Featured Application
Current study evaluated the possibility of using melting profiles obtained by differential scanning calorimetry (DSC) for cold-pressed hemp seed oil authentication. It was shown that during phase transition, the heating rate had a significant influence on the thermal behavior of the oil, as manifested by a different shape of the melting profile. Only in the case of a higher scanning rate of 5 °C/min was the similarity of curve shapes for all Henola cultivar oils observed, which can be utilized in analytics for profiling. Multivariate data analysis, such as PCA and LDA, showed that DSC parameters enabled distinction of the oils in terms of the seed quality used for pressing (fresh vs. stored).
Abstract
Among the variety of edible cold-pressed oils on the market, hemp seed oil is becoming increasingly popular among scientists and consumers due to its plethora of nutritional compounds. In this study, the goal was to examine the thermal characteristics of cold-pressed hemp seed oil pressed from seeds of the Henola cultivar procured by five different suppliers in two different seasons. This aim of this research was to establish how various scanning rates can affect the unique thermal profile of cold-pressed hemp seed oil in terms of an authenticity assessment. The melting transition was manifested by curves with four peaks for all hemp seed oils; however, they differed for each scanning rate in terms of the shape and peak intensity. Comparing the curves obtained at heating rates of 1 and 2 °C/min, noticeable differences were observed in the melting transition parameters between hemp seed oils, showing that small differences in fatty acid composition can cause changes in DSC profiles. In contrast, at a scanning rate 5 °C/min, the melting curves were similar for all hemp seed oils. It was also observed that for all the scanning rates, there was a strong negative correlation between the total content of polyunsaturated fatty acids (ƩPUFAs) and the peak temperature of the three peaks (Tm2, Tm3, and Tm4). The most abundant fatty acids were PUFAs, i.e., linoleic acid (C18:2), with contents ranging from 47 to 55%; and α-linolenic acid (C 18:3 n–3), with contents ranging from 17 to 25%. The application of linear discriminant analysis (LDA) enabled a discriminant model to be built based on the DSC data obtained for differentiation of oils pressed from fresh and stored seeds.
1. Introduction
Cold-pressed hemp seed oil has become one of the most sought-after non-traditional plant oils in recent years. Among a variety of other commodity oils, hemp seed oil has been popular amongst researchers due to its many applications in the food and medical industries. Commercially, the tagline ‘cold-pressed’ oils has managed to engage more consumers in the market. At the same time, the traceability control of these recently popular oils remains a bottleneck for authorities from the economic point of view. Hemp seed oil is generally produced as a byproduct of industrial hemp plant production of Cannabis sativa L. (family Cannabaceae), which is an annual herbaceous plant known for its utility as an emblematic multipurpose crop. Seeds acquired from the hemp plant can yield a significant quantity of oil between 28 and 35 g/100 g [1]. Compositional features are the most pivotal factor for hemp seed oils, since such oils are mainly composed of triacylglycerols (TAGs), with 1–2% non-TAGs as unsaponifiable fractions of minor components [2,3]. The uniquely balanced and nutritionally beneficial fatty acid composition of this oil has contributed to its consumer value. An abundant quantity of polyunsaturated fatty acids (PUFAs) of between 80 and 90% has been reported, with the most pertinent ratio of n−6 to n−3 fatty acids reported as 3:1 [4,5,6]. However, apart from these, several other bioactive compounds are also present in hemp seed oils, i.e., tocopherols and polyphenols, which act as antioxidants and protect the oil from oxidation damage. These compositional characteristics vary depending upon several factors, i.e., region of production [7], type of cultivars and quality of seeds [5,8], and the genetic predisposition of varieties based on tillage preparation [9] and extraction procedure [10]. In the past, long bast fiber and straw production was the primary method used to cultivate hemp crop; therefore, the harvested seeds (commonly as a byproduct) varied in quality, with different contents of chlorophyll and moisture influencing the physicochemical properties of cold-pressed oils [11]. Hemp seed oils are most commonly produced as cold-pressed oils (often referred to as virgin hemp seed oil), which contributes to their specific nutty taste and light-to-dark green color. Since cold-pressing does not require chemicals or organic solvents but is performed using a screw/mechanical press or a hydraulic press [12], it has been reported that numerous bioactive compounds, along with a wide array of essential amino acids, are retained in the final product, without any chemical contamination [13]. The high nutritional quality of this oil is associated with higher consumer demands for a genuine product. Currently, differential scanning calorimetry (DSC) is widely used to characterize the thermal properties of fats and oils in food analytics, including for the assessment of authenticity. Characterization of compounds using the DSC technique can provide sophisticated information on the thermal behavior of the sample, i.e., phase transition profiles of the crystallization and melting process. An analysis of heat-related phenomena reveals the most rudimentary changes taking place in a sample. Thus, phase transition analysis of fats or oils with different heating and cooling programs can produce different exothermic or endothermic curves, which can be analyzed further to explain the unique thermodynamic traits associated with triacylglycerols (TAGs) and fatty acid composition. One of the most important aspects of DSC analysis is the scanning rate, which refers to the rate at which the temperature changes during the experiment. The heating rate during melting phase transition can affect the behavior of the fat or oil in terms of additional thermal events such as polymorphic transitions [14,15]. Depending on how fast the heating is carried out, these polymorphic transitions may occur with greater or lesser intensity for various types of oils. Cold-pressed hemp seed oil may manifest its thermal behavior in a specific way depending on the scanning rate used, which may be important for authentication due to the specific TAG composition.
Accordingly, the goal of this study was set to establish how different scanning rates can affect the unique thermal profile of cold-pressed hemp seed oil in terms of the authenticity assessment. The thermal characteristics of cold-pressed hemp seed oil were determined based on oil obtained from the ‘Henola’ cultivar procured from five different suppliers in two different seasons. Most importantly, to the best of the author’s knowledge, no literature has yet been published that explains the melting behavior of cold-pressed hemp seed oils by employing analytical measurement with a DSC instrument.
2. Materials and Methods
2.1. Materials
Samples used for this study were hemp seeds cultivated in two different seasons in the Greater Poland region. Seeds were purchased from different suppliers who cultivated Henola variety and harvested at the end of November 2018 (HP_HLC and HP_HLD) and at the end of November 2019 (HP_HLA, HP_HLB, and HP_HLE). At the beginning of 2020, cold pressing was performed by maintaining a temperature below 50 °C, later the oil was left for the 24 h decantation process. Following pressing, the oils were stored in brown glass bottles at a temperature of −80 °C until they were opened for analysis.
2.2. Methods
2.2.1. Fatty Acid Composition
A gas chromatograph with flame ionization detector (GC-FID) was used to determine the fatty acid composition. After dissolving 15 mg of oil in 1 mL of hexane (for HPLC, Sigma Aldrich, St. Louis, MO, USA), 1 mL of 0.4 N sodium methoxide was added. The solution was stirred and left for 15 min. In the next step, 5 mL of distilled water was added. The upper layer was separated from the mixture and taken off. A Trace 1300 chromatograph (Thermo Fisher Scientific, Waltham, MA, USA) was used to analyze the fatty acid methyl esters by following AOCS official method [16]. A Supelcowax 10 capillary column (30 m × 0.2 mm × 0.2 μm) was used for separation. A sample volume of 1 µL was injected in splitless mode. The initial furnace temperature was 160 °C and was increased from 12 °C/min to 220 °C. A temperature of 220 °C was maintained for 20 min, and hydrogen gas was used as the carrier gas. Fatty acid methyl esters were identified using 37-component FAME mix (Supelco) based on the retention times. All samples were analyzed in two replications. Fatty acid composition results are reported as % of the total area for all peaks.
2.2.2. Melting Profile Analysis using Differential Scanning Calorimetry
The phase transition of hemp seed oil was investigated based on the method described by Tomaszewska-Gras et al. [17]. A PerkinElmer DSC 8500 differential scanning calorimeter (Waltham, MA, USA) equipped with an Intracooler II and Pyris software (Perkin Elmer, Waltham, MA, USA) was used to examine the melting properties of the hemp seed oil. The DSC calorimeter was calibrated using indium (m.p. 156.6 °C, ∆Hf = 28.45 J/g) and n-dodecane (m.p. −9.65 °C, ∆Hf = 216.73 J/g). Then, 6–7 mg samples were weighed into 20 µL aluminum pans (Perkin Elmer, No. 0219–0062, Waltham, MA, USA) and hermetically sealed. The reference was an empty, hermetically sealed aluminum pan, with nitrogen (99.999% purity) used as the purge gas. Different scanning rates were used to obtain the melting profile, and the melting curves were obtained between −65 and 30 °C. In order to obtain the profile with a scanning rate 1 °C/min, the samples were first cooled at 1 °C/min, then heated at the same scanning rate. For the scanning rate, 2 °C/min and 5 °C/min analyses were carried out analogously. For each scanning rate, the calibration procedure was completed with the correct scanning rate. The temperature of each peak (Tm), the peak height (h), and the enthalpy of melting (∆Hm, J/g) were measured from the heating curves. All measurements were performed in triplicate for each sample.
2.2.3. Statistical Analysis of Results
The results are reported as means and standard deviations. The homogeneity of variances was checked with the Hartley–Cochran–Bartlett test. One-way ANOVA and Tukey’s test were used to form statistically homogeneous groups if the variances were homogeneous. A non-parametric test, namely ANOVA and the Kruskal–Wallis rank test, was used if not. PCA and CA were used as exploratory methods to reveal the connections between variables/objects and identify patterns among them. The dataset was reduced from a higher to a lower dimensional level through analysis. LDA was performed to determine whether there were parameters that could distinguish the tested oils based on the seed freshness (group 1 vs. group 20). Statistica 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA) was used for statistical analysis of the results at a significance level of α = 0.05.
3. Results
3.1. Fatty Acid Composition
The fatty acids (FAs) present in oils are crucial for the evaluation of oils, as they exert a substantial impact on the quality and application of oils. The FA profiles of all cold-pressed hemp seed oils are shown in Table 1. Oil pressed from the Henola cultivar had 88% to 90% UFA, with the dominating PUFA accounting for 74% to 76% and saturated fatty acids (SFAs) accounting for 10% to 11%. These results are comparable with the FA profile of another Polish hemp variety, ‘Bialobrzeskie’ [6].

Table 1.
Fatty acid composition of cold-pressed hemp seed oil of the Henola cultivar.
UFA, SFA, MUFA, and PUFA totals did not show notable differences in samples pressed from stored (HP_HLC an HP_HLD) and fresh (HP_HLA, HP_HLB, and HP_HLE) seeds. However, individual FA content showed significant differences (p ≤ 0.05) in terms of the most abundant PUFA linoleic acid (C18: 2; LA) and α-linolenic acid (C 18: 3 n−3; ALA). The lowest LA content for HP_HLA (47%) and the highest for both HP_HLC and HP_HLD (55%) were determined. Amongst other PUFAs, ALA ranged from 17 to 25%, with the highest concentration noted for HP_HLA. The presence of a unique acid was detected, i.e., stearidonic acid (C18: 4, SDA), in the range of 1.23 to 1.58%, which makes this oil nutritionally more valuable. A comparatively lower presence of stearidonic acid (1.2%) was previously reported by other researchers [18]. SDA acts as a biological precursor for longer-chain n−3 fatty acids, which can be rapidly synthesized in the human body, i.e., eicosapentaenoic acid (EPA; C20: 5 n−3) and docosahexaenoic acids (DHA; C22: 6 n−3) [19]. Oleic acid (C18: 1) was found in the concentration range of 12 to 14%. The most abundant SFAs were found to be palmitic acid (C16: 0) and stearic acid (C18: 0), accounting for a range of 6.59–6.99% and 2.69–2.71% of total FAs, respectively. Among the remaining fatty acids, none exceeded a 1% contribution to the total FA content. The ratio of n−6 to n−3 fatty acids ranged from 2: 1 to 3: 1, as shown in Table 1. A similar result regarding the n−6/n−3 ratio was obtained for the same Henola variety by Teleszko et al. [6] at the level of 2.87 and for commercial hemp seed oils from 2.96 to 3.27 by Ustun-Argon [20]; however a lower quantity of PUFAs was reported.
3.2. DSC Melting Profile Analysis
For the samples analyzed in this study, different scanning rates (1, 2, 5 °C/min) were used to explore melting phenomena during phase transitions. It should be mentioned here that during cooling, the crystallization data were also collected; however, in this study, they were not taken into consideration. We decided to focus on melting curves, which provide more complex data at each scanning rate, as they can substantially articulate a unique profile for hemp seed oil. The melting curves obtained for each scanning rate are presented in Figure 1A (1 °C/min), 1B (2 °C/min), and 1C (5 °C/min). As three curves are presented for each hemp seed oil sample, it can be seen that they are highly repeatable.
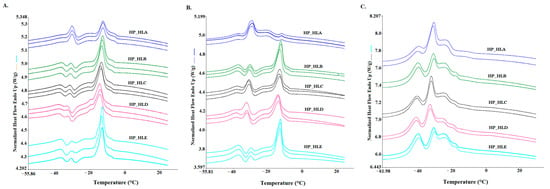
Figure 1.
DSC melting curves of cold-pressed hemp seed oils of the Henola cultivar obtained with various heating rates: (A) heating rate of 1 °C/min; (B) heating rate of 2 °C/min; (C) heating rate of 5 °C/min.
However, different heating rates imply changes in peak shape and size, since differences were observed between curves obtained for the same samples with different scanning rates. To quantitatively explain these differences in endothermic curves, several parameters were evaluated, as presented in Table 2, Table 3 and Table 4, i.e., peak temperature (Tm, °C), peak height (h, W/g), and enthalpy (ΔHm, J/g).

Table 2.
DSC parameters of melting profiles of cold-pressed hemp seed oils of the Henola cultivar obtained at a scanning rate of 1 °C/min.

Table 3.
DSC parameters of melting profiles of cold-pressed hemp seed oils of the Henola cultivar obtained at a scanning rate of 2 °C/min.

Table 4.
DSC parameters of melting profiles of cold-pressed hemp seed oils of the Henola cultivar obtained at a scanning rate of 5 °C/min.
Figure 1A presents the thermal transition of hemp oil samples during the melting process at a scanning rate of 1 °C/min. Four peaks were identified on all curves of hemp seed oil as a result of the endothermic process, since the melting of lipids occurred as a function of increased temperature. Among the four identified peaks, the fourth peak (Tm∽−16 °C) was the highest, while the first (Tm∽−41 °C), second (Tm∽−31 °C), and third (Tm∽−24 °C) peaks were much lower, except for sample HP_HLA, for which the second peak was higher than for the rest of the samples. Table 2 shows quantitative data regarding the peak temperature (Tm), peak height (h), and peak area (ΔHm). Amongst all four peaks, the highest peak height for all hemp seed oil samples was noted for the fourth peak (h4). However, among all the samples, there were significant differences in the h4 values (p ≤ 0.05). The highest h4 value was observed for HP_HLE (0.27 W/g), with the lowest value recorded for HP_HLA (0.13 W/g). As shown in Table 2, among all four peaks, the area for the fourth peak, which is expressed as partial enthalpy (ΔHm4), was the highest with the HP_HLD showing the highest value of 86.05 J/g among all hemp seed oils (p ≤ 0.05). The most prominent differences were observed in thermal behavior of the HP_HLA sample, especially with regard to the values of peak height and peak area. Other authors who also investigated cold-pressed hemp seed oils using the DSC technique with a 1 °C/min scanning rate obtained two major endothermic peaks at −18.12 and −40.10 °C [21].
To demonstrate the melting profile more diversely, hemp seed oils were treated with two more heating rates, which are illustrated in Figure 1B for scanning rates of 2 °C/min 5 °C/min (Figure 1C). Visual differences can be observed between the profiles obtained at scanning rates of 1, 2, and 5 °C/min.
Especially noticeable differences can be observed for sample HP_HLA at a scanning rate of 2 °C/min, for which the profile exhibits a different curve shape and peak heights compared to the other samples. It is worth noting that this sample (HP_HLA) contains the lowest total content of polyunsaturated fatty acids (ƩPUFA), i.e., 74.14%, compared to the other oil samples (Table 1), which could be the cause of the differences in DSC profiles. For the rest of the samples, four peaks were also recorded at a heating rate of 2 °C/min, as for a scanning rate of 1 °C/min. The first peak was observed at temperature Tm1 around −41 °C, and there were no significant differences except for sample HP_HLD. For the Tm2 of the second peak determined at around −31 °C, statistically significant differences were observed between the samples (p ≤ 0.05). The lowest Tm2 value was observed for the HP_HLD sample at a temperature of −35.95 °C, and the highest value was recorded for HP_HLA at a temperature of −32.50 °C. The third peak on the curves appeared as a shoulder for all samples, for which the peak temperature (Tm3) was found to range from −22 °C to −26 °C. Similar to the 1°C/min heating rate, the fourth peak was the main peak (considering peak height and enthalpy values), except for the HP_HLA sample, for which the second peak dominated, with significantly different highest values of h2 and ΔHm2 (p ≤ 0.05). The fourth peak (Tm4) for all samples was detected between −14 °C and −17 °C, whereas for the HP_HLD, sample the lowest peak temperature was observed, i.e., −17.36 °C; however, for the HP_HLE sample, the highest h4 value was obtained (0.34 W/g), which was statistically different (p ≤ 0.05) from the other oil samples.
Based on the results obtained with scanning rates of 1 and 2 °C/min, we also carried out an experiment with a faster heating rate of 5 °C/min, which resulted in a relatively fast analysis and prominent repeatability amongst the hemp seed oil samples (Figure 1C). Just as for the previous scanning rates, four peaks are visible, and the second peak is the most prominent for all samples. A comparison of the curves obtained at all scanning rates shows that the curves of all hemp seed oils are the most similar at a heating rate of 5 °C/min.
Table 4 shows the values of the DSC parameters (Tm, h, and ΔHm) calculated from curves obtained at a scanning rate of 5 °C/min. The first peak temperature (Tm1) appeared when the melting curves reached a temperature around −41°C, which was not significantly different for all oil samples. The second and major peak provides significantly different values (p ≤ 0.05) for peak temperature (Tm2) and enthalpy (ΔHm2) between stored seed oils (HP_HLC and HP_HLD) and fresh seed oils (HP_HLA, HP_HLB, and HP_HLE), whereas for the first group of oils, Tm2 values were lower than for the second group of oils, which is a similar observation to that at a scanning rate of 2 °C/min. The peak height regarding values (h2) and enthalpy ΔHm2 values were the highest for the HP_HLA sample, i.e., 0.42 W/g and 30.14 J/g, respectively, and the lowest for the HP_HLE variety (0.3 W/g and 19.48 J/g, respectively). The third peak was generated when the temperature (Tm3) reached a value around −24 °C for all samples. In the case of assessing the fourth peak, the peak temperature (Tm4) was found to be the highest for the HP_HLA sample (−12.03 °C), while for the rest of the samples, it was between −17 and −19 °C. The peak height (h4) and enthalpy (ΔHm4) of the fourth peak were also the lowest and significantly different for those of HP_HLA, confirming that the lowest total content of polyunsaturated fatty acids (ƩPUFA), i.e., 74.14%, compared to the rest of oils samples can play a pivotal role in the shape of the DSC profile, especially in the case of the second and fourth peaks.
Analysis of all the melting curves and parameters presented in Table 2, Table 3 and Table 4 for all three scanning rates (i.e., 1, 2, and 5 °C/min) shows that it was possible to determine the elemental thermal attributes of cold-pressed hemp seed oils. Regardless of the scanning rate, four endothermic peaks were identified for all samples. On the other hand, other authors performed melting analysis at 10 °C/min scanning rate and detected three sharp peaks at temperatures of −16.99, −20.65, and −37.06 ° using microwave-assisted extraction and one peak at a temperature of −32.26 in hexane-extracted hemp seed oils. This observation may indicate that the melting profile is not only scanning-rate-dependent but can also be affected by different extraction methods [22]. However, in the present study, it was shown that the scanning rate affected the shape of curves, as well as DSC parameters such as peak temperature, peak height, and enthalpy. The most similar DSC curves for all hemp seed oil samples were observed at a scanning rate 5 °C/min. This finding may contribute significantly to the prospect of fingerprinting hemp seed oil pressed from the seeds of the Henola cultivar.
4. Discussion
Multivariate Data Analysis
Since two types of seeds were used in this study, i.e., fresh seeds (from 2019) and stored seeds (from 2018), an additional goal was undertaken, namely, to examine the possibility of identifying DSC parameters to distinguish between oils pressed from stored and fresh seeds. In the first stage, before performing any multivariate analysis, the raw data (number of samples × number of variables) were standardized in order to obtain scores on the same scale. Principal component analysis was used as the first step of multivariate data analysis, since it enables the differences between objects and the relationship between variables to be visualized. For visual analysis, a two-dimensional projection of samples is usually constructed with the axes (principal components) as the factors. Based on graphical criteria, five PCs were used for the analysis. Each PC is a linear combination of the original responses, and PCs are orthogonal to each other. Figure 2 shows the relationship between variables describing the quality of oils (ƩSFA, total saturated acids; ƩMUFA, total monounsaturated acids; ƩPUFA, total polyunsaturated acids) and all DSC parameters for the melting profiles obtained at three scanning rates (1 °C/min (Figure 2A,D), 2 °C/min (Figure 2B,E), and 5 °C/min (Figure 2C,F)). The first component explains 56.51, 59.09. and 53.08% of the variance, while the second component explains 25.03, 25.46, and 35.3% of the variance for scanning rates of 1, 2, and 5 °C/min, respectively. In general, the first principal component described by h4, Hm4, ƩPUFA, Tm3, and Tm4 separates HP_HLA oils from the others. The highest loadings values of Hm2, h2, and Tm1 were noted in the second principal component, separating HP_HLD and HP_HLC oils from HP_HLB and HP_HLE. Generally, strong negative correlations were found between ƩPUFA and the Tm2, Tm3, and Tm4 parameters, i.e., −0.92, −0.95, and −0.76 at a scanning rate 1 °C/min and −0.73, −0.78, and −0.78, respectively at a scanning rate 5 °C/min.
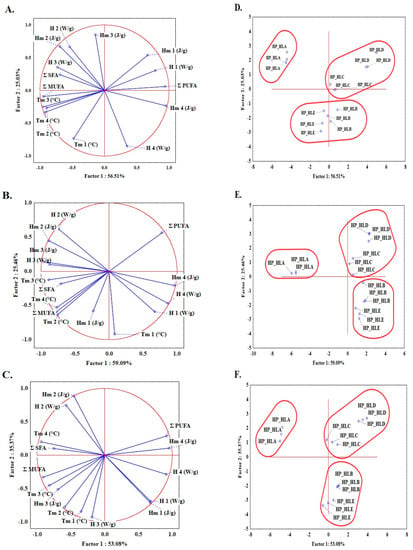
Figure 2.
PCA analysis and loading plots for PC1 and PC2 with projection of the variables: ƩSFA—total saturated fatty acids; ƩMUFA, total monounsaturated fatty acids; ƩPUFA, total polyunsaturated fatty acids. DSC parameters: Tm1, Tm2, Tm3, and Tm4—peak temperatures; h1, h2, h3, and h4—peak heights; Hm1, Hm2, Hm3, and Hm4—peak enthalpies determined at 1 °C/min (A), 2 °C/min (B), and 5 °C/min (C) and projection of cases showing distribution of hemp seed oil samples analyzed at 1 °C/min (D), 2 °C/min (E), and 5 °C/min (F).
Another unsupervised pattern recognition method applied in this study was cluster analysis (CA). The Ward method, known as the minimum variance method with a Euclidean distance between centroids, was applied. Figure 3 shows a dendrogram as the result of the cluster analysis performed, with three clusters obtained for each scanning rate (1 °C/min (Figure 3A), 2 °C/min (Figure 3B), and 5 °C/min (Figure 3C)). The first cluster includes HP_HLA oils, which are definitely different from the others (the longest binding distance); the second cluster includes HP_HLB and HP_HLC oils; and the third cluster includes HLC and HLD oils. The results of the cluster analysis are consistent with the results of the principal component analysis (Figure 2). Thus, principal component analysis, as well as cluster analysis, makes it possible to distinguish a group of lower freshness oils (HP_HLD and HP_HLC) from fresh oils (HP_HLA, HP_HLB, and HP_HLE). This allows for the assumption that it is possible to build a model that discriminates oils according to their freshness. To determine the rules for classifying oil samples, linear discriminant analysis (LDA) was used. LDA is a common method for supervised pattern recognition. LDA identifies the linear functions that best separate the classes by maximizing the between-class variation and minimizing the within-class variation. A data matrix with 45 rows (one for each oil sample) and 12 columns (one for each DSC parameter) [15] was prepared for LDA analysis. The functions were selected using the forward stepwise se-lection (SLDA) method, which chooses the variable with the highest F value and the lowest Wilks’ λ. The lower the Wilks’ λ and the higher F value, the better the variable discriminates. Table 5 shows the DSC parameters that had the best discrimination power according to this criterion.
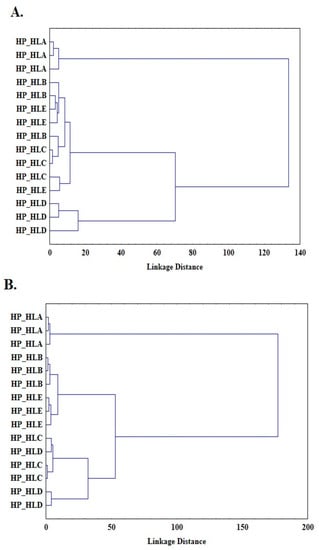
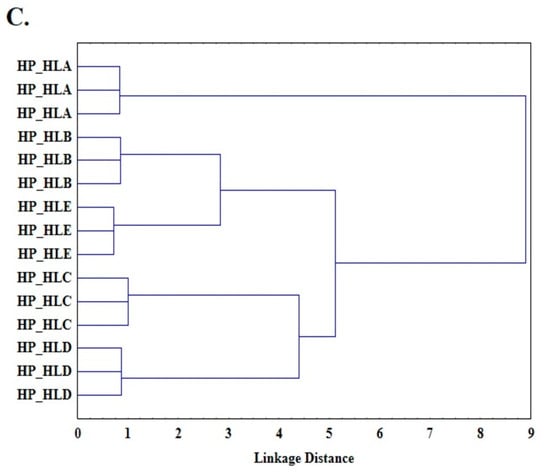
Figure 3.
Cluster analysis (CA) and loading plots representing results obtained at 1 °C/min (A), 2 °C/min (B), and 5 °C/min (C).

Table 5.
Wilks’ λ and F tests of group means.
As can be seen in Table 5, the ΔHm2 parameter has the highest discriminatory power, and the h3 parameter has the lowest discriminatory power. Since the model was built to differentiate between two groups, a single statistically significant (p < 0.05) discriminant function was obtained (Equation (1)), with a high canonical correlation coefficient (R = 0.96).
The analysis resulted in two classification functions:
Using these classification functions (Equations (2) and (3)), all oil samples were correctly classified. Thus, the ability to build discriminant model based on obtained data was 100%. It is obvious that in order for it to be possible to create a universal model estimating the freshness of oil, a larger number of samples is needed (seeds from different regions and different years); however, as shown in this paper, DSC parameters can be successfully used for such a purpose.
5. Conclusions
This study presents the applicability of differential scanning calorimetry (DSC) to characterize the melting phase transition of hemp seed oil from the Henola cultivar. Four endothermic peaks were detected at three different scanning rates during melting transition for all hemp oils. Regardless of the heating rate, the same number and position of four peaks were observed at temperatures averaged for all heating rates at −41, −32.5, −24, and 16.5 °C. However, the peaks differed in shape and intensity as measured by peak height and peak area expressed as enthalpy for each scanning rate. It was observed that lower heating rates (1, 2 °C/min) showed differences between hemp seed oils resulting from small differences in the composition of fatty acids. In the case of a higher scanning rate of 5 °C/min, the curves for all samples were similar, which could be utilized in analytics for profiling to assess the authenticity of hemp seed oil, considering relatively fast DSC analysis without using any chemicals. Additionally, it was shown that a multivariate statistical linear discriminant analysis (LDA) tool enabled a discriminant model to be built based on the DSC data obtained for differentiation of oils pressed from fresh and stored seeds.
Author Contributions
Conceptualization, M.I. and J.T.-G.; methodology, M.I., J.T.-G., and M.R.; formal analysis, M.I., J.T.-G., and M.R.; investigation, M.I. and J.T.-G.; resources, J.T.-G.; data curation, M.I., J.T.-G., and A.K.; writing—original draft preparation, M.I., J.T.-G., and A.K.; writing—review and editing, M.I. and J.T.-G.; visualization, M.I., A.K., and J.T.-G.; supervision, J.T.-G., project administration, J.T.-G.; funding acquisition, J.T.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Centre, Poland (grant number 2018/31/B/NZ9/02762).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matthäus, B.; Schumann, E.; Brühl, L.; Kriese, U. Hempseed Oil—Influence of the Genotype on the Composition in a Two-Year Study. J. Ind. Hemp. 2006, 10, 45–65. [Google Scholar] [CrossRef]
- Liang, J.; Appukuttan Aachary, A.; Hollader, U.T. Hemp seed oil: Minor components and oil quality. Lipid Technol. 2015, 27, 231–233. [Google Scholar] [CrossRef]
- Banskota, A.H.; Jones, A.; Hui, J.P.M.; Stefanova, R. Triacylglycerols and Other Lipids Profiling of Hemp By-Products. Molecules 2022, 27, 2339. [Google Scholar] [CrossRef] [PubMed]
- Da Porto, C.; Decorti, D.; Natolino, A. Potential Oil Yield, Fatty Acid Composition, and Oxidation Stability of the Hempseed Oil from Four Cannabis sativa L. Cultivars. J. Diet. Suppl. 2015, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Fawzi Mahomoodally, M. A comparative study of seed yield and oil composition of four cultivars of Hemp (Cannabis sativa L.) grown from three regions in northern Iran. Ind. Crops Prod. 2020, 152, 112397. [Google Scholar]
- Teleszko, M.; Zając, A.; Rusak, T. Hemp Seeds of the Polish ‘Bialobrzeskie’ and ‘Henola’ Varieties (Cannabis sativa L. var. sativa) as Prospective Plant Sources for Food Production. Molecules 2022, 27, 1448. [Google Scholar] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 323–329. [Google Scholar] [CrossRef]
- Dimić, E.; Romanić, R.; Vujasinović, V. Essential fatty acids, nutritive value and oxidative stability of cold pressed hempseed (Cannabis sativa L.) oil from different varieties. Acta Aliment. 2009, 38, 229–236. [Google Scholar] [CrossRef]
- Mediavilla, V.; Jonquera, M.; Schmid-Slembrouck, I.; Soldati, A. Decimal code for growth stages of hemp (Cannabis sativa L.). J. Int. Hemp Assoc. 1998, 5, 68–74. [Google Scholar]
- Devi, V.; Khanam, S. Optimization of the Ratio of ω-6 Linoleic and ω-3 α-Linolenic Fatty Acids of Hemp Seed Oil with Jackknife and Bootstrap Resampling. Chem. Prod. Process Model. 2019, 15, 20190028. [Google Scholar] [CrossRef]
- Oomah, B.D.; Busson, M.; Godfrey, D.V.; Drover, J.C.G. Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem. 2002, 76, 33–43. [Google Scholar] [CrossRef]
- FAO/WHO Joint Publications. Standard for Edible Fats and Oils Not Covered By Individual Standards. In Codex Aliment Food Agric Organ United Nations; FAO/WHO Joint Publications: Rome, Italy, 2015; pp. 2–7. [Google Scholar]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Islam, M.; Bełkowska, L.; Konieczny, P.; Fornal, E.; Tomaszewska-Gras, J. Differential scanning calorimetry for authentication of edible fats and oils—What can we learn from the past to face the current challenges? J. Food Drug Anal. 2022, 30, 185–201. [Google Scholar] [CrossRef]
- Sun, X.; Lee, K.O.; Medina, M.A.; Chu, Y.; Li, C. Melting temperature and enthalpy variations of phase change materials (PCMs): A differential scanning calorimetry (DSC) analysis. Phase Transit. 2018, 91, 667–680. [Google Scholar] [CrossRef]
- AOCS. AOCS Official Method Preparations of Methyl Esters of Fatty Acids; AOCS: Denver, CO, USA, 1997; pp. 2–66. [Google Scholar]
- Tomaszewska-Gras, J.; Islam, M.; Grzeca, L.; Kaczmarek, A.; Fornal, E. Comprehensive thermal characteristics of different cultivars of flaxseed oil (Linum usittatissimum L.). Molecules 2021, 26, 1958. [Google Scholar] [CrossRef] [PubMed]
- Tura, M.; Ansorena, D.; Astiasarán, I.; Mandrioli, M.; Toschi, T.G. Evaluation of Hemp Seed Oils Stability under Accelerated Storage Test. Antioxidants 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L. Stearidonic acid (18:4n-3): Metabolism, nutritional importance, medical uses and natural sources. Eur. J. Lipid Sci. Technol. 2007, 109, 1226–1236. [Google Scholar] [CrossRef]
- Ustun-Argon, Z. Phenolic Compounds, Antioxidant Activity and Fatty Acid Compositions of Commercial Cold-Pressed Hemp Seed (Cannabis sativa L.) Oils From Turkey. Int. J. Sci. Eng. Res. 2019, 10, 166–173. [Google Scholar]
- Teh, S.S.; Birch, J. Physicochemical and quality characteristics of cold-pressed hemp, flax and canola seed oils. J. Food Compos. Anal. 2013, 30, 26–31. [Google Scholar] [CrossRef]
- Rezvankhah, A.; Emam-Djomeh, Z.; Safari, M.; Askari, G.; Salami, M. Microwave-assisted extraction of hempseed oil: Studying and comparing of fatty acid composition, antioxidant activity, physiochemical and thermal properties with Soxhlet extraction. J. Food Sci. Technol. 2019, 56, 4198–4210. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).