Artificial Intelligence-Based Patient Selection for Deep Inspiration Breath-Hold Breast Radiotherapy from Respiratory Signals
Abstract
1. Introduction
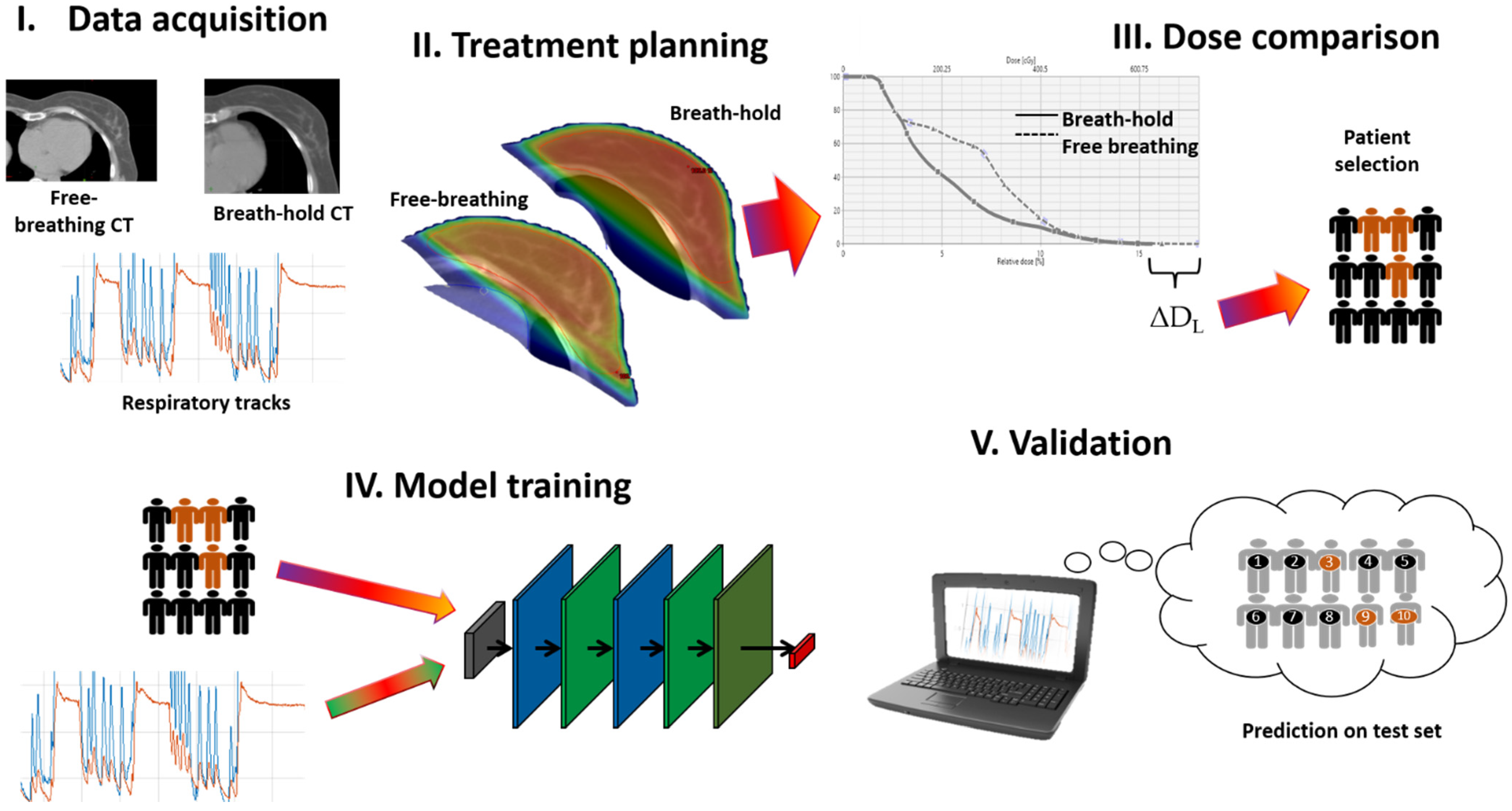
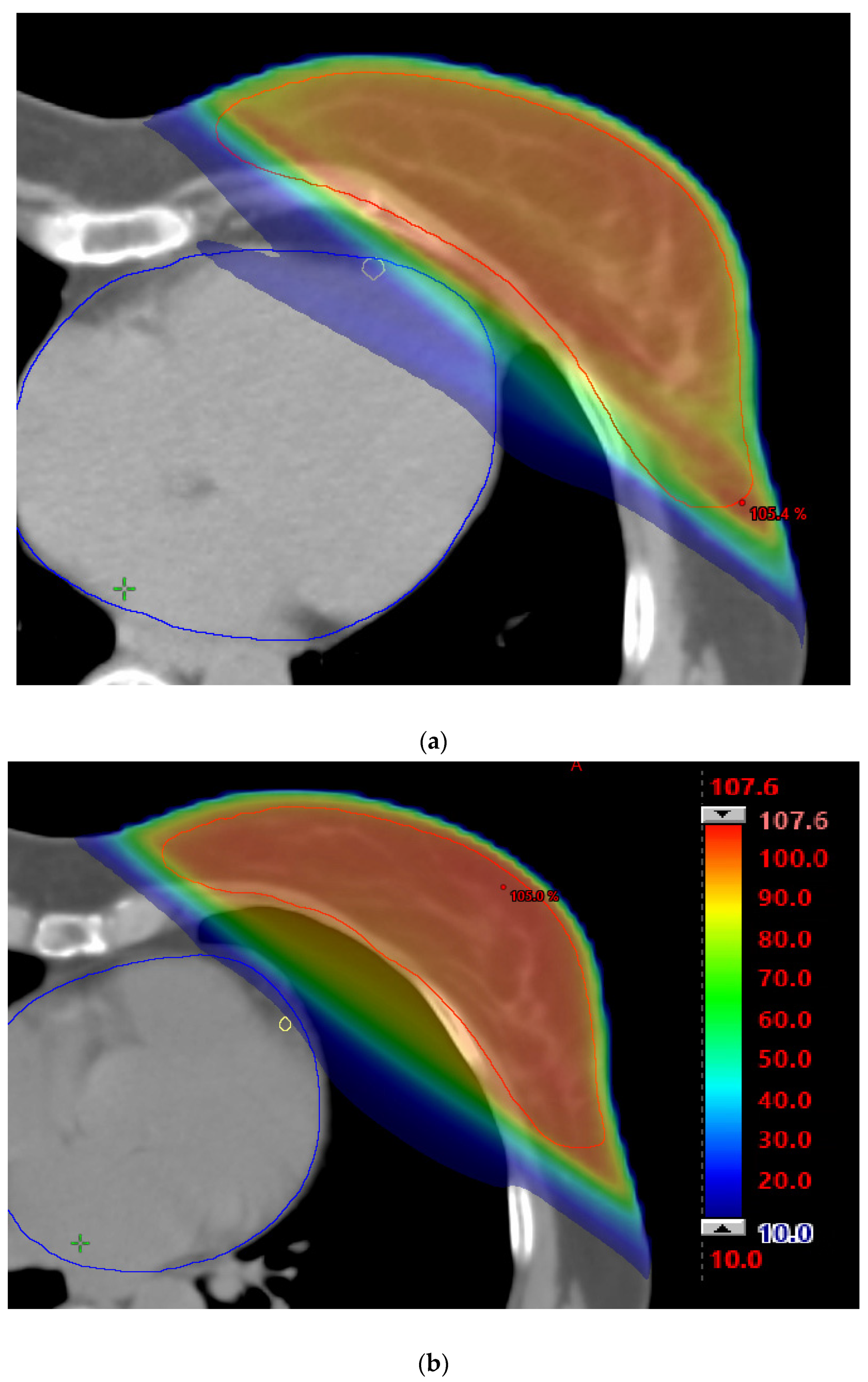
2. Methods
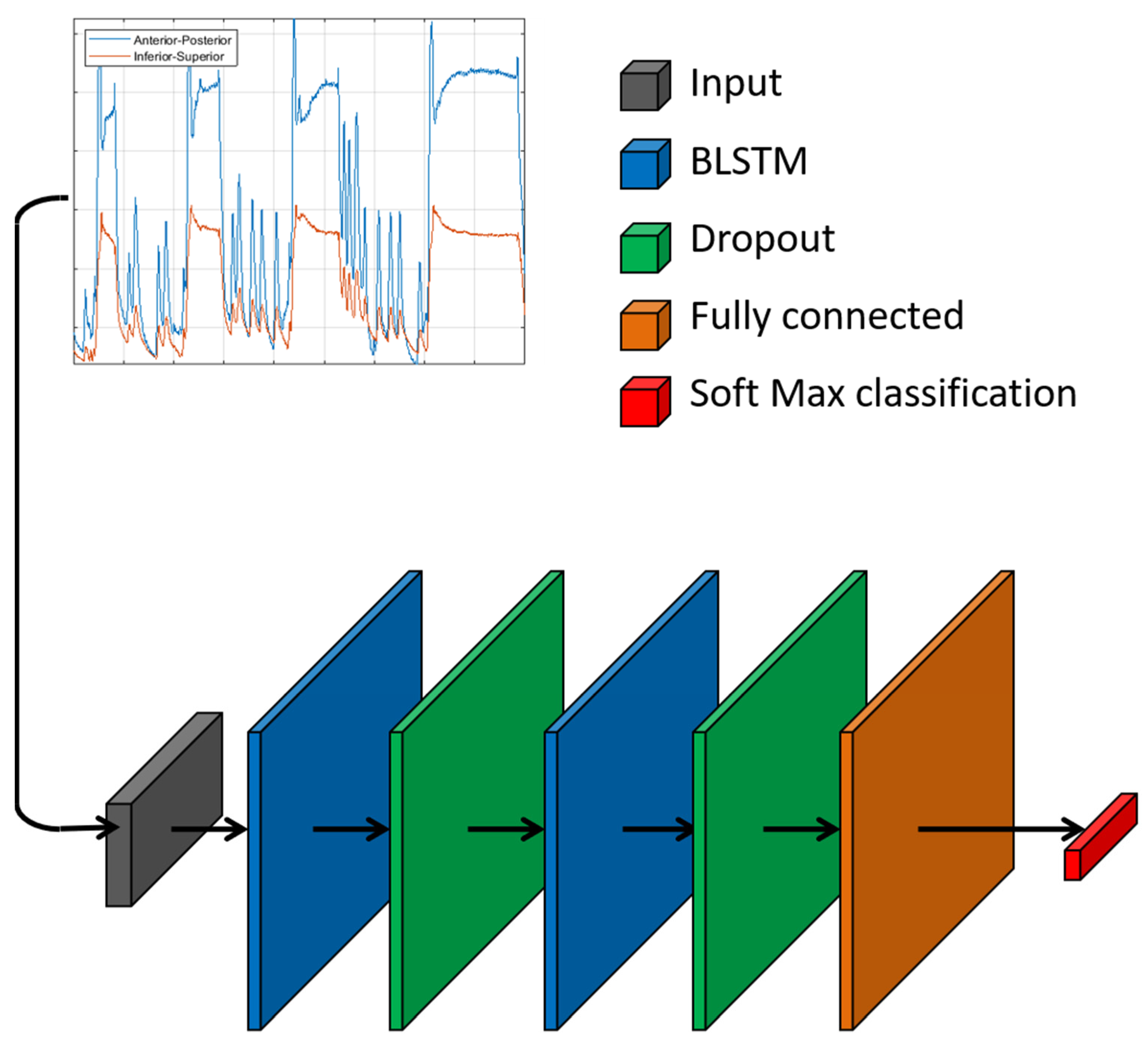
2.1. Neural Network
2.2. Training of Classification Model
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries and 25 Major Cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Sardaro, A.; Petruzzelli, M.F.; D’Errico, M.P.; Grimaldi, L.; Pili, G.; Portaluri, M. Radiation-Induced Cardiac Damage in Early Left Breast Cancer Patients: Risk Factors, Biological Mechanisms, Radiobiology, and Dosimetric Constraints. Radiother. Oncol. 2012, 103, 133–142. [Google Scholar] [CrossRef]
- Kabel, A.M.; Baali, F.H. Breast Cancer: Insights into Risk Factors, Pathogenesis, Diagnosis and Management. J. Cancer Res. Treat. 2015, 3, 28–33. [Google Scholar] [CrossRef]
- Bleicher, R.J. Timing and Delays in Breast Cancer Evaluation and Treatment. Ann. Surg. Oncol. 2018, 25, 2829–2838. [Google Scholar] [CrossRef]
- Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; Godwin, J.; et al. Effect of Radiotherapy after Breast-Conserving Surgery on 10-Year Recurrence and 15-Year Breast Cancer Death: Meta-Analysis of Individual Patient Data for 10 801 Women in 17 Randomised Trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Carlson, L.E.; Watt, G.P.; Tonorezos, E.S.; Chow, E.J.; Yu, A.F.; Woods, M.; Lynch, C.F.; John, E.M.; Mellemkjær, L.; Brooks, J.D.; et al. Coronary Artery Disease in Young Women After Radiation Therapy for Breast Cancer: The WECARE Study. JACC CardioOncology 2021, 3, 381–392. [Google Scholar] [CrossRef]
- Milo, M.L.H.; Thorsen, L.B.J.; Johnsen, S.P.; Nielsen, K.M.; Valentin, J.B.; Alsner, J.; Offersen, B.V. Risk of Coronary Artery Disease after Adjuvant Radiotherapy in 29,662 Early Breast Cancer Patients: A Population-Based Danish Breast Cancer Group Study. Radiother. Oncol. 2021, 157, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Darby, S.C.; McGale, P.; Taylor, C.W.; Peto, R. Long-Term Mortality from Heart Disease and Lung Cancer after Radiotherapy for Early Breast Cancer: Prospective Cohort Study of about 300 000 Women in US SEER Cancer Registries. Lancet Oncol. 2005, 6, 557–565. [Google Scholar] [CrossRef]
- Aznar, M.; Korreman, S.S.; Pedersen, A.N.; Persson, G.F.; Josipovic, M.; Specht, L. Evaluation of Dose to Cardiac Structures during Breast Irradiation. Br. J. Radiol. 2011, 84, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The Management of Respiratory Motion in Radiation Oncology Report of AAPM Task Group 76. Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef]
- Oechsner, M.; Düsberg, M.; Borm, K.J.; Combs, S.E.; Wilkens, J.J.; Duma, M.N. Deep Inspiration Breath-Hold for Left-Sided Breast Irradiation: Analysis of Dose-Mass Histograms and the Impact of Lung Expansion. Radiat. Oncol. 2019, 14, 1–10. [Google Scholar] [CrossRef]
- Rice, L.; Goldsmith, C.; Green, M.M.L.; Cleator, S.; Price, P.M. An Effective Deep-Inspiration Breath-Hold Radiotherapy Technique for Left-Breast Cancer: Impact of Post-Mastectomy Treatment, Nodal Coverage, and Dose Schedule on Organs at Risk. Breast Cancer Targets Ther. 2017, 9, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Latty, D.; Stuart, K.E.; Wang, W.; Ahern, V. Review of Deep Inspiration Breath-Hold Techniques for the Treatment of Breast Cancer. J. Med. Radiat. Sci. 2015, 62, 74–81. [Google Scholar] [CrossRef]
- Mast, M.E.; Van Kempen-Harteveld, L.; Heijenbrok, M.W.; Kalidien, Y.; Rozema, H.; Jansen, W.P.A.; Petoukhova, A.L.; Struikmans, H. Left-Sided Breast Cancer Radiotherapy with and without Breath-Hold: Does IMRT Reduce the Cardiac Dose Even Further? Radiother. Oncol. 2013, 108, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Holmberg, L.; Garmo, H.; Duvernoy, O.; Sjögren, I.; Lagerqvist, B.; Blomqvist, C. Distribution of Coronary Artery Stenosis after Radiation for Breast Cancer. J. Clin. Oncol. 2012, 30, 380–386. [Google Scholar] [CrossRef]
- Maraldo, M.V.; Brodin, N.P.; Aznar, M.C.; Vogelius, I.R.; Munck af Rosenschöld, P.; Petersen, P.M.; Specht, L. Estimated Risk of Cardiovascular Disease and Secondary Cancers with Modern Highly Conformal Radiotherapy for Early-Stage Mediastinal Hodgkin Lymphoma. Ann. Oncol. 2013, 24, 2113–2118. [Google Scholar] [CrossRef]
- Yamauchi, R.; Mizuno, N.; Itazawa, T.; Saitoh, H.; Kawamori, J. Dosimetric Evaluation of Deep Inspiration Breath Hold for Left-Sided Breast Cancer: Analysis of Patient-Specific Parameters Related to Heart Dose Reduction. J. Radiat. Res. 2020, 61, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Bertholet, J.; Knopf, A.; Eiben, B.; McClelland, J.; Grimwood, A.; Harris, E.; Menten, M.; Poulsen, P.; Nguyen, D.T.; Keall, P.; et al. Real-Time Intrafraction Motion Monitoring in External Beam Radiotherapy. Phys. Med. Biol. 2019, 64, 15TR01. [Google Scholar] [CrossRef]
- Brandner, E.D.; Chetty, I.J.; Giaddui, T.G.; Xiao, Y.; Huq, M.S. Motion Management Strategies and Technical Issues Associated with Stereotactic Body Radiotherapy of Thoracic and Upper Abdominal Tumors: A Review from NRG Oncology. Med. Phys. 2017, 44, 2595–2612. [Google Scholar] [CrossRef]
- Rochet, N.; Drake, J.I.; Harrington, K.; Wolfgang, J.A.; Napolitano, B.; Sadek, B.T.; Shenouda, M.N.; Keruakous, A.R.; Niemierko, A.; Taghian, A.G. Deep Inspiration Breath-Hold Technique in Left-Sided Breast Cancer Radiation Therapy: Evaluating Cardiac Contact Distance as a Predictor of Cardiac Exposure for Patient Selection. Pract. Radiat. Oncol. 2015, 5, e127–e134. [Google Scholar] [CrossRef]
- Wang, W.; Purdie, T.G.; Rahman, M.; Marshall, A.; Liu, F.F.; Fyles, A. Rapid Automated Treatment Planning Process to Select Breast Cancer Patients for Active Breathing Control to Achieve Cardiac Dose Reduction. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 386–393. [Google Scholar] [CrossRef]
- Zanca, F.; Hernandez-Giron, I.; Avanzo, M.; Guidi, G.; Crijns, W.; Diaz, O.; Kagadis, G.C.; Rampado, O.; Lønne, P.I.; Ken, S.; et al. Expanding the Medical Physicist Curricular and Professional Programme to Include Artificial Intelligence. Phys. Med. 2021, 83, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Gagliardi, V.; Stancanello, J.; Blanck, O.; Pirrone, G.; El Naqa, I.; Revelant, A.; Sartor, G. Combining Computed Tomography and Biologically Effective Dose in Radiomics and Deep Learning Improves Prediction of Tumor Response to Robotic Lung Stereotactic Body Radiation Therapy. Med. Phys. 2021, 48, 6257–6269. [Google Scholar] [CrossRef]
- Zanca, F.; Brusasco, C.; Pesapane, F.; Kwade, Z.; Beckers, R.; Avanzo, M. Regulatory Aspects of the Use of Artificial Intelligence Medical Software. Semin. Radiat. Oncol. 2022, 32, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Mancosu, P.; Lambri, N.; Castiglioni, I.; Dei, D.; Iori, M.; Loiacono, D.; Russo, S.; Talamonti, C.; Villaggi, E.; Scorsetti, M.; et al. Applications of Artificial Intelligence in Stereotactic Body Radiation Therapy. Phys. Med. Biol. 2022, 67, 16TR01. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Trianni, A.; Botta, F.; Talamonti, C.; Stasi, M.; Iori, M. Artificial Intelligence and the Medical Physicist: Welcome to the Machine. Appl. Sci. 2021, 11, 1691. [Google Scholar] [CrossRef]
- Avanzo, M.; Pirrone, G.; Vinante, L.; Caroli, A.; Stancanello, J.; Drigo, A.; Massarut, S.; Mileto, M.; Urbani, M.; Trovo, M.; et al. Electron Density and Biologically Effective Dose (BED) Radiomics-Based Machine Learning Models to Predict Late Radiation-Induced Subcutaneous Fibrosis. Front. Oncol. 2020, 10, 490. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Pirrone, G.; Mileto, M.; Massarut, S.; Stancanello, J.; Baradaran-Ghahfarokhi, M.; Rink, A.; Barresi, L.; Vinante, L.; Piccoli, E.; et al. Prediction of Skin Dose in Low-KV Intraoperative Radiotherapy Using Machine Learning Models Trained on Results of in Vivo Dosimetry. Med. Phys. 2019, 46, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, T.; Shi, C.; Petillion, S.; Kindts, I.; Weltens, C.; Depuydt, T.; Song, Y.; Saleh, Z.; Xu, X.G.; et al. Feasibility Study of Individualized Optimal Positioning Selection for Left-Sided Whole Breast Radiotherapy: DIBH or Prone. J. Appl. Clin. Med. Phys. 2018, 19, 218–229. [Google Scholar] [CrossRef]
- Fukushima, K. Neocognitron: A Self-Organizing Neural Network Model for a Mechanism of Pattern Recognition Unaffected by Shift in Position. Biol. Cybern. 1980, 36, 193–202. [Google Scholar] [CrossRef]
- Fukushima, K. Neocognitron: A Hierarchical Neural Network Capable of Visual Pattern Recognition. Neural Netw. 1988, 1, 119–130. [Google Scholar] [CrossRef]
- Dechter, R. Learning While Searching in Constraint-Satisfaction-Problems. In Proceedings of the Fifth AAAI National Conference on Artificial Intelligence, Philadelphia, PA, USA, 11–15 August 1986; pp. 178–183. [Google Scholar]
- Lombardo, E.; Kurz, C.; Marschner, S.; Avanzo, M.; Gagliardi, V.; Fanetti, G.; Franchin, G.; Stancanello, J.; Corradini, S.; Niyazi, M.; et al. Distant Metastasis Time to Event Analysis with CNNs in Independent Head and Neck Cancer Cohorts. Sci. Rep. 2021, 11, 6418. [Google Scholar] [CrossRef] [PubMed]
- Antropova, N.; Huynh, B.Q.; Giger, M.L. A Deep Feature Fusion Methodology for Breast Cancer Diagnosis Demonstrated on Three Imaging Modality Datasets. Med. Phys. 2017, 44, 5162–5171. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Robicquet, A.; Ramsundar, B.; Kuleshov, V.; DePristo, M.; Chou, K.; Cui, C.; Corrado, G.; Thrun, S.; Dean, J. A Guide to Deep Learning in Healthcare. Nat. Med. 2019, 25, 24–29. [Google Scholar] [CrossRef]
- Castiglioni, I.; Ippolito, D.; Interlenghi, M.; Monti, C.B.; Salvatore, C.; Schiaffino, S.; Polidori, A.; Gandola, D.; Messa, C.; Sardanelli, F. Artificial Intelligence Applied on Chest X-Ray Can Aid in the Diagnosis of COVID-19 Infection: A First Experience from Lombardy, Italy. Eur. Radiol. Exp. 2021, 5, 7. [Google Scholar] [CrossRef]
- Brunese, L.; Mercaldo, F.; Reginelli, A.; Santone, A. Explainable Deep Learning for Pulmonary Disease and Coronavirus COVID-19 Detection from X-Rays. Comput. Methods Programs Biomed. 2020, 196, 105608. [Google Scholar] [CrossRef]
- Eo, T.; Jun, Y.; Kim, T.; Jang, J.; Lee, H.-J.; Hwang, D. KIKI-Net: Cross-Domain Convolutional Neural Networks for Reconstructing Undersampled Magnetic Resonance Images. Magn. Reson. Med. 2018, 80, 2188–2201. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.M.; Goatman, K.A.; Siebert, J.P. Learning Rigid Image Registration—Utilizing Convolutional Neural Networks for Medical Image Registration. In Proceedings of the 5th International Conference on Bioimaging, Funchal, Portugal, 19–21 January 2018; pp. 89–99. [Google Scholar]
- Fourcade, C.; Ferrer, L.; Moreau, N.; Santini, G.; Brennan, A.; Rousseau, C.; Lacombe, M.; Fleury, V.; Colombié, M.; Jézéquel, P.; et al. Deformable Image Registration with Deep Network Priors: A Study on Longitudinal PET Images. Phys. Med. Biol. 2022, 67, 155011. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, F.; Ling, X.; Liu, Q.; Zhao, P. Intelligent Health Care: Applications of Deep Learning in Computational Medicine. Front. Genet. 2021, 12, 444. [Google Scholar] [CrossRef] [PubMed]
- Hochreiter, S.; Schmidhuber, J. Long Short-Term Memory. Neural Comput. 1997, 9, 1735–1780. [Google Scholar] [CrossRef]
- Denysyuk, H.V.; Pinto, R.J.; Silva, P.M.; Duarte, R.P.; Marinho, F.A.; Pimenta, L.; Gouveia, A.J.; Gonçalves, N.J.; Coelho, P.J.; Zdravevski, E.; et al. Algorithms for Automated Diagnosis of Cardiovascular Diseases Based on ECG Data: A Comprehensive Systematic Review. Heliyon 2023, 9, e13601. [Google Scholar] [CrossRef]
- Lombardo, E.; Rabe, M.; Xiong, Y.; Nierer, L.; Cusumano, D.; Placidi, L.; Boldrini, L.; Corradini, S.; Niyazi, M.; Belka, C.; et al. Offline and Online LSTM Networks for Respiratory Motion Prediction in MR-Guided Radiotherapy. Phys. Med. Biol. 2022, 67, 095006. [Google Scholar] [CrossRef]
- Bergom, C.; Currey, A.; Desai, N.; Tai, A.; Strauss, J.B. Deep Inspiration Breath Hold: Techniques and Advantages for Cardiac Sparing During Breast Cancer Irradiation. Front. Oncol. 2018, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, G.; Mosquera, C.; Nápoles, G. A Review on the Long Short-Term Memory Model. Artif. Intell. Rev. 2020, 53, 5929–5955. [Google Scholar] [CrossRef]
- Gers, F.A.; Schmidhuber, J.; Cummins, F. Learning to Forget: Continual Prediction with LSTM. Neural Comput. 2000, 12, 2451–2471. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bao, Z.; Li, L.; Zhao, Z. Exploring Temporal Representations by Leveraging Attention-Based Bidirectional LSTM-RNNs for Multi-Modal Emotion Recognition. Inf. Process. Manag. 2020, 57, 102185. [Google Scholar] [CrossRef]
- Graves, A.; Mohamed, A.R.; Hinton, G. Speech Recognition with Deep Recurrent Neural Networks. In Proceedings of the 2013 IEEE International Conference on Acoustics, Speech and Signal Processing, Vancouver, BC, Canada, 26–31 May 2013; Volume 3, pp. 45–49. [Google Scholar]
- Huang, Z.; Xu, W.; Yu, K. Bidirectional LSTM-CRF Models for Sequence Tagging. arXiv 2015, arXiv:1508.01991. [Google Scholar]
- Graves, A.; Schmidhuber, J. Framewise Phoneme Classification with Bidirectional LSTM and Other Neural Network Architectures. Neural Netw. 2005, 18, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Nguyen, D.; Zhou, Z.; Jiang, S.B.; Dong, B.; Jia, X. An Introduction to Deep Learning in Medical Physics: Advantages, Potential, and Challenges. Phys. Med. Biol. 2020, 65, 05TR01. [Google Scholar] [CrossRef]
- Hayden, A.J.; Rains, M.; Tiver, K. Deep Inspiration Breath Hold Technique Reduces Heart Dose from Radiotherapy for Left-Sided Breast Cancer. J. Med. Imaging Radiat. Oncol. 2012, 56, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Ferdinand, S.; Mondal, M.; Mallik, S.; Goswami, J.; Das, S.; Manir, K.S.; Sen, A.; Palit, S.; Sarkar, P.; Mondal, S.; et al. Dosimetric Analysis of Deep Inspiratory Breath-Hold Technique (DIBH) in Left-Sided Breast Cancer Radiotherapy and Evaluation of Pre-Treatment Predictors of Cardiac Doses for Guiding Patient Selection for DIBH. Tech. Innov. Patient Support Radiat. Oncol. 2021, 17, 25–31. [Google Scholar] [CrossRef]
- Borst, G.R.; Sonke, J.-J.; den Hollander, S.; Betgen, A.; Remeijer, P.; van Giersbergen, A.; Russell, N.S.; Elkhuizen, P.H.M.; Bartelink, H.; van Vliet-Vroegindeweij, C. Clinical Results of Image-Guided Deep Inspiration Breath Hold Breast Irradiation. Int. J. Radiat. Oncol. *Biol. *Phys. 2010, 78, 1345–1351. [Google Scholar] [CrossRef]
- Pandeli, C.; Smyth, L.M.L.; David, S.; See, A.W. Dose Reduction to Organs at Risk with Deep-Inspiration Breath-Hold during Right Breast Radiotherapy: A Treatment Planning Study. Radiat. Oncol. 2019, 14, 223. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brnønum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Vuong, W.; Garg, R.; Bourgeois, D.J.; Yu, S.; Sehgal, V.; Daroui, P. Dosimetric Comparison of Deep-Inspiration Breath-Hold and Free-Breathing Treatment Delivery Techniques for Left-Sided Breast Cancer Using 3D Surface Tracking. Med. Dosim. 2019, 44, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Malone, C.; Fennell, L.; Folliard, T.; Kelly, C. Using a Neural Network to Predict Deviations in Mean Heart Dose during the Treatment of Left-Sided Deep Inspiration Breath Hold Patients. Phys. Med. 2019, 65, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Czeremszyńska, B.; Drozda, S.; Górzyński, M.; Kępka, L. Selection of Patients with Left Breast Cancer for Deep-Inspiration Breath-Hold Radiotherapy Technique: Results of a Prospective Study. Rep. Pract. Oncol. Radiother. 2017, 22, 341–348. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD Statement. BMC Med. 2015, 13, 1-014-0241-z. [Google Scholar] [CrossRef]
- Ledsom, D.; Reilly, A.J.; Probst, H. Assessment of Deep Inspiration Breath Hold (DIBH) Amplitude and Reduction in Cardiac Dose in Left Breast Cancer Patients. Radiography 2018, 24, 98–103. [Google Scholar] [CrossRef]
- Meyer, P.; Noblet, V.; Mazzara, C.; Lallement, A. Survey on Deep Learning for Radiotherapy. Comput. Biol. Med. 2018, 98, 126–146. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Artificial Intelligence in Radiotherapy. Rep. Pract. Oncol. Radiother. 2020, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Montero, A.; Javaid, U.; Valdés, G.; Nguyen, D.; Desbordes, P.; Macq, B.; Willems, S.; Vandewinckele, L.; Holmström, M.; Löfman, F.; et al. Artificial Intelligence and Machine Learning for Medical Imaging: A Technology Review. Phys. Med. 2021, 83, 242–256. [Google Scholar] [CrossRef]
- Vandewinckele, L.; Claessens, M.; Dinkla, A.; Brouwer, C.; Crijns, W.; Verellen, D.; van Elmpt, W. Overview of Artificial Intelligence-Based Applications in Radiotherapy: Recommendations for Implementation and Quality Assurance. Radiother. Oncol. 2020, 153, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Al, N.P.B. A Quantitative Clinical Decision-Support Strategy Identifying Which Oropharyngeal Head and Neck Cancer Patients May Benefit the Most from Proton Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 176, 139–148. [Google Scholar] [CrossRef]
- Ma, M.; Liu, C.; Wei, R.; Liang, B.; Dai, J. Predicting Machine’s Performance Record Using the Stacked Long Short-Term Memory (LSTM) Neural Networks. J. Appl. Clin. Med. Phys. 2022, 23, e13558. [Google Scholar] [CrossRef]
- Johnson, J.M.; Khoshgoftaar, T.M. Survey on Deep Learning with Class Imbalance. J. Big Data 2019, 6, 27. [Google Scholar] [CrossRef]
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The Practical Implementation of Artificial Intelligence Technologies in Medicine. Nat. Med. 2019, 25, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zihni, E.; Madai, V.I.; Livne, M.; Galinovic, I.; Khalil, A.A.; Fiebach, J.B.; Frey, D. Opening the Black Box of Artificial Intelligence for Clinical Decision Support: A Study Predicting Stroke Outcome. PLoS ONE 2020, 15, e0231166. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Shao, Y.; Ding, B. LSTM-Convolutional-BLSTM Encoder-Decoder Network for Minimum Mean-Square Error Approach to Speech Enhancement. Appl. Acoust. 2021, 172, 107647. [Google Scholar] [CrossRef]
- Pirrone, G.; Matrone, F.; Chiovati, P.; Manente, S.; Drigo, A.; Donofrio, A.; Cappelletto, C.; Borsatti, E.; Dassie, A.; Bortolus, R.; et al. Predicting Local Failure after Partial Prostate Re-Irradiation Using a Dosiomic-Based Machine Learning Model. J. Pers. Med. 2022, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- Mahadevaiah, G.; Rv, P.; Bermejo, I.; Jaffray, D.; Dekker, A.; Wee, L. Artificial Intelligence-Based Clinical Decision Support in Modern Medical Physics: Selection, Acceptance, Commissioning, and Quality Assurance. Med. Phys. 2020, 47, e228–e235. [Google Scholar] [CrossRef] [PubMed]


| Variable | Value |
|---|---|
| Sex | Female |
| Age mean (95% CI) | 52 (41, 69.5) |
| Prescribed dose | 40.05 Gy/15 fractions |
| Sequential boost | 18/36 |
| Variable | Mean Value (95% CI) | Wilcoxon Signed Rank’s p | |
|---|---|---|---|
| FB | DIBH | ||
| Number of fields | 2.6 (2–5.3) | 2.6 (2–4) | 0.65 |
| PTV V95% (%) | 95.0 (90.3–97.7) | 96.5 (93.9–98.7) | 0.002 |
| PTV Dmax (Gy) | 42.9 (41.6–45.5) | 42.6 (41.6–43.7) | 0.051 |
| Distance of LAD to PTV in BEV (cm) | 0.08 (−1.62,1.25) | 2.19 (1.44,3.75) | <<0.001 |
| LAD Dmax (Gy) | 11.11 (0.49–18.90) | 0.56 (0.28–0.92) | <<0.001 |
| Heart Dmax (Gy) | 19.66 (0.61–30.20) | 0.93 (0.39–17.00) | <<0.001 |
| Contralateral Breast Dmax (Gy) | 0.41 (0.11–0.87) | 0.50 (0.12–16.59) | 0.62 |
| Lung V15% (Gy) | 0.44 (0.19–12.61) | 0.63 (0.33–12.26) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vendrame, A.; Cappelletto, C.; Chiovati, P.; Vinante, L.; Parvej, M.; Caroli, A.; Pirrone, G.; Barresi, L.; Drigo, A.; Avanzo, M. Artificial Intelligence-Based Patient Selection for Deep Inspiration Breath-Hold Breast Radiotherapy from Respiratory Signals. Appl. Sci. 2023, 13, 4962. https://doi.org/10.3390/app13084962
Vendrame A, Cappelletto C, Chiovati P, Vinante L, Parvej M, Caroli A, Pirrone G, Barresi L, Drigo A, Avanzo M. Artificial Intelligence-Based Patient Selection for Deep Inspiration Breath-Hold Breast Radiotherapy from Respiratory Signals. Applied Sciences. 2023; 13(8):4962. https://doi.org/10.3390/app13084962
Chicago/Turabian StyleVendrame, Alessandra, Cristina Cappelletto, Paola Chiovati, Lorenzo Vinante, Masud Parvej, Angela Caroli, Giovanni Pirrone, Loredana Barresi, Annalisa Drigo, and Michele Avanzo. 2023. "Artificial Intelligence-Based Patient Selection for Deep Inspiration Breath-Hold Breast Radiotherapy from Respiratory Signals" Applied Sciences 13, no. 8: 4962. https://doi.org/10.3390/app13084962
APA StyleVendrame, A., Cappelletto, C., Chiovati, P., Vinante, L., Parvej, M., Caroli, A., Pirrone, G., Barresi, L., Drigo, A., & Avanzo, M. (2023). Artificial Intelligence-Based Patient Selection for Deep Inspiration Breath-Hold Breast Radiotherapy from Respiratory Signals. Applied Sciences, 13(8), 4962. https://doi.org/10.3390/app13084962





