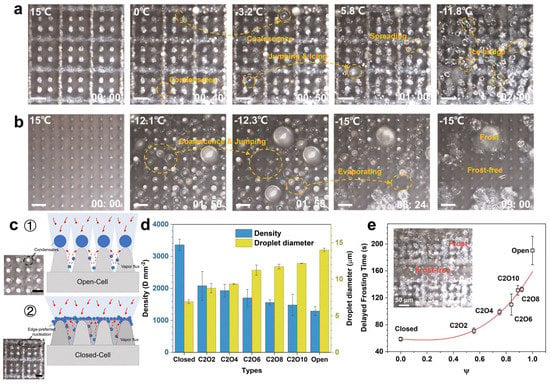
Figure 13.
Delayed frosting phenomena on the ULSASs with different microstructures. (a,b) The frosting processes on the closed-cell microstructures and open-cell microstructures. (c) Schematics describing the differences of condensates nucleating and growing on different microstructures. (d) The densities and diameters of condensate droplets on the ULSASs with different microstructures. (e) Delayed frosting times versus different microstructures. (reproduced with permission from [118]. Copyright (2023) Royal Society of Chemistry).
Although much progress has been made in delaying icing/frosting of ULSASs, the icing/frosting cannot still be completely avoided. Especially in some extremely low-temperature and high-humidity environments, numerous droplets initiate and adhere to the micro-nano valleys, and then rapidly freeze [145,146]. How to inhibit the icing/frosting on ULSASs in extremely freezing environments is a great challenge, which needs to be further explored in structures, chemistry, and other areas.
4.1.2. Ultralow Ice Adhesion Strengths
Besides delaying ice embryo nucleation, lowering ice adhesion strengths is also one of the most important properties of ULSASs [147]. In some severe icing environments (e.g., ice wind tunnel conditions), freezing processes inevitably occur on SHSs [81]. How to lower ice adhesion strengths is the key for avoiding ice accretion.
Centrifuge adhesion tests and push-off adhesion tests are the two most common methods [148,149]. The former determines the adhesion strengths by measuring the maximum shear forces or pulling forces at the moment of ice shedding during the centrifugal rotating while the latter records the maximum shear forces of the ice column detachment by the horizontal pushing. Although the reported ice adhesion strengths in literature cannot be compared accurately owing to the differences in test methods, it is widely regarded that the surfaces with ice adhesion strengths below 100 kPa are icephobic surfaces, and the ice adhesion strength of 20 kPa is the benchmark for passive anti-icing surfaces, where ice could be removed by wind blow or slight vibration [30,150]. To date, many ULSASs have been designed and fabricated with ultralow ice adhesion strengths [37,125,151,152,153]. Milles et al. tested the ice adhesion strengths of different ULSASs under various icing conditions and found that the cross-like DLIP pattern with the size of 2.6 μm showed the lowest ice adhesion strengths of 6~10 kPa [154]. As shown in Figure 14a, the accumulated ice layer on laser-structured surfaces could be easily removed by vibration. Considering that the solidification of environmental-related mixed liquids always took place in practical applications, we adopted a femtosecond laser to fabricate the superomniphobic surface showing excellent repellent ability for multiple liquids from deionized water to mixed solutions and then to organic liquids [78]. Figure 14b shows the ice adhesion strengths of different liquids on the designed surfaces. It was found that our fabricated surfaces had ultralow ice adhesion strengths for various liquids. Even after multiple deicing cycles, the ice adhesion strengths could be maintained below 20 kPa. However, some challenges still exist [11,30], such as poor icephobic durability (discussed in the next section), the poor environmental adaptivity, and the difficulty of large-scale fabrication. These issues greatly limit ULSASs for practical applications.
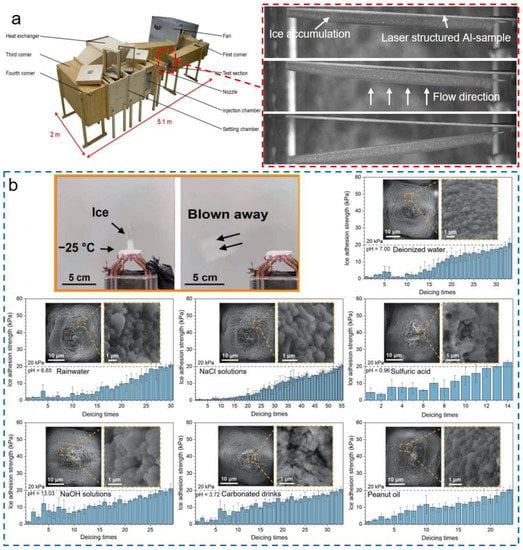
Figure 14.
Low ice adhesion on the ULSASs. (a) Images of the ice easy-removal of ULSAS with vibration. Ice was formed and accumulated in the icing wind tunnel condition (reproduced with permission from [154]. Copyright (2021) MDPI). (b) Low ice adhesion of ULSASs for different liquids (reproduced with permission from [78]. Copyright (2022) Springer).
4.1.3. Durable Icephobicity
Icephobic durability directly determines the lifespan of ULSASs in practical applications. Based on the discussion in Section 3.2.2, to enhance the icephobic durability, both the micro-nanostructure durability and the coating durability need to be enhanced.
On SHSs, microstructures work as the robust skeleton while nanostructures endow surfaces with better superhydrophobicity [155]. Although the closed microstructures could greatly enhance the room-temperature superhydrophobic durability, the open microstructures exhibit higher icephobic durability at low temperatures [118]. Hence, maintaining the open structures and further introducing more durable nanostructures become a good strategy for improving the micro-nanostructure durability. Chen et al. utilized ultrafast laser ablation and chemical oxidation to fabricate the micro-nano-nanowire triple-scale structures, where the Cu(OH)2 nanowires with an average length of 6–8 μm and a width of 50 nm in situ grew on the microcones (Figure 15a). After continuous deicing tests, it was found that the surface icephobicity was progressively enhanced with an increase in deicing cycles, showing a unique property that the surfaces could become more and more icephobic after wear. The phenomenon was mainly ascribed to the side nanowires and the flat microcones constructing the moderate top roughness after the top nanowires were damaged, which decreased the ice-solid contact area and further led to the lower ice adhesion strengths. The lowest ice adhesion strength on the prepared surfaces reached 12.2 kPa and could maintain 17.3 kPa even after 60 deicing cycles. To date, it is the lowest ice adhesion strength after so many deicing cycles among all reported ULSASs [156].
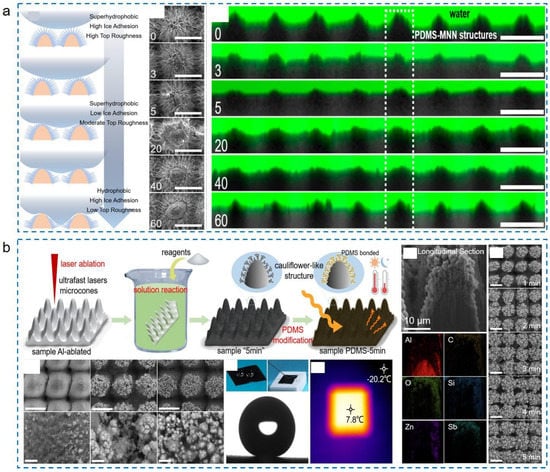
Figure 15.
Enhanced icephobic durability. (a) Enhanced micro-nanostructure durability by fabricating the micro-nano-nanowire triple-scale structures (reproduced with permission from [156]. Copyright (2022) American Chemical Society). (b) Enhanced low-surface-energy coating durability by using the method of PDMS spin coating (reproduced with permission from [82]. Copyright (2022) Elsevier).
To provide more durable low-surface-energy coatings, many durable coating materials have been developed and tested [157,158,159]. Among these, PDMS materials show great application potential owing to strong bonding strength and an easy preparation process. Many durable superhydrophobic surfaces have been fabricated by the PDMS spin coating method instead of the vapor deposition of fluorosilanes [157,160,161]. Based on these properties, Chen et al. fabricated a cauliflower-like micro-nanostructure combined with PDMS coatings by taking advantage of ultrafast laser ablation and wet chemical reactions [82]. As shown in Figure 15b, the prepared surfaces exhibited excellent superhydrophobicity and highly effective photothermal properties. Adopting FIB and EDS techniques to analyze the cross-sectional elements, it was found that the PDMS solutions could cover and bond the cauliflower-like structures well, forming an integrated structure. The infiltrated depth of PDMS in micro-nanostructures could reach the μm-scale, which meant that the low-surface-energy coating could still be preserved to maintain the qualified superhydrophobicity after structure damage. With the robust coatings and abundant micro-nanostructures, surface icephobicity could be well maintained after 25 abrasion cycles or 1.5-hour water impact or 500 tape peeling cycles.
Overall, the improvement of micro-nanostructures and low-surface-energy coatings contributes well to the enhancement of icephobic durability. However, most of the reported surfaces are far away from real industrial applications. Further developing durable icephobic surfaces to meet various demands is still needed.
4.2. Passive/Active Combined Superhydrophobic Anti-Icing Surfaces
In some severe application scenarios, the single passive anti-icing of SHSs is still limited to eliminating ice formation and accretion. Combining with other active anti-icing technologies to achieve passive/active combined anti-icing could overcome the disadvantages [162,163,164,165]. Among various hybrid anti-icing methods, electrothermal/superhydrophobic and photothermal/superhydrophobic anti-icing surfaces have been developed most widely. Here we introduce the main advances in the two methods.
4.2.1. Electrothermal/Superhydrophobic Anti-Icing Surfaces
In terms of different heating methods, electrothermal/superhydrophobic anti-icing surfaces are divided into two main types [166,167]: one is achieved by directly installing heating elements under SHSs; the other is enabled by electricity power input to transform from electricity to heat. The former has no requirement for the electrical conductivity of SHSs while the latter requires surfaces with good conductivity and electro-to-heat conversion performances. Alamri et al. used an ultrafast laser to fabricate the hierarchical micro-nanostructures on the NACA 0012 airfoil [151]. After chemical treatment, the surfaces showed strong water repellence with a contact angle of and a roll-off angle of . Combining the SHSs and their bottom-heating elements (Figure 16a), the electrothermal/superhydrophobic deicing tests were conducted in the icing wind tunnel environment (TAS (true air speed) = 65 m s−1; SAT (static air temperature) = −10 °C/−20 °C; LWC (liquid water content) = 0.2 g m−3 and AoA (angle of attack) = 3°). Figure 16b shows the plot of the required deicing time as a function of heating power density. The power density and time of deicing on the reference surfaces were significantly higher than on the laser-fabricated SHSs. For the SATs of −10 °C and −20 °C, the deicing power densities on the reference surfaces reached up to ~2.1 and ~2.5 W cm−2, respectively, while those on the SHSs decreased to ~0.5 and ~1.1 W cm−2, respectively. Sun et al. also compared the electrothermal/superhydrophobic deicing properties of pristine surfaces and laser-fabricated SHSs in the icing wind tunnel conditions (TAS = 0~200 m/s; SAT = −35 °C; LWC = 0.2~3.9 g m−3) [168]. They found that although external heating could avoid icing on the heating zone of the pristine surface, the runback ice would be accreted more severely at the tailing edge of airfoils. In contrast to the icing phenomena on pristine surfaces, the liquid water could quickly roll away from the laser-fabricated SHSs to prevent the runback icing (Figure 16c).
For the second electrothermal deicing technology, some new phases with electrothermal properties are usually added to substrates to reinforce the thermal conductivity and electro-to-heat conversion ability. The common electrothermal materials include carbon-based materials (e.g., graphene, carbon nanotube, etc.), Ag-based materials, and some conducting polymers [169]. Xue et al. mixed multi-walled carbon nanotubes (MWCNT) and N-methylpyrrolidone solutions to prepare the MWCNT/PEI nanocomposite film [170]. An ultrafast laser was adopted to fabricate the micro-nanostructures, realizing the superhydrophobicity with a contact angle of 156.6°. Under 12 V, the three-dimensional conductive network constructed by MWCNT could rapidly rise the temperature to 109 °C within 370 s. With excellent electrothermal and superhydrophobic properties, ice on the surfaces could completely melt in 170 s (Figure 16d).
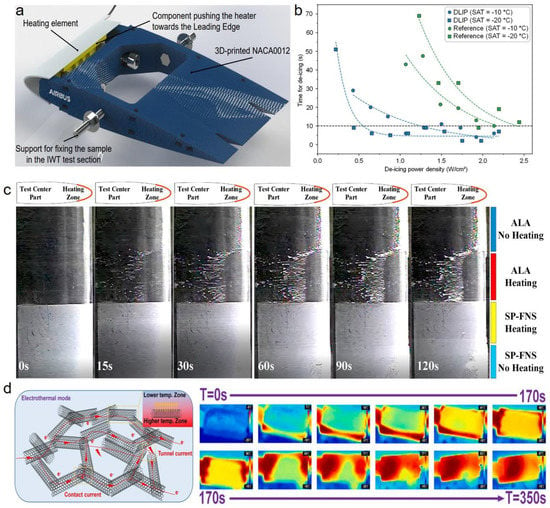
Figure 16.
Electrothermal/superhydrophobic combined anti-icing surfaces. (a) Schematics showing the NACA0012 support and the heating element. (b) Comparison of the deicing time and deicing power on pristine surfaces and ULSASs (reproduced with permission from [151]. Copyright (2020) John Wiley and Sons). (c) Image sequences of electrothermal deicing on different surfaces in the icing wind tunnel conditions (reproduced with permission from [168]. Copyright (2021) Elsevier). (d) Schematic showing the electrothermal mechanisms when the current is directly applied on materials, and the thermographic image sequences of electrothermal deicing processes on ULSASs (reproduced with permission from [170]. Copyright (2023) Elsevier).
4.2.2. Photothermal/Superhydrophobic Anti-Icing Surfaces
In contrast to electrothermal deicing methods, the photothermal/superhydrophobic anti-icing technology mainly utilizes solar energy in nature to convert into heat, eventually realizing passive/active combined deicing [171]. It is an environmentally friendly deicing technique without consuming any fossil fuel. Based on the photothermal conversion principles, photothermal SHSs can also be divided into two types: (i) the SHSs with structural light traps [82,172]; and (ii) the SHSs with the new phases that own the high solar-to-heat conversion efficiency [163,173,174]. Adopting various micro-nanostructures and the generated new matters enabled by ultrafast lasers, the two types of methods have been widely explored. Zhao et al. were inspired by moth eyes to fabricate the biomimetic micro-nanostructures with a femtosecond laser [175], as shown in Figure 17a. The surfaces were composed of micro-mountain arrays with micro-nanoparticles, which constructed a well-structured light trap. Under one-sun illumination, the surface temperature can rise rapidly and exceed 80 °C in 300 s. Even a large ice cube could melt and shed off the surfaces within 240 s under sunlight. Zhang et al. considered that the adhered condensates in extremely high-humidity conditions could hinder the photothermal properties and induce condensation freezing. Hence, they fabricated hierarchically structured materials by ultrafast pulsed laser deposition (PLD) technology (Figure 17b) [176]. The generated iron oxide during laser irradiation was deposited densely on substrates, not only constructing the strong light traps but also further contributing to the photothermal conversion by the thermoplasmonic effect of iron oxide nanoparticles. In the extreme environment with a temperature of −50 °C and a supersaturation degree of ~260, the photothermal anti-icing properties of the surfaces could be well maintained and the equilibrium temperature could exceed 0 °C under one-sun illumination owing to their superior condensate self-removing and high photothermal conversion capability.
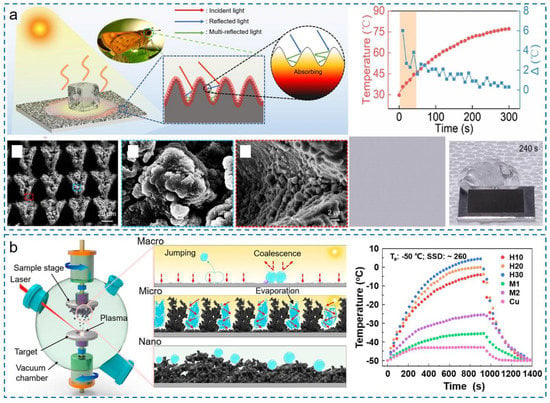
Figure 17.
Photothermal/superhydrophobic anti-icing surfaces. (a) Highly effective photothermal/superhydrophobic anti-icing properties enabled by the biomimetic laser-fabricated structures (reproduced with permission from [175]. Copyright (2021) Elsevier B.V.). (b) Solar anti-icing surfaces with enhanced condensate self-jumping in extremely low-temperature and high-humidity environments (reproduced with permission from [176]. Copyright (2021) PNAS).
Compared to the single passive superhydrophobic anti-icing method, the passive/active combined anti-icing methods could better withstand complicated and volatile environments. However, how to realize low-cost large-scale fabrication is still a key problem for practical applications [11,13]. Meanwhile, different application scenarios also propose more requirements, such as the specific surface roughness and the bonding between surfaces and heating elements in the aviation field.
4.3. Exploiting Superhydrophobic Anti-Icing Surfaces in Real Applications
Based on the excellent anti-icing properties of ULSASs, many outdoor tests have been conducted to exploit their feasibility for practical applications. Vercillo et al. installed a laser-fabricated superhydrophobic Ti64 metal sheet on an A350 port-side slat for a real flight test [177]. The total flight duration was 171.2 h, traveling mostly across Europe and experiencing two long flights to New Zealand and the Philippines. By measuring the contact angle changes and roughness, they found that surface superhydrophobicity was mostly lost after the flight. The degrading of superhydrophobicity was mainly ascribed to the failure of the low-surface-energy coating under long-time UV exposure. Boinovich et al. conducted outdoor tests with laser-fabricated SHSs [178]. The test went from September 2016 to the end of March 2019 in Moscow, where regular snowfalls, sleet, and freezing rain accompanied by high humidity and frequent air temperature changes occurred frequently. They found that the bare aluminum surfaces had significant pitting corrosion in outdoor conditions, which greatly increased the snow/ice adhesion. For the SHSs, their icephobicity could be well maintained after continuous long-time rain and could effectively keep snow from accumulating when the wind speed exceeded 3–5 m/s, as shown in Figure 18. Even at a lower wind speed, the buildup of snow cups could still induce the spontaneous shedding of snow by their gravity. Although the practical test results also revealed some problems and limitations of ULSASs, these attempts and advances provided tremendous reference values for guiding ULSASs designs and accelerating their applications.
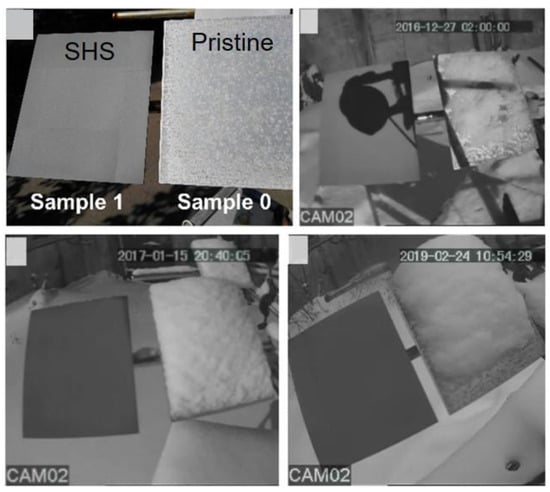
Figure 18.
Long-term outdoor anti-icing tests of ULSASs and pristine surfaces (reproduced with permission from [178]. Copyright (2019) American Chemical Society).
5. Conclusions
Ultrafast lasers, as powerful and promising manufacturing tools, have colossal value in accelerating the development and applications of superhydrophobic anti-icing surfaces. To eliminate the undesired ice, much attention has been paid to ultrafast laser-fabricated superhydrophobic anti-icing surfaces (ULSASs), and related research has been comprehensively conducted in recent years. Here, we summarized the recent development of ULSASs, mainly including three aspects: fabricating, mechanisms, and advances and challenges. With respect to fabricating, we introduced fundamental wettability. Combining exquisite micro-nanostructures and low-surface-energy matters, biomimetic superhydrophobicity can be endowed on various substrate materials. Next, the advantages and mechanisms of ultrafast laser micro-nano fabricating were explained. Compared to other micro-nano fabricating strategies, the ultrafast laser-fabricating method possesses the significant advantages of being highly programmable, having the nm-accuracy, being contact-free, and with non-limitation for materials. Based on the light-matter interaction, many unique micro-nanostructures have been widely developed, providing more opportunities and possibilities for exploring the superhydrophobic anti-icing mechanisms and applications. In mechanisms, we introduced some recent discoveries and thus extended understanding of anti-icing on the laser-fabricated micro-nanostructures, and further elucidated the relationship between superhydrophobicity and icephobicity that superhydrophobicity is one necessary but not insufficient condition of icephobicity. Meanwhile, three common failure mechanisms of ULSASs were analyzed, including Cassie–Baxter stability, surface durability, and environmental adaptivity. Essentially, structures and coatings were two crucial factors to avoid the failure of ULSASs. In advances and challenges, we summarized the recent progress and the respective challenges of ULSASs in passive anti-icing, passive/active combined anti-icing, and some practical outdoor applications. The passive anti-icing of ULSASs mainly lies in delaying icing time, decreasing ice adhesion strengths, and achieving durable anti-icing, while the passive/active combined anti-icing technologies were introduced with the electrothermal- or photothermal-assisted methods. However, in contrast to lab-scale freezing environments, the practical application environments were more complicated and volatile, which brings tremendous challenges for the performances of ULSASs (icephobicity, durability, stability, etc.) and their engineering feasibility (large-scale fabrication, surface installation, device matching, etc.). As for the future development of ULSASs, the following opportunities are envisioned:
- Further investigations and deepened understanding of icing-related phenomena on superhydrophobic surfaces need to be explored.
- More design and optimization of structures and chemical coatings are demanded for developing more durable ULSASs.
- More novel ultrafast laser-fabrication technologies and more advanced ultrafast laser instruments are expected to meet the strictly industrial application requirements.
Author Contributions
Writing—original draft preparation, L.W.; writing—review and editing, H.Z. (Huanyu Zhao), D.Z., L.Y. and H.Z. (Hongjun Zhang); supervision, P.F. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge funding support from the National Key R&D Program of China (Grant No. 2017YFB1104300 and 2022YFE0203700, M.Z.), the Tsinghua University Initiative Scientific Research Program (Grant No. 2018Z05JZY009, M.Z.), and the National Natural Science Foundation of China (Grant Nos. 51575309 and 51210009, M.Z.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We acknowledge the Liaoning Provincial Key Laboratory of Aircraft Ice Protection, Joint Research Center for Advanced Materials & Anti-icing of Tsinghua University (SMSE)-AVIC ARI, and the China-Italy Joint Laboratory on Green Aviation Technology for the support for this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wong, T.-S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Graeber, G.; Köhme, M.; Poulikakos, D. Spontaneous droplet trampolining on rigid superhydrophobic surfaces. Nature 2015, 527, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low–interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yu, M.; Shang, Y.; Zhou, P.; Song, R.; Xu, X.; Chen, X.; Wang, Z.; Yao, S. Suppressing Ice Nucleation of Supercooled Condensate with Biphilic Topography. Phys. Rev. Lett. 2018, 120, 075902. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Chen, C.; Fan, P.; Zhu, D.; Tian, Z.; Zhao, H.; Wang, L.; Peng, R.; Zhong, M. Crack-Initiated Durable Low-Adhesion Trilayer Icephobic Surfaces with Microcone-Array Anchored Porous Sponges and Polydimethylsiloxane Cover. ACS Appl. Mater. Interfaces 2023, 15, 6025–6034. [Google Scholar] [CrossRef]
- Grizen, M.; Tiwari, M.K. Icephobic Surfaces. In Ice Adhesion: Mechanism, Measurement and Mitigation; Scrivener Publishing LLC: Beverly, MA, USA, 2020. [Google Scholar]
- Schultz, P.; Politovich, M.K. Toward the improvement of aircraft icing forecasts for the continental United States. Weather. Forecast. 1992, 7, 491–500. [Google Scholar] [CrossRef]
- Cebeci, T.; Kafyeke, F. Aircraft Icing. Annu. Rev. Fluid Mech. 2003, 35, 11–21. [Google Scholar] [CrossRef]
- Chu, F. Condensed and Melting Droplet Behavior on Superhydrophobic Surfaces; Springer Nature: Berlin, Germany, 2020. [Google Scholar]
- Huang, W.; Huang, J.; Guo, Z.; Liu, W. Icephobic/anti-icing properties of superhydrophobic surfaces. Adv. Colloid Interface Sci. 2022, 304, 102658. [Google Scholar] [CrossRef]
- Esmeryan, K.D. From Extremely Water-Repellent Coatings to Passive Icing Protection—Principles, Limitations and Innovative Application Aspects. Coatings 2020, 10, 66. [Google Scholar] [CrossRef]
- Wang, F.; Zhuo, Y.; He, Z.; Xiao, S.; He, J.; Zhang, Z. Dynamic Anti-Icing Surfaces (DAIS). Adv. Sci. 2021, 8, 2101163. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, W.; Kwon, Y.S.; Li, W.; Yao, S.; Huang, B. Solar Deicing Nanocoatings Adaptive to Overhead Power Lines. Adv. Funct. Mater. 2022, 32, 2113297. [Google Scholar] [CrossRef]
- Jiang, S.; Diao, Y.; Yang, H. Recent advances of bio-inspired anti-icing surfaces. Adv. Colloid Interface Sci. 2022, 308, 102756. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, Y.; Tian, Y.; Zhang, D.; Song, J.; Crick, C.R.; Carmalt, C.J.; Parkin, I.P.; Lu, Y. Robust and durable liquid-repellent surfaces. Chem. Soc. Rev. 2022, 51, 8476–8583. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, D.; Sun, Z.; Song, J.; Deng, X. Robust superhydrophobicity: Mechanisms and strategies. Chem. Soc. Rev. 2021, 50, 4031–4061. [Google Scholar] [CrossRef]
- Liu, Y.; Moevius, L.; Xu, X.; Qian, T.; Yeomans, J.M.; Wang, Z. Pancake bouncing on superhydrophobic surfaces. Nat. Phys. 2014, 10, 515–519. [Google Scholar] [CrossRef]
- Jin, M.; Shen, Y.; Luo, X.; Tao, J.; Xie, Y.; Chen, H.; Wu, Y. A combination structure of microblock and nanohair fabricated by chemical etching for excellent water repellency and icephobicity. Appl. Surf. Sci. 2018, 455, 883–890. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; He, F.; Lv, C.; Hao, P. How micropatterns affect the anti-icing performance of superhydrophobic surfaces. Int. J. Heat Mass Transf. 2022, 195, 123196. [Google Scholar] [CrossRef]
- Lv, C.; Hao, P.; Zhang, X.; He, F. Dewetting Transitions of Dropwise Condensation on Nanotexture-Enhanced Superhydrophobic Surfaces. ACS Nano 2015, 9, 12311–12319. [Google Scholar] [CrossRef]
- Tian, X.; Verho, T.; Ras, R.H.A. Moving superhydrophobic surfaces toward real-world applications. Science 2016, 352, 142–143. [Google Scholar] [CrossRef]
- Lee, S.H.; Seong, M.; Kwak, M.K.; Ko, H.; Kang, M.; Park, H.W.; Kang, S.M.; Jeong, H.E. Tunable Multimodal Drop Bouncing Dynamics and Anti-Icing Performance of a Magnetically Responsive Hair Array. ACS Nano 2018, 12, 10693–10702. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Z.; Sett, S.; Oh, J.; Cha, H.; Li, L.; Feng, L.; Wu, Y.; Zhao, C.; Orejon, D.; et al. Atmosphere-mediated superhydrophobicity of rationally designed micro/nanostructured surfaces. ACS Nano 2019, 13, 4160–4173. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; He, M.; Li, K.; Cui, D.; Zhang, Q.; Zeng, X.; Zhang, Y.; Wang, J.; Song, Y. Superhydrophobic surfaces cannot reduce ice adhesion. Appl. Phys. Lett. 2012, 101, 111603. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Kulinich, S.; Farhadi, S.; Nose, K.; Du, X.-W. Superhydrophobic Surfaces: Are They Really Ice-Repellent? Langmuir ACS J. Surf. Colloids 2011, 27, 25–29. [Google Scholar] [CrossRef]
- Li, Y.; Quéré, D.; Lv, C.; Zheng, Q. Monostable superrepellent materials. Proc. Natl. Acad. Sci. USA 2017, 114, 3387–3392. [Google Scholar] [CrossRef]
- Tang, W.; Liu, L.; Ruan, Q.; Wu, Z.; Yang, C.; Cui, S.; Ma, Z.; Fu, R.K.Y.; Tian, X.; Chu, P.K.; et al. Dynamic changes of hydrophobic behavior during icing. Surf. Coat. Technol. 2020, 397, 126043. [Google Scholar] [CrossRef]
- Kreder, M.J.; Alvarenga, J.; Kim, P.; Aizenberg, J. Design of anti-icing surfaces: Smooth, textured or slippery? Nat. Rev. Mater. 2016, 1, 15003. [Google Scholar] [CrossRef]
- Fan, K.; Jin, Z.; Zhu, X.; Wang, Q.; Sun, J. A facile electrochemical machining process to fabricate superhydrophobic surface on iron materials and its applications in anti-icing. J. Dispers. Sci. Technol. 2021, 42, 457–464. [Google Scholar] [CrossRef]
- Dong, Z.; Vuckovac, M.; Cui, W.; Zhou, Q.; Ras, R.H.A.; Levkin, P.A. 3D Printing of Superhydrophobic Objects with Bulk Nanostructure. Adv. Mater. 2021, 33, 2106068. [Google Scholar] [CrossRef]
- Guo, P.; Zheng, Y.; Wen, M.; Song, C.; Lin, Y.; Jiang, L. Icephobic/Anti-Icing Properties of Micro/Nanostructured Surfaces. Adv. Mater. 2012, 24, 2642–2648. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.T.; Liu, E.J.; Wilson, P.; Chen, Z. Ice nucleation behaviour on sol–gel coatings with different surface energy and roughness. Phys. Chem. Chem. Phys. Pccp 2015, 17, 21492–21500. [Google Scholar] [CrossRef]
- Dong, Z.; Schumann, M.F.; Hokkanen, M.J.; Chang, B.; Welle, A.; Zhou, Q.; Ras, R.H.A.; Xu, Z.; Wegener, M.; Levkin, P.A. Superoleophobic Slippery Lubricant-Infused Surfaces: Combining Two Extremes in the Same Surface. Adv. Mater. 2018, 30, 1803890. [Google Scholar] [CrossRef] [PubMed]
- Ngo, C.-V.; Chun, D.-M. Control of laser-ablated aluminum surface wettability to superhydrophobic or superhydrophilic through simple heat treatment or water boiling post-processing. Appl. Surf. Sci. 2018, 435, 974–982. [Google Scholar] [CrossRef]
- Raja, R.S.S.; Selvakumar, P.; Babu, P.D. A novel fabrication of superhydrophobic surfaces on aluminium substrate by picosecond pulsed laser. J. Mech. Sci. Technol. 2020, 34, 1667–1674. [Google Scholar] [CrossRef]
- Samanta, A.; Wang, Q.; Shaw, S.K.; Ding, H. Nanostructuring of laser textured surface to achieve superhydrophobicity on engineering metal surface. J. Laser Appl. 2019, 31, 022515. [Google Scholar] [CrossRef]
- Fan, P.; Pan, R.; Zhong, M. Ultrafast Laser Enabling Hierarchical Structures for Versatile Superhydrophobicity with Enhanced Cassie–Baxter Stability and Durability. Langmuir 2019, 35, 16693–16711. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, J.; Liu, H.; Zhao, Y.; Pan, R. A short review on functionalized metallic surfaces by ultrafast laser micromachining. Int. J. Adv. Manuf. Technol. 2022, 119, 6919–6948. [Google Scholar] [CrossRef]
- Phillips, K.C.; Gandhi, H.H.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of materials: A review. Adv. Opt. Photon. 2015, 7, 684–712. [Google Scholar] [CrossRef]
- Fadeeva, E.; Chichkov, B. Biomimetic Liquid-Repellent Surfaces by Ultrafast Laser Processing. Appl. Sci. 2018, 8, 1424. [Google Scholar] [CrossRef]
- Yang, X.; Song, R.; He, L.; Wu, L.; He, X.; Liu, X.; Tang, H.; Lu, X.; Ma, Z.; Tian, P. Optimization mechanism and applications of ultrafast laser machining towards highly designable 3D micro/nano structuring. RSC Adv. 2022, 12, 35227–35241. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell Meets Marangoni—A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photonics Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Jiang, G.; Tian, Z.; Wang, L.; Luo, X.; Chen, C.; Hu, X.; Peng, R.; Zhang, H.; Zhong, M. Anisotropic Hemiwicking Behavior on Laser Structured Prismatic Microgrooves. Langmuir 2022, 38, 6665–6675. [Google Scholar] [CrossRef]
- Jiang, G.; Tian, Z.; Luo, X.; Chen, C.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Ultrathin aluminum wick with dual-scale microgrooves for enhanced capillary performance. Int. J. Heat Mass Transf. 2022, 190, 122762. [Google Scholar] [CrossRef]
- Fan, P.; Bai, B.; Zhong, M.; Zhang, H.; Long, J.; Han, J.; Wang, W.; Jin, G. General Strategy toward Dual-Scale-Controlled Metallic Micro–Nano Hybrid Structures with Ultralow Reflectance. ACS Nano 2017, 11, 7401–7408. [Google Scholar] [CrossRef]
- Liu, W.; Luo, X.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Directional anchoring patterned liquid-infused superamphiphobic surfaces for high-throughput droplet manipulation. Lab A Chip 2021, 21, 1373–1384. [Google Scholar] [CrossRef]
- Sabbah, A.; Youssef, A.; Damman, P. Superhydrophobic Surfaces Created by Elastic Instability of PDMS. Appl. Sci. 2016, 6, 152. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, Y.; Liu, N.; Li, Y.; Wang, P.; Dai, C.; Wei, Y. Multi-applicable, durable superhydrophobic anti-icing coating through template-method and chemical vapor deposition. Surf. Interfaces 2022, 32, 102100. [Google Scholar] [CrossRef]
- Long, J.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Superhydrophobic Surfaces Fabricated by Femtosecond Laser with Tunable Water Adhesion: From Lotus Leaf to Rose Petal. ACS Appl. Mater. Interfaces 2015, 7, 9858–9865. [Google Scholar] [CrossRef]
- Long, J.; Pan, L.; Fan, P.; Gong, D.; Jiang, D.; Zhang, H.; Li, L.; Zhong, M. Cassie-State Stability of Metallic Superhydrophobic Surfaces with Various Micro/Nanostructures Produced by a Femtosecond Laser. Langmuir 2016, 32, 1065–1072. [Google Scholar] [CrossRef]
- Cai, M.; Liu, W.; Luo, X.; Chen, C.; Pan, R.; Zhang, H.; Zhong, M. Three-Dimensional and In Situ-Activated Spinel Oxide Nanoporous Clusters Derived from Stainless Steel for Efficient and Durable Water Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 13971–13981. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tian, Z.; Luo, X.; Chen, C.; Jiang, G.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Wetting behavior of gallium-based room temperature liquid metal (LM) on nanosecond-laser-structured metal surfaces. Surf. Interfaces 2022, 32, 102180. [Google Scholar] [CrossRef]
- Luo, X.; Tian, Z.; Chen, C.; Jiang, G.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Laser-textured High-throughput Hydrophobic/Superhydrophobic SERS platform for fish drugs residue detection. Opt. Laser Technol. 2022, 152, 108075. [Google Scholar] [CrossRef]
- Liu, W.; Fan, P.; Cai, M.; Luo, X.; Chen, C.; Pan, R.; Zhang, H.; Zhong, M. An integrative bioinspired venation network with ultra-contrasting wettability for large-scale strongly self-driven and efficient water collection. Nanoscale 2019, 11, 8940–8949. [Google Scholar] [CrossRef]
- Correa, D.S.; Almeida, J.M.P.; Almeida, G.F.B.; Cardoso, M.R.; De Boni, L.; Mendonça, C.R. Ultrafast Laser Pulses for Structuring Materials at Micro/Nano Scale: From Waveguides to Superhydrophobic Surfaces. Photonics 2017, 4, 8. [Google Scholar] [CrossRef]
- Tang, B.-H.; Wang, Q.; Han, X.-C.; Zhou, H.; Yan, X.-J.; Yu, Y.; Han, D.-D. Fabrication of anti-icing/de-icing surfaces by femtosecond laser. Front. Chem. 2022, 10, 1073473. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-Step Fabrication of Robust Superhydrophobic Steel Surfaces with Mechanical Durability, Thermal Stability, and Anti-icing Function. ACS Appl. Mater. Interfaces 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Volpe, A.; Gaudiuso, C.; Di Venere, L.; Licciulli, F.; Giordano, F.; Ancona, A. Direct Femtosecond Laser Fabrication of Superhydrophobic Aluminum Alloy Surfaces with Anti-icing Properties. Coatings 2020, 10, 587. [Google Scholar] [CrossRef]
- Xia, A.; He, L.; Qie, S.; Zhang, J.; Li, H.; He, N.; Hao, X. Fabrication of an Anti-Icing Aluminum Alloy Surface by Combining Wet Etching and Laser Machining. Appl. Sci. 2022, 12, 2119. [Google Scholar] [CrossRef]
- Cai, M.; Fan, P.; Long, J.; Han, J.; Lin, Y.; Zhang, H.; Zhong, M. Large-Scale Tunable 3D Self-Supporting WO3 Micro-Nano Architectures as Direct Photoanodes for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 17856–17864. [Google Scholar] [CrossRef]
- Eklund, P.C.; Pradhan, B.K.; Kim, U.J.; Xiong, Q.; Fischer, J.E.; Friedman, A.D.; Holloway, B.C.; Jordan, K.; Smith, M.W. Large-Scale Production of Single-Walled Carbon Nanotubes Using Ultrafast Pulses from a Free Electron Laser. Nano Lett. 2002, 2, 561–566. [Google Scholar] [CrossRef]
- Yuan, D.; Li, J.; Huang, J.; Wang, M.; Xu, S.; Wang, X. Large-Scale Laser Nanopatterning of Multiband Tunable Mid-Infrared Metasurface Absorber. Adv. Opt. Mater. 2022, 10, 2200939. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Yang, J.; Yue, Y.; Zhang, H. Fabrication of superhydrophobic surface on stainless steel by two-step chemical etching. Chem. Phys. Lett. 2022, 797, 139567. [Google Scholar] [CrossRef]
- Guo, F.; Duan, S.; Wu, D.; Matsuda, K.; Wang, T.; Zou, Y. Facile etching fabrication of superhydrophobic 7055 aluminum alloy surface towards chloride environment anticorrosion. Corros. Sci. 2021, 182, 109262. [Google Scholar] [CrossRef]
- Talesh Bahrami, H.R.; Ahmadi, B.; Saffari, H. Preparing superhydrophobic copper surfaces with rose petal or lotus leaf property using a simple etching approach. Mater. Res. Express 2017, 4, 055014. [Google Scholar] [CrossRef]
- Ma, C.; Chen, L.; Wang, L.; Tong, W.; Chu, C.; Yuan, Z.; Lv, C.; Zheng, Q. Condensation droplet sieve. Nat. Commun. 2022, 13, 5381. [Google Scholar] [CrossRef]
- Shi, S.; Lv, C.; Zheng, Q. Temperature-regulated adhesion of impacting drops on nano/microtextured monostable superrepellent surfaces. Soft Matter 2020, 16, 5388–5397. [Google Scholar] [CrossRef]
- Niu, H.; Zhang, H.; Yue, W.; Gao, S.; Kan, H.; Zhang, C.; Zhang, C.; Pang, J.; Lou, Z.; Wang, L.; et al. Micro-Nano Processing of Active Layers in Flexible Tactile Sensors via Template Methods: A Review. Small 2021, 17, 2100804. [Google Scholar] [CrossRef]
- Gong, D.; Long, J.; Fan, P.; Jiang, D.; Zhang, H.; Zhong, M. Thermal stability of micro–nano structures and superhydrophobicity of polytetrafluoroethylene films formed by hot embossing via a picosecond laser ablated template. Appl. Surf. Sci. 2015, 331, 437–443. [Google Scholar] [CrossRef]
- He, Q.; Xu, Z.; Li, A.; Wang, J.; Zhang, J.; Zhang, Y. Study on hydrophobic properties of fluororubber prepared by template method under high temperature conditions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125837. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, X. A novel and facile fabrication of superhydrophobic surfaces on copper substrate via machined operation. Mater. Lett. 2017, 190, 115–118. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Z.; Zhang, H.; Yang, C.; Cheng, P. Promote anti- /de- frosting by suppressing directional ice bridging. Int. J. Heat Mass Transf. 2021, 165, 120609. [Google Scholar] [CrossRef]
- Dong, Z.; Levkin, P.A. 3D Microprinting of Super-Repellent Microstructures: Recent Developments, Challenges, and Opportunities. Adv. Funct. Mater. 2023, 2213916. [Google Scholar] [CrossRef]
- Luo, X.; Weng, Y.; Wang, S.; Du, J.; Wang, H.; Xu, C. Superhydrophobic and oleophobic textiles with hierarchical micro-nano structure constructed by sol–gel method. J. Sol-Gel Sci. Technol. 2019, 89, 820–829. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Novel superamphiphobic surfaces based on micro-nano hierarchical fluorinated Ag/SiO2 structures. Appl. Surf. Sci. 2018, 445, 262–271. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Luo, X.; Chen, C.; Jiang, G.; Hu, X.; Peng, R.; Zhang, H.; Zhong, M. Superomniphobic surfaces for easy-removals of environmental-related liquids after icing and melting. Nano Res. 2023, 16, 3267–3277. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 378. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, H.; Zhong, M. Triple-Scale Superhydrophobic Surface with Excellent Anti-Icing and Icephobic Performance via Ultrafast Laser Hybrid Fabrication. ACS Appl. Mater. Interfaces 2020, 13, 1743–1753. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, L.; Zhu, D.; Chen, C.; Zhao, H.; Peng, R.; Zhang, H.; Fan, P.; Zhong, M. Passive Anti-Icing Performances of the Same Superhydrophobic Surfaces under Static Freezing, Dynamic Supercooled-Droplet Impinging, and Icing Wind Tunnel Tests. ACS Appl. Mater. Interfaces 2023, 15, 6013–6024. [Google Scholar] [CrossRef]
- Chen, C.; Tian, Z.; Luo, X.; Jiang, G.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Cauliflower-like micro-nano structured superhydrophobic surfaces for durable anti-icing and photothermal de-icing. Chem. Eng. J. 2022, 450, 137936. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids philosophical transactions. R. Soc. Publ. 2022, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Trans. Faraday Soc. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Q.; Hokkanen, M.J.; Zhang, C.; Lin, F.-Y.; Liu, Q.; Zhu, S.-P.; Zhou, T.; Chang, Q.; He, B.; et al. Design of robust superhydrophobic surfaces. Nature 2020, 582, 55–59. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Feng, X.-Q.; Gao, X.; Wu, Z.; Jiang, L.; Zheng, Q.-S. Superior Water Repellency of Water Strider Legs with Hierarchical Structures: Experiments and Analysis. Langmuir 2007, 23, 4892–4896. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Li, P.; Zhang, B.; Zhao, H.; Zhang, L.; Wang, Z.; Xu, X.; Fu, T.; Wang, X.; Hou, Y.; Fan, Y.; et al. Unidirectional Droplet Transport on the Biofabricated Butterfly Wing. Langmuir 2018, 34, 12482–12487. [Google Scholar] [CrossRef]
- Gao, X.; Yan, X.; Yao, X.; Xu, L.; Zhang, K.; Zhang, J.; Yang, B.; Jiang, L. The Dry-Style Antifogging Properties of Mosquito Compound Eyes and Artificial Analogues Prepared by Soft Lithography. Adv. Mater. 2007, 19, 2213–2217. [Google Scholar] [CrossRef]
- Franta, B.; Mazur, E.; Sundaram, S.K. Ultrafast laser processing of silicon for photovoltaics. Int. Mater. Rev. 2018, 63, 227–240. [Google Scholar] [CrossRef]
- Chichkov, B.N.; Momma, C.; Nolte, S.; von Alvensleben, F.; Tünnermann, A. Femtosecond, picosecond and nanosecond laser ablation of solids. Appl. Phys. A 1996, 63, 109–115. [Google Scholar] [CrossRef]
- Wolfgang, K.; Joerg, K. Femtosecond pulse laser ablation of metallic, semiconducting, ceramic, and biological materials. In Proceedings of the Laser Materials Processing: Industrial and Microelectronics Applications, Vienna, Austria, 3–8 April 1994; pp. 600–611. [Google Scholar]
- Ihlemann, J.; Wolff, B.; Simon, P. Nanosecond and femtosecond excimer laser ablation of fused silica. Appl. Phys. A 1992, 54, 363–368. [Google Scholar] [CrossRef]
- Ahmmed, K.M.T.; Grambow, C.; Kietzig, A.-M. Fabrication of Micro/Nano Structures on Metals by Femtosecond Laser Micromachining. Micromachines 2014, 5, 1219–1253. [Google Scholar] [CrossRef]
- von der Linde, D.; Sokolowski-Tinten, K.; Bialkowski, J. Laser–solid interaction in the femtosecond time regime. Appl. Surf. Sci. 1997, 109–110, 1–10. [Google Scholar] [CrossRef]
- Fan, P.; Bai, B.; Jin, G.; Zhang, H.; Zhong, M. Patternable fabrication of hyper-hierarchical metal surface structures for ultrabroadband antireflection and self-cleaning. Appl. Surf. Sci. 2018, 457, 991–999. [Google Scholar] [CrossRef]
- Fan, P.; Zhong, M.; Bai, B.; Jin, G.; Zhang, H. Tuning the optical reflection property of metal surfaces via micro–nano particle structures fabricated by ultrafast laser. Appl. Surf. Sci. 2015, 359, 7–13. [Google Scholar] [CrossRef]
- Rajan, R.A.; Ngo, C.-V.; Yang, J.; Liu, Y.; Rao, K.S.; Guo, C. Femtosecond and picosecond laser fabrication for long-term superhydrophilic metal surfaces. Opt. Laser Technol. 2021, 143, 107241. [Google Scholar] [CrossRef]
- Rajab, F.H.; Liu, Z.; Li, L. Long term superhydrophobic and hybrid superhydrophobic/superhydrophilic surfaces produced by laser surface micro/nano surface structuring. Appl. Surf. Sci. 2019, 466, 808–821. [Google Scholar] [CrossRef]
- Luo, X.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Femtosecond laser micro-nano structured Ag SERS substrates with unique sensitivity, uniformity and stability for food safety evaluation. Opt. Laser Technol. 2021, 139, 106969. [Google Scholar] [CrossRef]
- Cai, M.; Pan, R.; Liu, W.; Luo, X.; Chen, C.; Zhang, H.; Zhong, M. Pulsed laser-assisted synthesis of defect-rich NiFe-based oxides for efficient oxygen evolution reaction. J. Laser Appl. 2020, 32, 022032. [Google Scholar] [CrossRef]
- Yin, K.; Wang, L.; Deng, Q.; Huang, Q.; Jiang, J.; Li, G.; He, J. Femtosecond Laser Thermal Accumulation-Triggered Micro-/Nanostructures with Patternable and Controllable Wettability Towards Liquid Manipulating. Nano-Micro Lett. 2022, 14, 97. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Wang, H.; Zhou, L.; Zhang, Z.; Zhou, Z. Temperature-Responsive, Femtosecond Laser-Ablated Ceramic Surfaces with Switchable Wettability for On-Demand Droplet Transfer. ACS Appl. Mater. Interfaces 2023, 15, 13740–13752. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, X.; Liu, Z.; Qin, Y.; Chang, W.; Sun, Y. Product and process fingerprint for nanosecond pulsed laser ablated superhydrophobic surface. Micromachines 2019, 10, 177. [Google Scholar] [CrossRef]
- Zhong, M.; Fan, P. Ultrafast Laser Enabling Versatile Fabrication of Surface Micro-nano Structures. In Laser Micro-Nano-Manufacturing and 3D Microprinting; Hu, A., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 75–112. [Google Scholar]
- Shen, Y.; Xie, X.; Tao, J.; Chen, H.; Cai, Z.; Liu, S.; Jiang, J. Mechanical Equilibrium Dynamics Controlling Wetting State Transition at Low-Temperature Superhydrophobic Array-Microstructure Surfaces. Coatings 2021, 11, 522. [Google Scholar] [CrossRef]
- Shi, S.; Lv, C.; Zheng, Q. Drop Impact on Two-Tier Monostable Superrepellent Surfaces. ACS Appl. Mater. Interfaces 2019, 11, 43698–43707. [Google Scholar] [CrossRef]
- Wu, H.; Yang, Z.; Cao, B.; Zhang, Z.; Zhu, K.; Wu, B.; Jiang, S.; Chai, G. Wetting and Dewetting Transitions on Submerged Superhydrophobic Surfaces with Hierarchical Structures. Langmuir 2017, 33, 407–416. [Google Scholar] [CrossRef]
- Tavakoli, F.; Kavehpour, H.P. Cold-Induced Spreading of Water Drops on Hydrophobic Surfaces. Langmuir 2015, 31, 2120–2126. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.; Yan, R.; Wang, H. Cassie State Stability and Gas Restoration Capability of Superhydrophobic Surfaces with Truncated Cone-Shaped Pillars. Langmuir 2021, 37, 12897–12906. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Chen, T.; Sun, X.; Mei, X.; Cui, J. Wettability and Stability of Wetting States for the Surfaces with Reentrant Structures. J. Phys. Chem. C 2020, 124, 28479–28487. [Google Scholar] [CrossRef]
- Pan, R.; Cai, M.; Liu, W.; Luo, X.; Chen, C.; Zhang, H.; Zhong, M. Extremely high Cassie-Baxter state stability of superhydrophobic surfaces via precisely tunable dual-scale and triple-scale micro-nano structures. J. Mater. Chem. A 2019, 7, 18050–18062. [Google Scholar] [CrossRef]
- Luo, X.; Pan, R.; Cai, M.; Liu, W.; Chen, C.; Jiang, G.; Hu, X.; Zhang, H.; Zhong, M. Atto-Molar Raman detection on patterned superhydrophilic-superhydrophobic platform via localizable evaporation enrichment. Sens. Actuators B Chem. 2021, 326, 128826. [Google Scholar] [CrossRef]
- Pan, R.; Cai, M.; Liu, W.; Luo, X.; Chen, C.; Zhang, H.; Zhong, M. Ultrafast laser hybrid fabrication of hierarchical 3D structures of nanorods on microcones for superhydrophobic surfaces with excellent Cassie state stability and mechanical durability. J. Laser Appl. 2020, 32, 022047. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, G.; Tian, Z.; Chen, C.; Hu, X.; Peng, R.; Zhang, H.; Fan, P.; Zhong, M. Superhydrophobic microstructures for better anti-icing performances: Open-cell or closed-cell? Mater. Horiz. 2023, 10, 209–220. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What do we need for a superhydrophobic surface? A review on the recent progress in the preparation of superhydrophobic surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Shi, S.; Lv, C.; Zheng, Q. Microskeleton-Nanofiller Composite with Mechanical Super-Robust Superhydrophobicity against Abrasion and Impact. Adv. Funct. Mater. 2020, 30, 1910665. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, H.; Huang, J.; Guo, Z. Review on the recent development of durable superhydrophobic materials for practical applications. Nanoscale 2021, 13, 11734–11764. [Google Scholar] [CrossRef] [PubMed]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The Lowest Surface Free Energy Based on −CF3 Alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Liu, L.; Charlton, L.; Song, Y.; Li, T.; Li, X.; Yin, H.; He, T. Scaling resistance by fluoro-treatments: The importance of wetting states. J. Mater. Chem. A 2022, 10, 3058–3068. [Google Scholar] [CrossRef]
- Li, R.; Tian, S.; Tian, Y.; Wang, J.; Xu, S.; Yang, K.; Yang, J.; Zhang, L. An Extreme-Environment-Resistant Self-Healing Anti-Icing Coating. Small 2022, 19, 2206075. [Google Scholar] [CrossRef] [PubMed]
- Vercillo, V.; Tonnicchia, S.; Romano, J.-M.; García-Girón, A.; Aguilar-Morales, A.I.; Alamri, S.; Dimov, S.S.; Kunze, T.; Lasagni, A.F.; Bonaccurso, E. Design Rules for Laser-Treated Icephobic Metallic Surfaces for Aeronautic Applications. Adv. Funct. Mater. 2020, 30, 1910268. [Google Scholar] [CrossRef]
- Walker, C.; Lerch, S.; Reininger, M.; Eghlidi, H.; Milionis, A.; Schutzius, T.M.; Poulikakos, D. Desublimation Frosting on Nanoengineered Surfaces. ACS Nano. 2018, 12, 8288–8296. [Google Scholar] [CrossRef]
- Bai, G.; Gao, D.; Liu, Z.; Zhou, X.; Wang, J. Probing the critical nucleus size for ice formation with graphene oxide nanosheets. Nature 2019, 576, 437–441. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, S.; Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 2002, 416, 409. [Google Scholar] [CrossRef]
- Long, J.; Wu, Y.; Gong, D.; Fan, P.; Jiang, D.; Zhang, H.; Zhong, M. Femtosecond laser fabricated superhydrophobic copper surfaces and their anti-icing properties. Zhongguo Jiguang/Chin. J. Lasers 2015, 42, 156–163. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, H.; Zhong, M. Ultrafast Laser Hybrid Fabrication and Ice -Resistance Performance of a Triple-Scale Micro/Nano Superhydrophobic Surface. Chin. J. Lasers-Zhongguo Jiguang 2021, 48, 0202009. [Google Scholar] [CrossRef]
- Ge, C.; Yuan, G.; Guo, C.; Ngo, C.-V.; Li, W. Femtosecond laser fabrication of square pillars integrated Siberian-Cocklebur-like microstructures surface for anti-icing. Mater. Des. 2021, 204, 109689. [Google Scholar] [CrossRef]
- Xing, W.; Li, Z.; Yang, H.; Li, X.; Wang, X.; Li, N. Anti-icing aluminum alloy surface with multi-level micro-nano textures constructed by picosecond laser. Mater. Des. 2019, 183, 108156. [Google Scholar] [CrossRef]
- Hong, Z.; Wang, W.; Ma, Z.; Lu, M.; Pan, S.; Shi, E.; Chen, Z.; Zhang, C. Anti-icing ceramics surface induced by femtosecond laser. Ceram. Int. 2022, 48, 10236–10243. [Google Scholar] [CrossRef]
- Wan, Y.; Yan, C.; Yu, H.; Wang, B. Anti-Icing Performance of Superhydrophobic Surface with Square-Ring Structure Prepared by Nanosecond Laser. Adv. Eng. Mater. 2021, 23, 2100190. [Google Scholar] [CrossRef]
- Gaddam, A.; Sharma, H.; Karkantonis, T.; Dimov, S. Anti-icing properties of femtosecond laser-induced nano and multiscale topographies. Appl. Surf. Sci. 2021, 552, 149443. [Google Scholar] [CrossRef]
- Zhao, M.; Yin, Y.; He, Q.; Zhao, X. Anti-icing capability of textured silicone rubber surfaces via laser processing. Mater. Manuf. Process. 2021, 36, 979–986. [Google Scholar] [CrossRef]
- Boreyko, J.B.; Collier, C.P. Delayed Frost Growth on Jumping-Drop Superhydrophobic Surfaces. ACS Nano 2013, 7, 1618–1627. [Google Scholar] [CrossRef]
- Sun, X.; Rykaczewski, K. Suppression of Frost Nucleation Achieved Using the Nanoengineered Integral Humidity Sink Effect. ACS Nano 2017, 11, 906–917. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-W.; Sahoo, V.; Lu, M.-C. Control of Ice Formation. ACS Nano 2017, 11, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing Coating with an Aqueous Lubricating Layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Cui, D.; Zhang, Q.; Zhang, Y.; Xu, F.; Zhou, X.; Wang, J.; Song, Y.; Jiang, L. Robust Prototypical Anti-icing Coatings with a Self-lubricating Liquid Water Layer between Ice and Substrate. ACS Appl. Mater. Interfaces 2013, 5, 4026–4030. [Google Scholar] [CrossRef]
- He, J.-G.; Zhao, G.-L.; Dai, S.-J.; Li, M.; Zou, G.-S.; Wang, J.-J.; Liu, Y.; Yu, J.-Q.; Xu, L.-F.; Li, J.-Q.; et al. Fabrication of Metallic Superhydrophobic Surfaces with Tunable Condensate Self-Removal Capability and Excellent Anti-Frosting Performance. Nanomaterials 2022, 12, 3655. [Google Scholar] [CrossRef]
- Long, J.; Zhou, P.; Huang, Y.; Xie, X. Enhancing the Long-Term Robustness of Dropwise Condensation on Nanostructured Superhydrophobic Surfaces by Introducing 3D Conical Microtextures Prepared by Femtosecond Laser. Adv. Mater. Interfaces 2020, 7, 2000997. [Google Scholar] [CrossRef]
- Byun, S.; Jeong, H.; Kim, D.R.; Lee, K.-S. Frost layer growth behavior on ultra-low temperature surface with a superhydrophobic coating. Int. Commun. Heat Mass Transf. 2021, 128, 105641. [Google Scholar] [CrossRef]
- Park, H.; Ahmadi, F.; Boreyko, J. Using Frost to Promote Cassie Ice on Hydrophilic Pillars. Phys. Rev. Lett. 2021, 127, 044501. [Google Scholar] [CrossRef]
- Mittal, K.L.; Choi, C.H. Ice Adhesion—Mechanism, Measurement and Mitigation; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Fortin, G.; Beisswenger, A.; Perron, J. Centrifuge Adhesion Test to Evaluate Icephobic Coatings. In Proceedings of the AIAA Atmospheric and Space Environments Conference, Toronto, ON, Canada, 2–5 August 2010. [Google Scholar]
- Irajizad, P.; Al-Bayati, A.; Eslami, B.; Shafquat, T.; Nazari, M.; Jafari, P.; Kashyap, V.; Masoudi, A.; Araya, D.; Ghasemi, H. Stress-localized durable icephobic surfaces. Mater. Horiz. 2019, 6, 758–766. [Google Scholar] [CrossRef]
- Work, A.; Lian, Y. A critical review of the measurement of ice adhesion to solid substrates. Prog. Aerosp. Sci. 2018, 98, 1–26. [Google Scholar] [CrossRef]
- Alamri, S.; Vercillo, V.; Aguilar-Morales, A.I.; Schell, F.; Wetterwald, M.; Lasagni, A.F.; Bonaccurso, E.; Kunze, T. Self-Limited Ice Formation and Efficient De-Icing on Superhydrophobic Micro-Structured Airfoils through Direct Laser Interference Patterning. Adv. Mater. Interfaces 2020, 7, 2001231. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.; Wang, W.; Xu, C.; Ren, L. Superhydrophobic Copper Surface Textured by Laser for Delayed Icing Phenomenon. Langmuir 2020, 36, 1075–1082. [Google Scholar] [CrossRef]
- Belaud, C.; Vercillo, V.; Kolb, M.; Bonaccurso, E. Development of nanostructured icephobic aluminium oxide surfaces for aeronautic applications. Surf. Coat. Technol. 2021, 405, 126652. [Google Scholar] [CrossRef]
- Milles, S.; Vercillo, V.; Alamri, S.; Aguilar, A.; Kunze, T.; Bonaccurso, E.; Lasagni, A. Icephobic Performance of Multi-Scale Laser-Textured Aluminum Surfaces for Aeronautic Applications. Nanomaterials 2021, 11, 135. [Google Scholar] [CrossRef]
- Pan, R.; Zhong, M. Fabrication of superwetting surfaces by ultrafast lasers and mechanical durability of superhydrophobic surfaces. Chin. Sci. Bull. 2019, 64, 1268–1289. [Google Scholar] [CrossRef]
- Chen, C.; Tian, Z.; Luo, X.; Jiang, G.; Hu, X.; Wang, L.; Peng, R.; Zhang, H.; Zhong, M. Micro–Nano-Nanowire Triple Structure-Held PDMS Superhydrophobic Surfaces for Robust Ultra-Long-Term Icephobic Performance. ACS Appl. Mater. Interfaces 2022, 14, 23973–23982. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Alsaid, Y.; Wu, D.; Hua, M.; Yan, Y.; Yao, B.; Ma, Y.; Zhu, X.; He, X. Superhydrophobic photothermal icephobic surfaces based on candle soot. Proc. Natl. Acad. Sci. USA 2020, 117, 11240–11246. [Google Scholar] [CrossRef]
- Jin, Y.; He, Z.; Guo, Q.; Wang, J. Control of Ice Propagation by Using Polyelectrolyte Multilayer Coatings. Angew. Chem. Int. Ed. 2017, 56, 11436–11439. [Google Scholar] [CrossRef]
- Niu, W.; Chen, G.Y.; Xu, H.; Liu, X.; Sun, J. Highly Transparent and Self-Healable Solar Thermal Anti-/Deicing Surfaces: When Ultrathin MXene Multilayers Marry a Solid Slippery Self-Cleaning Coating. Adv. Mater. 2022, 34, 2108232. [Google Scholar] [CrossRef]
- Sun, W.; Lv, K.; Lou, Y.; Zeng, D.; Lin, X. Highly durable superhydrophobic surfaces based on a protective frame and crosslinked PDMS-candle soot coatings. Mater. Res. Express 2022, 9, 096401. [Google Scholar] [CrossRef]
- Guo, X.-J.; Zhang, D.; Xue, C.-H.; Liu, B.-Y.; Huang, M.-C.; Wang, H.-D.; Wang, X.; Deng, F.-Q.; Pu, Y.-P.; An, Q.-F. Scalable and Mechanically Durable Superhydrophobic Coating of SiO2/Polydimethylsiloxane/Epoxy Nanocomposite. ACS Appl. Mater. Interfaces 2023, 15, 4612–4622. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yao, T.; Li, Z.; Wei, W.; Xie, Q.; Duan, W.; Han, H. A superhydrophobic/electrothermal synergistically anti-icing strategy based on graphene composite. Compos. Sci. Technol. 2020, 198, 108307. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, L.; Zhang, S.; Huang, H. Superhydrophobic SiC/CNTs Coatings with Photothermal Deicing and Passive Anti-Icing Properties. Acs Appl. Mater. Interfaces 2018, 10, 36505–36511. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, H.; Geng, Y.; Li, M.; Deng, Q.; Tian, Y.; Chen, R.; Zhu, X.; Liao, Q. Carbon-Based Photothermal Superhydrophobic Materials with Hierarchical Structure Enhances the Anti-Icing and Photothermal Deicing Properties. ACS Appl. Mater. Interfaces 2021, 13, 48308–48321. [Google Scholar] [CrossRef]
- Xie, H.; Wei, J.; Duan, S.; Zhu, Q.; Yang, Y.; Chen, K.; Zhang, J.; Li, L.; Zhang, J. Non-fluorinated and durable photothermal superhydrophobic coatings based on attapulgite nanorods for efficient anti-icing and deicing. Chem. Eng. J. 2022, 428, 132585. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.; Zhu, D.; Yuan, L.; Zhong, M. Anti-Icing Research and Development of Superhydrophobic Surfaces. Aerodyn. Res. Exp. 2022, 34, 1–26. [Google Scholar]
- Chu, Z.; Jiao, W.; Huang, Y.; Ding, G.; Zhong, X.; Yan, M.; Zheng, Y.; Wang, R. FDTS-Modified SiO2/rGO Wrinkled Films with a Micro-Nanoscale Hierarchical Structure and Anti-Icing/Deicing Properties under Condensation Condition. Adv. Mater. Interfaces 2020, 7, 1901446. [Google Scholar] [CrossRef]
- Sun, H.; Lin, G.; Jin, H.; Bu, X.; Cai, C.; Jia, Q.; Ma, K.; Wen, D. Experimental investigation of surface wettability induced anti-icing characteristics in an ice wind tunnel. Renew. Energy 2021, 179, 1179–1190. [Google Scholar] [CrossRef]
- Hao, T.; Zhu, Z.; Yang, H.; He, Z.; Wang, J. All-Day Anti-Icing/Deicing Film Based on Combined Photo-Electro-Thermal Conversion. ACS Appl. Mater. Interfaces 2021, 13, 44948–44955. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y.; Wang, Y.; Liang, W.; Wang, F.; Zhu, D.; Zhao, H. Functionalized superhydrophobic MWCNT/PEI nanocomposite film with anti-icing and photo-/electrothermal deicing properties. Mater. Chem. Phys. 2023, 297, 127385. [Google Scholar] [CrossRef]
- Ma, L.; Wang, J.; Zhao, F.; Wu, D.; Huang, Y.; Zhang, D.; Zhang, Z.; Fu, W.; Li, X.; Fan, Y. Plasmon-mediated photothermal and superhydrophobic TiN-PTFE film for anti-icing/deicing applications. Compos. Sci. Technol. 2019, 181, 107696. [Google Scholar] [CrossRef]
- Fan, P.X.; Wu, H.; Zhong, M.L.; Zhang, H.J.; Bai, B.F.; Jin, G.F. Large-scale cauliflower-shaped hierarchical copper nanostructures for efficient photothermal conversion. Nanoscale 2016, 8, 14617–14624. [Google Scholar] [CrossRef]
- Ma, W.; Li, Y.; Chao, C.Y.H.; Tso, C.Y.; Huang, B.; Li, W.; Yao, S. Solar-assisted icephobicity down to −60 °C with superhydrophobic selective surfaces. Cell Rep. Phys. Sci. 2021, 2, 100384. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, A.; Zhou, L.; Li, Y.; Kang, J.; Tang, J.; Han, Y.; Liu, H. Facilely Fabricated Self-Lubricated Photothermal Coating with Long-Term Durability and External-Replenishing Property for Anti-Icing/Deicing. ACS Appl Mater Interfaces 2022, 14, 8537–8548. [Google Scholar] [CrossRef]
- Zhao, W.; Xiao, L.; He, X.; Cui, Z.; Fang, J.; Zhang, C.; Li, X.; Li, G.; Zhong, L.; Zhang, Y. Moth-eye-inspired texturing surfaces enabled self-cleaning aluminum to achieve photothermal anti-icing. Opt. Laser Technol. 2021, 141, 107115. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, G.; Wu, S.; Alsaid, Y.; Zhao, W.; Yan, X.; Liu, L.; Zou, G.; Lv, J.; He, X.; et al. Solar anti-icing surface with enhanced condensate self-removing at extreme environmental conditions. Proc. Natl. Acad. Sci. USA 2021, 118, e2100978118. [Google Scholar] [CrossRef]
- Vercillo, V. Durable Laser Patterned Metal Surfaces with Enhanced Icephobic Properties for Aerospace Applications. Ph.D. Thesis, Technische Universität Dresden, Dresden, Germany, 2020. [Google Scholar]
- Boinovich, L.B.; Emelyanenko, A.M.; Emelyanenko, K.A.; Modin, E.B. Modus Operandi of Protective and Anti-icing Mechanisms Underlying the Design of Longstanding Outdoor Icephobic Coatings. ACS Nano 2019, 13, 4335–4346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).