Advanced CT Imaging, Radiomics, and Artificial Intelligence to Evaluate Immune Checkpoint Inhibitors’ Effects on Metastatic Renal Cell Carcinoma
Abstract
1. Introduction
2. Methods
3. Discussion
3.1. RECIST Criteria
- -
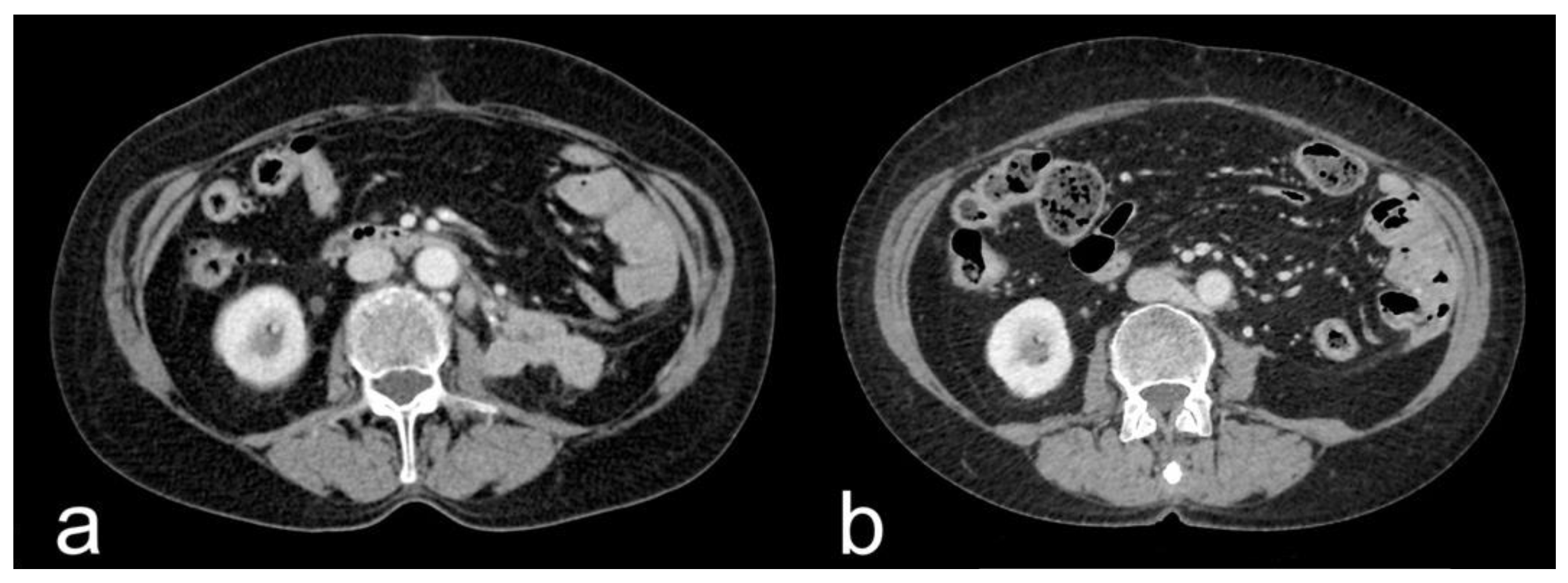
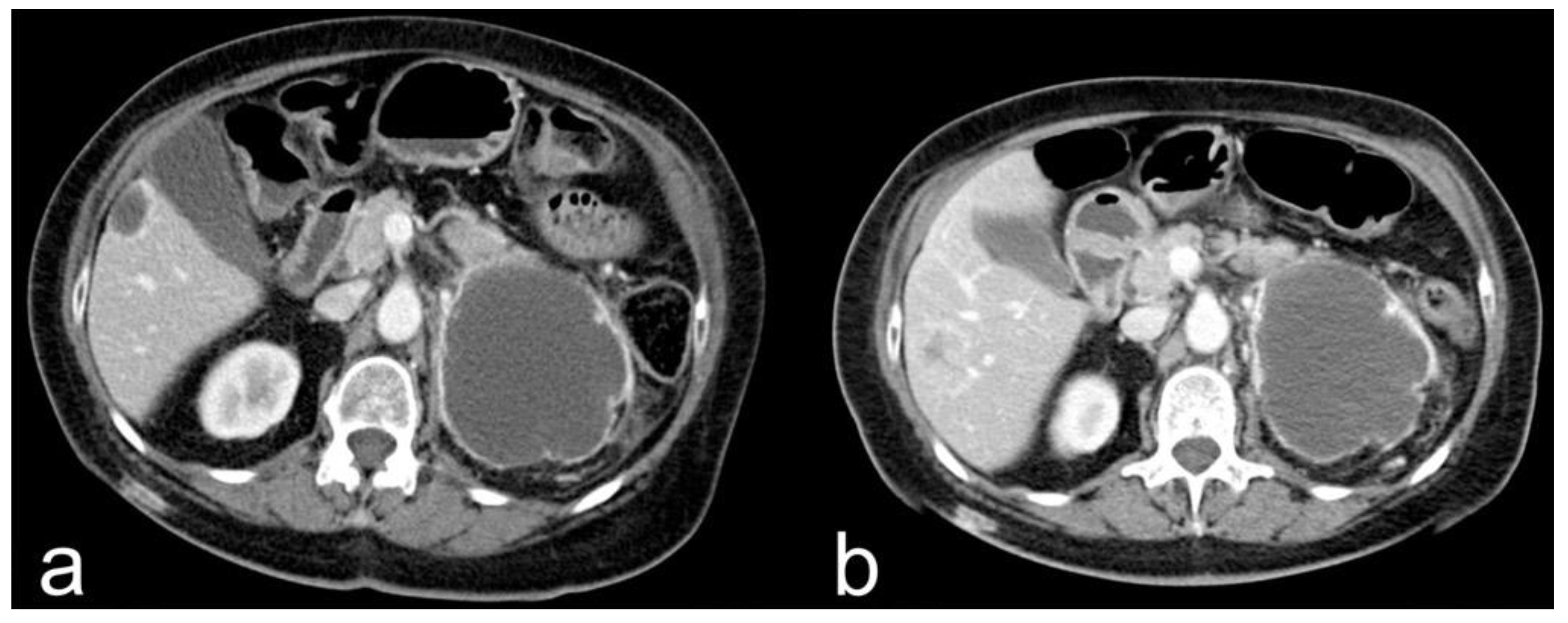
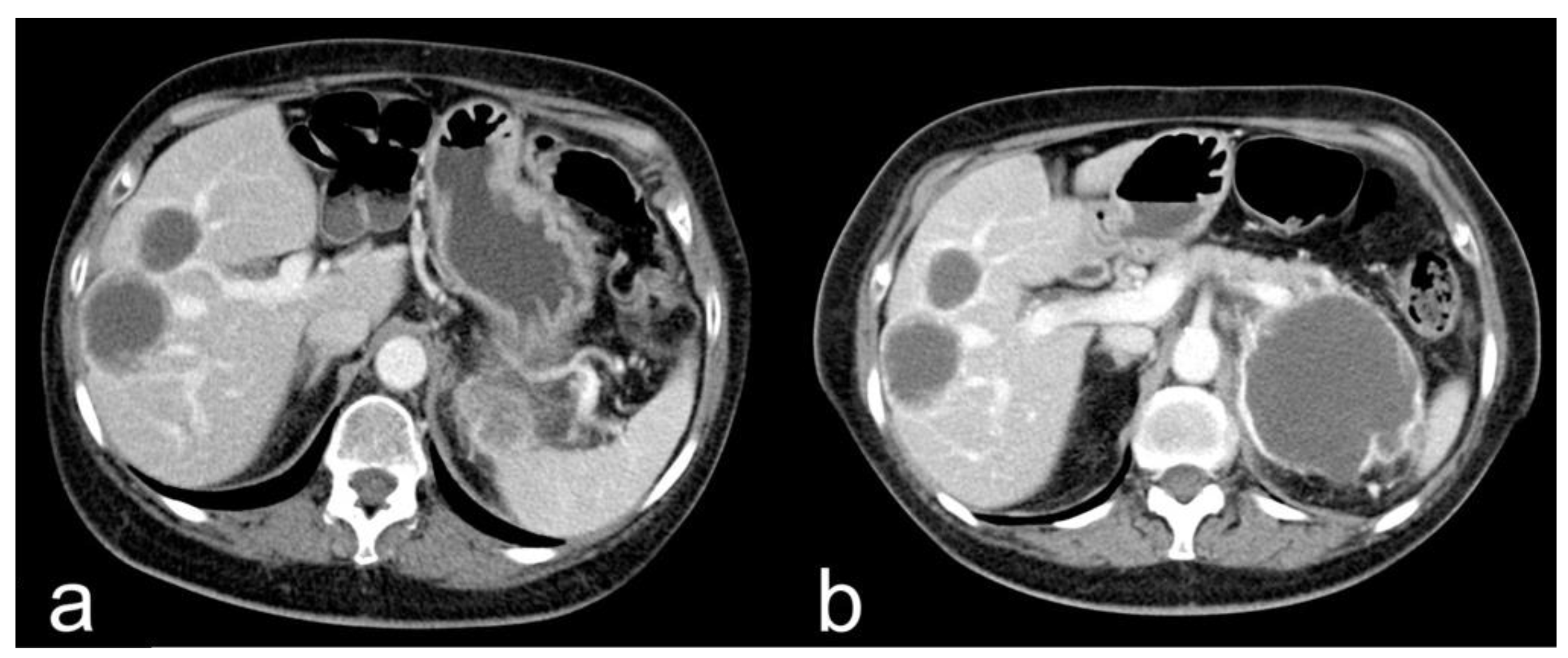
- Complete response (CR): disappearance of all target lesions together with any pathological lymph nodes (target or non-target) with short axis < 10 mm (Figure 1).
- -
- Partial response (PR): ≥30% reduction in the sum of the target lesions’ diameters.
- -
- Progressive disease (PD): ≥20% increase in the sum of the target lesions’ diameters, with the lower sum considered as the reference, plus an absolute ≥ 5 mm increment in the sum.
- -
3.2. Radiomics and Artificial Intelligence
3.3. Atypical Response Patterns
3.4. Body Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Gruenwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, T.Z.; Case, K.; Olsen, T.A.; Brown, J.T.; Carthon, B.C.; Kucuk, O.; Goldman, J.; Harris, W.; Bilen, M.A.; Nazha, B. Metastatic Clear-Cell Renal Cell Carcinoma in the Era of Immune Checkpoint Inhibitors: Therapies and Ongoing Trials. Cancers 2022, 14, 2867. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.C.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Vascular Endothelial Growth Factor–Targeted Agents: Results from a Large, Multicenter Study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Surveillance, Epidemology, and End Results Program. Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Available online: https://seer.cancer.gov/statfacts/html/kidrp.html (accessed on 17 October 2021).
- Pignon, J.C.; Jegede, O.; Shukla, S.A.; Braun, D.A.; Horak, C.E.; Wind-Rotolo, M.; Ishii, Y.; Catalano, P.J.; Grosha, J.; Flaifel, A.; et al. irRECIST for the evaluation of candidate biomarkers of response to nivolumab in metastatic clear cell renal cell carcinoma: Analysis of a phase II prospective clinical trial. Clin. Cancer Res. 2019, 25, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Flaifel, A.; Xie, W.; Braun, D.A.; Ficial, M.; Bakouny, Z.; Nassar, A.H.; Jennings, R.B.; Escudier, B.; George, D.J.; Motzer, R.J.; et al. PD-L1 expression and clinical outcomes to cabozantinib, everolimus, and sunitinib in patients with metastatic renal cell carcinoma: Analysis of the randomized clinical trials METEOR and CABOSUN. Clin. Cancer Res. 2019, 25, 6080–6088. [Google Scholar] [CrossRef]
- Miao, D.; Margolis, C.A.; Gao, W.; Voss, M.H.; Li, W.; Martini, D.J.; Norton, C.; Bossé, D.; Wankowicz, S.M.; Cullen, D.; et al. Genomic correlates of response to immune checkpoint therapies in clear cell renal cell carcinoma. Science 2018, 359, 801–806. [Google Scholar] [CrossRef]
- Motzer, R.J.; Robbins, P.B.; Powles, T.; Albiges, L.; Haanen, J.B.; Larkin, J.; Mu, X.J.; Ching, K.A.; Uemura, M.; Pal, S.K.; et al. Avelumab plus axitinib versus sunitinib in advanced renal cell carcinoma: Biomarker analysis of the phase 3 JAVELIN Renal 101 trial. Nat. Med. 2020, 26, 1733–1741. [Google Scholar] [CrossRef]
- Tannir, N.M.; Signoretti, S.; Choueiri, T.K.; McDermott, D.F.; Motzer, R.J.; Flaifel, A.; Pignon, J.C.; Ficial, M.; Frontera, O.A.; George, S.; et al. Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin. Cancer Res. 2021, 27, 78–86. [Google Scholar] [CrossRef]
- Park, H.J.; Qin, L.; Bakouny, Z.; Krajewski, K.M.; Van Allen, E.M.; Choueiri, T.K.; Shinagare, A.B. Computed Tomography Texture Analysis for Predicting Clinical Outcomes in Patients with Metastatic Renal Cell Carcinoma Treated With Immune Checkpoint Inhibitors. Oncologist 2022, 27, 389–397. [Google Scholar] [CrossRef]
- Greco, F.; Cirimele, V.; Mallio, C.A.; Beomonte Zobel, B.; Grasso, R.F. Increased visceral adipose tissue in male patients with clear cell renal cell carcinoma. Clin. Cancer Investig. J. 2018, 7, 132–136. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A.; Cirimele, V.; Grasso, R.F.; Beomonte Zobel, B. Subcutaneous adipose tissue as a biomarker of pancreatic cancer: A pilot study in male patients. Clin. Cancer Investig. J. 2019, 8, 114–118. [Google Scholar] [CrossRef]
- Lee, E.K.; Dickstein, R.J.; Kamta, A.M. Imaging of urothelial cancers: What the urologist needs to know. AJR Am. J. Roentgenol. 2011, 196, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, M.H.; Ahlman, M.A.; Lindenberg, L.; Turkbey, B.; Lin, J.; Cahid Civelek, A.; Malayeri, A.A.; Agarwal, P.K.; Choyke, P.L.; Folio, L.R.; et al. Advances in medical imaging for the diagnosis and management of common genitourinary cancers. Urol. Oncol. 2017, 35, 473–491. [Google Scholar] [CrossRef]
- Brufau, B.P.; Cerqueda, C.S.; Villalba, L.B.; Izquierdo, R.S.; González, B.M.; Molina, C.N. Metastatic renal cell carcinoma: Radiologic findings and assessment of response to targeted antiangiogenic therapy by using multidetector CT. Radiographics 2013, 33, 1691–1716. [Google Scholar] [CrossRef]
- Bianchi, M.; Sun, M.; Jeldres, C.; Trinh, Q.D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; Menon, M. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef]
- Queirolo, P.; Spagnolo, F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: A systematic review. Cancer Treat. Rev. 2017, 59, 71–78. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.; Curti, B. Immunotherapy in metastatic urothelial carcinoma: Focus on immune checkpoint inhibition. Nat. Rev. Urol. 2018, 15, 112–124. [Google Scholar] [CrossRef]
- Champiat, S.; Dercle, L.; Ammari, S.; Massard, C.; Hollebecque, A.; Postel-Vinay, S.; Chaput, N.; Eggermont, A.; Marabelle, A.; Soria, J.C.; et al. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin. Cancer Res. 2017, 23, 1920–1928. [Google Scholar] [CrossRef]
- Trebeschi, S.; Drago, S.G.; Birkbak, N.J.; Kurilova, I.; Cǎlin, A.M.; Delli Pizzi, A.; Lalezari, F.; Lambregts, D.M.J.; Rohaan, M.W.; Parmar, C.; et al. Predicting response to cancer immunotherapy using noninvasive radiomic biomarkers. Ann. Oncol. 2019, 30, 998–1004. [Google Scholar] [CrossRef]
- Navani, V.; Ernst, M.; Wells, J.C.; Yuasa, T.; Takemura, K.; Donskov, F.; Basappa, N.S.; Schmidt, A.; Pal, S.K.; Meza, L.; et al. Imaging Response to Contemporary Immuno-oncology Combination Therapies in Patients with Metastatic Renal Cell Carcinoma. JAMA Netw. Open 2022, 5, e2216379. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Mushti, S.L.; Mulkey, F.; Sridhara, R. Evaluation of overall response rate and progression-free survival as potential surrogate endpoints for overall survival in immunotherapy trials. Clin. Cancer Res. 2018, 24, 2268–2275. [Google Scholar] [CrossRef] [PubMed]
- Navani, V.; Graves, M.C.; Bowden, N.A.; Van Der Westhuizen, A. Immune checkpoint blockade in solid organ tumours: Choice, dose and predictors of response. Br. J. Clin. Pharmacol. 2020, 86, 1736–1752. [Google Scholar] [CrossRef] [PubMed]
- Jajodia, A.; Goel, V.; Patnaik, N.; Pasricha, S.; Gupta, G.; Batra, U.; Talwar, V. Analysis of Spatial Heterogeneity of Responses in Metastatic Sites in Renal Cell Carcinoma Patients Treated with Nivolumab. Tomography 2022, 8, 1363–1373. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Zheng, B.; Shin, J.H.; Li, H.; Chen, Y.; Guo, Y.; Wang, M. Comparison of Radiological Tumor Response Based on iRECIST and RECIST 1.1 in Metastatic Clear-Cell Renal Cell Carcinoma Patients Treated with Programmed Cell Death-1 Inhibitor Therapy. Korean J. Radiol. 2021, 22, 366–375. [Google Scholar] [CrossRef]
- Hermansen, C.K.; Donskov, F. Outcomes based on age in patients with metastatic renal cell carcinoma treated with first line targeted therapy or checkpoint immunotherapy: Older patients more prone to toxicity. J Geriatr. Oncol. 2021, 12, 827–833. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Malone, E.R.; Sim, H.W.; Stundzia, A.; Pierre, S.; Metser, U.; O’Malley, M.; Sacher, A.G.; Sridhar, S.S.; Hansen, A.R. Predictive radiomics signature for treatment response to nivolumab in patients with advanced renal cell carcinoma. Can. Urol. Assoc. J. 2022, 16, E94–E101. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Artificial intelligence and abdominal adipose tissue analysis: A literature review. Quant. Imaging Med. Surg. 2021, 11, 4461–4474. [Google Scholar] [CrossRef] [PubMed]
- Greco, F.; Salgado, R.; Van Hecke, W.; Del Buono, R.; Parizel, P.M.; Mallio, C.A. Epicardial and pericardial fat analysis on CT images and artificial intelligence: A literature review. Quant. Imaging Med. Surg. 2022, 12, 2075–2089. [Google Scholar] [CrossRef]
- Khene, Z.E.; Kokorian, R.; Mathieu, R.; Gasmi, A.; Nathalie, R.L.; Solène-Florence, K.J.; Shariat, S.; de Crevoisier, R.; Laguerre, B.; Bensalah, K. Metastatic clear cell renal cell carcinoma: Computed tomography texture analysis as predictive biomarkers of survival in patients treated with nivolumab. Int. J. Clin. Oncol. 2021, 26, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Khene, Z.E.; Mathieu, R.; Peyronnet, B.; Kokorian, R.; Gasmi, A.; Khene, F.; Rioux-Leclercq, N.; Kammerer-Jacquet, S.F.; Shariat, S.; Laguerre, B.; et al. Radiomics can predict tumour response in patients treated with Nivolumab for a metastatic renal cell carcinoma: An artificial intelligence concept. World J. Urol. 2021, 39, 3707–3709. [Google Scholar] [CrossRef]
- Wong, A.; Vellayappan, B.; Cheng, L.; Zhao, J.J.; Muthu, V.; Asokumaran, Y.; Low, J.L.; Lee, M.; Huang, Y.Q.; Kumarakulasinghe, N.B.; et al. Atypical Response Patterns in Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors-Navigating the Radiologic Potpourri. Cancers 2021, 13, 1689. [Google Scholar] [CrossRef] [PubMed]
- Mallio, C.A.; Napolitano, A.; Castiello, G.; Giordano, F.M.; D’Alessio, P.; Iozzino, M.; Sun, Y.; Angeletti, S.; Russano, M.; Santini, D.; et al. Deep Learning Algorithm Trained with COVID-19 Pneumonia Also Identifies Immune Checkpoint Inhibitor Therapy-Related Pneumonitis. Cancers 2021, 13, 652. [Google Scholar] [CrossRef]
- Martini, D.J.; Olsen, T.A.; Goyal, S.; Liu, Y.; Evans, S.T.; Magod, B.; Brown, J.T.; Yantorni, L.; Russler, G.A.; Caulfield, S.; et al. Body Composition Variables as Radiographic Biomarkers of Clinical Outcomes in Metastatic Renal Cell Carcinoma Patients Receiving Immune Checkpoint Inhibitors. Front. Oncol. 2021, 11, 707050. [Google Scholar] [CrossRef]
- Ueki, H.; Hara, T.; Okamura, Y.; Bando, Y.; Terakawa, T.; Furukawa, J.; Harada, K.; Nakano, Y.; Fujisawa, M. Association between sarcopenia based on psoas muscle index and the response to nivolumab in metastatic renal cell carcinoma: A retrospective study. Investig. Clin. Urol. 2022, 4, 415–424. [Google Scholar] [CrossRef]
- Ged, Y.; Sanchez, A.; Patil, S.; Knezevic, A.; Stein, E.; Petruzella, S.; Weiss, K.; Duzgol, C.; Chaim, J.; Akin, O.; et al. Associations between Pretreatment Body Composition Features and Clinical Outcomes among Patients with Metastatic Clear Cell Renal Cell Carcinoma Treated with Immune Checkpoint Blockade. Clin. Cancer Res. 2022, 28, 5180–5189. [Google Scholar] [CrossRef]
- Quattrocchi, C.C.; Giona, A.; Di Martino, A.; Gaudino, F.; Mallio, C.A.; Errante, Y.; Occhicone, F.; Vitali, M.A.; Beomonte Zobel, B.; Denaro, V. Lumbar subcutaneous edema and degenerative spinal disease in patients with low back pain: A retrospective MRI study. Musculoskelet. Surg. 2015, 99, 159–163. [Google Scholar] [CrossRef]
- Greco, F.; Mallio, C.A. Relationship between visceral adipose tissue and genetic mutations (VHL and KDM5C) in clear cell renal cell carcinoma. Radiol. Med. 2021, 126, 645–651. [Google Scholar] [CrossRef] [PubMed]
| Authors | Navani et al. (2022) [22] | Jajodia et al. (2022) [26] | Zheng et al. (2020) [28] |
|---|---|---|---|
| Objective | To compare the likelihood of objective imaging response to IOIO vs. IOVE therapies | Evaluate the different organ responses to nivolumab in surgically treated mRCC by describing response patterns and discrepancies between the RECIST 1.1 and iRECIST criteria | To compare the iRECIST and RECIST 1.1 criteria for radiological tumor response in mccRCC patients treated with programmed cell death-1 inhibitor therapy |
| Number of patients | 899 patients | 21 patients | 30 patients |
| Histotypes | 702 ccRCC 197 non-ccRCC | 18 ccRCC 2 pRCC 1 MiT family translocation renal cell carcinoma | 30 ccRCC |
| RECIST 1.1 | CR: 37 patients PR: 344 patients SD: 315 patients PD: 203 patients | CR: 0 patients PR: 3 patients SD: 4 patients PD: 14 patients | CR: 5 patients PR: 4 patients SD: 5 patients PD: 15 patients |
| iRECIST | - | iCR: 0 patients iPR: 3 patients iSD: 8 patients iPD: 10 patients (8 iUPD -> 8 iCPD) | iCR: 6 patients iPR: 9 patients iSD: 5 patients iPD: 8 patients (2 iUPD -> 2 iCPD) |
| IMDC favorable risk group (NR–R) | 59–68 patients | - | 4 |
| IMDC intermediate risk group (NR–R) | 244–198 patients | - | 23 |
| IMDC poor risk group (NR–R) | 147–78 patients | - | 3 |
| Lung metastasis (NR–R) | 329 NR, S–289 R, S | 26 L | Not specified |
| Lymph node metastasis (NR–R) | 239 NR, S–195 R, S | 20 L | Not specified |
| Bone metastasis (NR–R) | 181 NR, S–114 R, S | - | Not specified |
| Liver metastasis (NR–R) | 80 NR, S–57 R, S | 10 L | Not specified |
| Brain metastasis (NR–R) | 33 NR, S–16 R, S | 5 L | Not specified |
| Adrenal gland metastasis (NR–R) | 81 NR, S– 57 R, S | 4 L | Not specified |
| Spleen metastasis (NR–R) | 50 NR, S–31 R, S | - | Not specified |
| Soft tissue metastasis | - | 7 L | Not specified |
| Peritoneum metastasis | - | 4 L | Not specified |
| Best overall response in the IMDC intermediate and poor risk groups, IOIO therapy | CR: 20 patients PR: 180 patients SD: 183 patients PD: 145 patients | - | - |
| Best overall response in the IMDC intermediate and poor risk groups, IOVE therapy | CR: 4 patients PR: 72 patients SD: 46 patients PD: 17 patients | - | - |
| Results | Increased likelihood of obtaining response from IOVE therapy compared to IOIO therapy (OR, 1.89; 95% CI, 1.26–2.81; p = 0.002). Increased likelihood of response is associated with the presence of lung metastasis (OR, 1.49; 95% CI, 1.01–2.20), receipt of cytoreductive nephrectomy (OR, 1.59; 95% CI, 1.04–2.43), and favorable IMDC risk (OR, 1.93; 95% CI, 1.10–3.39) | Different responses were found between organ categories (Kruskal–Wallis p = 0.003). The response rates differed significantly among the lesion groups according to the location (Fisher p = 0.02) | The objective response rate during therapy was 30% for RECIST 1.1 (95% confidence interval (CI): 13.6–46.4) and 50% for iRECIST (95% confidence interval (CI): 13.6–46.4) Significant differences between iRECIST and RECIST 1.1 were shown (p < 0.001) |
| Level of evidence * | 2 | 2 | 2 |
| Authors | Malone et al. (2021) [31] | Khene et al. (2021) [34] | Khene et al. (2021) [35] | Park et al. (2022) [11] |
|---|---|---|---|---|
| Objective | To predict tumor response in mRCC patients treated with nivolumab through CT signature texture analysis | To predict progression-free survival and overall survival in mRCC patients treated with nivolumab through CT signature texture analysis | To predict tumor response in mRCC patients treated with nivolumab through CT signature texture analysis | To predict oncological outcomes in mRCC patients treated with ICIs through CT signature texture analysis |
| Number of patients | 27 patients | 48 patients | 48 patients | 68 patients |
| Histotypes | 24 ccRCC 3 non-ccRCC | 48 ccRCC | 48 ccRCC | 46 ccRCC 9 pRCC 5 chRCC 8 others RCC |
| RECIST 1.1 | CR, PR or SD: 19 patients PD: 8 patients | CR: 0 patients PR: 9 patients SD: 20 patients PD: 19 patients | CR: 0 patients PR: 9 patients SD: 20 patients PD: 19 patients | CR or PR: 17 patients SD: 19 patients PD: 27 patients Unknown: 5 patients |
| IMDC favorable risk group | 8 patients | 17 patients | 17 patients | 9 patients |
| IMDC intermediate risk group | 16 patients | 22 patients | 22 patients | 39 patients |
| IMDC poor risk group | 3 patients | 9 patients | 9 patients | 20 patients |
| Lung metastasis (L or S) | 24 L | 20 S | 20 S | 51 L |
| Lymph node metastasis (L or S) | 62 L | 28 S | 28 S | 97 L |
| Bone metastasis (L or S) | - | 18 S | 18 S | - |
| Liver metastasis (L or S) | - | 15 S | 15 S | 41 L |
| Brain metastasis (L or S) | - | 3 S | 3 S | |
| Renal or adrenal gland metastasis (L or S) | 18 L | 12 (only adrenal) S | 12 (only adrenal) S | 17 renal and 22 adrenal L |
| Pancreas metastasis (L or S) | 3 S | 3 S | 11 L | |
| Muscle metastasis (L or S) | - | - | - | 10 L |
| Peritoneum metastasis (L or S) | - | - | - | 40 L |
| Pleural metastasis (L or S) | - | - | - | 9 L |
| Results | Texture analysis demonstrated poor ability to predict nivolumab responders from non-responders, with 69% overall accuracy for baseline CT scans (AUC = 0.46, p = 0.30) and with 66% overall accuracy on the first post-treatment CT scans (AUC = 0.51, p = 0.40) | The skewness texture parameter was confirmed as an independent predictor of progression-free survival by multivariate Cox regression analysis (HR (95% CI) 1.49 [1.21–1.85], p < 0.001). The S22 entropy texture parameter was confirmed as an independent predictor of overall survival by multivariate Cox regression analysis (HR (95% CI) 1.68 (1.31–2.14), p < 0.001). | Radiomic analysis demonstrated the accuracy scores of the k-nearest neighbor, random forest, logistic regression, and support-vector machine predictive models to be 0.82, 0.71, 0.91, and 0.81, respectively, with area under the receiver operating characteristic curve scores of 0.79, 0.67, 0.92, and 0.71, respectively. | Radiomic analysis distinguished longer- and shorter-term survivors for both overall survival (p = 0.048) and progression-free survival (p = 0.003). The follow-up texture models distinguished longer- and shorter-term overall survivors (p = 0.008). The combined clinical–texture model predicted the overall survival (p = 0.03) and progression-free survival (p = 0.04). |
| Level of evidence * | 2 | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, F.; Beomonte Zobel, B.; Di Gennaro, G.; Mallio, C.A. Advanced CT Imaging, Radiomics, and Artificial Intelligence to Evaluate Immune Checkpoint Inhibitors’ Effects on Metastatic Renal Cell Carcinoma. Appl. Sci. 2023, 13, 3779. https://doi.org/10.3390/app13063779
Greco F, Beomonte Zobel B, Di Gennaro G, Mallio CA. Advanced CT Imaging, Radiomics, and Artificial Intelligence to Evaluate Immune Checkpoint Inhibitors’ Effects on Metastatic Renal Cell Carcinoma. Applied Sciences. 2023; 13(6):3779. https://doi.org/10.3390/app13063779
Chicago/Turabian StyleGreco, Federico, Bruno Beomonte Zobel, Gianfranco Di Gennaro, and Carlo Augusto Mallio. 2023. "Advanced CT Imaging, Radiomics, and Artificial Intelligence to Evaluate Immune Checkpoint Inhibitors’ Effects on Metastatic Renal Cell Carcinoma" Applied Sciences 13, no. 6: 3779. https://doi.org/10.3390/app13063779
APA StyleGreco, F., Beomonte Zobel, B., Di Gennaro, G., & Mallio, C. A. (2023). Advanced CT Imaging, Radiomics, and Artificial Intelligence to Evaluate Immune Checkpoint Inhibitors’ Effects on Metastatic Renal Cell Carcinoma. Applied Sciences, 13(6), 3779. https://doi.org/10.3390/app13063779










