A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process
Abstract
Featured Application
Abstract
1. Introduction
1.1. Level of Silicon Contamination
1.2. EDX Analysis of Silicon Contamination
1.3. History of Cleaning Agents in the Semiconductor Industry
2. Materials and Methods
2.1. Challenges of Cleaning the Contamination
2.2. Types of Cleaning Agents for Wafer Dicing on the Market
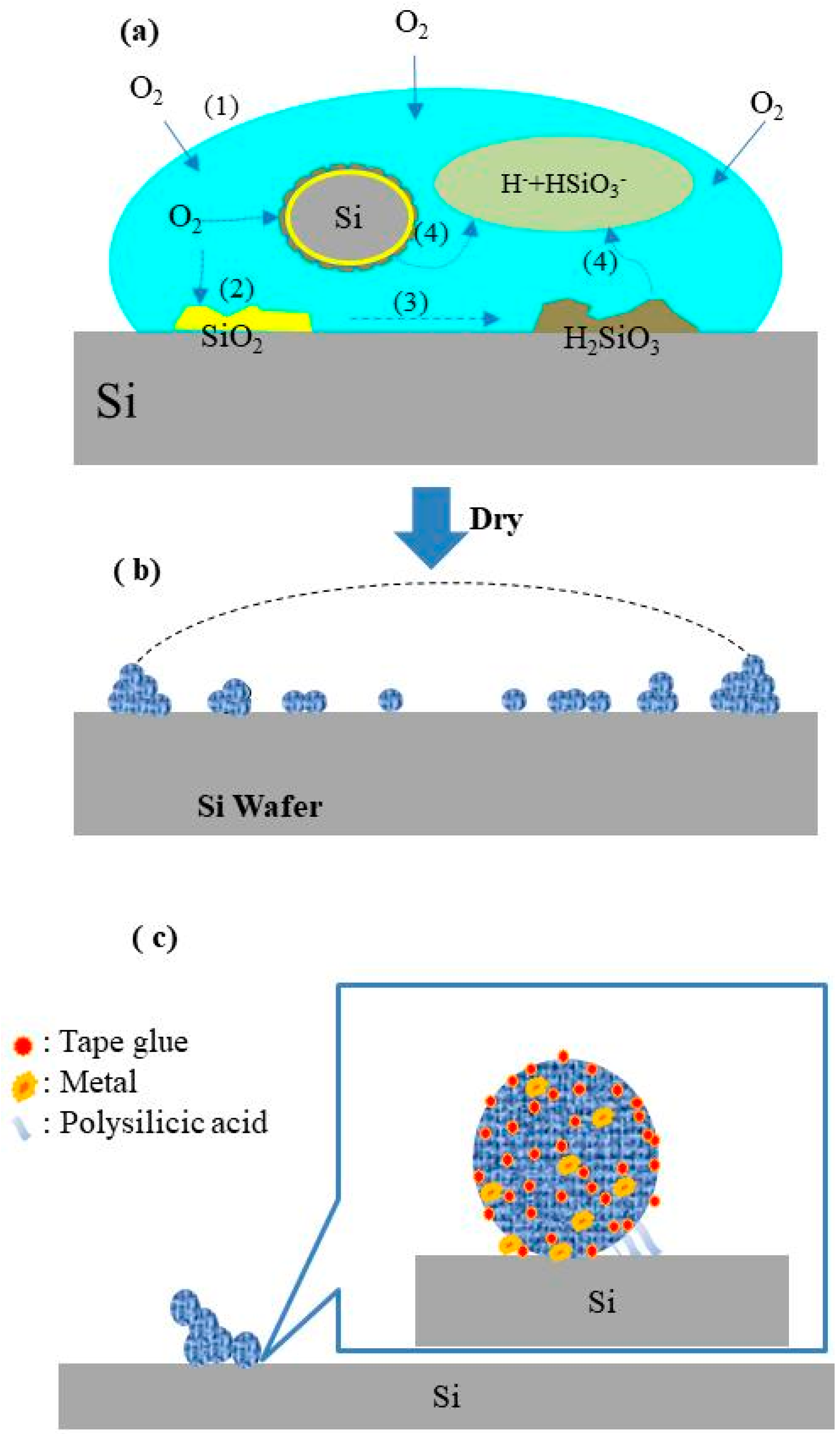
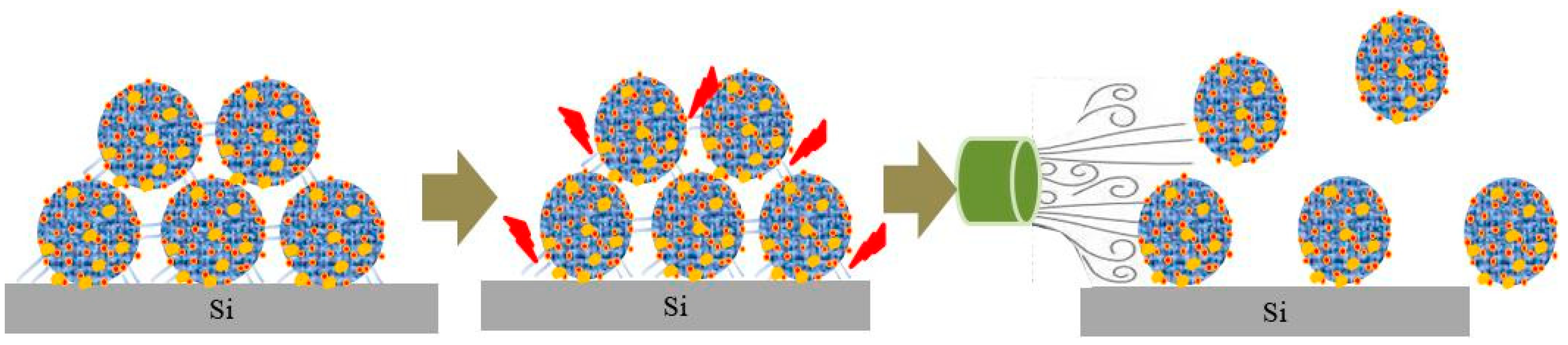
2.3. Formation of Contaminants
2.4. Mechanism of Contaminant Cleaning
- Silicic acid bonding
- Glue adhesive residue
- van der Waals forces
2.5. Development of a New Cleaning Agent
- Full cleaning of the silicon contamination (Levels 1 to 3)
- No IC trace (Al) corrosion remaining after cleaning process
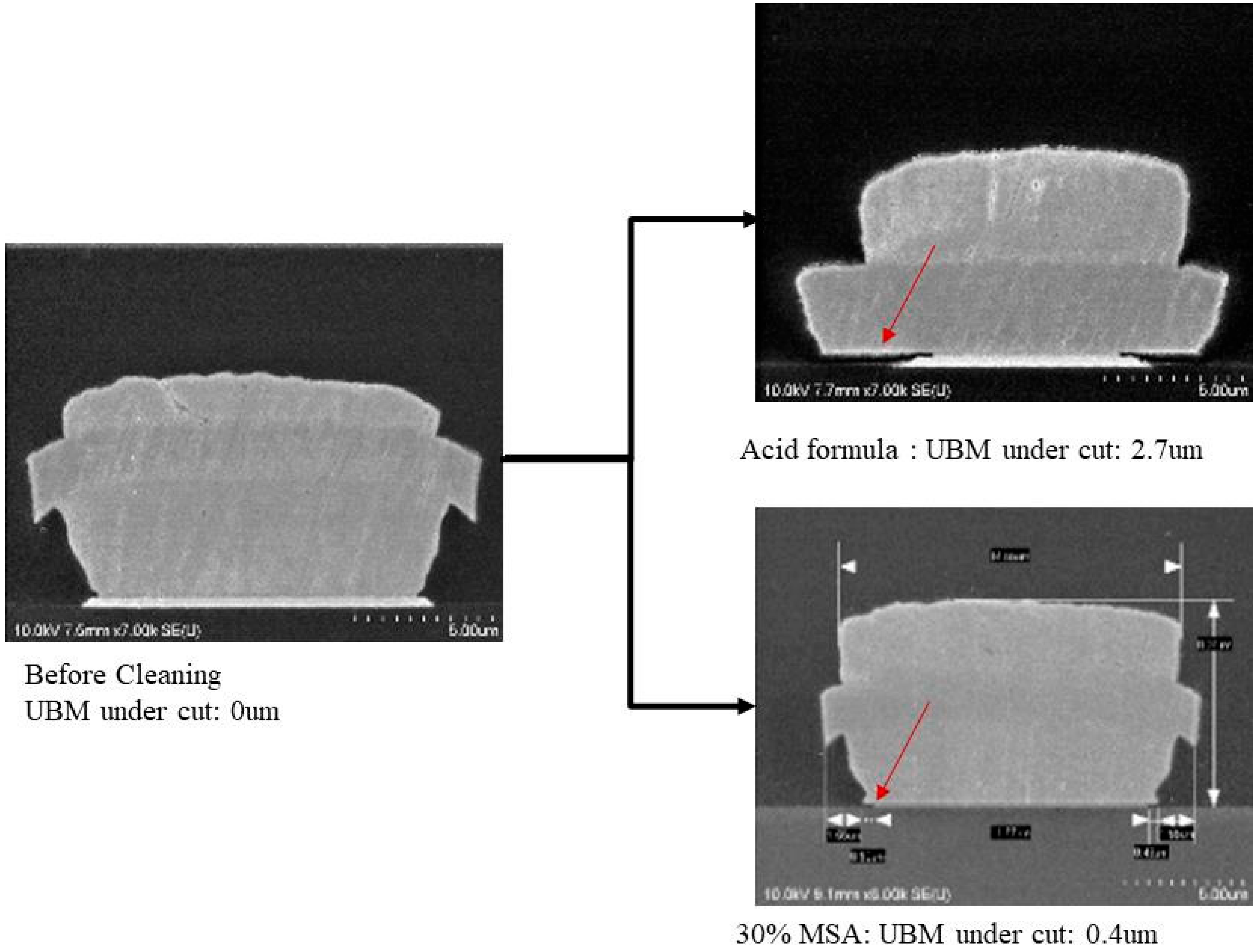
- No more UBM under cut to enhance
- No gel residue
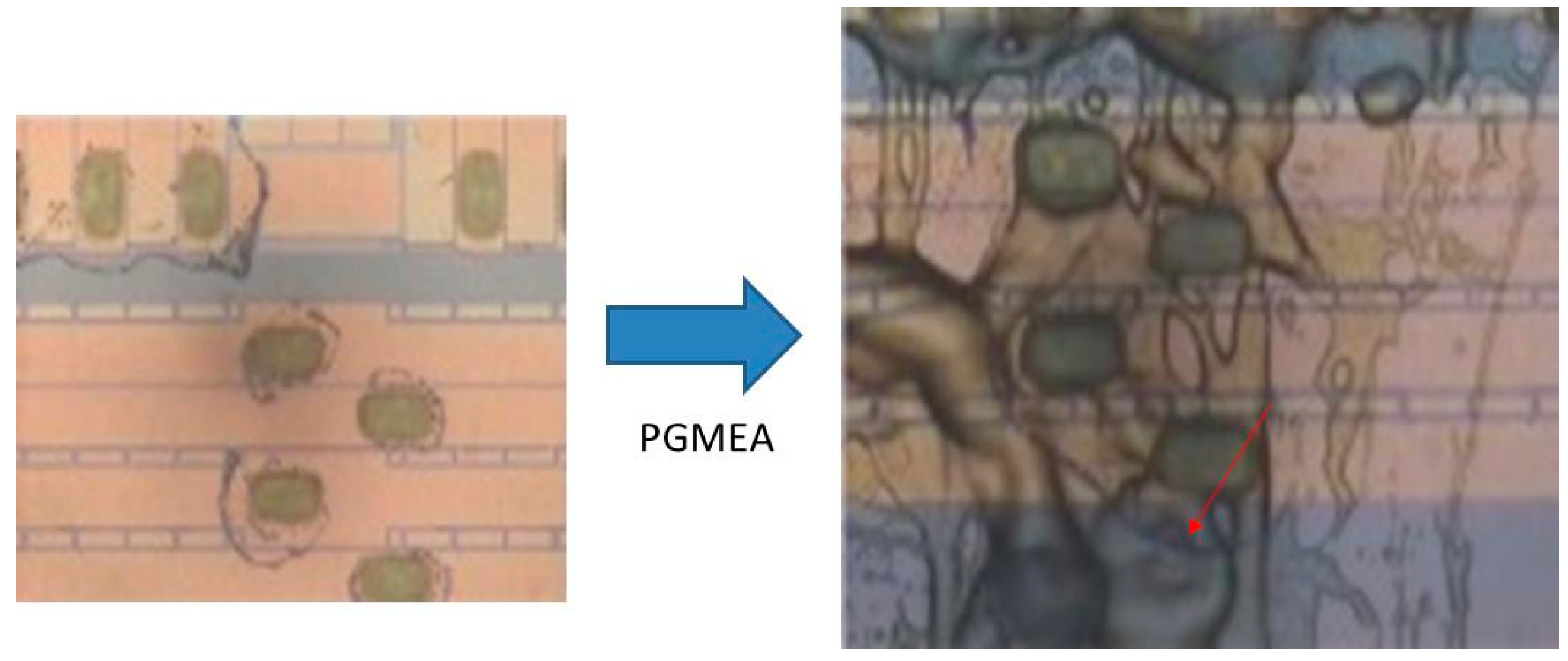
- In mark area, residue is more than 80% and is readable.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kern, W.; Puotinen, D.A. Cleaning solutions based on hydrogen peroxide for use in silicon semiconductor technology. RCA Rev. 1970, 31, 187–206. [Google Scholar]
- Vig, J.B. UV ozone cleaning of surfaces. J. Vac. Sci. Technol. A 1985, 3, 1027–1034. [Google Scholar] [CrossRef]
- Oehrlein, G.S.; Scilla, G.J.; Jeng, S.J. Efficiency of oxygen plasma cleaning of reactive ion damaged silicon surfaces. Appl. Phys Lett. 1988, 52, 907–909. [Google Scholar] [CrossRef]
- Brachmann, E.; Seifert, M.; Oswald, S.; Menzel, S.B.; Gemming, T. Evaluation of surface cleaning procedures for CTGS substrates for SAW technology with XPS. Materials 2017, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Affrossman, S.; Daviot, J.; Holmes, D.; Pethrick, R.A.; Wilson, M. Molecular design for inhibition of titanium corrosion in resist cleaner systems. Corros. Sci. 2001, 43, 939–950. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Zhang, S.; Isoya, S.; Shimao, S. Research on Surface Watermark Analysis of Wafer Cleaning Process. In Proceedings of the 2012 Conference on Society of Manufacturing Engineering—SME, Notre Dame, IN, USA, 4–8 June 2012. [Google Scholar]
- Sharafeev, S.M.; Pogrebenkov, V.M. Phase Formation Processes in Natural Magnesium Silicates of Various Structures by Ammonium Fluoride Treatment. Refract. Ind. Ceram. 2020, 61, 200–206. [Google Scholar] [CrossRef]
- Liu, X.; Liu, C.; Meng, C. Oligomerization of Silicic Acids in Neutral Aqueous Solution: A First-Principles Investigation. Int. J. Mol. Sci. 2019, 20, 3037. [Google Scholar] [CrossRef]
- Putz, M.V.; Russo, N.; Sicilia, E. On the applicability of the hsab principle through the use of improved computational schemes for chemical hardness evaluation. J. Comput. Chem. 2004, 25, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.V. Density functionals of chemical bonding. Int. J. Mol. Sci. 2008, 9, 1050–1095. [Google Scholar] [CrossRef]
- Putz, M.V. Maximum hardness index of quantum acid-base bonding. MATCH Commun. Math. Comput. Chem. 2008, 60, 845–868. [Google Scholar]
- Putz, M.V. Chemical action concept and principle. MATCH Commun. Math. Comput. Chem. 2011, 66, 35–63. [Google Scholar]
- Mondal, B.; Ghosh, D.; Das, A.K. Thermochemistry for silicic acid formation reaction: Prediction of new reaction pathway. Chem. Phys. Lett. 2009, 478, 115–119. [Google Scholar] [CrossRef]
- Goto, K. Effect of pH on Polymerization of Silicic Acid. J. Phys. Chem. 1956, 60, 1007–1008. [Google Scholar] [CrossRef]
- Houston, M.R.; Maboudian, R.; Howe, R.T. Ammonium Fluoride Anti-Stiction Treatments for Polysilicic Microstructures. In Proceedings of the 8th international Conference on Solid-State Sensors and Actuators, and Eurosensors IX, Stockholm, Sweden, 25–29 June 1995. [Google Scholar]
- Williams, K.R.; Muller, R.S. Etching Rates for Micromachining Processing. IEEE J. Microelectromechanical Syst. 1996, 5, 256–269. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Borazio, A.; Greef, R.; Peter, L.M.; Stumper, J. Electrochemical and Optical Studies of Silicon Dissolution in Ammonium Fluoride Solutions. Electrochem. Acta Print. Great Br. 1992, 37, 889–896. [Google Scholar] [CrossRef]
- Niwano, M.; Takeda, Y.; Ishibashi, Y.; Kurita, K.; Miyamoto, N. Miyamoto Morphology of hydrofluoric acid and ammonium fluoride-treated silicon surfaces studied by surface infrared spectroscopy. J. Appl. Phys. 1992, 71, 5646–5649. [Google Scholar] [CrossRef]
- Kalem, S. Synthesis of ammonium silicon fluoride crypto crystals on silicon by dry etching. Appl. Surf. Sci. 2004, 236, 336–341. [Google Scholar] [CrossRef]
- Gerischer, H.; Lübke, M. On the Etching of Silicon by Oxidants in Ammonium Fluoride Solution. J. Electrochem. Soc. 1988, 135, 2782. [Google Scholar] [CrossRef]
- Bahri, C.N.A.C.Z.; Ismail, A.F.; Majid, A.A. Synthesis of thorium tetra fluoride (ThF4) by ammonium hydrogen bifluoride (NH4HF2). Nucl. Eng. Technol. 2018, 51, 792–799. [Google Scholar] [CrossRef]
- Huang, J.J.; Lin, C.H.; Ho, Y.R.; Chang, Y.H. A study of aluminum oxide passivation films by liquid phase deposition and its application for ultraviolet solid-liquid hetero junction photodetectors. Surf. Coat. Technol. 2019, 391, 125684. [Google Scholar] [CrossRef]
- Mirhashemihaghighi, S.; Światowska, J.; Maurice, V.; Seyeux, A.; Zanna, S.; Salmi, E.; Ritala, M.; Marcus, P. Corrosion protection of aluminum by ultra-thin atomic layer deposited alumina coatings. Corros. Sci. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Chang, W.; Hsuan, T.T. Study on Anti-Degradation Methods of Silicon Cutting and Wafer Cleaning after Cutting; National Taipei University of Technology of Resource Engineering: Taipei, Taiwan, 2020. [Google Scholar]
- Peng, B.; Zheng, D.; Yu, Y. Method to remove wafer surface particles. IEEE J. Semicond. 2017, 38, 096004. [Google Scholar] [CrossRef]
- Ku, C.M.; Cheng, S. Factor Design for the Oxide Etching Process to Reduce Edge Particle Contamination in Capacitively Coupled Plasma Etching Equipment. Appl. Sci. 2022, 12, 5684. [Google Scholar] [CrossRef]
- Barkhudarov, P.M.; Shah, P.B.; Watkins, E.B.; Doshi, D.A.; Brinker, C.J.; Majewski, J. Corrosion inhibition using super hydrophobic films. Corros. Sci. 2008, 50, 897–902. [Google Scholar] [CrossRef]
- Tamura, H.; Ito, N.; Kitano, M.; Takasaki, S. A kinetic model of the dissolution of copper (II) oxide in EDTA solutions considering the coupling of metal and oxide ion transfer. Corros. Sci. 2001, 43, 1675–1691. [Google Scholar] [CrossRef]
- Said, M.; Nazeri, M.F.M.; Sharif, N.M.; Kheawhom, S.; Mohamad, A.A. Corrosion properties of Cu/Sn–3.0Ag–0.5Cu/Cu solder butt joints fabricated by conventional reflow and microwave hybrid heating. Corros. Sci. 2022, 208, 110641. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, H.; Wang, J.; Sun, R.; Wu, L.; Liu, Y.; Xu, J.; Liu, J.; Peng, K.Q. Carbon induced galvanic etching of silicon in aerated HF/H2O vapor. Corros. Sci. 2019, 157, 268–273. [Google Scholar] [CrossRef]
- Hossain, S.T.; Johra, F.T.; Jung, W.G. Fabrication of Silicon Carbide from Recycled Silicon Wafer Cutting Sludge and Its Purification. Appl. Sci. 2018, 8, 1841. [Google Scholar] [CrossRef]

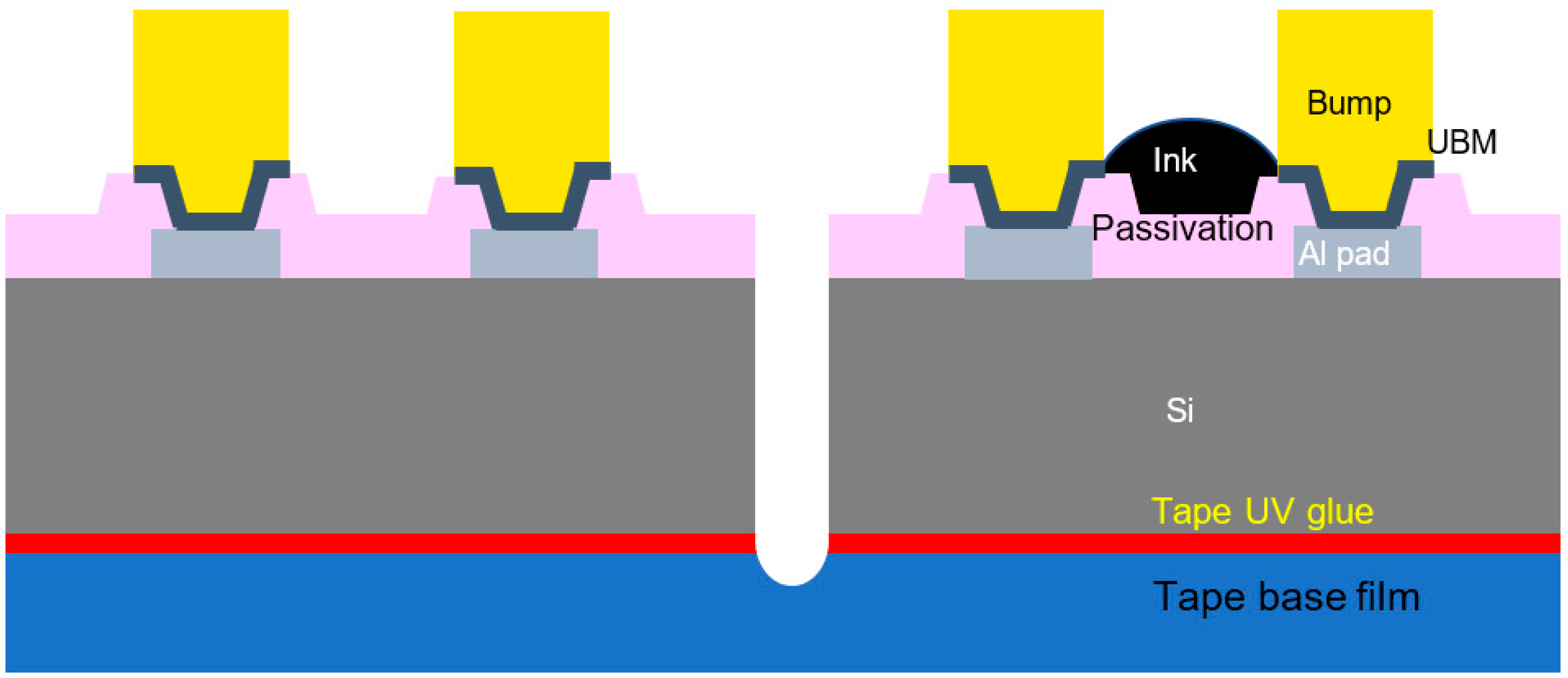
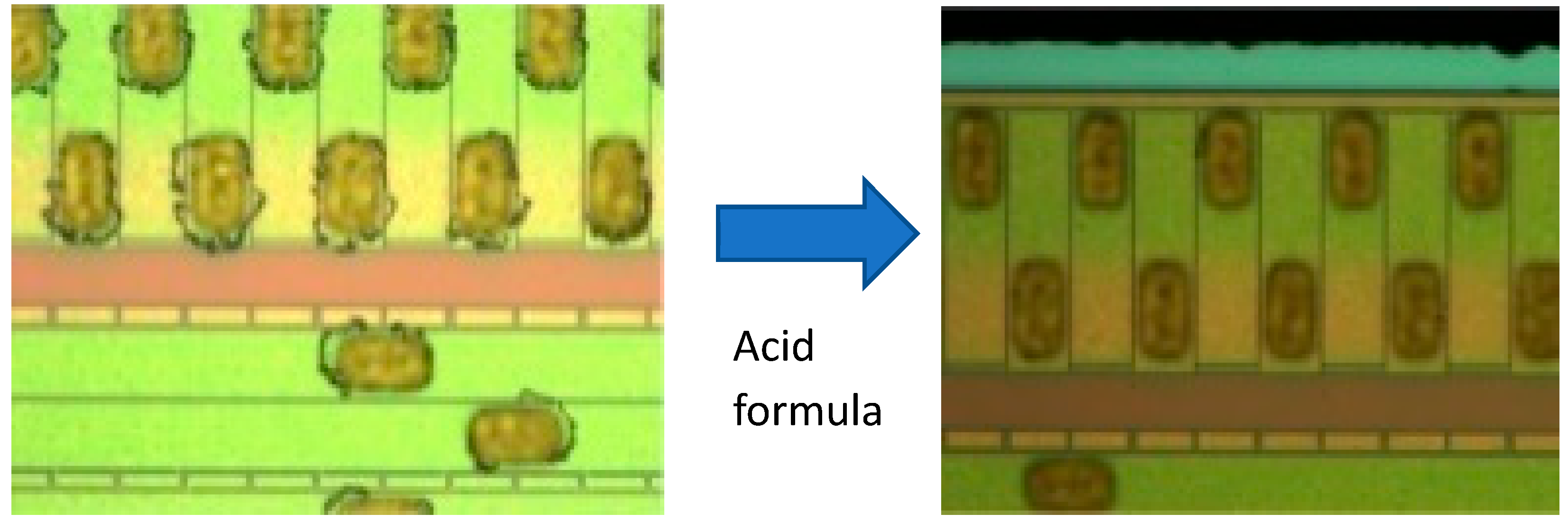
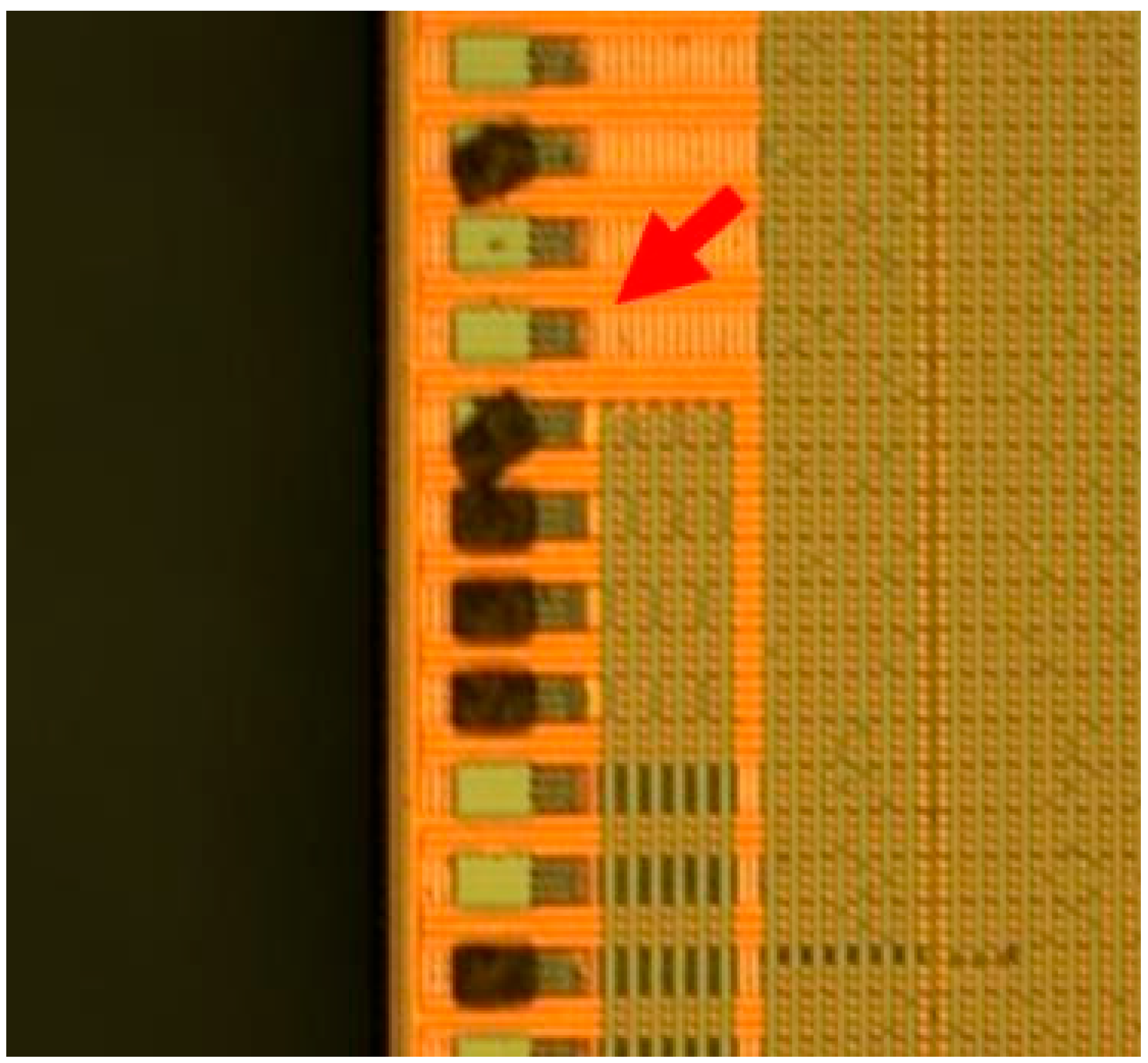
| Level 1 | Level 2 | Level 3 |
|---|---|---|
Circular around bumps | Deposit at non-bump area  | Water mark |
| Type | Solvent-Based | Alkaline-Based | Alkaline + Surfactant | Stripper (Photoresist) |
|---|---|---|---|---|
| Products | Triton: X-100 | Developer (D05) | GDC03 (Alkaline) | Stripper (SP-700) |
| Formula | 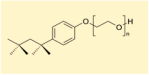 | 0.5% TMAH 0.05% non-ionic surfactant | Petroleum ether Amine base surfactant | 8% TMAH (25%) 85% DMSO 7% Sorbitol |
| Test | Type of Cleaner | Performance | ||||||
| ||||||||
| Silicon Contamination (Si) | Al | UBM | Gel | Ink Mark | ||||
| Level 1 | Level 2 | Level 3 | ||||||
| 1 | Triton X-100 | NG | NG | OK | OK | OK | OK | OK |
| 2 | Developer (D05) | NG | OK | OK | NG | OK | OK | OK |
| 3 | Alkaline + Surfactant (GDC03) | 40% NG | OK | OK | 20% NG | OK | OK | NG |
| 4 | Stripper (SP-700) | OK | OK | OK | OK | 50% NG | NG | NG |
| 5 | SC-1 | NG | NG | OK | NG | NG | NG | NG |
| 6 | SC-2 | NG | NG | OK | NG | NG | OK | OK |
| 7 | BOE (HF buffer) | OK | OK | OK | NG | NG | OK | OK |
| Test | Composition | Performance | ||||
|---|---|---|---|---|---|---|
| Si Level 1 | Al Corrosion | UBM Under Cut | Gel Residue | Ink Marks | ||
| 1 | 40% MEA + 4% NH4F-HF + 56% H2O | NG | OK | OK | OK | OK |
| 2 | 40% PGMEA + 4% NH4F-HF + 56% H2O | NG | OK | OK | NG | NG |
| 3 | 10% H2SO4 + 4% NH4F-HF + 86% H2O | OK | OK | NG | OK | OK |
| 4 | 15% H2SO4 + 1% NH4F-HF + 84% H2O | NG | OK | NG | OK | OK |
| 5 | 15% H2SO4 + 4% C NH4F-HF + 7% non-ionic surfactant + 74% H2O | NG | NG | NG | NG | NG |
| 6 | 20% H2SO4 + 6% NH4F-HF + 74% H2O | OK | OK | NG | OK | OK |
| 7 | 20% H2SO4 + 3% NH4F-HF + 77% MSA | NG | OK | OK | NG | NG |
| 8 | 20% H2SO4 + 3% NH4F-HF + 30% MSA + 47% H2O | OK | NG | △OK | OK | NG |
| 9 | 10% H2SO4 + 1.5% NH4F-HF + 8.5% MSA + 80% H2O | OK | OK | △OK | OK | OK |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, T.-H.; Wang, C.-Y. A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process. Appl. Sci. 2023, 13, 5294. https://doi.org/10.3390/app13095294
Tsai T-H, Wang C-Y. A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process. Applied Sciences. 2023; 13(9):5294. https://doi.org/10.3390/app13095294
Chicago/Turabian StyleTsai, Teh-Hua, and Chen-Yu Wang. 2023. "A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process" Applied Sciences 13, no. 9: 5294. https://doi.org/10.3390/app13095294
APA StyleTsai, T.-H., & Wang, C.-Y. (2023). A Study of Ammonium Bifluoride as an Agent for Cleaning Silicon Contamination in the Wafer Dicing Process. Applied Sciences, 13(9), 5294. https://doi.org/10.3390/app13095294




