Abstract
IoT-based wearable devices are significantly contributing to personalized and pervasive modern healthcare. Traditional healthcare systems are handicapped by several factors, including shortages of physicians, nurses, healthcare devices, hospital beds, healthcare cost, etc. Conventional therapy is carried out either at a hospital or at home by certified therapists which is not affordable for many in developing nations. In this research work, we present IoT-based monitoring and an evaluation of key parameters and indicators of a wearable device used during the rehabilitation process in stroke patients’ hand therapy which can be operated by the users at home without the need for therapists. Sensors along with a controller board are used for signal acquisition, processing, and monitoring. The efficiency of therapy can be increased through real-time follow-up and feedback from therapists. Three different control methods are proposed and studied: smartphone-based speech, smartphone-based touch, and Internet of Things (IoT)-based dashboard. In addition, four different architectures, including: therapist therapy wearable, dual therapy wearable, user therapy wearable, and multiuser therapy wearable architectures are discussed. A rehabilitation therapy-based case study using the proposed wearable device with multiple volunteers was conducted. Therapists can remotely operate the device and train the users. Users can benefit and save on costs without the need to visit hospitals or therapy centers or hire therapists. Based on the results of the experiments with volunteers, we can confidently say that the proposed IoT-based wearable device can enhance the quality of life and well-being of the users.
1. Introduction
Internet of Things (IoT) has enormous applications in various fields and has become a major wireless/wired communication paradigm in providing data analytics with the huge amounts of data collected over cloud. The COVID-19 pandemic, which is one of the few events in our lifetime to create a profound impact in each of our lives, accelerated the adoption of IoT-based technologies and automation. IoT, coupled with artificial intelligence and machine-learning algorithms, can play a significant role in the healthcare industry [1]. IoT technologies can help build fully automated medical equipment and systems for tracing, tracking, alerting, identification, responding on time, etc., in modern healthcare applications [2]. Telemedicine in healthcare can be improved by incorporating IoT technologies in hospitals and nursing homes [3]. IoT is enhancing telemedicine solutions by combining software and hardware and bringing doctors and patients closer in various ways for the improvement of public health. Access to IoT-enabled healthcare devices can significantly reduce healthcare costs which would benefit millions of people in developing and underdeveloped nations [4].
IoT technologies can be used to network various ‘things’ that can share data and information anywhere in the world [5,6]. The modern healthcare sector is one of the critical areas where the use of IoT can lead to early diagnosis and treatment of various diseases with the added advantage of cost reduction [7]. The COVID-19 pandemic, which led to the loss of millions of lives, has taught us the limitations of the traditional healthcare system in terms of shortages in physicians, nurses, healthcare devices, hospital beds, etc. [1]. Engineers, doctors, healthcare workers, and governments have understood the need to integrate IoT technology in medical equipment.
IoT-based wearable devices can play a huge role in future health data analytics, healthcare devices control and monitoring, and feedback requirements [8]. They can help in reducing healthcare costs and improve the quality of life of people in poor countries and developing nations. Many IoT devices in the healthcare sector are wearables or wearable devices which can be worn on the human body as attachments and accessories [9]. Some are even implants. They also come in a variety of shapes and sizes as smart watches, smart belts, smart eye-wears, smart earbuds, etc. [10]. Even smartphones can be used as a wearable device. The authors in the research paper [11] define five properties of wearable device data collected, including volume, variety, veracity, velocity, and value. There are a lot of technological challenges when building a wearable device [12].
Paralysis is one of the common disorders resulting from stroke and Spinal Cord Injury (SCI). Most stroke survivors have some type of paralysis instantly after stroke. Stroke survival patients can regain their motor function after rigorous therapy and rehabilitation [13]. There are around 84 to 262 stroke patients per 1 million people in populations in rural areas and 334 to 424 stroke patients per 1 million people in populations in urban areas in India and there are similar rates in low- and middle-income countries [14]. In developing countries, many stroke patients cannot afford to visit rehabilitation centers for treatment. The majority of them are from remote areas [15]. Although there are many exoskeleton models, they are too heavy [16] for a stroke patient to help them in picking up objects for their daily usage. Exoskeleton models-based therapy is costly. Many systems do not have feedback to measure the effort required by the patient and to accordingly adjust torque of the system [17]. In addition, many of the conventional exoskeleton models are not smart enough to store any information about the patient’s therapy at a rehabilitation center [16,17], thereby denying the benefits of data analytics to the users and the medical practitioners.
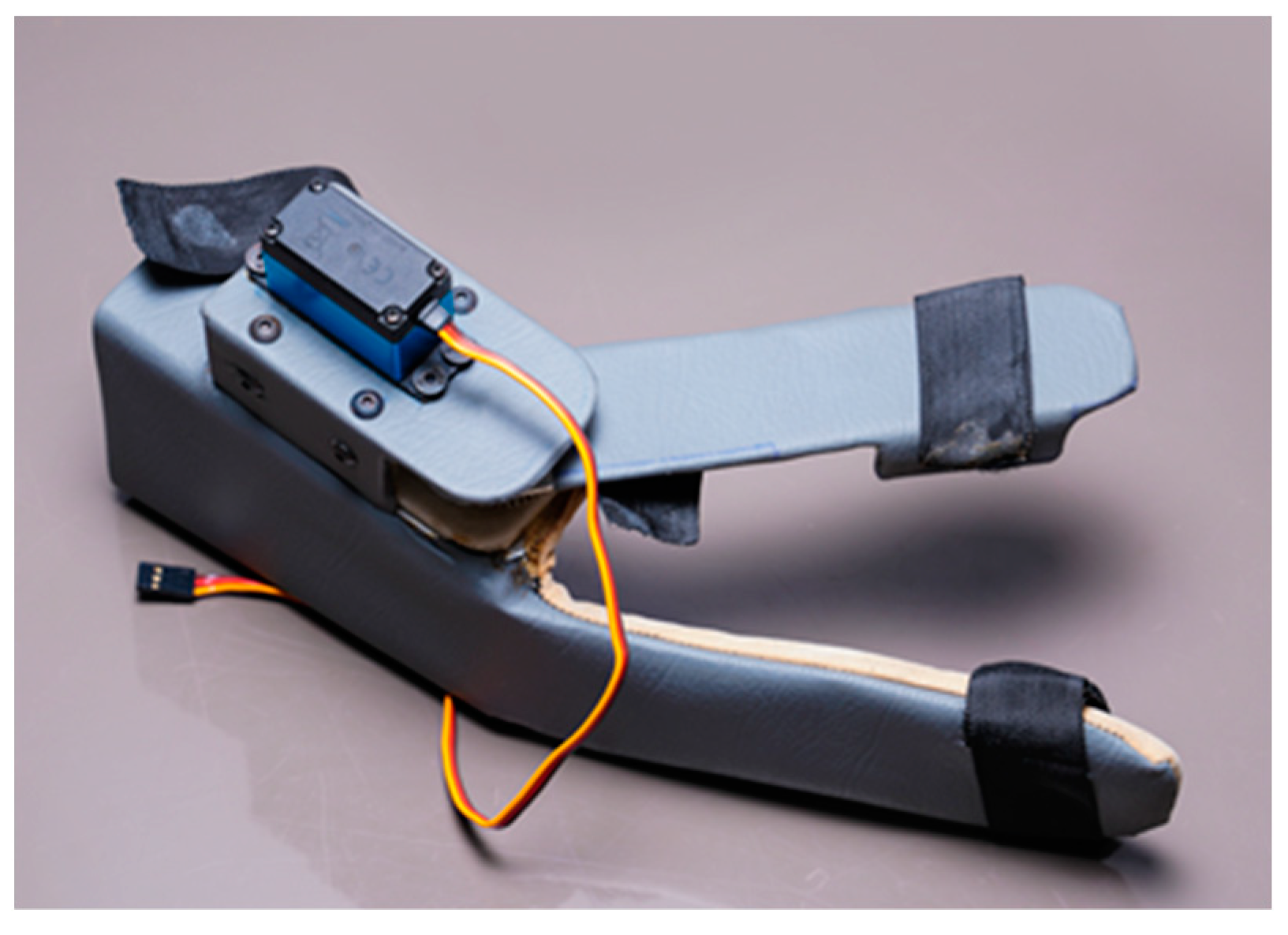
In this research work, a lightweight, easy-to-wear, low-cost, multi-control wearable device with IoT-based monitoring and evaluation of key parameters is proposed for the hand therapy of people affected by stroke. The device is shown in Figure 1. In addition to health data storage and analysis, IoT technology integrated into the device allows for real-time follow-up and feedback from therapists which increases the efficiency of the therapy. The device has the wearable part, the controller has the fixed gateway, sensors, an actuator, and a smartphone has the mobile gateway. It can be controlled by three different methods, including smartphone app-based speech, smartphone app-based touch, and IoT-based dashboard control. The sensor data is transmitted via Wi-Fi-enabled controller. The Bluetooth interface of the smartphone allows for the direct transmission of control commands for operation of the device to the controller which is also Bluetooth-enabled. A timestamp is inserted into the wearable data before the gateway forwards them to the cloud server. The device is portable so that it can be used even while travelling.
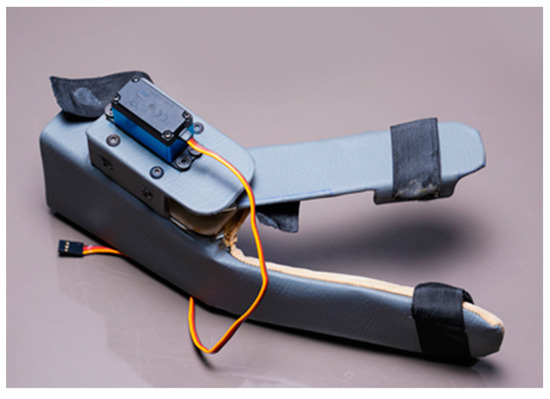
Figure 1.
The proposed wearable device for hand therapy.
The remainder of this paper is organized as follows. Section 2 presents the state-of-the-art wearable healthcare devices and platforms with IoT technology. This is followed by Section 3 which describes the hardware design, different architectures, and multiple control methods. Section 4 presents the technical background, experimental setup, and the case study involving volunteers. Section 5 elaborates on the results and discussion of the results which is followed by conclusion in Section 6.
2. Related Works
Over the past few decades, there has been quite a lot of work being conducted in this field of hand rehabilitation robotics. According to recent surveys by World Health Organization (WHO), more than 15% of people in the world are suffering from physical or mental disabilities, among which a huge number of them are suffering especially from motor impairments due to various stroke and spinal cord injuries. An outline and analyses of the elemental reasons for motor impairments and the necessity of developing rehabilitation devices are discussed in [18]. A hand extension robot orthosis glove is proposed in the research paper [19] for testing with stroke survivors with severe hand impairment. The patients showed significant improvement when performing different functional tasks. A significant increase in extension of the index finger of the patient was also observed after using the device. The authors’ focus is on development and testing of the glove and hence, IoT-based cloud benefits are not considered. The paper [20] presents an EMG (Electromyogram)-based wearable hand robot with user-driven functional movement training for stroke patients. The device aided the patient with performing everyday activities and hand exercises. This device also does not log user exercise data into cloud storage via IoT for future analysis. A neuromuscular-electrical-stimulation-based wearable system for hand function restoration is presented in [21]. The device helped the impaired patients in restoring their grasping function. The device was able to provide stimulation to both intrinsic and extrinsic muscles of the hand, which improved the clinical efficacy and flexibility of the device. The wearable is designed for the forearm with electrodes attached to the palm and is quite complex to wear and use. In addition, there is no provision for live sensor data storage using IoT. In [22], the authors proposed a wearable, spring-powered hand exoskeleton to be used in the therapy of stroke patients. This exoskeleton device helped in significantly improving both range of motion and maximum extension angles of the hand. As this device operation is spring-based and no electronics are involved for sensing or control, it was relatively cheaper. However, the disadvantage is that there was no possibility of storage of user exercise data. An EMG-based, IoT-enabled, smart wearable armband, along with real-time assistance from a robot hand, is used in the therapy of stroke patients [23]. The system also helped in successfully identifying gestures of the user. The cost of the proposed device could be very high. One research paper [24] presents a wearable hand rehabilitation system with two soft gloves, one to be worn on the non-affected hand and the other to be worn on the affected hand. This is a complex system with sophisticated sensors, actuators, and controllers along with machine-learning algorithms to recognize the gestures of the non-affected hand and to facilitate rehabilitation tasks for the affected hand. A five-fingered exoskeleton, which is made by poly-pyrrole strain sensors, allows for 19 Degree of Freedom (DOF) of independent movement for each phalange [25]. The novel strain sensors made the device portable and also made the exoskeleton, a lightweight one. Authors of the research work [26] are successful in designing a well-developed mechanical device for control of speed, position, and torque of actuators for each finger joint. In [27], mimicking individual hand movements of a healthy person to control the hand orthotic device is proposed. This system can provide a grasping sensation to the operator.
A voice-based hand orthotic device is presented in [28]. A wearable, low-cost hand orthotic device with a graphical user interface control is proposed in [29]. As we see in all the research work above, the focus is on making a successful wearable device for hand therapy but integration of the system with IoT cloud is not considered. Our goal is not only to develop a successful, lightweight, easy-to-use, wearable device, but also to integrate the system with IoT and cloud for feedback, control, and future analysis of the stored hand exercise data. Over time, some researchers worked on the security aspect of wearable devices [30,31]. An overview of the solutions for security in the IoT ecosystem is presented by the Internet Engineering Task Force, in which constrained application protocol (COAP) and Datagram Transport Layer Security are tested [32]. The research paper [33] presented an authentication procedure for a distributed healthcare-based IoT system. This system protocol depends on Body Sensor Networks (BSN), which consist of healthcare-oriented smart objects. A novel device, which provides an independent extension to each individual finger while also allowing them to participate in full arm motion of the hand, is proposed in [34]. This device is used to train patients to grasp various objects. A robotic arm control through mimicking of a miniature robotic arm and through Wearable Arm Robot Steering (WARS) is described in [35] and [36], respectively.
3. System Architecture for Hand Therapy
Hand therapy for stroke patients is a historically proven approach in physical medicine and rehabilitation. Monitoring the activities of the users of hand therapy is carried out by visual inspection of the therapist. However, visual inspection is not enough to capture certain important and finer details of hand movement. On the other hand, camera-based monitoring systems are expensive and complex. Wearable devices can overcome both these shortcomings. Wearable devices should be easy to wear, lightweight, and comfortable for users to wear. The device has the wearable part, the controller (master), sensors, an actuator, and a smartphone. The actuator and sensors are connected to the master controller which is used for signal processing and transmission. We are using an integrated encoder sensor that is part of the actuator for position estimation. The rigid structure can be easily attached to the hand and fastened to the arm and fingers using Velcro®, as shown in Figure 2. The control of the wearable device, processing of signals from sensors, and analysis and feedback of the hand exercise results are performed using multiple control methods and using multiple architectures and gateways. In this section, we present the hardware and the three different control methods: (1) Smartphone-based touch, (2) Smartphone-based speech, and (3) Internet of Things (IoT)-based dashboard. This is followed by the introduction of four different architectures: therapist therapy wearable, dual therapy wearable, user therapy wearable, and multiuser therapy wearable.
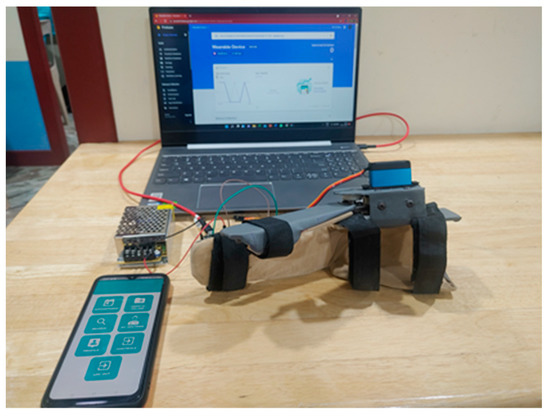
Figure 2.
The wearable device system. Both a mobile gateway (smartphone) and a fixed gateway using NodeMCU, which is a microcontroller unit, can connect to the IoT-based cloud for data storage and analysis.
3.1. Wearable and Master Controller
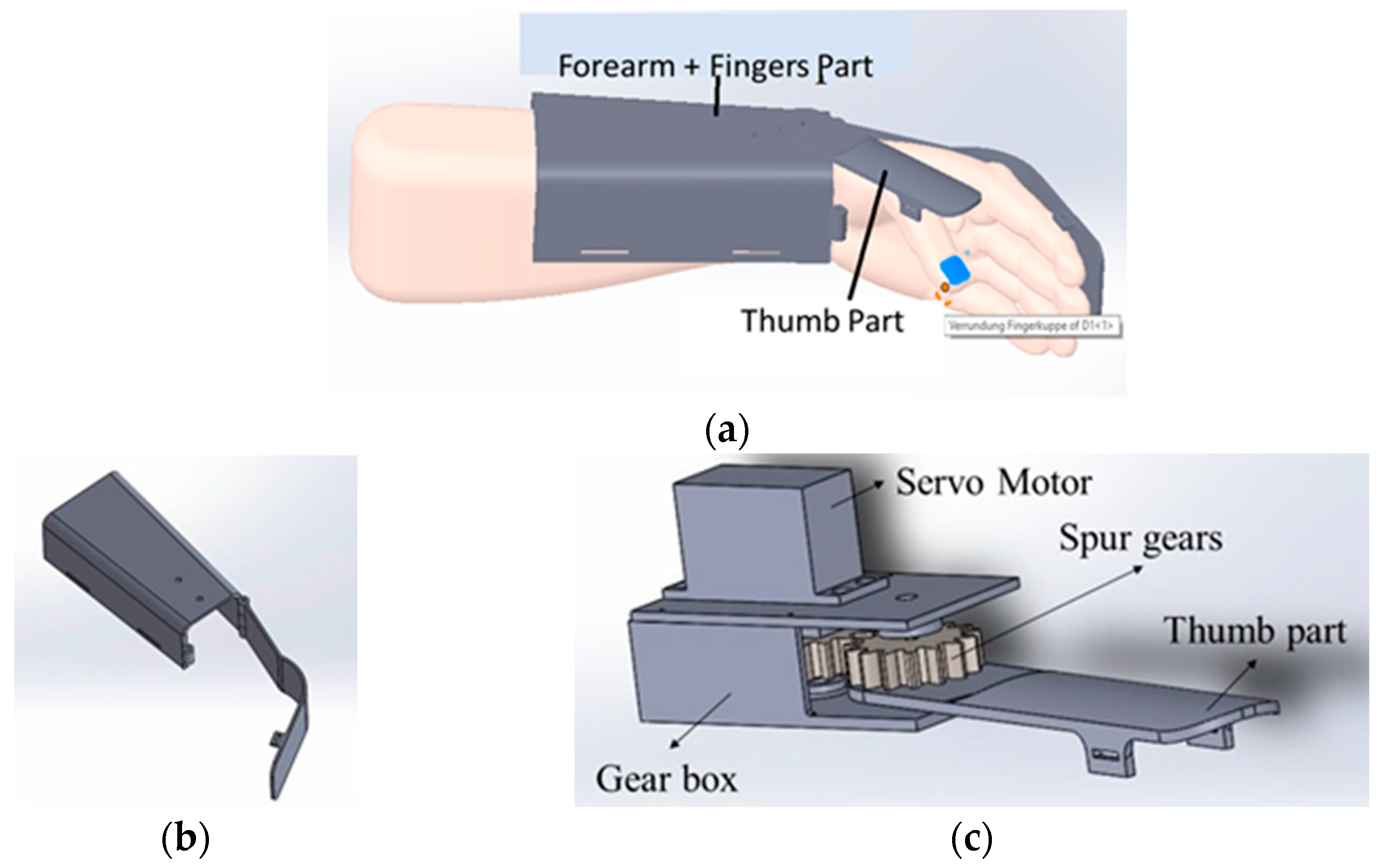
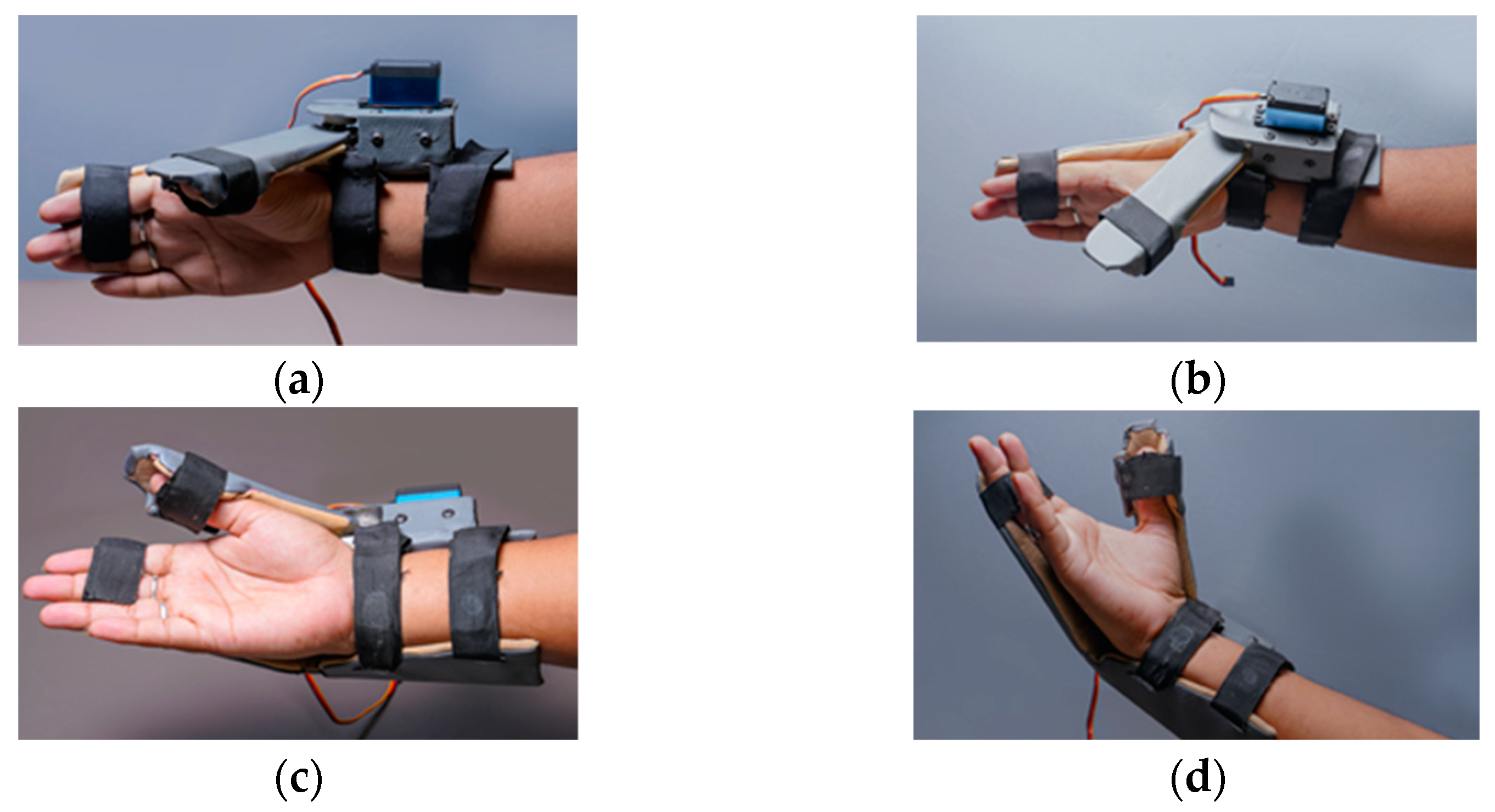
Figure 3a shows the wearable device which covers a part of the forearm and fingers. This device is made up of two parts: the main part as shown in the Figure 3b and the thumb part as shown in the Figure 3c. The thumb part is attached to a servo motor which is used to connect both the parts for opening and closing of the wearable. This helps the users to do hand exercises and also practice grasping and gripping of objects and displacement of objects in their process of rehabilitation. For this purpose, we have introduced the multiple control methods.
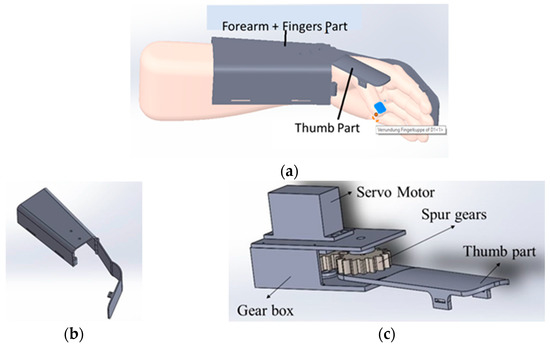
Figure 3.
Wearable Device Model (a) shows the wearable device worn over the hand and part of the forearm. It has two parts; (b) the part covering the forearm and fingers excluding the thumb, (c) the part covering the thumb attached to a servo motor. Parts shown in (a,b) are attached via servo motor.
The proposed wearable can be used for both the right and left hand, which can fit an average human hand. It is fabricated using polypropylene material to make it lightweight at 270 g. For motion transmission from the servo motor, two spur gears in a 1:1 ratio are used so that the torque transfer is the same. Each spur gear has fifteen teeth. The master controller is a NodeMCU, which is used to control the servo motor via the commands from the smartphone app or IoT-based dashboard and get the sensor feed. It is also used to wirelessly connect to the cloud, where the sensor data along with the key parameters, indicators, and control commands can be stored and monitored by therapists. NodeMCU is a low-cost microcontroller unit (MCU) which uses MCU ESP8266 with integrated Bluetooth and Wi-Fi facilities and provides one of the best platforms for cheaper IoT application development. It is similar to an Arduino board but is lesser in size compared with the Arduino. The commands from the user while operating the wearable device are sent to the NodeMCU via the Bluetooth wireless communication interface. The NodeMCU, along with current sensors, makes the electronics part of the wearable.
The wearable uses a current sensor to measure currents drawn by the servo motor when the user operates the device. The power supply is managed via two sources: Switched-mode Power Supply (SMPS) and rechargeable batteries. SMPS is useful when the user is at any facility during the therapy and batteries are handy when the user is travelling and has time for therapy. Apart from sending data, key parameters, indicators, and control commands to the cloud, the wearable system via servo motor can move the thumb of the user to perform open/close actions of the hand. Based on open/close actions, there are three key parameters that are monitored, including the count, width, and speed. The indicators refer to the grasp and grip indicators which are obtained from current sensor data. An ultra-torque (35 kg·cm) metal gear servo that weighs only 64 g is used for the wearable to keep the overall weight of the wearable device as low as possible and provide enough torque for the user to open/close the hand, grasp and grip objects, etc. The device operates in the range of 6–8.4 V and consumes 3.2–3.5 A, stall current at lock. The current sensor ACS70331 can provide a base sensitivity of 800 mV/A at a supply voltage of 3.3 V/5 V.
3.2. Wearable and Multiple Control Methods
The device supports three control methods for the users and the therapist: (1) Smartphone-based speech, (2) Smartphone-based touch, and (3) Internet of Things (IoT)-based dashboard. Considering the ease of use and comfort of the users, multiple control methods are provided. In addition, they are provided considering the financial background and affordability of users in under-developed and developing nations. The smartphone-based speech and touch control methods are cheaper and simpler for users from low-income countries. The IoT-based dashboard control is an option provided to the therapist to remotely control the wearable device. The user can set the speed and number of cycles through the mobile app or the IoT framework as per the instructions from the doctor. The goal of each control method is to open/close the wearable thumb part when attached to the user’s hand, so that the user can perform hand exercises and hold an object to perform useful tasks such as eating, drinking water, writing, etc.
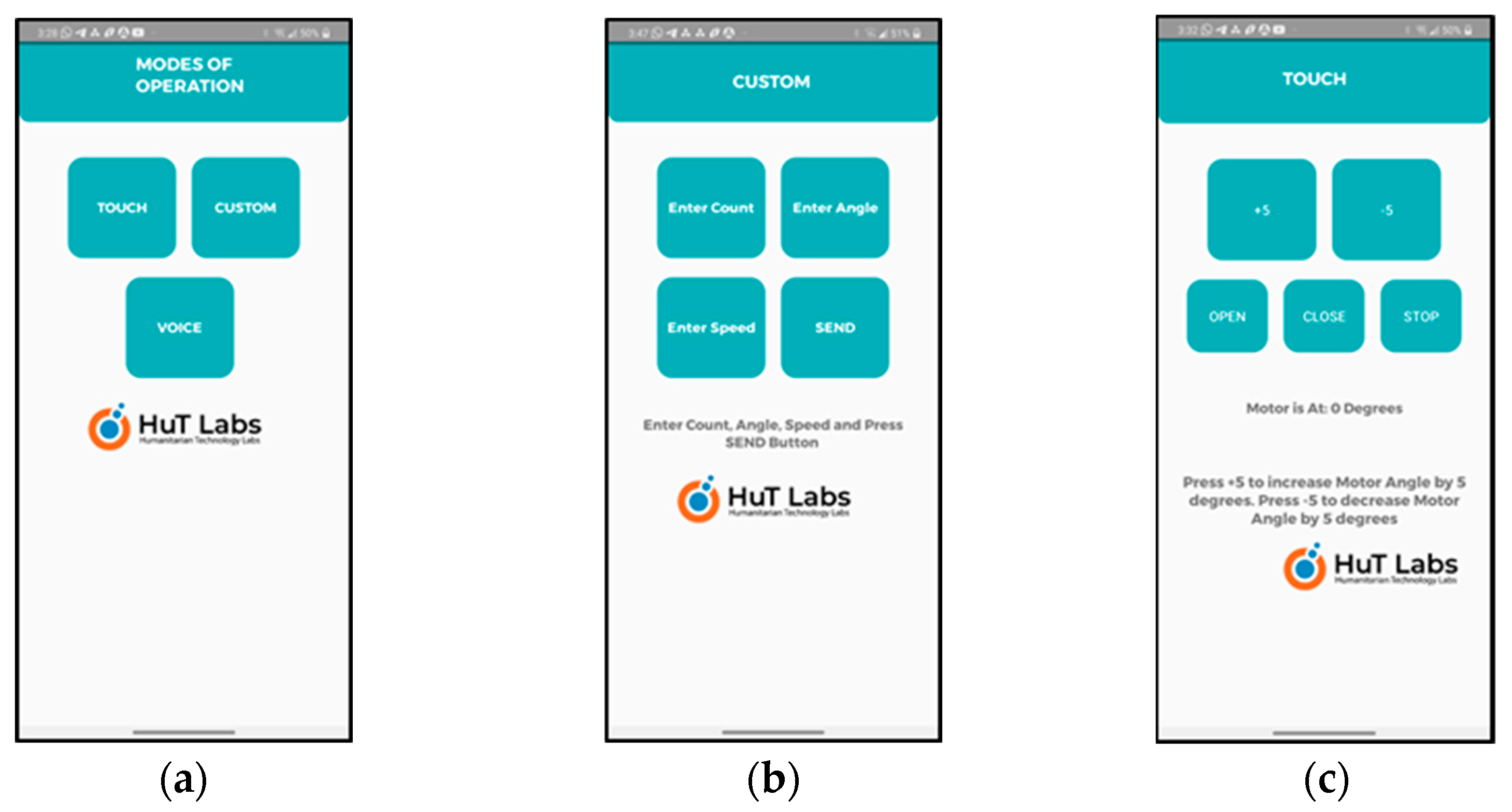
- Smartphone-based Speech Control: The user can use the speech/voice commands and control the wearable via a smartphone app which is exclusively designed and developed for this purpose, as shown in Figure 4. The speech commands are sent from the smartphone using the Bluetooth wireless interface. Only two simple voice commands are used: OPEN and CLOSE. In the beginning of therapy, the therapists can use the voice commands to operate the wearable. The user, along with the therapy, can easily learn how to use the wearable with simple voice commands. Refer to Video S1: HOD_1;
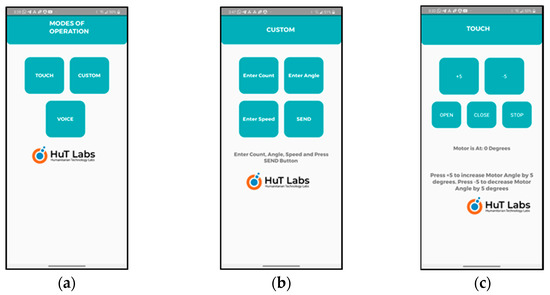 Figure 4. Developed smartphone app. (a) Three modes of operation supported: touch, voice, and custom. The custom mode is part of the touch mode of operation and can be considered as auto mode. (b) Using custom mode, the therapist/user can enter the control parameters: count, angle, and speed. (c) Using touch mode, the therapist/user can specify the angle and use OPEN/CLOSE buttons for operation of the wearable device.
Figure 4. Developed smartphone app. (a) Three modes of operation supported: touch, voice, and custom. The custom mode is part of the touch mode of operation and can be considered as auto mode. (b) Using custom mode, the therapist/user can enter the control parameters: count, angle, and speed. (c) Using touch mode, the therapist/user can specify the angle and use OPEN/CLOSE buttons for operation of the wearable device. - Smartphone-based Touch Control: This is similar to the speech-based app except that it uses a touch-based app of the smartphone. Using the touch-based app, the therapist or the user can choose the angle control parameters and touch the OPEN/CLOSE (control command) button as shown in Figure 3c to operate the wearable. In the initial phase of the therapy, the therapist can use this option to carry out the therapy slowly and steadily and train the users. Once the therapy reaches a stage where the users can operate the wearable themselves, then they can begin the operation with the touch control and gradually move to voice control or custom control which is similar to autonomous control.
- The smartphone app can track the three control parameters including count, angle, and speed when open and close commands are used. The therapist/user has the option to set all these three parameters in the custom control mode. The count tracks the number of times that the wearable opens and closes. This can be compared with auto mode. The angle parameter is set to specify how many degrees (width of open/close) the wearable can open or close. The angle can be set in the range of 0 to 90°. Initially, the therapy starts with a low angle and the angle can increase as the therapy progresses. Similarly, the speed parameter can be set to specify the speed with which the wearable can open/close. The count is user-programmable with a maximum value of 30 and hence, can be changed according to the progress in the therapy of the user. The speed change is in terms of the number of steps to reach the chosen angle as the servo doesn’t have any speed control.
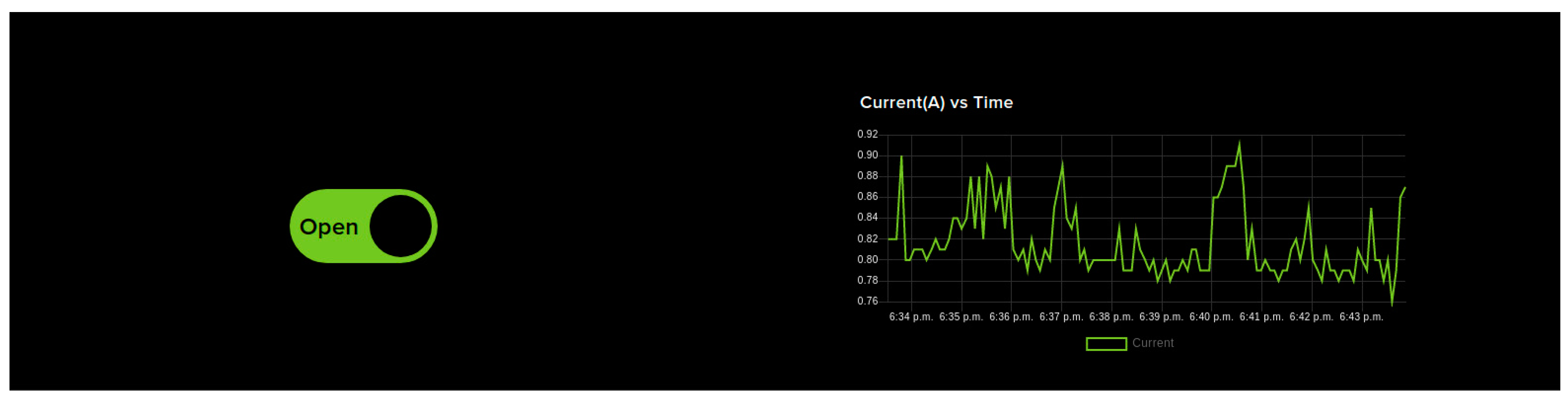
- IoT Dashboard Control: Figure 5 shows the IoT dashboard which can send control signals to NodeMCU via IoT cloud to open and close the wearable device. The dashboard contains OPEN/CLOSE switches used for opening and closing the wearable device when the user is undergoing therapy. This is particularly useful when the therapist is remotely monitoring the user. If the user has any issues in controlling the wearable device and wants some assistance from the therapist, IoT dashboard control can be used. It is a very simple interface and has only two switches which makes it easier for the therapist to use without the need to spend a lengthy amount of time getting trained. Unlike the smartphone-based control, this control mode might see a delay in controlling the device as the command has to reach the NodeMCU via the internet. The live current sensor data is shown as a graph on the right. These sensor feeds are also saved in the cloud for future reference.
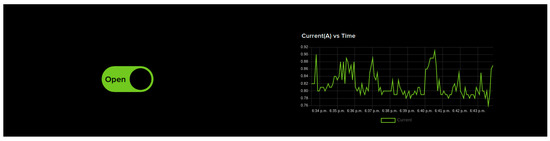 Figure 5. IOT dashboard-based control. OPEN/CLOSE switches are provided to help open and close the wearable device so that the user can perform hand exercises as part of the therapy. The dashboard also displays live sensor feeds from the device.
Figure 5. IOT dashboard-based control. OPEN/CLOSE switches are provided to help open and close the wearable device so that the user can perform hand exercises as part of the therapy. The dashboard also displays live sensor feeds from the device.
3.3. Wearable and Multiple Architectures
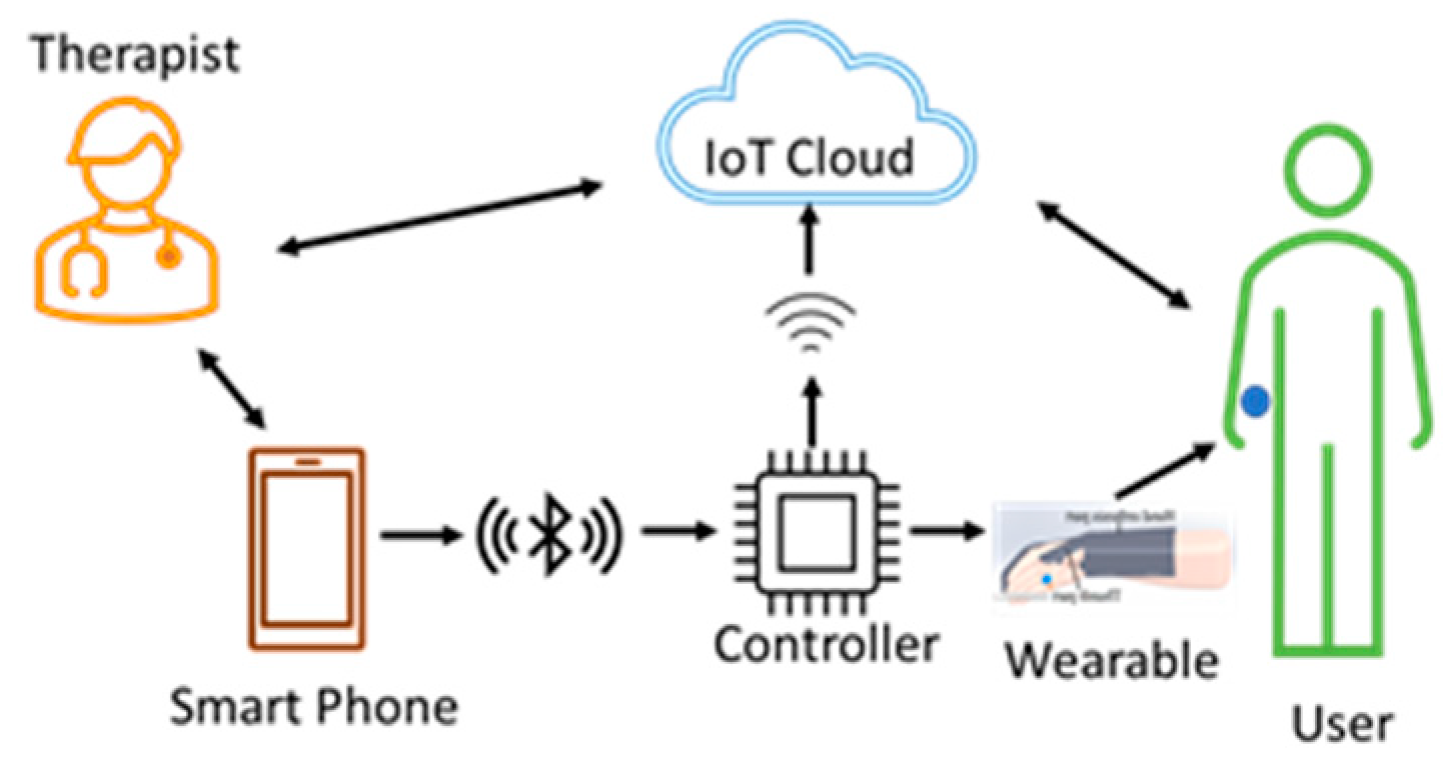
3.3.1. Therapist Therapy Wearable Architecture
Therapy is needed for the stroke patients to regain their hands’ lost motor function and to carry out day-to-day activities without the need for external help [19]. Most of the patients start therapy at the hospitals as part of the rehabilitation. Even after getting discharged from the hospital, the patients need to continue with the therapy at regular intervals. They have to periodically visit the hospitals or therapy centers, or the therapists visit the patient’s homes for the therapy for which the patients have to pay fees.
This architecture, shown in Figure 6, can be used by therapists at the hospitals where therapy begins for the patients. The therapist teaches and trains the users with smartphone-based control methods so that the users can continue with therapy on their own when they move to their residence. Training and allowing the users to continue with the therapy, using a wearable at their residence, reduces the financial burden on users to a great extent. The data from the sensors, the control commands, and the key parameters are stored in the cloud for future reference and analysis.
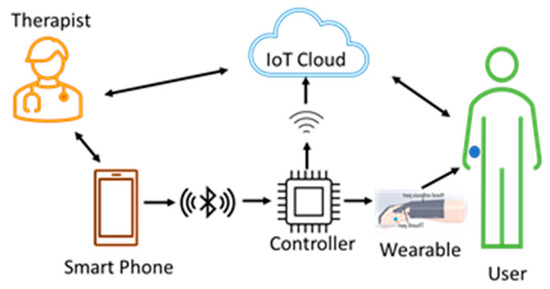
Figure 6.
Therapist therapy wearable architecture. Both the user and the therapist are present in a single room during the therapy. The user wears the device, and the therapist uses smartphone-based voice or touch control methods and starts the therapy. The therapist can observe the user and give oral feedback. The wearable device sends data from the sensors to the cloud for storage and analysis.
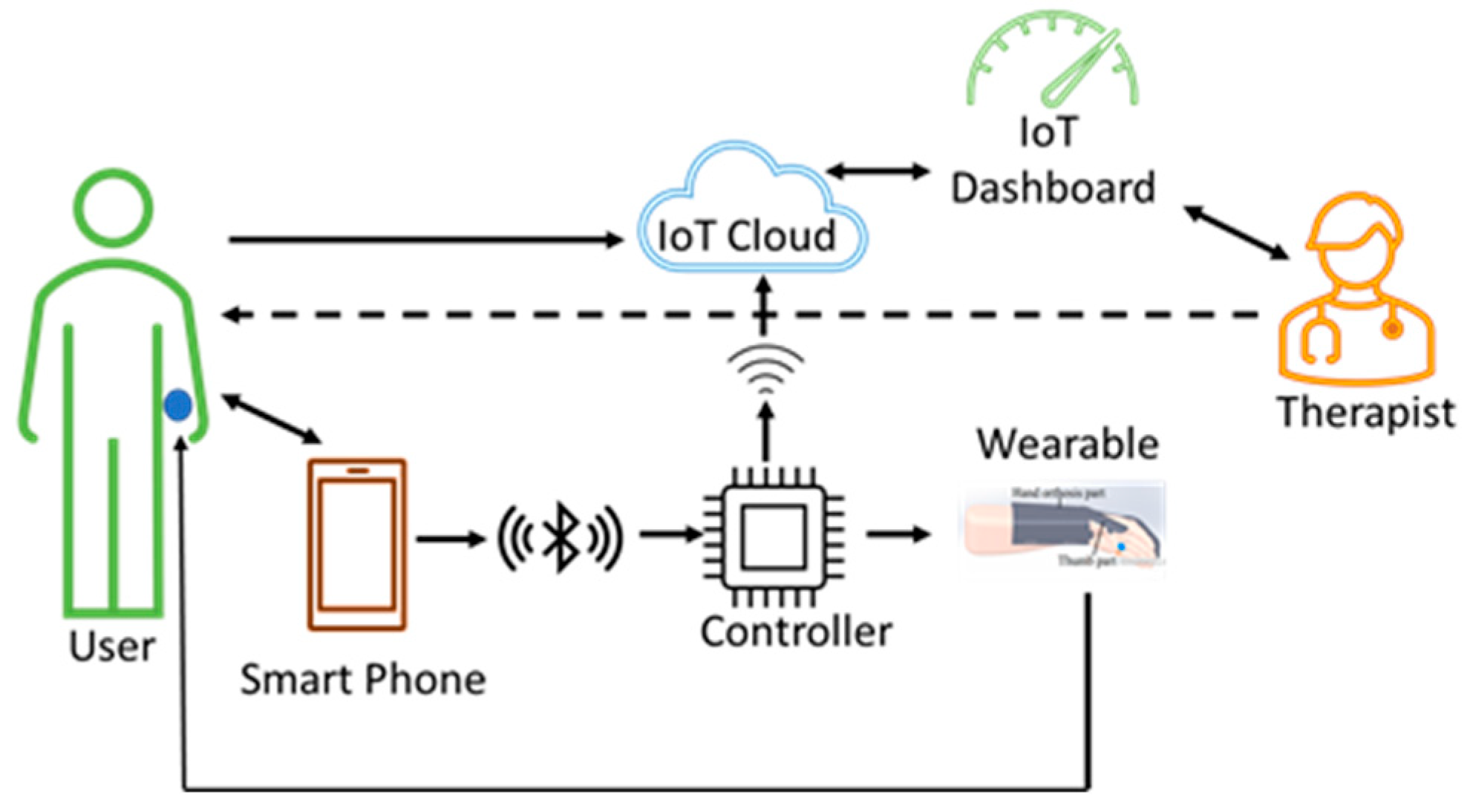
3.3.2. Dual Therapy Wearable Architecture
In this architecture, both the user and the therapist can control the wearable device during the rehabilitation process. The users can be at their residences and continue with the therapy and the therapist can be at a remote place.
This architecture allows the therapist to supervise and operate the wearable from a remote place via cloud using the IoT dashboard in case the user has some doubts. The cloud being part of the system offers some advantages. The therapist can remotely monitor the therapy session and make sure that the users are doing the therapy in the right way and at the right time. The therapist can give feedback from the cloud data directly to the user as shown in Figure 7. Even though there would be some delay when the wearable is operated by the therapist from a remote place, it has the following benefits: (1) the delay might be only a few seconds, (2) saves money and time for the user, and (3) saves time for the therapist. The control commands, signals from sensors, and data collected by the controller, along with the control parameters, are stored in the cloud with access to both users and the therapist for analysis.
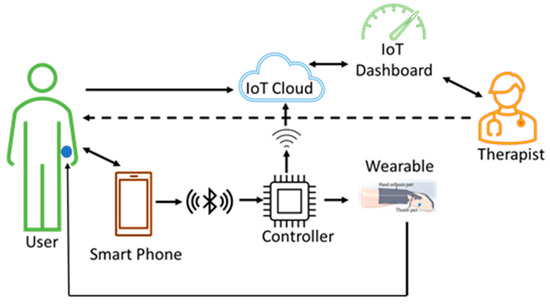
Figure 7.
Dual therapy wearable architecture. The user continues with the therapy at his/her place of residence without the need for therapist supervision. In addition, it allows the therapist to also control the device from a remote place whenever there is a need. The user wearable device sends data from the sensors to the cloud for processing. The therapist can access the cloud data simultaneously and can give feedback directly to the user.
Such a dual therapy, along with the supervision of the therapist, is supported in order to enable users who are discharged from the hospital after initial treatment and therapy to continue with their therapy at home.
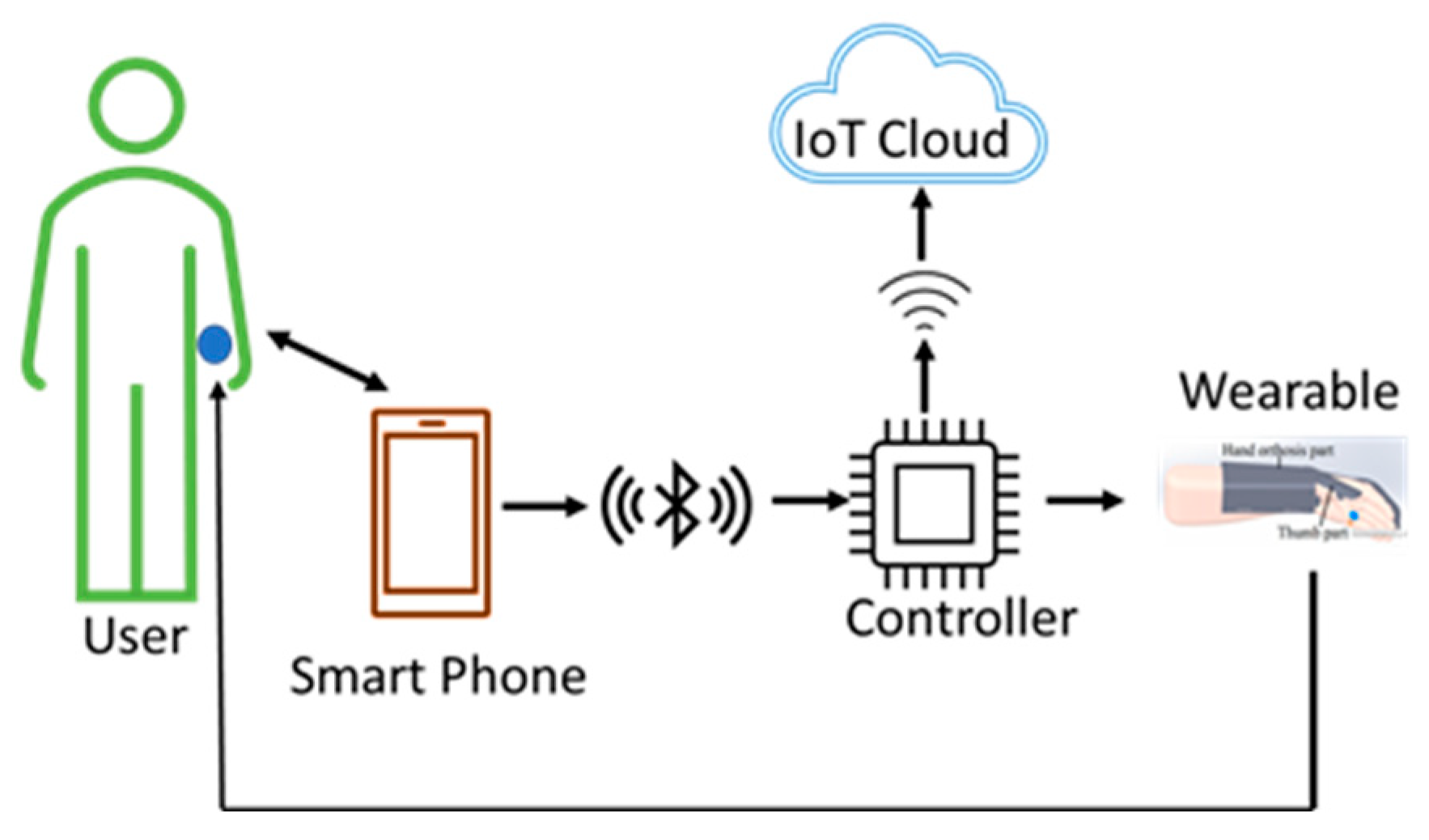
3.3.3. User Therapy Wearable Architecture
This architecture is a standalone architecture where the therapist is neither physically nor remotely present during the rehabilitation process. In this architecture, shown in Figure 8, the wearable device is in total control of the user which is suitable for advanced users. This may be considered for advanced users who feel confident enough to continue the therapy without the need for supervision from a therapist either physically or via cloud. As the user is already trained, as discussed in the previous two architectures, the user should know the correct and appropriate exercises and the steps while using the wearable.
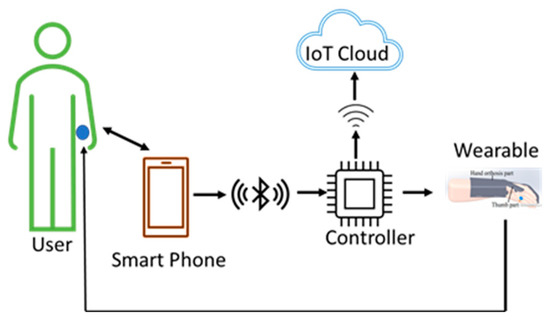
Figure 8.
User therapy wearable architecture. The user can autonomously use the wearable device without the need for direct supervision and feedback from a therapist. The user is a learned and trained user who has learnt the steps and the exercises while using the devices from the training given by the therapist, as discussed in the previous two architectures.
Users are saving the time of therapists who can focus on other users. Simultaneously, the users are also making progress in their rehabilitation processes. This makes the healthcare system much more efficient. As the data is stored in the cloud during therapy, there is a possibility of future analysis by the therapist.
3.3.4. Multiuser Therapy Wearable Architecture
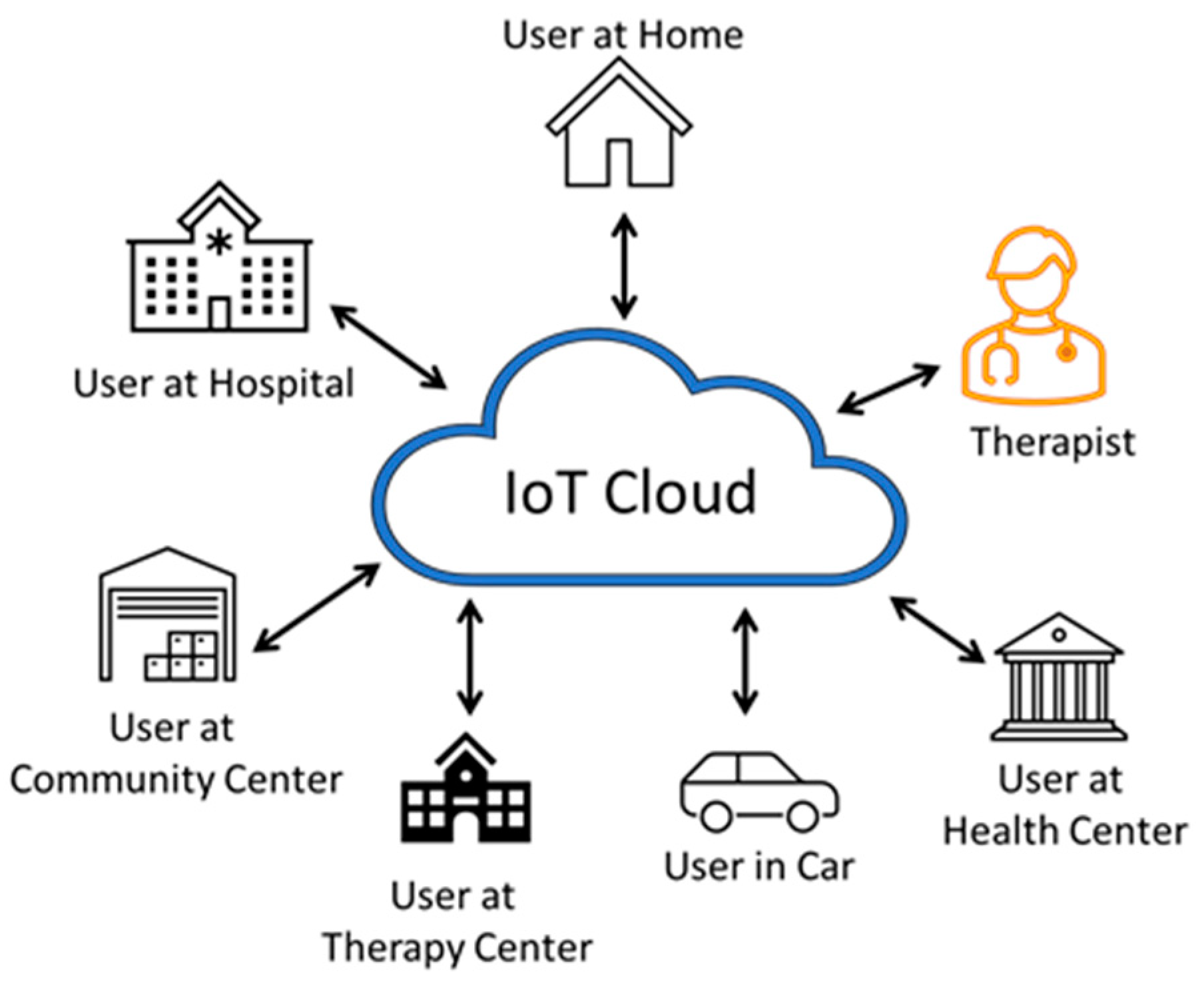
A large number of users with the wearable devices can be part of this multiuser therapy wearable system, as shown in Figure 9. In this architecture, several users can send their exercise data to the cloud in real-time. The users can be at multiple locations such as at home, the hospital, a community center, therapy center, health center, etc. The user can even be travelling, and the therapist can be at a remote place. Users of this architecture are assumed to be advanced users who received enough training and feel confident to use the device on their own. The sensor data and the performance indicators from each of the users are stored in the cloud.
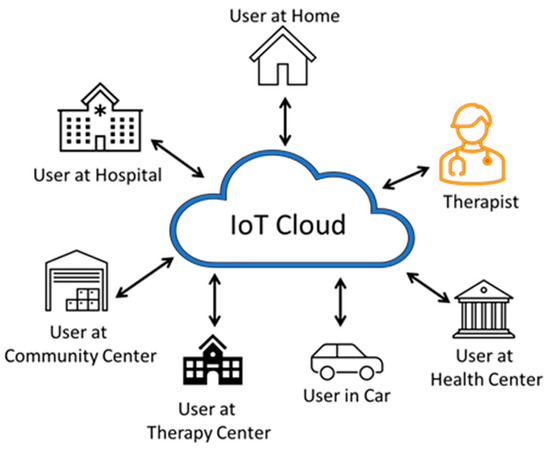
Figure 9.
Multiuser therapy wearable architecture. A large number of users can use the wearable device and autonomously operate without the need for direct supervision and feedback from a therapist. The users can be anywhere and can even be traveling.
3.4. IoT and Complexity of the Proposed Architectures
The data from the wearable device is sent to the cloud using Message Queuing Telemetry Transport (MQTT) protocol. The MQTT protocol and the COAP is used to address the targeted needs of IoT applications [37]. MQTT is much suited for the multiple architectures proposed for our wearable device compared with that of COAP. COAP uses one-to-one protocol whereas MQTT uses many-to-many protocol for the exchange of data between clients and servers. MQTT is a publish-subscribe message protocol that works on top of Transmission Control Protocol (TCP)/Internet Protocol (IP). MQTT needs a very low bandwidth for communication, which helps in controlling the wearable even when the internet speed is slow.
The complexity of the proposed architectures varies depending on the single user or multiuser system. As real time feedback is expected in the therapist therapy and user therapy architectures, the complexity of these systems should be as low as possible. In the case of battery-operated wearables, the power consumption and energy requirements of the system should be optimal. This can be achieved by avoiding computationally complex operations by measuring only the required parameters.
4. Technological Background and Experimental Setup
As mentioned earlier, the motivation is to provide a lightweight, easy-to-wear, low-cost, multi-control wearable device to improve the quality of life, well-being, and health of people affected by stroke with the help of current innovations in technology. We are developing the wearable device based on multiple architectures that include IoT, cloud, data analysis, Wi-Fi, Bluetooth, and other cutting-edge technologies.
4.1. Technological Background
The research works in this field discuss methods to improve the hand function of stroke patients by usage of the affected hand expressed as Intensity of Hand Use (IHU) and Duration of Hand Use (DHU) in daily tasks based on an accelerometer. IHU is related to hand movement intensity during the therapy and DHU is the total amount of time in which there is activity in one or both hands. It is shown that the IHU and DHU significantly improved hand functions with both uni-manual and bimanual tasks during the rehabilitation process. A Concise Arm and Hand Rehabilitation Approach in Stroke (CARAS), specifically designed for therapists on new treatment techniques for the restoration of arm-hand function and arm-hand skill performance in stroke patients, is presented in the research work [38]. They discuss three programs designed as part of CARAS based on severely, moderately, and mildly impaired hands of users. Program-1 is for the skill performance of the hands of users severely affected by stroke and whose hands became non-functional due to inactivity, spasticity, or stiffness. Program-2 and Program-3 are designed for moderately to mildly affected patients and encompasses high-intensity, task-oriented hand training, including gross motor grip tasks, passive and active fixation tasks, grasp and displace tasks, etc.
Our approach is to integrate IoT technology with the hand therapy of stroke survivors with a simple wearable device with benefits for both therapists and users in terms of time and costs with good performance, particularly in developing and underdeveloped nations. The control parameters introduced earlier handle the intensity and duration of hand use. Intensity of hand use can be associated with the speed and angle control parameters. The count control parameter is used for the duration of hand use. Apart from the therapy related to intensity and duration of hand use, the users are also taught to perform grasping and gripping tasks, including holding various objects and performing actions such as drinking, eating, writing, etc.
4.2. Test Setup
The test setup includes the wearable device and the control circuit including the sensor, a smartphone, Bluetooth connectivity, internet connectivity, and a power source. The wearable has two parts, the hand and the thumb, and has provisions to fasten the thumb part to the thumb and the hand part to the forearm and the fingers. The wearable can be custom designed to use either on the right or left hand. Assistance is required to fasten the wearable to the hand during initial training. When powered up, the user establishes connectivity between the smartphone and the controller via Bluetooth and internet connectivity via the controller and Wi-Fi. We confirmed that a single current sensor is enough to track the amount of force used for grasping and gripping tasks. The control parameter angle of the wearable is achieved by sending pulses for tiny changes in position for each pulse till the actuator reaches the final position. This simultaneously can change the control parameter speed of the wearable device. The count parameter can be updated each time that the actuator reaches the desired position, thereby attaining the desired angle.
5. Testing and Evaluation
5.1. Evaluation of the IoT Architectures
In this section, we discuss the evaluation of the proposed IoT architectures in different conditions. In addition, multiuser therapy wearable architecture is evaluated in terms of latency concerning the number of users, the distance between users, and speed of the vehicle in which the user is travelling (when the user is using the HOD during travel). This project was approved by the Amrita Ethics Committee for Engineering Research Projects (AECERP) on 31 October 2019 with project identification code AU/AM/HUT/2019-03. As a device that is using cloud for data storage and control (IoT framework-based control), latency, data loss, and type of network are the three important parameters considered in this evaluation. All these tests are performed by the four volunteers (healthy subjects, male, age between 18–22). In addition to the network, all the results are also influenced by the hardware that we used for IoT. We used the same hardware for the entire evaluation as discussed in the section System Architecture for Hand Therapy.
The time required and data loss are measured for the following actions:
- T1—Data reception through cloud by therapist
- T2—HOD control by therapist
- T3—HOD control by patient
- T31—HOD control by patient using voice
- T32—HOD control by patient using touch
- T4—Data reception through cloud by patient (mobile application)
The four IoT architectures are represented in the table as:
- A1—Therapist therapy wearable architecture
- A2—Dual therapy wearable architecture
- A3—User therapy wearable architecture
- A4—Multiuser therapy wearable architecture (two users in two different rooms)
In Table 1, the latency for different operations in the proposed IoT architectures are compared. We know that the latency is higher in 3G than 4G in all the cloud-based operations in these architectures. The touch control works better than voice control in terms of latency. In voice control, the conversion time and misinterpretations cause some delay in the control. User therapy wearable architecture has a minimum latency of 1.65 s and 1.38 s to send data to the patient’s mobile app through cloud. The multiuser therapy wearable architecture has high latency to receive the data through cloud. For patients to receive data, it took 2.76 s using 3G and 2.21 s using 4G. Similarly, for the therapist to receive the data from the cloud, it took 2.71 s using 3G and 2.27 using 4G.

Table 1.
Latency comparison in different IoT architectures.
The data loss in the four proposed IoT architectures in various sampling rates are given in Table 2, Table 3, Table 4 and Table 5. The data loss maximum was 1050 samples/s and the minimum was 750 samples/s. The data loss was higher when we were using the 3G network than when we were using the 4G network. In terms of data loss, multiuser therapy wearable architecture gives a similar performance compared with the other three architectures, if two users are considered and were in two different rooms in a house. Compared with other architectures, latency in the multiuser therapy architecture depends on a lot of factors. From Table 6, Table 7 and Table 8, we checked this variation with respect to the number of users, distance between the users, and the speed of the vehicle in which the user was traveling. From Table 6, we found that the number of users (in a house) was not dependent on the latency. The latency we got was less when four users were using the device compared with when it was three. From Table 7, we observed that the latency was proportional to the distance between the users but was not very significant. A few millisecond delay was observed when the distance was increased by 50 m. The latency increased with respect to the speed of the vehicle (as shown in Table 8) in which the user was travelling in both 3G and 4G. A ten-kilometer variation in speed caused a delay of 0.4 s to receive the data from clouds.

Table 2.
Data loss in therapist therapy wearable architecture.

Table 3.
Data loss in dual therapy wearable architecture.

Table 4.
Data loss in user therapy wearable architecture.

Table 5.
Data loss in multiuser therapy wearable architecture.

Table 6.
Latency variation in the multiuser therapy architecture concerning the number of users in a single house.

Table 7.
Latency variation in the multiuser therapy architecture concerning the distance between two users.

Table 8.
Latency variation in the multiuser therapy architecture concerning the speed of the vehicle in which the user was travelling.
5.2. Evaluation of the HOD
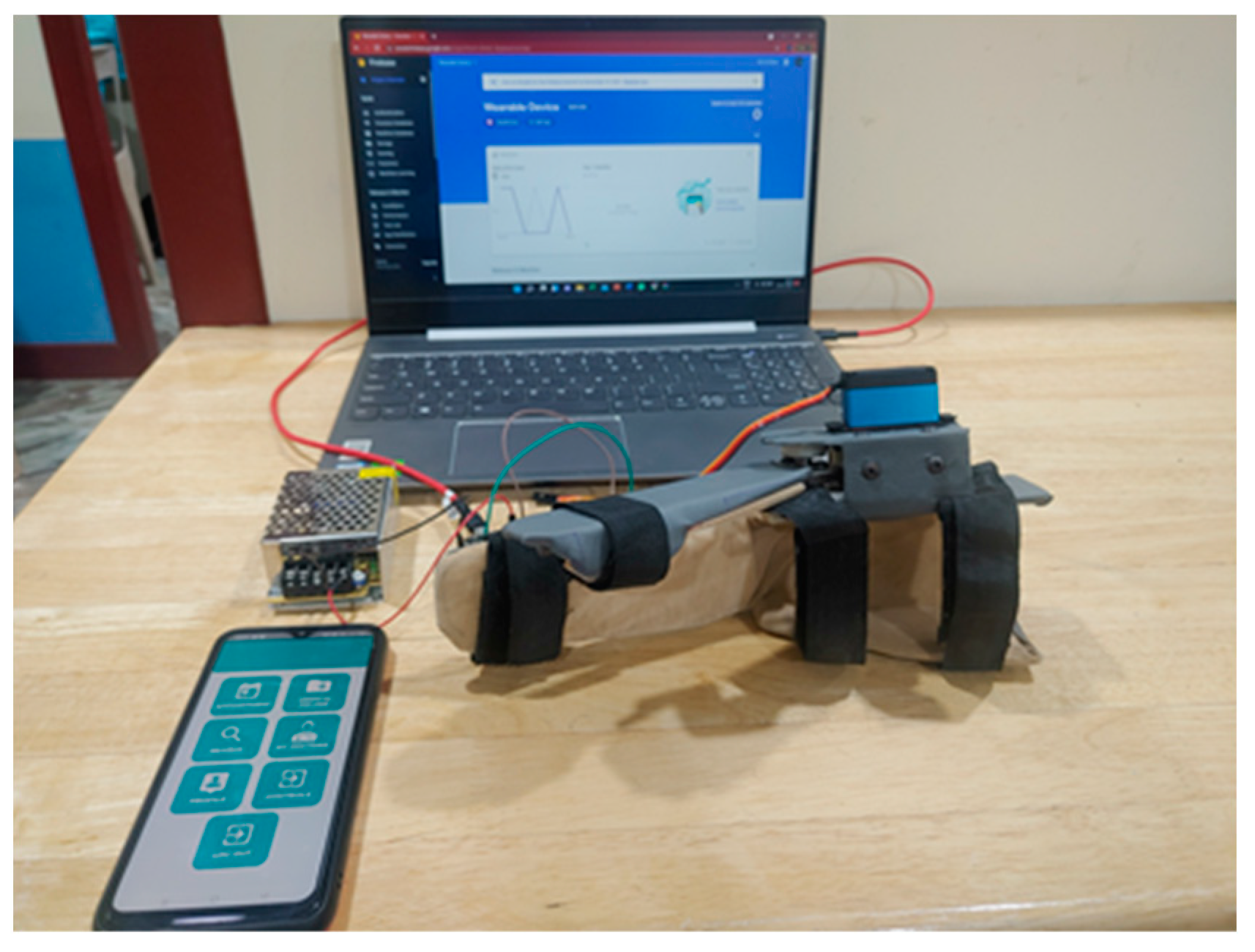
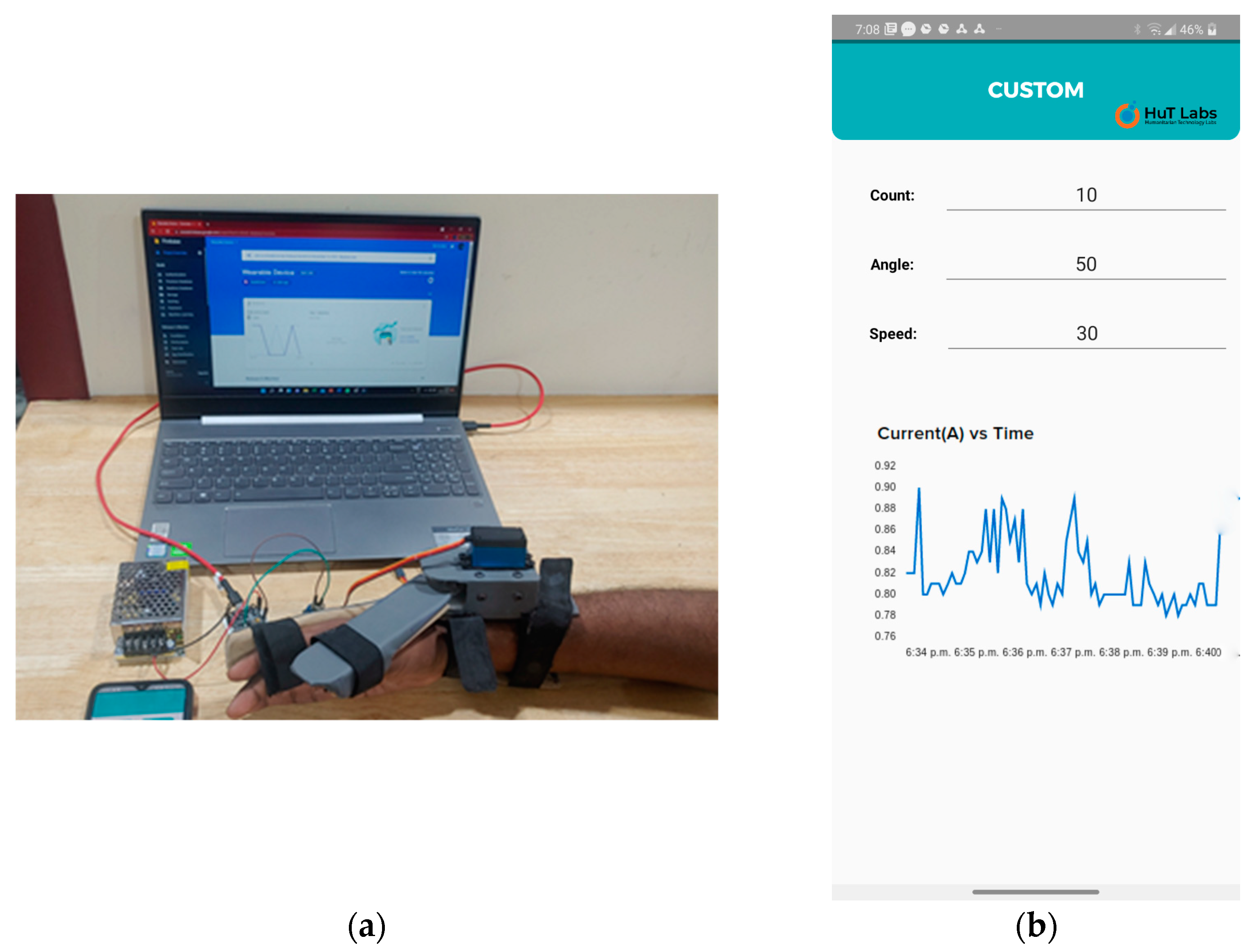
The tests were designed so that the intensity and duration of hand use were included. By using the count, angle, and speed control parameters, the intensity and duration of hand use tests could be conducted. The variation and monitoring of control parameters such as intensity and duration of hand use tests and current measurement tests were carried out. We had chosen four volunteers for these tests. The test setup is shown in Figure 10.
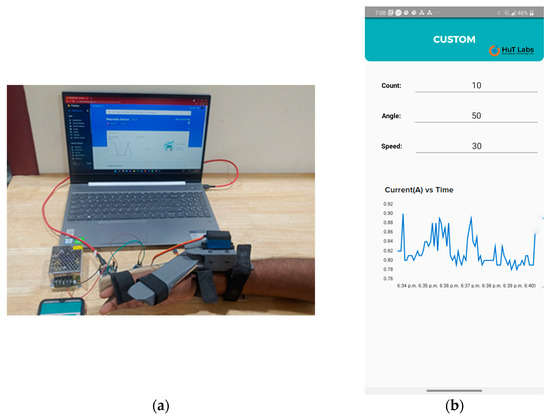
Figure 10.
(a) The wearable device with a user’s hand, along with fixed and mobile gateways. (b) Live feedback from the smartphone while the wearable device was in operation. The control parameters and the current sensor graph are displayed.
Four volunteers took part in the tests. The first test was to measure the time taken by the wearable device to open to the angle specified through the smartphone app, along with measuring speed and count. This was carried out through control parameters’ variation and monitoring. The user/volunteer was asked to choose any one of the five angle values 15, 30, 45, 60, and 80° at a constant count value of 25. For each angle, the user had to repeat the tests at five different speeds 66 deg/s (S1), 42 deg/s (S2), 26 deg/s (S3), 17 deg/s (S4), and 13 deg/s (S5). The maximum speed for the actuator was predefined by the actuator manufacturer, which was 66 deg/s. Count value cannot be increased to a high number as the increased duration of hand use tests might cause stress to the hand. Table 9 gives information about the average time taken by the thumb part of the wearable to reach the desired angle over a complete range of motion with different speeds. As speed decreases, the time taken to reach the desired angle increases, which is as expected. The speed parameter determines the intensity of the hand use, and the count parameter determines the duration of the hand use. Figure 11 shows various positions of the proposed wearable device. Refer to Video S2: HOD_2, Video S3: HOD_3, and Video S4: HOD_4.

Table 9.
Time taken by the HOD to reach the desired angle for different speeds.
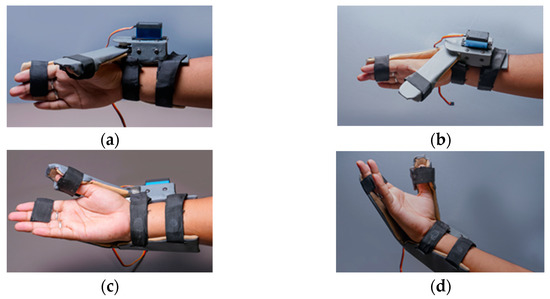
Figure 11.
Various positions of the wearable device (a,c) shows the wearable in closed position and (b,d) shows the open position.
Valuable information can be inferred using basic data analysis from the timing information given in Table 9. For example, intensity and duration of hand use are two important factors in determining hand improvement in the therapy of stroke patients [39]. As the speed of the proposed wearable device is controlled via the speed control parameter, the intensity of hand use during therapy can be achieved. Once the count is set and therapy started, the user can monitor the count parameter via smartphone. The processed data of the control parameters can be uploaded into the cloud and serve as feedback information for the therapist in hand rehabilitation therapy. As the user also has access to the data via cloud, this data can be valuable feedback to users undergoing the therapy.
The grasp, grip, and displace tests were carried out with three objects which included a ball, water bottle, and a spray paint bottle. The measurements of these objects are given in Table 10. We measured two parameters in these tests: the time taken to grasp an object after the command was issued, and the time taken to displace the object to a distance of 10 cm. Each of the users must grasp and grip the object first by issuing voice commands OPEN and CLOSE, and then displace the object 15 cm. The same tests were repeated with touch commands. For accurate results, the tests were carried out for 50 iterations, 25 times with voice command and 25 times with touch command. From Table 10, we can infer that the objects with smaller diameters take more time to grasp and grip when compared with the larger diameter objects. The time taken to displace an object was calculated, including the time to release an object from grip along with the displacement time.

Table 10.
Time taken to grasp, grip, and displace an object.
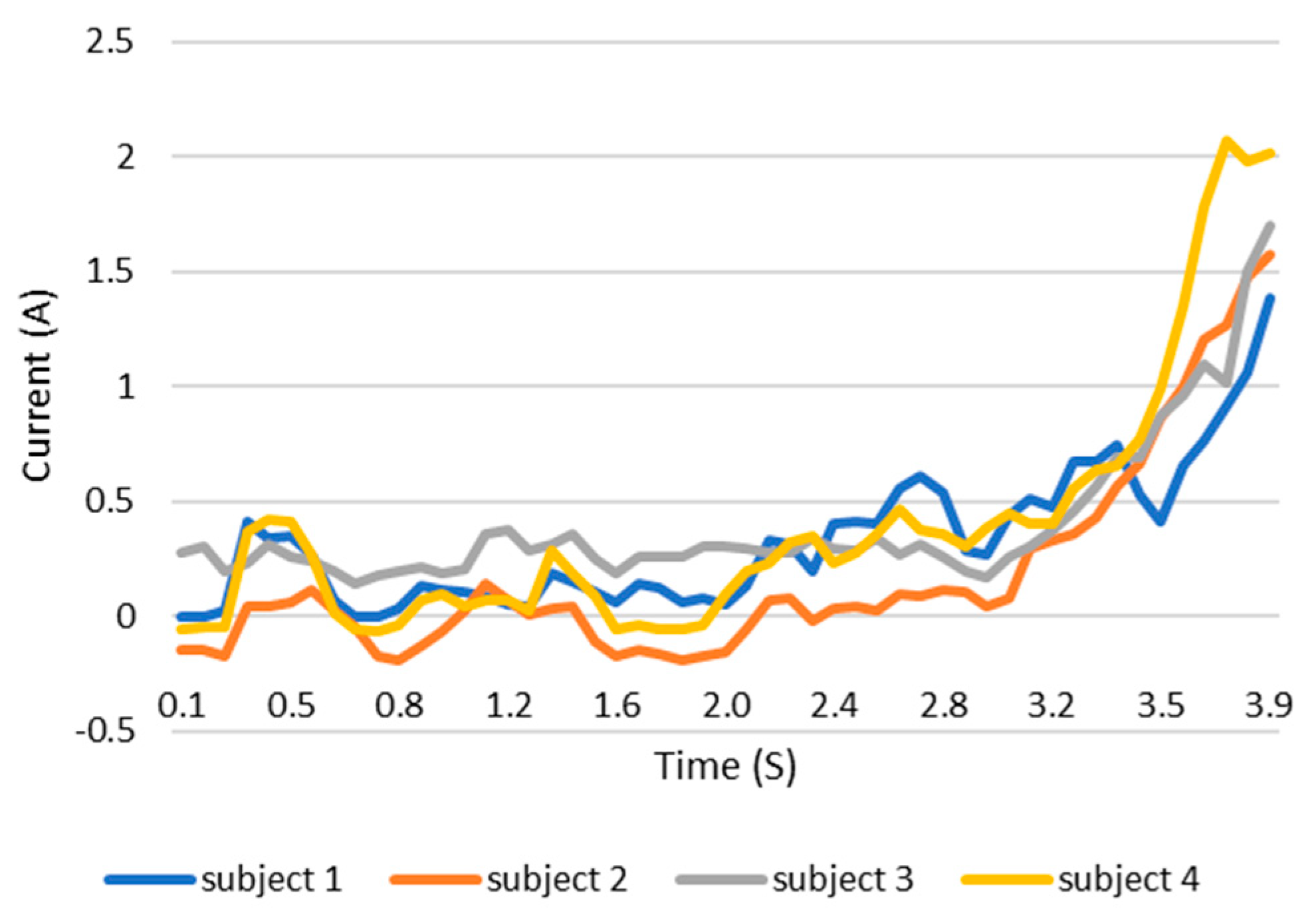
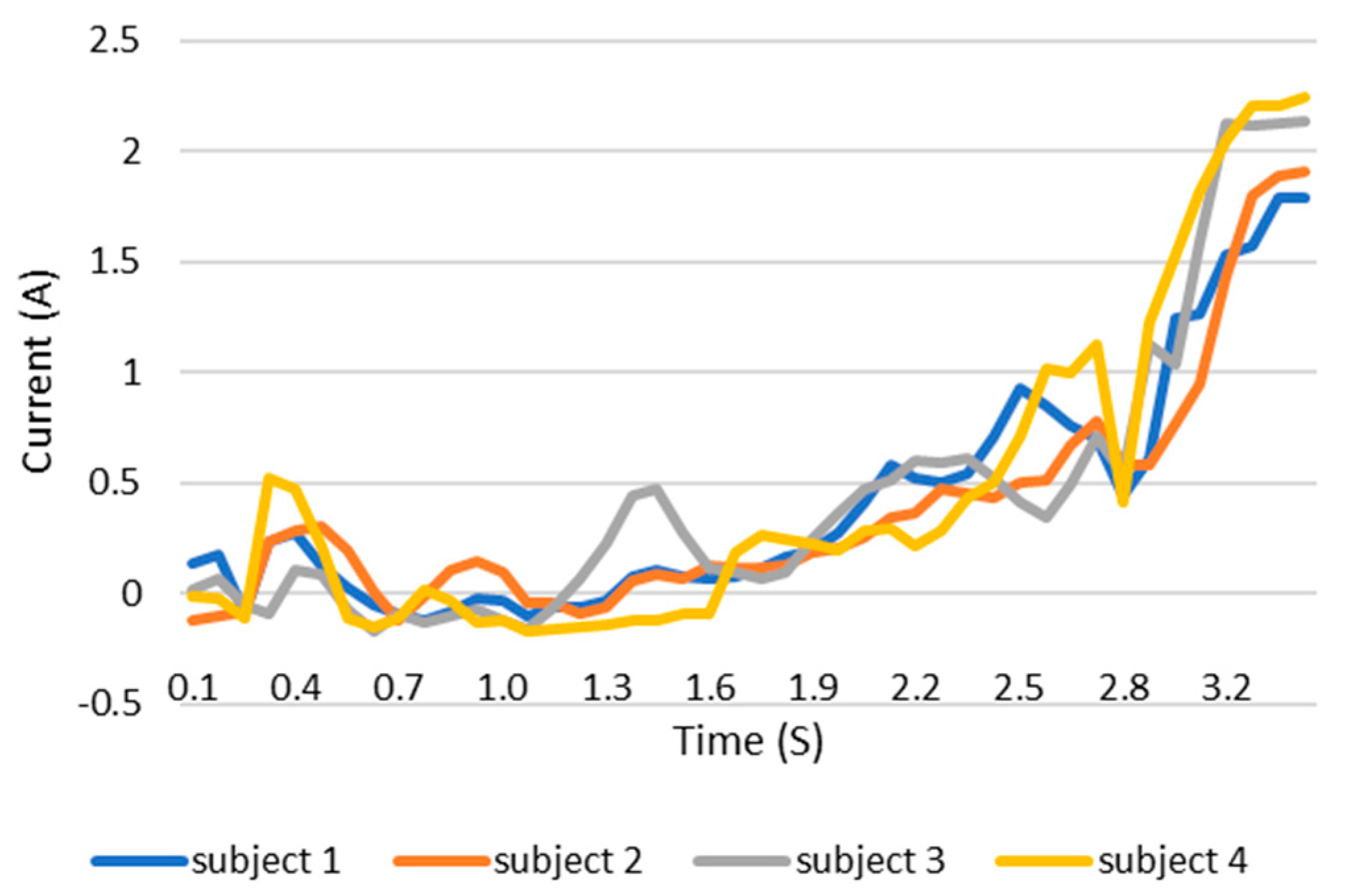
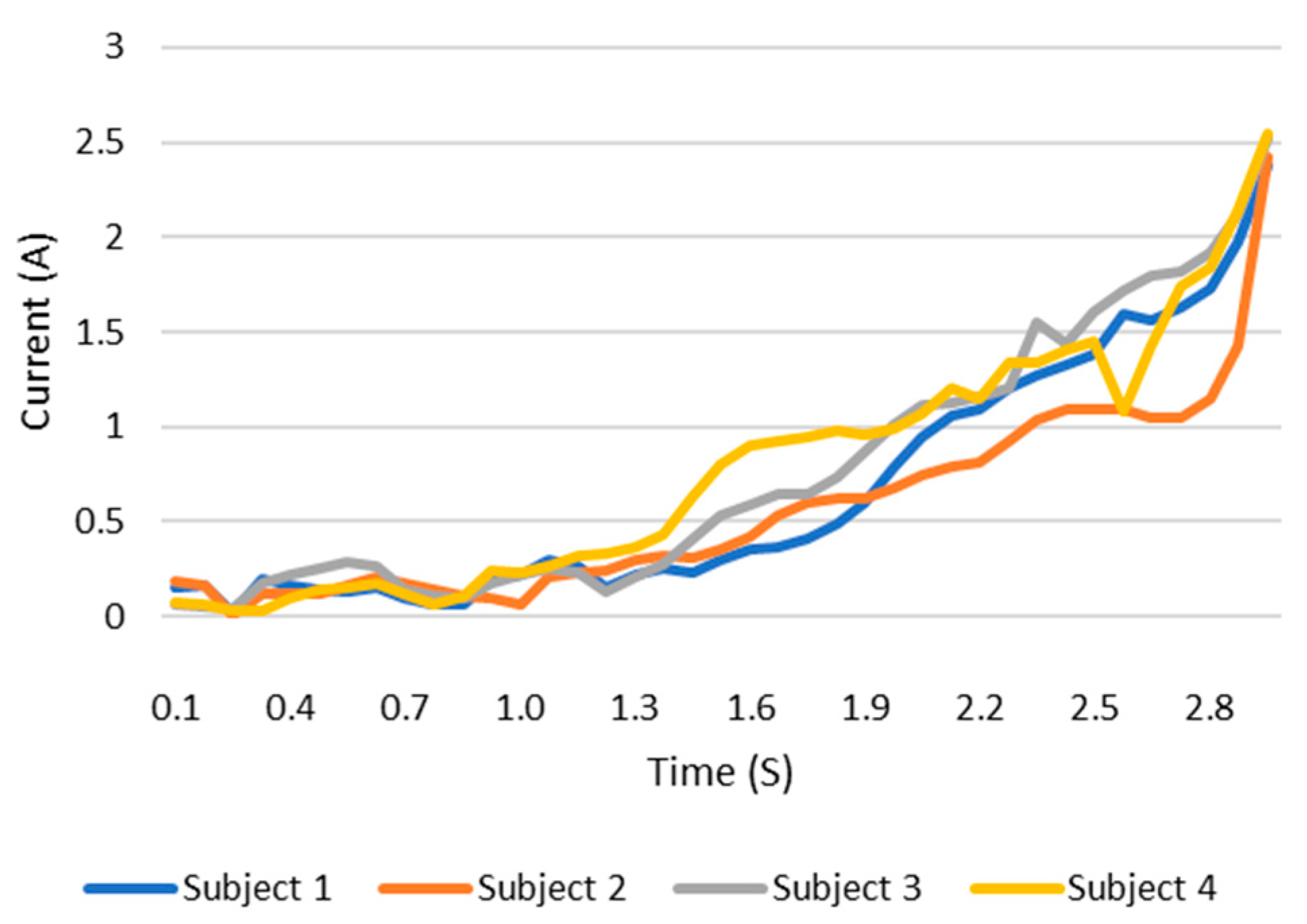
When users grip an object, the load current of the wearable system increases. This increase in current can be monitored and the grip force can be measured as the current can be related to the torque. This current, which is measured using a current sensor, is an indicator of whether the user is holding the object properly or not. The current measurement tests are carried out with the same three objects as in the previous test. The results are shown in the Figure 12, Figure 13 and Figure 14. These figures show the variation in the current of digital servo motor in amperes with respect to the angle of the thumb part, when the users are trying to grasp and grip an object. The sudden overshoot in the graphs helps in determining the presence of an object at a particular angle. We can observe that the overshoot in Figure 14 (for paint bottle) occurs as early as 37° compared with the other two objects’ (the ball and water bottle) current characteristics in Figure 12 and Figure 13. As the paint bottle is heavier than the other two objects, the current overshoot starts earlier. Figure 12, Figure 13 and Figure 14 were plotted by collecting the data for 200 iterations of each user picking each of the three objects. As the current measurement is independent of the control method, i.e., voice and touch, we only used the touch control method for this test.
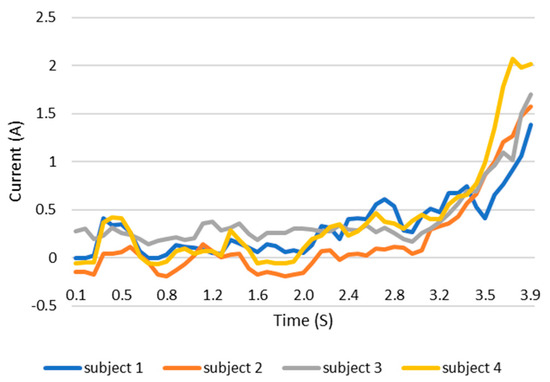
Figure 12.
Current vs. position graph of picking up a ball of weight 125 g.
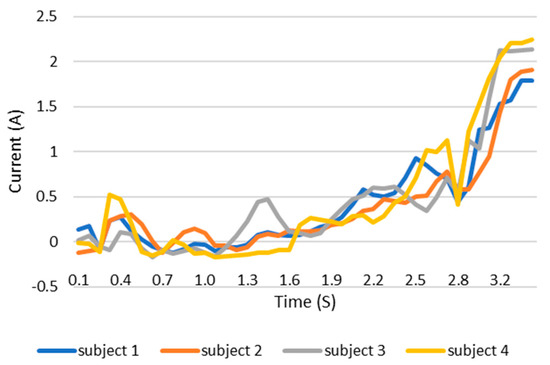
Figure 13.
Current vs. position graph of picking up a water bottle of weight 152 g.
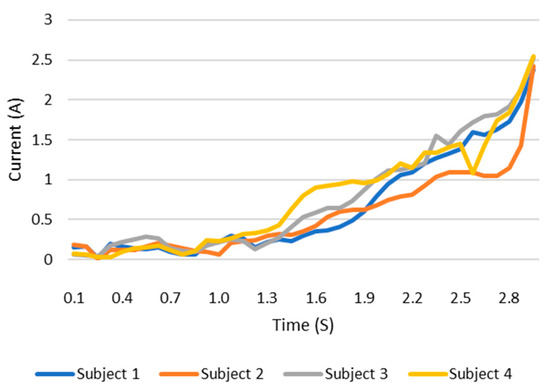
Figure 14.
Current vs. position graph of picking up a spray paint bottle of weight 226 g.
Compared with existing exoskeleton models [40,41,42], the weight of the proposed wearable is comparatively less. Our wearable device weighs about 270 g, which can be used for both hands when compared with other exoskeletons that can only be used for a single hand and weigh around 770 g [40], 430 g [41], and 731 g [42]. Like the previous tests, the data can be uploaded in the cloud for immediate feedback from the therapist. The data can be referenced at any time by both the therapist and the user for deciding the future course of action.
6. Conclusions
In this paper, an IoT-based, lightweight, easy-to-wear, low-cost, multi-control wearable device with integrated sensors for the hand therapy of people affected by stroke is proposed. Multiple architectures of the proposed wearable device are discussed, including therapist therapy wearable, dual therapy wearable, user therapy wearable, and multiuser therapy wearable architectures. Three types of control methods, including smartphone-based speech, smartphone-based touch, and IoT-based dashboards, are introduced. The wearable can be used both by physiotherapists and users; the former to train patients during rehabilitation and the latter to use the device without the need for a physiotherapist.
The therapists need not depend on the presence of a trained technical assistant for operation of the device. The users, after initial training from the therapists, should be able to use the system at their places of residence. This significantly reduces the overall cost of therapy for the users. Wider adoption of wearable hand therapy devices integrated with cloud systems, along with big data analytics, can offer additional advantages to healthcare and improve the quality of life of users. As part of our future work, we plan to apply machine-learning and deep learning algorithms to the cloud data and predict improvements in therapy, along with an advanced IoT-based healthcare platform.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13063976/s1.
Author Contributions
R.K.M. was responsible for conceptualization; i.e., ideas; formulation or evolution of overarching research goals and aims; he was also in charge of supervision, i.e., oversight and leadership responsibility for the research activity planning and execution, including mentorship external to the core team, not only the development or design of methodology; creation of models i.e., methodology, but also provision of study materials, reagents, materials, patients, laboratory samples, animals, instrumentation, computing resources, or other analysis tools i.e., resources. Additionally, R.K.M. handled the preparation, creation, and/or presentation of the published work, specifically writing the initial draft (including substantive translation) i.e., writing—original draft. R.K.M. executed management and coordination responsibility of the research activity planning and execution i.e., project administration and preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary, or revision—including pre- or post-publication stages i.e., writing—review & editing. S.K.M. was responsible for the development or design of methodology; creation of models i.e., methodology. S.K.M. performed application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data i.e., formal analysis but was also conducting management activities to annotate (produce metadata), scrub data, and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use i.e., data curation. S.K.M. conducted the validation i.e., verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs. Additionally, S.K.M. conducted preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary, or revision—including pre- or post-publication stages i.e., writing—review & editing. S.M.M. performed verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs i.e., validation. Additionally, S.M.M. conducted development or design of methodology; creation of models i.e., methodology. S.M.M. performed the writing—review & editing i.e., preparation, creation and/or presentation of the published work by those from the original research group, specifically critical review, commentary, or revision—including pre- or post-publication stages. C.P.K.R. worked on management activities to annotate (produce metadata), scrub data, and maintain research data (including software code, where it is necessary for interpreting the data itself) for initial use and later re-use i.e., data curation and application of statistical, mathematical, computational, or other formal techniques to analyze or synthesize study data i.e., formal analysis. E.V. worked on programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components i.e., software. E.V. performed verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs i.e., validation. P.N.V.K.N. worked on programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components i.e., software. Additionally, P.N.V.K.N. performed verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs i.e., validation. D.C. worked on programming, software development; designing computer programs; implementation of the computer code and supporting algorithms; testing of existing code components i.e., software. D.C. also performed verification, whether as a part of the activity or separate, of the overall replication/reproducibility of results/experiments and other research outputs i.e., validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Humanitarian Technology (HuT) Labs and the Department of Electronics and Communication of Amrita School of Engineering, Amrita Vishwa Vidyapeetham, Amritapuri, Kollam, Kerala, India for providing all the necessary lab facilities and a highly encouraging work environment which is a key factor in the completion of our research work.
Conflicts of Interest
The authors declare no conflict of interest that are relevant to the content of this article. The authors have no relevant financial or non-financial interests to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pathak, N.; Deb, P.K.; Mukherjee, A.; Misra, S. IoT-to-the-Rescue: A Survey of IoT Solutions for COVID-19-Like Pandemics. IEEE Internet Things J. 2021, 8, 13145–13164. [Google Scholar] [CrossRef]
- Poon, C.C.Y.; Lo, B.P.L.; Yuce, M.R.; Alomainy, A.; Hao, Y. Body sensor networks: In the era of big data and beyond. IEEE Rev. Biomed. Eng. 2015, 8, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhou, Z. Optimization of IoT-Based Artificial Intelligence Assisted Telemedicine Health Analysis System. IEEE Access 2021, 9, 85034–85048. [Google Scholar] [CrossRef]
- Wu, T.; Wu, F.; Qiu, C.; Redouté, J.-M.; Yuce, M.R. A Rigid-Flex Wearable Health Monitoring Sensor Patch for IoT-Connected Healthcare Applications. IEEE Internet Things J. 2020, 7, 6932–6945. [Google Scholar] [CrossRef]
- Lee, I.; Lee, K. The Internet of Things (IoT): Applications investments and challenges for enterprises. Bus. Horiz. 2015, 58, 431–440. [Google Scholar] [CrossRef]
- Wu, F.; Wu, T.; Yuce, M. An Internet-of-Things (IoT) network system for connected safety and health monitoring applications. Sensors 2019, 19, 21. [Google Scholar] [CrossRef]
- Haghi, M.; Neubert, S.; Geissler, A.; Fleischer, H.; Stoll, N.; Stoll, R.; Thurow, K. A Flexible and Pervasive IoT-Based Healthcare Platform for Physiological and Environmental Parameters Monitoring. IEEE Internet Things J. 2020, 7, 5628–5647. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Song, H.; Cao, X. Ubiquitous WSN for healthcare: Recent advances and future prospects. IEEE Internet Things J. 2014, 1, 311–318. [Google Scholar] [CrossRef]
- Andreu-Perez, J.; Leff, D.R.; Ip, H.M.; Yang, G.Z. From wearable sensors to smart implants—Toward pervasive and personalized healthcare. IEEE Trans. Biomed. Eng. 2015, 62, 2750–2762. [Google Scholar] [CrossRef]
- Umek, A.; Kos, A. Smart Equipment Design Challenges for Real Time Feedback Support in Sport. Facta Univ. Ser. Mech. Eng. 2018, 16, 389–403. [Google Scholar] [CrossRef]
- Viceconti, M.; Hunter, P.; Hose, R. Big data big knowledge: Big data for personalized healthcare. IEEE J. Biomed. Health Inform. 2015, 19, 1209–1215. [Google Scholar] [CrossRef]
- Williamson, J.; Liu, Q.; Lu, F.; Mohrman, W.; Li, K.; Dick, R.; Shang, L. Data sensing and analysis: Challenges for wearables. In Proceedings of the IEEE 20th Asia and South Pacific Design Automation Conference (ASP-DAC), Chiba, Japan, 12 March 2015; pp. 136–141. [Google Scholar] [CrossRef]
- Disability Statistics Annual Report. 2017. Available online: https://disabilitycompendium.org/sites/default/files/user-uploads/2017_AnnualReport_2017_FINAL.pdf (accessed on 1 January 2019).
- National Library of Medicine. Rehabilitation Needs of Stroke Survivors after Discharge from Hospital in India. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5813710 (accessed on 5 January 2019).
- National Library of Medicine. Disability and Rehabilitation Services in India: Issues and Challenges. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24479007 (accessed on 1 February 2019).
- Brokaw, E.B.; Black, I.; Holley, R.J.; Lum, P.S. Hand Spring Operated Movement Enhancer (HandSOME): A Portable, Passive Hand Exoskeleton for Stroke Rehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 391–399. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/disabilities/world_report/2011/report/en/ (accessed on 1 February 2019).
- Demain, S.; Metcalf, C.D.; Merrett, G.V.; Zheng, D.; Cunningham, S. A narrative review on haptic devices: Relating the physiology and psychophysical properties of the hand to devices for rehabilitation in central nervous system disorders. Disabil. Rehabil. Assist. Technol. 2013, 8, 181–189. [Google Scholar] [CrossRef]
- Yurkewich, A.; Hebert, D.; Wang, R.H.; Mihailidis, A. Hand Extension Robot Orthosis (HERO) Glove: Development and Testing with Stroke Survivors with Severe Hand Impairment. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 916–926. [Google Scholar] [CrossRef]
- Park, S.; Fraser, M.; Weber, L.M.; Meeker, C.; Bishop, L.; Geller, D.; Stein, J.; Ciocarlie, M. User-Driven Functional Movement Training with a Wearable Hand Robot After Stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 2265–2275. [Google Scholar] [CrossRef]
- Crema, A.; Malesevic, N.; Furfaro, I.; Raschella, F.; Pedrocchi, A.; Micera, S. A Wearable Multi-Site System for NMES-Based Hand Function Restoration. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 428–440. [Google Scholar] [CrossRef]
- Casas, R.; Sandison, M.; Chen, T.; Lum, P.S. Clinical Test of a Wearable, High DOF, Spring Powered Hand Exoskeleton (HandSOME II). IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1877–1885. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Deng, J.; Pang, G.; Zhang, H.; Li, J.; Deng, B.; Pang, Z.; Xu, J.; Jiang, M.; Liljeberg, P.; et al. An IoT-Enabled Stroke Rehabilitation System Based on Smart Wearable Armband and Machine Learning. IEEE J. Transl. Eng. Health Med. 2018, 6, 2100510. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gong, L.; Wei, L.; Yeh, S.C.; Da Xu, L.; Zheng, L.; Zou, Z. A Wearable Hand Rehabilitation System with Soft Gloves. IEEE Trans. Ind. Inform. 2021, 17, 943–952. [Google Scholar] [CrossRef]
- Tjahyono, A.P.; Aw, K.C.; Devaraj, H.; Surendra, W.; Haemmerle, E.; Travas-Sejdic, J. A five-fingered hand exoskeleton driven by pneumatic artificial muscles with novel polypyrrole sensors. Ind. Robot Int. J. 2013, 40, 251–260. [Google Scholar] [CrossRef]
- Jones, C.L.; Wang, F.; Morrison, R.; Sarkar, N.; Kamper, D. Design and development of the cable actuated finger exoskeleton for hand rehabilitation following stroke. IEEE/ASME Trans. Mechatron. 2014, 19, 131–140. [Google Scholar] [CrossRef]
- Endo, T.; Tanimura, S.; Kawasaki, H. Development of tool-type devices for a multi-fingered haptic interface robot. IEEE Trans. Robot. 2013, 29, 68–81. [Google Scholar] [CrossRef]
- Megalingam, R.K.; Vijay, E.; Naveen, P.N.; Reddy, C.P.; Chandrika, D. Voice-based Hand Orthotic Device. In Proceedings of the 2019 International Conference on Communication and Signal Processing (ICCSP) Chennai, Melmaruvathur, India, 25 April 2019; pp. 496–500. [Google Scholar] [CrossRef]
- Megalingam, R.K.; Baburaj, A.M.; Mattathil, A.; Hari, A.; Sreekanthan, K.; Koppaka, G.S.A. Low cost hand orthotic device to improve hand function of stroke patients. Technol. Disabil. 2019, 30, 185–197. [Google Scholar] [CrossRef]
- Winderickx, J.; Bellier, P.; Duflot, P.; Mentens, N. Communication and Security Trade-Offs for Battery-Powered Devices: A Case Study on Wearable Medical Sensor Systems. IEEE Access 2021, 9, 67466–67476. [Google Scholar] [CrossRef]
- Zheng, G.; Shankaran, R.; Yang, W.; Valli, C.; Qiao, L.; Orgun, M.A.; Mukhopadhyay, S.C. A Critical Analysis of ECG-Based Key Distribution for Securing Wearable and Implantable Medical Devices. IEEE Sens. J. 2019, 19, 1186–1198. [Google Scholar] [CrossRef]
- Keoh, S.L.; Kumar, S.S.; Tschofenig, H. Securing the Internet of Things: A standardization perspective. IEEE Internet Things J. 2014, 1, 265–275. [Google Scholar] [CrossRef]
- Gope, P.; Hwang, T. BSN-care: A secure IoT-based modern healthcare system using body sensor network. IEEE Sens. J. 2016, 16, 1368–1376. [Google Scholar] [CrossRef]
- Amsüss, S.; Goebel, P.M.; Jiang, N.; Graimann, B.; Paredes, L.; Farina, D. Self-correcting pattern recognition system of surface EMG signals for upper limb prosthesis control. IEEE Trans. Biomed. Eng. 2014, 61, 1167–1176. [Google Scholar] [CrossRef]
- Megalingam, R.K.; Boddupalli, S.; Apuroop, K.G.S. Robotic arm control through mimicking of miniature robotic arm. In Proceedings of the 2017 4th International Conference on Advanced Computing and Communication Systems (ICACCS), Coimbatore, India, 24 August 2017; pp. 1–7. [Google Scholar] [CrossRef]
- Megalingam, R.K.; Bandyopadhyay, S.; Gedala, V.V.; Apuroop, K.G.; Sriteja, G.; Ashwin Kashyap, N.; Juned Rahi, M. Wearable Arm Robot Steering (WARS) Device for Robotic Arm Control Used in Unmanned Robotic Coconut Tree Climber and Harvester. In Applications of Artificial Intelligence Techniques in Engineering; Malik, H., Srivastava, S., Sood, Y., Ahmad, A., Eds.; Advances in Intelligent Systems and Computing; Springer: Singapore, 2019; Volume 698. [Google Scholar] [CrossRef]
- Thangavel, D.; Ma, X.; Valera, A.; Tan, H.-X.; Tan, C.K.-Y. Performance evaluation of MQTT and CoAP via a common middleware. In Proceedings of the IEEE 9th International Conference Intelligent Sensors, Sensor Networks and Information Processing. (ISSNIP), Singapore, 9 June 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Franck, J.A.; Smeets, R.J.E.M.; Seelen, H.A.M. Changes in actual arm-hand use in stroke patients during and after clinical rehabilitation involving a well-defined arm-hand rehabilitation program: A prospective cohort study. PLoS ONE 2019, 14, e0214651. [Google Scholar] [CrossRef]
- Lemmens, R.J.; Timmermans, A.A.; Janssen-Potten, Y.J.; Smeets, R.J.; Seelen, H.A. Valid and reliable instruments for arm-hand assessment at ICF activity level in persons with hemiplegia: A systematic review. BMC Neurol. 2012, 12, 21. [Google Scholar] [CrossRef]
- Arata, J.; Ohmoto, K.; Gassert, R.; Lambercy, O.; Fujimoto, H.; Wada, I. A new hand exoskeleton device for rehabilitation using a three-layered sliding spring mechanism. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 17 October 2013. [Google Scholar] [CrossRef]
- Ma, Z.; Ben-Tzvi, P.; Danoff, J. Hand Rehabilitation Learning System with an Exoskeleton Robotic Glove. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Gonzalez, O.; Jacinto-Villegas, J.; Herrera-Aguilar, I.; Portillo-Rodiguez, O.; Tripicchio, P.; Hernandez-Ramos, M.; Flores-Cuautle, A.; Avizzano, C. Design and Development of a Hand Exoskeleton Robot for Active and Passive Rehabilitation. Int. J. Adv. Robot. Syst. 2016, 13, 66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).