A Knowledge Graph Embedding Approach for Polypharmacy Side Effects Prediction
Abstract
1. Introduction
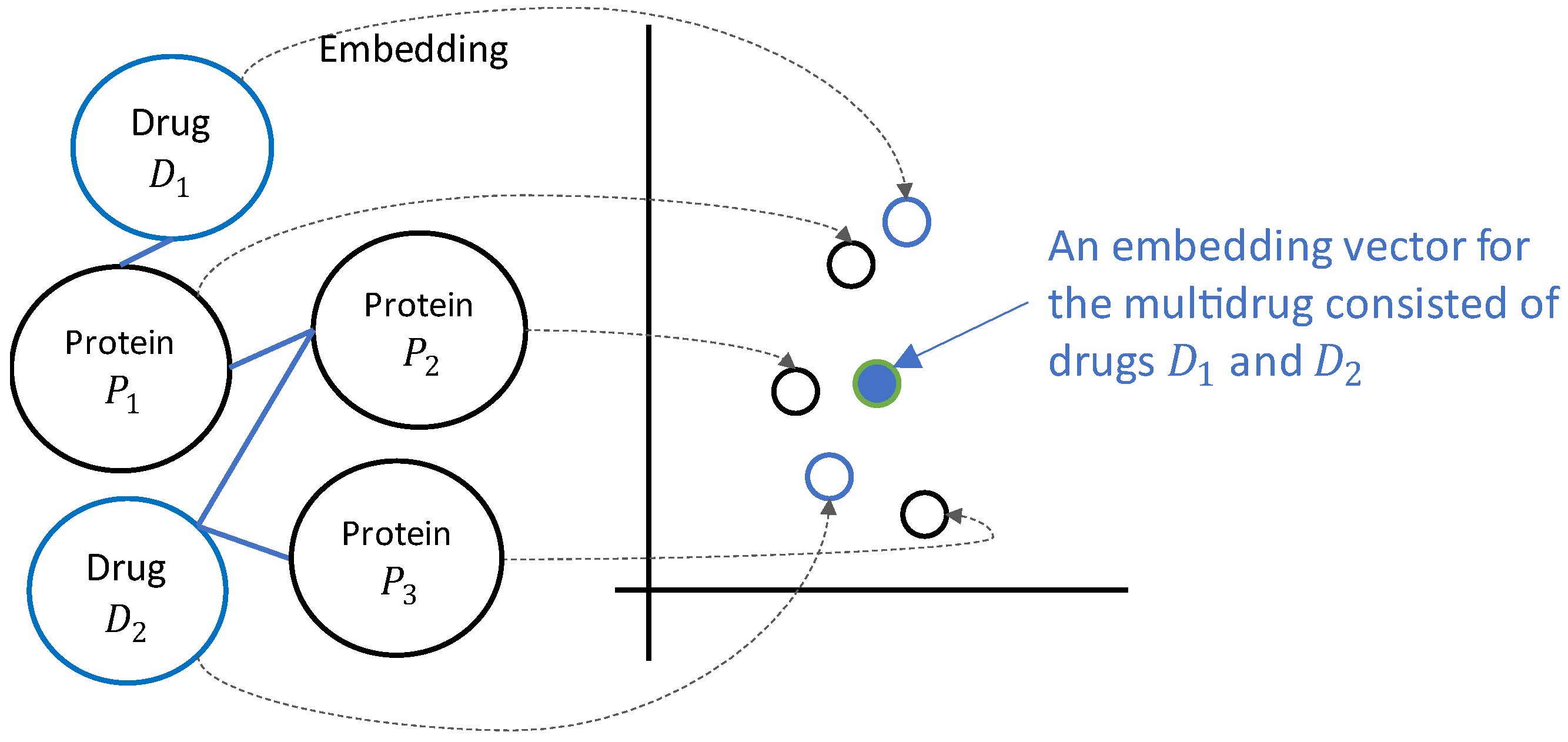
- We introduce a unified embedding approach to convert all graph nodes (representing single or multiple drugs or proteins) into a single embedding space. Side effects caused by a single drug often contribute to multidrug side effects in polypharmacy. This single drug side effect information can help predict the side effects of various multidrug combinations. Herein, we proposed a unified embedding approach that enables the learning of single drug side effect information along with that of multidrug combinations. In this approach, all biological entities represented by nodes in the knowledge graph are mapped to their corresponding n-dimensional vectors that exist in the same embedding space.
- We employ unified embedding vectors to leverage the side effect data related to single drugs as well as multidrug combinations. The embedding module in the proposed method generates the same n-dimensional vector for any given single or multidrug combination, regardless of the number of its constituent drugs. Thus, predictive model training is possible using data from single as well as multidrug combinations (labeled on the side effects that have occurred) represented by unified embedding vectors in the same latent space.
- We utilize relationships between nodes (including distant nodes) for model regularization. Another feature of the proposed model is its ability to utilize the relationships between drugs and all other biological entities shown over the knowledge graph. In particular, for each single or multidrug combination, we computed the values of its connectivity strength to proteins, which indicate how closely its constituent drugs are related to each protein in the graph. We then used these connectivity strengths to regularize the model.
- We use virtually created multidrug combinations for model regularization. To mitigate the risk of overfitting to a limited number of drug–drug samples labeled with side effects, we produced virtual multidrug combinations (actually virtual drug pairs) created with all possible pairs of single drugs and then used them for model regularization. Herein, a virtual multidrug is a combination of available single drugs with no label information. This allows our model to reflect the characteristics of all possible drug combinations as well as those of labeled drug–drug samples during the embedding process.
- We provide the interpretability of proteins significantly associated with multidrug side effects. The embedding vectors of multidrug combinations and proteins represented in a unified vector space enable us to identify proteins that are more influential in producing specific side effects. This can be achieved through the identification of proteins that are relatively close to multidrug combinations associated with a specific side effect, based on connectivity strengths between multidrug combinations and proteins in the embedding space.
2. Methods
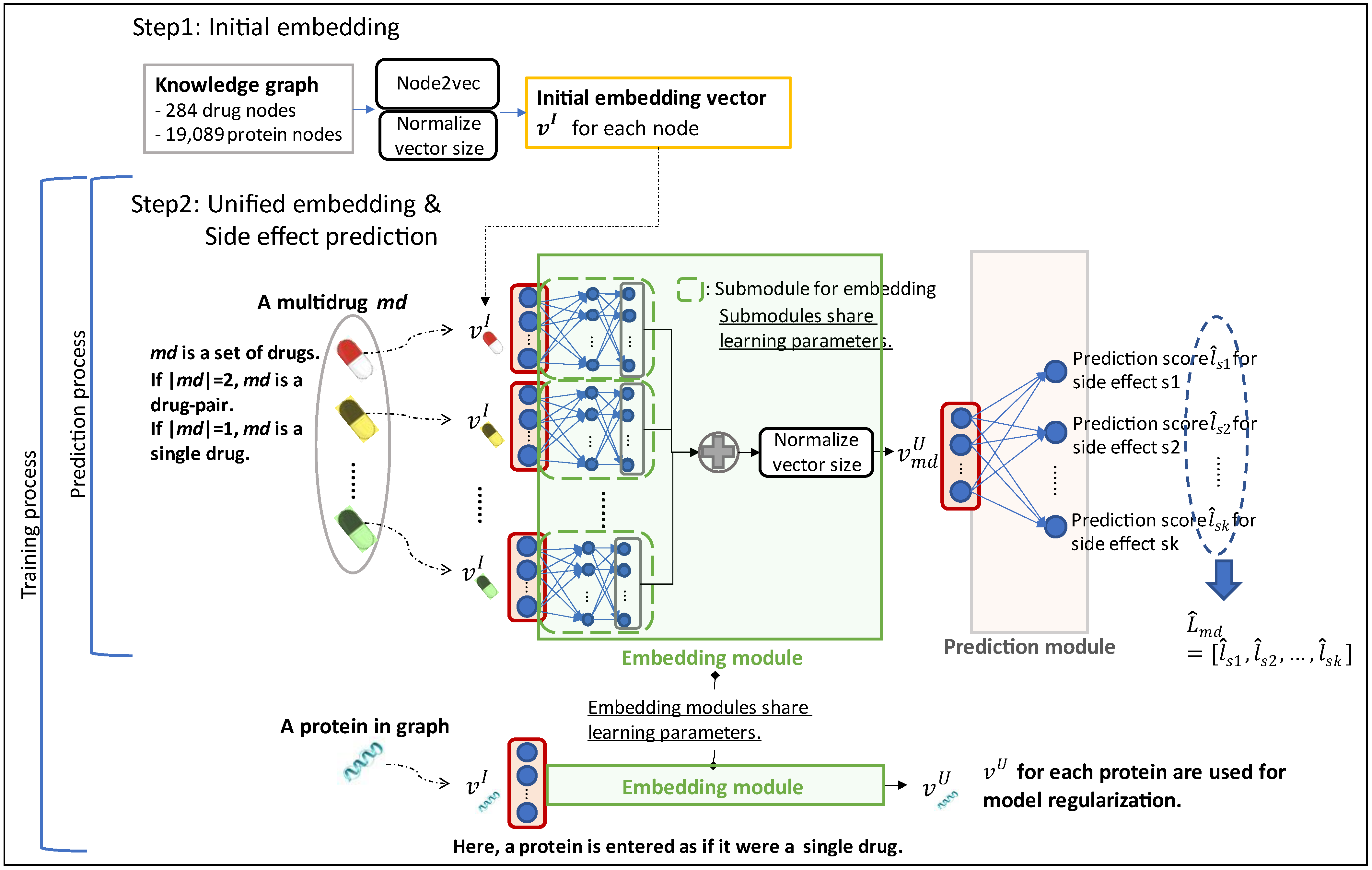
2.1. Initial Embedding
2.2. Unified Embedding
2.3. Model Architecture
2.4. Learning Objective and Loss Function
| Algorithm 1. Pseudo code for training the proposed model |
| G: The knowledge graph consisting of drug and protein nodes. Md: A multidrug (i.e., a set of one or more drugs). : Training data of multidrug combinations labeled with side effects. : Training data of multidrug combinations virtually generated. : An initial embedding vector of a node a. : A set of initial embedding vectors for given nodes. : A unified embedding vector of a multidrug md. : A set of side effect labels of a multidrug md. EmbNN: Neural networks of embedding module. PredNN: Neural networks of prediction module. Function EmbModule (x) Ifx is a multidrug do ElseIf x is a protein do ← EmbNN() EndIF Return normalize_vector_size() EndFunction Function get_loss_by_connectivity_strength(,) loss ← 0 For each protein p do ← (, EmbModule()) loss ← loss + EndFor Return loss EndFunction (, ) ← get_initial_embedding_vectors_for_all_nodes(G) Initialize the weights of EmbNN and PredNN randomly. Set as a positive value. For 1 to Iterations do loss ← 0 P ← Randomly select 1000 among all proteins. For each multidrug md do ← EmbModule() If md ∈ do ← PredNN () loss ← loss + get_loss_by_binary_cross_entropy(,) EndIF loss ← loss + *get_loss_by_connectivity_strength(,) EndFor Adjust the weights of EmbNN and PredNN to reduce the loss. EndFor |
3. Experiments and Results
3.1. Dataset
3.2. Experimental Setup
- DPU: Drug pair including drugs unused in labeled multidrug combinations included in training data.
- Non-DPU: Drug pair consisted of only drugs used in labeled multidrug combinations included in training data.
3.3. DPU Side Effect Prediction
3.4. Non-DPU Side Effect Prediction
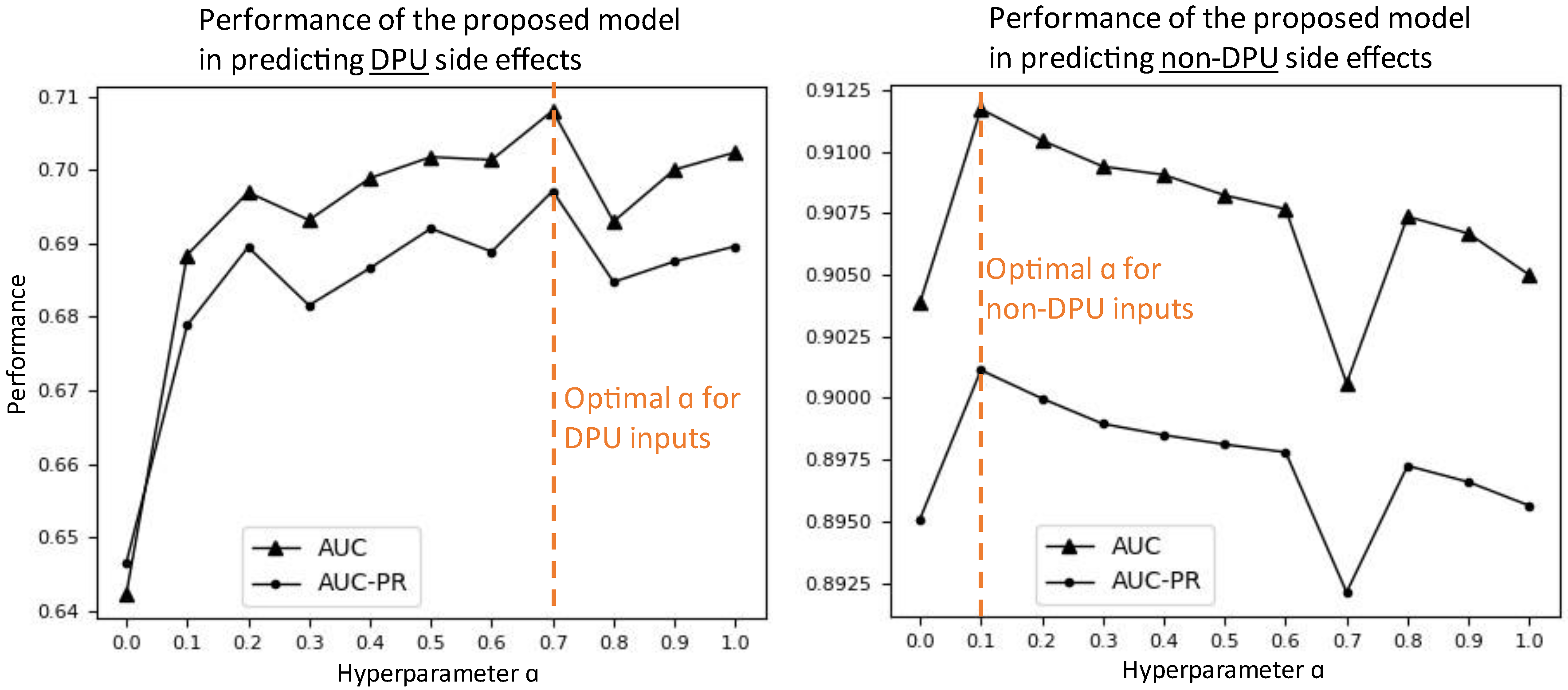
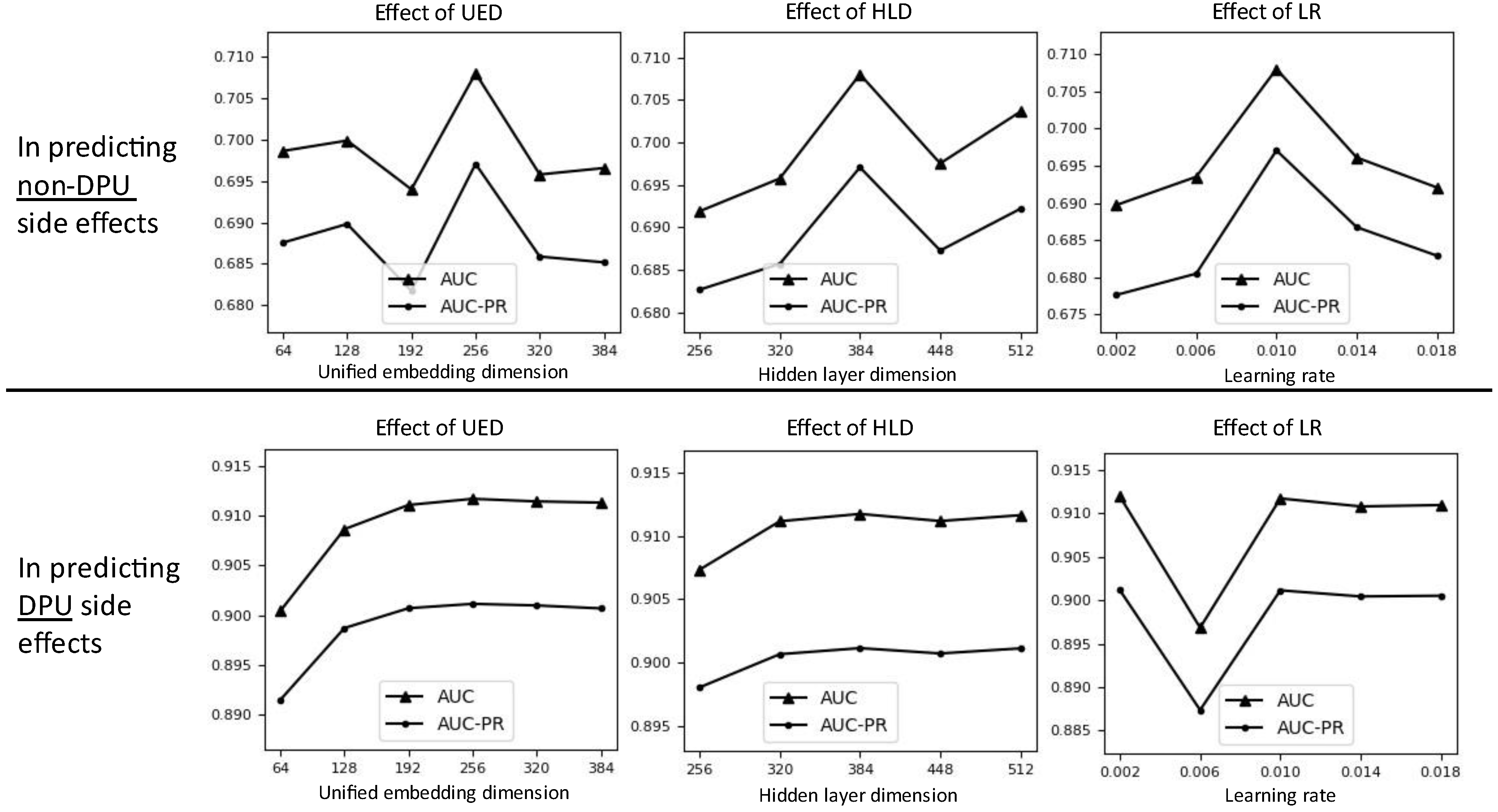
3.5. Effects of Hyperparameter Setting on Prediction Performance
4. A Case Study on Interpretability
4.1. Determining and Selecting Proteins Significant in a Side Effect
| Algorithm 2. Pseudo code of determining and selecting proteins significant in a side effect s. |
| : The set of significant proteins for the s. : All proteins in graph. : A set of positive drug pairs that cause s by polypharmacy effect. : A set of negative drug pairs that do not cause the s. : A group of connectivity strength (cs) values. : Threshold to determine significance. Function MannWhitneyU(, ) Return a p-value calculated by MannWhitney U test between two groups. (Alternative hypothesis: Protein p has larger cs values in positive drug pairs than in negative drug pairs.) EndFunction Initialize to empty set. For each protein do: The group of (dp, p) values for dp The group of (dp, p) values for dp p_valueI MannWhitneyU(, ) The group of (dp, p) values for dp The group of (dp, p) values for dp p_valueU MannWhitneyU(, ) If (p_valueI ≧ ) and (p_valueU < ) do: Include p into . EndIf |
4.2. Examining the Relationships between Significant Proteins and Side Effects
4.3. Results
5. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DPU | Drug pair including drugs unused in labeled multidrug combinations included in training data. |
| Non-DPU | Drug pair consisted of only drugs used in labeled multidrug combinations included in training data. |
| AUC | Area under the receiver operating characteristic curve |
| AUC-PR | Area under the curve of Precision-Recall |
| GO | Gene ontology |
References
- Dagli, R.J.; Sharma, A. Polypharmacy: A global risk factor for elderly people. J. Int. Oral. Health 2014, 6, i–ii. [Google Scholar]
- Hrestha, S.; Shrestha, S.; Khanal, S. Polypharmacy in elderly cancer patients: Challenges and the way clinical pharmacists can contribute in resource-limited settings. Aging Med. 2019, 2, 42–49. [Google Scholar] [CrossRef]
- Khezrian, M.; McNeil, C.J.; Murray, A.D.; Myint, P.K. An overview of prevalence, determinants and health outcomes of polypharmacy. Ther. Adv. Drug Saf. 2020, 11, 2042098620933741. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Kuhn, M.; Szklarczyk, D.; Franceschini, A.; Campillos, M.; von Mering, C.; Jensen, L.J.; Beyer, A.; Bork, P. STITCH 2: An interaction network database for small molecules and proteins. Nucleic Acids Res. 2010, 38, D552–D556. [Google Scholar] [CrossRef]
- Musa, A.; Tripathi, S.; Dehmer, M.; Emmert-Streib, F. L1000 Viewer: A Search Engine and Web Interface for the LINCS Data Repository. Front. Genet. 2019, 10, 557. [Google Scholar] [CrossRef]
- Liu, T.; Cui, J.; Zhuang, H.; Wang, H. Modeling polypharmacy effects with heterogeneous signed graph convolutional networks. Appl. Intell. 2021, 51, 8316–8333. [Google Scholar] [CrossRef]
- Yao, J.; Sun, W.; Jian, Z.; Wu, Q.; Wang, X. Effective knowledge graph embeddings based on multidirectional semantics relations for polypharmacy side effects prediction. Bioinformatics 2022, 38, 2315–2322. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Liu, X.; Ma, X.; Wang, B. Drug-Drug Interaction Predictions via Knowledge Graph and Text Embedding: Instrument Validation Study. JMIR Med. Inform. 2021, 9, e28277. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, K.; Zhang, C.; Glass, L.M.; Sun, J.; Xiao, C. SumGNN: Multi-typed drug interaction prediction via efficient knowledge graph summarization. Bioinformatics 2021, 37, 2988–2995. [Google Scholar] [CrossRef]
- Nováček, V.; Mohamed, S.K. Predicting Polypharmacy Side-effects Using Knowledge Graph Embeddings. AMIA Jt. Summits Transl. Sci. Proc. 2020, 2020, 449–458. [Google Scholar]
- Burkhardt, H.A.; Subramanian, D.; Mower, J.; Cohen, T. Predicting Adverse Drug-Drug Interactions with Neural Embedding of Semantic Predications. AMIA Annual Symposium proceedings. AMIA Symp. 2019, 2019, 992–1001. [Google Scholar]
- Bang, S.; Jhee, J.H.; Shin, H. Polypharmacy Side effect Prediction with Enhanced Interpretability Based on Graph Feature Attention Network. Bioinformatics 2021, 37, 2955–2962. [Google Scholar] [CrossRef]
- Zitnik, M.; Agrawal, M.; Leskovec, J. Modeling polypharmacy side effects with graph convolutional networks. Bioinformatics 2018, 34, 457–466. [Google Scholar] [CrossRef]
- Wang, R.; Li, T.; Yang, Z.; Yu, H. Predicting Polypharmacy Side Effects Based on an Enhanced Domain Knowledge Graph. In Proceedings of the Applied Informatics, Ota, Nigeria, 29–31 October 2020; pp. 89–103. [Google Scholar]
- Carletti, V.; Foggia, P.; Greco, A.; Roberto, A.; Vento, M. Predicting Polypharmacy Side Effects through a Relation-Wise Graph Attention Network. In Proceedings of the Structural, Syntactic, and Statistical Pattern Recognition, Padua, Italy, 21–22 January 2021; pp. 119–128. [Google Scholar]
- Dai, Y.; Wang, S.; Xiong, N.N.; Guo, W. A Survey on Knowledge Graph Embedding: Approaches, Applications and Benchmarks. Electronics 2020, 9, 750. [Google Scholar] [CrossRef]
- Wang, Q.; Mao, Z.; Wang, B.; Guo, L. Knowledge Graph Embedding: A Survey of Approaches and Applications. IEEE Trans. Knowl. Data Eng. 2017, 29, 2724–2743. [Google Scholar] [CrossRef]
- Bordes, A.; Usunier, N.; Garcia-Duran, A.; Weston, J.; Yakhnenko, O. Translating Embeddings for Modeling Multi-relational Data. Adv. Neural Inf. Process. Syst. 2013, 26, 2787–2795. [Google Scholar]
- Lin, H.; Liu, Y.; Wang, W.; Yue, Y.; Lin, Z. Learning Entity and Relation Embeddings for Knowledge Resolution. Procedia Comput. Sci. 2017, 108, 345–354. [Google Scholar] [CrossRef]
- Trouillon, T.; Welbl, J.; Riedel, S.; Gaussier, É.; Bouchard, G. Complex embeddings for simple link prediction. In Proceedings of the 33rd International Conference on International Conference on Machine Learning, New York, NY, USA, 20–22 June 2016; Volume 48, pp. 2071–2080. [Google Scholar]
- Yang, B.; Yih, W.-t.; He, X.; Gao, J.; Deng, L. Embedding entities and relations for learning and inference in knowledge bases. arXiv 2014, arXiv:1412.6575. [Google Scholar]
- Grover, A.; Leskovec, J. node2vec: Scalable Feature Learning for Networks. KDD 2016, 2016, 855–864. [Google Scholar] [CrossRef]
- Gao, Z.; Fu, G.; Ouyang, C.P.; Tsutsui, S.; Liu, X.Z.; Yang, J.; Gessner, C.; Foote, B.; Wild, D.; Ding, Y.; et al. edge2vec: Representation learning using edge semantics for biomedical knowledge discovery. BMC Bioinform. 2019, 20, 1–15. [Google Scholar] [CrossRef]
- Yadati, N.; Nitin, V.; Nimishakavi, M.; Yadav, P.; Louis, A.; Talukdar, P. NHP: Neural Hypergraph Link Prediction. In Proceedings of the 29th ACM International Conference on Information & Knowledge Management, Virtual Event, 19–23 October 2020; pp. 1705–1714. [Google Scholar]
- Chen, C.; Liu, Y.-Y. A survey on hyperlink prediction. arXiv 2022, arXiv:2207.02911. [Google Scholar]
- Klamt, S.; Haus, U.-U.; Theis, F. Hypergraphs and cellular networks. PLoS Comput. Biol. 2009, 5, e1000385. [Google Scholar] [CrossRef]
- Xu, B.; Wang, N.; Chen, T.; Li, M. Empirical evaluation of rectified activations in convolutional network. arXiv 2015, arXiv:1505.00853. [Google Scholar]
- Kingma, D.P.; Ba, J. Adam: A Method for Stochastic Optimization. arXiv 2014, arXiv:1412.6980. [Google Scholar]
- Hu, W.; Xiao, L.; Pennington, J. Provable benefit of orthogonal initialization in optimizing deep linear networks. arXiv 2020, arXiv:2001.05992. [Google Scholar]
- Tatonetti, N.P.; Ye, P.P.; Daneshjou, R.; Altman, R.B. Data-Driven Prediction of Drug Effects and Interactions. Sci. Transl. Med. 2012, 4, 125ra31. [Google Scholar] [CrossRef]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- van den Broek Best, O.; Vo, L.; Handmer, M.; Maclean, F.; Mancuso, P. Masquerading as A Stone: An Unusual Cause of Chronic Ureteric Obstruction. J. Urol. Surg. 2021, 8, 303–305. [Google Scholar] [CrossRef]
- Mantoo, S.; Hwang, J.; Chiang, G.; Tan, P.H. A rare case of localised AA-type amyloidosis of the ureter with spheroids of amyloid. Singap. Med. J. 2012, 53, e77–e79. [Google Scholar]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye 2015, 29, 1013–1026. [Google Scholar] [CrossRef]
- Lee, V.; Rekhi, E.; Kam, J.H.; Jeffery, G. Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol. Aging 2012, 33, 2382–2389. [Google Scholar] [CrossRef]
- Plewig, G.; Kligman, A.M. The Role of Demodex. In Acne: Morphogenesis and Treatment; Springer: Berlin/Heidelberg, Germany, 1975; pp. 267–269. [Google Scholar]
- Lehman, J.M.; Laag, E.; Michaud, E.J.; Yoder, B.K. An essential role for dermal primary cilia in hair follicle morphogenesis. J. Investig. Dermatol. 2009, 129, 438–448. [Google Scholar] [CrossRef]
- Somuncu, S.; Somuncu, Ö.S.; Ballıca, B.; Tabandeh, B. Deficiency of epithelial–mesenchymal transition causes child indirect inguinal hernia. J. Pediatr. Surg. 2020, 55, 665–671. [Google Scholar] [CrossRef]
- Diets, I.J.; van der Donk, R.; Baltrunaite, K.; Waanders, E.; Reijnders, M.R.F.; Dingemans, A.J.M.; Pfundt, R.; Vulto-van Silfhout, A.T.; Wiel, L.; Gilissen, C.; et al. De Novo and Inherited Pathogenic Variants in KDM3B Cause Intellectual Disability, Short Stature, and Facial Dysmorphism. Am. J. Hum. Genet. 2019, 104, 758–766. [Google Scholar] [CrossRef]
- de Freitas, L.C.C.; Castilho, R.M.; Squarize, C.H. Histone Modification on Parathyroid Tumors: A Review of Epigenetics. Int. J. Mol. Sci. 2022, 23, 5378. [Google Scholar] [CrossRef]
- Alvelos, M.I.; Mendes, M.; Soares, P. Molecular alterations in sporadic primary hyperparathyroidism. Genet. Res. Int. 2011, 2011, 275802. [Google Scholar] [CrossRef]
- van der Knaap, J.A.; Kumar, B.P.; Moshkin, Y.M.; Langenberg, K.; Krijgsveld, J.; Heck, A.J.; Karch, F.; Verrijzer, C.P. GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol. Cell 2005, 17, 695–707. [Google Scholar] [CrossRef]
- Zhao, Y.; Lang, G.; Ito, S.; Bonnet, J.; Metzger, E.; Sawatsubashi, S.; Suzuki, E.; Le Guezennec, X.; Stunnenberg, H.G.; Krasnov, A. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol. Cell 2008, 29, 92–101. [Google Scholar] [CrossRef]
- Casertano, A.; Rossi, A.; Fecarotta, S.; Rosanio, F.M.; Moracas, C.; Di Candia, F.; Parenti, G.; Franzese, A.; Mozzillo, E. An Overview of Hypoglycemia in Children Including a Comprehensive Practical Diagnostic Flowchart for Clinical Use. Front. Endocrinol 2021, 12, 684011. [Google Scholar] [CrossRef]
- Fanti, P.; Tosti, A.; Morelli, R.; Galbiati, G. Nail changes as the first sign of systemic amyloidosis. Dermatology 1991, 183, 44–46. [Google Scholar] [CrossRef]
- Renker, T.; Haneke, E.; Röcken, C.; Borradori, L. Systemic light-chain amyloidosis revealed by progressive nail involvement, diffuse alopecia and sicca syndrome: Report of an unusual case with a review of the literature. Dermatology 2014, 228, 97–102. [Google Scholar] [CrossRef]
- Derrick, E.K.; Price, M.L. Primary systemic amyloid with nail dystrophy. J. R. Soc. Med. 1995, 88, 290. [Google Scholar]
- Tausend, W.; Neill, M.; Kelly, B. Primary amyloidosis-induced nail dystrophy. Dermatol. Online J. 2014, 20, 21247. [Google Scholar] [CrossRef]
- af Klinteberg, B.; Oreland, L.; Hallman, J.; Wirsén, A.; Levander, S.E.; Schalling, D. Exploring the connections between platelet monoamine oxidase activity and behavior: Relationships with performance in neuropsychological tasks. Neuropsychobiology 1990, 23, 188–196. [Google Scholar] [CrossRef]
- Padmakumar, M.; Van Raes, E.; Van Geet, C.; Freson, K. Blood platelet research in autism spectrum disorders: In search of biomarkers. Res. Pract. Thromb. Haemost. 2019, 3, 566–577. [Google Scholar] [CrossRef]
- Xia, P.; Liu, Y.; Chen, J.; Coates, S.; Liu, D.X.; Cheng, Z. Inhibition of cyclin-dependent kinase 2 protects against doxorubicin-induced cardiomyocyte apoptosis and cardiomyopathy. J. Biol. Chem. 2018, 293, 19672–19685. [Google Scholar] [CrossRef]
- Rao, Z.; Ding, Y. Ubiquitin pathway and ovarian cancer. Curr. Oncol. 2012, 19, 324–328. [Google Scholar] [CrossRef]
- Meng, Y.; Qiu, L.; Zhang, S.; Han, J. The emerging roles of E3 ubiquitin ligases in ovarian cancer chemoresistance. Cancer Drug Resist. 2021, 4, 365–381. [Google Scholar] [CrossRef]
- Ando, H.; Ichihashi, M.; Hearing, V.J. Role of the ubiquitin proteasome system in regulating skin pigmentation. Int. J. Mol. Sci. 2009, 10, 4428–4434. [Google Scholar] [CrossRef]
- Qu, X.; Zhai, B.; Liu, Y.; Chen, Y.; Xie, Z.; Wang, Q.; Wu, Y.; Liu, Z.; Chen, J.; Mei, S.; et al. Pyrroloquinoline quinone ameliorates renal fibrosis in diabetic nephropathy by inhibiting the pyroptosis pathway in C57BL/6 mice and human kidney 2 cells. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 150, 112998. [Google Scholar] [CrossRef]
- Jia, D.; Duan, F.; Peng, P.; Sun, L.; Ruan, Y.; Gu, J. Pyrroloquinoline-quinone suppresses liver fibrogenesis in mice. PLoS ONE 2015, 10, e0121939. [Google Scholar] [CrossRef]
- Lacombe, A.; Lee, H.; Zahed, L.; Choucair, M.; Muller, J.M.; Nelson, S.F.; Salameh, W.; Vilain, E. Disruption of POF1B binding to nonmuscle actin filaments is associated with premature ovarian failure. Am. J. Hum. Genet. 2006, 79, 113–119. [Google Scholar] [CrossRef]
- Tucker, E.J.; Grover, S.R.; Bachelot, A.; Touraine, P.; Sinclair, A.H. Premature Ovarian Insufficiency: New Perspectives on Genetic Cause and Phenotypic Spectrum. Endocr. Rev. 2016, 37, 609–635. [Google Scholar] [CrossRef]
- Li, H.; Zhao, Z.; Ling, J.; Pan, L.; Zhao, X.; Zhu, H.; Yu, J.; Xie, B.; Shen, J.; Chen, W. USP14 promotes K63-linked RIG-I deubiquitination and suppresses antiviral immune responses. Eur. J. Immunol. 2019, 49, 42–53. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, Q.; Liuyu, T.; Xu, Z.; Zhang, M.-X.; Luo, M.-H.; Zeng, W.-B.; Zhu, Q.; Lin, D.; Zhong, B. USP49 negatively regulates cellular antiviral responses via deconjugating K63-linked ubiquitination of MITA. PLoS Pathog. 2019, 15, e1007680. [Google Scholar] [CrossRef]
- Klein, K.; Michel, B.; Gay, R.E.; Gay, S.; Ospelt, C. SAT0063|Functional analysis of the primary cilium in rheumatoid arthritis synovial fibroblasts. Ann. Rheum. Dis. 2013, 71, 491. [Google Scholar] [CrossRef]
- Rattner, J.B.; Sciore, P.; Ou, Y.; van der Hoorn, F.A.; Lo, I.K. Primary cilia in fibroblast-like type B synoviocytes lie within a cilium pit: A site of endocytosis. Histol. Histopathol. 2010, 25, 865–875. [Google Scholar] [CrossRef]
| Model Type | Labeled Data for Training | Data for Regularization | AUC | AUC-PR | Accuracy | F1-Score | ||
|---|---|---|---|---|---|---|---|---|
| Drug Pairs | Single Drugs | Drug Pairs Included in Training Data | Virtually Created Drug Pairs | |||||
| Baseline | O | X | X | X | 0.618 | 0.618 | 0.576 | 0.567 |
| Model 1 | O | O | X | X | 0.642 | 0.645 | 0.588 | 0.575 |
| Model 2 | O | O | O | X | 0.684 | 0.673 | 0.600 | 0.584 |
| Model 3 | O | X | O | X | 0.690 | 0.683 | 0.616 | 0.602 |
| Model 4 | O | X | O | O | 0.685 | 0.670 | 0.602 | 0.582 |
| Model 5 (Proposed) | O | O | O | O | 0.708 | 0.697 | 0.637 | 0.618 |
| Model Type | Labeled Data for Training | Data for Regularization | AUC | AUC-PR | Accuracy | F1-Score | |||
|---|---|---|---|---|---|---|---|---|---|
| Drug Pairs | Single Drugs | Drug Pairs Included in Training Data | Virtually Created Drug Pairs | ||||||
| Existing models | Decagon [14] | O | X | X | X | 0.531 | 0.529 | 0.498 | 0.492 |
| DistMult [22] | O | X | X | X | 0.577 | 0.581 | 0.531 | 0.520 | |
| Our model | Baseline | O | X | X | X | 0.618 | 0.618 | 0.576 | 0.567 |
| Proposed | O | O | O | O | 0.708 | 0.697 | 0.637 | 0.618 | |
| Model Type | Labeled Data for Training | Data for Regularization | AUC | AUC-PR | Accuracy | F1-Score | ||
|---|---|---|---|---|---|---|---|---|
| Drug Pairs | Single Drugs | Drug Pairs Included in Training Data | Virtually Created Drug Pairs | |||||
| Baseline | O | X | X | X | 0.893 | 0.887 | 0.821 | 0.818 |
| Model 1 | O | O | X | X | 0.904 | 0.895 | 0.827 | 0.821 |
| Model 2 | O | O | O | X | 0.912 | 0.900 | 0.835 | 0.824 |
| Model 3 | O | X | O | X | 0.912 | 0.901 | 0.837 | 0.827 |
| Model 4 | O | X | O | O | 0.912 | 0.901 | 0.836 | 0.827 |
| Model 5 (Proposed) | O | O | O | O | 0.912 | 0.901 | 0.837 | 0.828 |
| Study | Average of AUCs for Non-DPU Inputs | Targeted Side Effects (Ses) | Used Resources for Graph Construction | Used Data |
|---|---|---|---|---|
| Proposed model | 0.912 in all targeted Ses. 0.963 in the 10 Ses with the highest AUC. | 200 Ses | Drug-protein (DP), and Protein-Protein (PP) | 14,247 labeled drug pairs and 284 labeled single drugs. |
| Zitnik et al., (2018) [14] | 0.872 in all targeted Ses. | 964 Ses | DP, PP and Drug-Drug (DD) * | 63,473 labeled drug pairs and negative sampling |
| Wang et al., (2020) [15] | 0.97 in the 50 Ses with the highest AUC. | 50 or more Ses | PP, DP, DD *, and Drug-Enzyme | Labeled drug pairs from 389 drugs. |
| Nováček et al., (2020) [11] | Apparently 0.99 in all targeted Ses. | Apparently 964 Ses | DD * (and perhaps also PP and DP) | 63,473 labeled drug pairs and negative sampling. |
| Liu et al., (2021) [7] | 0.947 in all targeted Ses. | 964 Ses | DP, DD *, and Semantic data. | Labeled drug pairs from 645 drugs. |
| Bang et al., (2021) [13] | 0.94 in the 10 Ses with the highest AUC. | 10 or more Ses | DP and DD * | 14,247 labeled drug pairs. |
| Yu et al., (2021) [10] | 0.949 in all targeted Ses. | 200 Ses | PP *, DP *, DD *, and other ten links of multi-type | 46,221 labeled drug pairs and negative sampling. |
| Carletti et al., (2021) [16] | 0.998 in all targeted Ses. | 964 Ses | DP, PP and DD * | 63,473 labeled drug pairs. |
| Yao et al., (2022) [8] | 0.996 in all targeted Ses. | 200 Ses | DD * (and perhaps also DP and PP) | Apparently 63,473 labeled drug pairs and negative sampling. |
| Model Type | AUC | AUC-PR | Targeted Side Effects (Ses) | Used Resources for Graph Construction | Used Data |
|---|---|---|---|---|---|
| Proposed model | 0.912 | 0.901 | 200 Ses | Drug-protein (DP), and Protein-Protein (PP) | 14,247 labeled drug pairs and 284 labeled single drugs |
| Decagon [14] | 0.805 | 0.792 | PP, DP, and multi-typed Drug-Drug | 14,247 labeled drug pairs and negative sampling | |
| DistMult [22] | 0.812 | 0.806 |
| Side Effect ID | Significant GO Term | GO ID | Overlap * | Adjusted p-Value | Related Literatures and Notes |
|---|---|---|---|---|---|
| Calculus ureteric | Regulation of amyloid precursor protein biosynthetic process | GO:0042984 | 5/11 | 0.008806 | Ureteric calculus is related to amyloid [34,35]. |
| Eye injury | Regulation of amyloid precursor protein biosynthetic process | GO:0042984 | 5/11 | 0.007394 | Weaken of eye function is related to amyloid [36,37]. |
| Folliculitis | Intraciliary transport involved in cilium assembly | GO:0035735 | 10/40 | 0.00145 | Folliculitis is directly related to cilia (cilium) [38,39]. |
| Hernia inguinal | Histone lysine methylation | GO:0034968 | 10/34 | 0.003045 | Hernia is related to histone lysine methylation [40,41]. |
| Hyperparathyroidism | Histone ubiquitination | GO:0016574 | 7/21 | 0.009554 | Hyperparathyroidism is related to histone [42,43]. |
| Hyperpigmentation | Histone H2B ubiquitination | GO:0033523 | 5/10 | 0.003118 | Pigmentation is related to histone H2B in Drosophila [44,45]. |
| Hypoglycaemia neonatal | Protein serine/threonine kinase activator activity | GO:0043539 | 6/37 | 0.001837 | Neonatal hypoglycaemia is related to serine/threonine kinase [46]. |
| Ingrowing nail | Regulation of amyloid precursor protein biosynthetic process | GO:0042984 | 6/11 | 0.003511 | Nail growing is related to amyloid [47,48,49,50]. |
| Motor retardation | Platelet aggregation | GO:0070527 | 8/36 | 0.004725 | Psychomotor activity is related to platelet [51,52]. |
| Myocarditis | Cyclin/CDK positive transcription elongation factor complex | GO:0008024 | 4/6 | 0.001296 | Myocarditis is strongly related to cyclin/CDK [53]. |
| Ovarian cancer | Ubiquitin binding | GO:0043130 | 8/73 | 0.004612 | Ovarian cancer is related to ubiquitin [54,55]. |
| Pigmentation disorder | Ubiquitin binding | GO:0043130 | 8/73 | 0.006731 | Pigmentation is related to ubiquitin [56]. |
| Pleural fibrosis | Quinone biosynthetic process | GO:1901663 | 5/14 | 0.009926 | Fibrosis of liver and kidney is related to pyrroloquinoline-quinone [57,58]. |
| Premature menopause | Intermediate filament organization | GO:0045109 | 8/18 | 0.005834 | Premature menopause is strongly related to actin filament [59,60]. |
| Soft tissue infection | Protein K63-linked deubiquitination | GO:0070536 | 6/32 | 0.00143 | K63-linked deubiquitination is related to antiviral response [61,62]. |
| Synovitis | Intraciliary transport involved in cilium assembly | GO:0035735 | 10/40 | 0.000563 | Synovitis is related to cilia [63,64]. |
| Colitis pseudomembranous | DNA-templated transcription, elongation | GO:0006354 | 10/69 | 0.004319 | Not found related Literatures |
| Dysphemia | Intraciliary transport involved in cilium assembly | GO:0035735 | 11/40 | 0.00759 | |
| Flashing lights | Histone monoubiquitination | GO:0010390 | 8/24 | 0.005066 | |
| Furuncle | Coenzyme A metabolic process | GO:0015936 | 6/14 | 0.005619 | |
| Myasthenia gravis | Histone ubiquitination | GO:0016574 | 9/21 | 6.16E-05 | |
| Seborrhoeic keratosis | Prenyltransferase activity | GO:0004659 | 6/9 | 0.008161 | |
| Sinus headache | tRNA wobble base modification | GO:0002097 | 7/14 | 0.00292 | |
| Trigger finger | Positive regulation of protein export from nucleus | GO:0046827 | 7/15 | 0.003106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Shin, M. A Knowledge Graph Embedding Approach for Polypharmacy Side Effects Prediction. Appl. Sci. 2023, 13, 2842. https://doi.org/10.3390/app13052842
Kim J, Shin M. A Knowledge Graph Embedding Approach for Polypharmacy Side Effects Prediction. Applied Sciences. 2023; 13(5):2842. https://doi.org/10.3390/app13052842
Chicago/Turabian StyleKim, Jinwoo, and Miyoung Shin. 2023. "A Knowledge Graph Embedding Approach for Polypharmacy Side Effects Prediction" Applied Sciences 13, no. 5: 2842. https://doi.org/10.3390/app13052842
APA StyleKim, J., & Shin, M. (2023). A Knowledge Graph Embedding Approach for Polypharmacy Side Effects Prediction. Applied Sciences, 13(5), 2842. https://doi.org/10.3390/app13052842






