Featured Application
The findings of the present review study can be of great importance for European chestnut producers. The causal agent of brown rot causes significant economic losses and increases the amount of waste, not meeting sustainability principles.
Abstract
The European chestnut tree (Castanea sativa Mill.) has great economic importance, mostly due to the recognized nutritional value of its fruit. Thus, the development and improvement of the techniques of the production, preservation, and control of the diseases/pests of chestnut trees is a topic of great interest to producers, companies, researchers, and consumers to ensure the quality of this exceptional fruit. Recently, an emerging rot in chestnuts caused by the fungus Gnomoniopsis smithogilvyi (syn. Gnomoniopsis castaneae Tamietti) (Gnomoniaceae, Diaporthales) was reported both in Australia and Europe. Since then, the number of records of this pathogen in several countries of the world (Europe and Asia) where Castanea spp. is cultivated has been increasing. This disease, called “brown rot”, has been causing significant production losses, raising serious concerns for producers and the chestnut industry. This review describes the world distribution and life cycle of the causal agent of brown rot. The life cycle of G. smithogilvyi can involve primary infection, caused by ascospores, and secondary infection, related to the asexual phase of the fungus (conidia). Then, the analytical methods used to detect G. smithogilvyi are described. Furthermore, the incidences of the disease caused by G. smithogilvyi are presented, ranging from 5 to 94%, with high infection rates causing significant economic losses. The damages caused by G. smithogilvyi are discussed. In fact, it can act as an endophyte or as a pathogenic fungus, causing fruit rot, canker in several plant tissues, and necrosis in leaves, as well as in galls caused by the gall wasp Dryocosmus kuriphilus Yasumatsu. Possible pre- and post-harvest methods to mitigate the damage caused by moulds, and in particular G. smithogilvyi, are presented, including biocontrol agents and chemicals. Finally, some challenges and future prospects for a number of uncertainties related to the epidemiology, geographic distribution, spread, detection, and management of this disease are discussed.
1. Introduction
The chestnut is a fruit of great interest in many regions of the world, with a total production of approximately 2.3 million tonnes, with China being the largest producer, with 1,743,354 tonnes [1]. However, this country produces the species Castanea mollissima Blume, while European countries produce Castanea sativa Mill. In the worldwide and European chestnut productions, Portugal ranks in the seventh and fourth positions, respectively, with an annual production of 42,180 tonnes in 2020, distributed through 51,700 ha [1].
The production of chestnuts in Portugal has a significant economic impact, especially in the region of Trás-os-Montes, where the largest area of cultivation of the chestnut tree is concentrated (30,000 ha) [2]. However, in the last decades, the production of chestnuts in this region, as well as in other regions of the world, has greatly declined. Specifically, in the Trás-os-Montes region (Portugal), losses of 70% in the production of some chestnut varieties have been reported in some areas for the year 2022 [3]. Both abiotic and, particularly, biotic stress factors, such as pests (ex. Curculio spp. and Cydia spp.) and diseases (ex. canker), have been the main causes of this decline [4]. Curculio spp. and, in particular, Curculio elephas (an important pest of the European chestnut Castanea sativa [5]) may cause significant economic damages, approximately 25–30% per year in Turkey, as mentioned by Yaman et al. [6]. Furthermore, on average, 56.3 and 27.8% of the infested fruits contain one or two immatures (eggs and larvae) [7], showing the frequent presence of this chestnut weevil in the groves. More recently, when analyzing the loss of fruit traits due to damage by this weevil in four populations of healthy sweet chestnut trees in Turkey, Caliskan et al. [8] verified that the fruit weight loss percentages varied between 25% and 32%, demonstrating again the production and economic losses that this pest can cause to producers. Cydia splendana Hübner (Lepidoptera: Tortricidae) is another pest that chestnut producers face because the larvae feed on the fruits, reducing their quality and economic value. Significant attacks in Spain [9] and Hungary have been reported. Nevertheless, management strategies to control chestnut tortrix in Italy [10] and Hungary [11,12] have been developed.
Concerning diseases, the chestnut blight fungus Cryphonectria parasitica causes cankers, lesions caused by the growth of mycelia within the bark tissue of the host plant, which in some extreme situations may result in the death of all plant tissue distal to the canker [13]. This disease is frequently controlled using hypovirulent strains [14]. However, some challenges must be surpassed because it is known that the presence of other fungi in cankers may inhibit the hypovirulent form of the pathogen more than the virulent form, intensifying the canker development [13]. Moreover, ink disease is caused by the oomycete Phytophthora cinnamomi, which is a dangerous pathogen that causes root rot, threatening the production of chestnuts worldwide [15,16].
In the Trás-os-Montes region, this situation becomes even worse due to the newly introduced pest Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) and, more recently, the opportunistic fungal pathogen Gnomoniopsis smithogilvyi (syn. Gnomoniopsis castaneae) [4,17]. Dobry and Campbell [18] reported that the origin of the disease is not known. In Portugal, the presence of this fungus in chestnuts was reported for the first time in 2020 by Possamai [19] and then by Coelho and Gouveia [20]. Despite being an emerging disease in chestnuts, the presence of this fungus has already been reported in several European countries, as well as in Asia and Australia, being mainly responsible for the brown rot of chestnut tissues. The disease caused by brown rot in chestnut tissues is still difficult to understand. The fungus presence is not visible outside the fruit, even after it has already rotted inside. Nevertheless, G. smithogilvyi can be present in chestnut tissues without causing symptoms. However, due to the high incidence of the disease and because it is difficult to control and manage, G. smithogilvyi is now recognized as an emerging pathogen, threatening the production of chestnuts and challenging researchers, policymakers, and chestnut producers on a global scale. Dobry and Campbell [18] referred to this fungus as a ubiquitous endophyte; however, it may be shifted to pathogenic activity due to climate change. Under this premise, the purpose of this review is to provide a comprehensive view of the state of the art of the causal agent of this disease, highlighting the incidences of the disease and damages caused by this fungus, as well as to present potential strategies that can be applied for its management.
2. Gnomoniopsis smithogilvyi—Biology, Epidemiology, Symptomatology, and Identification
G. smithogilvyi is an ascomycete with a wide distribution, occurring in several countries in Europe, Asia, Australia, and America [21,22,23]. It has been reported to exhibit diverse lifestyles as an endophyte, inhabiting different asymptomatic chestnut tree plant tissues, and as a pathogen, causing “brown rot” in fruits [22,24,25]. G. smithogilvyi can also cause cankers, as reported by Dar and Rai [26] in India, Lewis et al. [27] in the United Kingdom, Trapiello et al. [28] in Spain, O´Loinsigh et al. [29] in Ireland, and Aglietti et al. [30]. In fact, the bark canker caused by G. smithogilvyi shows a symptomatology very similar to that caused by the chestnut blight pathogen Cryphonectria parasitica, which is the causal agent of chestnut canker [31], but the severity may be distinct. G. smithogilvyi has also been reported as a pathogen of several other species, affecting mainly nuts (hazelnuts (Corylus avellana L.), manna ash (Fraxinus ornus L.), holm oak (Quercus ilex L.), Turkey oak (Quercus cerris L.), and maritime pine (Pinus pinaster Aiton)), causing fruit rot, cankers, and necrosis in branches and leaves [18,21,22,24,25,31,32].
This fungus was initially identified independently by Shuttleworth et al. [33] and Visentin et al. [21] as G. smithogilvyi and Gnomoniopsis castaneae (originally called G. castanea), respectively. However, morphological and phylogenetic analyses (based on DNA sequencing) showed the synonymy between the two taxons [34]. Currently, G. smithogilvyi is the name adopted for this fungus [35]. Nevertheless, as stated by Sillo et al. [36], more data on the phylogeography of this fungus are needed. In fact, its origin and taxonomical features still remain unclear, causing debate about the possible existence of different lineages and the legitimate name of the species.
The life cycle of G. smithogilvyi and the process of how to infect chestnut have not yet been fully known. According to Shuttleworth and Guest [37], the primary infection is caused by ascospores that spend the winter on the infected plant debris lying on the soil surface of chestnut groves. During the flowering period, these ascospores are transported to flowers, leaves, and branches by wind and wind-driven rain and insects [22] (Figure 1). The fungus infects only the female part of the flower (pistil) through the stigma–style and not through the ovary wall [37]. The appearance of multiple embryos in the fruit, where one is rotted and the other healthy, suggests that the pellicle surrounding each embryo is impervious to the fungus [37]. Additionally, the infection of female flowers may also occur via G. smithogilvyi-infected pollen. The main means of entry into the other plant organs, such as branches and leaves, is through wounds caused by mechanical or natural injuries [26].
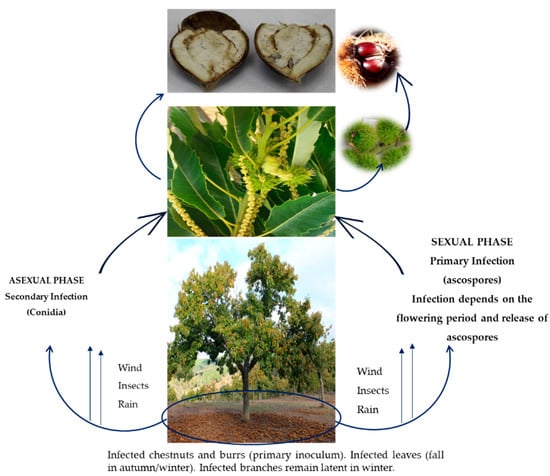
Figure 1.
Process of infection of the fungus Gnomoniopsis smithogilvyi. Source: Adapted from Shuttleworth and Guest [37].
Ascospore chestnut blossom infection is affected by several abiotic and biotic factors. The abiotic factors include temperature, rain, relative humidity, and wind. For example, an increase in temperature was reported to lead to a higher incidence of brown rot [38]. Likewise, Lione et al. [39] found that the propagule deposition rate of the G. smithogilvyi varies across seasons, being positively correlated with temperature, growing degree days at 0 and 5 °C thresholds, and wind gusts. Thus, it is expected that rising temperatures and strong winds due to climate change may increase the spread of the fungus in the future. This can be a problem because Arunrat et al. [40] predict that precipitation and the maximum and minimum temperatures will increase during three future periods, namely, near (2015–2039), mid (2040–2069), and far future (2070–2100), which may increase the incidence of the disease. These authors even suggest which major crops should be grown in order to reduce the negative impact of future climate changes, suggesting that the adaptation strategies of the cropping systems must be considered. Furthermore, Gullino et al. [41] reported that higher temperatures will allow the introduction and settlement of pests. Moreover, market globalization and transport, also linked with rising temperatures, had created favourable conditions for those pests. Therefore, in managing these pests, sustainability must always be considered.
Secondary infection, related to the asexual phase of the fungus (conidia), is not yet sufficiently studied. However, it is thought to be related to the attacks of the wasp D. kuriphilus, known as the chestnut gall wasp, and responsible for forming galls in the branches and leaves of chestnut trees [42,43]. Indeed, there are several reports of the presence of G. smithogilvyi conidia in these galls, which can be an inoculum source and thus promote fungus dispersion [42,43]. Although the role of this wasp on the G. smithogilvyi epidemiology is not yet documented, it was suggested that the stress induced on chestnut by D. kuriphilus attacks could lead to the transition of the endophyte G. smithogilvyi, naturally present in chestnut tissues, from a latent mode to an active virulent pathogen [44]. Likewise, Turco et al. [45] reported that the endophytic lifestyle of G. smithogilvyi may play a fundamental role in its epidemiology, allowing the asymptomatic colonization of the host tissues by the fungus.
The detection and accurate identification of G. smithogilvyi are critical steps for its understanding and control. Table 1 shows the methods most used in the detection of G. smithogilvyi. Most of these methods require the isolation of the fungus from previously surface-disinfected asymptomatic or symptomatic fruits in culture media, such as MEA (malt extract agar), MYA (malt yeast agar), or PDA (potato dextrose agar) media [22]. Furthermore, the fungus isolation can also be carried out from D. kuriphilus galls, G. smithogilvyi fruiting bodies on galls, chestnut branch tissues, chestnut gems and flowers, and chestnut burrs. The fungal isolates obtained are then identified mainly through molecular techniques. The identification based on the morphological features of the fungus may not be conclusive due to the similarities in the vegetative and reproductive structures between G. smithogilvyi and other closely related species colonizing the same plant tissues. Nevertheless, some authors [34] reported the morphological-based identification by analyzing and measuring conidia, fruitbodies, and mycelium.

Table 1.
Methods of diagnosis of G. smithogilvyi in European chestnut (Castanea sativa Mill.).
The molecular identification of G. smithogilvyi is mostly based on the sequencing of a segment of the encoding ribosomal RNA genes, which includes the entire internal transcribed spacer region (ITS) (ITS1-5.8S-ITS2), or the 28S gene of the large ribosomal subunit (LSU) (Table 1). The sequencing of various protein-coding genes has been used together with the ITS region. Among protein-coding markers, the second largest (RPB2) subunits of RNA polymerase, translation elongation factor 1-alpha (EF1-α), calmodulin, and beta-tubulin have been used to identify G. smithogilvyi reliably (Table 1). The technique of real-time PCR (qPCR) also proved to be effective in detecting and quantifying G. smithogilvyi in symptomatic and/or asymptomatic fruits, leaves, and branches of the chestnut tree [45,50]. More recently, the Loop-mediated isothermal AMPlification (LAMP) technique has been successfully used for the detection of G. smithogilvyi on chestnuts. Compared with the PCR and qPCR methods, the advantages of LAMP are: (i) it can be applied in the field [18]; (ii) it is faster and more user-friendly [18]; (iii) it requires less reagents; and (iv) it is more resistant to inhibitions, allowing for crude extract processing. As for the qPCR method, LAMP does not require prior isolation of the pathogen in a culture medium to detect it in the infected tissues [18,50]. Recently, a multiplex PCR was developed by Silva-Campos et al. [49] to detected the presence of G. smithogilvyi in chestnut fruit. This new method, based on the amplification of ITS, EF1-α, and beta-tubulin, is used as an internal control of the C. sativa gene petD in order to confirm that the negative results are due to the absence of G. smithogilvyi gDNA and not due to a reaction failure.
This fungus was identified by our research group this year (2022) in burrs and fruit shells collected in the Trás-os-Montes region (northeast of Portugal) (Figure 2). The identification of the fungus was made by sequencing the entire ITS region (ITS1-5.8S-ITS2), using the universal primers ITS1 and ITS4 [51].
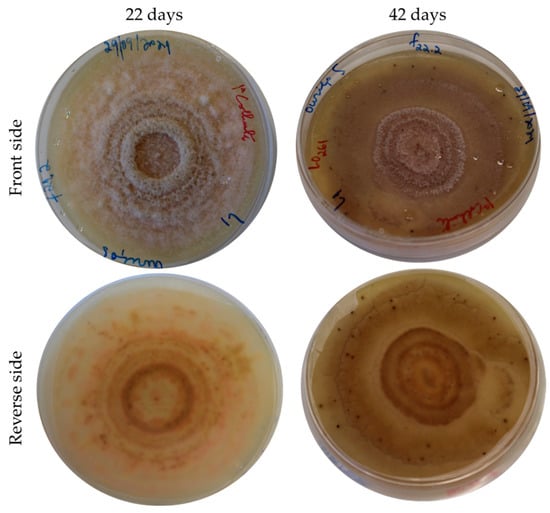
Figure 2.
Gnomoniopsis smithogilvyi isolated from the fruit shell after 22 and 42 days of cultivation in a PDA medium.
Table 2 compiles the main symptoms associated with this fungus when exhibiting pathogenic features, but also highlights the common presence of this fungus within several asymptomatic host tissues as an endophyte. This fact can lead to an underestimation of the infection values when the diagnosis is made solely by a visual inspection of the plant tissues/fruits, not including isolation or molecular detection methods.

Table 2.
Host of G. smithogilvyi in several species of Castanea, observed symptoms/asymptomatic, and incidence of the disease (%) (Source: Adapted from Lione et al. [22] and updated).
3. Incidences of the Disease Caused by G. smithogilvyi
G. smithogilvyi is already recognized today as one of the most severe threats to chestnut production due to the significant economic losses that this fungus can cause, mainly in post-harvest [21,22,55]. Table 2 compiles the incidence of the disease reported for G. smithogilvyi, ranging from 5 to 94%, in Chile and Italy, respectively. Specifically in Europe, the highest infection rates of fruit rot caused by G. smithogilvyi were reported in Switzerland and Italy (54 to 91% and 20 to 94%, respectively). Portugal still has a low value, less than 10%. Outside Europe, G. smithogilvyi has been reported to cause high levels of infection in Australia (around 72%), followed by India (33–58%) and Chile (5%).
The disease is mainly expressed after fruit harvesting, although there are already reports of its presence in fruits in the pre-harvest phase when fruits are still in the tree [21,38,42,47].
In Portugal, Driss [67] identified the presence of the fungus G. smithogilvyi in industrial samples of Portuguese chestnuts, having determined an infection rate of 6.4%. Likewise, Coelho and Gouveia [20] isolated and identified this fungus in commercial chestnut samples in 2017, 2018, and 2019, with a fruit infection ratio of 8.0, 5.3, and 5.0%, respectively. In five chestnut groves in the Trás-os-Montes region (northeast of Portugal), in 2018, the same authors found an incidence of the disease by G. smithogilvyi between 0 and 4.3%. Lione et al. [38], in twelve groves sampled in 2011 in northern Italy, found an incidence of the disease ranging from 20 to 94% in the fruits analyzed. Another study, which reports for the first time the presence of G. smithogilvyi in Chile, identified an average incidence of the disease of 4.8% (range equal to 3.7–6.5%) in 31,851 fruits surveyed from the market during 2018, 2019, and 2020 [23]. In Northern India (Kashmir), an incidence of the disease by G. smithogilvyi ranged from 33 to 58% in chestnuts harvested randomly from 2009 to 2013 [26]. The differences reported for the countries can be related to the climate [38], the occurrence of drought periods, and the presence of D. kuriphilus, which are stress factors for the chestnut trees [42] and may induce higher incidences of the disease. In fact, higher temperatures are associated with higher incidences of the disease [38]. Furthermore, it seems that areas that are more isolated may be a barrier to the settlement of this fungus [26], decreasing the disease incidence.
Therefore, these results demonstrate the possibility of high percentages of G. smithogilvyi infection in the near future, which will cause significant economic losses. In fact, producers are paid, taking into account the quality of the product supplied to the industry. The product is randomly sampled when it is delivered to the manufacturing facilities. Therefore, fruits with a high rot rate will entail a lower price paid to the producer, which has associated costs (request for labour, rental of harvesting machines, fuel, etc.) that may not be fully supported with the product sale. Thus, this activity can be abandoned due to a lack of profitability.
4. Damages Caused by G. smithogilvyi
Infection with the fungus G. smithogilvyi is responsible for the degradation of texture, dehydration, and alteration of the interior colour of the fruits, causing the loss of quality and commercial value of this nut [18,22,43].
The symptoms caused by the pathogenic fungus G. smithogilvyi have been reported mainly in Australia, Italy, and France. These include pre- and mostly post-harvest fruit rot, canker in various plant tissues (e.g., shoots and branches), and necrosis in leaves, as well as in galls caused by D. kuriphilus (Table 2).
Although the fungus G. smithogilvyi is already considered a danger to the production and transformation of chestnut, its presence is not yet widely disseminated in Portugal. Thus, it is essential to study this fungus to alert producers and find effective solutions for its control as soon as possible.
At the production level, producers have also faced the problem of the chestnut gall wasp (D. kuriphilus Yasumatsu) that causes severe damage to chestnut cultivation worldwide [17]. This insect attacks plants of the genus Castanea, inducing the formation of galls in the buds and leaves. These galls can reduce tree growth and nut production, leading to a significant reduction in fruit production and quality [69,70]. As previously reported, G. smithogilvyi has been observed in association with galls induced by D. kuriphilus [25,27,43,45,60,62,64,65,71]. In Spain, Fernández et al. [71] isolated G. smithogilvyi from necrotic galls, being the fungi with the highest frequency, closely followed by Fusarium avenaceum. However, there are contradictory results regarding the type of association G. smithogilvyi and chestnut gall wasp established, pointing in some cases to synergism [44] while in others to antagonism [43]. For example, Lione et al. [44] observed that the number of emerging adults of D. kuriphilus was significantly higher in galls colonized with G. smithogilvyi, suggesting a possible synergy between the pathogen and the pest. Vinale et al. [61] observed that G. smithogilvyi produced abscisic acid (ABA) and 1′,4′-trans-diol ABA inside necrotic galls. ABA has been considered a negative regulator of disease resistance [62]. Therefore, the colonisation of the pathogen in oviposited buds would be favoured. In contrast, Vannini et al. [43] observed an increase in the compaction and hardness of the necrotic galls due to the infection of G. smithogilvyi, which prevented the exit of the D. kuriphilus adults, who remained trapped. Thus, these results show the need to conduct further studies on this topic.
5. Strategies to Mitigate the Damage Caused by Moulds and, in Particular, G. smithogilvyi
Some works have been carried out at both the pre-harvest and post-harvest levels of the European chestnut to increase the shelf life of the fruits. Table 3 compiles the studies carried out so far on this topic.

Table 3.
Methods to prevent and control G. smithogilvyi during pre-harvest and post-harvest.
Applying good management practices in the orchards is essential for reducing the spread and inoculum density of G. smithogilvyi. Dobry and Campbell [18] suggest removing the litter after harvesting to reduce the inoculum. Nevertheless, as stated by the authors, more studies need to be carried out to show a positive relationship between the presence of litter and the incidence of the disease.
At the pre-harvest level, Pasche et al. [72] found that both Bacillus amyloliquefaciens and Trichoderma atroviride are promising biocontrol agents of G. smithogilvyi. In fact, the inoculation of the chestnut incisions of the cultivar Monti Cimini of Italy with suspensions of B. amyloliquefaciens or T. atroviride before grafting inhibited the growth of G. smithogilvyi in the bark tissues [72]. The same authors also observed a reduction in the symptoms of bark canker when these antagonists were applied [72]. It was now found that Trichoderma spp. isolates were able to suppress G. smithogilvyi growth through the production of volatile compounds (VOCs) and non-volatile compounds (nVOCs) [73]. On the other hand, Bacillus subtilis was also able to inhibit the fungus growth; however, its main effect was through nVOCs [73].
Bastianelli et al. [74] reports that the application of 300 mL/hL of Kalex Zn (crown spray) during blooming and burr formation efficiently controlled the disease. This product is a fertilizer of Alba Milagro®, composed of zinc phosphonate, potassium phosphite, and phosphonic acid, with a low environmental impact. However, since July 2022, all phosphonates were eliminated from the European fertilizers database as indicated in Regulation (EU) 2019/1009 of the European Parliament and the Council of 5 June 2019 [78]. However, new guidelines are expected regarding the application of these products in the field, considering their role in the orchard, and for which products they can be applied. Good results were also reported after the application of the fungicide Tebuconazole [74] (Mystic® 430 SC from Nufarm Italia Ltd., Milano, Italy). Silva-Campos et al. [75] studied the effectiveness of several fungicides by in vitro assays. Moreover, they verified that the most effective were those based on pyraclostrobin and difenoconazole, both effective in suppressing the conidial germination and mycelial growth of G. smithogilvyi. In the field, the treatment with both compounds simultaneously reduced the percentage of infected nuts. However, the use of this mixture should be used with care. It must be performed in rotation with other fungicides to avoid inducing resistance in G. smithogilvyi or other fungal populations [75].
Regarding post-harvest, the number of studies carried out until now to control the infections caused by G. smithogilvyi is also very scarce. Moreover, most of these studies focused on the control of the total microbial and fungal loads, with the species responsible for the symptoms on fruits not being specified. According to Donis-González et al. [77], fungi are responsible for significant economic losses in the post-harvest period. Fruit contamination takes place before and at the time of harvest, increasing during transport and storage. These authors, when studying the effect of several chemical disinfectants to mitigate the damage caused by moulds, found that the use of 2700 ppm of hydrogen peroxide + 200 ppm of peracetic acid (StorOx®), and 0.15 ppm of trifloxistrobin (Flint®) significantly reduced the presence of moulds in the bark and the incidence of the rot of the kernel. Among the treatments studied by Donis-González et al. [76], the combined use of chemical disinfectants with good agricultural practices and good post-harvest management was shown to be the most efficient for protecting chestnuts against the development of moulds and the appearance of rot in the post-harvest period. Moreover, Donis-González et al. [77], when studying the microbial contamination in peeled chestnuts, verified that the bacteria Rahnella spp. and Curtobacterium spp. and yeast Candida spp. were always isolated from vacuum-packaged samples, the methods with 2700 ppm hydrogen dioxide + 200 ppm peracetic acid, warm water at 65 °C, and X-ray irradiation being the most effective in reducing microbial counts. Nevertheless, some concerns arise when using chemical substances in high quantities because they may cause human health and environmental impacts. As stated by Toolkiattiwong et al. [79], even though the application of some pesticides may not exceed the recommended dose, the monitoring of some substances should be performed. This situation will depend on the chemical properties of the substance and its long-term health effects [79]. Furthermore, the use and the management of fertilizers must be improved because they are essential for reducing the crop water scarcity footprint and the water eutrophication footprint by decreasing the nitrogen leaching into waters [80].
Ozone application may be a good alternative to chemical disinfectants. Vettraino et al. [66] verified that ozone is an appropriate and economical tool to maximize the quality of chestnut shelf life, enabling it to be stored for long periods. Nevertheless, further studies on the ozone-based treatment of fruits must be carried out. In industry, the chestnuts are generally subjected to a hot-water treatment, called “curatura”, with the main objective of killing the larvae of pests, mainly Curculio spp. and Cydia spp. However, the addition of enzymes to this water, with the ability to lysate fungal cell walls, has been tested to control moulds in chestnuts during storage. For example, Ruocco et al. [63] showed that the addition of a mixture of enzymes obtained from the fungus Trichoderma harzianum Rifai strain T22 to the treatment with hot water (50 °C, 45 min) significantly reduced the incidence of rot caused by G. smithogilvyi. Additionally, they found that in chestnuts subject to this treatment, after two months of storage at room temperature, 50% remained in good condition, as opposed to 15% in untreated fruits.
Morales-Rodriguez et al. [81], when using hot water at different temperatures for the treatment of chestnut in the post-harvest period, found that the application of a temperature of 50 °C for a time of 30 or 45 min completely inactivated G. smithogilvyi, regardless of the level of colonization and stage of rot. However, this treatment did not reduce the impact of other moulds responsible for the chestnut’s external contamination and mycotoxin production. However, the same authors [81] observed that the increase in temperature and time of the treatment caused significant losses in fruit quality. In Portugal, all the chestnut fruits for exportation must be subjected to a hot-water treatment of at least 48 °C for 45 min in a continuous system (and 50 °C for 45 min in the case of exports to Canada) [82]. This treatment aims to eliminate insects or live larvae of Cydia pomonella, Cydia flagiglandana, Cydia splendana, and Curculio elephas. However, this process requires a large consumption of water and energy, and its efficacy in the inactivation of G. smithogilvyi is unknown.
In general, these methods show the importance of applying the correct pre- and post-harvest methods, and both must be considered and carried out together. On the one hand, it is essential to reduce the microbial load and dispersion in the orchards. Therefore, the application of agricultural practices and the addition of fertilizers and fungicides must be adequately performed. However, the possible creation of resistance to fungicides must be considered and always avoided. On the other hand, concerning the post-harvest methods, it is crucial to study the effect of common disinfectants, ozone, and hot-water treatment (with and without enzymes) in controlling G. smithogilvyi because the role and impact of these methodologies on this fungus are not well-known.
6. Future Prospects
Based on the literature review, it was found that more studies need to be performed, namely in:
- -
- the epidemiology of the disease caused by G. smithogilvyi, particularly the elucidation of the factors that determine the development of the disease. Indeed, it is crucial to understand the role played by biotic (pests, pathogens, etc.) and abiotic (climate change, chestnut grove management, etc.) factors on the transition of the endophyte lifestyle to the active virulent pathogen;
- -
- the geographic distribution and frequency of G. smithogilvyi. The presence of this fungus has already been reported in various regions of the world, but further studies should be carried out in other countries that are important producers of chestnuts, to better know its distribution in the world;
- -
- the relationships of G. smithogilvyi with other fungi, bacteria, cankers, and necrosis of the chestnut tissues. It is essential to find antagonists to this fungus and better understand the metabolic processes that originate cankers and necrosis in leaves and branches. Furthermore, it is necessary to understand the mechanisms that cause the shift from the endophytic to the pathogenic phase;
- -
- the relationship between the wasp D. kuriphilus and G. smithogilvyi, in order to better understand the role played by this insect on the incidence of the disease;
- -
- the identification of methods and/or tools to manage the disease caused by G. smithogilvyi. These may include the identification of C. sativa cultivars that are resistant or at least more tolerant to G. smithogilvyi or improve host plant resistance to the pathogen through breeding programs. This information will be of great interest to producers to help them choose the plant material to use in new plantations as well as to prevent the disease’s development in new plantations. A potential additional measure includes the identification of biocontrol agents, such as micro-organisms, with efficacy against G. smithogilvyi. Furthermore, several cultural practices, such as the removal of fallen burrs (in which the teleomorph stage of the fungus develops) as well as of severely infected plant residues in the field, may all have important implications on the spread of G. smithogilvyi and disease management;
- -
- the identification of the means of spread of the pathogen, either at short or long distances. Indeed, G. smithogilvyi could potentially spread at short distances via natural means (e.g., wind, water splash, and insects) and/or at long distances via human-assisted means (e.g. movement of infected host plants for planting), similarly to other pathogenic fungi;
- -
- the development of methods for the early detection G. smithogilvyi in the groves;
- -
- the elucidation of the mycotoxigenic potential of G. smithogilvyi. This work is extremely important, since its potential is not known;
- -
- the identification of post-harvest management methods for reducing G. smithogilvyi growth, such as the use of disinfectants.
7. Conclusions
There is still a long way to obtain more knowledge on G. smithogilvyi.
It is important to: (i) understand its biological cycle; (ii) elucidate the infection processes that cause canker and necrosis in leaves and branches; (iii) know its role on the galls of D. kuriphilus; (iv) understand the mechanisms that trigger the change from the endophytic to pathogenic phase; (v) understand what ecological factors can be the precursors of outbreaks of this fungus; and (vi) find post-harvest technologies to manage this pathogen, such as the use of disinfectants, ozone, and hot-water treatment (with and without enzymes) in controlling G. smithogilvyi.
This fungus is able to depreciate the quality of chestnut very significantly and affects its production. Thus, all potential biotic interactions must be analyzed. In addition, the precautionary principle should be considered when considering the risk associated with this pathogen on a global or local scale.
Author Contributions
Conceptualization, E.R. and P.B.; investigation, F.L.; resources, E.R., P.B. and C.O.; data curation, F.L.; writing—original draft preparation, F.L.; writing—review and editing, E.R., P.B. and C.O.; supervision, E.R., P.B. and C.O.; project administration, E.R., P.B. and C.O.; funding acquisition, E.R., P.B. and C.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal), which gave financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020), SusTEC (LA/P/0007/2021), and LEAF (UID/AGR/04129/LEAF). Filipe Lema acknowledge the Portuguese Foundation for Science and Technology (FCT, Portugal) for the financial support provided by the research grant PRT/BD/154025/2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAOSTAT. Food and Agriculture Organization of the United Nations (FAO). ‘Production of Chestnut by Countries’, Statistics Division. 2020. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 21 November 2022).
- Laranjo. Manual Técnico Castanheiro: Estado da Produção; Centro Nacional de Competências dos Frutos Secos: Bragança, Portugal, 2017. [Google Scholar]
- Viva Douro, 2022. Produção de Castanha Martaínha cai 70% em 2022. Newsletter of 18 November 2022. Available online: http://www.public.vivadouro.org/informacao/sociedade/producao-de-castanha-martainha-cai-70-em-2022/#:~:text=Produ%C3%A7%C3%A3o%20de%20castanha%20Marta%C3%ADnha%20cai%2070%25%20em%202022,aponta-se%20para%20uma%20quebra%20de%2070%25%20na%20produ%C3%A7%C3%A3o (accessed on 6 February 2023).
- Ministério da Agricultura. Sub-Fileira: Castanha. Gabinete de Planeamento e Políticas; Castel—Publicações E Edições, Sa: Lisbon, Portugal, 2007; p. 17. [Google Scholar]
- Menu, F.; Debouzie, D. Larval development variation and adult emergence in the chestnut weevil Curculio elephas Gyllenhal (Col., Curculionidae). J. Appl. Entomol. 1995, 119, 279–284. [Google Scholar] [CrossRef]
- Yaman, M.; Demirbag, Z.; Beldüz, A.O. Investigations on the bacterial flora as a potential biocontrol agent of chestnut weevil, Curculio elephas (Coleoptera: Curculionidae) in Turkey. Biologia 1999, 54, 677–681. [Google Scholar]
- Desouhant, E. Selection of fruits for oviposition by the chestnut weevil, Curculio elephas. Entomol. Exp. Et Appl. 1998, 86, 71–78. [Google Scholar] [CrossRef]
- Caliskan, S.; Göltaş, M.; Aslan, V.; Özer, G.; Tandoğan, M.; Sezgin, G.; Cebeci, H. Variation in fruit traits and infestation ratios in natural sweet chestnut (Castanea sativa) populations under chestnut weevil (Curculio elephas) damage. Biologia 2020, 75, 2287–2294. [Google Scholar] [CrossRef]
- Cuestas, M.I.; Martin, M.A.; Aldebis, H.K.; Mena, J.D.; Martin, L.M.; Vargas-Osuna, E. Differential response among chestnut traditional varieties to the attack of Cydia splendana. Neth. Entomol. Soc. Entomol. Exp. Et Appl. 2020, 168, 259–265. [Google Scholar] [CrossRef]
- Ferracini, C.; Pogolotti, C.; Rama, F.; Lentini, G.; Saitta, V.; Mereghetti, P.; Mancardi, P.; Alma, A. Pheromone-Mediated Mating Disruption as Management Option for Cydia spp. in Chestnut Orchard. Insects 2021, 12, 905. [Google Scholar] [CrossRef]
- Jósvai, J.K.; Voigt, E.; Tóth, M. A pear ester-based female-targeted synthetic lure for the chestnut tortrix, Cydia splendana. Entomol. Exp. Et Appl. 2016, 159, 370–374. [Google Scholar] [CrossRef]
- Karagoz, M.; Gulcub, B.; Hazirb, S.; Kaya, H.K. Laboratory evaluation of Turkish entomopathogenic nematodes forsuppression of the chestnut pests, Curculio elephas (Coleoptera: Curculionidae) and Cydia splendana (Lepidoptera: Tortricidae). Biocontrol Sci. Technol. 2009, 19, 755–768. [Google Scholar] [CrossRef]
- Kolp, M.; Fulbright, D.W.; Jarosz, A.M. Inhibition of virulent and hypovirulent Cryphonectria parasitica growth in dual culture by fungi commonly isolated from chestnut blight cankers. Fungal Biol. 2018, 122, 935–942. [Google Scholar] [CrossRef]
- Ježić, M.; Kolp, M.; Prospero, S.; Karin-Kujundžić, V.; Idžojtić, M.; Sotirovski, K.; Risteski, M.; Rigling, D.; Double, M.L.; Ćurković-Perica, M. Diversity of parasitica in callused chestnut blight cankers on European and American chestnut. For. Pathol. 2019, 49, e12566. [Google Scholar] [CrossRef]
- Camisón, Á.; Martín, M.Á.; Sánchez-Bel, P.; Flors, V.; Alcaide, F.; Morcuende, D.; Pinto, G.; Solla, A. Hormone and secondary metabolite profiling in chestnut during susceptible and resistant interactions with Phytophthora cinnamomi. J. Plant Physiol. 2019, 241, 153030. [Google Scholar] [CrossRef] [PubMed]
- Zhebentyayeva, T.N.; Sisco, P.H.; Georgi, L.L.; Jeffers, S.N.; Perkins, M.T.; James, J.B.; Hebard, F.V.; Saski, C.; Nelson, C.D.; Abbott, A.G. Dissecting Resistance to Phytophthora cinnamomi in Interspecific Hybrid Chestnut Crosses Using Sequence-Based Genotyping and QTL Mapping. Phytopathology 2019, 109, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- INE. Estatísticas Agrícolas 2020; Instituto Nacional de Estatística: Lisbon, Portugal, 2020; ISSN 0079-4139. [Google Scholar]
- Dobry, E.; Campbell, M. Gnomoniopsis castaneae: An emerging plant pathogen and global threat to chestnut systems. Plant Pathol. 2023, 72, 218–231. [Google Scholar] [CrossRef]
- Possamai, G. Podridão da Castanha em Trás-os-Montes: Caracterização Morfológica, Ecofisiológica e Molecular do Agente Causal Gnomoniopsis smithogilvyi. Master’s Thesis, Biotechnological Engineering, Instituto Politécnico de Bragança, Lisbon, Portugal, 2020; p. 85. Available online: https://bibliotecadigital.ipb.pt/bitstream/10198/23171/4/Possamai_Guilherme_Podrida%cc%83o_da_Castanha_em_Tra%cc%81s_os_Montes.pdf (accessed on 20 March 2022).
- Coelho, V.; Gouveia, E. Gnomoniopsis smithogilvyi, the causal agent of chestnut brown rot reported from Portugal. New Dis. Rep. 2021, 43, e12007. [Google Scholar] [CrossRef]
- Visentin, I.; Gentile, S.; Valentino, D.; Gonthier, P.; Tamietti, G.; Cardinale, F. Gnomoniopsis castanea sp. nov. (Gnomoniaceae, Diapothales) as the causal agent of nut rot in sweet chestnut. J. Plant Pathol. 2012, 94, 411–419. [Google Scholar]
- Lione, G.; Danti, R.; Fernandez-Conradi, P.; Ferreira-Cardoso, J.V.; Lefort, F.; Marques, G.; Meyer, J.B.; Prospero, S.; Radócz, L.; Robin, C.; et al. The emerging pathogen of chestnut Gnomoniopsis castaneae: The challenge posed by a versatile fungus. Eur. J. Plant Pathol. 2019, 153, 671–685. [Google Scholar] [CrossRef]
- Cisterna-Oyarce, V.; Carrasco-Fernandez, J.; Castro, J.F.; Santelices, C.; Munoz-Reyes, V.; Millas, P.; Alan, G.; Buddie, A.G.; France, A. Gnomoniopsis smithogilvyi: Identification, characterisation and incidence of the main pathogen causing brown rot in postharvest sweet chestnut fruits (Castanea sativa) in Chile. Australas. Plant Dis. Notes 2022, 17, 3. [Google Scholar] [CrossRef]
- Fernandez-Conradi, P. Diversité des Arbres et Résistance des Forêts Aux Invasions Biologiques: Application au Châtaignier et Son Complexe de Bioagresseurs Exotiques, Chancre (Cryphonectria parasitica) et Cynips (Dryocosmus kuriphilus). Interactions Entre Organismes. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2017. [Google Scholar]
- Fernandez-Conradi, P.; Borowiec, N.; Capdevielle, X.; Castagneyrol, B.; Maltoni, A.; Robin, C.; Selvi, F.; Halder, I.V.; Ve´tillard, F.; Jactel, H. Plant neighbour identity and invasive pathogen infection affect associational resistance to an invasive gall wasp. Biol. Invasions 2017, 20, 1459–1473. [Google Scholar] [CrossRef]
- Dar, M.A.; Rai, M.K. Gnomoniopsis smithogilvyi a canker causing pathogen on Castanea sativa: First report. Mycosphere 2015, 6, 327–336. [Google Scholar] [CrossRef]
- Lewis, A.; Gorton, C.; Rees, H.; Webber, J.; Pérez-Sierra, A. First report of Gnomoniopsis smithogilvyi causing lesions and cankers of sweet chestnut in the United Kingdom. New Dis. Rep. 2017, 35, 20. [Google Scholar] [CrossRef]
- Trapiello, E.; Feito, I.; González, A.J. First report of Gnomoniopsis castaneae causing canker on hybrid plants of Castanea sativa x C. crenata in Spain. Plant Dis. 2017, 102, 1040. [Google Scholar] [CrossRef]
- O´Loinsigh, B.; McAuley, D.; Bréchon, A.L.; Lopez Vernaza, M.; Ryan, C.; Destefanis, M.L.; O’Hanlon, R. First report of the fungus Gnomoniopsis smithogilvyi causing cankers on sweet chestnut (Castanea sativa) in Ireland. New Dis. Rep. 2022, 45, e12072. [Google Scholar] [CrossRef]
- Aglietti, C.; Cappelli, A.; Andreani, A. From Chestnut Tree (Castanea sativa) to Flour and Foods: A Systematic Review of the Main Criticalities and Control Strategies towards the Relaunch of Chestnut Production Chain. Sustainability 2022, 14, 12181. [Google Scholar] [CrossRef]
- Pasche, S.; Calmin, G.; Auderset, G.; Crovadore, J.; Pelleteret, P.; Mauch-Mani, B.; Barja, F.; Paul, B.; Jermini, M.; Lefort, F. Gnomoniopsis smithogilvyi causes chestnut canker symptoms in Castanea sativa shoots in Switzerland. Fungal Genet. Biol. 2016, 87, 9–21. [Google Scholar] [CrossRef] [PubMed]
- EPPO—European and Mediterranean Plant Protection Organization (2017). EPPO Reporting Service No. 02–2017 Num. Article: 2017/047. Available online: http://archives.eppo.int/EPPOReporting/2017/Rse-1702.pdf (accessed on 25 February 2022).
- Shuttleworth, L.A.; Liew, E.C.Y.; Guest, D.I. Fungal Planet Description Sheet 108: Gnomoniopsis smithogilvyi. Persoonia 2012, 28, 142–143. Available online: http://www.fungalplanet.org/content/pdf-files/FungalPlanet108.pdf (accessed on 25 February 2022).
- Shuttleworth, L.A.; Walker, D.M.; Guest, D.I. The chestnut pathogen Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) and its synonyms. Mycotaxon 2015, 130, 929–940. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Guest, D.I.; Walker, D.M. The fungus the Code and the mysterious publication date: Why Gnomoniopsis smithogilvyi is still the correct name for the chestnut rot fungus. IMA Fungus 2018, 9, A78–A79. [Google Scholar] [CrossRef]
- Sillo, F.; Giordano, L.; Zampieri, E.; Lione, G.; De Cesare, S.; Gonthier, P. HRM analysis provides insights on the reproduction mode and the population structure of Gnomoniopsis castaneae in Europe. Plant Pathol. 2017, 66, 293–303. [Google Scholar] [CrossRef]
- Shuttleworth, L.A.; Guest, D.I. The infection process of chestnut rot, an important disease caused by Gnomoniopsis smithogilvyi (Gnomoniaceae, Diaporthales) in Oceania and Europe. Aust. Plant Pathol. 2017, 46, 397–405. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Sillo, F.; Gonthier, P. Testing and modelling the effects of climate on the incidence of the emergent nut rot agent of chestnut Gnomoniopsis castanea. Plant Pathol. 2015, 64, 852–863. [Google Scholar] [CrossRef]
- Lione, G.; Giordano, L.; Sillo, F.; Brescia, F.; Gonthier, P. Temporal and spatial propagule deposition patterns of the emerging fungal pathogen of chestnut Gnomoniopsis castaneae in orchards of north-western Italy. Plant Pathol. 2021, 70, 2016–2033. [Google Scholar] [CrossRef]
- Arunrat, N.; Sereenonchai, S.; Chaowiwat, W.; Wang, C. Climate change impact on major crop yield and water footprint under CMIP6 climate projections in repeated drought and flood areas in Thailand. Sci. Total Environ. 2022, 807, 150741. [Google Scholar] [CrossRef]
- Gullino, M.L.; Albajes, R.; Al-Jboory, I.; Francislene Angelotti, F.; Chakraborty, S.; Garrett, K.A.; Hurley, B.P.; Juroszek, P.; Lopian, R.; Makkouk, K.; et al. Climate Change and Pathways Used by Pests as Challenges to Plant Health in Agriculture and Forestry. Sustainability 2022, 14, 12421. [Google Scholar] [CrossRef]
- Maresi, G.; Oliveira-Longa, C.M.; Turchetti, T. Brown rot on nuts of Castanea sativa Mill: An emerging disease and its causal agent. Iforest—Biogeosciences For. 2013, 6, 294–301. [Google Scholar] [CrossRef]
- Vannini, A.; Vettraino, A.M.; Martignoni, D.; Morales-Rodriguez, C.; Contarini, M.; Caccia, R.; Paparatti, B.; Speranza, S. Does Gnomoniopsis castanea contribute to the natural biological control of chestnut gall wasp? Fungal Biol. 2017, 121, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Lione, G.; Giordano, L.; Ferracini, C.; Alma, A.; Gonthier, P. Testing ecological interactions between Gnomoniopsis castaneae and Dryocosmus kuriphilus. Acta Oecologica 2016, 77, 10–17. [Google Scholar] [CrossRef]
- Turco, S.; Bastianelli, G.; Morales-Rodrìguez, C.; Vannini, A.; Mazzaglia, A. Development of a TaqMan qPCR assay for the detection and quantification of Gnomoniopsis castaneae in chestnut tissues. For. Pathol. 2021, 51, e12701. [Google Scholar] [CrossRef]
- Aguín, O.; Rial, C.; Piñón, P.; Sainz, M.J.; Mansilla, J.P.; Salinero, C. First Report of Gnomoniopsis smithogilvyi Causing Chestnut Brown Rot on Nuts and Burrs of Sweet Chestnut in Spain. Plant Dis. 2023, 107, 218. [Google Scholar] [CrossRef]
- Dennert, F.G.; Broggini, G.A.L.; Gessler, C.; Storari, M. Gnomoniopsis castanea is the main agent of chestnut nut rot in Switzerland. Phytopathol. Mediterr. 2015, 54, 199–211. [Google Scholar] [CrossRef]
- Meyer, J.B.; Trapiello, E.; Senn-Irlet, B.; Sieber, T.N.; Cornejo, C.; Aghayeva, D.; González, A.J.; Prospero, S. Phylogenetic and phenotypic characterisation of Sirococcus castaneae comb. nov. (synonym Diplodina castaneae), a fungal endophyte of European chestnut. Fungal Biol. 2017, 121, 625–637. [Google Scholar] [CrossRef]
- Silva-Campos, M.; Nadiminti, P.; Cahill, D. Rapid and Accurate Detection of Gnomoniopsis smithogilvyi the Causal Agent of Chestnut Rot, through an Internally Controlled Multiplex PCR Assay. Pathogens 2022, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, A.M.; Luchi, N.; Rizzo, D.; Pepori, A.L.; Pecori, F.; Santini, A. Rapid diagnostics for Gnomoniopsis smithogilvyi (syn. Gnomoniopsis castaneae) Chestnut Nuts: New Chall. By Using LAMP Real-Time PCR Methods. AMB Express 2021, 11, 105. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, S.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Medina-Mora, C.M.; Kolp, M.; Fulbright, D.W. First report of Gnomoniopsis smithogilvyi causing chestnut brown rot on chestnut fruit in Michigan. Plant Dis. 2019, 103, 2134. [Google Scholar] [CrossRef]
- Smith, H.C.; Agri, M. The life cycle, pathology and taxonomy of two different nut rot fungi in chestnut. Aust. Nutgrow. 2008, 22, 11–15. [Google Scholar]
- Smith, H.C.; Ogilvyi, D. Nut rot in chestnuts. Aust. Nutgrow. 2008, 2, 10–15. [Google Scholar]
- Shuttleworth, L.A.; Liew, E.C.Y.; Guest, D.I. Survey of the incidence of chestnut rot in south-eastern Australia. Aust. Plant Pathol. 2012, 42, 63–72. [Google Scholar] [CrossRef]
- Çakar, D.; Şimşek, S.A. First report of Gnomoniopsis smithogilvyi causing nut rot on Castanea sativa in Turkey. New Dis. Rep. 2022, 46, e12105. [Google Scholar] [CrossRef]
- Chandelier, A.; Massot, M.; Fabreguettes, O.; Gischer, F.; Teng, F.; Robin, C. Early detection of Cryphonectria parasitica by real-time PCR. Eur. J. Plant Pathol. 2018, 153, 29–46. [Google Scholar] [CrossRef]
- Ivić, D.; Novak, A. Fungi associated with nut rot in sweet chestnut, with the first record of Gnomoniopsis smithogilvyi in Croatia. Pomologia Croatica 2018, 22, 13–22. [Google Scholar] [CrossRef]
- Gentile, S.; Valentino, D.; Visentin, I.; Tamietti, G. Discula pascoe infections of sweet chestnut fruits in North-West Italy. Aust. Nutgrow. 2009, 23, 23–25. [Google Scholar]
- Magro, P.; Speranza, S.; Stacchiotti, M.; Martignoni, D.; Paparatti, B. Gnomoniopsis associated with necrosis of leaves and chestnut galls induced by Dryocosmus kuriphilus. Plant Pathol. 2010, 59, 1171. [Google Scholar] [CrossRef]
- Vinale, F.; Ruocco, M.; Manganiello, G.; Guerrieri, E.; Bernardo, U.; Mazzei, P.; Piccolo, A.; Sannino, F.; Caira, S.; Woo, S.L.; et al. Metabolites produced by Gnomoniopsis castanea associated with necrosis of chestnut galls. Chem. Biol. Technol. Agric. 2014, 1, 294. [Google Scholar] [CrossRef]
- Lione, G.; Gonthier, P. A permutation-randomization approach to test the special distribution of plant diseases. Phytopathology 2016, 106, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Varlese, R.; Aliberti, A.; Carpenito, S.; Woo, S.L.; Scala, F.; Lorito, M. New tools to improve the shelf life of chestnut fruit during storage. Acta Hortic. 2016, 1144, 309–316. [Google Scholar] [CrossRef]
- Seddaiu, S.; Cerboneschi, A.; Sechi, C.; Mello, A. Gnomoniopsis castaneae associated with Dryocosmus kuriphilus galls in chestnut stands in Sardinia (Italy). iForest 2017, 10, 440–445. [Google Scholar] [CrossRef]
- Vannini, A.; Morales-Rodriguez, C.; Aleandri, M.; Bruni, N.; Dalla Valle, M.; Mazzetto, T.; Martignoni, D.; Vettraino, A. Emerging new crown symptoms on Castanea sativa (Mill.): Attempting to model interactions among pests and fungal pathogens. Fungal Biol. 2018, 122, 911–917. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Bianchini, L.; Caradonna, V.; Forniti, R.; Goffi, V.; Zambelli, M.; Testa, A.; Vinciguerra, V.; Botondi, R. Ozone gas as a storage treatment to control Gnomoniopsis castanea, preserving chestnut quality. J. Sci. Food Agric. 2019, 99, 6060–6065. [Google Scholar] [CrossRef]
- Driss, J.O. Chestnut Rots: Disease Incidence and Molecular Identification of Causal Agents. Master’s Thesis, Biotechnological Engineering, Instituto Politécnico de Bragança, Bragança, Portugal, 2019; p. 44. Available online: https://bibliotecadigital.ipb.pt/bitstream/10198/20487/1/pauta-relatorio-17.pdf (accessed on 20 March 2021).
- Meyer, J.B.; Gallien, L.; Prospero, S. Interaction between two invasive organisms on the European chestnut: Does the chestnut blight fungus benefit from the presence of the gall wasp? FEMS Microbiol. Ecol. 2015, 91, fiv122. [Google Scholar] [CrossRef][Green Version]
- EPPO—European and Mediterranean Plant Protection Organization Data sheets on quarantine pests—Dryocosmus kuriphilus. EPPO Bull. 2005, 35, 422–424.
- EFSA Risk assessment of the oriental chestnut gall wasps Dryocosmus kuriphilus for the EU territory and identification and evaluation of risk management options. EFSA J. 2010, 8, 114.
- Fernández, M.M.; Bezos, D.; Diez, J.J. Fungi associated with necrotic galls of Dryocosmus kuriphilus (Hymenoptera: Cynipidae) in northern Spain. Silva Fenn. 2018, 52, 9905. [Google Scholar] [CrossRef]
- Pasche, S.; Crovadore, J.; Pelleteret, P.; Jermini, M.; Mauch-Mani, B.; Oszako, T.; Lefort, F. Biological control of the latent pathogen Gnomoniopsis smithogylvyi in European chestnut grafting scions using Bacillus amyloliquefaciens and Trichoderma atroviride. Dendrobiology 2016, 75, 113–122. [Google Scholar] [CrossRef]
- Silva-Campos, M.; Callahan, D.L.; Cahill, D.M. Metabolites derived from fungi and bacteria suppress in vitro growth of Gnomoniopsis smithogilvyi, a major threat to the global chestnut. Metabolomics 2022, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Bastianelli, G.; Morales-Rodríguez, C.; Caccia, R.; Turco, S.; Rossini, L.; Mazzaglia, A.; Thomidis, T.; Vannini, A. Use of Phosphonate Salts to Control Chestnut ‘Brown Rot’ by Gnomoniopsis castaneae in Fruit Orchards of Castanea sativa. Agronomy 2022, 12, 2434. [Google Scholar] [CrossRef]
- Silva-Campos, M.; Islam, M.T.; Cahill, D.M. Fungicide control of Gnomoniopsis smithogilvyi, causal agent of chestnut rot in Australia. Australas. Plant Pathol. 2022, 51, 483–494. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Fulbright, D.W.; Ryser, E.T.; Guyer, D. Efficacy of postharvest treatments for reduction of molds and decay in fresh Michigan chestnuts. Acta Hortic. 2010, 866, 563–570. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Jeong, S.; Guyer, D.E.; Fulbright, D.W. Microbial contamination in peeled chestnuts and the efficacy of postprocessing treatments for microbial spoilage management. J. Food Process. Preserv. 2017, 41, e12874. [Google Scholar] [CrossRef]
- Regulamento (EU) 2019/1009 do Parlamento Europeu e do Conselho de 05 de Junho de 2019 que estabelece regras relativas à disponibilização no mercado de produtos fertilizantes EU e que altera os Regulamentos (CE) n.º 1069/2009 e (CE) n.º 1107/2009 e revoga o Regulamento (CE) n.º 2003/2003. J. Off. Da União Eur. 2019, 170, 1–114.
- Toolkiattiwong, P.; Arunrat, N.; Sereenonchai, S. Environmental, Human and Ecotoxicological Impacts of Different Rice Cultivation Systems in Northern Thailand. Int. J. Environ. Res. Public Health 2023, 20, 2738. [Google Scholar] [CrossRef]
- Huang, J.; Xu, C.; Ridoutt, B.G.; Chen, F. Reducing agricultural water footprints at the farm scale: A case study in the Beijing region. Water 2015, 7, 7066–7077. [Google Scholar] [CrossRef]
- Morales-Rodriguez, C.; Bastianelli, G.; Caccia, R.; Bedini, G.; Massantini, R.; Moscetti, R.; Thomidis, T.; Vanninia, A. Impact of ‘brown rot’ caused by Gnomoniopsis castanea on chestnut fruits during the postharvest process: Critical phases and proposed solutions. J. Sci. Food Agric. 2022, 102, 680–687. [Google Scholar] [CrossRef] [PubMed]
- de Agricultura e Veterinária, D.G. (Ed.) DGAV Manual de Procedimentos—Exportação de Castanha em Fresco Submetida a Tratamento Com água Quente em Sistema Contínuo—Procedimento a Adotar nas Centrais de Armazenagem e Embalagem (CAE) de Castanha; Direção Geral de Agricultura e Veterinária: Lisbon, Portugal, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).