Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of C. incana
2.3. Preparation of C. incana Ethanolic Extract
2.4. Identification of Chemical Compounds
2.5. Cell Culture
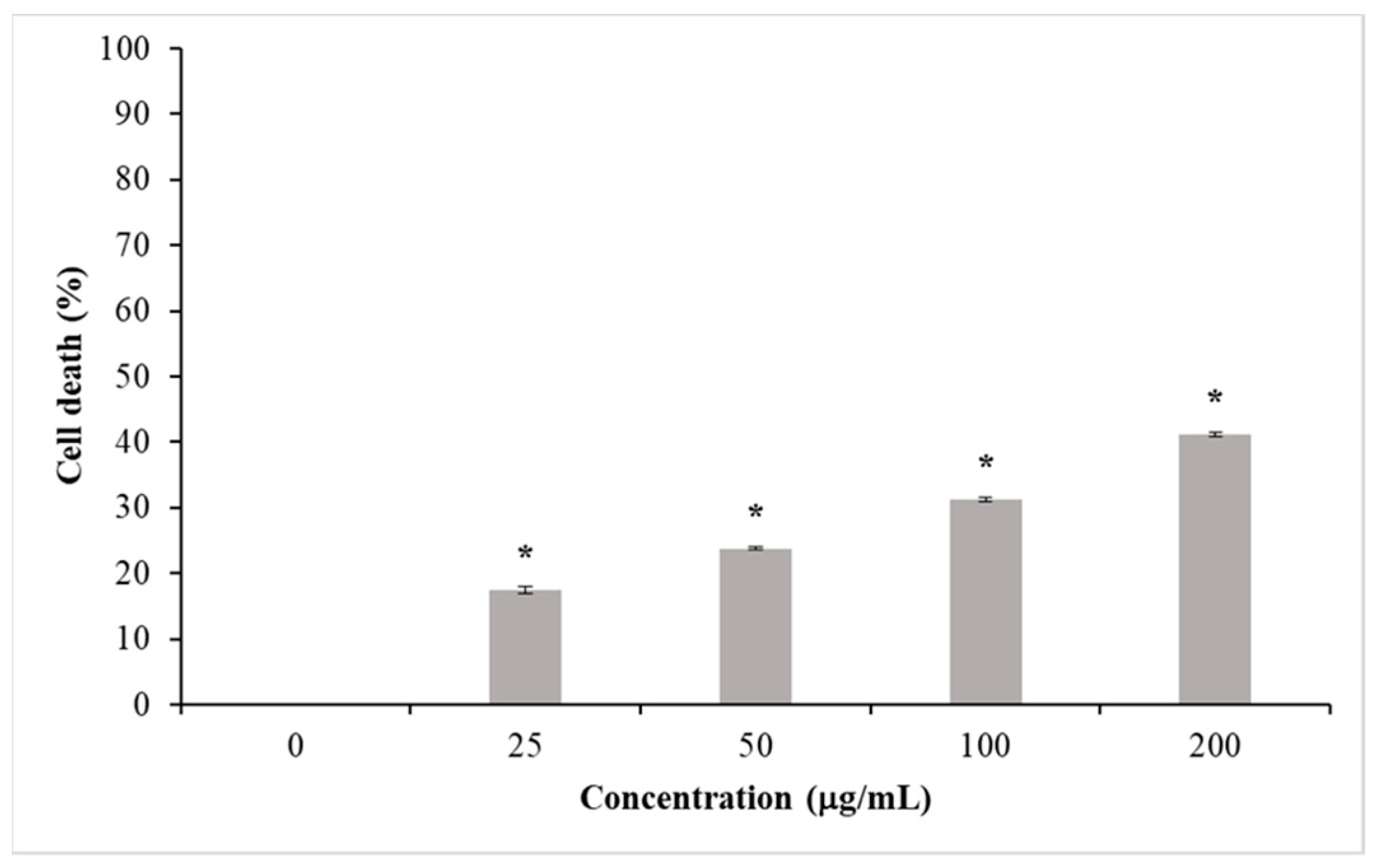
Cytotoxicity Assay
2.6. Antioxidant Activity
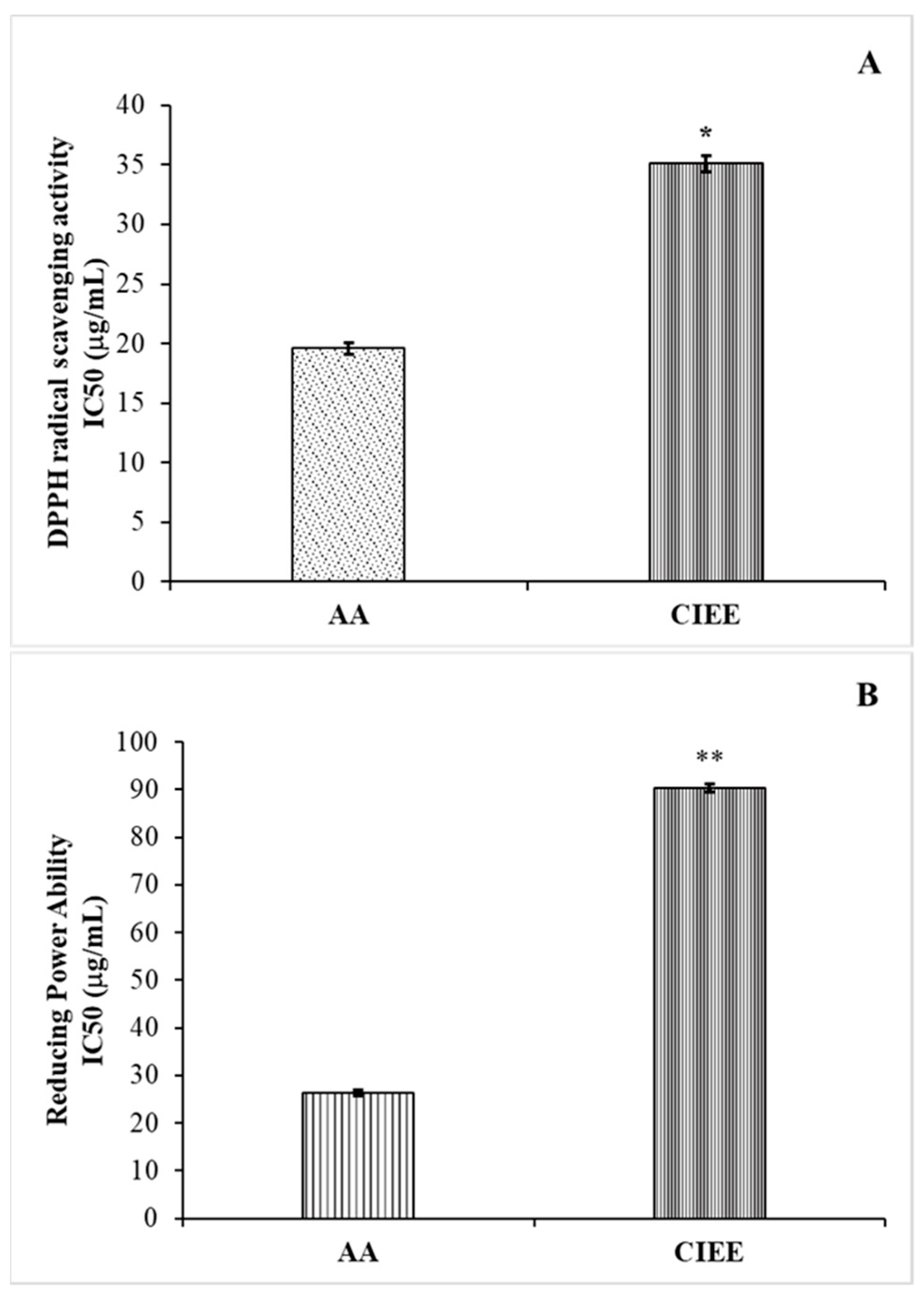
2.6.1. DPPH Assay
2.6.2. Reducing Power Assay
2.7. Antidiabetic Activity
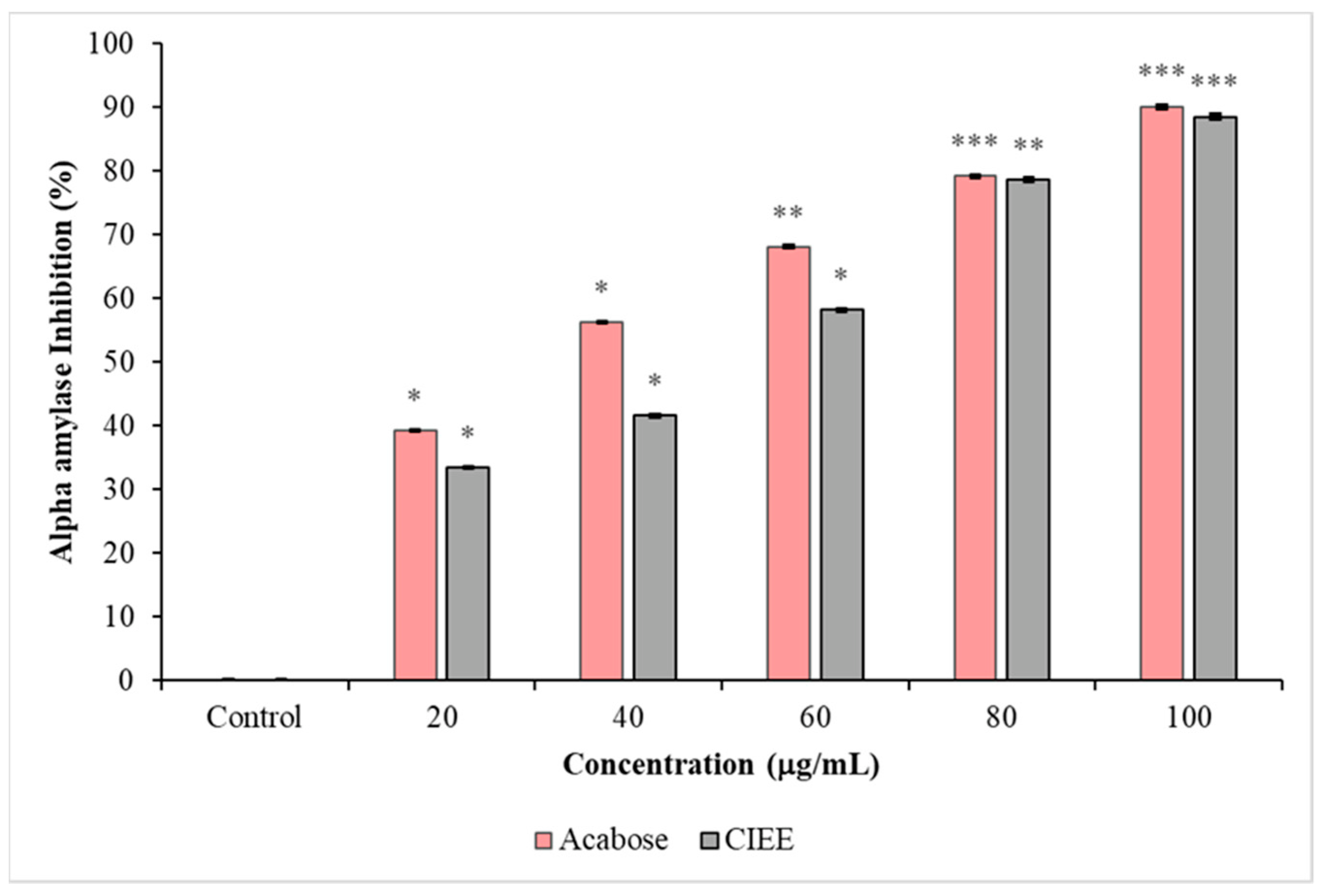
2.7.1. α-Amylase Inhibition Assay
2.7.2. α-Glucosidase Inhibition Assay
2.7.3. Pancreatic Lipase Inhibition Test
2.7.4. Dipeptidyl Peptidase IV (DPP-IV) Inhibition Test
2.8. Statistical Analysis
3. Results
3.1. Phytochemical Analysis of C. incana Ethanolic Extract
3.2. Cytotoxicity
3.3. Antioxidant Activity
3.3.1. DPPH Assay
3.3.2. Reducing Power Assay
3.4. Antidiabetic Activity
3.4.1. α-Amylase Inhibition Assay
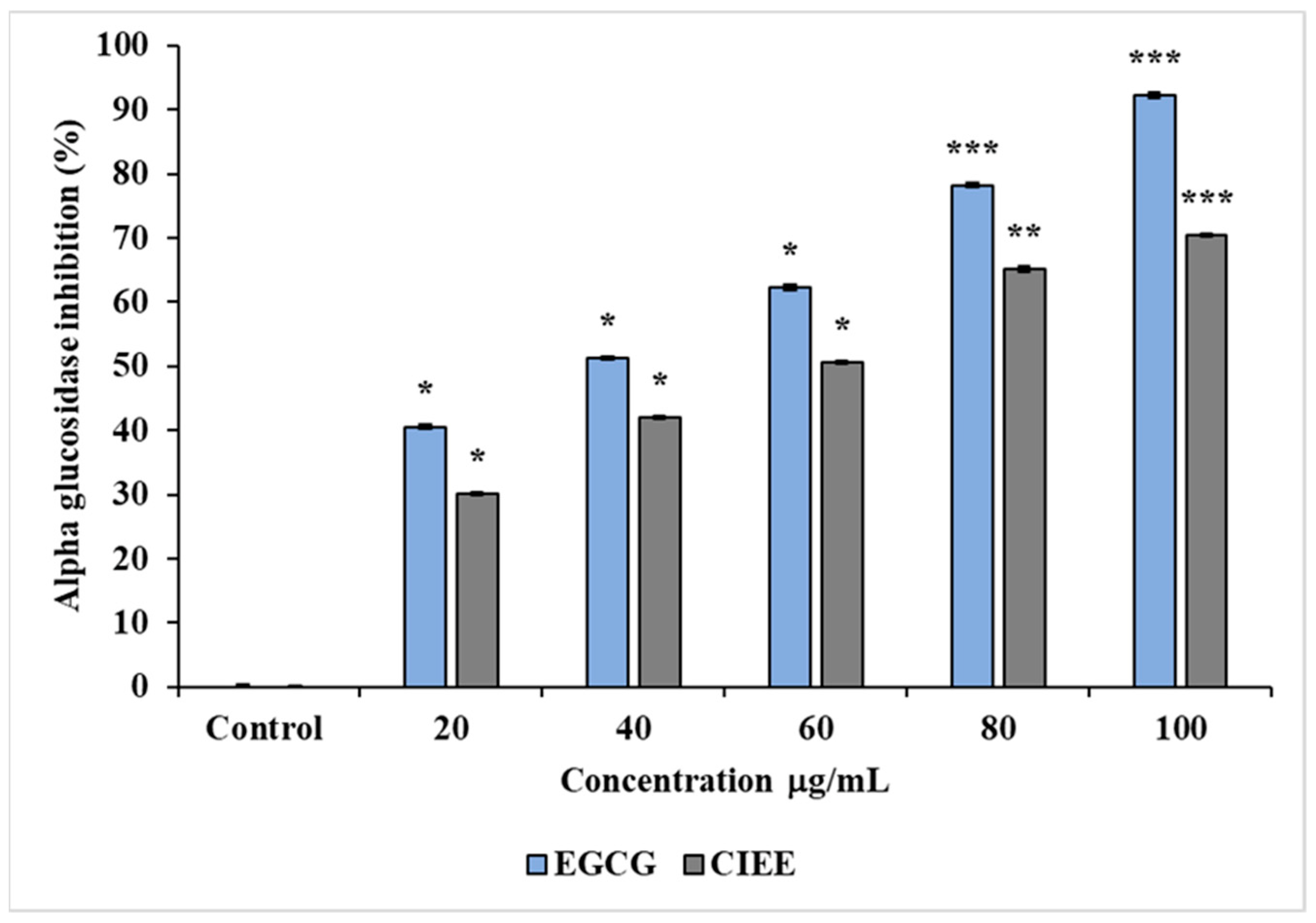
3.4.2. α-Glucosidase Inhibition Assay
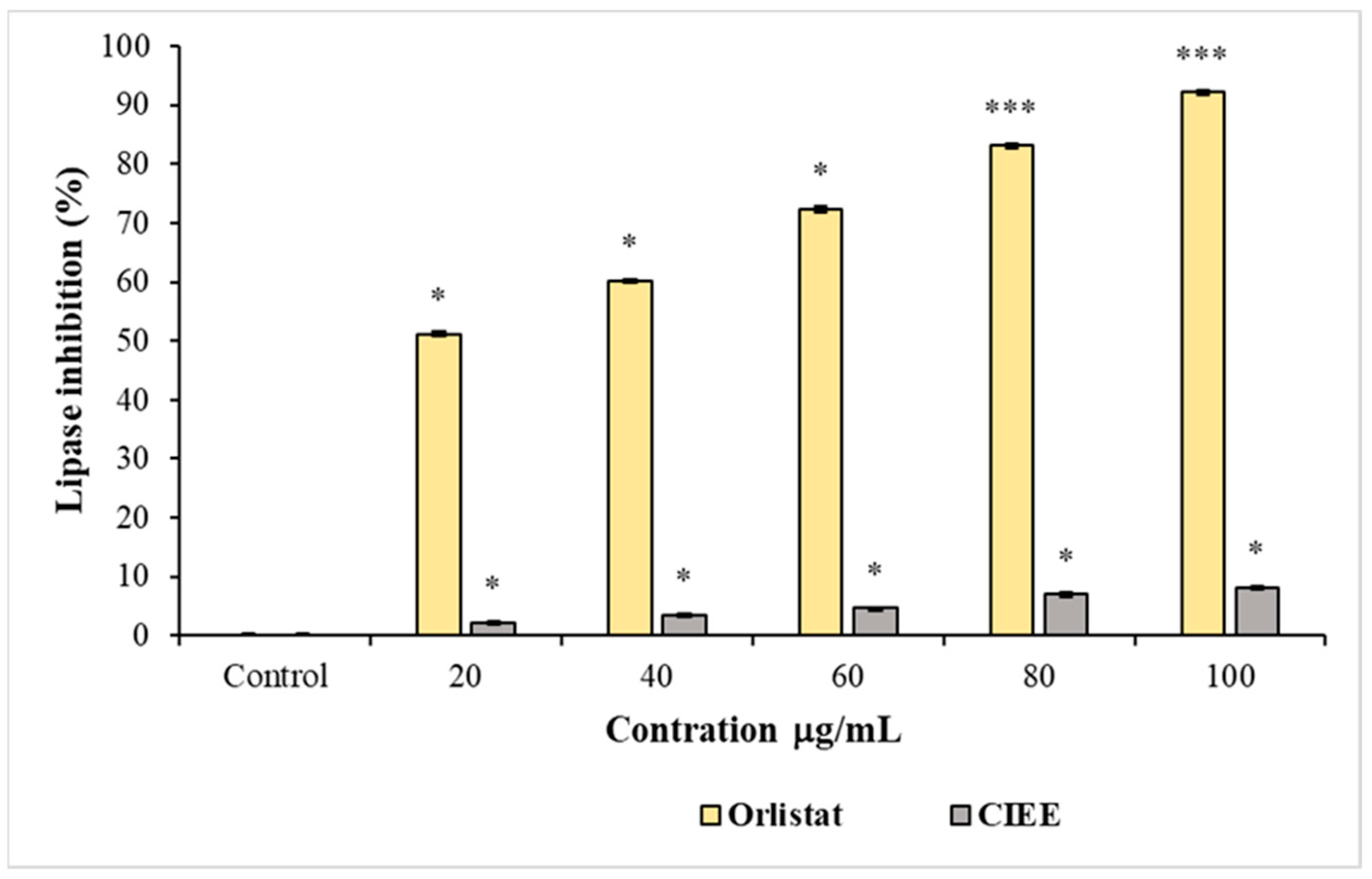
3.4.3. Pancreatic Lipase Inhibition Assay
3.4.4. Dipeptidyl Peptidase-IV (DPP-IV) Inhibition Assay
4. Discussion
4.1. Phytochemical Analysis of C. incana Ethanolic Extract
4.2. Cytotoxicity
4.3. Antioxidant Activity
4.4. Antidiabetic Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Bustanji, Y.K. Phytochemical Analysis, In Vitro Assessment of Antioxidant Properties and Cytotoxic Potential of Ruta Chalepensis L. Essential Oil. J. Essent. Oil Bear. Plants 2020, 23, 1409–1421. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Ma, L.; Yao, L. Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.A.; Usman, L.A.; Akolade, J.O.; Idowu, O.A.; Abdulazeez, A.T.; Amuzat, A.O. Antidiabetic Potentials of Citrus Aurantifolia Leaf Essential Oil. Drug Res. 2019, 69, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kim, H.C.; Ko, Y.J.; Kim, J.S. Antioxidant, α-Glucosidase Inhibitory and Anti-Inflammatory Effects of Aerial Parts Extract from Korean Crowberry (Empetrum Nigrum Var. Japonicum). Saudi J. Biol. Sci. 2016, 23, 181–188. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Mukhtar, Y.; Galalain, A.; Yunusa, U. A Modern Overview on Diabetes Mellitus: A Chronic Endocrine Disorder. Eur. J. Biol. 2020, 5, 1–14. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Kooti, W.; Farokhipour, M.; Asadzadeh, Z.; Ashtary-Larky, D.; Asadi-Samani, M. The Role of Medicinal Plants in the Treatment of Diabetes: A Systematic Review. Electron. Physician 2016, 8, 1832–1842. [Google Scholar] [CrossRef]
- Ademiluyi, A.O.; Oboh, G. Soybean Phenolic-Rich Extracts Inhibit Key-Enzymes Linked to Type 2 Diabetes (α-Amylase and α-Glucosidase) and Hypertension (Angiotensin I Converting Enzyme) in Vitro. Exp. Toxicol. Pathol. 2013, 65, 305–309. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Koorbanally, N.A.; Islam, M.S. Antioxidative Activity and Inhibition of Key Enzymes Linked to Type-2 Diabetes (α-Glucosidase and α-Amylase) by Khaya Senegalensis. Acta Pharm. 2014, 64, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Striegel, L.; Kang, B.; Pilkenton, S.J.; Rychlik, M.; Apostolidis, E. Effect of Black Tea and Black Tea Pomace Polyphenols on α-Glucosidase and α-Amylase Inhibition, Relevant to Type 2 Diabetes Prevention. Front. Nutr. 2015, 2, 3. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus-Screening for Pancreatic Lipase and α-Amylase Inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.P.V.; Balasuriya, N.; Wang, Y. Prevention of Type 2 Diabetes by Polyphenols of Fruits. Nutr. Antioxid. Ther. Treat. Perspect. 2018, 447–466. [Google Scholar] [CrossRef]
- Sewidan, N.; Khalaf, R.A.; Mohammad, H.; Hammad, W. In-Vitro Studies on Selected Jordanian Plants as Dipeptidyl Peptidase-IV Inhibitors for Management of Diabetes Mellitus. Iran. J. Pharm. Res. IJPR 2020, 19, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, R.A.; Alqazaqi, S.; Aburezeq, M.; Sabbah, D.; Albadawi, G.; Sheikha, G.A. Phenanthridine Sulfonamide Derivatives as Potential DPP-IV Inhibitors: Design, Synthesis and Biological Evaluation. Curr. Comput. Aided. Drug Des. 2020, 18, 9–25. [Google Scholar] [CrossRef]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An Overview on Antidiabetic Medicinal Plants Having Insulin Mimetic Property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Bustanji, Y.K. Chemical Composition, in Vitro Evaluation of Antioxidant Properties and Cytotoxic Activity of the Essential Oil from Calamintha Incana (Sm.) Helder (Lamiaceae). Trop. J. Nat. Prod. Res. 2021, 5, 1333–1339. [Google Scholar] [CrossRef]
- Božović, M.; Ragno, R.; Tzakou, O. Calamintha Nepeta (L.) Savi and Its Main Essential Oil Constituent Pulegone: Biological Activities and Chemistry. Molecules 2017, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Oran, S.A. The status of Medicinal Plants in Jordan. J. Agric. Sci. Technol. 2014, 4, 461–467. [Google Scholar]
- Abu-Irmaileh, B.E.; Afifi, F.U. Herbal Medicine in Jordan with Special Emphasis on Commonly Used Herbs. J. Ethnopharmacol. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Mimica-Dukić, N.; Couladis, M.; Tzakou, O.; Jančić, R.; Slavkovska, V. Essential Oil of Calamintha Sylvatica Bromf. and Calamintha Vardarensis Šilic. J. Essent. Oil Res. 2004, 16, 219–222. [Google Scholar] [CrossRef]
- Popović-Djordjević, J.; Cengiz, M.; Ozer, M.S.; Sarikurkcu, C. Calamintha Incana: Essential Oil Composition and Biological Activity. Ind. Crops Prod. 2019, 128, 162–166. [Google Scholar] [CrossRef]
- Oran, S.A.; Althaher, A.R.; Al Shhab, M.A. Chemical Composition, In Vitro Assessment of Antioxidant Properties and Cytotoxicity Activity of Ethanolic and Aqueous Extracts of Ajuga Orientalis L. (Lamiaceae). J. Pharm. Pharmacogn. Res. 2022, 10, 486–495. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant Activities of Polysaccharides Obtained from Chlorella Pyrenoidosa via Different Ethanol Concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef]
- Ekin, S.; Bayramoglu, M.; Goktasoglu, A.; Ozgokce, F.; Kiziltas, H. Antioxidant Activity of Aqueous and Ethanol Extracts of Crataegus Meyeri Pojark Leaves and Contents of Vitamin, Trace Element. J. Chil. Chem. Soc. 2017, 62, 3661–3667. [Google Scholar] [CrossRef]
- Sagbo, I.J.; Van De Venter, M.; Koekemoer, T.; Bradley, G. In Vitro Antidiabetic Activity and Mechanism of Action of Brachylaena Elliptica (Thunb.) DC. Evid. Based. Complement. Alternat. Med. 2018, 2018, 4170372. [Google Scholar] [CrossRef]
- Sancheti, S.; Sancheti, S.; Seo, S. Evaluation of Antiglycosidase and Anticholinesterase Activities of Boehmeria Nivea. Pak. J. Pharm. Sci. 2010, 23, 236–240. [Google Scholar]
- Lewis, D.R.; Liu, D.J. Direct Measurement of Lipase Inhibition by Orlistat Using a Dissolution Linked In Vitro Assay. Clin. Pharmacol. Biopharm. 2012, 1, 1000103. [Google Scholar] [CrossRef]
- Vawhal, P.K.; Jadhav, S.B. Design, Synthesis, and Biological Evaluation of 3-Chloro-2-Oxo-N-(Arylcarbamoyl)-2H-1-Benzopyran-6-Sulfonamide Derivatives as Potential DPP-IV Inhibitors. Int. J. Health Sci. 2022, 6, 373–392. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Telagari, M.; Hullatti, K. In-Vitro α-Amylase and α-Glucosidase Inhibitory Activity of Adiantum Caudatum Linn. and Celosia Argentea Linn. Extracts and Fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [CrossRef]
- Sathish, M.; Nandhini, V.; Suresh, R. In Vitro Alpha Amylase and Alpha Glucosidase Enzyme Inhibition of Leaf Extracts of Jatropha Glandulifera Roxb. Res. J. Pharm. Technol. 2022, 15, 2493–2497. [Google Scholar] [CrossRef]
- Helen, P.A.M.; Bency, B.J. Inhibitory Potential of Amaranthus Viridis on α-Amylase and Glucose Entrapment Efficacy In Vitro. Res. J. Pharm. Technol. 2019, 12, 2089. [Google Scholar] [CrossRef]
- Chen, L.; Lu, X.; El-Seedi, H.; Teng, H. Recent Advances in the Development of Sesquiterpenoids in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 88, 46–56. [Google Scholar] [CrossRef]
- Inthongkaew, P.; Chatsumpun, N.; Supasuteekul, C.; Kitisripanya, T.; Putalun, W.; Likhitwitayawuid, K.; Sritularak, B. α-Glucosidase and Pancreatic Lipase Inhibitory Activities and Glucose Uptake Stimulatory Effect of Phenolic Compounds from Dendrobium Formosum. Rev. Bras. Farm. J. Pharmacogn. 2017, 27, 480–487. [Google Scholar] [CrossRef]
- Denisenko, Y.K.; Kytikova, O.Y.; Novgorodtseva, T.P.; Antonyuk, M.V.; Gvozdenko, T.A.; Kantur, T.A. Lipid-Induced Mechanisms of Metabolic Syndrome. J. Obes. 2020, 2020, 5762395. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; De Cássia Freitas, K.; De Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty Acids Consumption: The Role Metabolic Aspects Involved in Obesity and Its Associated Disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef]
- Zhou, H.; Yu, B.; Sun, J.; Liu, Z.; Chen, H.; Ge, L.; Chen, D. Short-chain fatty acids can improve lipid and glucose metabolism independently of the pig gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 61. [Google Scholar] [CrossRef]
- Takato, T.; Iwata, K.; Murakami, C.; Wada, Y.; Sakane, F. Chronic administration of myristic acid improves hyperglycaemia in the Nagoya–Shibata–Yasuda mouse model of congenital type 2 diabetes. Diabetologia 2017, 60, 2076–2083. [Google Scholar] [CrossRef]
- Hamilton, J.S.; Klett, E.L. Linoleic acid and the regulation of glucose homeostasis: A review of the evidence. Prostaglandins Leukot. Essent. Fat. Acids 2021, 175, 102366. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No “Strain”, No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Carradori, S.; Cairone, F.; Garzoli, S.; Fabrizi, G.; Iazzetti, A.; Giusti, A.M.; Menghini, L.; Uysal, S.; Ak, G.; Zengin, G.; et al. Phytocomplex Characterization and Biological Evaluation of Powdered Fruits and Leaves from Elaeagnus angustifolia. Molecules 2020, 25, 2021. [Google Scholar] [CrossRef]
- Al-Mrabeh, A. β-Cell Dysfunction, Hepatic Lipid Metabolism, and Cardiovascular Health in Type 2 Diabetes: New Directions of Research and Novel Therapeutic Strategies. Biomedicines 2021, 9, 226. [Google Scholar] [CrossRef]
- Qin, X.Y.; Hou, X.D.; Zhu, G.H.; Xiong, Y.; Song, Y.Q.; Zhu, L.; Zhao, D.F.; Jia, S.N.; Hou, J.; Tang, H.; et al. Discovery and Characterization of the Naturally Occurring Inhibitors Against Human Pancreatic Lipase in Ampelopsis Grossedentata. Front. Nutr. 2022, 9, 844195. [Google Scholar] [CrossRef]
- Marrelli, M.; Morrone, F.; Argentieri, M.P.; Gambacorta, L.; Conforti, F.; Avato, P. Phytochemical and Biological Profile of Moricandia arvensis (L.) DC.: An Inhibitor of Pancreatic Lipase. Molecules 2018, 23, 2829. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa, L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Mastinu, A.; Pira, M.; Pani, L.; Pinna, G.A.; Lazzari, P. NESS038C6, a novel selective CB1 antagonist agent with anti-obesity activity and improved molecular profile. Behav. Brain Res. 2012, 234, 192–204. [Google Scholar] [CrossRef]
- Lazzari, P.; Pau, A.; Tambaro, S.; Asproni, B.; Ruiu, S.; Pinna, G.; Mastinu, A.; Curzu, M.M.; Reali, R.; Bottazzi, M.E.; et al. Synthesis and pharmacological evaluation of novel 4-alkyl-5-thien-2′-yl pyrazole carboxamides. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 254–276. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Sanna, A.; Mara, L.; Pilichi, S.; Mastinu, A.; Chessa, F.; Pani, L.; Dattena, M. Oct4 expression in in-vitro-produced sheep blastocysts and embryonic-stem-like cells. Cell Biol. Int. 2009, 34, 53–60. [Google Scholar] [CrossRef]
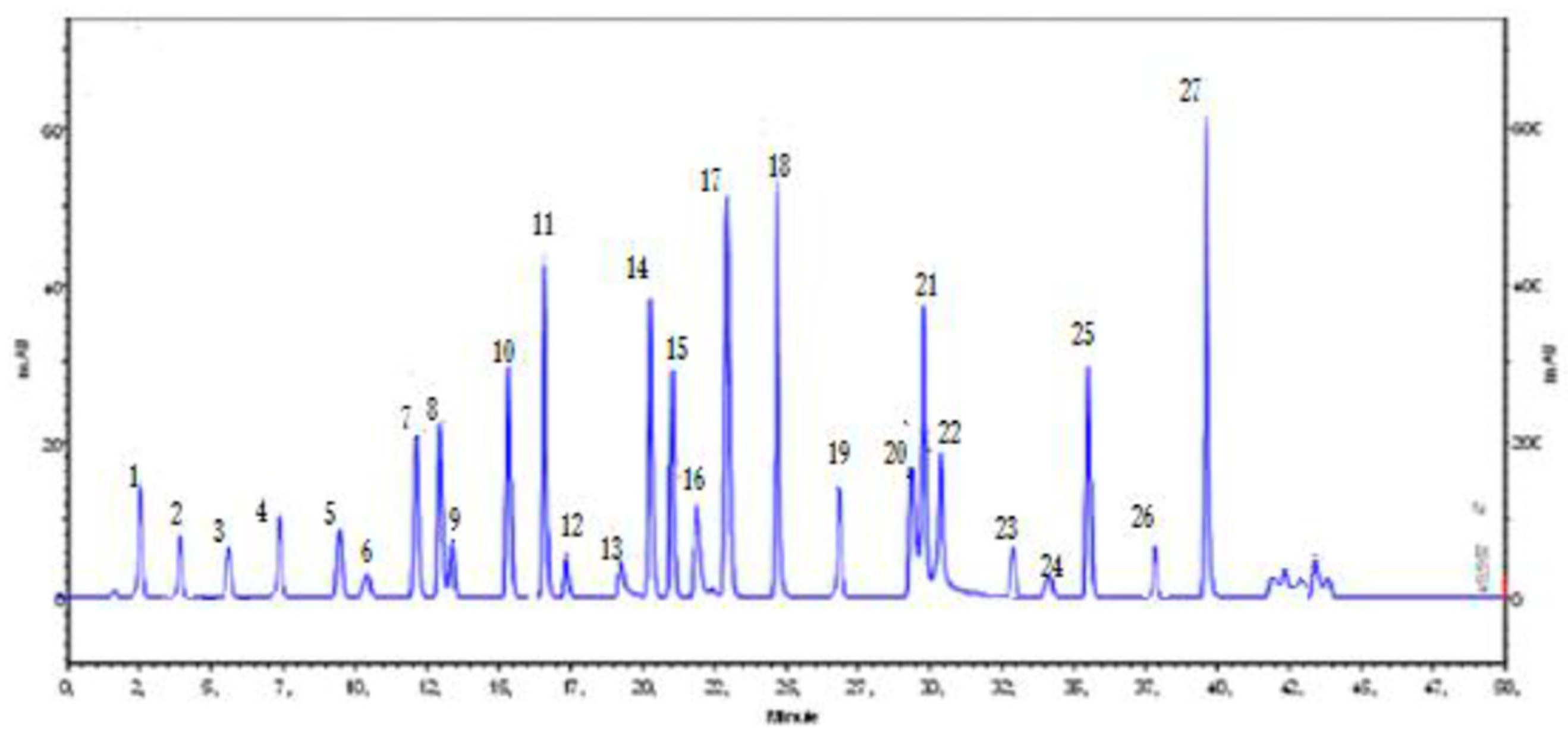

| Number | RT (min) | Compounds | Peak Area% |
|---|---|---|---|
| 1 | 2.0 | Azulene | 0.2 |
| 2 | 3.5 | 3-Octanol | 4.3 |
| 3 | 5.5 | p-cymene | 10.5 |
| 4 | 7.0 | Limonene | 2.1 |
| 5 | 9.5 | 3-nonanone | 0.2 |
| 6 | 10.2 | Unknown | 1.2 |
| 7 | 11.5 | Camphor | 3.5 |
| 8 | 12.1 | 1,8-Cineole | 0.2 |
| 9 | 12.5 | Unknown | 3.7 |
| 10 | 15.3 | p-Coumaric acid | 0.6 |
| 11 | 16.0 | Jasmone | 2.8 |
| 12 | 17.0 | Piperitone oxide | 4.3 |
| 13 | 19.5 | Unknown | 2.3 |
| 14 | 20.2 | Gallic acid | 1.1 |
| 15 | 20.5 | Caffeic acid | 0.8 |
| 16 | 21.8 | beta-Damascenone | 0.1 |
| 17 | 23.0 | Calamenene | 0.7 |
| 18 | 25.0 | Caryophyllene | 2.2 |
| 19 | 26.8 | δ-cadinene | 6.1 |
| 20 | 29.8 | Spathulenol | 7.4 |
| 21 | 30.0 | α-bisabolol | 4.7 |
| 22 | 30.8 | Myristic acid | 12.1 |
| 23 | 32.5 | Palmitic acid | 6.0 |
| 24 | 34.0 | Linolenic acid | 13.2 |
| 25 | 35.5 | Catechin | 0.4 |
| 26 | 38.0 | Quercetin | 2.5 |
| 27 | 40.0 | Chlorogenic acid | 5.5 |
| Total identified compounds % | 91.5% | ||
| RT: Retention Time | |||
| Fatty acids | 31.3% | ||
| Sesquiterpenes | 23.9% | ||
| Monoterpenes | 20.7% | ||
| Phenols | 7.2% | ||
| Alcohol | 4.3% | ||
| Flavonoids | 2.9% | ||
| Aromatic acid | 0.8% | ||
| Non-benzenoid aromatic hydrocarbon | 0.2% | ||
| Ketone | 0.2% | ||
| Ethanolic Extract of C. incana |
Positive Control (Acarbose) | |
|---|---|---|
| IC50 values of α-amylase (μg/mL) | 46.3 ± 0.2 | 33.5 ± 0.1 |
| Ethanolic extract of C. incana |
Positive control (Epigallocatechin gallate) | |
| IC50 values of α- glucosidase (μg/mL) | 56.8 ± 0.1 | 37.1 ± 0.2 |
| Ethanolic extract of C. incana |
Positive control (Orlistat) | |
| IC50 values of pancreatic lipase (μg/mL) | 639.9 ± 0.1 | 19.1 ± 0.2 |
| Ethanolic extract of C. incana |
Positive control (Sitagliptin) | |
| IC50 values of DPP-IV (μg/mL) | >600 | 18.6 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althaher, A.R.; Mastinu, A. Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action. Appl. Sci. 2023, 13, 3966. https://doi.org/10.3390/app13063966
Althaher AR, Mastinu A. Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action. Applied Sciences. 2023; 13(6):3966. https://doi.org/10.3390/app13063966
Chicago/Turabian StyleAlthaher, Arwa R., and Andrea Mastinu. 2023. "Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action" Applied Sciences 13, no. 6: 3966. https://doi.org/10.3390/app13063966
APA StyleAlthaher, A. R., & Mastinu, A. (2023). Calamintha incana (Sm.) Helder: A New Phytoextract with In Vitro Antioxidant and Antidiabetic Action. Applied Sciences, 13(6), 3966. https://doi.org/10.3390/app13063966







