Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients
Abstract
1. Introduction
- Section 2 describes the methodology in relation to the design and implementation of the UE training by means of the Inrobics Rehab Clinic platform; the characteristics of the participants; the experimental protocol and the content of each experimental session; and the variables analyzed;
- Section 3 presents the results in relation to the variables described at baseline and at ending the UE training;
2. Materials and Methods
2.1. Study Design and Participants
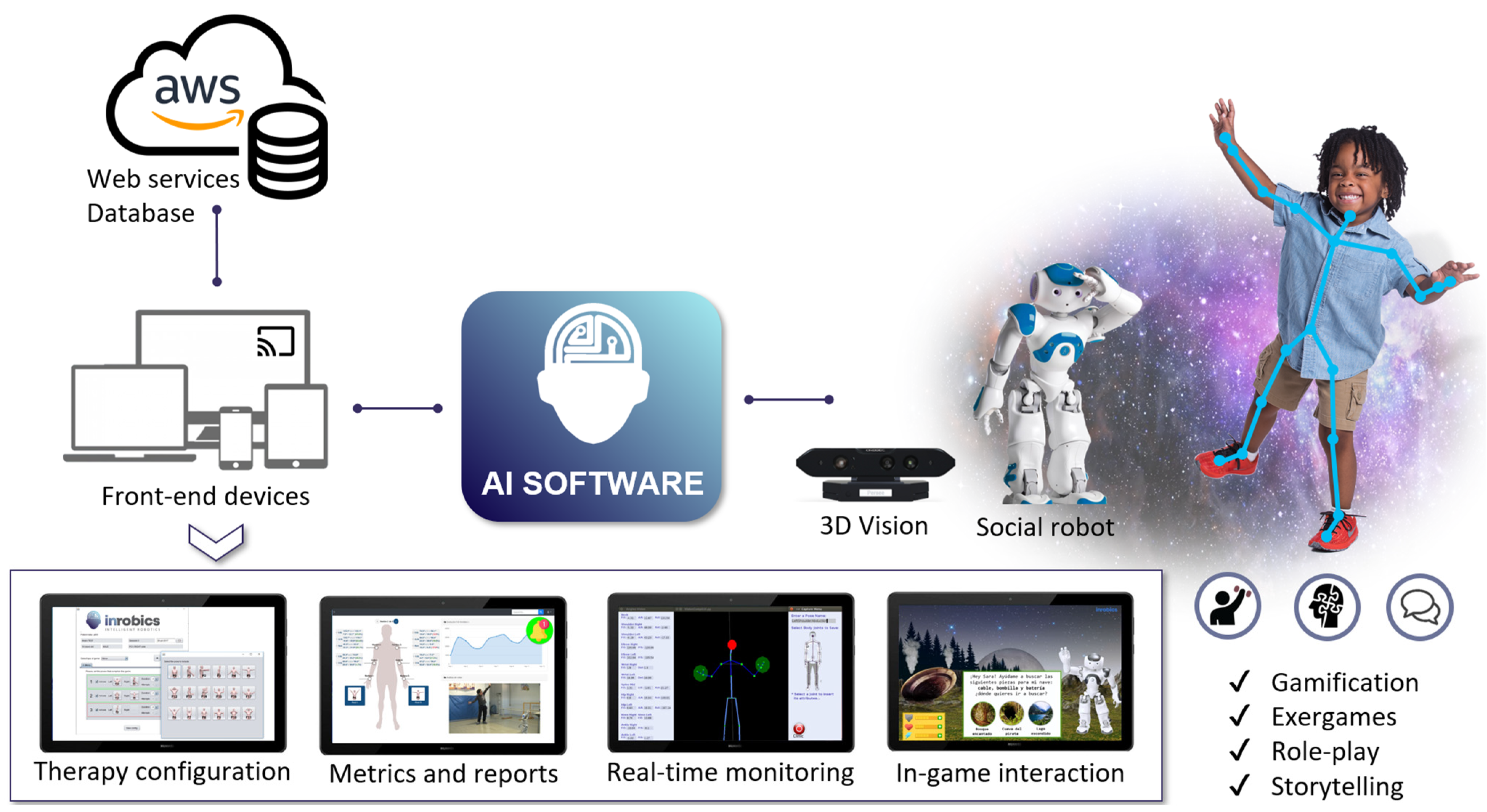
2.2. Upper Extremity Training
2.3. Outcome Variables
2.4. Data Analysis
3. Results
Comparison between Groups and between Baseline and Ending Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- New, P.W.; Lee, B.B.; Cripps, R.; Vogel, L.C.; Scheinberg, A.; Waugh, M.C. Global mapping for the epidemiology of paediatric spinal cord damage: Towards a living data repository. Spinal Cord 2019, 57, 183–197. [Google Scholar] [CrossRef]
- Silvestri, J. Effects of chronic shoulder pain on quality of life and occupational engagement in the population with chronic spinal cord injury: Preparing for the best outcomes with occupational therapy. Disabil. Rehabil. 2017, 39, 82–90. [Google Scholar] [CrossRef]
- Huang, V.S.; Krakauer, J.W. Robotic neurorehabilitation: A computational motor learning perspective. J. Neuroeng. Rehabil. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Pisano, F.; Mazzone, A.; Delconte, C.; Micera, S.; Carrozza, M.C.; Dario, P.; Minuco, G. Design strategies to improve patient motivation during robot-aided rehabilitation. J. Neuroeng. Rehabil. 2007, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Guneysu, A.; Arnrich, B. Socially assistive child-robot interaction in physical exercise coaching. In Proceedings of the 2017 26th IEEE International Symposium on Robot and Human Interactive Communication (RO-MAN), Lisbon, Portugal, 28 August–1 September 2017; pp. 670–675. [Google Scholar]
- Casas, J.; Irfan, B.; Senft, E.; Gutiérrez, L.; Rincon-Roncancio, M.; Munera, M.; Belpaeme, T.; Cifuentes, C.A. Social assistive robot for cardiac rehabilitation: A pilot study with patients with angioplasty. In Proceedings of the Companion of the 2018 ACM/IEEE International Conference on Human-Robot Interaction, Chicago, IL, USA, 5-8 March 2018; pp. 79–80. [Google Scholar]
- Pulido, J.C.; Suarez-Mejias, C.; Gonzalez, J.C.; Ruiz, A.D.; Ferri, P.F.; Sahuquillo, M.E.M.; De Vargas, C.E.R.; Infante-Cossio, P.; Calderon, C.L.P.; Fernandez, F. A socially assistive robotic platform for upper-limb rehabilitation: A longitudinal study with pediatric patients. IEEE Robot. Autom. Mag. 2019, 26, 24–39. [Google Scholar] [CrossRef]
- Rohrer, B.; Fasoli, S.; Krebs, H.I.; Hughes, R.; Volpe, B.; Frontera, W.R.; Stein, J.; Hogan, N. Movement smoothness changes during stroke recovery. J. Neurosci. 2002, 22, 8297–8304. [Google Scholar] [CrossRef]
- De los Reyes-Guzmán, A.; Dimbwadyo-Terrer, I.; Pérez-Nombela, S.; Monasterio-Huelin, F.; Torricelli, D.; Pons, J.L.; Gil-Agudo, A. Novel kinematic indices for quantifying movement agility and smoothness after cervical Spinal Cord Injury. NeuroRehabilitation 2016, 38, 199–209. [Google Scholar] [CrossRef]
- de Los Reyes-Guzmán, A.; Dimbwadyo-Terrer, I.; Pérez-Nombela, S.; Monasterio-Huelin, F.; Torricelli, D.; Pons, J.L.; Gil-Agudo, A. Novel kinematic indices for quantifying upper limb ability and dexterity after cervical spinal cord injury. Med. Biol. Eng. Comput. 2017, 55, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Kirshblum, S.; Waring, W. Updates for the International Standards for Neurological Classification of Spinal Cord Injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 505–517. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Stucki, G.; Cieza, A.; Ewert, T.; Kostanjsek, N.; Chatterji, S.; Ustun, T.B. Application of the International Classification of Functioning, Disability and Health (ICF) in clinical practice. Disabil. Rehabil. 2002, 24, 281–282. [Google Scholar] [CrossRef]
- Cahill, B.R. Proceedings of the Conference on Strength Training and the Prepubescent; American Orthopaedic Society for Sports Medicine: Rosemont, IL, USA, 1988; pp. 1–14. [Google Scholar]
- Faigenbaum, A.D.; Kraemer, W.J.; Blimkie, C.J.; Jeffreys, I.; Micheli, L.J.; Nitka, M.; Rowland, T.W. Youth resistance training: Updated position statement paper from the national strength and conditioning association. J. Strength Cond. Res. 2009, 23, 60–79. [Google Scholar] [CrossRef] [PubMed]
- National Strength and Conditioning Association. Strength & Conditioning Professional Standards & Guidelines; National Strength and Conditioning Association: Colorado Springs, CO, USA, 2001; Available online: https://www.nsca.com/education/articles/nsca-strength-and-conditioning-professional-standards-and-guidelines/ (accessed on 10 October 2022).
- Faigenbaum, A.D. Strength training for children and adolescents. Clin. Sport. Med. 2000, 19, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Fragala-Pinkham, M.A.; Haley, S.M.; Rabin, J.; Kharasch, V.S. A fitness program for children with disabilities. Phys. Ther. 2005, 85, 1182–1200. [Google Scholar] [CrossRef]
- Leap Motion Controller. Available online: https://www.ultraleap.com/product/leap-motion-controller/ (accessed on 13 February 2023).
- de Los Reyes-Guzmán, A.; Lozano-Berrio, V.; Alvarez-Rodríguez, M.; López-Dolado, E.; Ceruelo-Abajo, S.; Talavera-Díaz, F.; Gil-Agudo, A. RehabHand: Oriented-tasks serious games for upper limb rehabilitation by using Leap Motion Controller and target population in spinal cord injury. NeuroRehabilitation 2021, 48, 365–373. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. Off. Publ. Am. Occup. Ther. Assoc. 1985, 39, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Peljovich, A.E.; von Bergen, T.N.; Bryden, A.M.; Bohn, A.; Keith, M.W. Surgical restoration of the hand in tetraplegia: Tendon and nerve transfers. In Spinal Cord Medicine, 3rd ed.; Kirshblum, S., Lin, V.W., Eds.; Demos Medical Publishing: New York, NY, USA, 2019; pp. 487–515. [Google Scholar]
- Latash, M.L. Fundamentals of Motor Control, 1st ed.; Elsevier: London, UK, 2012. [Google Scholar]
- Muceli, S.; Boye, A.T.; d’Avella, A.; Farina, D. Identifying representative synergy matrices for describing muscular activation patterns during multidirectional reaching in the horizontal plane. J. Neurophysiol. 2010, 103, 1532–1542. [Google Scholar] [CrossRef]
- McBurney, H.; Taylor, N.F.; Dodd, K.J.; Graham, H.K. A qualitative analysis of the benefits of strength training for young people with cerebral palsy. Dev. Med. Child. Neurol. 2003, 45, 658–663. [Google Scholar] [CrossRef]
- Taylor, A.W.; McDonell, E.; Brassard, L. The effects of an arm ergometer training programme on wheelchair subject. Paraplegia 1986, 24, 105–114. [Google Scholar] [CrossRef]
- Yim, S.Y.; Cho, K.J.; Park, C.I.; Yoon, T.S.; Han, D.Y.; Kim, S.K.; Lee, H.L. Effects of wheelchair ergometer training on spinal cord injured paraplegics. Yonsei Med. J. 1993, 34, 278–286. [Google Scholar] [CrossRef]
- Nightingale, T.E.; Walhin, J.-P.; Thomson, D.; Bilzon, J.L.J. Impact of exercise on cardiometabolic component risk in spinal cord injured humans. Med. Sci. Sport. Exerc. 2017, 49, 2469. [Google Scholar] [CrossRef]
- DiCarlo, S.E. Effects of arm ergometry training on wheelchair propulsion endurance of individuals with quadriplegia. Phys. Ther. 1988, 68, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S.; Bilzon, J.L.J. Therapeutic Exercise after Spinal Cord Injury. In Spinal Cord Medicine, 3rd ed.; Kirshblum, S., Lin, V.W., Eds.; Demos Medical Publishing: New York, NY, USA, 2019; pp. 831–848. [Google Scholar]
- Graybiel, A.M. The basal ganglia: Learning new tricks and loving it. Curr. Opin. Neurobiol. 2005, 15, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L. Stage in learning motor synergies: A view based on the equilibrium-point hypothesis. Hum. Mov. Sci. 2010, 29, 642–654. [Google Scholar] [CrossRef] [PubMed]



| Variables | Sample Analyzed | |
|---|---|---|
| Tetraplegic Patients (n = 5) | Paraplegic Patients (n = 5) | |
| Sex (Male) * | 1.00 ± 20.00 | 3.00 ± 60.00 |
| Age (Years) + | 9.67 ± 4.04 | 10.67 ± 2.08 |
| Weight (kg) | 39.66 ± 7.50 | 36.03 ± 12.22 |
| Height (cm) | 138.66 ± 11.06 | 140.33 ± 16.92 |
| Etiology Injury (Traumatic) | 1.00 ± 20.00 | 1.00 ± 20.00 |
| Time since injury (months) | 3.66 ± 2.51 | 10.00 ± 3.00 |
| Injury Level | C1: 1.00 ± 20.00 | T3: 1.00 ± 20.00 |
| C2: 1.00 ± 20.00 | T12: 1.00 ± 20.00 | |
| C5: 1.00 ± 20.00 | L1: 1.00 ± 20.00 | |
| C6: 1.00 ± 20.00 | L2: 1.00 ± 20.00 | |
| C7: 1.00 ± 20.00 | L3: 1.00 ± 20.00 | |
| AIS Classification | ||
| A | - | - |
| B | 3.00 ± 60.00 | - |
| C | 1.00 ± 20.00 | 3.00 ± 60.00 |
| D | 1.00 ± 20.00 | 2.00 ± 40.00 |
| Upper Extremity Motor Score | 31.33 ± 15.27 a | 50.00 ± 0.00 a |
| Variables | Tetraplegic Patients (n = 5) | Paraplegic Patients (n = 5) | p2 | p3 | ||||
|---|---|---|---|---|---|---|---|---|
| At Baseline | At Ending | p1 | At Baseline | At Ending | p1 | |||
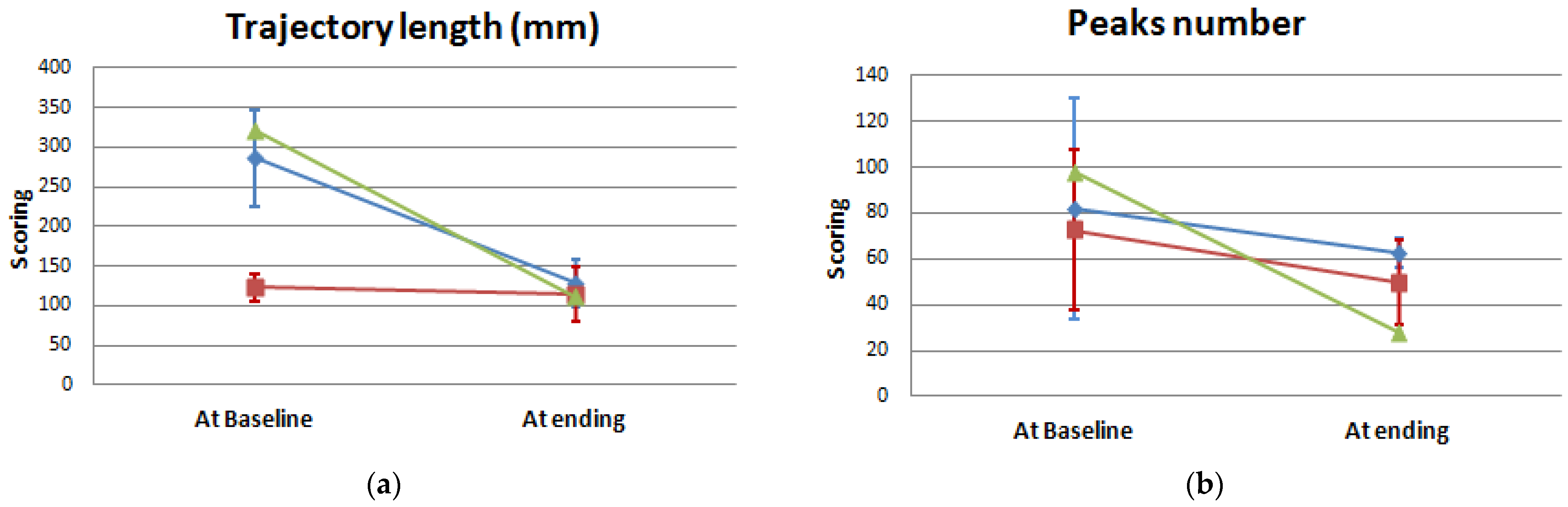
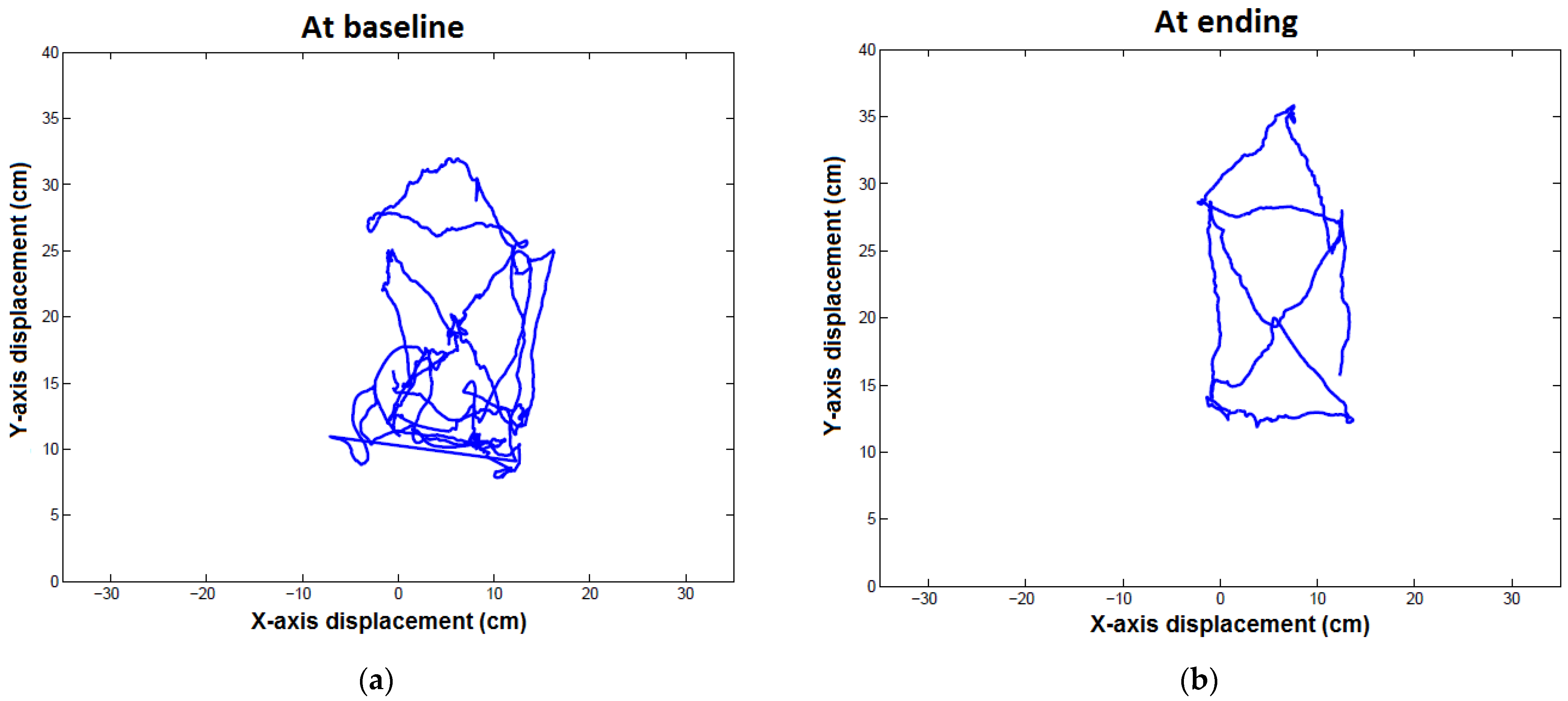
| Trajectory length (mm) | 286.01 ± 59.87 a | 128.73 ± 30.07 | 0.109 | 123.61 ± 17.14 a | 114.13 ± 34.59 | 0.285 | 0.004 | 0.700 |
| Peaks number (units) | 81.67 ± 48.21 | 62.67 ± 6.65 | 0.285 | 79.00 ± 31.34 | 44.25 ± 18.57 | 0.285 | 0.700 | 0.700 |
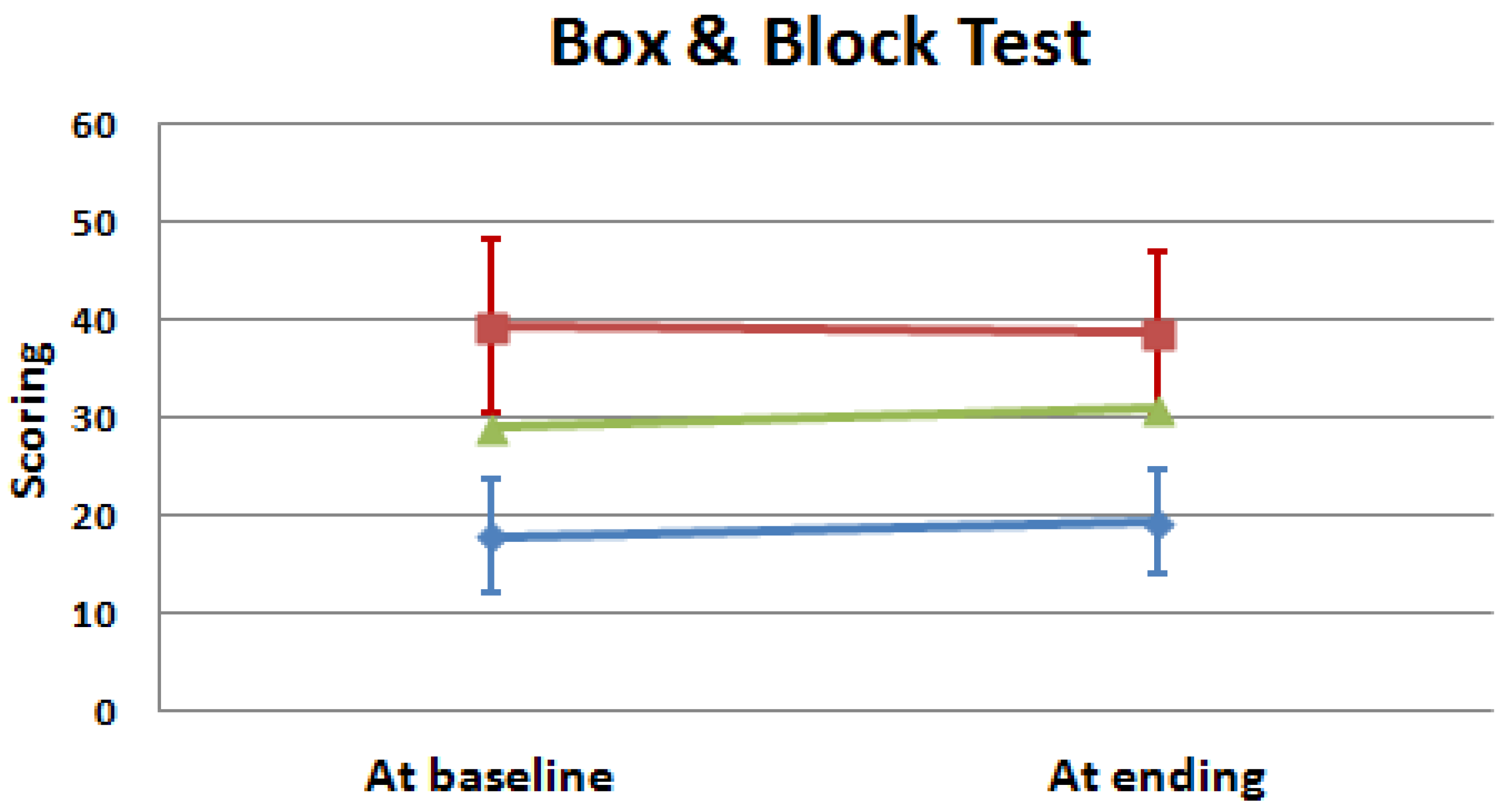
| Box and Block Test (blocks) | 17.83 ± 5.72 a | 19.40 ± 5.41 b | 0.066 | 39.40 ± 9.02 a | 38.75 ± 8.38 b | 0.496 | 0.008 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Monedero, M.; Cereijo-Herranz, V.; DelosReyes-Guzmán, A.; Pérez-Borrego, Y.; Gil-Agudo, A.; García-Martín, F.; Pulido-Pascual, J.-C.; López-Dolado, E. Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients. Appl. Sci. 2023, 13, 4979. https://doi.org/10.3390/app13084979
Salas-Monedero M, Cereijo-Herranz V, DelosReyes-Guzmán A, Pérez-Borrego Y, Gil-Agudo A, García-Martín F, Pulido-Pascual J-C, López-Dolado E. Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients. Applied Sciences. 2023; 13(8):4979. https://doi.org/10.3390/app13084979
Chicago/Turabian StyleSalas-Monedero, Miriam, Víctor Cereijo-Herranz, Ana DelosReyes-Guzmán, Yolanda Pérez-Borrego, Angel Gil-Agudo, Fuensanta García-Martín, José-Carlos Pulido-Pascual, and Elisa López-Dolado. 2023. "Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients" Applied Sciences 13, no. 8: 4979. https://doi.org/10.3390/app13084979
APA StyleSalas-Monedero, M., Cereijo-Herranz, V., DelosReyes-Guzmán, A., Pérez-Borrego, Y., Gil-Agudo, A., García-Martín, F., Pulido-Pascual, J.-C., & López-Dolado, E. (2023). Smoothness and Efficiency Metrics Behavior after an Upper Extremity Training with Robic Humanoid Robot in Paediatric Spinal Cord Injured Patients. Applied Sciences, 13(8), 4979. https://doi.org/10.3390/app13084979






