The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Drone Brood Collection
2.3. Chicken Eggshell Collection and Processing
2.4. Preparation of a Dietary Supplement Based on Drone Brood and Eggshell Powder
2.5. Analysis of the Mineral Composition of Eggshells Using the ICP-OES
2.6. Calcium Assay Test
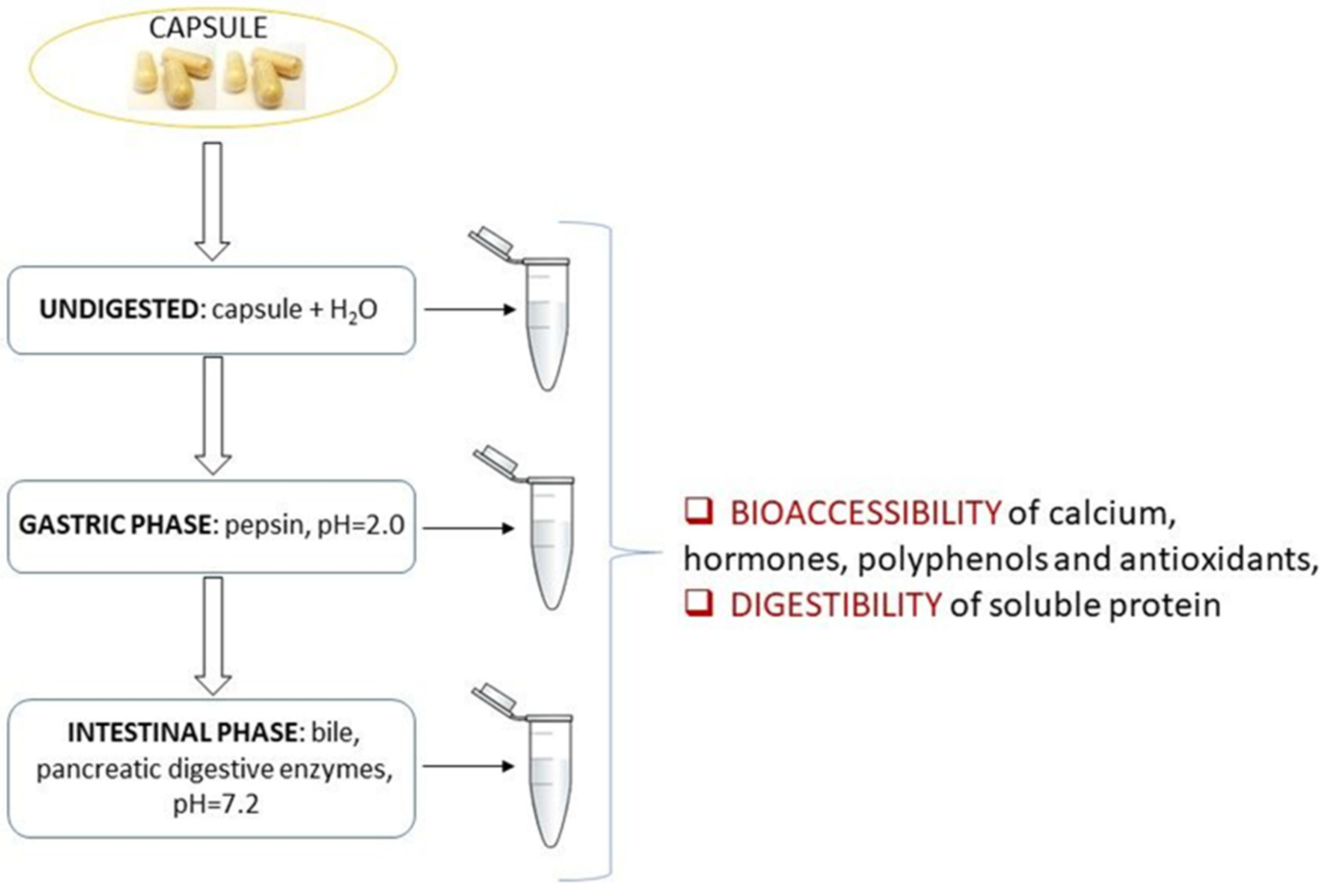
2.7. In Vitro Bioaccessibility of the Supplement Components
2.8. Soluble Protein Assay
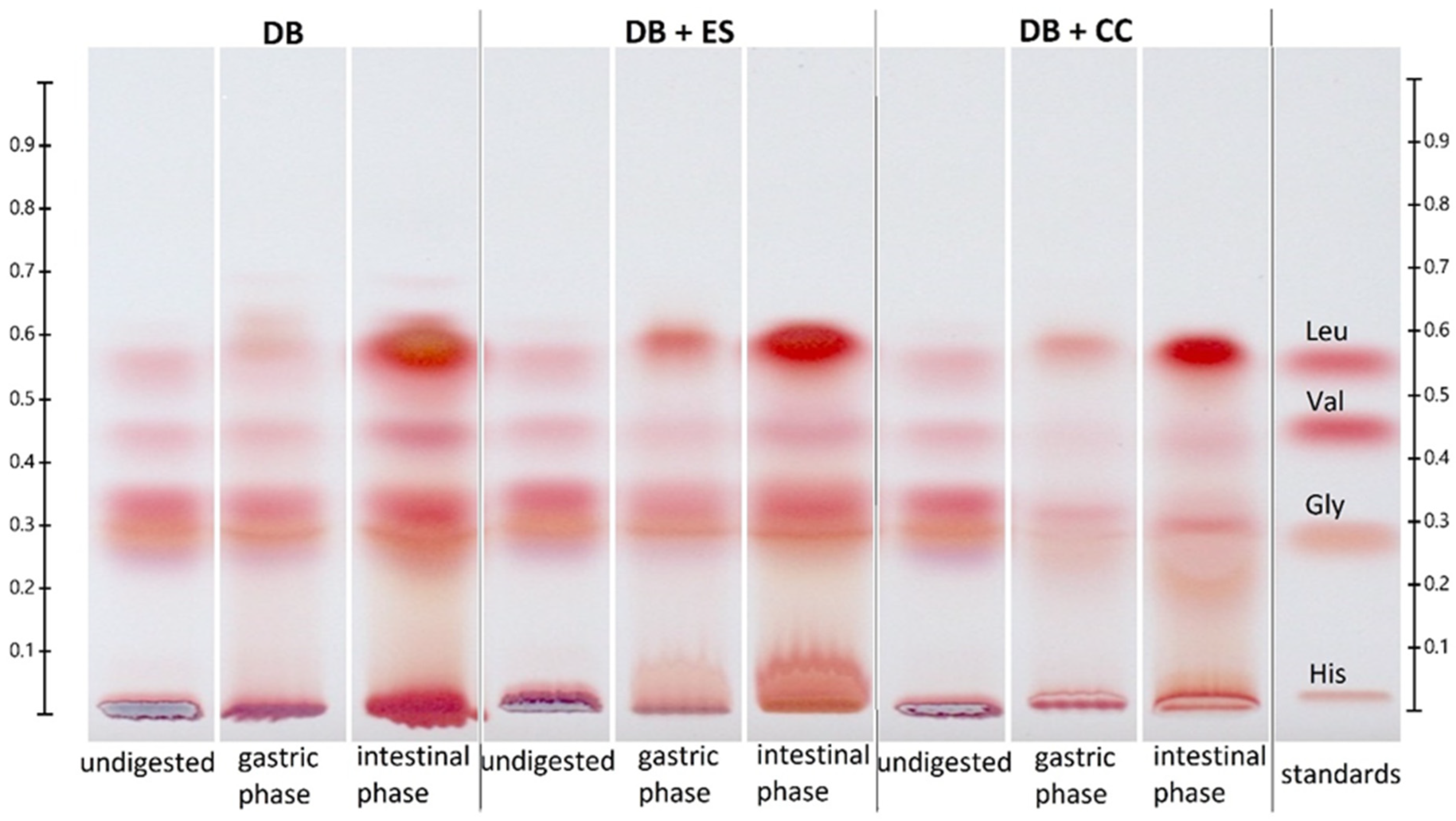
2.9. Protein Profiling by SDS-PAGE
2.10. Hormonal Activity Determination
2.11. Antioxidants Assay
2.11.1. FRAP Test
2.11.2. Total Phenolic Content (TPC) Assay
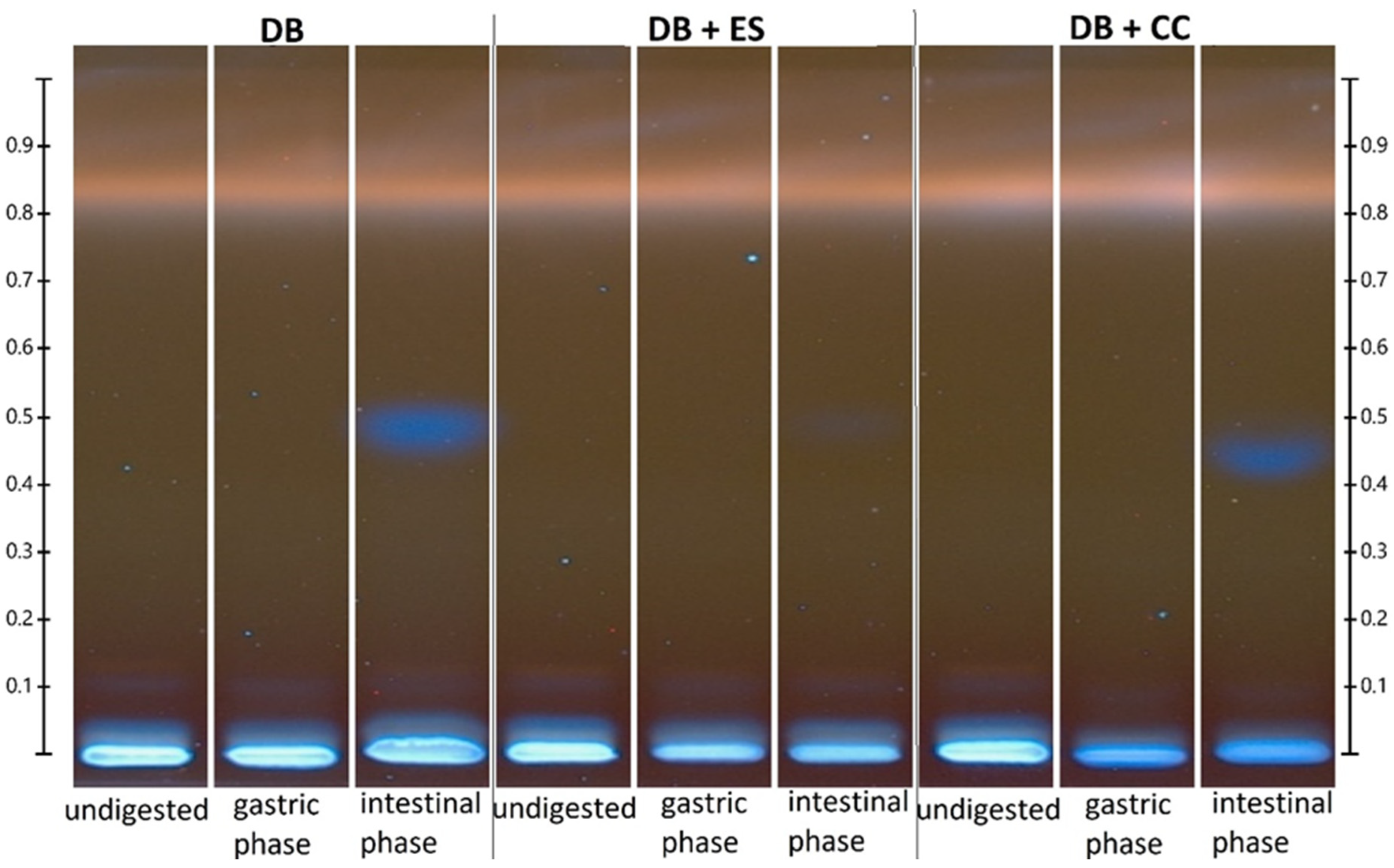
2.12. HPTLC Analysis
2.13. Statistical Calculations
3. Results and Discussion
3.1. Eggshell Quality Analysis
3.2. Supplement Design
3.3. In Vitro Digestion Study
3.4. Calcium
3.5. Steroid Hormones
3.6. Protein
3.7. Free Amino Acids
3.8. Antioxidants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rutka, I.; Galoburda, R.; Galins, J.; Galins, A. Bee Drone Brood Homogenate Chemical Composition, Stabilization and Application: A Review. Res. Rural Dev. 2021, 36, 96–103. [Google Scholar] [CrossRef]
- Kędzia, B.; Hołderna-Kędzia, E. Mniej Znane Produkty Pszczele; Sądecki Bartnik: Stróże, Poland, 2017; pp. 58–89. [Google Scholar]
- Sawczuk, R.; Karpińska, J.; Miltyk, W. What do we need to know about drone brood homogenate and what is known. J. Ethnopharmacol. 2019, 245, 111581. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, L. Physico-Chemical and Biological Appreciation of Drone Brood. Ph.D. Thesis, Ryazan Medical University, Ryazan, Russia, 1999. (In Russian). [Google Scholar]
- Wyszyńska, M.; Kabała-Dzik, A.; Szaflarska-Stojko, E. Observations on the hepatoprotective effect of DNA extract from bee brood. Farm. Przegl. Nauk. 2008, 4, 21–23. [Google Scholar]
- Kabała-Dzik, A.; Smagacz, O.; Marquard, W. Shielding effect of bee brood in relation to embryotoxic compounds-acetylsalicylic acid. Proc. Sci. Beekeep. Conf. 2007, 44, 138–139. (In Polish) [Google Scholar]
- Meda, A.; Lamien, C.E.; Millogo, J. Therapeutic uses of honey and honeybee larvae in central Burkina Faso. J. Ethnopharmacol. 2004, 95, 103–107. [Google Scholar] [CrossRef]
- Osnicewa, L.A.; Efanowa, N.W.; Kabyszewa, W.W. Homogenate of drone in the diet of dogs. Beekeeping 2009, 10, 50–51. (In Ukrainian) [Google Scholar]
- Bieljajew, W.A.; Safonowskaja, J.W. Adaptogenic relationship training based on drone brood. Beekeeping 2009, 6, 51–52. (In Russian) [Google Scholar]
- Peters, B.S.E.; Martini, L.A. Nutritional aspects of the prevention and treatment of osteoporosis. Arq. Bras. Endocrinol. Metabol. 2010, 54, 179–185. [Google Scholar] [CrossRef]
- Becheva, M.; Taneva, D. Prevention and treatment of osteoporosis. Pharmacia 2020, 67, 181–185. [Google Scholar] [CrossRef]
- Prentice, A. Diet, nutrition and the prevention of osteoporosis. Public Health Nutr. 2004, 7, 227–243. [Google Scholar] [CrossRef]
- Pouresmaeili, F.; Kamilidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview of osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed]
- Canarella, R.; Barbagallo, F.; Condorelli, S.A.; Aversa, A.; La Vignera, S.; Calogero, A.E. Osteoporosis from an endocrine perspective: The role of hormonal changes in the elderly. J. Clin. Med. 2019, 8, 1564. [Google Scholar] [CrossRef] [PubMed]
- Quattrini, S.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Brandi, M.L. The Mediterranean Diet in Osteoporosis Prevention: An Insight in a Peri- and Post-Menopausal Population. Nutrients 2021, 13, 531. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Babikova, M.; Omelka, R. Pharmacological agents and natural compounds: Available treatments for osteoporosis. J. Physiol. Pharmacol. 2020, 71, 307–320. [Google Scholar]
- Oh, S.-M.; Chung, K.-H. Estrogenic activities of Ginkgo biloba extracts. Life Sci. 2004, 74, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Bolland, M.J. Calcium supplementation in osteoporosis: Useful or harmful? Eur. J. Endocrinol. 2018, 178, D13–D25. [Google Scholar] [CrossRef]
- Rovenský, J.; Stancíková, M.; Masaryk, P.; Svík, K.; Istok, R. Eggshell calcium in the prevention and treatment of osteoporosis. Int. J. Clin. Pharmacol. Res. 2003, 23, 83–92. [Google Scholar]
- Brun, L.R.; Lupo, M.; Delorenzi, D.A.; Di Loreto, V.E.; Rigalli, A. Chicken eggshell as suitable calcium source at home. Int. J. Food Sci. Nutr. 2013, 64, 740–743. [Google Scholar] [CrossRef]
- Shahnila, A.S.; Pasha, I.; Iftikhar, H.; Mehak, F.; Sultana, R. Effects of eggshell powder supplementation on nutritional and sensory attributes of biscuits. Czech J. Food Sci. 2022, 40, 26–32. [Google Scholar] [CrossRef]
- Ray, S.; Kumar Barman, A.; Kumar Roy, P.; Kumar Singh, B. Chicken eggshell powder as dietary calcium source in chocolate cakes. Pharma Innov. J. 2017, 6, 1–4. [Google Scholar]
- Dolińska, B.; Jelińska, M.; Szulc-Musioł, B.; Ryszka, F. Use of eggshells as a raw material for production of calcium preparations. Czech J. Food Sci. 2016, 34, 313–317. [Google Scholar] [CrossRef]
- Bartter, J.; Diffey, H.; Yeung, Y.H.; O’Leary, F.; Häsler, B.; Maulaga, W.; Alders, R. Use of chicken eggshell to improve dietary calcium intake in rural sub-Saharan Africa. Matern. Child Nutr. 2018, 14, e12649. [Google Scholar] [CrossRef] [PubMed]
- Aditya, S.; Stephen, J.; Radhakrishnan, M. Utilization of eggshell waste in calcium-fortified foods and other industrial applications: A review. Trends Food Sci. Technol. 2021, 115, 422–432. [Google Scholar] [CrossRef]
- Sakai, S.; Hien, V.T.T.; Tuyen, L.D.; Duc, H.A.; Masuda, Y.; Yamamoto, S. Effects of eggshell calcium supplementation on bone mass in postmenopausal Vietnamese women. J. Nutr. Sci. Vitaminol. 2017, 63, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Omelka, R.; Martiniakova, M.; Svik, K.; Slovak, L.; Payer, J.; Oppenbergerova, I.; Kovacova, V.; Babikova, M.; Soltesova-Prnova, M. The effects of eggshell calcium (Biomin H®) and its combinations with alfacalcidol (1α-hydroxyvitamin D3) and menaquinone-7 (vitamin K2) on ovariectomy-induced bone loss in a rat model of osteoporosis. J. Anim. Physiol. Anim. Nutr. 2021, 105, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Vitali, D.; Dragojević, I.V.; Šebečić, B. Effects of incorporation of integral raw materials and dietary fibre on the selected nutritional and functional properties of biscuits. Food Chem. 2009, 114, 1462–1469. [Google Scholar] [CrossRef]
- Sidor, E.; Miłek, M.; Zaguła, G.; Bocian, A.; Dżugan, M. Searching for Differences in Chemical Composition and Biological Activity of Crude Drone Brood and Royal Jelly Useful for Their Authentication. Foods 2021, 10, 2233. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, E.; Navia Lomban, B.; Lopez-Sobaler, A.M.; Ortega Anta, R.M. Review and future perspectives on recommended calcium intake. Nutr. Hosp. 2010, 25, 366–374. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frigola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Corraud, J.; Berger, J.; Cristol, J.P.; Avallone, S. Stability and bioaccessibility of different forms of carotenoids and vitamin A during in vitro digestion. Food Chem. 2013, 136, 871–877. [Google Scholar] [CrossRef]
- Kusumi, N.; Nakamura, M.; Tando, Y.; Suda, T.; Kudo, K. Egg–shell calcium solubility in stomach. Jpn. J. Nutr. Assess. 1999, 16, 291–296. [Google Scholar]
- Kobus-Cisowska, J.; Szymanowska-Powałowska, D.; Szymandera-Buszka, K.; Rezler, R.; Jarzębski, M.; Szczepaniak, O.; Marciniak, G.; Jędrusek-Golińska, A.; Kobus-Moryson, M. Effect of fortification with calcium from eggshells on bioavailability, quality, and rheological characteristics of traditional Polish bread spread. J. Dairy Sci. 2020, 103, 6918–6929. [Google Scholar] [CrossRef] [PubMed]
- Meiron, O.E.; Bar-David, E.; Aflalo, E.D.; Shechter, A.; Stepensky, D.; Berman, A.; Sagi, A. Solubility and bioavailability of stabilized amorphous calcium carbonate. J. Bone Miner. Res. 2011, 26, 364–372. [Google Scholar] [CrossRef]
- Sharp, M.; Dohme, B.V. Stable Formulations of Testosterone Undecanoate. CN106456782B, 18 February 2020. [Google Scholar]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Cauley, J.A. Estrogen and bone health in men and women. Steroids 2015, 99, 11–15. [Google Scholar] [CrossRef]
- Humphries, K.H.; Gill, S. Risks and benefits of hormone replacement therapy: The evidence speaks. CMAJ 2003, 168, 1001–1010. [Google Scholar]
- Kosum, N.; Yucel, B.; Kandemir, C.; Taskin, T.; Duru, M.E.; Kucukayadin, S.; Margaoan, R.; Cornea-Cipcigan, M. Chemical Composition and Androgenic Effect of Bee Drone Larvae (Apilarnil) for Goat Male Kids. Chem. Biodivers. 2022, 19, e202200548. [Google Scholar] [CrossRef]
- Kistanova, E.; Zdoroeva, E.; Nevitov, M.; Nosov, A.; Vysokikh, M.; Sukhanova, I.; Vishnyakova, P.; Abadijeva, D.; Ankova, D.; Rashev, P.; et al. Drone brood fed suplement impacts on the folliculogenesis in growing gilts. Vet. Arh. 2020, 90, 583–592. [Google Scholar] [CrossRef]
- Abdelouahab, N.; Nabila, B.; Roza, S.; Slimane, B.; Dako, E.; Pascal, A.; Mouloud, B.M. Molecular weight determination of a protease extracted from Mucor pusillus: Comparison methods. Food Nutr. Sci. 2015, 6, 348–354. [Google Scholar]
- Hui, T.; Tang, T.; Gu, X.; Yuan, Z.; Xing, G. Comparison on Protein Bioaccessibility of Soymilk Gels Induced by Glucono-δ-Lactone and Lactic Acid Bacteria. Molecules 2022, 27, 6202. [Google Scholar] [CrossRef]
- Margaoan, R.; Marghitas, L.A.; Dezmirean, D.S.; Bobis, O.; Bonta, V.; Catana, C.; Urcan, A.; Muresan, C.I.; Margin, M.G. Comparative Study on Quality Parameters of Royal Jelly, Apilarnil and Queen Bee Larvae Triturate. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2017, 74, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Delmas, D.; Hichami, A.; Rebe, C.; Ghiringhelli, F. Immunomodulation and Anti-inflammatory Roles of Polyphenols as Anticancer Agents. Anti-Cancer Agents Med. 2012, 12, 852–873. [Google Scholar]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as Plant-Based Nutraceuticals: Health Effects, Encapsulation, Nano-Delivery, and Application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]


| Supplement | Code | Drone Brood | Eggshell of Organic Eggs | Calcium Carbonate |
|---|---|---|---|---|
| Organic Ca | ES | - | 250 mg (=95 mg Ca) | - |
| Inorganic Ca | CC | - | - | 250 mg (=100 mg Ca) |
| Hormones | DB | 300 mg =0.0024 nmol testosterone =1.99 nmol estradiol | - | - |
| Hormones + Organic Ca (3.2:1) | DB+ES | 300 mg | 250 mg | - |
| Hormones + Inorganic Ca (3:1) | DB+CC | 300 mg | - | 250 mg |
| Identified Group | Mobile Phase (v:v:v) | Derivatization Reagent | Used Standards |
|---|---|---|---|
| Amino acids | 1-propanol, H2O (7:3) | ninhydrin | valine, histidine, leucine, glycine, aspartic acid, proline, lysine, glutamic acid |
| Polyphenols | ethyl acetate, methanol, water, formic acid (50:4:4:2.5) | NP reagent, PEG 400 | ferulic acid, ellagic acid, vanillin, kaempferol, apigenin 7-glucoside |
| Cage Rearing [mg/100 g d.m. ± SD] | Organic Farming [mg/100 g d.m. ± SD] | Average Content [mg/100 g d.m. ± SD] | Percentage [%] | |
|---|---|---|---|---|
| Al | 0.12 ± 0.29 a | 0.48 ± 0.30 b | 0.30 ± 0.11 | 0.0009 |
| As | 0.04 ± 0.07 a | 0.03 ± 0.04 a | 0.04 ± 0.009 | 0.0001 |
| Ca | 32,362.79 ± 503.94 a | 31,417.52 ± 678.54 a | 31,890.15 ± 473.06 | 96.15 |
| Cd | 0.002 ± 0.004 a | 0.002 ± 0.001 a | 0.002 ± 0.00 | 0.0001 |
| Cr | 0.12 ± 0.08 b | 0.004 ± 0.008 a | 0.08 ± 0.00 | 0.0002 |
| Cu | 0.13 ± 0.03 b | 0.05 ± 0.030 a | 0.09 ± 0.02 | 0.0002 |
| Fe | 4.30 ± 1.69 a | 4.86 ± 1.33 a | 4.58 ± 1.33 | 0.14 |
| K | 48.24 ± 0.75 a | 56.67 ± 1.40 a | 52.45 ± 2.75 | 0.16 |
| Mg | 508.73 ± 4.95 b | 457.49 ± 8.15 a | 483.11 ± 3.60 | 1.45 |
| Mn | 0.20 ± 0.09 a | 0.21 ± 0.103 a | 0.20 ± 0.06 | 0.0006 |
| Mo | n.d. | n.d. | n.d. | n.d. |
| Na | 87.26 ± 0.54 a | 104.99 ± 1.52 b | 96.12 ± 0.54 | 0.02 |
| Ni | n.d. | n.d. | n.d. | n.d. |
| P | 194.49 ± 2.83 b | 139.68 ± 4.35 a | 167.08 ± 2.83 | 0.57 |
| Pb | n.d. | n.d. | n.d. | n.d. |
| S | 445.83 ± 3.14 a | 448.15 ± 12.47 a | 446.99 ± 3.14 | 1.34 |
| Sr | 20.93 ± 0.17 a | 32.15 ± 1.31 b | 23.28 ± 1.31 | 0.07 |
| Zn | 0.48 ± 0.53 a | 0.44 ± 0.02 a | 0.44 ± 0.01 | 0.001 |
| TOTAL | 33,673.82 | 32,662.23 | 33,164.91 | 100% |
| Sample | Tested Fraction before/after Digestion | Proteins [mg/Capsule ± SD] | Calcium [mg/Capsule ± SD | Testosterone [nmol/Capsule ± SD] | Estradiol [nmol/Capsule ± SD] | TPC [mgGAE/Capsule ± SD] | FRAP [µmolTE/Capsule ± SD] |
|---|---|---|---|---|---|---|---|
| ES (250 mg/capsule) | Undigested | 6.09 ± 2.13 | 78.7 ± 15.52 | n.t. | n.t. | n.d. | n.d. |
| Gastric phase | 4.22 ± 0.67 * | 72.45 ± 0.37 | n.t. | n.t. | n.d. | n.d. | |
| Intestinal phase | 0.88 ± 1.53 * | 82.60 ± 1.24 | n.t. | n.t. | n.d. | n.d. | |
| CC (250 mg/capsule) | Undigested | n.t | 100.00 ± 17.102 | n.t. | n.t. | n.d. | n.d. |
| Gastric phase | n.t. | 9.20 ± 0.12 * | n.t. | n.t. | n.d. | n.d. | |
| Intestinal phase | n.t. | 10.28 ± 0.04 * | n.t. | n.t. | n.d. | n.d. | |
| DB (300 mg/capsule) | Undigested | 114.22 ± 3.84 | 0.10 ± 0.02 | 0.011 ± 0.00 | 1.28 ± 0.07 | 2.28 ± 0.30 | 8.37 ± 0.56 |
| Gastric phase | 18.18 ± 2.63 * | 0.29 ± 0.03 * | 0.0019 ± 0.00 * | n.d. | 1.74 ± 0.35 * | 3.52 ± 0.36 * | |
| Intestinal phase | 13.62 ± 2.96 * | 0.72 ± 0.10 * | 0.0063 ± 0.00 * | 0.35 ± 0.02 * | 2.12 ± 0.60 | 4.41 ± 0.56 * | |
| DB + ES (300 mg + 250 mg/capsule) | Undigested | 45.19 ± 5.49 | 43.34 ± 4.12 | 0.0060 ± 0.00 | 1.08 ± 0.03 | 2.56 ± 0.11 | 3.54 ± 0.06 |
| Gastric phase | 7.62 ± 1.15 * | 38.96 ± 1.01 * | 0.0025 ± 0.01 * | n.d. | 1.56 ± 0.19 * | 2.81 ± 0.25 * | |
| Intestinal phase | 2.99 ± 0.36 * | 45.57 ± 0.11 | 0.0098 ± 0.03 * | 0.23 ± 0.07 * | 1.34 ± 0.30 * | 2.88 ± 0.09 * | |
| DB + CC (300 mg + 250 mg/capsule) | Undigested | 40.95 ± 2.00 | 55.05 ± 3.41 | 0.012 ± 0.0 0 | 1.11 ± 0.04 | 1.97 ± 0.08 | 3.47 ± 0.13 |
| Gastric phase | 22.10 ± 4.25 * | 5.55 ± 0.50 * | 0.0048 ± 0.03 * | n.d. | 1.42 ± 1.40 * | 4.21 ± 1.54 * | |
| Intestinal phase | 14.23 ± 10.57 * | 6.66 ± 0.13 * | 0.0136 ± 0.03 | 0.30 ± 0.08 * | 1.22 ± 1.75 * | 3.33 ± 0.60 | |
| Control (capsule alone) | Undigested | <0.035 | n.t. | n.t. | n.t. | 0.05. | n.d. |
| Gastric phase | n.d. | n.t. | n.t. | n.t. | n.d. | n.d. | |
| Intestinal phase | n.d. | n.t. | n.t. | n.t. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dżugan, M.; Sidor, E.; Miłek, M.; Tomczyk, M. The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy. Appl. Sci. 2023, 13, 4687. https://doi.org/10.3390/app13084687
Dżugan M, Sidor E, Miłek M, Tomczyk M. The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy. Applied Sciences. 2023; 13(8):4687. https://doi.org/10.3390/app13084687
Chicago/Turabian StyleDżugan, Małgorzata, Ewelina Sidor, Michał Miłek, and Monika Tomczyk. 2023. "The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy" Applied Sciences 13, no. 8: 4687. https://doi.org/10.3390/app13084687
APA StyleDżugan, M., Sidor, E., Miłek, M., & Tomczyk, M. (2023). The Possibility of Using Bee Drone Brood to Design Novel Dietary Supplements for Apitherapy. Applied Sciences, 13(8), 4687. https://doi.org/10.3390/app13084687






