An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study
Abstract
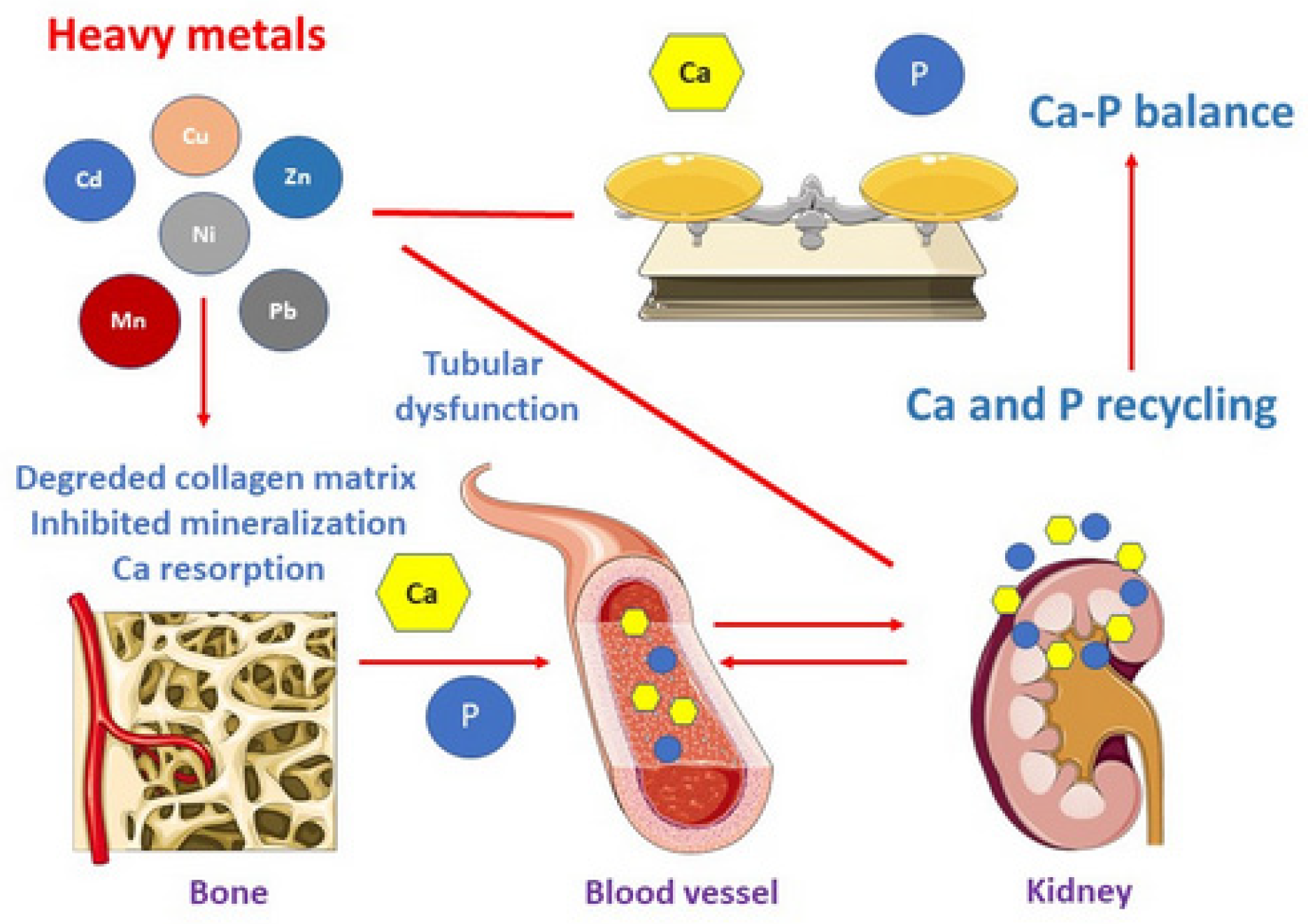
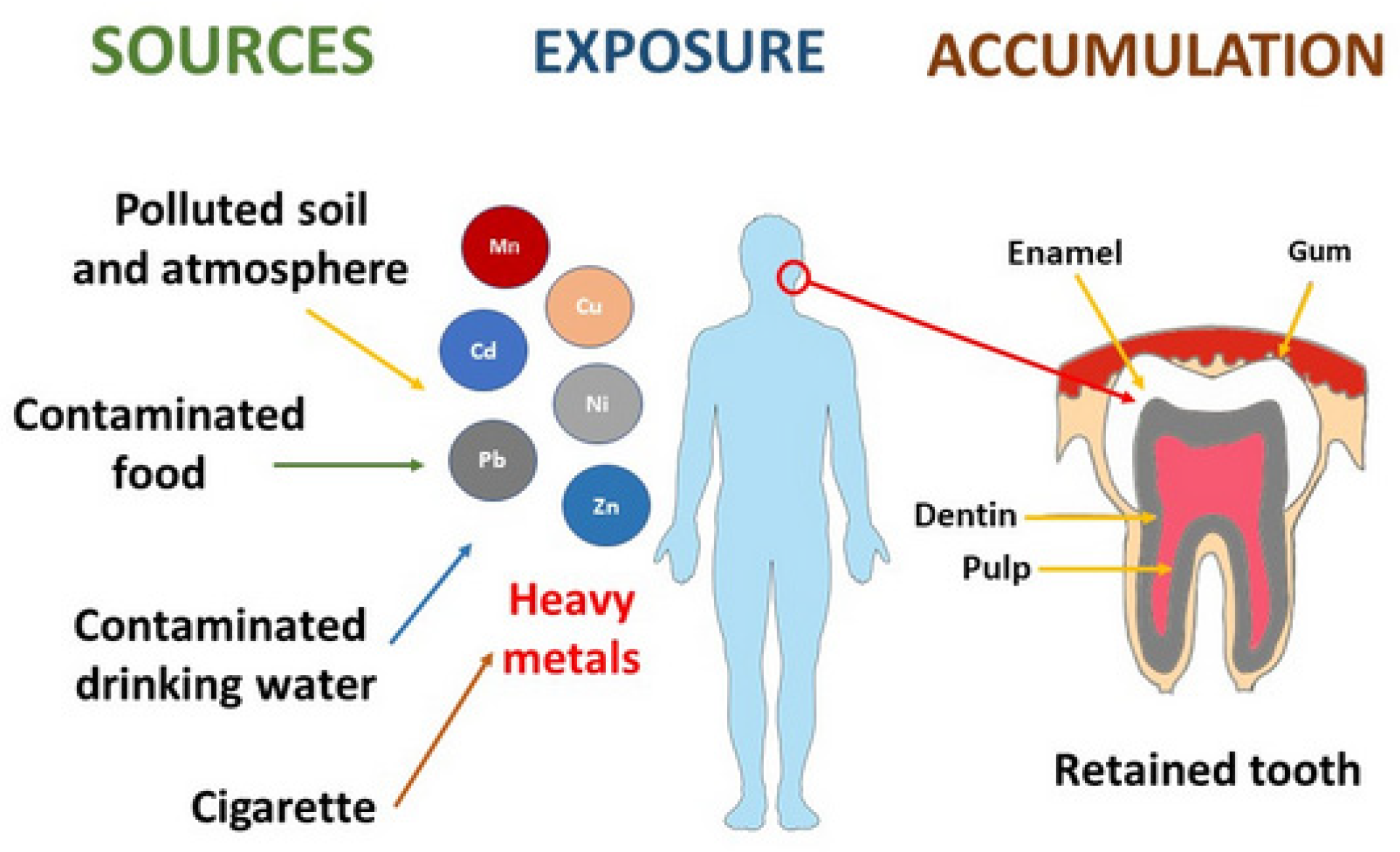
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Determination of Toxic Metals in the Study Material
2.3. Mineralization of Examined Material
2.4. Examination of Toxic Metals in the Samples
- Determination of trace elements—Determination of lead, cadmium, zinc, copper, iron, and chromium by atomic absorption spectrometry (AAS) after dry mineralization [18]—Pb, Cd, Zn, Cu, Fe, and Cr—PN-EN 14082: 2004 Food products.
- Determination of heavy metal content by atomic emission spectrometry—Determination of nickel content [19]—Ni—PN-A-86939-6: 1998 Vegetable and animal oils and fats.
- Determination of calcium, copper, iron, magnesium, manganese, potassium, sodium, and zinc content—Atomic absorption spectrometry method [20]—Mn—PN-EN ISO 6869: 2002 Feed
2.5. Methodology of Blood Collection and Determination of Vitamin D3
2.6. Statistical Analysis
3. Results
3.1. Basic Characteristic of Patients
3.2. The Content of Toxic Metals in the Removed Teeth in Patients from the LG Copper Area and in Control Group
3.3. Risk Factors Determining the Accumulation of Toxic Metals in the Teeth of Patients Residing in the LG District
3.4. Content of Manganese
3.5. Content of Chromium and Nickel
3.6. Content of Copper
3.7. Content of Iron
3.8. Content of Cadmium
3.9. Content of Lead
3.10. Content of Zinc
4. Discussion
5. Conclusions
6. The Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsz-Shan Lum, J.; Chan, Y.-N.; Sze-Yin Leung, K. Current applications and future perspectives on elemental analysis of non-invasive samples for human biomonitoring. Talanta 2021, 234, 122683. [Google Scholar] [CrossRef]
- Esteban, M.; Castaño, A. Non-invasive matrices in human biomonitoring: A review. Environ. Int. 2009, 35, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Barton, H.J. Advantages of the use of deciduous teeth, hair, and blood analysis for lead and cadmium bio-monitoring in children. A study of 6-year-old children from Krakow (Poland). Biol. Trace Elem. Res. 2011, 143, 637–658. [Google Scholar] [CrossRef]
- Alomary, A.; Al-Momani, I.F.; Obeidat, S.M.; Massadeh, A.M. Levels of lead, cadmium, copper, iron, and zinc in deciduous teeth of children living in Irbid, Jordan by ICP-OES: Some factors affecting their concentrations. Environ. Monit. Assess. 2013, 185, 3283–3295. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Wiechuła, D.; Przybyła-Misztela, C. Changes of concentrations of elements in deciduous teeth with age. Biol. Trace Elem. Res. 2013, 154, 427–432. [Google Scholar] [CrossRef]
- Tvinnereim, H.M.; Eide, R.; Riise, T. Heavy metals in human primary teeth: Some factors influencing the metal concentration. Sci. Total Environ. 2000, 255, 21–27. [Google Scholar] [CrossRef]
- Leventouri, T.; Antonakos, A.; Kyriacou, A.; Venturelli, R.; Liarokapis, E.; Perdikatsis, V. Crystal structure studies of human dental apatite as a function of age. Int. J. Biol. Mater. 2009, 2009, 1–17. [Google Scholar] [CrossRef]
- Bryła, E.; Dobrzyński, M.; Konkol, D.; Kuropka, P.; Styczyńska, M.; Korczyński, M. Toxic Metals Content in Impacted Third Molars and Adjacent Bone Tissue in Different Groups of Patients. Materials 2021, 14, 793. [Google Scholar] [CrossRef]
- Krawiec, M.; Dominiak, M. Prospective evaluation of vitamin D levels in dental treated patients: A screening study. Dent. Med. Probl. 2021, 58, 321–326. [Google Scholar] [CrossRef]
- Moon, J. The role of vitamin D in toxic metal absorption: A review. J. Am. CollNutr. 1994, 13, 559–564. [Google Scholar] [CrossRef]
- Ślebioda, Z.; Szponar, E.; Dorocka-Bobkowska, B. Vitamin D and its relevance in the etiopathogenesis of oral cavity diseases. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Malara, P.; Fischer, A.; Malara, B. Selected toxic and essential heavy metals in impacted teeth and the surrounding mandibular bones of people exposed to heavy metals in the environment. J. Occup. Med. Toxicol. 2016, 11, 56. [Google Scholar] [CrossRef]
- Kołacz, R.; Dobrzański, Z.; Kupczyński, R.; Cwynar, P.; Opaliński, S.; Pogoda-Sewerniak, K. Impact of the copper industry on the content of selected heavy metals and biochemical indicators in the blood of dairy cows. Med. Weter 2017, 73, 171–175. [Google Scholar] [CrossRef]
- Strzelec, Ł.; Niedźwiecka, W. Natural environment in the area of copper smelter plants. Trend Chang. Med. Sr. Environ. Med. 2012, 15, 21–31. [Google Scholar]
- Tsushima, F.; Sakurai, J.; Shimizu, R.; Kayamori, K.; Harada, H. Oral lichenoid contact lesions related to dental metal allergy may resolve after allergen removal. J. Dent. Sci. 2022, 17, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Dolci, A.; Minervini, G.; Salerno, C.; DIStasio, D.; Minervini, G.; Laino, L.; Silvestre, F.; Serpico, R. Vulvovaginal gingival lichen planus: Report of two cases and review of literature. Oral. Implantol. 2016, 9, 54–60. [Google Scholar] [CrossRef]
- PN-EN 13805:2003; Food Products-Determination of Trace Elements-Pressure Mineralization. European Commission: Brussels, Belgium, 2003.
- PN-EN 14082:2004; Food Products-Determination of Trace Elements-Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium by Atomic Absorption Spectrometry (AAS) after Dry Mineralization. European Commission: Brussels, Belgium, 2004.
- PN-A-86939-6:1998; Vegetable and Animal Oils and Fats-Determination of Heavy Metal Content by Atomic Emission Spectrometry-Determination of Nickel Content. European Commission: Brussels, Belgium, 1998.
- PN-EN-ISO 6869:2002; Determination of Calcium, Copper, Iron, Magnesium, Manganese, Potassium, Sodium and Zinc Content-Atomic Absorption Spectrometry Method. European Commission: Brussels, Belgium, 2002.
- Sławińska-Ochla, T.; Ignasiak, Z.; Fugiel, J.; Pokrywka, J. Lead concentration in blood of school children from copper mining area and the level of somatic development at birth. Med. Environ. Med. 2011, 14, 42–48. [Google Scholar]
- Dembicka, D.; Pastuszek, B.; Strugała-Stawik, H.; Zaręba, A. Monitoring biologiczny stopnia narażenia na ołów i skutków zdrowotnych u dzieci z byłego województwa legnickiego (1996–1999). [W:] Z. Rudkowski (red.) Materiały Konferencji Naukowej nt. Dziecko w środowisku zagrożonym ekologicznie- profilaktyka i problemy zdrowotne. Legnica 1999, 42–44. [Google Scholar]
- Charkiewicz, A.E.; Backstrand, J.R. Lead toxicity and pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef]
- Krzywy, I.; Krzywy, E.; Pastuszak-Gabinowska, M.; Brodkiewicz, A. Lead—Is there something to be afraid of? Ann. Acad. Med. Stetin. 2010, 56, 118–128. [Google Scholar]
- Rzymski, P.; Tomczyk, K.; Rzymski, P.; Poniedziałek, B.; Opala, T.; Wilczak, M. Impact of heavy metals on the female reproductive system. Ann. Agric. Environ. Med. 2015, 22, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, K.; Drzazga, Z.; Kaszuba, M. Role of trace elements (Zn, Sr, Fe) in bone development: Energy dispersive X-ray fluorescence study of rat bone and tooth tissue. Biofactors 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Wychowański, P.; Małkiewicz, K. Evaluation of metal ion concentration in hard tissues of teeth in residents of central Poland. BioMed. Res. Int. 2017, 2017, 6419709. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; DanutaWiechuła, D. Age-Dependent Changes in Pb Concentration in Human Teeth. Biol. Trace Elem. Res. 2016, 173, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.F.; Harris, C.; Kaye, J.A.; Lind, B.; Carter, R.; Anekonda, T.; Ralle, M. Gender effects on plasma and brain copper. Int. J. Alzheimers Dis. 2011, 2011, 150916. [Google Scholar] [CrossRef] [PubMed]
| Determined Element | Wavelength (nm) | Gap (nm) | Air-Acetylene Flow (L/min) | Background Correlation | Characteristic Concentration c (mg/L) | r |
|---|---|---|---|---|---|---|
| Ni | 341.5 | 0.2 | 13.50/2.00 | on | 0.005 | 0.9997 |
| Fe | 372.0 | 0.2 | 13.50/2.00 | on | 0.011 | 1.0000 |
| Cu | 327.4 | 0.2 | 13.50/2.00 | on | 0.015 | 0.9999 |
| Mn | 279.5 | 0.2 | 13.50/2.00 | on | 0.009 | 0.9996 |
| Cd | 228.8 | 0.5 | 13.50/2.00 | on | 0.005 | 0.9998 |
| Pb | 217.0 | 1.0 | 13.50/2.00 | on | 0.005 | 0.9997 |
| Zn | 213.9 | 1.0 | 13.50/2.00 | on | 0.012 | 1.0000 |
| Variable | Total (n = 69) | LG Copper Area—No (n = 20) | LG Copper Area—Yes (n = 49) | p-Value |
|---|---|---|---|---|
| Gender: | 0.763 | |||
| male | 16 (23.2) | 4 (20.0) | 12 (24.5) | |
| female | 53 (76.8) | 16 (80.0) | 37 (75.5) | |
| Age (years old) | 27.0 (22.0–31.0) [25.6–30.9] | 25.0 (24.0–27.5) [23.7–27.6] | 29.0 (22.0–32.0) [25.8–32.0] | 0.305 |
| Age (years old): | 0.092 | |||
| 16–26 | 34 (49.3) | 12 (60.0) | 22 (44.9) | |
| 27–37 | 29 (42.0) | 8 (40.0) | 21 (42.9) | |
| 38–45 | 6 (8.7) | 0 (0.0) | 6 (12.2) | |
| Smoking | 0.682 | |||
| no | 61 (88.4) | 17 (85.0) | 44 (89.8) | |
| yes | 8 (11.6) | 3 (15.0) | 5 (10.2) | |
| Occupation: | 0.245 | |||
| student | 20 (28.9) | 5 (25.0) | 15 (30.6) | |
| worker | 10 (14.5) | 1 (5.0) | 9 (18.4) | |
| white-collar worker | 39 (56.5) | 14 (70.0) | 25 (51.0) | |
| Years of residence in the LG copper district (n = 49): | - | - | ||
| ≤20 years old | 17 (34.7) | 17 (34.7) | ||
| 21–30 years old | 19 (38.8) | 19 (38.8) | ||
| ≥31 years old | 13 (26.5) | 13 (26.5) | ||
| Thyroid and parathyroid glands diseases: | 1.000 | |||
| no | 62 (89.9) | 18 (90.0) | 44 (89.8) | |
| yes | 7 (10.1) | 2 (10.0) | 5 (10.2) | |
| Cardiac diseases: | 0.498 | |||
| no | 67 (97.1) | 19 (95.0) | 48 (98.0) | |
| yes | 2 (2.9) | 1 (5.0) | 1 (2.0) | |
| Dietary supplements: | 0.453 | |||
| no | 46 (66.7) | 12 (60.0) | 34 (69.4) | |
| yes | 23 (33.3) | 8 (40.0) | 15 (30.6) | |
| Reason of extraction: | 0.020 * | |||
| surgical | 54 (78.3) | 20 (100.0) | 34 (69.4) | |
| orthodontic | 8 (11.6) | 0 (0.0) | 8 (16.3) | |
| inflammation | 7 (10.1) | 0 (0.0) | 7 (14.3) | |
| Vit. D3 (ng/mL) | 27.0 (19.8–29.5) [22.5–32.1] | 27.8 (25.5–32.2) [24.9–37.6] | 26.4 (18.5–28.4) [20.9–31.8] | 0.135 |
| Vit. D3 (ng/mL): | 0.173 | |||
| <20—big deficiency | 18 (26.1) | 3 (15.0) | 15 (30.6) | |
| 20–29—deficiency | 34 (49.3) | 9 (45.0) | 25 (51.0) | |
| 30–50—norm | 11 (15.9) | 6 (30.0) | 5 (10.2) | |
| 51–100—above the norm | 6 (8.7) | 2 (10.0) | 4 (8.2) |
| Variable | Total (n = 69) | LG Copper Area —No (n = 20) | LG Copper Area —Yes (n = 49) | p-Value |
|---|---|---|---|---|
| Mn (µg/g) | 0.19 (0.13–0.28) [0.19–0.28] | 0.17 (0.14–0.25) [0.14–0.34] | 0.22 (0.13–0.30) [0.19–0.28] | 0.796 |
| Cr (µg/g) | 0.0 (0.00–0.00) [0.03–0.16] | 0.00 (0.00–0.00) [0.00–0.29] | 0.00 (0.00–0.00) [0.03–0.14] | 0.667 |
| Ni (µg/g) | 0.0 (0.00–0.00) [0.00–0.32] | 0.0 (0.00–0.00) [0.00–0.13] | 0.00 (0.00–0.04) [0.00–0.43] | 0.183 |
| Cu (µg/g) | 0.12 (0.05–0.24) [0.09–0.25] | 0.08 (0.01–0.15) [0.01–0.17] | 0.13 (0.06–0.28) [0.10–0.26] | 0.103 |
| Fe (µg/g) | 3.59 (1.82–5.88) [3.56–6.14] | 1.81 (1.23–4.79) [1.23–6.03] | 3.86 (2.51–6.23) [3.82–6.28] | 0.016 * |
| Cd (µg/g) | 0.06 (0.05–0.09) [0.05–0.09] | 0.06 (0.05–0.08) [0.05–0.08] | 0.07 (0.05–0.09) [0.05–0.09] | 0.117 |
| Pb (µg/g) | 0.47 (0.30–0.66) [0.34–0.68] | 0.31 (0.22–0.48) [0.25–0.48] | 0.53 (0.37–0.75) [0.39–0.76] | 0.002 * |
| Zn (µg/g) | 100.36 (90.24–150.69) [93.29–141.26] | 99.70 (81.40–114.95) [88.63–118.51] | 103.80 (90.31–155.17) [94.06–151.67] | 0.311 |
| (A) | ||||||||
| Variable | Mn (µg/g) | p-Value | Cr (µg/g) | p-Value | Ni (µg/g) | p-Value | Cu (µg/g) | p-Value |
| Gender: | 0.633 | 0.485 | 0.192 | 0.463 | ||||
| male (n = 12) | 0.21 (0.15–0.29) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.15 (0.07–0.32) | ||||
| female (n = 37) | 0.22 (0.12–0.30) | 0.00 (0.00–0.00) | 0.00 (0.00–0.10) | 0.12 (0.06–0.24) | ||||
| Age (years old): | 0.328 | 0.669 | 0.655 | 0.061 # | ||||
| 16–26 (n = 22) | 0.15 (0.12–0.23) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.07 (0.05–0.15) | ||||
| 27–37 (n = 21) | 0.25 (0.13–0.31) | 0.00 (0.00–0.39) | 0.00 (0.00–0.55) | 0.16 (0.10–0.36) | ||||
| 38–45 (n = 6) | 0.24 (0.13–0.33) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.26 (0.03–0.40) | ||||
| Smoking | 0.436 | 0.940 | 0.785 | 0.778 | ||||
| no (n = 44) | 0.22 (0.13–0.31) | 0.00 (0.00–0.00) | 0.00 (0.00–0.05) | 0.12 (0.06–0.28) | ||||
| yes (n = 5) | 0.13 (0.12–0.22) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.14 (0.07–0.20) | ||||
| Occupation: | 0.043 * | 0.085 # | 0.072 # | 0.569 | ||||
| student (n = 15) | 0.14 (0.10–0.16) 1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.07 (0.05–0.42) | ||||
| worker (n = 9) | 0.27 (0.19–0.31) | 0.00 (0.00–0.27) | 0.00 (0.00–0.39) | 0.12 (0.01–0.16) | ||||
| white-collar worker (n = 25) | 0.25 (0.13–0.37) 1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.45) | 0.15 (0.07–0.28) | ||||
| Years of residence in the LG Copper Area: | 0.624 | 0.233 | 0.221 | 0.389 | ||||
| ≤20 years (n = 17) | 0.19 (0.11–0.23) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.08 (0.06–0.28) | ||||
| 21–30 years old (n = 19) | 0.26 (0.13–0.31) | 0.00 (0.00–0.00) | 0.00 (0.00–0.39) | 0.13 (0.04–0.20) | ||||
| ≥31 years old (n = 13) | 0.21 (0.13–0.28) | 0.00 (0.00–0.27) | 0.00 (0.00–0.45) | 0.22 (0.07–0.40) | ||||
| Thyroid and parathyroid glands diseases: | 0.381 | 0.509 | 0.620 | 0.655 | ||||
| no (n = 44) | 0.21 (0.13–0.29) | 0.00 (0.00–0.00) | 0.00 (0.00–0.02) | 0.12 (0.05–0.28) | ||||
| yes (n = 5) | 0.28 (0.13–0.33) | 0.00 (0.00–0.27) | 0.00 (0.00–0.45) | 0.15 (0.03–0.22) | ||||
| Dietary supplements: | 0.182 | 0.189 | 0.250 | 0.887 | ||||
| no (n = 34) | 0.17 (0.10–0.28) | 0.0 (0.00–0.00) | 0.0 (0.00–0.00) | 0.12 (0.05–0.29) | ||||
| yes (n = 15) | 0.26 (0.13–0.33) | 0.00 (0.00–0.44) | 0.00 (0.00–0.52) | 0.15 (0.06–0.23) | ||||
| Reason of extraction: | 0.563 | 0.797 | 0.694 | 0.121 | ||||
| surgical (n = 34) | 0.19 (0.13–0.28) | 0.00 (0.00–0.00) | 0.00 (0.00–0.06) | 0.15 (0.06–0.36) | ||||
| orthodontic (n = 8) | 0.21 (0.10–0.34) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.06 (0.02–0.13) | ||||
| inflammation (n = 7) | 0.27 (0.12–0.38) | 0.00 (0.00–0.27) | 0.00 (0.00–0.45) | 0.08 (0.05–0.24) | ||||
| Vit. D3 (ng/mL): | 0.012 * | 0.034 * | 0.112 | 0.818 | ||||
| <20 (n = 15) | 0.23 (0.16–0.30) | 0.00 (0.00–0.42) | 0.00 (0.00–0.55) | 0.08 (0.04–0.29) | ||||
| 20–29 (n = 25) | 0.13 (0.09–0.23) 1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.15 (0.07–0.28) | ||||
| 30–50 (n = 5) | 0.28 (0.25–0.40) 1 | 0.00 (0.00–0.00) | 0.00 (0.00–0.03) | 0.06 (0.05–0.23) | ||||
| 51–100 (n = 4) | 0.27 (0.19–0.32) | 0.00 (0.00–0.13) | 0.00 (0.00–0.31) | 0.09 (0.04–0.28) | ||||
| (B) | ||||||||
| Variable | Fe (µg/g) | p-Value | Cd (µg/g) | p-Value | Pb (µg/g) | p-Value | Zn (µg/g) | p-Value |
| Gender: | 0.311 | 0.037 * | 0.843 | 0.492 | ||||
| male (n = 12) | 6.72 (1.93–8.11) | 0.05 (0.04–0.07) | 0.52 (0.29–0.75) | 122.27 (87.10–170.66) | ||||
| female (n = 37) | 3.72 (2.57–5.67) | 0.08 (0.05–0.10) | 0.53 (0.38–0.68) | 102.57 (92.31–150.72) | ||||
| Age (years old): | 0.497 | 0.736 | 0.032 * | 0.088 # | ||||
| 16–26 (n = 22) | 3.41 (1.94–5.77) | 0.07 (0.04–0.09) | 0.51 (0.33–0.59) 1 | 96.79 (76.67–129.16) | ||||
| 27–37 (n = 21) | 3.90 (2.97–6.23) | 0.08 (0.07–0.10) | 0.47 (0.33–0.76) | 120.94 (93.68–157.41) | ||||
| 38–45 (n = 6) | 4.87 (2.57–7.28) | 0.06 (0.04–0.09) | 0.77 (0.56–1.03) 1 | 149.99 (96.73–179.69) | ||||
| Smoking | 0.888 | 0.714 | 0.453 | 0.459 | ||||
| no (n = 44) | 3.88 (2.51–6.54) | 0.07 (0.05–0.09) | 0.54 (0.35–0.75) | 103.82 (91.31–158.02) | ||||
| yes (n = 5) | 3.72 (3.59–4.04) | 0.06 (0.03–0.09) | 0.47 (0.47–0.53) | 93.68 (84.49–114.73) | ||||
| Occupation: | 0.504 | 0.116 | 0.203 | 0.100 # | ||||
| student (n = 15) | 3.72 (2.33–7.34) | 0.06 (0.05–0.10) | 0.41 (0.33–0.66) | 94.70 (75.72–108.77) | ||||
| worker (n = 9) | 4.90 (3.20–5.88) | 0.09 (0.07–0.10) | 0.56 (0.51–0.90) | 132.22 (95.18–163.22) | ||||
| white-collar worker (n = 25) | 3.62 (2.51–5.98) | 0.06 (0.04–0.08) | 0.53 (0.30–0.68) | 129.16 (93.68–158.62) | ||||
| Years of residence in the LG Copper Area: | 0.956 | 0.719 | 0.740 | 0.088 # | ||||
| ≤20 years (n = 17) | 3.91 (1.94–5.98) | 0.09 (0.06–0.10) | 0.54 (0.30–0.66) | 95.18 (84.49–103.79) | ||||
| 21–30 years old (n = 19) | 3.62 (2.51–6.35) | 0.07 (0.06–0.09) | 0.51 (0.33–0.68) | 108.77 (92.31–155.17) | ||||
| ≥31 years old (n = 13) | 3.86 (2.61–5.88) | 0.07 (0.05–0.09) | 0.53 (0.41–0.92) | 146.21 (96.73–167.76) | ||||
| Thyroid and parathyroid glands diseases: | 0.346 | 0.631 | 0.457 | 0.934 | ||||
| no (n = 44) | 3.81 (2.54–6.54) | 0.07 (0.04–0.10) | 0.52 (0.35–0.71) | 103.82 (87.37–156.29) | ||||
| yes (n = 5) | 3.86 (1.63–4.56) | 0.08 (0.06–0.09) | 0.62 (0.47–0.92) | 96.73 (95.69–132.22) | ||||
| Dietary supplements: | 0.580 | 0.196 | 0.273 | 0.887 | ||||
| no (n = 34) | 3.58 (2.33–6.23) | 0.06 (0.04–0.09) | 0.49 (0.30–0.74) | 100.72 (84.49–163.22) | ||||
| yes (n = 15) | 3.90 (2.88–6.35) | 0.08 (0.06–0.10) | 0.59 (0.42–0.76) | 114.73 (92.87–150.69) | ||||
| Reason of extraction: | 0.152 | 0.984 | 0.899 | 0.455 | ||||
| surgical (n = 34) | 4.73 (2.99–6.72) | 0.07 (0.05–0.09) | 0.52 (0.38–0.74) | 106.28 (90.24–163.22) | ||||
| orthodontic (n = 8) | 2.56 (1.69–7.05) | 0.07 (0.04–0.09) | 0.52 (0.26–0.73) | 96.79 (75.40–127.28) | ||||
| inflammation (n = 7) | 2.57 (1.82–3.90) | 0.09 (0.03–0.10) | 0.59 (0.37–0.92) | 132.22 (95.69–158.62) | ||||
| Vit. D3 (ng/mL): | 0.693 | 0.783 | 0.722 | 0.008 * | ||||
| <20 (n = 15) | 3.62 (2.33–7.22) | 0.06 (0.04–0.09) | 0.51 (0.30–0.74) | 158.62 (99.13–174.00) 1 | ||||
| 20–29 (n = 25) | 4.90 (2.61–6.23) | 0.08 (0.04–0.10) | 0.54 (0.33–0.77) | 95.18 (76.67–108.77) 1 | ||||
| 30–50 (n = 5) | 2.88 (2.57–2.99) | 0.07 (0.07–0.10) | 0.63 (0.57–0.68) | 146.21 (90.24–155.17) | ||||
| 51–100 (n = 4) | 4.09 (1.80–5.78) | 0.06 (0.06–0.08) | 0.47 (0.44–0.51) | 142.45 (108.36–165.77) | ||||
| Toxic Metal (µg/g) | Age (Years) | Vit. D3 (ng/mL) | ||
|---|---|---|---|---|
| Correlation Coefficient (rho) | p-Value | Correlation Coefficient (rho) | p-Value | |
| Mn | 0.23 | 0.097 # | −0.04 | 0.791 |
| Cr | 0.29 | 0.044 * | −0.29 | 0.038 * |
| Ni | 0.17 | 0.240 | −0.25 | 0.071 # |
| Cu | 0.31 | 0.027 * | 0.02 | 0.840 |
| Fe | 0.15 | 0.303 | −0.07 | 0.638 |
| Cd | 0.04 | 0.768 | 0.16 | 0.271 |
| Pb | 0.19 | 0.181 | 0.04 | 0.760 |
| Zn | 0.46 | <0.001 * | −0.13 | 0.377 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayad, S.; Dobrzyński, M.; Kuźniarski, A.; Styczyńska, M.; Diakowska, D.; Gedrange, T.; Klimas, S.; Gębarowski, T.; Dominiak, M. An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study. Appl. Sci. 2023, 13, 2904. https://doi.org/10.3390/app13052904
Rayad S, Dobrzyński M, Kuźniarski A, Styczyńska M, Diakowska D, Gedrange T, Klimas S, Gębarowski T, Dominiak M. An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study. Applied Sciences. 2023; 13(5):2904. https://doi.org/10.3390/app13052904
Chicago/Turabian StyleRayad, Sadri, Maciej Dobrzyński, Amadeusz Kuźniarski, Marzena Styczyńska, Dorota Diakowska, Tomasz Gedrange, Sylwia Klimas, Tomasz Gębarowski, and Marzena Dominiak. 2023. "An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study" Applied Sciences 13, no. 5: 2904. https://doi.org/10.3390/app13052904
APA StyleRayad, S., Dobrzyński, M., Kuźniarski, A., Styczyńska, M., Diakowska, D., Gedrange, T., Klimas, S., Gębarowski, T., & Dominiak, M. (2023). An In-Vitro Evaluation of Toxic Metals Concentration in the Third Molars from Residents of the Legnica-Głogów Copper Area and Risk Factors Determining the Accumulation of Those Metals: A Pilot Study. Applied Sciences, 13(5), 2904. https://doi.org/10.3390/app13052904











