Abstract
Background: Root canal sealers and repair materials should have the desirable physical, chemical, and biological characteristics, and an antibacterial effect if possible. There is little information available on the biocompatibility of new sealers on the market. Fourier transform infrared spectroscopy (FTIR) can offer trustworthy data to examine chemical structures; another technique for revealing the elements in the constituents that may contribute to the cytotoxicity of these sealers is scanning electron microscopy (SEM), with the goal of elemental mapping utilizing energy-dispersive X-ray spectroscopy (EDX). Methodology: All the root canal sealers were mixed as per the manufacturers’ instructions and allowed to set in molds for 24 h. Then, the samples were placed into an incubator (Memmert GmbH + Co. KG, Schwabach, Germany for 72 h, in a moist environment to allow complete chemical setting of the sealers. The organic and inorganic components of the sample were identified using FTIR with the wavelength length in the infra-red region measuring 400–450 nm. The finely crushed samples were coated with gold metal; following that, the sealer samples were examined under a scanning electron microscope (SEM) at 5000×, 10,000×, and 20,000× magnification, followed by energy-dispersive X-ray spectroscopy. Results: The surfaces of BioRoot and DiaRoot sealers revealed a relatively uniform distribution of irregular micro-sized particles aggregated in clusters, with the particle size ranging from 1 to 65 µm and 0.4 to 55 µm, respectively. OneFill, iRoot, and CeraSeal demonstrated irregularly shaped particles with particle sizes of 0.5 to 105 µm, 0.5 to 195 µm, and 0.3 to 68 µm, respectively. The EDX microanalysis revealed that oxygen, calcium, and carbon were found in all the tested sealer materials. Silicone and zirconium were absent in DiaRoot, but DiaRoot contained fluoride and ytterbium. Moreover, aluminum was noted in DiaRoot, One Fill, and CeraSeal, and chloride was only observed in BioRoot. FTIR analysis revealed strong absorption bands at 666 cm−1 and 709 cm−1 in BioRoot. Bands at 739 cm−1, 804 cm−1, 863 cm−1, 898 cm−1, and 1455 cm−1 were observed in DiaRoot. Bands at 736 cm−1 and 873 cm−1 in OneFill suggested the presence of C-H bending. Similarly, bands were observed at 937 cm−1, 885 cm−1, 743 cm−1, and 1455 cm−1 in iRoot, representing C-H stretching. Conclusions: All root canal sealers had diverse surface morphologies that contained irregular, micro-sized particles that were uniformly distributed, and they lacked heavy metals. All the experimental sealers comprised mainly calcium, oxygen, and carbon.
1. Introduction
Endodontic sealers are utilized in the obturation process of root canal treatment to improve a fluid-tight or hermetic seal throughout the canal. This includes the apical foramen as well as any canal unevenness or minor discrepancies that exist between the root canal wall and the core filling material [1,2]. Root canal sealers and repair materials should have the desirable physical, chemical, and biological characteristics, and an antibacterial effect if possible. Root canal sealers are used to prevent bacterial infiltration and the formation of harmful byproducts in the periapical tissues, hence facilitating tissue regeneration and preventing the progression of periapical diseases [1]. Several methods, such as single master cone, cold lateral compaction, the Thermafil system, hybrid Tagger’s, and continuous wave, have been suggested as possible approaches to the obturation of root canals. Gutta-percha is typically selected as the core material associated with endodontic sealers that have a variety of compositions [3]. Therefore, the best endodontic sealers should prevent leakage and reduce the chance of bacterial invasion through the periapical tissues. An endodontic sealer should be dimensionally stable, biocompatible, slow setting to allow for ample working time, seal well once set, and adhere well to canal walls. Additionally, it should not dissolve in body fluids [2]. The chemical nature of the substances present in root canal sealers may define significant relationships with tissue tolerability, bond strength with dentin, and antibacterial capabilities. Each new root canal sealer should offer suitable options that are well defined for their intended use and environmental contexts [4].
The characteristics of root canal sealers influence the overall quality of the root filling that is placed in the canal. It is possible to gain an understanding of the clinical behavior of a root canal sealer as well as its handling characteristics by conducting laboratory experiments on their physical and chemical properties [5,6]. There are a variety of laboratory tests that may be performed to investigate the clinical and physical characteristics of sealers. To meet the standards set by the American National Standards Institute and the American Dental Association (ANSI/ADA), a sealer must have a radiopacity of at least 3 mm aluminum thickness, less than 3% solubility, more than 20% flowability, no more than 50 µm film thickness, and a setting time that does not exceed 10% of the time prescribed by the manufacturer’s declaration [7,8]. Endodontic sealers come in a variety of forms, including glass ionomer, silicone, resin, calcium hydroxide, and zinc oxide eugenol, as well as a sealer made from a bioceramic resin, which can be used for apexification, retro-filling, direct pulp capping, apexogenesis, and perforation repair [9]. Portland sealer is related to the radiopacifier, known as MTA, and the bioceramic resin [10]. These bioceramic materials in particular are produced by mixing calcium silicate and calcium phosphate, which are frequently employed in the medical and dentistry fields [11]. Their physical and biological characteristics, such as their alkaline pH and chemical stability in the biological environment, as well as the fact that they are biocompatible, have received a great deal of attention. These materials also have the benefit of forming hydroxyapatite during the fixing procedure, which creates a link between the filling material and dentin [12].
However, all root canal sealers demonstrate some level of toxicity in their active ingredients. Because of this, the cytotoxicity of sealers continues to be an issue despite the fact that newer sealers have been produced due to the great biocompatibility of these sealers [13,14]. There is little information available on the biocompatibility of new sealers on the market. Fourier transform infrared spectroscopy (FTIR) can offer trustworthy data to examine chemical structures in order to determine the functional groups present in the sealers that may be responsible for the cytotoxicity of the endodontic sealers. Another technique for revealing the elements in the constituents that may contribute to the cytotoxicity of these sealers is scanning electron microscopy (SEM) with the goal of elemental mapping utilizing energy-dispersive X-ray spectroscopy (EDX) [15]. Therefore, the purpose of this study was to assess the physicochemical characteristics of five commercially available endodontic sealers using FTIR, SEM, and EDX to describe the physicochemical constituents found on the surface of root canal sealers.
2. Materials and Methods
Five root canal sealers were used in the current study; the brand names, contents, and batch numbers are presented in Table 1. The purpose of this study was to characterize the five commercially available root canal sealers.

Table 1.
Details of root canal sealers used in the current study.
2.1. Sample Preparations
Silicon molds were prepared and used in the current study to set the root canal sealer materials. All the root canal sealers were mixed as per the manufacturers’ instructions and allowed to set in the molds for 24 h. Then, the samples were placed into an incubator (Memmert GmbH + Co. KG, Schwabach, Germany) for 72 h, in a moist environment to allow complete chemical setting of the sealers. These samples were used for further analysis of the root canal sealers.
2.2. FTIR Analysis
Fourier transform infrared microscopy (Nicolet Summit FTIR Spectrometer; Thermo Fisher Scientific, Waltham, MA, USA) is the most broadly used vibrational technique to identify the organic and inorganic components of a sample. The molded samples were crushed finely in a mortar and pestle, and then KBr pellet was made using potassium bromide and the samples in a 1:10 ratio under pressure until transparent pellet was made. FTIR measures the wavelength length in the infra-red region measuring 400–450 nm.
2.3. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray Spectroscopy (EDS)
Scanning electron microscopy examines the objects at a finer scale to yield knowledge about the morphology, topography, composition, and surface irregularities. The sealer samples were demolded and crushed into a fine powder with a mortar and pestle, and then the samples were incubated at 37 °C and 100% humidity in an incubator (Memmert GmbH + Co. KG, Schwabach, Germany) for 24 h (Figure 1).

Figure 1.
Crushed samples of the five root canal sealers.
After incubating, the samples were coated with gold metal prior to imaging. This is mainly performed to convert the non-conductive samples into electrically conducting samples to prevent the artifacts of images, which is most likely to happen when non-conducting samples are tested in an electron microscope as they are likely to be charged by the beam. After gold plating, the sealer samples were examined under a scanning electron microscope (SEM-EDS, Quanta FEG 450 USM) at 5000× and 10,000× magnification, followed by energy-dispersive X-ray spectroscopy (Figure 2). EDS is the technique which is used to provide elemental composition, chemical analysis, and quantitative compositional data of the samples. It mainly generates X-rays according to the nature of the elements present in the sample and provides a quantitative analysis of respective samples [16].
Figure 2.
(A) Gold plating of samples and the (B) scanning electron microscope.
3. Results
The scanning electron micrographs of each sealer group at 5000× and 10,000× magnification are illustrated in Figure 3. The surface of the BioRoot sealer revealed a relatively uniform distribution of irregular micro-sized particles aggregated in clusters, with the particle size ranging from 1 µm to 65 µm under 5000× magnification. A similar pattern was observed in DiaRoot with smaller particle sizes ranging from 0.4 µm to 55 µm. The distinctive surface morphology of crystalline structures can also be seen as rectangular, sharp-edged particles embedded in the matrix under 10,000× magnification. OneFill demonstrated irregularly shaped particles interspersed within a rich and dense matrix with particle surface structures barely able to be defined. The particle sizes were approximately 0.5 µm to 105 µm. The SEM image of iRoot revealed irregular micro-sized particles aggregated in clusters with particle sizes ranging from 0.5 µm to 195 µm. CeraSeal exhibited smaller round-shaped particles in clusters discernible in the matrix with varying sizes ranging from 0.3 µm to 68 µm. The smaller particles were zirconium adhering to larger calcium-based particles (Figure 3).
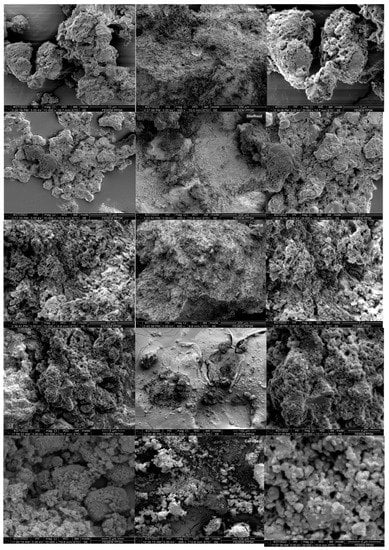
Figure 3.
Scanning electron micrographs of root canal sealers at 5000×, 10,000×, and 20,000× magnification.
The EDX microanalysis revealed oxygen, calcium, and carbon in all the tested sealer materials (Table 2). Both silicone and zirconium were absent in DiaRoot, but DiaRoot contained fluoride and ytterbium. Moreover, aluminum was noted in DiaRoot, One Fill, and CeraSeal, and chloride was only observed in BioRoot. It can be inferred that DiaRoot, OneFill, and CeraSeal comprise portlandite cement, while BioRoot and iRoot are pure calcium silicate cement. None of the tested sealer materials included bismuth or other heavy metals such as lead, chromium, cobalt, copper, zinc, and manganese. In general, OneFill, iRoot, and CeraSeal contained higher contents of carbon, oxygen, and zirconium, while BioRoot had higher amounts of calcium and silicone compared to the others.

Table 2.
Percentage of mass (weight %) of different elements presented among the tested root canal sealers.
FTIR analysis showed strong absorption bands at 666 cm−1 and 709 cm−1 in BioRoot. These bands suggested the presence of C-Cl stretching, which is attributed to the presence of calcium chloride in the sealer material itself. The bands at 1296 cm−1 implied the stretching mode of C-N, which is associated with the presence of povidone. Both bands at 1431 cm−1 and 1483 cm−1 reflected the stretching of C-H groups, while the band at 1740 cm−1 showed the stretching of C=O carboxylic groups, confirming the presence of polycarboxylate in BioRoot. Meanwhile, numerous strong bands 739 cm−1, 804 cm−1, 863 cm−1, 898 cm−1, and 1455 cm−1 in DiaRoot suggested the stretching of C-H groups. The band at 1098 cm−1 showed the stretching of C-O groups, attributed to the presence of calcium carbonate (CaCO3). Furthermore, bands at 1260 cm−1 and 1352 cm−1 represent C-F bending, while bands at 2874 cm−1 and 3656 cm−1 represent O-H stretching due to the hydration of cement.
Bands at 736 cm−1 and 873 cm−1 in OneFill suggested the presence of C-H bending, whereas bands at 965 cm−1 showed C=C bending. Moreover, a band at 1301 cm−1 revealed C-O stretching, suggesting the possible existence of CaCO3. Bands at 1412 cm−1 and 1639 cm−1 represented S=O and C=O stretching, respectively, while bands at 2880 cm−1 and 3402 cm−1 represented O-H stretching, explaining the possible calcium hydroxide (CaOH2) formation. On the other hand, similar bands were observed at 937 cm−1, 885 cm−1, 743 cm−1, and 1455 cm−1 in iRoot, representing C-H stretching. The two strong bands at 1069 cm−1 and 1350 cm−1 appeared to be the existence of S=O bending. In addition, a band at 1786 cm−1 showed C=O stretching, which could possibly be due to the monobasic found in iRoot, whereas the two strong bands at 2876 cm−1 and 3403 cm−1 suggested O-H stretching of CaOH2. The bands that appeared in CeraSeal were similar to those of iRoot with bands at 732 cm−1, 874 cm−1, 945 cm−1, and 1459 cm−1 showing the presence of C-H bending, while the band at 1350 cm−1 represented S=O stretching. The bands at 987 cm−1 and 1091 cm−1 showed evidence of C=C bending and C-O stretching, respectively. Furthermore, the band at 1648 cm−1 represented C=O stretching, while bands at 2876 cm−1 and 3405 cm−1 represented O-H stretching groups, which signify the formation of CaOH2 (Figure 4).
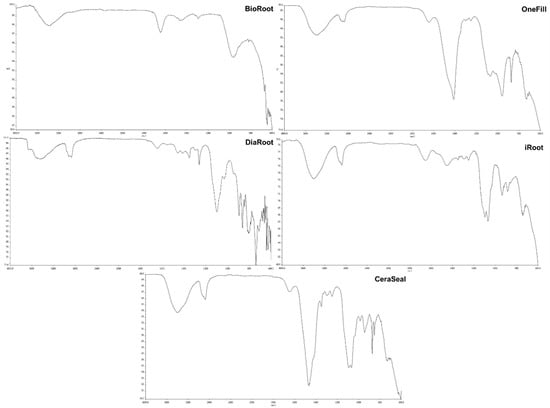
Figure 4.
FTIR analysis of root canal sealers.
4. Discussion
The primary purpose of the obturation process is to provide a seal in all three dimensions. This helps to prevent the reappearance of infection in the root canals and maintains the good condition of the periapical tissues. Gutta-percha is the most commonly used material for obturating root canals due to the many benefits it provides, including its non-toxicity, biocompatibility, and non-allergenic characteristics, as well as the ease with which it can be retrieved from the root canal in the event that retreatment is necessary [17]. The obturation of root canals with root canal sealers and gutta-percha is the accepted standard for endodontic treatment [18]. Root canal sealers have several advantages, but they also have some disadvantages as well, the most notable of which being their inability to completely connect with the dentin that surrounds the root canals. Due to the hydrophobic nature of gutta-percha, the sealer may also need to adjust away from it throughout the setting process [19]. Since gutta-percha does not adhere to the root canal walls, it cannot be used alone to create a hermetic seal. To prevent bacteria from colonizing the empty spaces between the gutta-percha cones and the dentinal walls of the root canal, a sealer is used [20]. As a result, it appears challenging to achieve a hermetic seal of the root canal, even when employing a combination of gutta-percha and a root canal sealer, as is the case in routine clinical practice [20]. It is essential for any root canal filler material to be able to adhere to root dentin at the dentin–sealer interface [21]. In the present study, we assessed the physicochemical characteristics of five root canal sealers (BioRoot, IRoot, CeraSeal, OneFill, and DiaRoot) using FTIR, SEM, and EDX.
4.1. Characterization of Root Canal Sealers through SEM
The surface morphology and particle sizes of the sealer materials were analyzed with the help of images obtained from SEM. Surface regularity is essential in determining whether a substance is biocompatible since it affects the ability of cells to adhere to the surface of the material [22]. According to the findings of our research, all the sealers we tested produced irregular micro-sized particles that aggregated in clusters with particle sizes ranging from 0.5 µm to 200 µm. However, CeraSeal produced smaller round-shaped particles in clusters that were discernible in the matrix with varying sizes ranging from 0.3 µm to 68 µm. The smaller particles were zirconium adhering to larger calcium-based particles. Several studies support the idea that using smaller sealer particles can produce a thinner film thickness and increase dentinal tubule penetration [16,23,24,25]. However, it was claimed that bioceramic-based root canal sealers produced more sealer tags because of their smaller particle size, increased fluidity, and hydrophilicity upon contact with the root dentinal walls [16]. The use of root canal sealers that provide regular surfaces should, as a result, be anticipated to yield superior results in terms of cell adhesion. Surface regularity should not be examined in isolation because other parameters, such as chemical composition, can also influence cell adhesion and biocompatibility [6]. The penetrating ability of root canal sealers is determined by factors such as particle size of the components and physicochemical properties of the root canal sealer [26]. It is ideal for the root canal sealers to provide a regular surface to penetrate into the dentine tubules because doing so will enhance the sealing efficiency and the stability of the material; moreover, it may entomb any bacteria that may be present, and the chemical constituents of the sealers may exhibit an antibacterial effect [27]. According to a few authors, particle size is crucial because it influences many material characteristics [28,29]. A study by Akcay et al., 2016 [30], supports the idea that smaller particles may enter dentinal tubules more effectively. In this study, we assessed the physicochemical characteristics of five root canal sealers, namely BioRoot, IRoot, CeraSeal, OneFill, and DiaRoot. The CeraSeal sealer exhibited rounder particles that were smaller in size than those exhibited by other sealers. This can be attributed to the very small particle diameter sizes that the CeraSeal sealer possessed, which ranged from 0.3 µm to 68 µm. Because of their greater surface-to-volume ratio, smaller particles hydrate more quickly than larger ones, making them well suited for dental material and potentially enhancing the clinical performance of root canal fillings [31].
4.2. Characterization of Root Canal Sealers through EDX
The results of the EDX microanalysis from our study showed that all the tested sealer materials contained oxygen, calcium, and carbon. This finding is consistent with findings from earlier studies [6,16,32,33,34,35], which reported that calcium plays an active role in the periapical repair process. Calcium ions are responsible for the formation of calcite crystals, which play a direct role in the construction of a mineralized barrier [32]. According to Seux et al., 1991 [34], fibronectin has an affinity for calcite crystals, which in turn promotes cell adhesion and differentiation, which ultimately leads to the accumulation of hard tissue. Therefore, materials with high calcium content may result in better filling. On the other hand, the capacity of the sealer to induce periapical repair should be expected to be lower if it contains significantly lower levels of calcium. To induce periapical repair and the formation of a mineralized barrier in the root apex, calcium hydroxide is often used prior to root canal filling. Its higher calcium and hydroxyl ion content and mechanical action as a matrix may protect against overfilling and justify its use [6]. When in contact with simulated body fluids such as Hank’s balanced salt solution, calcium silicate cement has been shown in previous research to be capable of producing a surface layer of apatite [36,37]. This apatite layer might be able to help improve the biological activity at the bone–periapical level by fostering the formation of a barrier and inducing the activation and differentiation of the apical cells [38]. In addition to this, the apatite deposits might fill the porosity and any gaps that are still present, which would result in an additional layer of external sealing [39]. According to the results of our study, none of the sealer materials tested contained bismuth or other heavy metals such as lead, chromium, cobalt, copper, zinc, or manganese. This finding is in line with a study by Lin et al., 2022 [16], which found that none of the sealers tested contained any bismuth or other heavy metals such as lead, chromium, cobalt, copper, zinc, or manganese. Furthermore, OneFill, iRoot, and CeraSeal contained higher contents of zirconium and oxygen. In order to reduce the amount of harmful heavy metals present in calcium silicate-based products, a new radiopacifier called zirconium oxide has been developed and implemented. Zirconium oxide’s adequate radiopacity and lack of interference with the hydration of calcium silicate-based materials has made it a popular choice [31]. When compared to bismuth oxide, zirconium oxide is more biocompatible and does not result in tooth discoloration [29]. Controlled amounts of zirconium oxide are reported to increase radiopacity without affecting the setting of tricalcium silicate cements [40].
4.3. Characterization of Root Canal Sealers through FTIR
FTIR spectroscopy is able to provide information that is reliable for assessing the chemical structures of the endodontic sealers [35]. The FTIR spectrum analysis of root canal sealers has the potential to assist in the identification of some of the causal agents that are responsible for the cytotoxicity of the sealers. According to the results of the FTIR analysis, the BioRoot sealer exhibited prominent absorption bands at 666 cm−1 and 709 cm−1. The presence of these bands indicated the existence of C-Cl stretching, which can be attributed to the presence of calcium chloride in the sealer material itself. Similar findings were observed in a study by Lin, Chan [24]. Furthermore, C-H stretching vibration can be attributed to the emergence of new absorption bands at 1431 cm−1 and 1483 cm−1 in BioRoot and DiaRoot sealers, indicating the synthesis of carbonated apatite similar to apatite found in bone and dental hard tissues. The existence of polycarboxylate in BioRoot was demonstrated by a band at 1740 cm−1, indicating the stretching of C=O carboxylic groups due to the presence of povidone, as stated by the manufacturer. There is a possibility that the C=O shows strong bonding between the water in the liquid of BioRoot and DiaRoot sealers, which could perhaps explain why there was a drop in the strength of the hydroxyl peak in both the sealers.
Furthermore, the C-F bending was depicted by bands at 1260 cm−1 and 1352 cm−1, which is consistent with the results of the EDX microanalysis, showing that DiaRoot is composed of fluoride. Meanwhile, the peaks at 1098 cm−1 in DiaRoot, 1301 cm−1 in OneFill, and 1091 cm−1 in CeraSeal revealed C-O stretching, which may be caused by the particles mineralizing in the environment with water and carbon dioxide [41]. Moreover, the potential formation of calcium hydroxide can account for the existence of strong O-H bands in all sealer groups in the range of 3000 cm−1 to 4000 cm−1. It is plausible to predict that the sealer will interact with water molecules to create calcium silicate hydrate, which will solidify and produce Ca(OH)2 [42]. The O-H bending seen in all bioceramic-based sealer groups is likewise projected to gradually diminish over time once the sealers are fully set [43]. Nonetheless, the O-H bending intensity varied between all sealer groups, which could be because the specimens’ surfaces were examined during testing and not the materials’ bulk [44].
5. Limitations
Five commercially available root canal sealers were characterized physiochemically in this laboratory-based study. For pertinent responses to the queries we posed, an in vivo environment should be provided. Future research may build on our work to create an ex vivo root canal model to evaluate the physical, chemical, and antibacterial characteristics of sealants. Our capacity to make clinical conclusions would significantly increase with the use of an ex vivo model.
6. Conclusions
SEM, FTIR, and EDX analyses of five commercially available endodontic sealers revealed their physical and chemical characteristics and also assisted in the identification of the materials present in the sealers. All root canal sealers had diverse surface morphologies that contained irregular, micro-sized particles that were uniformly distributed, and they lacked heavy metals. All the experimental sealers comprised mainly calcium, oxygen, and carbon. The manufacturers should provide evidence and knowledge of the characteristics of physical and chemical constituents of root canal sealers for their cytotoxic effects. These findings have clinical implications that can be used to advance the choice of materials by dental professionals for improved patient outcomes in clinical settings. Because of their physicochemical characteristics and biocompatibility, this can also improve the predicted clinical outcomes.
Author Contributions
Conceptualization, A.A.A. and T.Y.N.; Data curation, R.B. and N.T.S.; Formal analysis, G.A.S.; Funding acquisition, A.A.A.; Investigation, M.I.K., G.S.S.L., R.B. and N.T.S.; Methodology, A.A.A., M.I.K., G.S.S.L. and T.Y.N.; Project administration, M.I.K. and T.Y.N.; Resources, A.A.A., A.M.L. and K.P.S.; Supervision, T.Y.N.; Validation, G.A.S.; Visualization, G.S.S.L. and G.A.S.; Writing—original draft, A.A.A., M.I.K., G.S.S.L., R.B. and N.T.S.; Writing—review and editing, A.M.L., K.P.S., G.A.S. and T.Y.N. All authors have read and agreed to the published version of the manuscript.
Funding
The research was funded by a grant from KSA and Global Dental Research Consultants (304/PPSG/6150237.K159).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset used in the current study will be made available upon reasonable request.
Acknowledgments
The authors would like to thanks Nora Binti Aziz and Nik Fakurudin Nik Ali for their technical support in research procedures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Branstetter, J.; Von Fraunhofer, J. The physical properties and sealing action of endodontic sealer cements: A review of the literature. J. Endod. 1982, 8, 312–316. [Google Scholar] [CrossRef]
- Abu Zeid, S.T.; Alamoudi, R.A.; Mokeem Saleh, A.A. Impact of Water Solubility on Chemical Composition and Surface Structure of Two Generations of Bioceramic Root Canal Sealers. Appl. Sci. 2022, 12, 873. [Google Scholar] [CrossRef]
- Furtado, T.C.; de Bem, I.A.; Machado, L.S.; Pereira, J.R.; Só, M.V.R.; da Rosa, R.A. Intratubular penetration of endodontic sealers depends on the fluorophore used for CLSM assessment. Microsc. Res. Tech. 2021, 84, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Estrela, C.; Sousa-Neto, M.D.; Alves, D.R.S.; Alencar, A.H.G.; Santos, T.O.; Pécora, J.D. A preliminary study of the antibacterial potential of cetylpyridinium chloride in root canals infected by E. faecalis. Braz. Dent. J. 2012, 23, 645–653. [Google Scholar] [CrossRef]
- Zhou, H.-m.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.-f.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, F.C.; Alencar, A.H.G.d.; Guedes, O.A.; Veloso, H.H.P.; Santos, T.O.d.; Estrela, C. Chemical elements characterization of root canal sealers using scanning electron microscopy and energy dispersive X-ray analysis. Oral. Health Dent. Manag. 2014, 13, 27–34. [Google Scholar] [PubMed]
- Marciano, M.A.; Guimarães, B.M.; Ordinola-Zapata, R.; Bramante, C.M.; Cavenago, B.C.; Garcia, R.B.; Bernardineli, N.; Andrade, F.B.; Moraes, I.G.; Duarte, M.A. Physical properties and interfacial adaptation of three epoxy resin–based sealers. J. Endod. 2011, 37, 1417–1421. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Ghani, N.R.N.A.; Noorani, T.Y. Physicochemical properties of methacrylate resin, calcium hydroxide, calcium silicate, and silicon-based root canal sealers. J. Stomatol. 2021, 74, 153–159. [Google Scholar]
- Savannah, G. Bioceramic technology–the game changer in endodontics. Endod. Pract. 2009, 13, 3–17. [Google Scholar]
- Duarte, M.; Alves de Aguiar, K.; Zeferino, M.; Vivan, R.; Ordinola-Zapata, R.; Tanomaru-Filho, M.; Weckwerth, P.; Kuga, M.C. Evaluation of the propylene glycol association on some physical and chemical properties of mineral trioxide aggregate. Int. Endod. J. 2012, 45, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.A.; Brave, G.D.; Nasseh, A.A. Bioceramic technology: Closing the endo-restorative circle, part 2. Dent. Today 2010, 29, 98–100. [Google Scholar]
- de Miranda Candeiro, G.T.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- Fonseca, D.A.; Paula, A.B.; Marto, C.M.; Coelho, A.; Paulo, S.; Martinho, J.P.; Carrilho, E.; Ferreira, M.M. Biocompatibility of root canal sealers: A systematic review of in vitro and in vivo studies. Materials 2019, 12, 4113. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Sielker, S.; Hanisch, M.R.; Libricht, V.; Schäfer, E.; Dammaschke, T. Cytotoxic effects of four different root canal sealers on human osteoblasts. PLoS ONE 2018, 13, e0194467. [Google Scholar] [CrossRef]
- Khan, M.T.; Moeen, F.; Safi, S.Z.; Said, F.; Mansoor, A. The Structural, Physical, and In Vitro Biological Performance of Freshly Mixed and Set Endodontic Sealers. Eur. Endod. J. 2021, 6, 98. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.S.S.; Sim, D.H.H.; Luddin, N.; Lai, J.C.H.; Abd Ghani, H.; Noorani, T.Y. Fabrication and characterisation of novel algin incorporated bioactive-glass 58S calcium-silicate-based root canal sealer. J. Dent. Sci. 2022. [Google Scholar] [CrossRef]
- Drukteinis, S.; Peciuliene, V.; Shemesh, H.; Tusas, P.; Bendinskaite, R. Porosity distribution in apically perforated curved root canals filled with two different calcium silicate based materials and techniques: A micro-computed tomography study. Materials 2019, 12, 1729. [Google Scholar] [CrossRef]
- Patri, G.; Agrawal, P.; Anushree, N.; Arora, S.; Kunjappu, J.J.; Shamsuddin, S.V. A scanning electron microscope analysis of sealing potential and marginal adaptation of different root canal sealers to dentin: An in vitro study. J. Contemp. Dent. Pract. 2020, 21, 73–77. [Google Scholar]
- Tyagi, S.; Mishra, P.; Tyagi, P. Evolution of root canal sealers: An insight story. Eur. J. Gen. Dent. 2013, 2, 199–218. [Google Scholar] [CrossRef]
- Vujašković, M.; Teodorović, N. Analysis of sealing ability of root canal sealers using scanning electronic microscopy technique. Srp. Arh. Za Celok. Lek. 2010, 138, 694–698. [Google Scholar] [CrossRef] [PubMed]
- Barbizam, J.V.B.; Trope, M.; Tanomaru-Filho, M.; Teixeira, E.C.N.; Teixeira, F.B. Bond strength of different endodontic sealers to dentin: Push-out test. J. Appl. Oral Sci. 2011, 19, 644–647. [Google Scholar] [CrossRef]
- Balto, H.; Al-Nazhan, S. Attachment of human periodontal ligament fibroblasts to 3 different root-end filling materials: Scanning electron microscope observation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2003, 95, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive glass-based endodontic sealer as a promising root canal filling material without semisolid core materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef]
- Lin, G.S.S.; Chan, D.Z.K.; Leong, J.Z.; Kan, Z.; Xuan, W.M.; Tee, V. Dentinal tubule penetration of bioceramic-based versus epoxy resin-based root canal sealers: A systematic review and meta-analysis. G. Ital. Di Endod. 2021, 36, 1–21. [Google Scholar]
- Baghdadi, I.; AbuTarboush, B.J.; Zaazou, A.; Skienhe, H.; Özcan, M.; Zakhour, M.; Salameh, Z. Investigation of the structure and compressive strength of a bioceramic root canal sealer reinforced with nanomateri-als. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211014747. [Google Scholar]
- Viapiana, R.; Guerreiro-Tanomaru, J.; Tanomaru-Filho, M.; Camilleri, J. Interface of dentine to root canal sealers. J. Dent. 2014, 42, 336–350. [Google Scholar] [CrossRef]
- Heling, I.; Chandler, N.P. The antimicrobial effect within dentinal tubules of four root canal sealers. J. Endod. 1996, 22, 257–259. [Google Scholar] [CrossRef]
- Możyńska, J.; Metlerski, M.; Lipski, M.; Nowicka, A. Tooth discoloration induced by different calcium silicate–based cements: A systematic review of in vitro studies. J. Endod. 2017, 43, 1593–1601. [Google Scholar] [CrossRef]
- Walsh, R.M.; He, J.; Schweitzer, J.; Opperman, L.A.; Woodmansey, K.F. Bioactive endodontic materials for everyday use: A review. Gen. Dent. 2018, 66, 48–51. [Google Scholar] [PubMed]
- Akcay, M.; Arslan, H.; Durmus, N.; Mese, M.; Capar, I.D. Dentinal tubule penetration of AH Plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: A confocal microscopic study. Lasers Surg. Med. 2016, 48, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Reszka, P.; Nowicka, A.; Dura, W.; Marek, E.; Lipski, M. SEM and EDS study of TotalFill BC Sealer and GuttaFlow Bioseal root canal sealers. Dent. Med. Probl. 2019, 56, 167–172. [Google Scholar] [CrossRef]
- Estrela, C.; Sydney, G.B.; Bammann, L.L.; Felippe Junior, O. Mechanism of the action of calcium and hydroxy ions of calcium hydroxide on tissue and bacteria. Braz. Dent. J. 1995, 6, 85–90. [Google Scholar]
- Holland, R.; Pinheiro, C.E.; de Mello, W.; Nery, M.J.; de Souza, V. Histochemical analysis of the dogs’ dental pulp after pulp capping with calcium, barium, and strontium hydroxides. J. Endod. 1982, 8, 444–447. [Google Scholar] [CrossRef]
- Seux, D.; Couble, M.; Hartmann, D.; Gauthier, J.; Magloire, H. Odontoblast-like cytodifferentiation of human dental pulp cells in vitro in the presence of a calcium hydroxide-containing cement. Arch. Oral Biol. 1991, 36, 117–128. [Google Scholar] [CrossRef]
- Abu Zeid, S.; Edrees, H.Y.; Mokeem Saleh, A.A.; Alothmani, O.S. Physicochemical properties of two generations of MTA-based root canal sealers. Materials 2021, 14, 5911. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.; Taddei, P.; Tinti, A.; Prati, C. Apatite-forming ability (bioactivity) of ProRoot MTA. Int. Endod. J. 2010, 43, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Ciapetti, G.; Perut, F.; Taddei, P.; Modena, E.; Rossi, P.L.; Prati, C. Biomimetic calcium-silicate cements aged in simulated body solutions. Osteoblast response and analyses of apatite coating. J. Appl. Biomater. Biomech. 2009, 7, 160–170. [Google Scholar] [PubMed]
- Gandolfi, M.G.; Ciapetti, G.; Taddei, P.; Perut, F.; Tinti, A.; Cardoso, M.V.; Van Meerbeek, B.; Prati, C. Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent. Mater. 2010, 26, 974–992. [Google Scholar] [CrossRef] [PubMed]
- Prati, C.; Siboni, F.; Polimeni, A.; Bossu’, M.; Gandolfi, M.G. Use of calcium-containing endodontic sealers as apical barrier in fluid-contaminated wide-open apices. J. Appl. Biomater. Funct. Mater. 2014, 12, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Uğur Aydin, Z.; Akpinar, K.E.; Hepokur, C.; Erdönmez, D. Assessment of toxicity and oxidative DNA damage of sodium hypochlorite, chitosan and propolis on fibroblast cells. Braz. Oral Res. 2018, 32, 1–8. [Google Scholar] [CrossRef]
- Nabian, N.; Jahanshahi, M.; Rabiee, S.M. Synthesis of nano-bioactive glass–ceramic powders and its in vitro bioactivity study in bovine serum albumin protein. J. Mol. Struct. 2011, 998, 37–41. [Google Scholar] [CrossRef]
- Okamura, T.; Chen, L.; Tsumano, N.; Ikeda, C.; Komasa, S.; Tominaga, K.; Hashimoto, Y. Biocompatibility of a High-Plasticity, Calcium Silicate-Based, Ready-to-Use Material. Materials 2020, 13, 4770. [Google Scholar] [CrossRef] [PubMed]
- Abu Zeid, S.T.; Mokeem Saleh, A.A.; Khafagi, M.G.E.-D.; Abou Neel, E.A. Setting reaction of new bioceramic root canal sealers. Spectrosc. Lett. 2018, 51, 426–430. [Google Scholar] [CrossRef]
- Cañaveral, S.; Morales, D.; Vargas, A.F. Synthesis and characterization of a 58S bioglass modified with manganese by a sol-gel route. Mater. Lett. 2019, 255, 126575. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).