Abstract
A low-permeability reservoir contains many fine pore throat structures, which result in excessive injection pressure of the water injection well and difficult water injection in the production process of a low-permeability reservoir. In this study, a new silane coupling agent was synthesized via the ring-opening reaction between dodecyl amine and KH-560 (γ-propyl trimethoxysilane). The modified KH-560 was reacted with nano-SiO2 to synthesize the modified nano-SiO2 as an antihypertensive additive. Fourier infrared spectroscopy, thermogravimetric analysis and laser scattering were used to characterize this modified nano-SiO2. The results show that the particle size of the modified nano-SiO2 is less than 60 nm. The test results of the water contact angle show that the dispersion system can increase the rock contact angle from 37.34° to 136.36°, which makes the rock surface transform from hydrophilicity to hydrophobicity and reduce the binding effect of rock with water. The dispersion test shows that the modified nano-SiO2 has good dispersion stability under alkaline conditions with TX-100 (Polyethylene glycol octylphenyl ether) as the dispersant. The antiswelling test results show that the antiswelling rate of this modified nano-SiO2 is 42.9%, which can efficiently prevent the clay expansion in the formation to reduce the injection pressure. The core displacement test results show that its depressurization rate reaches 49%. The depressurization rate still maintains 46% at a 20 PV water flow rate, indicating that its depressurization effect is remarkable and it has excellent erosion resistance.
1. Introduction
In recent years, given the newly added geological reserves in China, low-permeability and very-low-permeability geological reserves have increased yearly [1,2]. Based on previous statistical results, the porosity of low-permeability reservoirs is less than 15% and their permeability is less than 20 mD [3]. Many fine pore throat structures exist in low-permeability reservoirs [4]. Owing to the existence of microcapillary forces, the solid–liquid interface and the electrochemistry effect, a start-up pressure difference exists in low-permeability reservoirs [5,6]. Moreover, the clay in the formation is easy to expand [7,8], which leads to the high pressure of the water injection well. So, it directly affects the efficiency of water injection, increases the burden of the water injection system and causes high energy consumption. In addition, a casing rupture will occur when the water injection system is under a long-term high-pressure condition, which would result in a reduction in sweep efficiency and oil recovery efficiency [9]. Therefore, the development of high-efficiency pressure-reducing and injection-enhancing agents is key to the exploitation of low-permeability reservoirs.
The pressure-decreasing and augmented injection in low-permeability reservoirs have been extensively studied. At present, the technology of pressure-decreasing and augmented injection has two main methods: reservoir modification and surface modification [10]. Reservoir reconstruction technology is used to expand the effective diameter of rock pores by acidizing plugging removal and hydraulic fracturing [11,12] to reduce injection resistance to achieve the result of reduced pressure and increased injection [13,14,15]. In the process of hydraulic fracturing and acidification, it is easy for the reservoir to be injured by the intrusion of external fluids, resulting in a serious decline in the exploitation degree [16,17,18]. Therefore, how to reduce the damage to the reservoir and the equipment during the use of reservoir reconstruction technology is also a main problem that needs to be solved urgently. Surface modification technology is utilized to change the rock surface properties of pores [19] and prevent clay from swelling by injecting modifiers into rock pores [20], so as to achieve the effect of reducing pressure and increasing injection [21,22,23,24].
Hydrophobically modified nano-SiO2, as a modifier to reduce pressure and increase injection, is a newly developing technology. The hydrophobic nano-SiO2 dispersion is injected into the formation to achieve the pressure-decreasing and augmented injection of the reservoir. The surface of the modified nano-SiO2 particles has unsaturated bonds and strong adsorption capacity. After entering the formation, the nano-SiO2 adsorbs onto the surface of the rock pores, replacing the existing hydration film and forming a new adsorption layer, making the rock pores hydrophobic. It reduces the flow resistance and the contact between the water and the rock pores, achieving the effect of the pressure-decreasing and augmented injection [25,26]. Liu [27] synthesized an environmentally responsive modified nano-SiO2, which was covalently modified by organic compounds containing hydrophobic groups and double bonds. The chemical adsorption method was used to cover a layer of hydrophilic organic matter on the surface of the modified nano-SiO2 to ensure its good dispersibility in water. Owing to the change in the environment (temperature, pH and salinity), the nano-SiO2 would be released from the dispersion and adsorbed onto the rock pores when the nano-SiO2 enters the injection well from the normal environment, thereby achieving the effect of reduced pressure and increased injection. Dai [28] used dimethyldichlorosilane to modify nano-SiO2. The core-flow experiment shows that the modified nano-SiO2 exerts a better effect on depressurization and injection in low-permeability reservoirs, and the depressurization rate reaches 45%. Zhao [29] used n-propyl trichlorosilane to modify nano-SiO2 and evaluated the effect of the pressure-decreasing and augmented injection through core-flow experiments. The results showed that the depressurization rate of the modified nano-SiO2 in ultra-low permeability reservoirs reached 31.47%. A roughness reduction mechanism was proposed to explain their pressure reduction mechanism. Hydrophobic silica nanoparticles can adsorb onto the sand surface, reduce the roughness of the flow channel, partially change the wettability of the flow channel and reduce its water wettability. Flow resistance and injection pressure are reduced accordingly.
The above-mentioned pressure-reducing and injecting agent has an unsatisfactory depressurization effect under the condition of ultra-low-permeability (less than 10 mD). Based on this problem, a new modified nano-SiO2, used as a depressurization and injection enhancement agent, was synthesized in this study, which has excellent pressure-decreasing and augmented injection effects under the formation condition with permeability less than 10 mD. The structure and particle size of prepared nano-SiO2 were characterized using infrared spectrometry and dynamic light scattering. The wettability, thermal stability, dispersion stability and antiswelling properties of the modified nano-SiO2 were simultaneously studied in this study. The performance of the pressure-decreasing and augmented injection of modified nano-SiO2 was evaluated via an indoor core displacement experiment. This study hoped to provide a new modification strategy for solving the problem of excessive injection pressure in low-permeability reservoirs.
2. Experimental Sections
2.1. Experimental Materials and Instruments
Materials: nano-SiO2, Shanghai Aladdin Biochemical Technology Co., Ltd.; lauryl amine, AR, Shanghai Xianding Biological Technology Co., Ltd.; γ-propyl trimethoxysilane (KH560), BR, Shanghai Yuanye Bio-Technology Co., Ltd.; sodium hydroxide (NaOH), absolute ethanol (C2H5OH) are both AR, Chengdu Kelong Chemicals Co., Ltd.; Polyethylene glycol octylphenyl ether (TX-100), CP, Chengdu Kelong Chemicals Co., Ltd.; kerosene, Unified Petrochemical Co., Ltd.; bentonite, Chengdu Hua Chemical Reagent Co., Ltd.; artificial core.
Instruments: heat-collecting-type constant-temperature-heating magnetic stirrer, Beijing Kewei Yongxing Instrument Co., Ltd.; Analytical Balances, Mettler-Toledo Instruments Co., Ltd.; constant temperature drying oven, Chengdu Test Instrument Co., Ltd.; ultrasonic cleaner, Shanghai Kedao Ultrasonic Instrument Co., Ltd.; centrifuge, Shanghai Medical Devices Co., Ltd.; rotary evaporator, Gongyi Yuhua Instrument Co., Ltd.; infrared spectrometer, Thermo Electron Corporation; contact angle measuring instrument, German Kruss Scientific Instruments Shanghai Co., Ltd.; synchronous integrated thermal analyzer, Netzsch Group; zeta potential analyzer, American CD Company; laser scattering system, Brookhaven Instruments, Inc.
2.2. Experimental Method
2.2.1. Preparation of Modified Nano-SiO2
Owing to the hydrophobicity of a long carbon chain, it is often used to endow hydrophobicity to nano-materials. Wang [30] declaimed that the ring-opening reaction could occur between amino groups and epoxy groups. In this article, the silane coupling agent (KH560) was used as an intermediate to connect a long carbon chain and nano-SiO2.
Specifically, dodecyl amine was utilized as a modifier to graft long carbon onto the KH560, and then the modified KH560 containing a long carbon chain was branched to nano-SiO2. After the experiment mentioned above, the nano-SiO2 with hydrophobicity was prepared. The experimental steps were as follows.
- (1)
- Dodecyl amine (2.2242 g) was dissolved in 10 mL of absolute ethanol, and then it was placed into a three-hole round-bottom flask and stirred in a thermostatic heating magnetic stirrer at 60 °C. At the same time, 2.83 g of KH560 was added into the three-hole round-bottom flask drop by drop and it reacted for 2 h. Then, the cooled dispersion liquid was placed in a rotary evaporator at 50 °C for 20 min until the ethanol rotary evaporation was complete. Finally, the viscous oil-like liquid (3.67 g) was obtained, which was modified KH-560. Additionally, the yield was 72.6%.
- (2)
- About 0.8 g of nano-SiO2 was dried at 120 °C for 2 h. Then, it was added to 20 mL of absolute ethanol. The above liquid was ultrasonically processed in an ultrasonic cleaner for 30 min to ensure the uniform dispersion of the nano-SiO2. Subsequently, 1.6 g of modified KH560 was dissolved in 20 mL of absolute ethanol and stirred evenly, and it was added to the nano-SiO2 dispersion slowly. The mixture was stirred in a constant-temperature-heating magnetic stirrer for 10 min to mix it evenly. Then, the thermostatic heating magnetic stirrer was adjusted to 60 °C, and the thermostatic reaction continued for 4 h under the condition of nitrogen gas. The reaction dispersion liquid was centrifuged at a high speed for 5 min and washed with absolute ethanol three times until the remaining modified KH560 was removed. The precipitation in the centrifugal tube was taken and dried for 4 h in a drying oven at 120 °C. Finally, the modified nano-SiO2 was obtained.
2.2.2. Infrared-Spectroscopy Analysis
A Fourier infrared spectrometer was used to characterize the modified nano-SiO2. Small amounts of modified nano-SiO2 and KBr were mixed uniformly and pressed into tablets after grinding. Then, they were placed in an infrared spectrometer to obtain the infrared spectrum of modified nano-SiO2. The measured wavenumbers ranged from 0 cm–1 to 4000 cm–1, the wavenumber precision was 0.01 cm–1 and the resolution was 4 cm–1.
2.2.3. Wettability of Modified Nano-SiO2
The wettability of modified nano-SiO2 was evaluated by measuring the contact angle with the German KRUSS DSA30S interface parameter-measuring instrument (Shanghai KRUSS Scientific Instrument Co., Ltd., Shanghai, China). First, the modified nano-SiO2 with different concentration gradients was dispersed in TX-100. Subsequently, the core was cut into thin slices (3 mm) and soaked in the prepared mixture for 24 h. Then, the prepared sample was put into a constant-temperature drying oven and dried at 120 °C. Finally, the contact angle of the water droplet and core was measured using an interface surface parameter-measuring instrument.
2.2.4. Dynamic Light Scattering Test
The particle size of the modified nano-SiO2 was analyzed via dynamic light scattering. The particle size distribution was measured using a laser scattering system from Brookhaven Instruments, USA. First, the modified nano-SiO2 was dispersed in TX-100, and then the particle size distribution of modified nano-SiO2 was obtained via dynamic light scattering.
2.2.5. Thermogravimetric Analysis (TGA)
The TGA of the nano-SiO2 and modified nano-SiO2 was performed using a thermogravimetric analyzer from 0 °C to 1000 °C. Under nitrogen flow, the samples were continuously heated to 1000 °C with a step size of 10 °C/min, and the data of the change in sample weight with temperature were collected. By analyzing the weight change in the samples at each stage, the amount of groups grafted onto the nano-SiO2 was quantitatively evaluated.
2.2.6. Zeta Potential Test
Equal amounts of modified nano-SiO2 were dispersed in TX-100 and NaOH-regulated TX-100, respectively. The zeta potential of the two dispersions was measured using a Zeta PALS 190 Plus Potential Analyzer (Brookhaven Instruments, Austin, TX, USA). Zeta potential was used to evaluate the stability of the two dispersions.
2.2.7. Dispersibility and Stability
The modified nanosilica was dispersed in water, TX-100, ethanol, kerosene and NaOH-adjusted TX-100. The dispersion conditions of the five kinds of dispersion liquids were observed. After storing them for 30 days, the dispersion conditions of the five kinds of dispersion liquids were observed again to judge the stability of the dispersion liquids.
2.2.8. Antiswelling Test
The antiswelling rate of modified mano-SiO2 was evaluated using the centrifugal method. The antiswelling ratio was evaluated by measuring the swelling volume of bentonite in kerosene, modified nano-SiO2 dispersions and pure water. Bentonite (1 g) was added into 20 mL of kerosene, water and modified nano-SiO2 dispersions, respectively, before shaking at room temperature for 2 h. After storing it for 24 h, the swelling volume of bentonite was recorded.
The antiswelling rate was calculated as follows:
where B1 is the antiswelling rate, %; V0 is the swelling volume of bentonite in kerosene, mL; V1 is the swelling volume of bentonite in modified nano-SiO2 dispersion, mL, and V2 is the swelling volume of bentonite in pure water volume, mL.
2.2.9. Evaluation of the Effect of Blood Pressure Reduction and Injection
The prepared physical parameters of the low-permeability cores are shown in Table 1.

Table 1.
Physical parameters of core.
The confining pressure of the core was set as 8 MPa, and the core was vacuum dried at 100 °C for 24 h. First, the mass (W1) of the dry core was measured, and it was immersed in water for 24 h. Then, the mass (W2) of the core under a wet state was measured. As shown in Figure 1, the pipeline of the displacement experiment was connected. Brine was injected into the core at a constant flow rate of 0.1 mL/min, and the displacement pressure was recorded until the pressure P1 stabilized. Water permeability was then calculated. The modified nano-SiO2 dispersion of 0.5 PV was injected at a constant flow rate of 0.1 mL/min and aged at 60 °C for 48 h. During the subsequent water flooding, brine was injected at a constant flow rate of 0.1 mL/min until the pressure was stable after water flooding, and the stable pressure P2 was recorded. Subsequently, 30 PV of brine was injected at a constant flow rate of 0.1 mL/min to flush the core, and the pressure during displacement was recorded to evaluate the depressurization and boosting effect as well as the erosion resistance of the modified nano-SiO2.
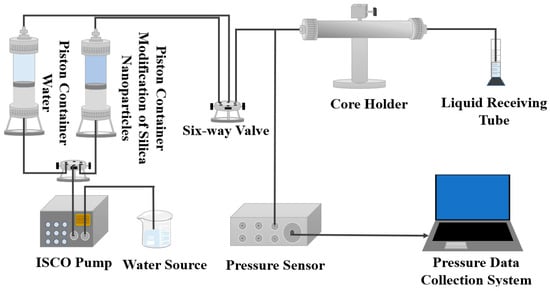
Figure 1.
Physical model of displacement experiment.
The core parameters were calculated as follows:
where ρ is the density of water, g/cm3; Q is the flow rate of fluid passing through the core per unit time, cm3/s; μ is the viscosity of liquid, mPa/s; l is the core length, cm; Δp is the pressure difference before and after the liquid passes through the core, MPa, and A is the cross-sectional area of the liquid passing through the core, cm2.
3. Results and Analysis
3.1. Characterization of Modified Nano-SiO2
The FR-IR spectra of nano-SiO2, modified KH-560 and modified nano-SiO2 are presented in Figure 2. The characteristic peaks at 472 and 1105 cm−1 belong to the bending vibration and stretching vibration of the Si-O. The antisymmetric tensile vibration absorption peak and bending vibration absorption peak of the hydroxyl emerged at 3446 and 1633 cm−1. Compared with the data of nano-SiO2, the peak intensity at 3446 cm−1 of the modified nano-SiO2 decreased, indicating that the hydroxyl on the nano-SiO2 had reacted. In addition, the characteristic peaks of methyl and methylene can be found at 2925 and 2858 cm−1. The two characteristic peaks mentioned above can be simultaneously observed in the spectra of modified nano-SiO2 and modified KH-560, indicating that the modified KH-560 successfully grafted onto the modified nano-SiO2.
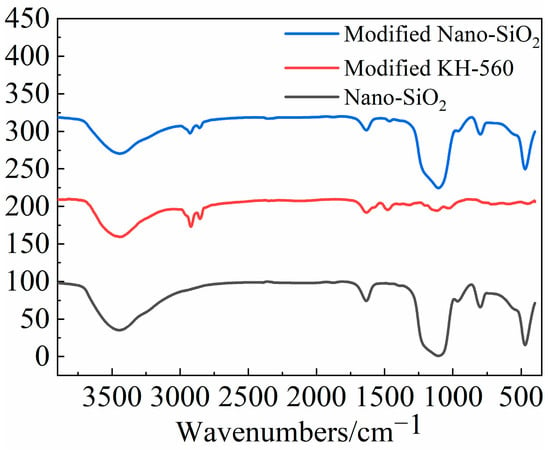
Figure 2.
Infrared spectra of nano-SiO2 before and after modification.
3.1.1. Dynamic Light Scattering
Nano-SiO2 has strong hydrophilic properties, and it easily agglomerates between particles, thereby enlarging the particle radius. Figure 3 displays the particle size distribution of modified nano-SiO2. All of the particle sizes of the modified nano-SiO2 were less than 60 nm, and the median particle size was 15 nm, in line with the nanoparticle size range. The modified nano-SiO2 had less agglomeration, and the obtained particles had smaller and uniform particle size. When it was injected into low-permeability oil reservoirs, it was not easy to block and it more easily adsorbed onto the surface of rock pores evenly.
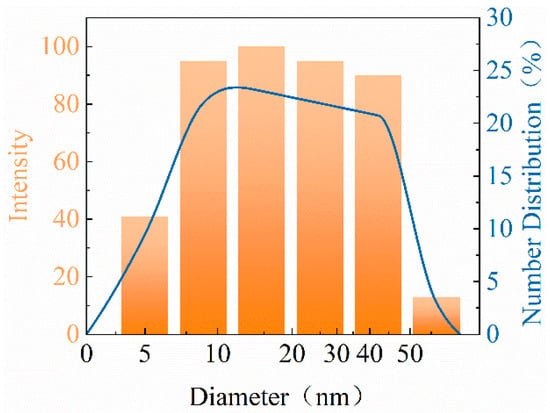
Figure 3.
Particle size distribution.
3.1.2. TGA
Figure 4 shows the TGA of SiO2 before and after modification. With increased temperature, the weight of the sample gradually decreased, and the mass change with temperature was divided mostly into two stages. In the first stage, with increased temperature from 25 °C to 120 °C, the mass of the sample decreased from 100% to 98%, which was primarily caused by the loss in adsorbed water on the sample surface. In the second stage, with increased temperature from 120 °C to 1000 °C, the weight of nano-SiO2 decreased from 98% to 92%, which was due to the weight change caused by the dehydration and polycondensation of the hydroxyl groups on the nano-SiO2 surface. However, the weight of the modified nano-SiO2 decreased from 98% to 89% with increased temperature. On the one hand, this was due to the dehydration and polycondensation of the unreacted hydroxyl groups on the surface of the modified nano-SiO2, the same as with the above-mentioned SiO2. On the other hand, the weight of the modified KH560 grafted onto the surface of SiO2 was further reduced due to the fracture of the modified KH560 owing to the excessive temperature. At 1000 °C, the weight of SiO2 before and after modification differed by 3%, indicating that 3% of KH560 was grafted onto the surface of SiO2.
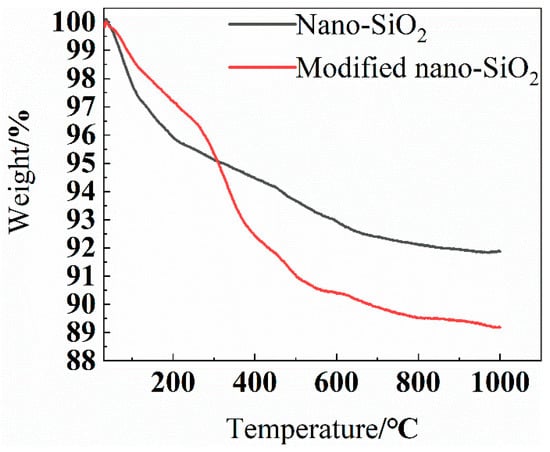
Figure 4.
Thermogravimetric curve.
3.2. Performance Evaluation of Modified Nano-SiO2
3.2.1. Contact Angle
Wettability is very important as it directly affects the effect of pressure reduction and injection. Core wettability was evaluated by measuring the contact angle. Figure 5 shows the contact angles of core slices immersed in modified nano-SiO2 dispersion and saline. In the control experiment of core sections soaked in salt water, the core contact angle was 37.34°, indicating that the initial surface wettability of the core was hydrophilic. However, the contact angle of the core slices immersed in the modified nano-SiO2 dispersion solution was 104.26°, indicating that the modified nano-SiO2 can change the core from hydrophilic wetting to hydrophobic wetting, and the change in wettability can reduce the adhesion force of the rock surface to water to promote water flow. Consequently, the effect of lowering the pressure and increasing injection was achieved. With an increased concentration of modified nano-SiO2, the contact angle increased from 104.26° to 136.36° and gradually became stable. This finding indicated that with increased concentration, the adsorption amount on the core surface increased, and the hydrophobic degree on the core surface also increased. However, with a further increased concentration of modified nano-SiO2, the adsorption gradually became saturated. The adsorption capacity was basically unchanged and the contact angle tended to be stable.
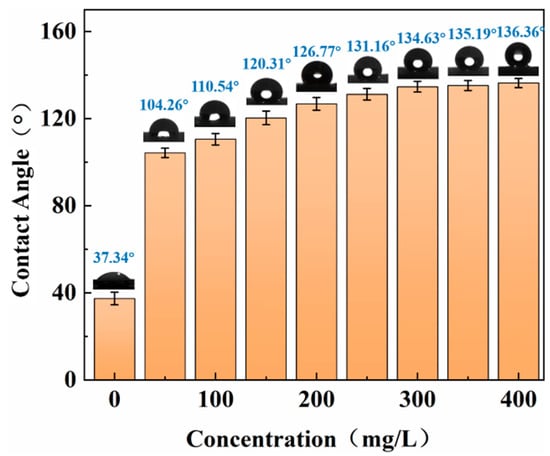
Figure 5.
Contact angle of core before and after adsorption of modified nano-SiO2.
3.2.2. Stability and Zeta Potential
Modified nano-SiO2 was dispersed in pure water, TX-100, ethanol and kerosene to explore the dispersibility of nano-SiO2. As shown in Figure 6, due to the large specific surface area and high surface energy of modified nano-SiO2, it was unable to disperse in water and agglomerate seriously. In ethanol solution, the dispersion effect was poor, and some modified nano-SiO2 agglomerated and precipitated. In TX-100, the dispersion effect of modified nano-SiO2 was better, but the solution was cloudy. The nano-SiO2 had good dispersibility in the kerosene dispersion system, and the solution was clear and transparent.
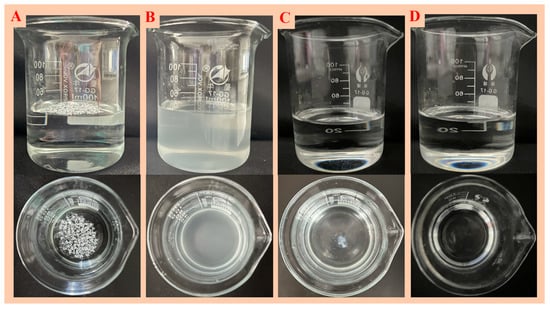
Figure 6.
Modified nano-SiO2 dispersion in water (A), Polyethylene glycol octylphenyl ether (TX-100) (B), ethanol (C) and kerosene (D).
To explore the dispersion stability, the dispersion liquid was stored for 30 days, and then the solution dispersion was observed. Figure 7 shows the dispersion situation of the above-mentioned solution. Compared with the dispersion solution not stored for 30 days, precipitation occurred in the surfactant aqueous dispersion system. The precipitation in the ethanol dispersion system increased significantly, but the kerosene dispersion system remained well dispersed.
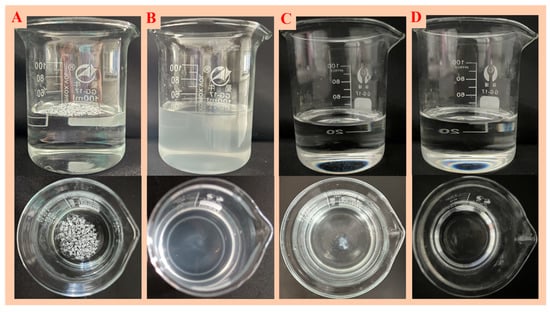
Figure 7.
State of modified nano-SiO2 after 30 days of dispersion in water (A), Polyethylene glycol octylphenyl ether (TX-100) (B), ethanol (C) and kerosene (D).
A large amount of working fluid is generally used on site; so, using kerosene as a dispersant is costly and has high safety risks during storage and transportation. Therefore, the dispersion system of adding NaOH to the surfactant aqueous solution needed to be explored. As shown in Figure 8, the dispersion of the aqueous surfactant dispersion system without NaOH was turbid, whereas the dispersion of the aqueous surfactant dispersion system with NaOH was well dispersed, and the dispersion was clear and transparent. Then, the dispersion before and after NaOH adjustment was stored for 30 days. As shown in Figure 7 and Figure 8, the precipitation formed in the dispersion system without NaOH, whereas the dispersion with NaOH remained clear and transparent.
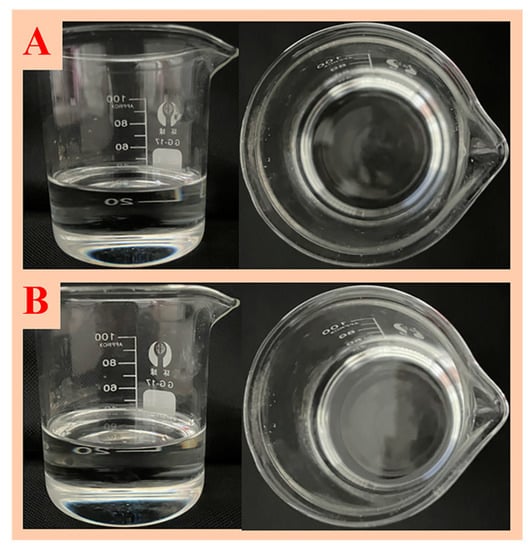
Figure 8.
Dispersion state of modified nano-SiO2 in NaOH-regulated Polyethylene glycol octylphenyl ether (TX-100) (A) and after 30 days of dispersion (B).
Nano-SiO2 dispersion is a colloidal system, and zeta potential is also an important parameter affecting its stability. In addition to the stability experiments mentioned above, the zeta potentials of the surfactant aqueous dispersions before and after the introduction of NaOH were also measured. The larger absolute value of the zeta potential indicated that the system was more stable. As shown in Figure 9 and Figure 10, after NaOH addition, the zeta potential of the system increased from −24.1 mV to 62.7 mV. Analysis revealed that surfactant TX-100 can form a steric hindrance effect through adsorption onto the surface of modified nano-SiO2, which can prevent mutual collision and coalescence between particles and improve the system stability. After adding NaOH, OH− adsorbed onto the surface of the modified nano-SiO2 through hydrogen bonds, whereas Na+ was arranged around the modified nano-SiO2 via diffusion, forming a diffusion electric double layer. Consequently, the charge on the surface of nano-SiO2 increased, which improved the repulsion between particles and the system stability.
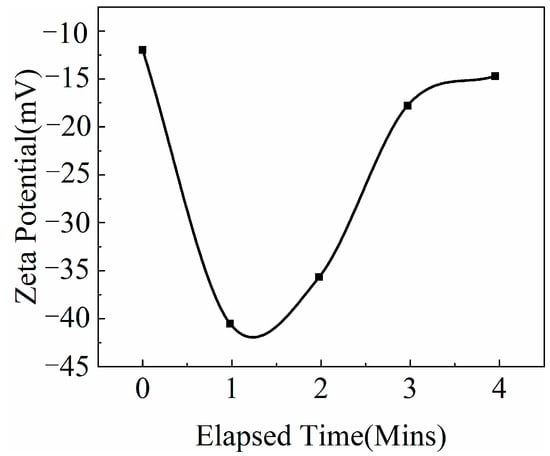
Figure 9.
Zeta potential of surfactant aqueous dispersion before NaOH conditioning.
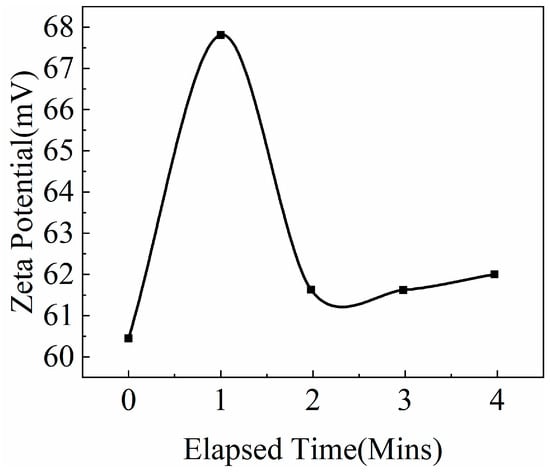
Figure 10.
Zeta potential of surface active aqueous dispersion after NaOH adjustment.
3.2.3. Antiswelling Test
The water expansion of bentonite in the rock channel reduced the effective diameter of the channel and increased the injection pressure. The antiswelling performance is also an important index affecting the influence of the modified nano-SiO2 dispersion under pressure reduction and injection. The swelling volumes of bentonite in kerosene, nano-SiO2 dispersion and water are, respectively, shown in Table 2. The swelling volume of bentonite in kerosene was 1 mL, the swelling volume of bentonite in nano-SiO2 was 1.8 mL and the swelling volume of bentonite in water was 2.4 mL. According to Equation (1), the antiswelling rate of modified nano-SiO2 was 42.9%, and the modified nano-SiO2 had a good antiswelling effect. After analysis, given that the modified nano-SiO2 was hydrophobic, the modified nano-SiO2 was adsorbed onto the surface of bentonite, and a hydrophobic layer formed on the bentonite surface, blocking the contact between water and bentonite to achieve the effect of antiswelling.

Table 2.
Swelling volume of bentonite in different solutions.
3.2.4. Depressurization and Injection Enhancement Performance
During the start-up process of water injection, given that the low-permeability core needed to overcome a certain start-up pressure, the pressure initially increased to the maximum value and then decreased to the equilibrium value. The low-permeability core surface had a layer of hydration boundary, and fluid flow was not easy. Smaller core permeability corresponded with a greater resistance to overcome the fluid flow and a greater starting pressure gradient. As shown in Figure 11, the initial injection pressure of the core was 0.141 MPa. After the injection of 0.5 PV nano-SiO2 dispersion solution with a concentration of 300 mg/L, the pressure of the subsequent water flooding was stable at 0.071 MPa, and the pressure reduction rate reached 49.6%. After flushing 20 PV water at a flow rate of 0.1 mL/min, the displacement pressure was 0.076 MPa, and the pressure reduction rate remained as high as 46%. This result indicated that the modified nano-SiO2 adsorbed onto the surface of the rock passage and can maintain a low injection pressure for a long time even after long-term water erosion, indicating long-term effective performance.
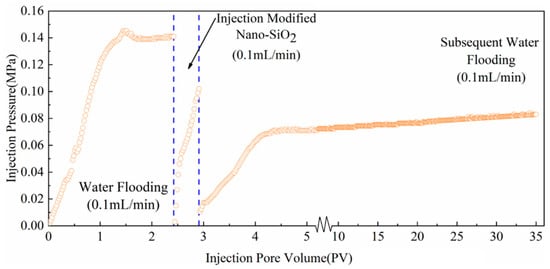
Figure 11.
Effect of modified nano-SiO2 on water injection pressure.
4. Analysis of Mechanism of Reduced Blood Pressure and Increased Injection
4.1. Reduced Solid–Liquid Adhesion
The adhesion of solid to liquid reflects the retention ability of the solid surface to liquid. The work of adhesion is the maximum work performed outwards by the system during solid–liquid adhesion. Greater adhesion performance corresponded with greater system stability and a stronger solid–liquid interface. Therefore, reducing the adhesion work of the solid–liquid interface can reduce the resistance of liquid flowing through solid surface. In 1952, Harkins [31] discussed the interface problem from the view of adhesion work. From the perspective of thermodynamics, he introduced the following solid–liquid adhesion work equation:
Combined with the Young equation, it is as follows:
It follows from Equations (4) and (5) that
where Wa is the solid–liquid work of adhesion, mJ/m2; cosθ is the contact angle of a liquid on a solid surface, °; γgl is the gas–liquid interfacial tension, mN/m; γgs is the gas–solid interfacial tension, mN/m, and γsl is the solid–liquid interfacial tension, mN/m.
According to Equation (6), the adhesion work between a liquid and solid can be obtained only by measuring the surface tension of liquid and the contact angle of liquid on the solid surface, as shown in Table 3. According to the results of wettability evaluation in Section 3.2.1, the solid–liquid adhesion work can be calculated.

Table 3.
Effect of modified nano-SiO2 on solid–liquid adhesion.
The modified nano-SiO2 induced a change in the rock surface from hydrophilic to hydrophobic. The contact angle increased, and the adhesion work of the rock to the water phase decreased from 129.49 mJ/m2 to 21.46 mJ/m2. These phenomena reduced the ability of the rock surface to bind to the water film and increased its fluidity and effective flow space. Thus, the injection capacity of the water phase improved and the injection pressure was reduced.
4.2. Prevention of Clay Swelling
The modified nano-SiO2 also exerted an antiswelling effect. As shown in Figure 12, when water flowed through the clay minerals on the surface of the rock pores, the clay particles absorbed water and expanded, thereby reducing the effective diameter of the rock pores and greatly increasing the injection pressure. As shown in Figure 12, after injecting the modified nano-SiO2, the modified nano-SiO2 adsorbed onto the surface of the clay mineral to form a hydrophobic layer and isolate the contact between the water and the clay particles, greatly reducing the probability of hydration expansion of the clay particles and increasing the effective diameter of the rock pores. Accordingly, reduced injection pressure and increased injection volume were also achieved.
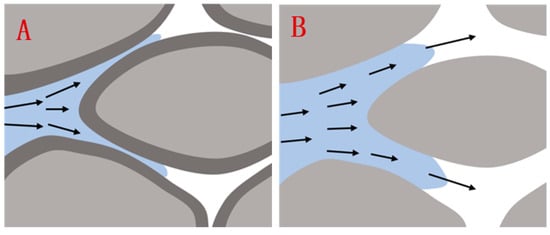
Figure 12.
Rock pore state before (A) and after (B) the adsorption of nano-SiO2.
5. Conclusions
For solving the problem of excessive high pressure in ultra-low permeability reservoirs, a new pressure-decreasing and augmented injection agent (modified nano-SiO2) was prepared. The structure and particle size of the prepared nano-SiO2 were characterized via infrared spectrometry and dynamic light scattering. Additionally, the test results showed that the particle size of modified nano-SiO2 was less than 60 nm, which contributed to making it pass through smaller cracks. Based on the water contact angle test results, the surface of the rock transformed from having hydrophilicity to hydrophobicity due to the modified nano-SiO2, which led to the weakened binding effect of the rocks with water to prevent the clay expansion of the formation. Under the ultra-low permeability condition, the depressurization efficiency of the modified nano-SiO2 could reach 49%. Additionally, it could be maintained at 46% at 20 PV water flow.
Author Contributions
Conceptualization, H.L. and W.S.; methodology, H.L.; validation, H.L. and N.L.; formal analysis, L.T.; investigation, H.L.; resources, N.L.; data curation, J.W.; writing—original draft preparation, H.L.; writing—review and editing, H.L. and W.S.; visualization, H.L.; supervision, J.W.; project administration, N.L.; funding acquisition, N.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding or This research was funded by [The project was supported by the Opening Fund of The Key Laboratory of Well Stability and Fluid and Rock Mechanics in Oil and Gas Reservoir of Shaanxi Province; The Opening Project of Oil and Gas Field Applied Chemistry Key Laboratory of Sichuan Province] grant number [no. WSFRM20210402001; no. YQKF202010].
Institutional Review Board Statement
This study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hou, Q.; He, H.; Li, J.; Yang, T. Recent progress and prospect of oil and gas exploration by PetroChina Company Limited. China Pet. Explor. 2018, 23, 1–13. [Google Scholar]
- Wei, H.; Wei, L.; Yin, J.H.; Yin, F.L.; Liu, J.H.; Li, H.H.; Wei, G.Q.; Liu, X.G.; Liu, W.D. Fractal Application in Micro Rock Mechanics of Low-Permeability Oil Reservoirs and Its Relationship with Enlarging Producing Reserves by Gemini Surfactant Flooding. Appl. Mech. Mater. 2010, 29, 170–176. [Google Scholar] [CrossRef]
- Li, Y. Technical advancement and prospect for CO2 flooding enhanced oil recovery in low permeability reservoirs. Pet. Geol. Recovery Effic. 2020, 27, 1–10. [Google Scholar]
- Yan, L.D.; Gao, Z.J.; Dai, F.G. Effective use model of low permeability oil reservoir. Int. Conf. Adv. Eng. Mater. Technol. 2013, 753, 53–57. [Google Scholar] [CrossRef]
- Feng, Y.L.; Zhang, Y.; Ji, B.Y.; Mu, W.Z. Micro-acting force in boundary layer in low-permeability porous media. Chin. Phys. Lett. 2011, 28, 024703. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Chen, S.; Mbia, E.; Wang, K.; Ren, H. Capillarity characters measurement and effects analysis in different permeability formations during waterflooding. Fuel 2017, 194, 129–143. [Google Scholar] [CrossRef]
- Ahmed Khan, R.; Murtaza, M.; Abdulraheem, A.; Kamal, M.S.; Mahmoud, M. Imidazolium-based ionic liquids as clay swelling inhibitors: Mechanism, performance evaluation, and effect of different anions. ACS Omega 2020, 5, 26682–26696. [Google Scholar] [CrossRef]
- Tariq, Z.; Murtaza, M.; Mahmoud, M.; Aljawad, M.S.; Kamal, M.S. Machine learning approach to predict the dynamic linear swelling of shales treated with different waterbased drilling fluids. Fuel 2022, 315, 123282. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, G.; Ge, J.; Feng, A.; Jiang, P.; Li, R.; Zhang, Y.; Fu, X. Laboratory studies of depressurization with a high concentration of surfactant in low-permeability reservoirs. J. Dispers. Sci. Technol. 2012, 33, 1589–1595. [Google Scholar] [CrossRef]
- Lai, N.; Nie, X.; Zheng, X.; Zhao, W.; Zhao, X.; Wang, Y. Experimental investigation on a novel polyelectrolyte molecular deposition film for improved injectivity in low-permeability reservoirs. ACS Omega 2020, 5, 29300–29311. [Google Scholar] [CrossRef] [PubMed]
- Tariq, Z.; Murtaza, M.; Kamal, M.S.; Shakil Hussain, S.M.; Mahmoud, M.; Al-Jawad, M. Clay Swelling Mitigation During Fracturing Operations Using Novel Magnetic Surfactants. Int. Pet. Technol. Conf. Riyadh 2022. [Google Scholar] [CrossRef]
- Tariq, Z.; Kamal, M.S.; Mahmoud, M.; Murtaza, M.; Abdulraheem, A.; Zhou, X. Dicationic surfactants as an additive in fracturing fluids to mitigate clay swelling: A petrophysical and rock mechanical assessment. ACS Omega 2021, 6, 15867–15877. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 15. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Mahmud, H.K.B.; Zahoor, M.K.; Shahid, A.S.; Rezaee, R.; Arif, M. Investigation of change in different properties of sandstone and dolomite samples during matrix acidizing using chelating agents. J. Pet. Explor. Prod. Technol. 2019, 9, 2793–2809. [Google Scholar] [CrossRef]
- Lufeng, Z.; Fujian, Z.; Shicheng, Z.; Zhun, L.; Jin, W.; Yuechun, W. Evaluation of permeability damage caused by drilling and fracturing fluids in tight low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2019, 175, 1122–1135. [Google Scholar] [CrossRef]
- Gao, P.; Feng, Q.; Chen, X.; Li, S.; Sun, Y.; Li, J.; Zhou, J.; Qian, F. Numerical simulation and field application of biological nano-technology in the low-and medium-permeability reservoirs of an offshore oilfield. J. Pet. Explor. Prod. Technol. 2022, 12, 3275–3288. [Google Scholar] [CrossRef]
- Tariq, Z.; Kamal, M.S.; Mahmoud, M.; Hussain, S.M.S.; Abdulraheem, A.; Zhou, X. Polyoxyethylene quaternary ammonium gemini surfactants as a completion fluid additive to mitigate formation damage. SPE Drill. Complet. 2020, 35, 696–706. [Google Scholar] [CrossRef]
- Hussain SM, S.; Kamal, M.S.; Murtaza, M. Synthesis of novel ethoxylated quaternary ammonium gemini surfactants for enhanced oil recovery application. Energies 2019, 12, 1731. [Google Scholar] [CrossRef]
- Tariq, Z.; Kamal, M.S.; Mahmoud, M.; Hussain, S.M.S.; Hussaini, S.R. Novel gemini surfactant as a clay stabilizing additive in fracturing fluids for unconventional tight sandstones: Mechanism and performance. J. Pet. Sci. Eng. 2020, 195, 107917. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Chen, S.; Guo, J.; Wang, K.; Luo, H. Effects of chemical additives on dynamic capillary pressure during waterflooding in low permeability reservoirs. Energy Fuels 2016, 30, 7082–7093. [Google Scholar] [CrossRef]
- Belhaj, A.F.; Elraies, K.A.; Mahmood, S.M.; Zulkifli, N.N.; Akbari, S.; Hussien, O.S. The effect of surfactant concentration, salinity, temperature, and pH on surfactant adsorption for chemical enhanced oil recovery: A review. J. Pet. Explor. Prod. Technol. 2020, 10, 125–137. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.A.; Sultan, A.S. Review on surfactant flooding: Phase behavior, retention, IFT, and field applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar] [CrossRef]
- Xiao, Z.; Dexin, L.; Lulu, L.; Yue, L.; Panhong, Y.; Jiaju, X.; Hua, Z.; Jie, Y. Synergistic effects of surfactants on depressurization and augmented injection in high salinity low-permeability reservoirs: Formula development and mechanism study. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127312. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, C.; Zhang, W.P. Application of nano-active depressurization and injection enhancement technology in low-permeability oilfields. Synth. Mater. Aging Appl. 2021, 50, 89–91. [Google Scholar]
- Zhai, H.; Qi, N.; Sun, X. Preparation and evaluation of a new type step-down augmented injection agent made of SiO2 nanoparticles. Mater. Rep. 2019, 33, 975–979. [Google Scholar]
- Liu, P.; Niu, L.; Li, X.; Zhang, Z. Environmental response nanosilica for reducing the pressure of water injection in ultra-low permeability reservoirs. J. Nanoparticle Res. 2017, 19, 390. [Google Scholar] [CrossRef]
- Dai, C.; Wang, S.; Li, Y.; Gao, M.; Liu, Y.; Sun, Y.; Zhao, M. The first study of surface modified silica nanoparticles in pressure-decreasing application. Rsc Adv. 2015, 5, 61838–61845. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, S.; Dai, C. A new insight into the pressure-decreasing mechanism of hydrophobic silica nanoparticles modified by n-propyltrichlorosilane. J. Surfactants Deterg. 2017, 20, 873–880. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, J.K.; Yu, B.R.; Xu, F.J. Construction of medical cationic polymer based on ring-opening reaction of amine group and epoxy. Acta Polym. Sin. 2022, 53, 828–841. [Google Scholar]
- Harkins, W.D. The Physical Chemistry of Surface Films; Reinhold Publishing Corporation: New York, MY, USA, 1952. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).