Abstract
Alien invasive species (AIS) and non-native species are a prominent and extended problem in a wide range of areas in Europe and around the world. Centered in the Umia’s riparian forest, in Galicia, we found at least three main AIS needing to be controlled and harvested to preserve the biodiversity of the area. Previous studies probed that leaves and bark of selected species—Tradescantia fluminensis, Arundo donax, and Eucalyptus globulus—have important antioxidant properties, suitable for use in pharmaceutical and industrial contexts. A comparison of four solid-liquid extraction methods—Soxhlet extraction, ultrasound assisted extraction, thermal agitator, and infusion—was conducted in order to define the most efficient method in correlation within antioxidant (anthocyanins and total phenols) extraction. Water was selected as solvent, providing a sustainable research background without implying any chemical additives. The best extraction yields were obtained with Soxhlet extraction for all raw matter, with best results for Tradescantia fluminensis (41.89%) and Eucalyptus globulus leaves (39.35%); followed by the ultrasonic assisted extraction method, with better yield performed with Eucalyptus leaves (27.07%). On the contrary, Tradescantia fluminensis showed better efficiency with thermal agitator (35.07% compared to 23.19% from ultrasound extraction). After extractions, identification and quantification of total phenolic compounds and anthocyanins were carried out using spectrophotometric determination and acid hydrolysis in butanol method. In general, the best extraction yield in correlation with higher antioxidant content was performed by thermal agitator method, and Eucalyptus globulus leaves were demonstrated to be the better anthocyanins (6.18 ± 0.82 mg CC/g) and total phenols containers (172.40 ± 44.53 mg GAE/g). Studies provided promising possibilities for the residues of the three non-native species analyzed, as a source of antioxidants, favoring circular economy systems, as well as taking care of biodiversity in affected environments.
1. Introduction
Invasive alien species and non-native species represent a serious risk to the biodiversity of an area. One of the most significant affectations of natural ecosystems is biological invasion, which represents a threat to biodiversity conservation [1]. Due to globalization and the expansion-annexation of territories, the presence of non-native species has become increasingly common [2]. The presence of non-native riparian species is a growing problem that modifies and disturbs native forests, causing major problems for both flora and fauna. These situations must be controlled by applying management programs, which often include burning that would also damage native species in the surrounding area and is not considered an environmentally friendly solution. Other management alternatives produce bio waste by cutting and cleaning fields, which are often neglected as a valuable resource [2]. For these reasons, knowing the potential application of vegetable waste, we oriented our research towards an opportunity for a sustainable alternative to by-products derived from non-native species processing. This study was focused on the Umia riparian vegetation in Galicia, an area in which the restoration and respect of its biodiversity is being promoted. Derived from the ecosystem management decisions of the council area, there are several unused residues generated from the extraction of three of the non-native species. This waste has been used for the extraction and analysis of bio-compounds of each species, which could be used in the arts as pigments or colorants, as well as in food or pharmaceutical industries, due to the high concentration of antioxidants present in the extractions [2,3,4] (Figure 1).
Figure 1.
Extraction methods and selected species used in the present study.
The selected species for this study were Eucalyptus globulus, Tradescantia fluminensis, and Arundo donax, some of which are considered to have the most fast-spreading capacities in Mediterranean and humid ecosystems such as the Galician river forest [1].
Eucalyptus globulus: The genus Eucalyptus, part of the Myrtaceae family, consists of more than 900 species and subspecies of trees and shrubs [5]. Due to its characteristics, the Eucalyptus genus has high resistance to fire, drought, and soil acidity which, combined with its fast growing capacities, makes it an important source of resources. The main subspecies of Eucalyptus are: globulus, maidenii, biscotata, and pseudo globulus. They all originate from Tasmania and Australia. Eucalyptus globulus expanded throughout the world, especially in Mediterranean, subtropical, and tropical climate areas where its development was thriving, between 1800 and 1850 [6], although the first Eucalyptus planted in Europe was in 1774 at Kew Botanic Gardens [7]. According to the catalog of the exotic flora of Galicia by W. Weber [8], the species was first recorded in 1955 for cultivation and was recognized as invasive. Nowadays, this situation has changed; currently it is not catalogued as an invasive species in Spain, but as a non-native species. Despite the popular belief that Eucalyptus globulus was introduced into Galicia by Fray Rosendo Salvado, it is known that it was introduced around 1850 in a plantation in Santa Marta de Ortigueira, according to Silva-Pando and Pino Pérez (2016) [7]. In Australia, Eucalyptus occupies 95% of the wooded areas and in Spain between 1973 and 1995 the area occupied by this species increased from 390,277 to 460,000 ha. In 2019, according to the study ‘Eucalyptus in Galicia: relevant environmental and socioeconomic aspects’ published by COSE (Confederation of Forestry Organizations of Spain), it comprised 20% of the wooded population of Galicia and accounted for 60% of the wood destined for forestry felling. Eucalyptus bark has a rich chemical composition, but it is usually discarded when the wood is used for timber, paper, or charcoal. The waste is usually destined to be burned for energy [9] despite several studies that showed it could be interesting to use it for other applications such as soil conditioner [10], potential sorbent for removal of pollutants from an aqueous phase [11], bioethanol [8], and green adhesive based on tannins extracted from bark [12]. Eucalyptus leaves are usually scrapped in the timber and paper industries as well, and considered by-products also used for soil amendment and burned for energy; however, it has been shown that this biomass has valuable bioactive properties within biorefinery [13].
Arundo donax L. is colloquially known as reed, common reed, reed canary grass, wild reed, and thistle. The Arundo donax is a herbaceous, aquatic, and rhizomatous perennial species, part of the Poaceae family [3,14] which is considered one of the most aggressive invasive species and is recognized as such in many warm regions, including Oceania, Africa, and the Americas [15]. Due to its spreading capacities, it was considered to have high economic potential and it was cultivated in some regions [16,17]. Arundo is considered a native species from the Mediterranean climate and sub-tropical Eurasia, but the origin of invasive populations remains unknown [15]. Unlike Eucalyptus, Arundo donax has little genetic variation across its range. However, it is this lack of genetic variety that has raised questions about its origin in the Mediterranean basin and, according to Hardion et al. (2014), this redirected the search for species native to Asia. In Galicia it was first recorded in 1852 as an invasive species intended for cultivation. According to the Spanish Catalog of Invasive Alien Species, its entry into Western Europe is considered to have taken place in the 16th century, when we find references to it in Italy. From that time onwards, its dispersal occurred naturally by its own dispersal mechanisms, vegetatively by means of rhizomes. It tends to form continuous root masses, growing around huge areas making it impossible for native species to grow [2]. The mechanical properties of the stem have excellent applications in carpentry [18,19]. Due to its growing capacities and apart from the environmental protection point of view, this species has been used for many different means, such as raw material for bioenergy or biochemistry [14]. However, it has been shown that its chemical compounds could be used in industrial and even medicinal fields, as a potential source of antimicrobial agents [20], and other studies consider that its chemical compounds could improve fertility in industrially polluted soils and water masses [16].
Tradescantia fluminensis velloso is colloquially known as Men’s love and Cat’s ear. This species, part of the Commelinaceae family, is a ground-smothering perennial herb [3]. Its native area is South America, from the south-east of Brazil to Argentina. It was first recorded in Galicia in 1951 as an invasive species intended for cultivation but it was originally introduced as an ornamental plant. Tradescantia’s capacity for fast spreading in low light and humid spots between canopies in the forest and riverside areas does not allow for the growth of native seedlings and inhibits indigenous forest regeneration because of its shade [21]. As stated in the Spanish Catalogue of Invasive Alien Species, it is a species typical of shady and humid environments, which is why its development is favored on the Mediterranean, Galician, and Cantabrian coasts. Containing the spread of T. fluminensis is difficult due to the plant’s chemical resistance to herbicides and its fast recovery growth [3]. In relation with previous chemical analysis applied to other genus of the Commelinaceae family, it has been shown that they used to be a good source of renewable bioactive compounds [22] and to have anti-inflammatory, febrifuge, and diuretic properties that could be applied in medical care [23].
Samples of the species’ leaves and bark (Table 1) were collected in the riparian forest area of the Umia river in Ribadumia, Galicia, Spain (42°30′53.6″ N 8°45′58.3″ W) in January and February 2022, and the experiment was carried out simultaneously using the fresh samples, which is why the humidity of the leaves/bark was also taken into account during analysis. Depending on the specific properties of the species, samples were divided into leaves, stems, and bark. For E. globulus, we analyzed the bark and the leaves separately, for A. donax only the leaves were analyzed, and for T. fluminensis leaves and stems were analyzed together. The aim of this study was to determine the most efficient pigment extraction method from the leaves and the bark of Eucalyptus globulus, the leaves of Arundo donax L., and the leaves and stem of Tradescantia fluminensis. We also assessed the correlation with extraction method, species used, and phenolic-anthocyanins compounds. For this purpose, four solid-liquid extraction methods have been studied: Soxhlet, thermal agitator, infusion, and ultrasound. As we did not want to introduce chemical compounds into the final bio-solutions, water was chosen for the solvent. We aimed to obtain a bio-compostable liquid that could be used in eco-design and arts, as well as in food and 149 pharmaceutical applications.

Table 1.
Taxonomy of the selected species.
Phenolic compounds are synthesized in plants partly as a response to ecological and physiological pressures such as pathogens and insect attacks, UV radiation, and wounding [24]. Chemically, phenolic compounds vary widely, although their common characteristic is the presence of one (simple phenolics) or more (polyphenols) aromatic rings with bound hydroxyl groups in their structures. Depending on their structure, they can be grouped into phenolic acids, flavonoids, stilbenoids, and lignan [25]. Phenolic acids with C6-C1 structure are widely distributed in the plant kingdom, with the exception of fungi and algae [25]. In general, phenolic acids are divided into hydroxybenzoic and hydroxycinnamic acids according to their structural characteristics. Polyphenols, which include flavonoids, have at least two phenolic subunits; compounds with three or more phenolic subunits are called tannins (hydrolysable and non-hydrolysable). The basic structure of flavonoids is C6-C3-C6, this basic structure allows a multitude of substitution patterns and variations on the “C” ring, giving rise to subclasses [26].
Total phenolic compounds are a diverse group of compounds that include phenolic acids, flavonoids, stilbenoids, and lignans. They are known to have antioxidant properties. Total phenolic content can be measured using various methods, including the Folin–Ciocalteu assay, the ferric reducing antioxidant power (FRAP) assay, and the oxygen radical absorbance capacity (ORAC) assay. They are expressed as milligrams of gallic acid per gram of extract ().
Anthocyanins are a subclass of flavonoids. They have been shown to have antioxidant, anti-inflammatory, and anti-cancer properties, and may also have neuroprotective and cardiovascular benefits. Anthocyanin content can be measured using spectrophotometry, such as pH differential and high-performance liquid chromatography (HPLC) after separation of molecules based on their chemical properties. They are expressed as milligrams of cyanine chloride per gram of extract.
Phenolic compounds have the potential to be utilized in various sectors, but their extraction needs to be optimized to achieve high yields that are suitable for large-scale industrial production. To this end, there is a need for further research to develop efficient extraction methods that can provide a high concentration of phenolic compound [27], in relation with the raw matter used in the study. Due to the high concentration of anthocyanins and total phenolic compounds present in the different plant organs of non-invasive plants, the valorization of the by-products of our focal species is promising. In addition to obtaining value-added antioxidant compounds, we would promote the reuse of the waste generated by the cleaning process of the basin forest, in order to favor the biodiversity of the area. The development of these technologies, as well as defining the most efficient one, promotes land restoration and innovative industrial development.
2. Materials and Methods
2.1. Sample Preparation
Raw material, leaves, bark, and stems from the selected species E. globulus, A. donax, and T. fluminensis (Table 2), were manually collected in the river Umia basin forest, in Ribadumia, Galicia in January 2022. It was prepared in the Wood Chemical Transformation Laboratory of the School of Forestry Engineering, in Pontevedra (Galicia) during January and February 2022. The fresh raw material was cleaned and cut into pieces of 1–2 cm. A small part of each sample, around 2.00 g, was used to obtain the humidity percentile. This content for each type of sample is shown in Table 2.

Table 2.
Humidity content of the fresh samples.
Once the moisture calculation was completed, the rest of fresh-cut material was stored in boxes at room temperature throughout the experiment.
Due to the initial premise of the experiment of using sustainable products and processes, water was selected as the unique and specific solvent. It has also been proved that it gives the most efficient extraction yield results [4]. Water () is a polar protic solvent whose dielectric constant (F/m) is 80. It has a high polarity, its boiling temperature is 100 °C, and its density () at 20 °C is 1000. Using distilled water as a unique solvent has several advantages such as its polarity, non-toxic and non-inflammable properties, its capacity to dissolve a huge range of substances, and its affordable price. On the other hand, it is necessary to note that it has its limitations, such as the facility and fast promotion of bacterial and mold growth. At the same time, the energy and resources needed to concentrate the extract is higher than other solvents such as ethanol [3].
2.2. Extraction Methods
As we can see in Figure 2, we selected four solid-liquid (SL) extraction methods, and each one of those was carried out three times with each sample in order to obtain a true and consistent result. All the experiments were made with a minimum of 20 g of raw material (bark/leaves/stems) and 250 mL of distilled water. The proportion of temperature (°C) and time (min) can be shown in the following Table 3. Temperatures were selected by comparison with other studies: Soxhlet extraction was developed at solvent boiling point, 100 °C. Ultrasound-assisted extraction was carried out at 70 °C in order to reduce time to 40 min [9,24]. Thermal agitator was fixed at 40 °C as it was the most efficient medium temperature used in the study of Miguez et al., 2022, obtaining favorable yield results around 24%. Infusion extraction temperature was determined by humidity and ambient temperature of the laboratory, estimated at 20 °C.
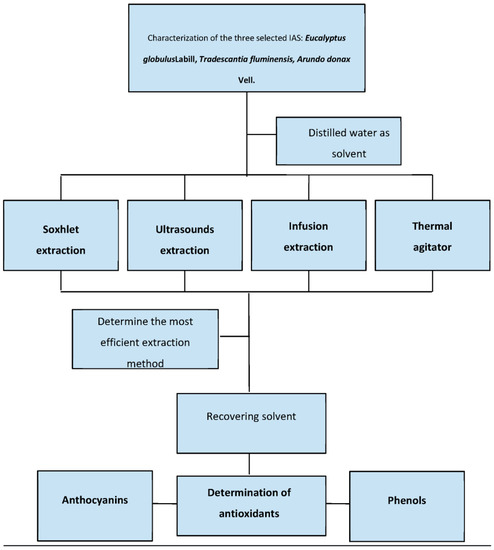
Figure 2.
Flowchart of the proposed methodology.

Table 3.
Experiments temperature and time.
2.2.1. Soxhlet Extraction (SOX)
The SOX was developed with a glass recipient and a cellulose cartridge into an extraction system and a heating mantle Nahita blue series 655. For this method, each sample of 20 g was placed in the thimble, with 250 mL of distilled water. It was developed in five cycles, which means, according to other studies [28], for each extraction of 2.5 h, one cycle runs in 30 min, and the temperature of the process was the boiling point of solvent, 100°. Other studies suggest that over 6 h higher yields are not obtained [29] and as cited in Álvarez et al. (2021), the solid-liquid extraction for Eucalyptus leaves with a Soxhlet extractor with water as solvent is around 30% for 7 h. On the contrary, our results showed that after 2.5 h the medium yield is 40%. Other studies have shown that Soxhlet extraction is the most effective method for the extraction of phenolic compounds [4].
After extraction, the cellulose cartridge, filled with the extracted solid sample, was introduced into the drying oven to calculate the final mass after extraction. This allowed us to obtain the yield taking into account the initial moisture.
2.2.2. Thermal Agitator Extraction (THA)
The shaking incubator used was a Benchtop Shaking Incubator Corning LSE model 6791 EU. This product is a combination of a shaker and a temperature chamber, designed for simultaneous shaking and heat treatment of samples. It works with a range of temperatures from 4 to 65 °C and a relative humidity of 85% without condensation. The shaking orbit is 19 mm and its temperature accuracy and stability is ±0.5 °C. Time can be set from 1 min to 99.0 h. This incubator allowed us to perform a solid-liquid extraction by applying heat and movement to four extractions at the same time. It was performed with four sample balloons with 20 g of raw matter and 250 mL of solvent each. Tests were developed by setting the machine at a temperature of 40 °C and in two cycles of 4 h each, finishing the full process after 8 h.
2.2.3. Ultrasound Extraction (ULS)
Ultrasound extraction was made with Bandelin Sonorex Super RK 102 H with a 35 kHz ultrasonic frequency, 1 to 15 min timer and continuous operation option and maximum temperature of 80 °C. SONOREX ultrasonic baths use the cavitation effect. Under the tank there are piezoelectric oscillating systems whose energy is transferred to the liquid, water, as mechanical oscillations with ultrasonic frequency. Microscopic bubbles are constantly generated in the liquid, which release energy when imploding and create local microcurrents.
Within the ULS machine characteristics it was possible to process two samples at once, working with 20 mg of sample mixed with 250 mL of the solvent each. In the study by Lima et al., temperature and time were set for 60 min at 50 °C for ethanol/water (50/50 v/v), and in the study Llorent-Martínez et al., less solvent was used (50 mL) and the time-temperature correlation was set at 60 min at 30 °C. In comparison with previous data, extraction temperature was set at 70 °C in order to reduce extraction time to 40 min, performing the fastest method of the study.
2.2.4. Infusion Extraction (IN)
The IN solid-liquid extraction took place by leaving 20 g of sample cut into small pieces in 250 mL of distilled water in a glass measuring cup, covered with a circular glass lid, for 48 h. Without heat or movement, after 48 h we filtered the dye from the residue of the samples.
2.2.5. Solvent Recovering
After the extraction, the liquid solutions were evaporated into the IKA RV 3V rotary evaporator (IKA, Staufen, Germany) at 100 °C until the liquid bio-solution was dense and concentrated, in order for this to be sent to the analysis performing laboratory CACTI. The extracted samples were finally dried in the oven with a temperature just under 100 °C, to verify the constant mass of the extract, then it was weighed in a pre-weighted holder and the total extraction yield was calculated.
2.3. Extraction Yield Calculation
Each total extraction yield was calculated by the Equation (1).
where:
Ytotal is the total extraction yield;
Hm is the humidity of the fresh sample;
Mraw is the fresh mass;
Ns is the number of samples of 20 gm × 250 mL in that experiment.
The extraction efficiency was proved to perform a pattern which allowed us to observe that Soxhlet extraction was the most efficient extraction method, followed by ultrasound extraction, thermal agitator and, in last place, infusion.
2.4. Chemical Characterization
The condensed samples were analyzed at the CACTI Laboratory in Ourense by spectrophotometric determination of total phenolic compounds and acid hydrolysis in butanol method for anthocyanins. Extractions were cataloged separately by plant species (Eucalyptus globulus, Tradescantia fluminensis, Arundo donax), type of sample (bark, leaves), and extraction methodology (SOX, THA, ULS, IN).
The total phenolics content were determined by the Folin–Ciocalteu method, using gallic acid as recommended [30]. This method works along with an alkaline medium and its transfer of electrons. It used the Cary-60 VIS-UV Agilent spectrophotometer and absorbance capacity was read at 760 nm; this result obtained a gallic calibration curve with five concentrations (0, 5, 10, 25, 50 mg/L). The absorbance was recorded for each extract and the total phenolic content was expressed as mg of gallic acid equivalent (GAE) per gram of plant matter. Results were expressed as TPC, mg GAE/g calculated with Equation (2).
where:
CGae is the concentration of total polyphenols (mg GAE/mL);
V is the volume of the extract (mL);
m is the part of the plant (g).
Anthocyanins measurements were developed by the acid hydrolysis in butanol method [31]. Absorbance was recorded at 530 nm and a cyanine calibration curve was prepared with five concentrations (30, 15, 10, 5, 0 mg/L). Following the standard method, 1 mg of commercial cyanine chloride was dissolved in 1 mL of methanol and distilled water was added in order to fill up the 5 mL flask to the mark. Results were expressed as mg cyanine chloride/g extract.
2.5. Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) 16.0 version (SPSS, Inc., Headquarters, Chicago, IL, USA) was used for the statistical analysis. All experiments were conducted in triplicates and their average was calculated and indicated in tables with the standard deviations. Comparisons were performed by Kruskal–Wallis test. The statistical results confirm the hypothesis that the differences between the results are either not significant (p > 0.05), significant (0.001 < p < 0.05), or highly significant (p < 0.001)
3. Results
The results obtained by the study were the correlation of efficiency of extraction method and raw matter used, as shown in Figure 3. The anthocyanins content in relation with the raw matter used and extraction method as shown in Table 4, and phenols content in relation with extraction method and raw material, as shown in Table 5.
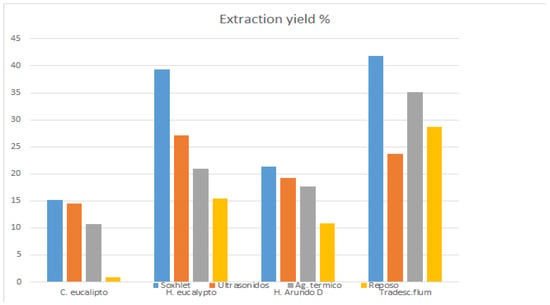
Figure 3.
Correlation of extraction yield efficiency by method and species.

Table 4.
Anthocyanins obtained, expressed as mg cyanine chloride/g extract.

Table 5.
Total phenolics obtained, expressed as mg gallic acid/g extract.
4. Discussion
A comparative study of different solvent extraction methods with six different Eucalyptus species [4] examined whether solid-liquid extraction by Soxhlet extraction is the most efficient method, compared to another study [28], in which sample size was also taken into account. Meanwhile, a literature review [3] probed distilled water as the most efficient non-polar solvent.
Our results illustrate that the Soxhlet extraction method was the most efficient for all types of sample, obtaining with the Tradescantia fluminensis Soxhlet extraction the highest yield level. These results are also confirmed by the Kruskal–Wallis tests performed. There were highly significant differences (p < 0.001) between Soxhlet extraction and the other extraction methods, which indicate the influence of this variable in the percentage of yields extracted (Figure 3). However, the Kruskal–Wallis tests showed significant differences between the concentrations for all species (0.001 < p < 0.05). These results open the discussion to analyze whether plant extractions are more efficient than tree extractions. Other authors suggested yields between 25% and 27% for Tradescantia fluminensis [3], making a distinction between leaves and stems, for thermal agitator extractions. In this study, thermal agitator extraction for Tradescantia yield is 35%, and it is higher with Soxhlet extraction, at 42%. Ultrasound extraction is the second most efficient method in contrast with another study [32], which proposes ultrasound-assisted extraction as the most efficient extraction method with water as solvent. Tradescantia fluminensis was the unique species that achieved better results with thermal agitator than ultrasound-assisted method, where it is under the medium level even after repetition of the experiment. Finally, the infusion method was confirmed to be the least efficient method for all samples (0.89–28.64%) [32]. The overall extraction was higher in Tradescantia fluminensis except for ultrasound extraction, where Eucalyptus globulus leaves obtained higher yields. Lowest yield results were in Eucalyptus globulus bark followed by Arundo donax.
Anthocyanins content was found in higher concentrations in Eucalyptus globulus leaves. As in another study [4] where Soxhlet extraction obtained 0.89 ± 0.01 mg CC/g, a medium content of 0.61 ± 0.07 mg CC/g was obtained. However, in comparison with Soxhlet extraction, higher results were obtained with thermal agitator extraction method, followed by ultrasound-assisted extraction and infusion (6.18 ± 0.82 mg CC/g; 3.18 ± 2.05 mg CC/g; 2.44 ± 0.09 mg CC/g). Eucalyptus bark extraction with ultrasound method also obtained remarkable results (2.64 ± 0.12 mg CC/g). Lower anthocyanins content was found in Tradescantia fluminensis, from Soxhlet extraction (0.13 ± 0.05 mg CC/g) compared to ultrasound extraction (0.27 ± 0.13 mg CC/g).
Eucalyptus globulus leaves demonstrate higher phenols concentration; thermal agitator obtained the highest results (172.40 ± 44.53 mg GAE/g), followed by ultrasound-assisted extraction and infusion extraction (94.27 ± 55.83 mg GAE/g; 57.39 ± 24.38 mg GAE/g), and with difference, Soxhlet extraction obtained the lowest results for Eucalyptus leaves (29.76 ± 1.86 mg GAE/g). In comparison with another study [4], where phenolic compounds with Soxhlet extraction using water as solvent for Eucalyptus globulus leaves were 7.68 ± 0.41 mg GAE/g, the developed experiments show better higher quantities.
In Eucalyptus globulus bark, results were also better for thermal agitator, followed by ultrasound extraction, Soxhlet extraction, and with lowest results, infusion.
Comparing obtained content for Tradescantia fluminensis with those obtained by study [3], for thermal agitator extraction at 45° in 6 h, (4.21 ± 1.65 mg GAE/g leaves and 1.96 ± 0.35 mg GAE/g stems) and [3] (1.90 ± 0.57 mg GAE/g leaves and 1.15 ± 0.31 mg GAE/g stems) results show that higher times and lower temperatures, and in-distinction of leaves and stems obtained higher phenols concentration (11.75 ± 0.60 mg GAE/g leaves and stems at 40° in 8 h). In relation with Arundo donax extraction, obtained results are higher than expected compared with study [2] (1.60 ± 0.22 mg GAE/g leaves 0.93 ± 0.42 mg GAE/g stems). Obtaining higher content with thermal agitator extraction (26.31 ± 0.00 mg GAE/g) followed by ultrasound extraction, Sohxlet, and infusion extraction (13.98 ± 0.00 mg GAE/g; 9.11 ± 0.00 mg GAE/g; 1.39 ± 0.00 mg GAE/g).
After analyzing all obtained results, it was demonstrated that there is a covariation between the extraction method and anthocyanins and phenolic content. Thermal agitator obtained the highest content in both anthocyanins and phenolic compounds. In contrast, Soxhlet and infusion extraction methods obtained the lowest concentrations, regardless of the plant organ.
In relation to raw matter and contents, Eucalyptus leaves have higher phenolic and anthocyanins content, and Tradescantia fluminensis have the lowest anthocyanin and phenolic concentration.
5. Conclusions
The Soxhlet (SO) method is confirmed as the most efficient method, but it is discarded as an option when it comes to extracting the antioxidant content from the plant residue, with the thermal agitator (THA) being the most efficient in relation to the content extracted. Tree species, such as Eucalyptus globulus, have a higher antioxidant content than herbaceous species, but this is mainly concentrated in the leaves.
The use of residual leaves from wood production with Eucalyptus globulus is presented as a good option for obtaining antioxidants that could be used in food or pharmaceutical industries. This would promote a circular economy in which the waste generated by forestry production could be used and not discarded first.
Author Contributions
Conceptualization, Á.C. and A.I.; methodology, Á.C. and A.I.; formal analysis, X.Á.; investigation, A.I.; writing—original draft preparation, A.I.; writing—review and editing, A.I. and Á.C.; visualization, X.Á.; supervision, Á.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Xunta de Galicia Predoctoral Grants 2021, grant number 290.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data collected for this article has been created under an ongoing doctoral thesis research. Due to this situation data base is not publish.
Acknowledgments
To the Engineering forestry school and its instalations and administrative and technical stuff for the technical support and materials for the experiments.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Bermúdez, X.; Abilleira, F. Conservación y Restauración del Bosque de Ribera. Impacto de Especies Invasoras, 1st ed.; Universidade de Vigo: Galicia, Spain, 2015. [Google Scholar]
- Míguez, C.; Cancela, A.; Sánchez, A.; Álvarez, X. Possibilities for Exploitation of Invasive Species, Arundo donax L., as a Source of Phenol Compounds. Waste Biomass Valorization 2022, 13, 4253–4265. [Google Scholar] [CrossRef]
- Míguez, C.; Cancela, A.; Álvarez, X.; Sánchez, A. The reuse of bio waste from invasive species Tradescantia fluminensis as a source of phenolic compounds. J. Clean. Prod. 2022, 336, 130293–130303. [Google Scholar] [CrossRef]
- Álvarez, X.; Cancela, Á.; Merchán, Y.; Sánchez, Á. Anthocyanins, Phenolic Compounds and Antioxidants from Extractions of Six Eucalyptus Species. Appl. Sci. 2021, 11, 9818. [Google Scholar] [CrossRef]
- Brooker, M.I.H.; Kleinig, D.A. Field Guide to Eucalyptus, 1st ed.; Inkata Press Pty Ltd.: Melbourne, Australia, 1983. [Google Scholar]
- Di Marco, E. Eucalyptus globulus sp. globulus Labill (eucalipto blanco) familia Myrtaceae. Prod. For. 2015, 5, 34–36. [Google Scholar]
- Silva-Pando, F.; Pino Pérez, R. Introduction of Eucalyptus into Europe. Aust. For. 2016, 79, 283–291. [Google Scholar] [CrossRef]
- Romero Buján, M.I. The alien flora of Galicia (Iberian northwest). Bot. Complut. 2007, 31, 113–125. [Google Scholar]
- Lima, M.A.; Lavorente, G.B.; Da Silva, H.K.; Bragatto, J.; Rezende, C.A.; Bernardinelli, O.D.; Polikarpov, I. Effects of pretreatment on morphology, chemical composition and enzymatic digestibility of eucalyptus bark: A potentially valuable source of fermentable sugars for biofuel production-part 1. Biotechnol. Biofuels 2013, 6, 75. [Google Scholar] [CrossRef]
- Yadav, K.R.; Sharma, R.K.; Kothari, R.M. Bioconversion of eucalyptus bark waste into soil conditioner. Bioresour. Technol. 2002, 81, 163–165. [Google Scholar] [CrossRef]
- Sen, A.; Pereira, H.; Olivella, A.; Villaescusa, I. Heavy metals removal in aqueous environments using bark as a biosorbent. Int. J. Environ. Sci. Technol. 2015, 12, 391–404. [Google Scholar] [CrossRef]
- Amari, M.; Khimeche, K.; Hima, A.; Checkout, R.; Mezroua, A. Synthesis of Green Adhesive with Tannin Extracted from Eucalyptus Bark for Potential Use in Wood Composites. J. Renew. Mater. 2020, 9, 463–475. [Google Scholar] [CrossRef]
- Rodrígues, V.; De Melo, M.; Portugal, I.; Silva, C. Extraction of Eucalyptus leaves using solvents of distinct polarity. Cluster analysis and extracts characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Corno, L.; Pilu, R.; Adani, F. Arundo donax L.: A non-food crop for bioenergy and bio-compound production. Biotechnol. Adv. 2014, 32, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Jámbor, A.; Török, Á. The economics of Arundo donax-A systematic literature review. Sustainability 2019, 11, 4225. [Google Scholar] [CrossRef]
- Nocentini, A.; Field, J.; Monti, A.; Paustian, K. Biofuel production and soil GHG emissions after land-use change to switchgrass and giant reed in the U.S Southeast. Food Energy Secur. 2018, 7, e00125. [Google Scholar] [CrossRef]
- Flores, J.A.; Pastor, J.J.; Martinez-Gabarron, A.; Gimeno-Blanes, F.J.; Rodríguez-Guisado, I.; Frutos, M.J. Arundo donax chipboard based on urea-for-maldehyde resin using under 4 mm particles size meets the standard criteria for indoor use. Ind. Crops Prod. 2011, 34, 1538–1542. [Google Scholar] [CrossRef]
- Pilu, R.; Badone, F.C.; Michela, L. Giant reed (Arund donax L.): A weed plant or a promising energy crop? Afr. J. Biotechnol. 2012, 11, 9163–9174. [Google Scholar]
- Licursi, D.; Antonetti, C.; Bernardini, J.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A.; Martinelli, M.; Raspolli Galleti, A.M. Characterization of the Arundo Donax L. solid residue from hydrothermal conversion: Comparison with technical lignins and applications perspectives. Ind. Crops Prod. 2015, 76, 1008–1024. [Google Scholar] [CrossRef]
- Standish, R.J.; Robertson, A.W.; Williams, P.A. The impact of an invasive weed Tradescantia fluminensis on native forest regeneration. J. Appl. Ecol. 2001, 38, 1253–1263. [Google Scholar] [CrossRef]
- Tan, J.B.L.; Yap, W.J.; Tan, S.Y.; Lim, Y.Y.; Lee, S.M. Antioxidant content, antioxidant activity, and antibacterial activity of five plants fro the commelinaceae family. Antioxidants 2014, 3, 758–769. [Google Scholar] [CrossRef]
- Alaba, C.S.M.; Chichioco-Hernandez, C.L. 15-Lipoxygenase inhibition of Commelina benghalensis, Tradescantia fluminensis, Tradescantia sebrina. Asian Pac. J. Trop. Biomed. 2014, 4, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.; Lourenço-Lopes, C.; Prieto, M.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Varela, P.; Álvarez, X.; Cancela, A.; Sánchez, A.; Valero, E. Use of Eucalyptus bark as tannin source. Eur. J. Sustain. Dev. 2015, 4, 9–14. [Google Scholar]
- Zhao, S.; Zhang, D. Supercritical CO2 extraction of Eucalyptus leaves oil and comparison with Soxhlet extraction and hydro-distillation methods. Sep. Purif. Technol. 2014, 133, 443–451. [Google Scholar] [CrossRef]
- Genwali, G.R.; Archarya, P.P.; Rajbhandari, M. Isolation of Galic Acid and estimation of total phenolic content in some medicinal plants and their antioxidant activity. J. Sci. Technol. 2013, 14, 95–102. [Google Scholar]
- Athomo, A.B.B.; Anris, S.E.; Safou-Tchiama, R.; Santiago-Medina, F.J.; Cabaret, T.; Pizzi, A.; Charrier, B. Chemical composition in African mahogany (K. ivorensis A. Chev) extractive and tannin structures of the bark by MALDI-TOF. Ind. Crops Prod. 2018, 113, 167–178. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.; Zengin, G.; Ibrahim Sinan, K.; Polat, R.; Canli, D.; Carene Nancy Picot-Allain, M.; Fawzi Mahomoodally, M. Impact of different extraction solvents and techniques on the biological activities of Cirsium yildizianum (Ateraceae: Cynareae). Ind. Crops Prod. 2020, 144, 112033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).