Abstract
Electroencephalograms (EEGs) of children with reading disorders (RDs) are characterized by a higher theta and a lower alpha than those of typically developing children. Neurofeedback (NFB) may be helpful for treating learning disorders by reinforcing a reduction in the theta/alpha ratio. Several studies have suggested that NFB may lead to EEG power normalization and cognitive improvements. To further explore brain changes in isolated areas, the aim of this study was to explore the effects of an NFB protocol on functional connectivity (coherence) among children with RDs. Twenty children with an RD and an abnormally high theta/alpha ratio underwent 30 NFB sessions, and five children with the same characteristics received a sham NFB treatment. On average, the children in the NFB group showed an increase in reading accuracy and comprehension scores; their coherence diminished in the delta, theta, and beta bands and increased in the alpha band, primarily the theta intrahemispheric coherences of the left hemisphere, which is closely associated with reading. In contrast, children who received the sham NFB treatment did not show reading changes and had few changes in their coherence patterns. These preliminary results suggest that NFB can positively impact reading-related functions in the brain networks of children with RDs.
Keywords:
EEG; qEEG; connectivity; coherence; neurofeedback; reading disorder; learning disorder; dyslexia 1. Introduction
Learning to read is a cognitive process that takes place at an early age. It is a process that requires formal training and depends on the development and coordination of multiple brain processes in different regions that participate in its acquisition [1,2,3].
Although most people learn to read easily, 5–17% of school-age children [4,5] have difficulties acquiring reading skills [6]. When these difficulties manifest in combination with low scores on standardized reading tests (1.5 standard deviations below the population mean for the child’s age), they provide diagnostic certainty of a specific learning disorder with impairment in reading (RD), which is considered specific because it is not attributable to intellectual disability, hearing disorders, vision disorders, neurological disorders, or motor disorders [7]. Although the term “dyslexia” has been widely used to refer to people with RD, according to the DSM-5, the diagnosis of dyslexia does not include deficits in reading comprehension. Both terms are used interchangeably in this work. Furthermore, RD is one of the three specific learning disorders described in the DSM-5 and has the highest prevalence [7].
Reading problems have been reported to be associated with structural and/or functional abnormalities, specifically in the left perisylvian regions [4]. Among individuals with reading disorders, abnormalities in cortical connectivity have been found. These could be due to alterations in neuronal migration [8], as reported by Galaburda et al. [9], who found evidence of cortical anomalies in the brains of people with dyslexia in postmortem studies, including neuronal ectopias and dysplasias located predominantly in the left perisylvian regions.
Using imaging techniques such as magnetic resonance imaging, structural differences between dyslexic patients and individuals with typical development have been found. Dyslexic adult patients have reduced brain volume and abnormal lateralization, and their white matter depth is greater than normal [10]. Among children with dyslexia, decreased cortical thickness has been reported not only in regions associated with reading (occipitotemporal and occipitoparietal areas of both hemispheres) but also in other brain areas, including the right orbitofrontal cortex and left anterior cingulate cortex [11]. Regarding gyrification, Casanova et al. [10] reported lower gyrification in dyslexic adults, while Williams et al. [11] reported higher gyrification in dyslexic children. The increase in gyrification serves to improve communication between neighboring areas; however, it comes at the cost of reducing effective long-range connectivity.
A cortical network in the left hemisphere involves three specialized regions that participate in reading: (1) the left dorsal temporoparietal circuit located around the area of Wernicke, including the extrastriate cortex, fusiform gyrus, and inferior temporal region, which specializes in phonological decoding; (2) the left ventral occipitotemporal circuit that houses the visual area of word form, to which visual and orthographic recognition based on memory is attributed; and (3) the left inferior frontal circuit, classically called Broca’s area, which is involved in the articulation of words [12]. It has been proposed that people with dyslexia exhibit dysfunction in this reading network, consisting of underactivation of the left temporoparietal region, involving the inferior parietal region, temporal lobe, and fusiform gyrus; underactivation of the left inferior frontal gyrus; and overactivation in the left primary motor cortex and in the anterior insula [13]. Among children with dyslexia, unlike adults with dyslexia, underactivation in the temporoparietal region has not been confirmed, and occipitotemporal underactivation is less extended than in adults [14]. Various studies have reported overactivation of the right hemisphere in individuals with dyslexia during reading, and this has been interpreted as a mechanism to compensate for deficits in the left hemisphere network associated with reading [15]. Furthermore, the activation of the right frontal region (homolog of Broca’s area) during reading and the greater integrity of the white matter of the right superior longitudinal fasciculus, which includes fibers that ipsilaterally connect the right ventrolateral prefrontal region with the temporoparietal region (homolog of Wernicke’s area), are able to predict reading improvement after 2.5 years in dyslexic children. A previous study highlighted that this is a specific phenomenon observed in dyslexia [16].
Numerous noninvasive techniques can provide information on brain function. Due to their high temporal resolution, electroencephalogram (EEG) recordings provide a useful tool for the study of brain dynamics [17,18,19,20] and for estimating connectivity [21].
1.1. EEG Characteristics in Children with RD
The human electroencephalogram (EEG) reflects the ongoing rhythmic electrical activity of the brain. EEG characteristics are different depending on recording conditions. In this section, references will be made exclusively to the EEG recorded at rest.
The most commonly reported resting-state EEG pattern in children with RDs is an excess of slow activity, primarily in the theta frequency range [22,23,24,25,26,27,28,29], and an alpha activity deficit [26,28,29,30,31,32] when compared to children with typical development. However, there is no consensus regarding what brain regions with atypical patterns are implicated. Some authors have found theta excess in almost all leads [23,26], and other studies have found theta excess in bilateral frontal lobes [22,24,30], the left temporal region [22,25], the right temporal region [27] or bilateral parietooccipital areas [25,28]. Regarding alpha power, some authors found alpha deficits in all leads except in the bilateral occipital and right centroparietal areas [26], while other studies have found deficits in the bilateral occipital area [22]. Most studies found alpha deficits in the parietooccipital area [25,28,30,32] with left predominance [25]. However, Harmony et al. [23] detected alpha deficits in right frontal and left frontocentral leads when they controlled for socioeconomic status, and Jäncke and Alahmadi [24] reported alpha deficits in the left frontal region of children with nonspecified learning disorders and the left centro–temporal–parietal region of children with learning disorders with verbal deficits.
The increased theta and decreased alpha activity pattern described in children with RDs correspond to a normal EEG pattern of a younger child; therefore, this finding has been interpreted as these children with RDs exhibiting a delay in electroencephalographic maturation [23,31].
The nervous system is organized into neural networks for information processing. Therefore, the study of brain connectivity is relevant. However, most studies exploring brain activity in children with learning disorders using EEGs have been based on EEG power analysis, and few have focused on functional connectivity measures. Functional connectivity is a way of measuring the strength of the connection between different structures. There is an increasing number of theoretical and empirical studies that approach the study of the function of the human brain from a network perspective. Specifically, in this study, it is of interest to examine the connection between the regions that constitute the reading network, namely, the posterior dorsal pathway, the posterior ventral pathway, and the anterior network, located in the left hemisphere, to evaluate processes involved in reading in children with RDs (phonological decoding, fast orthographic word recognition, and phonological and articulatory processes). Examining these connections may explain the behavioral deficits observed in accuracy, comprehension, and reading speed. Studying brain connectivity not only allows for the identification of isolated brain areas that participate in a certain function but also shows the neural networks involved and their interactions [33].
EEG coherence has been widely used to study the functional connectivity between different brain areas [34] because it provides a quantifiable measure of the synchrony between the electrical activity of structures beneath the recording electrodes on the scalp [35,36,37,38]. Currently, there are better measures to assess EEG connectivity [27]; however, in this study, the coherence between sensors was examined because it is the measure that is used in the clinical setting. Furthermore, coherence has been previously used to characterize the EEGs of children with RDs, and thus, it is possible to compare the results of the present study with previous results and validate their reproducibility.
Previous studies have reported that children with learning disorders have a different maturational process for EEG coherence than children with typical development [39]. Children with poor reading performance have shown higher coherence values, specifically in the delta [40], theta [28,40], and beta frequency bands [28,40,41,42]. In the alpha band, the results are not consistent. Marosi et al. [40] observed lower alpha coherence values in children with learning disorders than in controls; in contrast, Arns et al. [42] and Shiota et al. [41] found higher alpha coherence values. However, it must be taken into account that the study by Arns et al. [42] was the only one performed in a resting condition with eyes open; furthermore, in the study by Shiota et al. [41], the sample was very small and heterogeneous, since two of the seven children with dyslexia suffered from epilepsy and three suffered from ADHD. Usually, higher intrahemispheric coherence of the delta, theta, and beta bands occurs between regions of the left hemisphere, while lower intrahemispheric alpha coherence, described by Marosi et al. [40], occurs between leads of the right hemisphere. Regarding interhemispheric and intrahemispheric coherence, children with dyslexia are characterized by lower interhemispheric coherence and higher intrahemispheric coherence in the alpha frequency band compared to classmates of the same age [29].
1.2. Neurofeedback
Neurofeedback (NFB) is the most employed term to refer to the biofeedback of brain activity, and it is generally intended to normalize brain activity. There are two modalities: biofeedback based on EEG/MEG and biofeedback based on functional magnetic resonance imaging at rest. In this paper, the term NFB is used to refer to EEG-based biofeedback. NFB involves a type of learning called “operant conditioning,” where a given behavior (for example, an EEG event) receives a reward signal (positive or negative) as a consequence; as a result of pairing behavior with a reward, the probability of the occurrence of this EEG event increases.
Several NFB protocols have been used to treat RD. In children, protocols have been based on abnormal EEG characteristics described for RD populations: Nazari et al. [43] and Fernández et al. [44,45,46] trained power values, and Breteler et al. [47], Walker and Norman [48], and Coben et al. [49] trained power and coherence values. In some of these studies, the design of the NFB protocol also considered some neuropsychological aspects associated with reading, for example, its left lateralization. In this sense, the protocol that reinforces the reduction in the theta/alpha ratio has a limitation. However, there is a mitigating factor to this limitation, since the regions involved in reading and the connections between them may be different in children with RD and not specific to the left hemisphere.
There is consensus that children with learning disabilities have more theta activity (absolute and relative power) and a lower amount of alpha relative power (RP) when compared to typically developed children of the same age; however, this does not occur in specific regions. Furthermore, in neurotypical children [50] and young adults [51], a certain amount of resting alpha seems to be necessary for the correct performance of a mental task in the regions involved in that specific task. It should also be considered that Jäncke and Alahmadi [24] reported that the theta/alpha ratio could be an electroencephalographic marker of brain maturity and that in many studies, excessive theta/alpha ratios have been found in children with learning disorders [23,24,52,53]. Considering all this evidence, an NFB treatment that positively reinforces a reduction in the theta/alpha ratio at the lead, which presents the most abnormal theta/alpha ratio, was proposed, with the aim of normalizing the ratio and improving cognitive performance.
On the other hand, the theta/beta ratio has repeatedly been used to characterize EEG profiles in clinical populations [24]. A higher theta/beta ratio is characteristic of ADHD, and it has been proposed that an excess of the theta/beta ratio in frontal–central leads could be considered a biomarker for top-down control of attention [54]. Jäncke and Alahmadi [24] also reported that there is no difference between theta/alpha and theta/beta ratios in the eyes-open condition, but it is important to consider that having the eyes closed facilitates alpha production. A protocol based on the theta/beta ratio was not considered in this or previous studies because a consensus regarding the beta deficit has not been reached in studies comparing children with learning disorders and neurotypical control children. On the other hand, in all NFB studies on learning disorders carried out by the Fernández group, children with ADHD were excluded.
1.3. NFB Studies Using Theta/Alpha Reduction Protocol
Four experiments using the NFB protocol reinforcing a reduction in the theta/alpha ratio at the most abnormal lead have been reported by the Fernández group. All children had learning disorders, most of them with impairments in reading and abnormally higher theta/alpha ratios. The first study [44] was a control pseudorandomized single-blind design with five children receiving NFB and five children (three of them with IQs lower than eighty) receiving a sham NFB treatment. Cognitive scores only improved in the NFB group. Children showed an absolute power (AP) reduction in all bands at several leads, and only alpha RP at T6 increased. The two-year follow-up [55] showed that the EEG maturational lag in the control group increased, reaching abnormally high theta values; however, there were no positive behavioral changes, and the neurological diagnosis remained a learning disorder. Some children (more in the experimental group) showed improvements in memory, attention, self-esteem, and school achievement, as reported by parents. In contrast, in the NFB group, EEG maturation continued and was accompanied by positive behavioral changes, which were reflected in the remission of LD symptoms in four children. An unexpected result was the reduction in verbal IQ in the NFB group, which was attributed to their low socioeconomic status.
The second study [45] included eleven children receiving NFB treatment (six plus the previous five) and the previous five children receiving sham NFB treatment. All the children in the NFB group learned to decrease their theta/alpha ratios during the course of the NFB sessions, and their cognitive scores improved. In this study, EEG current sources were reported because they represent the brain generators of the EEG observed at the scalp, and by calculating current sources, two of the undesirable effects inherent in EEG recorded on the surface are notably corrected: the effect of the reference is removed, and the effect of the conductive volume is considerably reduced, which makes current sources a more reliable measure of brain activity. In addition to using a more reliable measure, this study aimed to confirm a phenomenon that was previously observed by this group [44] and reported by other authors [56,57]: immediately after NFB, changes in behavior were observed in the absence of changes in the EEG of the scalp. By using the current sources, Fernández et al. [45] were able to find some changes immediately after treatment in the NFB group (delta reduction in the right occipitotemporal gyrus and alpha increase in the left anterior cingulate and occipital gyri); however, two months later, not only were some of these changes sustained, but an important reduction in delta and theta frequencies emerged in the frontal, temporal, occipitotemporal, cingulate, and parahippocampal gyrus regions, mainly in the left hemisphere, as was an increase in alpha and beta in similar areas, but with right predominance. The number of changes observed coincided with the learning of the NFB since immediately after the NFB, only 4 of the 11 children managed to show a reduction in the theta/alpha ratio. However, two months later, it was achieved by seven children. In the control group, no significant differences were observed in the EEG current sources.
The aim of the third study [46] was to explore whether the sensory modality of the reinforcer (auditory or visual) induced differences in the outcomes of NFB treatment aimed at reducing the theta/alpha ratio. For this, 10 children received an auditory reward (with eyes closed), and another 10 were given a visual reward (with eyes open). In both NFB groups, the theta/alpha ratio in the most abnormal lead was reduced, AP and RP decreased in the delta and theta bands at several leads, and an increase in the alpha band was observed in almost all leads. In the beta band, the NFB-auditory group showed some increases, while the visual-NFB group only showed an increase in Cz. Cognitive scores significantly improved in the auditory group only. Two factors could be combined; on the one hand, an auditory stimulus usually takes 8–10 ms to reach the brain, whereas a visual stimulus takes between 20 and 40 ms [58]. This could explain why, in the auditory-NFB, the temporal contingency was greater. On the other hand, it is known that the eyes-closed condition promotes the production of alpha. Therefore, in the present study, auditory feedback was used.
In the last study [59], differences in working memory were explored by comparing 10 children with learning disorders who received NFB treatment against 8 who received a sham NFB treatment. Both groups reduced their theta/alpha ratio. The group treated with NFB increased their response speed in the working memory task and showed electroencephalographic changes, indicating an improved recruitment of neural resources related to this cognitive process that is relevant for reading.
In summary, the NFB protocol, which reinforces a reduction in the theta/alpha ratio at the lead with the most abnormal theta/alpha ratio, has been shown to reduce this ratio, indicating that children have acquired the learning that the NFB itself induces. However, this ratio has also been found to be reduced in children who received sham NFB treatment. However, this ratio has also been found to be reduced in children who received a sham NFB treatment, but this is not surprising. Various elements of an NFB protocol, such as the trained frequencies [60], the number of administered sessions [61], and the sensory modality of the reinforcer [46], are demonstrated to be essential for the outcomes. However, an NFB protocol and its associated sham NFB protocol share characteristics, such as the participants’ expectancy, the use of metacognitive strategies [62], or the placebo effect [63,64,65], that can produce changes even in the EEG [66,67].
Considering that the results of current sources are more reliable than scalp ones, it could be inferred that delta and theta activity diminished and alpha and beta activity increased after NFB using this protocol. This tendency toward electroencephalographic maturation, regardless of frequencies and regions trained, suggests a reorganization of the EEG [44]. As a consequence, cognitive improvement is observed whenever an auditory reward is used [44,45,46]. Although the cited studies are limited by their small samples, they were homogeneous because the participants were exquisitely selected by imposing rigorous inclusion criteria. This high homogeneity involves little intragroup variability. Although these restrictions allowed for a more profound study of learning disorders, the results cannot be generalized to the whole learning disorder population because a high comorbidity exists between learning disorders and other psychiatric pathologies.
However, both large samples and theoretically precise dependent measures are necessary to evaluate the efficacy of a psychophysiological treatment [68]. This was a problem in most of the previous studies addressed in the current study.
Criticisms about the low IQ of children with learning disorders are frequent. However, their low IQ should come as no surprise considering that these children not only have deficits in reading, writing, and arithmetic but also have alterations in executive functions, specifically in working memory [69], and all these abilities are considered in obtaining IQ scores. On the other hand, according to criterion D for classifying a learning disorder [7], an intellectual disability (IQ < 70 and impairment in everyday adaptive functioning) should be excluded.
In these prior studies, changes in the AP and RP of EEG signals were explored. However, they refer to changes in neuronal activation of isolated areas; currently, neural networks and connectivity have provided a more realistic perspective in cerebral function research [70]. Among children with RD treated with this NFB protocol, it would be interesting to explore whether it had a positive effect on electroencephalographic connectivity between brain areas related to the reading process.
The main goal of this study was to explore the effects of an NFB protocol that reinforces a reduction in the theta/alpha ratio on functional connectivity in children with RDs to evaluate whether this treatment can normalize the altered mechanisms that underlie reading in children with RD who also have an EEG maturational lag. It should be noted that the children with RDs included in this study had EEG maturational lags; however, there are no reports that specifically describe the atypical EEG coherence characteristics of children with RDs who also present this EEG feature. Therefore, the first stage of the present study consisted of exploring the differences between two groups of children who had received the same school instruction and came from the same socioeconomic and cultural environment: one group included children with RDs and delayed maturity shown on EEG, and the other group included children with typical development. It is possible that the results are similar to those of previous studies even though the study populations differ; that is, children with RD probably have higher coherence values in the delta, theta, and beta frequency bands and show higher values of intrahemispheric coherence and lower values of interhemispheric coherence in the alpha band than their typically developing peers.
However, to establish the hypotheses for the main objective, differences between children with RDs who had an excessive theta/alpha value and children with typical development (TD) were examined.
2. Materials and Methods
The Ethics Committee of the Institute of Neurobiology of the National Autonomous University of México approved the experimental protocol INEU/SA/CB/146 on 1 July 2015, which followed the Ethical Principles for Medical Research on Human Subjects established by the Declaration of Helsinki [71].
2.1. Design
Two designs were used in this study. In the first part, where a comparison of the coherences between children with a reading disorder and typically developing children was performed, a descriptive correlational design was used, including two independent groups. In the second part, a pretest–posttest design was used to explore the effects on coherence in children with a reading disorder who received NFB treatment.
2.2. Participants
Sample sizes were calculated with G*Power 3.1 software (https://www.psycholo-gie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower accessed on 7 February 2023). Based on an effect size (Cohen’s d) of 0.86 [40], a 1:1 ratio between the sample sizes of the two groups, a one-sided type I error rate of 0.05, and a power (1-β error probability) of 0.8, 18 participants per group were necessary for the comparison between independent groups in the first stage of this study. For the second stage of this study, four participants per group were required for a one-sided type I error rate of 0.05 and a power of 0.8, considering the results reported by Nazari et al. [43].
A total of 204 children from different public and private schools in Querétaro, Mexico, were evaluated. They were referred by social workers, who, based on the opinion of the teacher and the child’s school grades, identified children who had reading problems and children with good academic performance (children from the same classrooms who had grades of 85 or higher in all subjects). The parents of the identified children were summoned to meetings where the research project was explained; it was stressed that the child’s participation must be voluntary and that it was not appropriate to force the child. Parents of 144 children with reading problems and 60 children with good academic performance attended the meetings and later the Laboratory of Psychophysiology, where the project was explained again and doubts were solved. All the children and their parents interested in participating signed informed consent forms. In the first session, parents were given a structured interview, while the Wechsler Intelligence Scale for Children 4th edition [72] was administered to the child in a separate room. Children with reading problems with IQs less than 80 and children with good academic performance with IQs less than 90 were excluded to rule out intellectual disability, as specified by the DSM-5 in the D criterion, and to ensure that the children in the neurotypical control group had an average IQ.
Then, the following assessments were administered: a neuropediatric exam, the Mini-International Neuropsychiatric Interview for Children and Adolescents (MINI Kid) [73,74]; the reading scale of the Child Neuropsychological Assessment-2 (ENI-2) [75], standardized for the Mexican population; and a resting-state EEG under the eyes-closed condition.
In total, 43 right-handed volunteers aged 7 to 11 years were included in this study. In the first stage of this study, two groups were considered: one including 25 children diagnosed with a reading disorder (RD group, n = 25, age = 8.84 ± 1.06, 9 girls, 8 born preterm, of which 3 were early premature, IQ = 93.92 ± 13.12), in which the child had a percentile of 9 or less in at least one reading subscale, and the other including 18 children with typical development (TD group, n = 18, age = 8.83 ± 0.92, 9 girls, 1 late premature, IQ = 106.1 ± 8.7), in which all children had percentiles greater than 35 in all reading subscales. Differences in IQ were observed between groups (t = 3.7325, p = 0.0008, d = 0.955). No differences were found in age or gender ratio.
The children included had no severe socioeconomic or cultural disadvantages that could affect their EEG (mother or guardian had at least completed primary school, and the per capita family income exceeded 50% of the minimum wage) [76]. They had no hearing impairments, and those who had visual difficulties (five children in the RD group and one in the TD group) used lenses to correct them. No children presented neurological or psychiatric pathology, except for RD. No children were medicated.
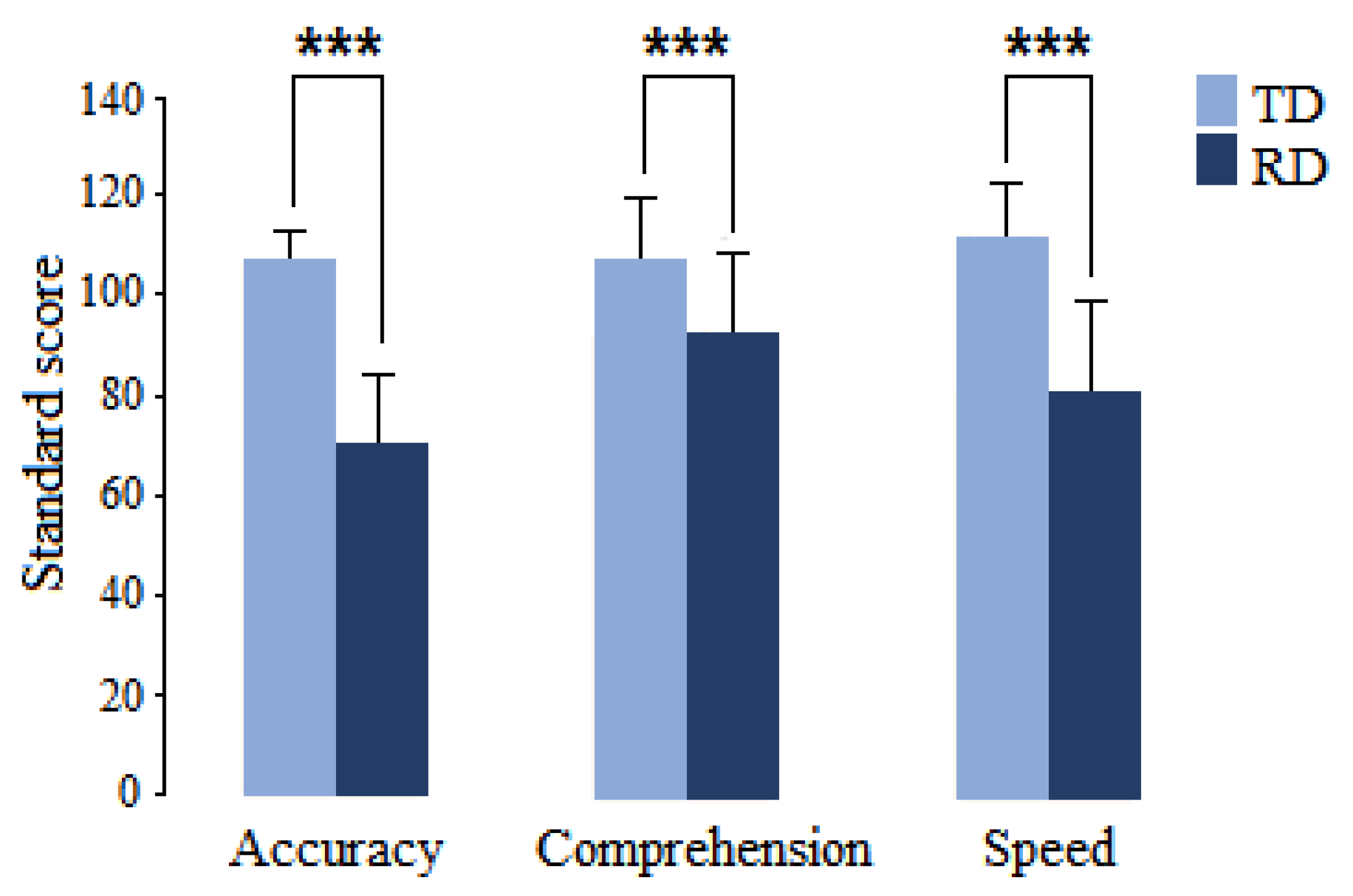
The groups displayed significant differences in standard scores on the three examined reading subdomains of the ENI-2 (accuracy: t = 1.0013, p = 0.0002, d = 2.656; comprehension: t = 4.2254, p = 0.0002, d = 1.068; and speed: t = 6.8140, p = 0.0002, d = 1.764), as shown in Figure 1.
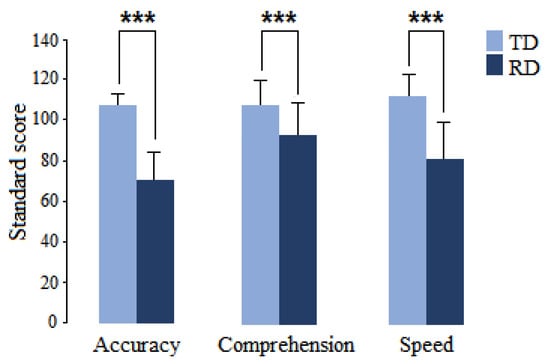
Figure 1.
Mean and standard deviation of the reading subdomains of the ENI-2 in children with RD (dark blue) and children with typical development (light blue). *** p < 0.001.
2.2.1. Children with RD and Delayed EEG Maturation
The teacher reported whether the child in question had reading problems. Reading skills were measured with the reading scale of the ENI-2, which includes three subscales: reading accuracy, reading comprehension, and reading speed; children included in the RD group had percentile scores <9 in at least one of these subscales.
All the participants in the RD group met the diagnostic criteria for RD according to the Diagnostic and Statistical Manual of Mental Disorders 5th Edition. These criteria were as follows: (a) the child showed persistent difficulties in acquiring and/or using academic skills related to reading (accuracy, comprehension, and/or speed); (b) reading difficulties began in the first years of schooling and not because the participants had intellectual disabilities (the IQ score obtained by each child was greater than or equal to 80); and (c) they did not have any neuropsychiatric disorder other than RD.
Although children had some attentional disabilities, they did not satisfy the criteria to be diagnosed with attention deficit hyperactivity disorder (ADHD) according to the DSM-5 [7].
All participants had an abnormally high EEG theta/alpha ratio compared to a normative database [77,78] in at least one lead. This is an essential requirement to be a candidate for NFB treatment. Additionally, if epileptiform activity is present, it should not be in the alpha frequency range.
For the second stage of this study, 20 out of the 25 children with RD were randomly assigned to an NFB group using nonreplacement random sampling, and the remaining 5 were assigned to a placebo control group (the Sham group).
2.2.2. Children with Typical Development
Teachers reported if the child in question did not have academic problems. Children included in the TD group had percentile scores >35 in all reading subscales of the ENI-2. They did not have any neuropsychiatric disorders.
All the children had a normal EEG, both by visual inspection and by quantitative analysis of EEG power; the theta/alpha ratio was also within normal limits according to a normative database [77,78].
2.3. EEG Recording and Analysis
The electroencephalogram was recorded in a resting condition with eyes closed from the 19 leads of the international 10–20 system (Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, O2, F7, F8, T3, T4, T5, T6, Fz, Cz, and Pz), referenced to the linked earlobes (A1A2). For this purpose, a Medicid™ IV instrument (Neuronic Mexicana, SA, Mexico City, Mexico) with a v2.0 Track Walker™ recording system was used. The amplifier’s bandwidth was set between 0.5 and 50 Hz. All electrode impedances were equal to or less than 5 kΩ, and the signal was amplified with a gain of 20,000. EEG data were sampled every 5 ms and edited offline. Overall, 24 artifact-free segments of 2.56 s were selected. EEG analysis was performed offline. The fast Fourier transform was applied to the data, and the cross-spectral matrices were obtained every 0.39 Hz to calculate the AP and coherence values of each of the bands (delta: 0.5–3.5 Hz, theta: 3.6–7.5 Hz, alpha: 7.6–12.5 Hz, and beta: 12.6–19 Hz).
2.4. Z Values for the Theta/Alpha Ratio
To calculate z values for the theta/alpha ratio, AP in theta and alpha bands was computed for each electrode, and the geometric power [79] was subtracted from the cross-spectral matrix.
The log value (theta AP/alpha AP) was computed, and z values for this logarithm were calculated using the equation:
where μ and σ are the mean value and the standard deviation, respectively, of a normative sample that is the same age as the subject [77,78]. Considering that the EEG of this population is characterized by having more theta activity and less alpha activity than children with typical development, having a z value greater than 1.645 (1-tailed distribution, p = 0.05) in at least one lead was also designated as an inclusion criterion. Treatment was delivered via the lead with the highest abnormal z value.
Z = [thetaAP/alphaAP − μ]/σ
2.5. Treatments
2.5.1. NFB Treatment
Only children with RD and abnormally high theta/alpha ratios received NFB treatment. The treatment was performed using an NFB program adapted for the Medicid IV registration system. A threshold level for the theta/alpha ratio lead with the highest abnormal z value was selected based on the resting-state EEG recording, with the subject obtaining a reward between 60% and 80% of the time. During NFB training, the children were in an eye-closed condition. Every 20 ms, the program performs the following operations: first, it calculates the theta/alpha ratio in an EEG segment of 1200 ms; second, it compares this ratio with the threshold value. Whenever the ratio is less than the threshold, the participant receives a reward. The stimulus used as a reward was a tone of 500 Hz at 60 dB. Every 3 min, the threshold level was updated based on the subject’s performance. If, within 3 min, the child receives the reward between 60% and 80% of the time, the threshold value remains the same; however, if he or she receives it less than 60% of the time, the threshold level is increased, or if he or she receives it more than 80% of the time, the threshold value is lowered. Each subject received 30 training sessions three times a week, and the duration of each session was 30 min.
2.5.2. Sham NFB Treatment
In placebo treatment, all conditions were exactly as in NFB, except that in this case, the reward was randomly delivered and was not noncontingent on EEG activity. After posttreatment evaluations, NFB treatment was applied to these children as stipulated by the Declaration of Helsinki.
2.6. Posttreatment Analysis
The Wechsler Intelligence Scale for Children 4th edition and the ENI-2 Child Neuropsychological Assessment were applied before NFB treatment for diagnostic purposes. After treatment, only the ENI-2 was applied to assess the cognitive effects of the treatment. The application manual of this test stipulates that it is necessary for a year to elapse between the first and second application; therefore, the second application was applied two months after treatment since recruitment lasted several months, and the treatment period included the same dates for all children. This timeline fulfilled the scheduling requirements.
In a session following the tests’ application, the posttreatment EEG was recorded for two purposes: (a) to assess whether NFB-induced learning occurred and (b) to evaluate the effects of NFB on coherence. It was considered that learning had been induced for a child if their z value decreased.
2.7. Coherence Analysis
EEG data were exported from Medicid IV to calculate coherence with Neuroguide 2.6.5 software. Intrahemispheric coherences were obtained considering all the leads except the midline (Fp1, F3, C3, P3, O1, F7, T3, and T5 in the left hemisphere and Fp2, F4, C4, P4, O2, F8, T4 and T6 in the right hemisphere), i.e., Fp1-F3, Fp1-C3, Fp1-P3, Fp1-O1, Fp1-F7, Fp1-T3, Fp1-T5, F3-C3, F3-P3, F3-O1, F3-F7, F3-T3, F3-T5, C3-P3, C3-O1, C3-F7, C3-T3, C3-T5, P3-O1, P3-F7, P3-T3, P3-T5, O1-F7, O1-T3, O1-T5, F7-T3, F7-T5, and T3-T5 for the left hemisphere; and Fp2-F4, Fp2-C4, Fp2-P4, Fp2-O2, Fp2-F8, Fp2-T4, Fp2-T6, F4-C4, F4-P4, F4-O2, F4-F8, F4-T4, F4-T6, C4-P4, C4-O2, C4-F8, C4-T4, C4-T6, P4-O2, P4-F8, P4-T4, P4-T6, O2-F8, O2-T4, O2-T6, F8-T4, F8-T6, and T4-T6 for the right hemisphere). Interhemispheric coherences were obtained considering only homologous derivations (Fp1-Fp2, F3-F4, C3-C4, P3-P4, O1-O2, F7-F8, T3-T4, and T5-T6). The z values of EEG coherence were calculated before and after treatment.
2.8. Statistical Analysis
Because the sample size was not very large, the Kolmogorov-Smirnoff test was performed to assess the normality of the distributions. None of the cognitive variables had a normal distribution in either of the two groups (TD and RD groups) in the first stage. In the second stage, none of the cognitive variables had a normal distribution in either of the two groups (NFB and Sham treatment groups) for either of the two conditions (before and after). The theta/alpha ratio did not satisfy the normality assumption for either of the two groups (NFB and Sham treatment groups) in either of the two conditions (before and after). Regarding the coherence variables, approximately 35% did not have a normal distribution. Considering that for multivariate normal data, the marginal distributions and linear combinations should also be normal, the use of parametric statistical analysis was not appropriate.
Nonparametric methods do not require theoretical distributions because the null-hypothesis distribution of statistical tests is created by iteratively shuffling the data [80]. The data of this study had a multivariate nature, so correction for multiple comparisons was necessary. Bonferroni and false discovery rate (FDR) approaches are more conservative methods to avoid the inflation of type I and type II errors [81,82] compared to the nonparametric permutation test implemented by Pascual-Marqui in the LORETA software [21].
In this study, a nonparametric permutation test [83] was used. The following comparisons were performed: (1) comparison between RD and TD groups, (2) comparison between NFB and Sham groups regarding the before vs. after changes, and (3) independent comparison before vs. after treatment in each of the treated groups (NFB or Sham).
Cohen’s d statistic was computed for all significant results.
2.8.1. RD Group vs. TD Group
Analyses were performed for the following variables or sets of variables: reading accuracy, reading comprehension, reading speed, interhemispheric coherence, and intrahemispheric coherence. Analyses were carried out using a nonparametric t-test for independent samples with 5000 permutations.
2.8.2. NFB vs. Sham Groups Regarding Pre-Post Treatment Changes in Children with RDs
Null hypotheses H0: A1–A2 = B1–B2 was tested, where A denotes the NFB group and B denotes the Sham group; 1 and 2 represent after and before treatment, respectively.
Variables or sets of variables tested were reading accuracy, reading comprehension, reading speed, theta/alpha ratio, interhemispheric coherence, and intrahemispheric coherence. Analyses were carried out using a nonparametric t-test for dependent samples with 5000 permutations.
2.8.3. Pre-Post Treatment Comparisons for Each Group (NFB or Sham)
Independent analyses for each group were performed for the following variables or sets of variables: reading accuracy, reading comprehension, reading speed, theta/alpha ratio, interhemispheric coherence, and intrahemispheric coherence. Analyses were carried out using a nonparametric t-test for dependent samples with 5000 permutations.
3. Results
3.1. Comparison between Children with RDs and Children with Typical Development
3.1.1. Intrahemispheric Coherence
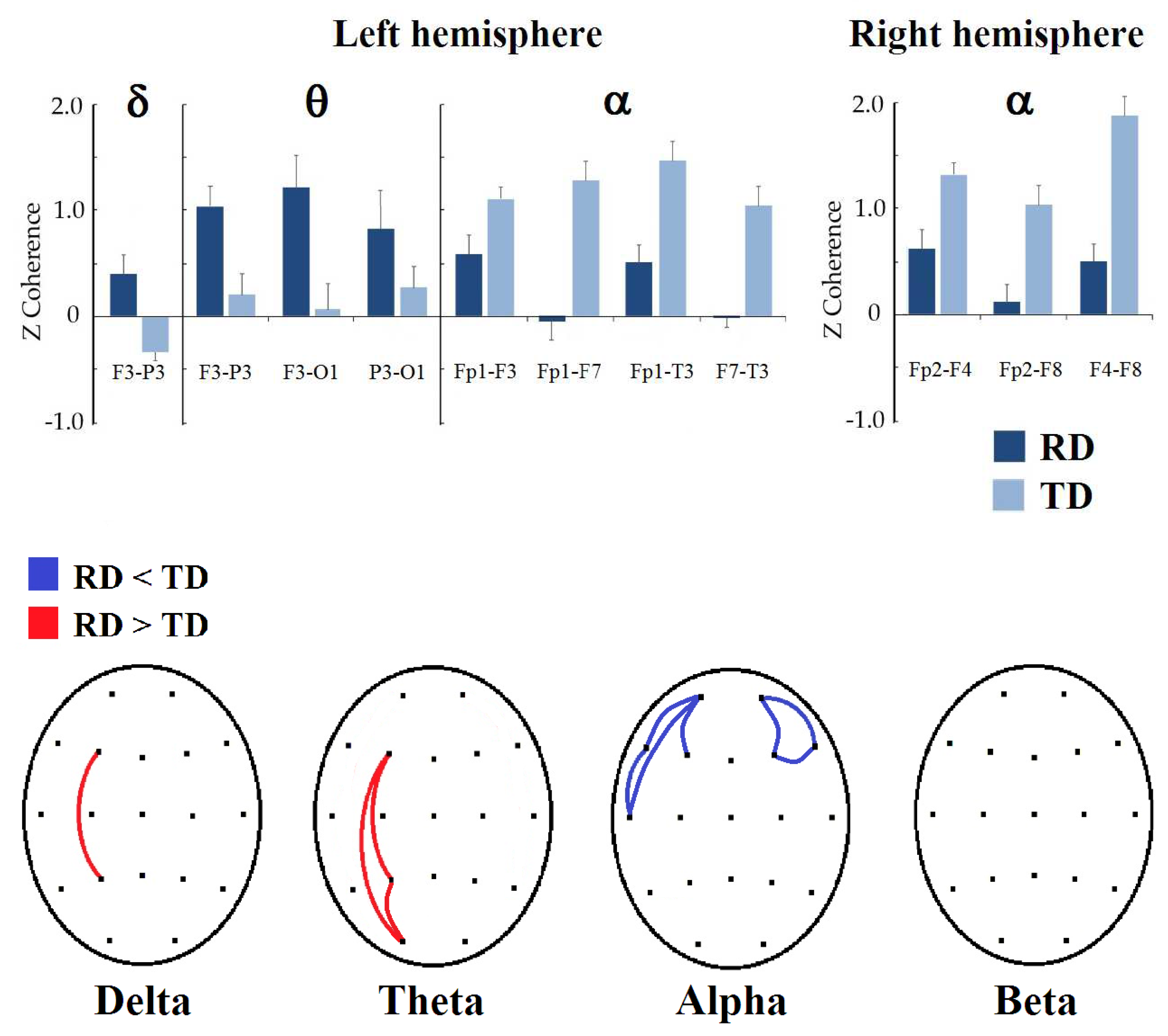
In Figure 2, the results of intrahemispheric coherence in both hemispheres can be observed. In the left hemisphere, the group made up of children with RDs obtained higher delta coherence values in F3-P3 (t = −2.9712, p = 0.0497, d = 0.702) than the children with TD. In the theta frequency band, the RD group also presented higher values than the TD group in F3-P3 (t = −3.0414, p = 0.0384, d = 0.724), F3-O1 (t = −3.1413, p = 0.0290, d = 0.728), and P3-O1 (t = −2.9475, p = 0.0472, d = 0.720). Contrary to the delta and theta bands, the RD group presented lower coherence values in the alpha frequency band in Fp1-F3 (t = 3.2876, p = 0.0200, d = −0.741), Fp1-F7 (t = 3.1549, p = 0.0324, d = −0.660), Fp1-T3 (t = 3.0781, p = 0.0374, d = −0.761), and F7-T3 (t = 3.1629, p = 0.0320, d = −0.684) than the TD group. In the beta band, there were no significant differences between groups.
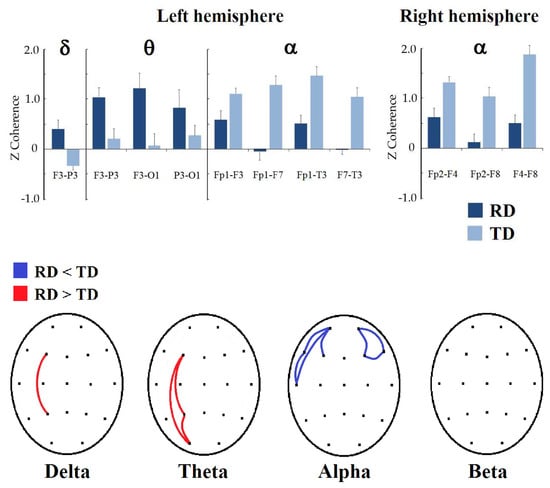
Figure 2.
Significant differences between the RD and TD groups in intrahemispheric coherence. (Top): Bar graphs showing the significant changes in coherence z values by frequency band (indicated at the top) when comparing intrahemispheric connectivity between RD and TD groups; left or right hemisphere is displayed below the graph. (Bottom): topographic representation of significant differences in z-coherence between groups; red color represents that coherence is higher in the RD group, and blue color means that it is lower compared to the TD group.
In the right hemisphere, significant differences were observed only in the alpha band. Children with reading disorders obtained lower coherence values in Fp2-F4 (t = 3.2664, p = 0.0238, d = −0.725), Fp2-F8 (t = 3.8603, p = 0.0038, d = −0.862), and F4-F8 (t = 3.2980, p = 0.0226, d = −0.764) in the alpha band than the TD group. In the delta, theta, and beta bands, no differences were observed between groups.
3.1.2. Interhemispheric Coherence
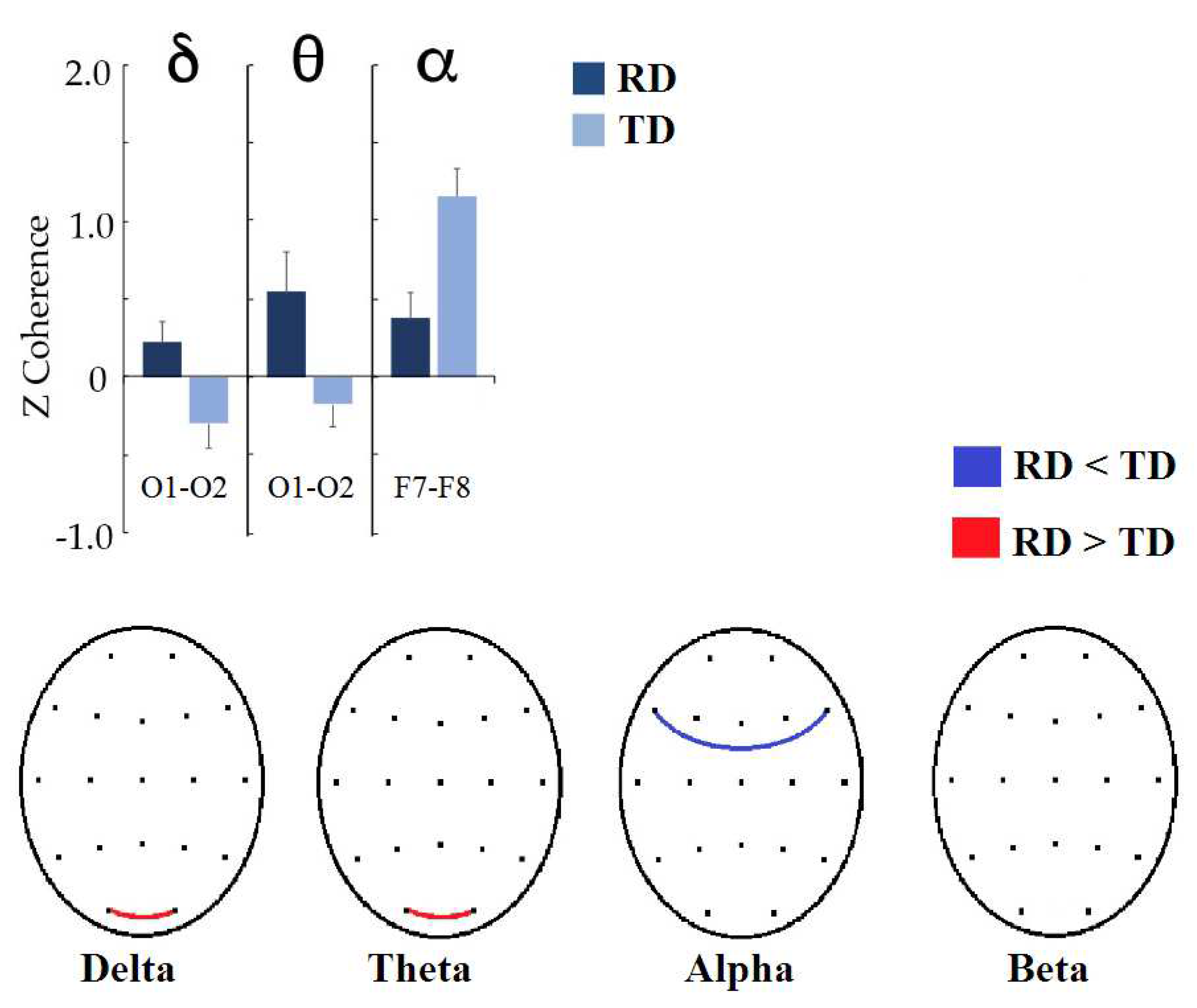
Coherences between homologous occipital leads were significantly higher in the RD group than in the TD group in the delta (t = −2.5737, p = 0.0428, d = 0.646) and theta (t = −2.8898, p = 0.0188, d = 0.637) frequency bands. In the alpha band, the RD group had lower coherences between the F7 and F8 leads (t = 3.3646, p = 0.0096, d = −0.853). The results are shown in Figure 3.

Figure 3.
Significant differences between the RD and TD groups in interhemispheric coherence between homologous pairs. (Top): Bar graph showing the significant changes in coherence z values by frequency band (indicated at the superior part). (Bottom): topographic representation of significant changes at the scalp; red lines represent that coherence is higher in the RD group, and the blue line indicates that coherence is lower in the RD group when compared with the TD group.
3.2. Comparison between NF and Sham Groups with Respect to Pre-Post Treatment Changes
No significant differences were found when the groups were compared regarding the changes produced by the respective treatment. This was true for treatment-induced learning, ENI reading scores, and coherence values. However, it is of interest to know if significant changes occurred in each group, considered independently.
3.2.1. Treatment-Induced Learning
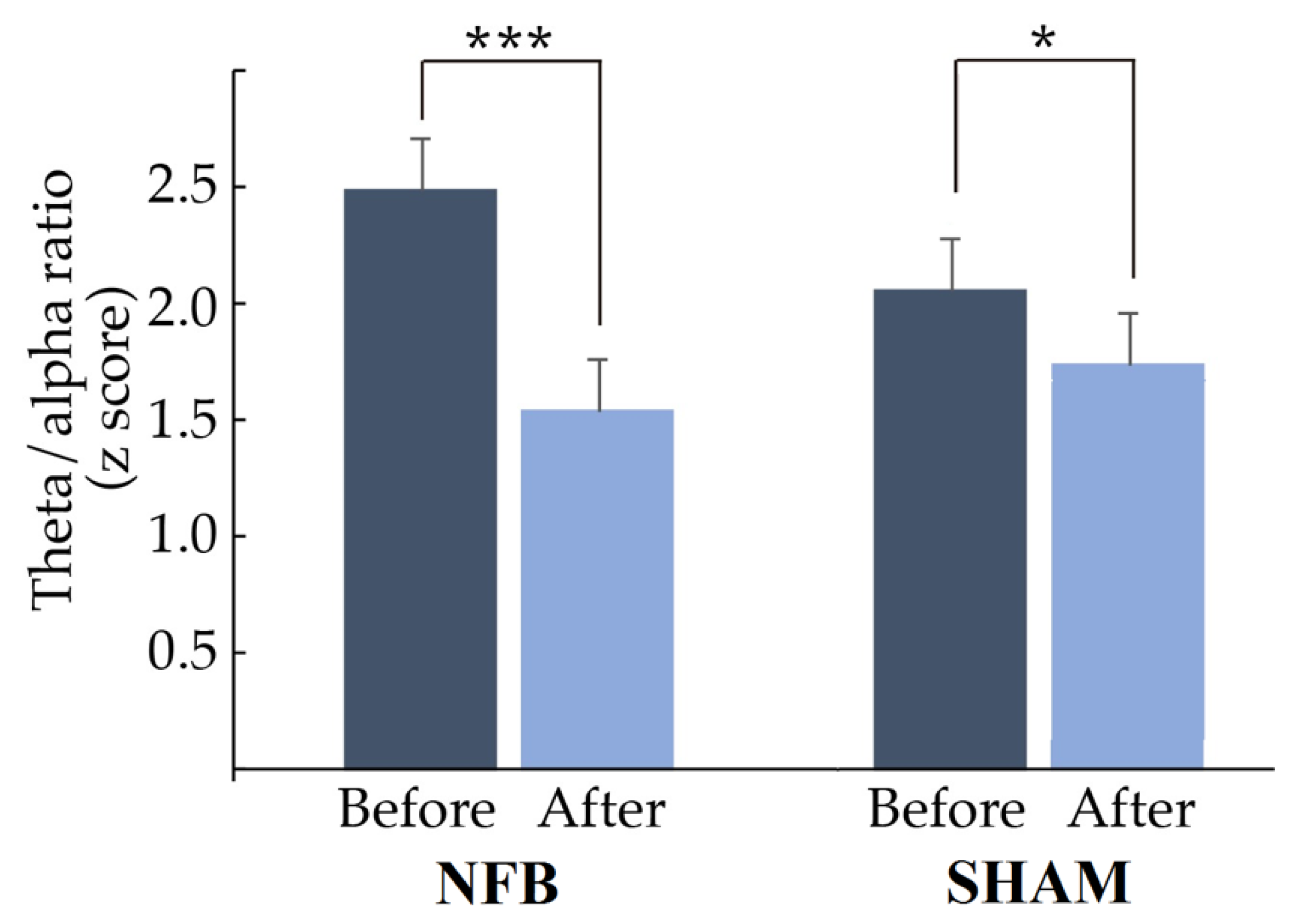
In Figure 4, it can be observed that the theta/alpha ratio of the most abnormal derivation for everyone decreased significantly after treatment (NFB: t = 4.4497, p = 0.0002, d = 0.803; Sham: t = 3.6769, p = 0.0346, d = 0.865). Furthermore, in the NFB group, 85% of the treated children normalized their theta/alpha ratio values (z < 1.96) in the derivation used to provide NFB, while in the Sham group, 80% normalized their ratio values. However, children in the NFB group had more abnormal values than children in the Sham group before treatment (mean ± SD: NFB: 2.48 ± 0.96; Sham: 2.09 ± 0.56).
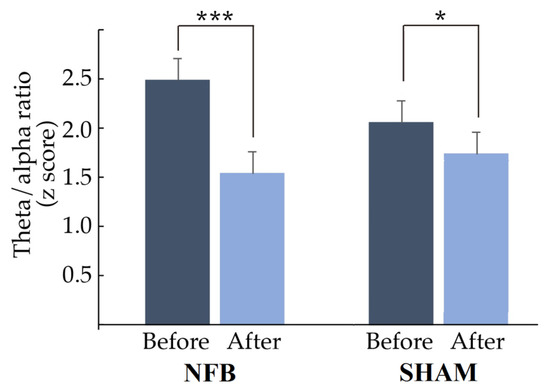
Figure 4.
Mean and standard deviation of the theta/alpha ratio before and after treatment (NF or Sham). The z score of the theta/alpha ratio in the lead with the most abnormal theta/alpha ratio decreased significantly after treatment in both groups. * p < 0.05, *** p < 0.001.
3.2.2. Behavioral Outcomes
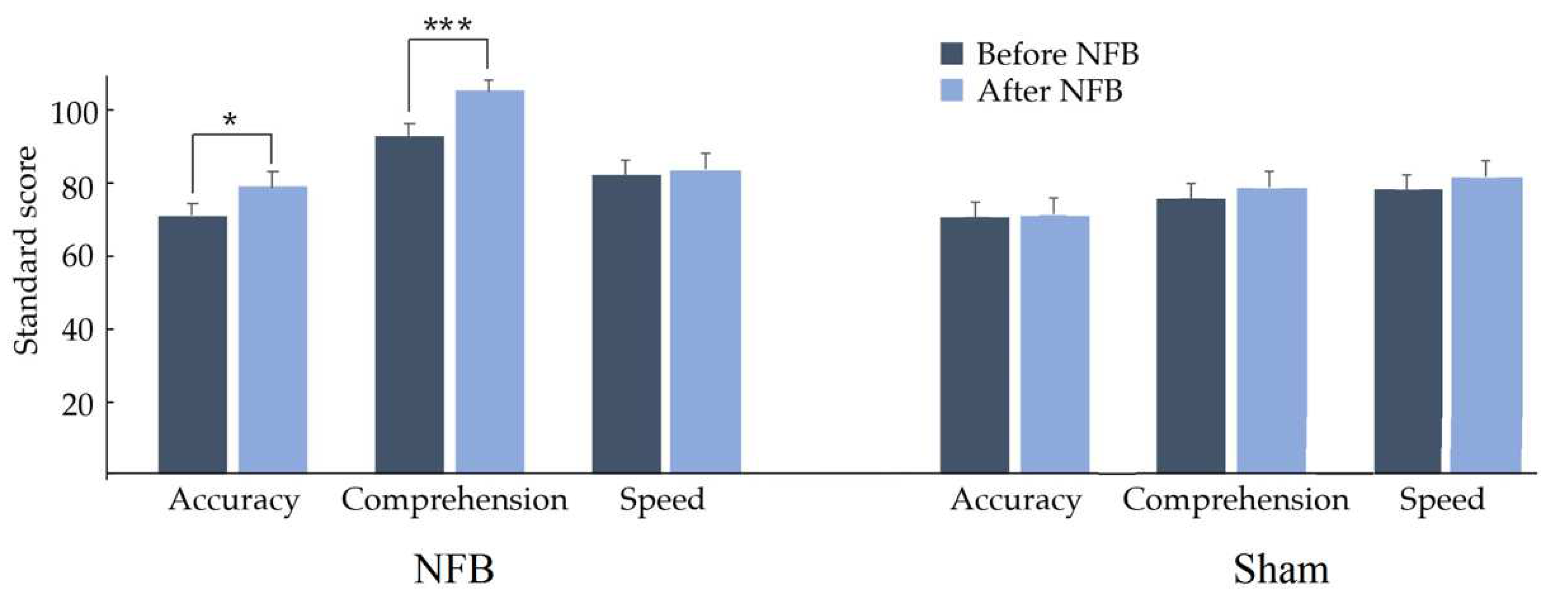
In the reading variables included in the ENI-2, significant increases were observed in accuracy (t = 2.1391, p = 0.0230, d = 0.381) and comprehension (t = −3.4554, p = 0.0006, d = 0.657) scores, as shown in Figure 5; this suggests that, on average, reading level increases, showing medium and large effect sizes for reading accuracy and reading comprehension, respectively. No significant change was found in speed reading when comparing results before vs. after NFB.
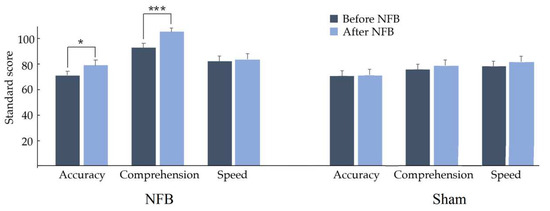
Figure 5.
Mean and standard deviation of the Child Neuropsychological Assessment-2 in the reading domain before (dark blue) and after (light blue) treatment (NFB or Sham). Reading accuracy and comprehension increased significantly (* p < 0.01, *** p < 0.001) only in the NFB group.
3.2.3. Functional Connectivity Changes: Intrahemispheric Coherence
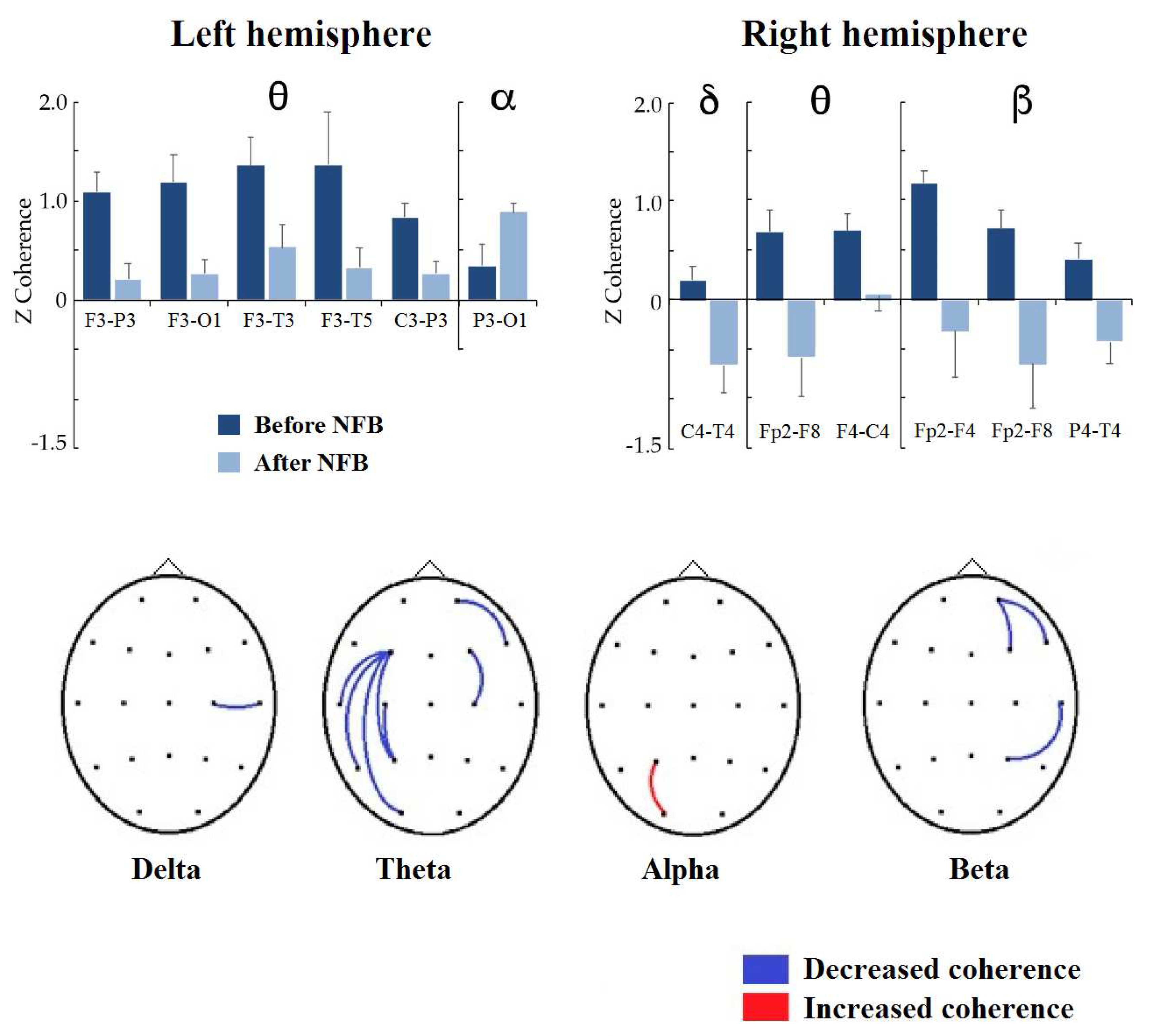
In Figure 6, the results of intrahemispheric coherence in both hemispheres for the NFB group can be observed. In the left hemisphere, P3-O1 coherence in the alpha frequency band increased significantly (t = 3.2083, p = 0.0310, d = 0.527). In the theta frequency band, the coherence values decreased significantly in F3-P3 (t = 3.6818, p = 0.0130, d = 0.876), F3-01 (t = 3.3866, p = 0.0236, d = 0.692), F3-T3 (t = 3.8031, p = 0.0096, d = 0.550), F3-T5 (t = 3.8038, p = 0.0096, d = 0.870), and C3-P3 (t = 4.9384, p = 0.0006, d = 0.774); in the delta and beta bands, no significant changes occurred in the intrahemispheric coherence of the left hemisphere. In the right hemisphere, the delta coherence C4-T4 decreased (t = 3.2628, p = 0.0366, d = 0.770), and the theta coherence decreased in FP2-F8 (t = 3.1543, p = 0.0424, d = 0.802) and F4-C4 (t = 3.7558, p = 0.0126, d = 0.693). In the beta band, the coherence values decreased in Fp2-F4 (t = 3.0484, p = 0.0476, d = 0.979), FP2-F8 (t = 3.1789, p = 0.0378, d = 0.818) and P4-T4 (t = 3.1180, p = 0.0416, d = 0.844). No significant changes were observed in the alpha band in the right hemisphere.

Figure 6.
Significant differences between before and after treatment in intrahemispheric coherence between homologous pairs in the NFB group. (Top): Bar graphs showing the significant changes in coherence z values by frequency band (indicated at the top) when comparing intrahemispheric connectivity before vs. after NFB treatment; the left or right hemisphere is indicated below the graph. (Bottom): topographic representation of significant changes in z-coherence after NFB treatment, showing the increase or decrease in red or blue, respectively.
In contrast, in the Sham group, only the F7-T3 intrahemispheric coherence in the theta band was reduced significantly (t = 7.3478, p = 0.0298, d = 0.230).
3.2.4. Functional Connectivity Changes: Interhemispheric Coherence (Homologous Pairs)
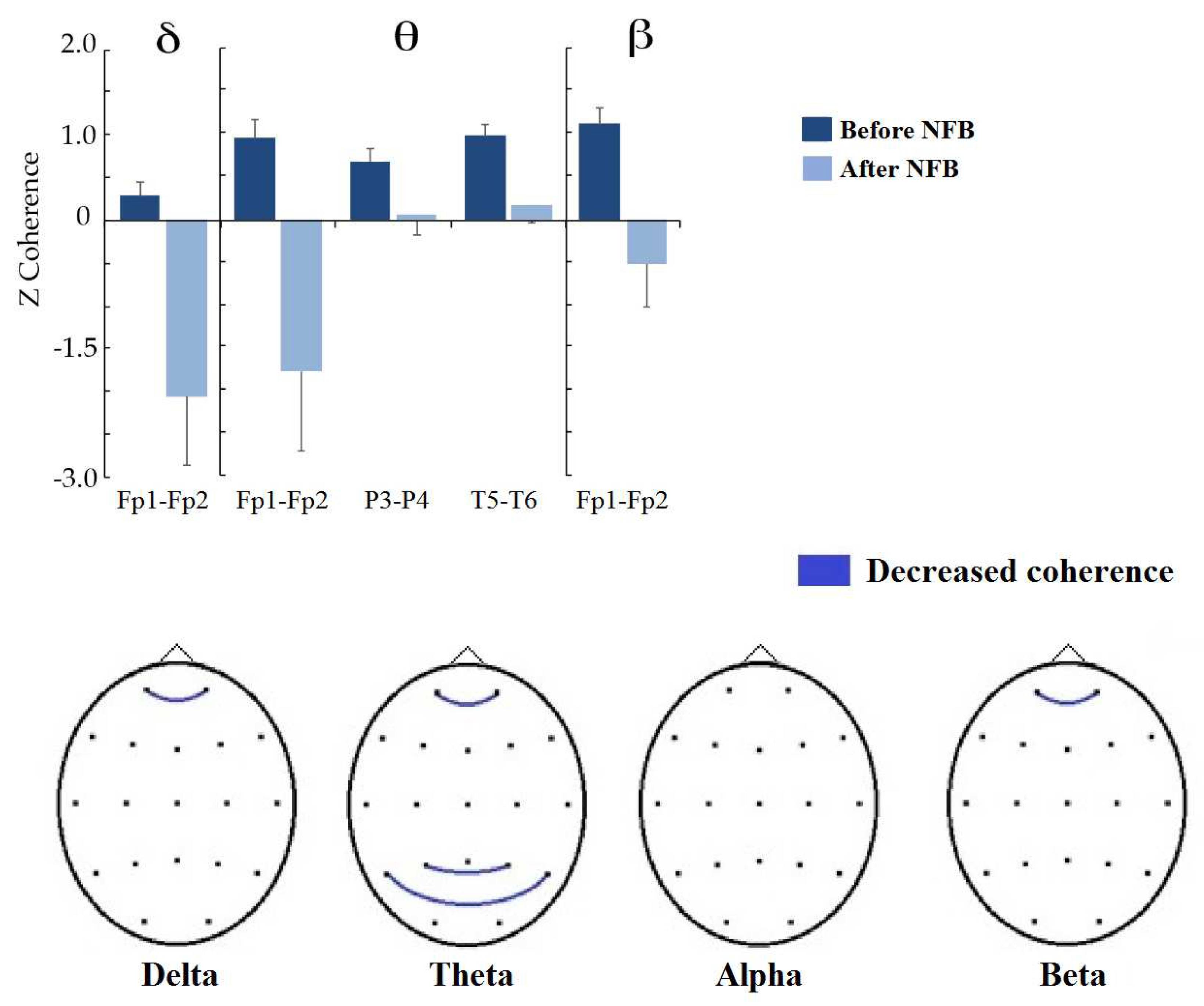
Figure 7 shows the changes in interhemispheric coherence between homologous pairs. In all bands, coherence values between frontopolar regions significantly decreased, except in the alpha band, where no changes were observed (delta Fp1-Fp2: t = 2.6653, p = 0.0370, d = 0.819; theta Fp1-Fp2: t = 2.7395, p = 0.0398, d = 0.901; beta Fp1-Fp2: t = 2.7592, p = 0.0322, d = 0.938). In addition, coherence between the posterior temporal (T5-T6: t = 3.3042, p = 0.0148, d = 0.592) and parietal regions (P3-P4: t = 3.1918, p = 0.0176, d = 0.513) decreased in the theta band.
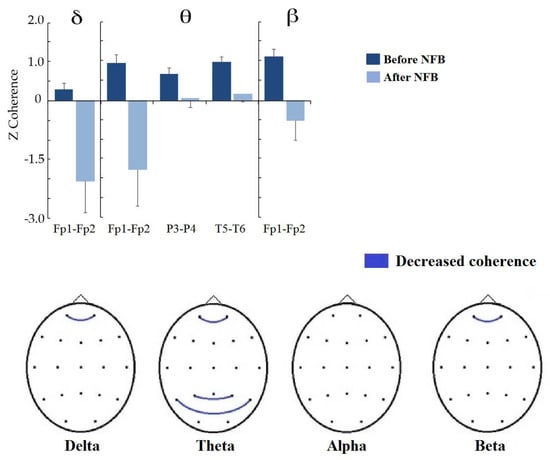
Figure 7.
Significant differences between before and after NBF treatment in interhemispheric coherence between homologous pairs. (Top): Bar graph showing the significant changes in coherence z values by frequency band (indicated at the superior part). (Bottom): topographic representation of significant changes at the scalp; blue lines indicate that coherence was reduced after treatment.
In contrast, in the Sham group, only the T5-T6 interhemispheric coherence in the beta band was reduced significantly (t = −5.1832, p = 0.03, d = −0.520).
4. Discussion
Our main objective in this study was to explore the changes in EEG coherence patterns in children with RDs and EEG maturational lag treated with NFB using a protocol that reinforces a reduction in the theta/alpha ratio.
In the first part of this section, the results obtained when comparing a sample of children with RDs and a sample of children with TD who came from the same schools, received similar academic instruction, and lived in the same sociocultural environment will be compared. The second part will address the observed effects on EEG coherence after applying the aforementioned NFB treatment and comparing the NFB effects on this group with the effects of the sham NFB treatment on a control group.
4.1. Children with RDs vs. Children with TD
Considering the information provided by studies comparing the resting-EEG coherence of children with RD with that of children with TD, it can be hypothesized that children with RDs would show higher coherence values in the delta, theta, and beta frequency bands; these differences could be expressed in areas related to reading, or perhaps children with RDs display compensatory functions. The results partially support this hypothesis because the RD group presented higher coherence values in the delta (left frontoparietal and occipito-occipital coherences) and theta (left frontoparietal, fronto-occipital, and parieto-occipital coherences; and occipito-occipital coherence) frequency bands than the TD group, but no differences between groups were observed in the beta band. Higher delta and theta coherences within regions of the left hemisphere coincide with those reported by Arns et al. [42], Marosi et al. [40] and Sklar et al. [28]. In addition, decreased frontoparietal delta and theta coherence could point to deficits in the attention-related fronto-parietal network [84,85]; this would support the fact that children with RDs not only have a specific deficit in reading but also deficits in general domain processes [69,86]. In the alpha band, it was expected that children with RDs would present higher values of intrahemispheric coherence [29,41,42] and lower values of interhemispheric coherence [28,29,40]. Among children with RDs and EEG maturational lags in this study, lower intrahemispheric alpha coherences (bilateral frontofrontal coherences and left frontotemporal coherences) and lower interhemispheric alpha coherence were found between the F7 and F8 leads. The results confirm those obtained by Marosi et al. [40] in a sample of 84 children with writing-reading disorders (20 of them with TD) and do not match the higher intrahemispheric alpha coherence observed in other studies in dyslexic children [31,32,33]. It is necessary to remember that the children included in this study did not have dyslexia but rather a specific reading disorder.
In studies conducted on the pediatric population and analyzing EEG coherence, the tendency has been to associate high values of coherence, mainly in the delta and theta bands, with cognitive dysfunction [87]. For example, Thatcher [88] reported high coherence in children with low IQ scores. In patients with ADHD, Chabot et al. [89], Barry et al. [90] and Clarke et al. [91] reported higher delta and theta coherences, mainly in frontal regions. Regarding the alpha band, higher IQ performance has been related to higher alpha coherence [88], and lower alpha coherence has been reported in ADHD children [89,91,92]. In contrast, in studies conducted in children with learning disorders, the results were not very consistent; this may be because children with learning disabilities are a heterogeneous population [93,94] and because they frequently have many comorbidities. These comorbidities were excluded from this study but have not been controlled for in other studies. It must be considered that the RD diagnostic criteria have been modified over the years and that all these children with RDs had delayed EEG maturation; the latter contributed to reducing the heterogeneity that could exist within the RD group.
The lower alpha coherences between the right frontal regions observed in this study may be explained by the right brain activation described as a compensatory mechanism in children with dyslexia [15,16]. This mechanism includes the right frontal region (homolog of Broca’s area).
These results led to hypothesize the effects of NFB in children with RDs and lagging maturation. NFB was expected to lead to a reorganization of brain interconnections that would manifest as a reduction in delta and theta intra- and interhemispheric coherence and an increase in alpha, intra-, and interhemispheric coherence. According to the reading network model, these changes could be observed in the left dorsal temporoparietal, left ventral occipito-temporal, and left inferior frontal networks; however, it is difficult to develop a hypothesis in this regard due to the possible compensatory phenomena that may take place and the poor spatial resolution of the EEG.
4.2. NFB Effects on Coherence in Children with RDs
Previous studies have reported that NFB-induced learning occurs in approximately 80% of treated individuals [45]. In this study, a significant reduction in the theta/alpha ratio was demonstrated in the lead used to guide NFB. In total, 90% of the participants exhibited a reduction in this quotient, and 55% achieved normalization. This suggests that most individuals achieved learning that was induced by the NFB treatment.
Additionally, in the Sham group, a significant reduction in the theta/alpha ratio was observed in the derivation with the most abnormal value; however, this reduction was significantly greater in the NFB group, suggesting that NFB-specific learning was responsible for a portion of this decrease in the NFB group. The remaining decrease found in both groups could be attributed to several uncontrolled factors, independent of the operant conditioning on which the NFB is based, for example, the use of metacognitive strategies, which have the potential to modify the EEG independently of the contingency between the EEG and the delivery of rewards [66,67]. Other factors involved could be an expectation effect [62] or a placebo effect [63,64,65]. Additionally, it is important to remember the lifestyle changes experienced by both groups as a result of their participation in the study, including a friendlier approach from parents and teachers. However, in this study, the placebo treatment did not resemble the effects produced by the real NFB treatment, as Thibault and Raz state [95].
Our results confirm the hypotheses regarding a reduction in delta and theta coherences in the NFB group. There was a significant reduction in delta coherence between right central and temporal regions and between frontopolar leads; furthermore, there was a significant reduction in the theta frequency band between the left frontal regions and other regions of the same hemisphere, between frontal areas of the right hemisphere, and between frontal, parietal, and posterior-temporal homologous areas. Although it was not proposed as a related hypothesis, a reduction was also observed in the beta band in the right hemisphere between frontal leads, parietal and temporal regions, and frontopolar homologous regions. However, when the involved regions were explored, the results only supported the theta coherence reduction between the left frontal and temporoparietal regions. The superior longitudinal fasciculus connects the frontal areas and temporoparietal areas. The integrity of these areas and their connection are related to adequate accuracy in reading words and pseudowords. As a consequence, this may produce better reading comprehension. In this study, children with RDs treated with NFB improved their reading accuracy and comprehension.
An increase in alpha coherence was also observed in the NFB group between the parietal and occipital areas of the left hemisphere. This finding could be explained by the relationship between specialized areas of the ventral posterior circuit, which includes the visual word form area (VWFA), and the left dorsal temporoparietal circuit, which is specialized in phonological functions. An improvement in these connections may also explain their better performance in reading accuracy.
In addition, a reduction in beta coherence between regions of the right hemisphere with frontal dominance was observed in the NFB group. This change, added to other changes in the right hemisphere or changes in the connections between homologous areas of the brain hemispheres, could be due to compensatory mechanisms necessary to improve reading. The role of the right hemisphere in prosody should not be forgotten; injury to the right temporal lobe has been referred to as a cause of deficits in reading rhythm [96,97], which could have a negative effect on reading comprehension.
As expected, NFB likely produces a reorganization of brain interconnections. Therefore, these results indicate a trend toward the normalization of coherence patterns in treated children. This coherence maturation can be associated with improved reading comprehension and accuracy scores on the ENI-2 neuropsychological test.
When comparing the changes that occurred in the NFB group with those that occurred in the Sham group, the changes in the NFB group are magnified, since in the Sham group, only an increase in beta coherence between T5 and T6 and a decrease in theta coherence between F7 and F8 leads were observed, without evidence of behavioral changes in reading. Although there are few reports on the placebo effect on EEG, some changes in EEG after placebo administration have been reported [64,98].
To our knowledge, there are few other NFB studies in children with RDs. The first report of NFB in dyslexia was conducted by Walker et al. [48], who described 12 clinical cases that had significant cognitive improvement after receiving a considerable number of NFB sessions of a personalized combination of various different protocols; these rarely included increases in alpha power. Breteler et al. [47] reported the first randomized controlled NFB study in dyslexia that included 19 dyslexic children; 10 of them received a personalized NFB protocol (different between subjects), and the remaining 9 constituted the control group; the main changes observed in the EEG recording at rest with eyes open of the treated children were an increased theta and lower alpha power and increased delta and alpha coherence; although improvement in spelling was observed, no changes were found in reading. More recently, Nazari et al. [43] reported a significant improvement in the reading skills and phonological awareness of six children who received twenty NFB sessions set to reduce delta and theta and increase beta in F7 and T3. Interestingly, the EEG analysis did not show noticeable changes in the power of the trained bands; however, they observed normalization of theta interhemispheric coherence between temporal areas, normalization of the delta band between anterior regions of the midline, and normalization of the beta band between the vertex and other areas.
It was difficult to compare the results obtained with those of other studies. In this study, a theta/alpha ratio protocol was used while the subject had their eyes closed. The most similar approach in other research has been theta AP reduction, but the alpha power increase was rarely trained. Two main factors explain the difficulties in comparing the results of neurofeedback: (1) EEG rhythms have different cerebral origins, and therefore, the reinforcement of particular frequencies will activate different brain networks; and (2) the eyes-open and eyes-closed conditions are related to different patterns of EEG frequencies recorded at the scalp. Eyes being closed produces an enhancement of the alpha rhythm, while eyes being open generates visual stimulation and activates a different network, thus decreasing the alpha rhythm. Despite the difficulty of using a different NFB protocol and the fact that electroencephalographic changes were not consistent between the studies, cognitive improvement was a common finding for all of them. A possible explanation for this improvement could be the multiple factors involved in NFB treatment.
One of the limitations of this study is its small sample size; in particular, the Sham group included only five subjects. Unfortunately, the COVID-19 pandemic prevented other children diagnosed with RD and EEG delayed maturation from receiving the placebo treatment. However, it should be mentioned that the sample size of the NFB group in this study is larger than that of the other NFB studies aimed at treating RD that preceded it.
In contrast, a strength of this study is that the sample of children with RDs was more homogeneous than those used in other studies because a stricter criterion was imposed: children with RDs also had an abnormally high theta/alpha ratio for their chronological age; however, this strength means that the results are less generalizable to the population of children with RDs since not all of them present with this electroencephalographic alteration. Although it could be thought that this constitutes an important clinical limitation, this is not the only alteration present in these children’s EEGs; therefore, in children with different EEG alterations, a protocol aimed at correcting them could be used. With this study, the possibilities have expanded since EEG abnormalities sometimes do not occur in specific sites but in the connection between them. In the exceptional case in which the child with RD has a normal EEG, then a therapy that considers theoretical neurobiological aspects related to this disorder, as Cancer et al. proposed [91], could be implemented. However, it is important to remember that RD could be associated with a more nonspecific deficit involving the frontoparietal network. Therefore, in future applications of NFB to treat children with RDs, both the neurobiological aspects of the reading and executive functions process and the EEG characteristics associated with this disorder that are present in the individual undergoing treatment should be considered.
In this study, a reduction in theta coherence was observed in areas of the left hemisphere spatially correlated with the previously described reading cortical network. However, as previously mentioned, EEG recordings are characterized by great temporal resolution, but they do not have a good spatial resolution. Therefore, it cannot be guaranteed that they corresponded precisely to the structures directly involved in the reading process. Future studies should perform analyses using other methods that may result in a more specific topographic localization.
Author Contributions
Conceptualization, L.A.-C. and T.F.; methodology, L.A.-C., J.S.-P., B.J.M.-B., J.B.-B. and T.F.; software, J.B.-B.; formal analysis, L.A.-C., J.S.-P. and T.F.; investigation, L.A.-C., J.S.-P., B.J.M.-B., J.B.-B. and T.F.; resources, L.A.-C., B.J.M.-B. and T.F.; data curation, L.A.-C., J.S.-P., B.J.M.-B., J.B.-B. and T.F.; writing—original draft preparation, L.A.-C.; writing—review and editing, L.A.-C., J.S.-P., B.J.M.-B., J.B.-B. and T.F.; visualization, L.A.-C. and J.S.-P.; supervision, J.S.-P., J.B.-B. and T.F.; project administration, T.F.; funding acquisition, T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by CONACYT under grant CB-2015-1-251309 and by grants IN204613, IN205520, and IN207520 from Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), DGAPA-UNAM, Mexico. Jorge Bosch-Bayard was supported by Brain Canada (243030), the Fonds de recherche du Québec (FRQ) HBHL FRQ/CCC Axix (246117), the CFREF/HBHL HIBALL, and Helmholtz (252428). During the realization of this work, Lucero Albarrán-Cárdenas (scholarship recipient: 626189) was a beneficiary of the CONACYT scholarship 473496.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Instituto de Neurobiología, Universidad Nacional Autónoma de México (INEU/SA/CB/146; 1 July 2015).
Informed Consent Statement
Informed consent was obtained from all children involved in the study and their parents.
Data Availability Statement
Data will be uploaded to figshare-url.
Acknowledgments
The authors are grateful for the children’s and parents’ cooperation in this study. The authors also acknowledge Fabiola García Martínez, Milene Roca-Stappung, Sonia Y. Cárdenas, Rodrigo Flores Gallegos, Paulina Rodríguez Leis, Bertha Elena Barrera Díaz, María Elena Juárez, Manuel Hinojosa Rodríguez, Héctor Belmont, Saulo Hernández, Eliseo Islas, Lourdes Cubero, Nuri Aranda, Leonor Casanova, Carlos Sair Flores Bautista, Teresa Álvarez, Eduardo González-Moreira, Juan José Ortiz Retana, Bertha Esquivel Quiroz, Marco Olguín Araujo, Joel Bernardino Peláez, Ricardo González, Antonio González Cruz, and Lourdes Lara for their technical assistance. This work was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (IN204613, IN205520, and IN207520) and the Consejo Nacional de Ciencia y Tecnología (CONACYT; CB-2015-01-251309). Lucero Albarrán is a beneficiary of a CONACYT scholarship (No. 473496).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Galaburda, A.; Camposano, S. Dislexia Evolutiva: Un Modelo Exitoso de Neuropsicología Genética. Rev. Chil. Neuropsicol. 2006, 1, 9–14. [Google Scholar]
- Puente, A.; Jiménez, V.; Ardila, A. Anormalidades Cerebrales En Sujetos Disléxicos. Rev. Latinoam. Psicol. 2009, 41, 27–45. [Google Scholar]
- Žarić, G.; Correia, J.M.; González, G.F.; Tijms, J.; van der Molen, M.W.; Blomert, L.; Bonte, M. Altered patterns of directed connectivity within the reading network of dyslexic children and their relation to reading dysfluency. Dev. Cogn. Neurosci. 2017, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cutting, L.E.; Clements-Stephens, A.; Pugh, K.R.; Burns, S.; Cao, A.; Pekar, J.J.; Davis, N.; Rimrodt, S.L.; Bailey, S.; Hoeft, F.; et al. Not All Reading Disabilities Are Dyslexia: Distinct Neurobiology of Specific Comprehension Deficits. Brain Connect. 2013, 3, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, J.D.E. Dyslexia: A New Synergy Between Education and Cognitive Neuroscience. Science 2009, 325, 280–283. [Google Scholar] [CrossRef]
- Démonet, J.-F.; Taylor, M.J.; Chaix, Y. Developmental dyslexia. Lancet 2004, 363, 1451–1460. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Philadelphia, PA, USA, 2013. [Google Scholar] [CrossRef]
- Dhar, M.; Been, P.H.; Minderaa, R.B.; Althaus, M. Reduced interhemispheric coherence in dyslexic adults. Cortex 2010, 46, 794–798. [Google Scholar] [CrossRef]
- Galaburda, A.M.; Sherman, G.F.; Rosen, G.D.; Aboitiz, F.; Geschwind, N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann. Neurol. 1985, 18, 222–233. [Google Scholar] [CrossRef]
- Casanova, M.F.; El-Baz, A.; Giedd, J.; Rumsey, J.M.; Switala, A. Increased White Matter Gyral Depth in Dyslexia: Implications for Corticocortical Connectivity. J. Autism Dev. Disord. 2010, 40, 21–29. [Google Scholar] [CrossRef]
- Williams, V.J.; Juranek, J.; Cirino, P.; Fletcher, J.M. Cortical Thickness and Local Gyrification in Children with Developmental Dyslexia. Cereb. Cortex 2018, 28, 963–973. [Google Scholar] [CrossRef]
- Martin, A.; Schurz, M.; Kronbichler, M.; Richlan, F. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Hum. Brain Mapp. 2015, 36, 1963–1981. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum. Brain Mapp. 2009, 30, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Richlan, F.; Kronbichler, M.; Wimmer, H. Meta-analyzing brain dysfunctions in dyslexic children and adults. Neuroimage 2011, 56, 1735–1742. [Google Scholar] [CrossRef]
- Meisler, S.L.; Gabrieli, J.D. A large-scale investigation of white matter microstructural associations with reading ability. Neuroimage 2022, 249, 118909. [Google Scholar] [CrossRef] [PubMed]
- Hoeft, F.; McCandliss, B.D.; Black, J.M.; Gantman, A.; Zakerani, N.; Hulme, C.; Lyytinen, H.; Whitfield-Gabrieli, S.; Glover, G.H.; Reiss, A.L.; et al. Neural systems predicting long-term outcome in dyslexia. Proc. Natl. Acad. Sci. USA 2011, 108, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hanlon, H.W.; Thatcher, R.W.; Cline, M.J. Gender Differences in the Development of EEG Coherence in Normal Children. Dev. Neuropsychol. 1999, 16, 479–506. [Google Scholar] [CrossRef]
- Haufe, S.; Nikulin, V.V.; Müller, K.-R.; Nolte, G. A critical assessment of connectivity measures for EEG data: A simulation study. Neuroimage 2013, 64, 120–133. [Google Scholar] [CrossRef]
- Marzetti, L.; Del Gratta, C.; Nolte, G. Understanding brain connectivity from EEG data by identifying systems composed of interacting sources. Neuroimage 2008, 42, 87–98. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M.; Vorbach, S.; Wheaton, L.; Bai, O.; Mari, Z.; et al. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L.; et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Fernández-Bouzas, A.; Silva, J.; Herrera, W.; Santiago-Rodriguez, E.; Sánchez, L. Sources of EEG activity in learning disabled children. Clin. Electroencephalogr. 2002, 33, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T.; Hinojosa, G.; Marosi, E.; Becker, J.; Rodriguez, M.; Reyes, A.; Rocha, C. Correlation Between Eeg Spectral Parameters and an Educational Evaluation. Int. J. Neurosci. 1990, 54, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jäncke, L.; Alahmadi, N. Resting State EEG in Children with Learning Disabilities. Clin. EEG Neurosci. 2016, 47, 24–36. [Google Scholar] [CrossRef] [PubMed]
- John, E.; Prichep, L.; Ahn, H.; Easton, P.; Fridman, J.; Kaye, H. Neurometric evaluation of cognitive dysfunctions and neurological disorders in children. Prog. Neurobiol. 1983, 21, 239–290. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.C.; Tedrus, G.M.; Chiodi, M.G.; Cerqueira, J.N.; Tonelotto, J.M. Quantitative EEG in children with learning disabilities: Analysis of band power. Arq. de Neuro-Psiquiatr. 2006, 64, 376–381. [Google Scholar] [CrossRef]
- Bosch-Bayard, J.; Girini, K.; Biscay, R.J.; Valdes-Sosa, P.; Evans, A.C.; Chiarenza, G.A. Resting EEG effective connectivity at the sources in developmental dysphonetic dyslexia. Differences with non-specific reading delay. Int. J. Psychophysiol. 2020, 153, 135–147. [Google Scholar] [CrossRef]
- Sklar, B.; Hanley, J.; Simmons, W.W. An EEG Experiment Aimed Toward Identifying Dyslexic Children. Nature 1972, 240, 414–416. [Google Scholar] [CrossRef]
- Leisman, G. Coherence of Hemispheric Function in Developmental Dyslexia. Brain Cogn. 2002, 48, 425–431. [Google Scholar] [CrossRef]
- Byring, R.F.; Salmi, T.K.; Sainio, K.O.; Örn, H.P. EEG in children with spelling disabilities. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 247–255. [Google Scholar] [CrossRef]
- Chabot, R.J.; Di Michele, F.; Prichep, L.; John, E.R. The Clinical Role of Computerized EEG in the Evaluation and Treatment of Learning and Attention Disorders in Children and Adolescents. J. Neuropsychiatry Clin. Neurosci. 2001, 13, 171–186. [Google Scholar] [CrossRef]
- Babiloni, C.; Stella, G.; Buffo, P.; Vecchio, F.; Onorati, P.; Muratori, C.; Miano, S.; Gheller, F.; Antonaci, L.; Albertini, G.; et al. Cortical sources of resting state EEG rhythms are abnormal in dyslexic children. Clin. Neurophysiol. 2012, 123, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, R.; Pflieger, M.; Ossadtchi, A. Connectivity measures applied to human brain electrophysiological data. J. Neurosci. Methods 2012, 207, 1–16. [Google Scholar] [CrossRef]
- Carson, A.M.; Salowitz, N.M.G.; Scheidt, R.A.; Dolan, B.K.; Van Hecke, A.V. Electroencephalogram Coherence in Children with and Without Autism Spectrum Disorders: Decreased Interhemispheric Connectivity in Autism. Autism Res. 2014, 7, 334–343. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; Heaven, P.C.; McCarthy, R.; Selikowitz, M.; Byrne, M.K. EEG coherence in adults with Attention-Deficit/Hyperactivity Disorder. Int. J. Psychophysiol. 2008, 67, 35–40. [Google Scholar] [CrossRef]
- Duffy, F.H.; Shankardass, A.; McAnulty, G.B.; Als, H. The relationship of Asperger’s syndrome to autism: A preliminary EEG coherence study. BMC Med. 2013, 11, 175. [Google Scholar] [CrossRef]
- Srinivasan, R.; Winter, W.R.; Ding, J.; Nunez, P.L. EEG and MEG coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J. Neurosci. Methods 2007, 166, 41–52. [Google Scholar] [CrossRef]
- Marosi, E.; Harmony, T.; Sánchez, L.; Becker, J.; Bernal, J.; Reyes, A.; de León, A.E.D.; Rodríguez, M.; Fernández, T. Maturation of the coherence of EEG activity in normal and learning-disabled children. Electroencephalogr. Clin. Neurophysiol. 1992, 83, 350–357. [Google Scholar] [CrossRef]
- Marosi, E.; Harmony, T.; Becker, J.; Reyes, A.; Bernal, J.; Fernández, T.; Rodríguez, M.; Silva, J.; Guerrero, V. Electroencephalographic coherences discriminate between children with different pedagogical evaluation. Int. J. Psychophysiol. 1995, 19, 23–32. [Google Scholar] [CrossRef]
- Shiota, M.; Koeda, T.; Takeshita, K. Cognitive and neurophysiological evaluation of Japanese dyslexia. Brain Dev. 2000, 22, 421–426. [Google Scholar] [CrossRef]
- Arns, M.; Peters, S.; Breteler, R.; Verhoeven, L. Different brain activation patterns in dyslexic children: Evidence from EEG power and coherence patterns for the double-deficit theory of dyslexia. J. Integr. Neurosci. 2007, 6, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.A.; Mosanezhad, E.; Hashemi, T.; Jahan, A. The Effectiveness of Neurofeedback Training on EEG Coherence and Neuropsychological Functions in Children with Reading Disability. Clin. EEG Neurosci. 2012, 43, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.; Herrera, W.; Harmony, T.; Díaz-Comas, L.; Santiago-Rodriguez, E.; Sánchez, L.; Bosch, J.; Fernández-Bouzas, A.; Otero, G.; Ricardo-Garcell, J.; et al. EEG and Behavioral Changes following Neurofeedback Treatment in Learning Disabled Children. Clin. Electroencephalogr. 2003, 34, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.; Harmony, T.; Fernández-Bouzas, A.; Díaz-Comas, L.; Prado-Alcalá, R.A.; Valdés-Sosa, P.; Otero, G.; Bosch, J.; Galán, L.; Santiago-Rodriguez, E.; et al. Changes in EEG Current Sources Induced by Neurofeedback in Learning Disabled Children. An Exploratory Study. Appl. Psychophysiol. Biofeedback 2007, 32, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Fernández, T.; Bosch-Bayard, J.; Harmony, T.; Caballero, M.I.; Díaz-Comas, L.; Galán, L.; Ricardo-Garcell, J.; Aubert, E.; Otero-Ojeda, G. Neurofeedback in Learning Disabled Children: Visual versus Auditory Reinforcement. Appl. Psychophysiol. Biofeedback 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Breteler, M.H.M.; Arns, M.; Peters, S.; Giepmans, I.; Verhoeven, L. Improvements in Spelling after QEEG-based Neurofeedback in Dyslexia: A Randomized Controlled Treatment Study. Appl. Psychophysiol. Biofeedback 2010, 35, 5–11. [Google Scholar] [CrossRef]
- Walker, J.E.; Norman, C.A. The Neurophysiology of Dyslexia: A Selective Review with Implications for Neurofeedback Remediation and Results of Treatment in Twelve Consecutive Patients. J. Neurother. 2006, 10, 45–55. [Google Scholar] [CrossRef]
- Coben, R.; Wright, E.K.; Decker, S.L.; Morgan, T. The Impact of Coherence Neurofeedback on Reading Delays in Learning Disabled Children: A Randomized Controlled Study. Neuroregulation 2015, 2, 168–178. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Silva, J.; Galín, L.; Díaz-Comas, L.; Bosch, J.; Rodríguez, M.; Fernández-Bouzas, A.; Yáñez, G.; Otero, G.; et al. Relationship of specific EEG frequencies at specific brain areas with performance. Neuroreport 1998, 9, 3680–3687. [Google Scholar] [CrossRef]
- Fernández, T.; Harmony, T.; Silva-Pereyra, J.; Fernández-Bouzas, A.; Gersenowies, J.; Galán, L.; Carbonell, F.; Marosi, E.; Otero, G.; Valdés, S.I. Specific EEG frequencies at specific brain areas and performance. Neuroreport 2000, 11, 2663–2668. [Google Scholar] [CrossRef]
- Gasser, T.; Verleger, R.; Bächer, P.; Sroka, L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 91–99. [Google Scholar] [CrossRef]
- Gasser, T.; Rousson, V.; Gasser, U.S. EEG Power and Coherence in Children with Educational Problems. J. Clin. Neurophysiol. 2003, 20, 273–282. [Google Scholar] [CrossRef]
- Arns, M.; Conners, C.K.; Kraemer, H.C. A Decade of EEG Theta/Beta Ratio Research in ADHD: A Meta-Analysis. J. Atten. Disord. 2013, 17, 374–383. [Google Scholar] [CrossRef]
- Becerra, J.; Fernández, D.T.; Harmony, T.; Caballero, M.; Garcia, F.; Fernández-Bouzas, A.; Santiago-Rodriguez, E.; Prado-Alcalá, R. Follow-Up Study of Learning-Disabled Children Treated with Neurofeedback or Placebo. Clin. EEG Neurosci. 2006, 37, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Sterman, M.B. Basic Concepts and Clinical Findings in the Treatment of Seizure Disorders with EEG Operant Conditioning. Clin. Electroencephalogr. 2000, 31, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lubar, J.F.; Swartwood, M.O.; Swartwood, J.N.; O’Donnell, P.H. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self. Regul. 1995, 20, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Pain, M.T.G.; Hibbs, A. Sprint starts and the minimum auditory reaction time. J. Sports Sci. 2007, 25, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Briones, B.; Bosch-Bayard, J.; Biscay-Lirio, R.; Silva-Pereyra, J.; Albarrán-Cárdenas, L.; Fernández, T. Effects of Neurofeedback on the Working Memory of Children with Learning Disorders—An EEG Power-Spectrum Analysis. Brain Sci. 2021, 11, 957. [Google Scholar] [CrossRef]
- Egner, T.; Sterman, M.B. Neurofeedback treatment of epilepsy: From basic rationale to practical application. Expert Rev. Neurother. 2006, 6, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Domingos, C.; Peralta, M.; Prazeres, P.; Nan, W.; Rosa, A.; Pereira, J.G. Session Frequency Matters in Neurofeedback Training of Athletes. Appl. Psychophysiol. Biofeedback 2021, 46, 195–204. [Google Scholar] [CrossRef]
- Schönenberg, M.; Weingärtner, A.-L.; Weimer, K.; Scheeff, J. Believing is achieving—On the role of treatment expectation in neurofeedback applications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 105, 110129. [Google Scholar] [CrossRef] [PubMed]
- Huneke, N.; Brown, C.A.; Burford, E.; Watson, A.; Trujillo-Barreto, N.; El-Deredy, W.; Jones, A. Experimental Placebo Analgesia Changes Resting-State Alpha Oscillations. PLoS ONE 2013, 8, e78278. [Google Scholar] [CrossRef] [PubMed]
- Leuchter, A.F.; Cook, I.A.; Witte, E.A.; Morgan, M.; Abrams, M. Changes in Brain Function of Depressed Subjects During Treatment with Placebo. Am. J. Psychiatry 2002, 159, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, H.; Ke, X.; Liu, X.; Yuan, Y.; Zhang, D.; Xiong, D.; Qiu, Y. Placebo Analgesia Changes Alpha Oscillations Induced by Tonic Muscle Pain: EEG Frequency Analysis Including Data during Pain Evaluation. Front. Comput. Neurosci. 2016, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, H.; Huang, J.; Duan, C.; Kim, J.J.; Ferrari, M.; Hu, C.S. Stronger resting-state neural oscillations associated with wiser advising from the 2nd- but not the 3rd-person perspective. Sci. Rep. 2020, 10, 12677. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.M.; Wells, A. Single Dose of the Attention Training Technique Increases Resting Alpha and Beta-Oscillations in Frontoparietal Brain Networks: A Randomized Controlled Comparison. Front. Psychol. 2018, 9, 2664. [Google Scholar] [CrossRef] [PubMed]
- La Vaque, T.J.; Hammond, D.C.; Trudeau, D.; Monastra, V.; Perry, J.; Lehrer, P.; Matheson, D.; Sherman, R. Template for Developing Guidelines for the Evaluation of the Clinical Efficacy of Psychophysiological Interventions. J. Neurother. 2002, 6, 11–23. [Google Scholar] [CrossRef]
- Swanson, H.L. Information Processing Theory and Learning Disabilities: A Commentary and Future Perspective. J. Learn. Disabil. 1987, 20, 155–166. [Google Scholar] [CrossRef]
- Morken, F.; Helland, T.; Hugdahl, K.; Specht, K. Reading in dyslexia across literacy development: A longitudinal study of effective connectivity. Neuroimage 2017, 144, 92–100. [Google Scholar] [CrossRef]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Wechsler, D.; Flanagan, D.; Kaufman, A. WISC-IV, Escala de Inteligencia de Wechsler Para Niños-IV. Manual Técnico y de Interpretación, 4th ed.; Publicaciones de psicología aplicada; TEA Ediciones: Madrid, Spain, 2010. [Google Scholar]
- Ferrando, L.; Bobes, J.; Gilbert, M.; Soto, M.; Soto, O. MINI International Neuropsychiatric Interview. Versión en Español 5.0.0. DSM-IV. Available online: https://www.academia.cat/files/425-7297-DOCUMENT/MinientrevistaNeuropsiquatribaInternacional.pdf (accessed on 1 August 2021).
- Sheehan, D.V.; Sheehan, K.H.; Shytle, R.D.; Janavs, J.; Bannon, Y.; Rogers, J.E.; Milo, K.M.; Stock, S.L.; Wilkinson, B. Reliability and Validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Matute, E.; Inozemtseva, O.; Gonzalez, A.L.; Chamorro, Y. La Evaluación Neuropsicológica Infantil (ENI): Historia y fundamentos teóricos de su validación. Un acercamiento práctico a su uso y valor diagnóstico. Rev. Neuropsicol. Neuropsiquiatr. Neurocienc. 2014, 14, 68–95. [Google Scholar]
- Harmony, T.; Marosi, E.; de León, A.E.D.; Becker, J.; Fernández, T. Effect of sex, psychosocial disadvantages and biological risk factors on EEG maturation. Electroencephalogr. Clin. Neurophysiol. 1990, 75, 482–491. [Google Scholar] [CrossRef]
- Szava, S.; Valdes, P.; Biscay, R.; Galan, L.; Bosch, J.; Clark, I.; Jimenez, J.C. High resolution quantitative EEG analysis. Brain Topogr. 1994, 6, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Valdés, P.; Biscay, R.; Galán, L.; Bosch, J.; Zsava, S.; Virués, T. High Resolution Spectral EEG Norms Topography. Brain Topogr. 1990, 3, 281–282. [Google Scholar]
- Hernández, J.L.; Valdés, P.; Biscay, R.; Virues, T.; Szava, S.; Bosch, J.; Riquenes, A.; Clark, I. A Global Scale Factor in Brain Topography. Int. J. Neurosci. 1994, 76, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X. Analyzing Neural Time Series Data: Theory and Practice; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Önder, H. Using Permutation Tests to Reduce Type I and II Errors for Small Ruminant Research. J. Appl. Anim. Res. 2007, 32, 69–72. [Google Scholar] [CrossRef]
- Galán, L.; Biscay, R.; Rodríguez, J.L.; Pérez-Abalo, M.C.; Rodríguez, R. Testing topographic differences between event related brain potentials by using non-parametric combinations of permutation tests. Electroencephalogr. Clin. Neurophysiol. 1997, 102, 240–247. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Human cortical mechanisms of visual attention during orienting and search. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1353–1362. [Google Scholar] [CrossRef]
- Taran, N.; Farah, R.; DiFrancesco, M.; Altaye, M.; Vannest, J.; Holland, S.; Rosch, K.; Schlaggar, B.L.; Horowitz-Kraus, T. The role of visual attention in dyslexia: Behavioral and neurobiological evidence. Hum. Brain Mapp. 2022, 43, 1720–1737. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, T.; Van Leeuwen, C. Reading as functional coordination: Not recycling but a novel synthesis. Front. Psychol. 2014, 5, 1046. [Google Scholar] [CrossRef] [PubMed]
- Murias, M.; Swanson, J.M.; Srinivasan, R. Functional Connectivity of Frontal Cortex in Healthy and ADHD Children Reflected in EEG Coherence. Cereb. Cortex 2006, 17, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Thatcher, R.; North, D.; Biver, C. EEG and intelligence: Relations between EEG coherence, EEG phase delay and power. Clin. Neurophysiol. 2005, 116, 2129–2141. [Google Scholar] [CrossRef]
- Chabot, R.J.; Serfontein, G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol. Psychiatry 1996, 40, 951–963. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M. EEG coherence in attention-deficit/hyperactivity disorder: A comparative study of two DSM-IV types. Clin. Neurophysiol. 2002, 113, 579–585. [Google Scholar] [CrossRef]
- Clarke, A.R.; Barry, R.J.; McCarthy, R.; Selikowitz, M.; Johnstone, S.; Abbott, I.; Croft, R.J.; Magee, C.A.; Hsu, C.-I.; Lawrence, C.A. Effects of methylphenidate on EEG coherence in Attention-Deficit/Hyperactivity Disorder. Int. J. Psychophysiol. 2005, 58, 4–11. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Hajos, M.; Dupuy, F.E.; McCarthy, R.; Selikowitz, M. EEG coherence and symptom profiles of children with Attention-Deficit/Hyperactivity Disorder. Clin. Neurophysiol. 2011, 122, 1327–1332. [Google Scholar] [CrossRef]
- Weiss, S.; Mueller, H.M. The contribution of EEG coherence to the investigation of language. Brain Lang. 2003, 85, 325–343. [Google Scholar] [CrossRef]
- Roca-Stappung, M.; Fernández, T.; Becerra, J.; Mendoza-Montoya, O.; Espino, M.; Harmony, T. Healthy aging: Relationship between quantitative electroencephalogram and cognition. Neurosci. Lett. 2012, 510, 115–120. [Google Scholar] [CrossRef]
- Thibault, R.T.; Raz, A. The psychology of neurofeedback: Clinical intervention even if applied placebo. Am. Psychol. 2017, 72, 679–688. [Google Scholar] [CrossRef]
- Adolphs, R.; Damasio, H.; Tranel, D. Neural systems for recognition of emotional prosody: A 3-D lesion study. Emotion 2002, 2, 23–51. [Google Scholar] [CrossRef]
- Wright, A.E.; Davis, C.; Gomez, Y.; Posner, J.; Rorden, C.; Hillis, A.E.; Tippett, D.C. Acute Ischemic Lesions Associated with Impairments in Expression and Recognition of Affective Prosody. Perspect. ASHA Spéc. Interes. Groups 2016, 1, 82–95. [Google Scholar] [CrossRef]
- Petersen, T.H.; Puthusserypady, S. Assessing TDCS Placebo Effects on EEG and Cognitive Tasks. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).