Aging of Polylactide Films Exposed to Plasma—Hydrophobic Recovery and Selected Application Properties
Abstract
1. Introduction
- aging in the context of changing the properties of the modified layer—hydrophobic recovery,
- additionally, aging (change in mechanical and optical properties) of the film as a result of plasma modification of its surface layer.
2. Materials and Methods
2.1. Materials
2.2. Preparing Materials for Testing—Plasma Activation of Samples
2.3. Testing of Film Properties
2.3.1. Changes in Contact Angle and Surface Free Energy
2.3.2. Changes in Mechanical, Typographic, and Weight Properties of Samples
2.3.3. Changes in Color and Gloss of the Sample
2.4. Aging of Samples Due to Storage
3. Results and Discussion
3.1. Changes in Film Wettability and Surface Free Energy—Effectiveness of Activation
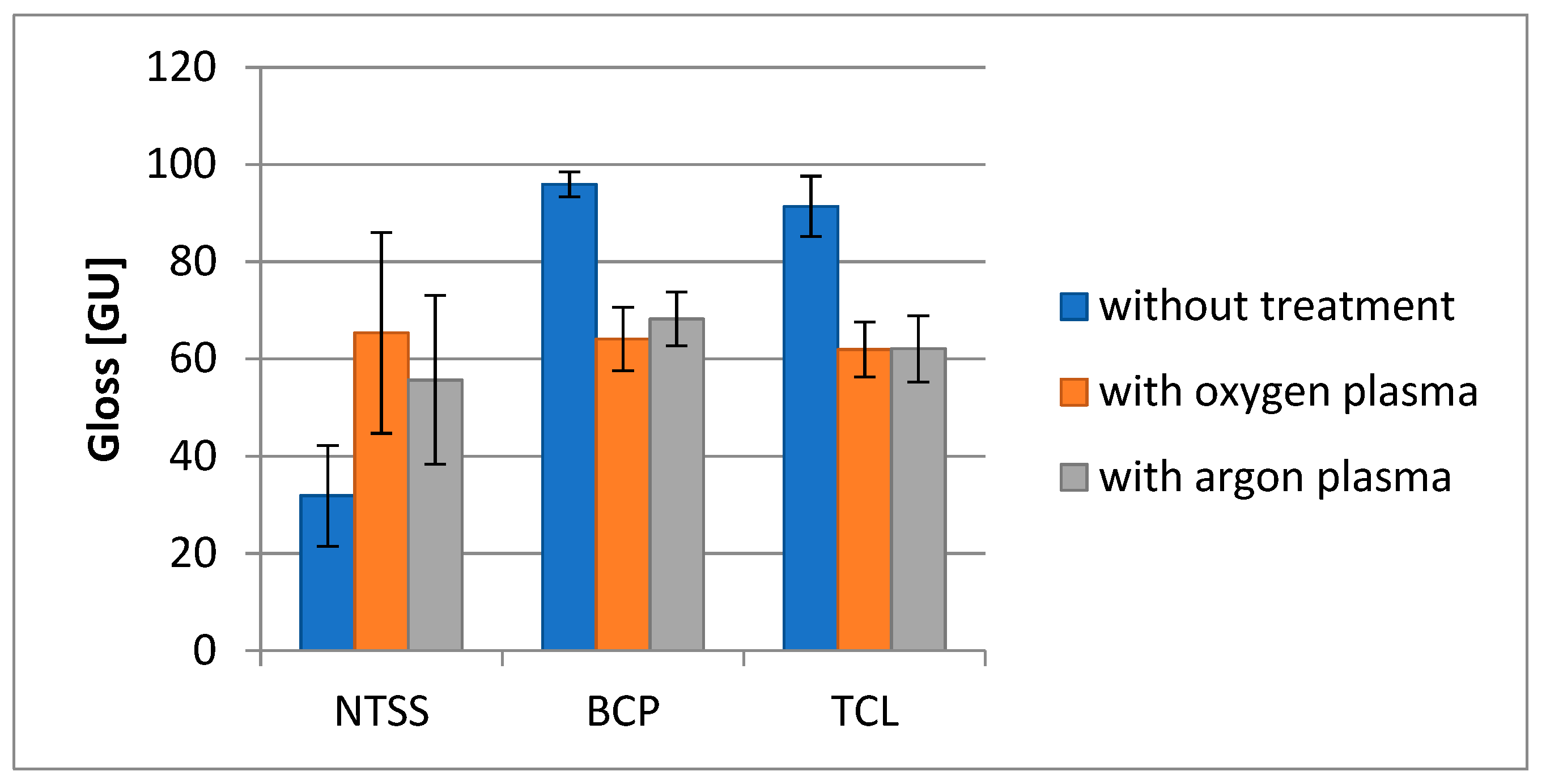
3.2. Changes in Selected Film Properties as a Result of Plasma Modification
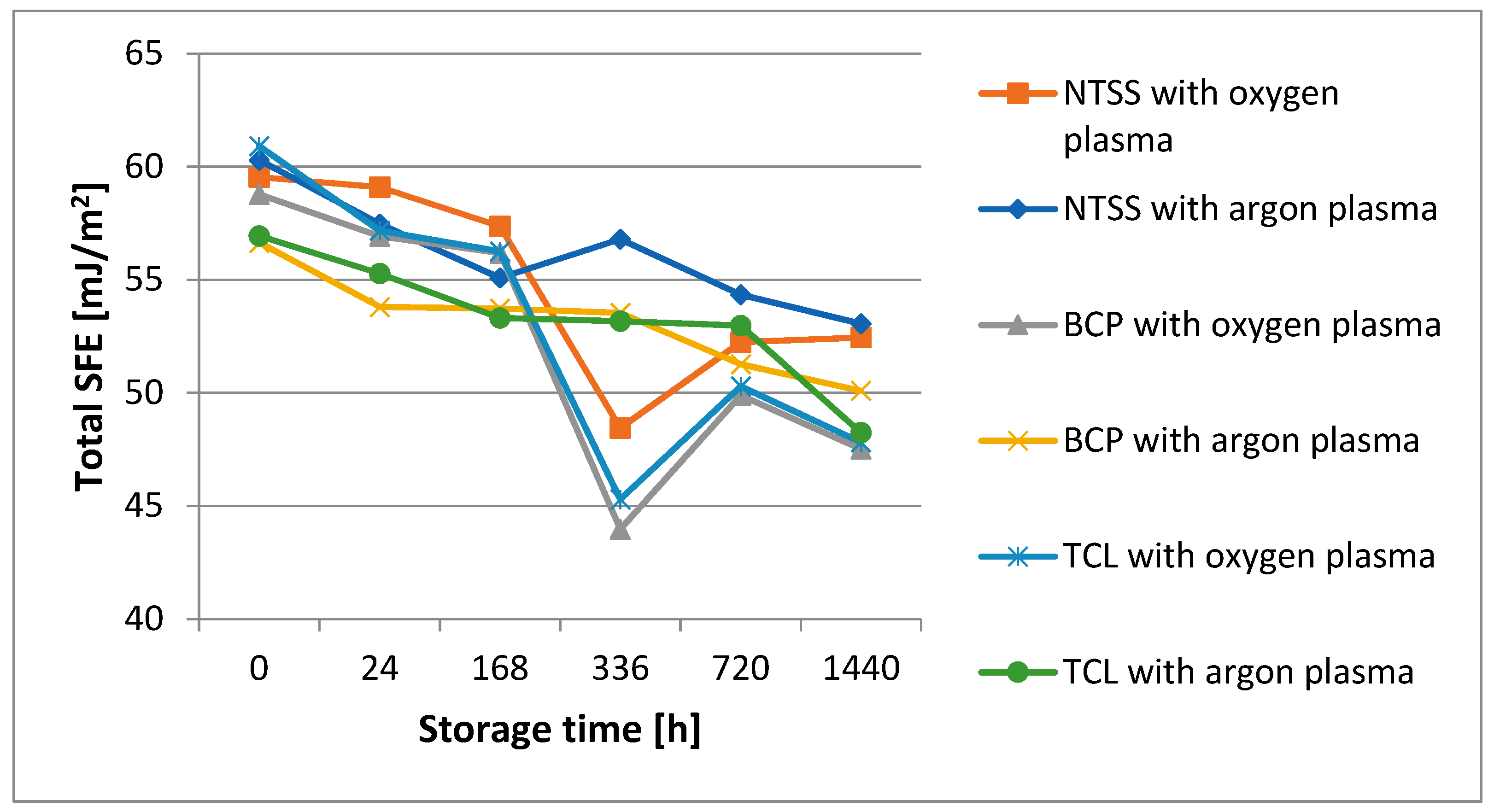
3.3. Aging of Samples Due to Storage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cangialosi, D.; Boucher, V.M.; Alegría, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Arabeche, K.; Delbreilh, L.; Baer, E. Physical aging of multilayer polymer films–Influence of layer thickness on enthalpy relaxation process, effect of confinement. J. Polym. Res. 2021, 28, 431. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Karasiewicz, T.; Jagodziński, B.; Rytlewski, P. The Effect of Accelerated Aging on Polylactide Containing Plant Extracts. Polymers 2019, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Izdebska, J. Chapter 22—Aging and Degradation of Printed Materials. In Printing on Polymers, 1st ed.; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 353–370. [Google Scholar]
- Erhard, D.P.; Lovera, D.; Giesa, R.; Altstädt, V.; Schmidt, H. Influence of physical aging on the performance of corona-charged amorphous polymer electrets. Polym. Phys. 2010, 48, 990–997. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Chapter 6—Physical Aging of Polymer Blends. In Encyclopedia of Polymer Blends; Isayev, A.I., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef]
- Thees, M.F.; Roth, C.B. Unexpected Molecular Weight Dependence to the Physical Aging of Thin Polystyrene Films Present at Ultra–High Molecular Weights. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1224–1238. [Google Scholar] [CrossRef]
- Lewis, E.A.; Stafford, C.M.; Vogt, B.D. Effect of adjacent hydrophilic polymer thin films on physical aging and residual stress in thin films of poly(butylnorbornene–ran–hydroxyhexafluoroisopropyl norbornene). J. Polym. Sci. Part B Polym. Phys. 2019, 57, 992–1000. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, R.; Tian, C.; Jiang, M. Optimizing physical aging in poly(ethylene terephthalate)-glycol (PETG). J. Non-Cryst. Solids 2018, 502, 15–21. [Google Scholar] [CrossRef]
- Pye, J.E.; Roth, C.B. Physical Aging of Polymer Films Quenched and Measured Free-Standing via Ellipsometry: Controlling Stress Imparted by Thermal Expansion Mismatch between Film and Support. Macromolecules 2013, 46, 9455–9463. [Google Scholar] [CrossRef]
- Monnier, X.; Nassar, S.F.; Domenek, S.; Guinault, A.; Sollogoub, C.; Dargent, E.; Delpouve, N. Reduced physical aging rates of polylactide in polystyrene/polylactide multilayer films from fast scanning calorimetry. Polymer 2018, 150, 1–9. [Google Scholar] [CrossRef]
- Popović, D.; Mozetič, M.; Vesel, A.; Primc, G.; Zaplotnik, R. Review on vacuum ultraviolet generation in low-pressure plasmas. Plasma Process. Polym. 2021, 18, 2100061. [Google Scholar] [CrossRef]
- Egghe, T.; Van Guyse, J.F.R.; Ghobeira, R.; Morent, R.; Hoogenboom, R.; De Geyter, N. Evaluation of cross-linking and degradation processes occurring at polymer surfaces upon plasma activation via size-exclusion chromatography. Polym. Degrad. Stab. 2021, 187, 109543. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Hegemann, D.; Brunner, H.; Oehr, C. Plasma treatment of polymers for surface and adhesion improvement. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 281–286. [Google Scholar] [CrossRef]
- Shenton, M.J.; Stevens, G.C. Surface modification of polymer surfaces: Atmospheric plasma versus vacuum plasma treatments. J. Phys. D Appl. Phys. 2001, 34, 2761. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Thomas, S.; Mozetič, M.; Cvelbar, U.; Špatenka, P.; Praveen, K.M. (Eds.) Non-Thermal Plasma Technology for Polymeric Materials, 1st ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]
- Izdebska-Podsiadły, J.; Dörsam, E. Effects of argon low temperature plasma on PLA film surface and aging behaviors. Vacuum 2017, 145, 278–284. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J.; Dörsam, E. The storage stability of the oxygen plasma modified PLA film. Bull. Mater. Sci. 2021, 44, 79. [Google Scholar] [CrossRef]
- Eddington, D.T.; Puccinelli, J.P.; Beebe, D.J. Thermal aging and reduced hydrophobic recovery of polydimethylsiloxane. Sens. Actuators B Chem. 2006, 114, 170–172. [Google Scholar] [CrossRef]
- Moraczewski, K.; Stepczyńska, M.; Malinowski, R.; Rytlewski, P.; Jagodziński, B.; Żenkiewicz, M. Stability studies of plasma modification effects of polylactide and polycaprolactone surface layers. Appl. Surf. Sci. 2016, 337, 228–237. [Google Scholar] [CrossRef]
- Rocca-Smith, J.R.; Chaua, N.; Champion, D.; Brachais, C.H.; Marcuzzo, E.; Sensidoni, A.; Piasente, F.; Karbowiak, T.; Debeaufort, F. Effect of the state of water and relative humidity on ageing of PLA films. Food Chem. 2017, 236, 109–119. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Hydrophobic Recovery of Plasma-Hydrophilized Polyethylene Terephthalate Polymers. Polymers 2022, 14, 2496. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Bouaziz, M. Polylactide Films with the Addition of Olive Leaf Extract—Physico-Chemical Characterization. Materials 2021, 14, 7623. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Quiles-Carrillo, L.; Garcia-Garcia, D.; Melendez-Rodriguez, B.; Balart, R.; Torres-Giner, S. Tailoring the Properties of Thermo-Compressed Polylactide Films for Food Packaging Applications by Individual and Combined Additions of Lactic Acid Oligomer and Halloysite Nanotubes. Molecules 2020, 25, 1976. [Google Scholar] [CrossRef]
- Lebreton, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Lavender Law, K.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleay, M.A. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Gao, X.-R.; Li, Y.; Huang, H.-D.; Xu, J.-Z.; Xu, L.; Ji, X.; Zhong, G.-J.; Li, Z.-M. Extensional Stress-Induced Orientation and Crystallization can Regulate the Balance of Toughness and Stiffness of Polylactide Films: Interplay of Oriented Amorphous Chains and Crystallites. Macromolecules 2019, 52, 5278–5288. [Google Scholar] [CrossRef]
- Song, Y.; Li, Y.; Song, W.; Yee, K.; Lee, K.-Y.; Tagarielli, V.L. Measurements of the mechanical response of unidirectional 3D-printed PLA. Mater. Des. 2017, 123, 154–164. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Dubey, S.P.; Thakur, V.K.; Krishnaswamy, S.; Abhyankar, H.A.; Marchante, V.; Brighton, J.L. Progress in environmental-friendly polymer nanocomposite material from PLA: Synthesis, processing and applications. Vacuum 2017, 146, 655–663. [Google Scholar] [CrossRef]
- Mesic, B.; Lestelius, M.; Engström, G. Influence of corona treatment decay on print quality in water-borne flexographic printing of low-density polyethylene-coated paperboard. Packag. Technol. Sci. 2006, 19, 61–70. [Google Scholar] [CrossRef]
- Izdebska, J. Chapter 1—Printing on Polymers: Theory and Practice. In Printing on Polymers, 1st ed.; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 1–20. [Google Scholar]
- Izdebska, J. Chapter 8—Corona Treatment. In Printing on Polymers, 1st ed.; Izdebska, J., Thomas, S., Eds.; William Andrew Publishing: Oxford, UK, 2016; pp. 123–142. [Google Scholar]
- Izdebska-Podsiadły, J. Effect of Plasma Surface Modification on Print Quality of Biodegradable PLA Films. Appl. Sci. 2021, 11, 8245. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J. Chapter 6—Application of Plasma in Printed Surfaces and Print Quality. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Oxford, UK, 2019; pp. 159–191. [Google Scholar]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of biodegradable material. Plasma. Process. Polym. 2016, 13, 1080–1088. [Google Scholar] [CrossRef]
- Luque-Agudo, V.; Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. Effect of plasma treatment on the surface properties of polylactic acid films. Polym. Test. 2021, 96, 107097. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Song, A.Y.; Oh, Y.A.; Roh, S.H.; Kim, J.K.; Min, S.C. Cold Oxygen Plasma Treatments for the Improvement of the Physicochemical and Biodegradable Properties of Polylactic Acid Films for Food Packaging. J. Food Sci. 2016, 81, E86–E96. [Google Scholar] [CrossRef]
- Chaiwong, C.; Rachtanapun, P.; Wongchaiya, P.; Auras, R.; Boonyawan, D. Effect of plasma treatment on hydrophobicity and barrier property of polylactic acid. Surf. Coat. Technol. 2010, 204, 2933–2939. [Google Scholar] [CrossRef]
- De Geyter, N.; Morent, R.; Desmet, T.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Plasma modification of polylactic acid in a medium pressure DBD. Surf. Coat. Technol. 2010, 204, 3272–3279. [Google Scholar] [CrossRef]
- Laput, O.; Vasenina, I.; Salvadori, M.C.; Savkin, K.; Zuza, D.; Kurzina, I. Low-temperature plasma treatment of polylactic acid and PLA/HA composite material. J. Mater. Sci. 2019, 54, 11726–11738. [Google Scholar] [CrossRef]
- Demina, T.S.; Piskarev, M.S.; Shpichka, A.I.; Gilman, A.B.; Timashev, P.S. Wettability and aging of polylactide films as a function of AC-discharge plasma treatment conditions. J. Phys. Conf. Ser. 2020, 1492, 012001. [Google Scholar] [CrossRef]
- Ivanova, N.M.; Filippova, E.O.; Aleinik, A.N.; Pichugin, V.F. Effect of Low-Temperature Plasma Treatment and γ Irradiation on the Surface Properties of Thin Films Based on Polylactic Acid. Inorg. Mater. Appl. Res. 2021, 12, 664–675. [Google Scholar] [CrossRef]
- Żołek-Tryznowska, Z.; Prica, M.; Pavlović, Ž.; Cveticanin, L.; Annusik, T. The influence of aging on surface free energy of corona treated packaging films. Polym. Test. 2020, 89, 106629. [Google Scholar] [CrossRef]
- Vandencasteele, N.; Reniers, F. Plasma-modified polymer surfaces: Characterization using XPS. J. Electron Spectrosc. Relat. Phenom. 2010, 178–179, 394–408. [Google Scholar] [CrossRef]
- Vesel, A.; Mozetic, M.; Zalar, A. XPS characterization of PTFE after treatment with RF oxygen and nitrogen plasma. Surf. Interface Anal. 2008, 40, 661–663. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Cuadri, A.A.; Martín-Alfonso, J.E. Thermal, thermo-oxidative and thermomechanical degradation of PLA: A comparative study based on rheological, chemical and thermal properties. Polym. Degrad. Stab. 2018, 150, 37–45. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Alkan, Ü. Influence of time and room temperature on mechanical and thermal degradation of PLA. Therm. Sci. 2019, 23, S383–S390. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater.Manuf.Eng. 2007, 24, 137–145. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Izdebska, J.; Podsiadło, H.; Harri, L. Influence of surface free energy of biodegradable films on optical density of ink coated fields of prints. In Advances in Printing and Media Technology, Proceedings of the 39th International Research Conference of IARIGAI, Ljubljana, Slovenia, 9–12 September 2012; The International Association of Research Organizations for the Information, Media and Graphic Arts Industries: Darmstadt, Germany, 2012; pp. 245–251. [Google Scholar]
- Tang, Z.; Hess, D.W.; Breedveld, V. Fabrication of oleophobic paper with tunable hydrophilicity by treatment with non-fluorinated chemicals. J. Mater. Chem. A 2015, 3, 14651. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsumae, T.; Kurashima, Y.; Takagi, H.; Suga, T.; Itoh, T.; Higurashi, E. Comparison of Argon and Oxygen Plasma Treatments for Ambient Room-Temperature Wafer-Scale Au–Au Bonding Using Ultrathin Au Films. Micromachines 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Więcek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Kuvaldina, E.V.; Rybkin, V.V.; Titov, V.A.; Shikova, T.G.; Shutov, D.A. Oxidation and Degradation of Polypropylene in an Oxygen Plasma. High Energy Chem. 2004, 38, 411–414. [Google Scholar] [CrossRef]
- Yellowing. In Encyclopedic Dictionary of Polymers; Gooch, J.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar] [CrossRef]
- Wu, C.; Meng, B.C.; Tam, L.-H.; He, L. Yellowing mechanisms of epoxy and vinyl ester resins under thermal, UV and natural aging conditions and protection methods. Polym. Test. 2022, 114, 107708. [Google Scholar] [CrossRef]
- Cai, H.; Dave, V.; Gross, R.A.; McCarthy, S.P. Effects of physical aging, crystallinity, and orientation on the enzymatic degradation of poly(lactic acid). Polym. Phys. 1996, 34, 2701–2708. [Google Scholar] [CrossRef]
- Darvish, F.; Sarkari, N.M.; Khani, M.; Eslami, E.; Shokri, B.; Mohseni, M.; Ebrahimi, M.; Alizadeh, M.; Dee, C.F. Direct plasma treatment approach based on non-thermal gliding arc for surface modification of biaxially-oriented polypropylene with post-exposure hydrophilicity improvement and minus aging effects. Appl. Surf. Sci. 2020, 509, 144815. [Google Scholar] [CrossRef]
- Guimond, S.; Wertheimer, M.R. Surface degradation and hydrophobic recovery of polyolefins treated by air corona and nitrogen atmospheric pressure glow discharge. J. Appl. Polym. Sci. 2004, 94, 1291–1303. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; Berthier, E.; Young, E.W.K.; Beebe, D.J. Induced hydrophobic recovery of oxygen plasma-treated surfaces. Lab Chip 2012, 12, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Vesel, A.; Mozetic, M. Surface modification and ageing of PMMA polymer by oxygen plasma treatment. Vacuum 2012, 86, 634–637. [Google Scholar] [CrossRef]
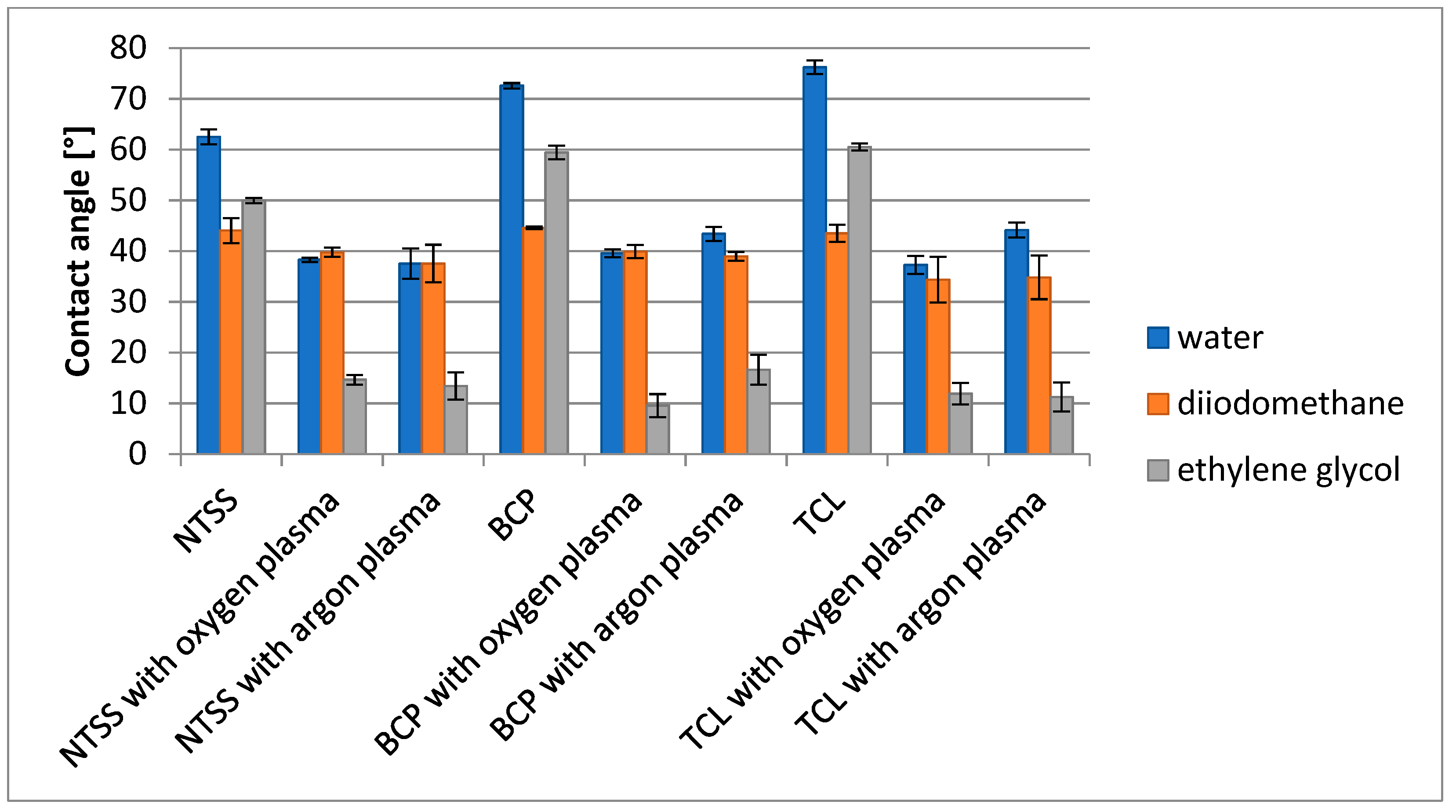

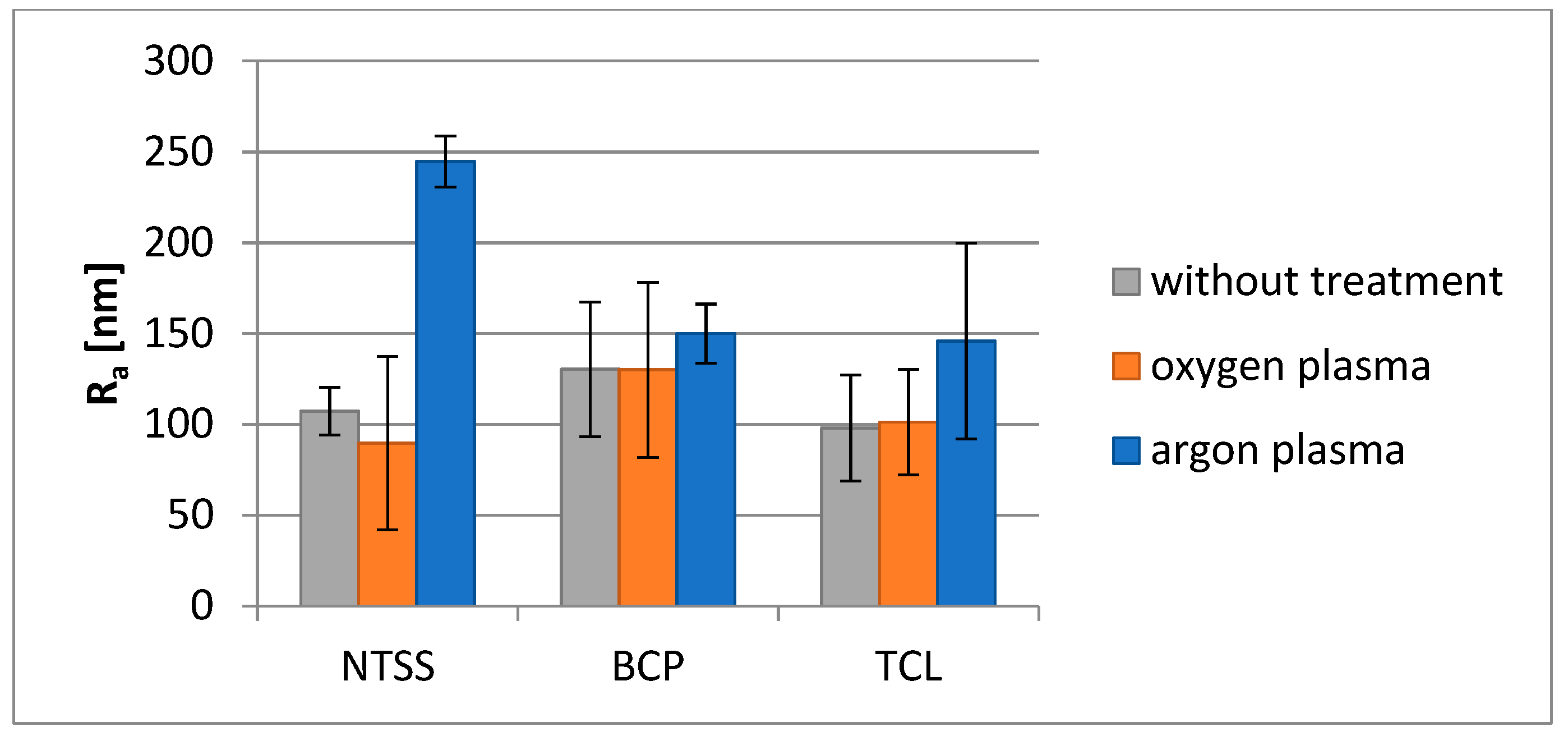
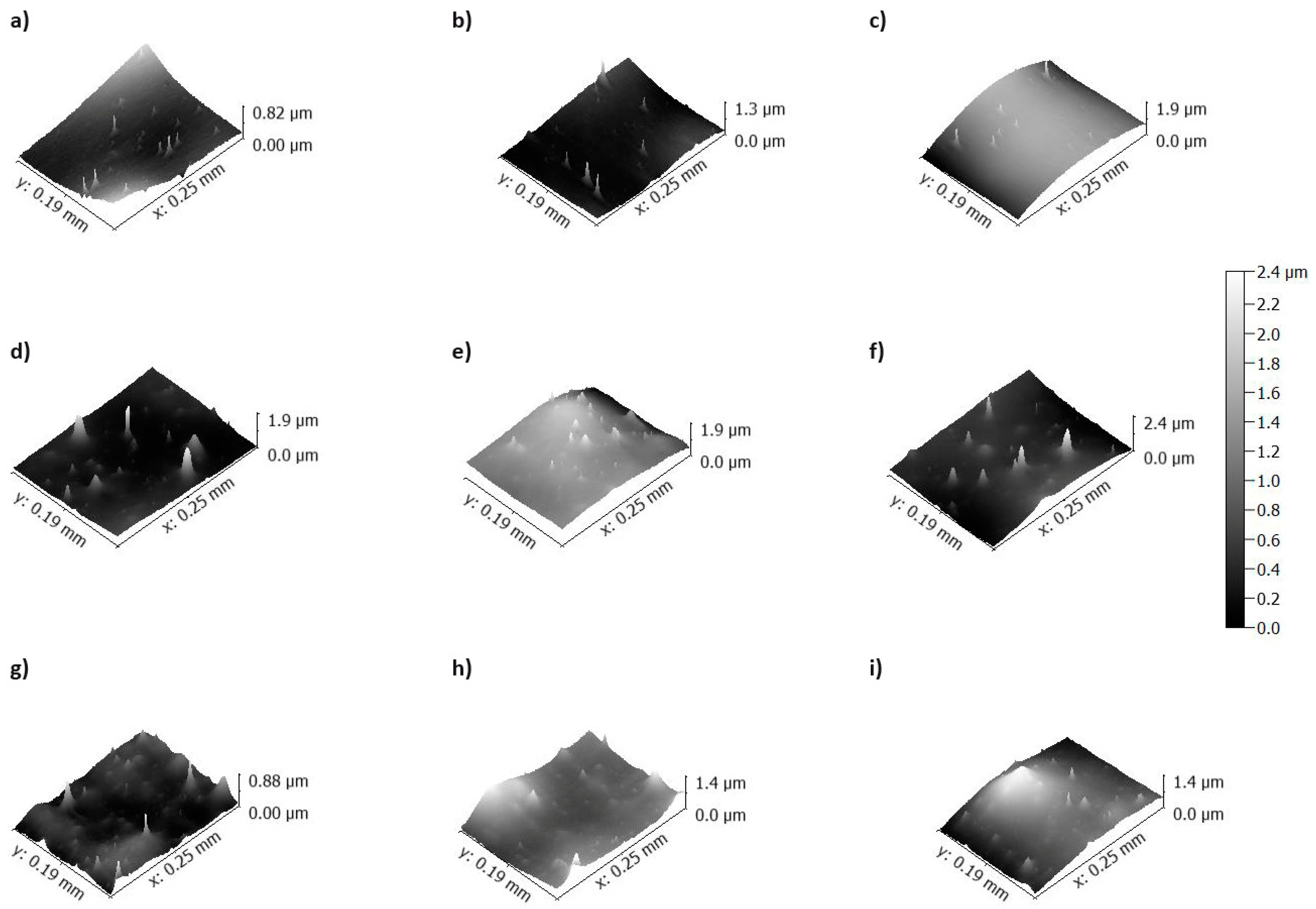


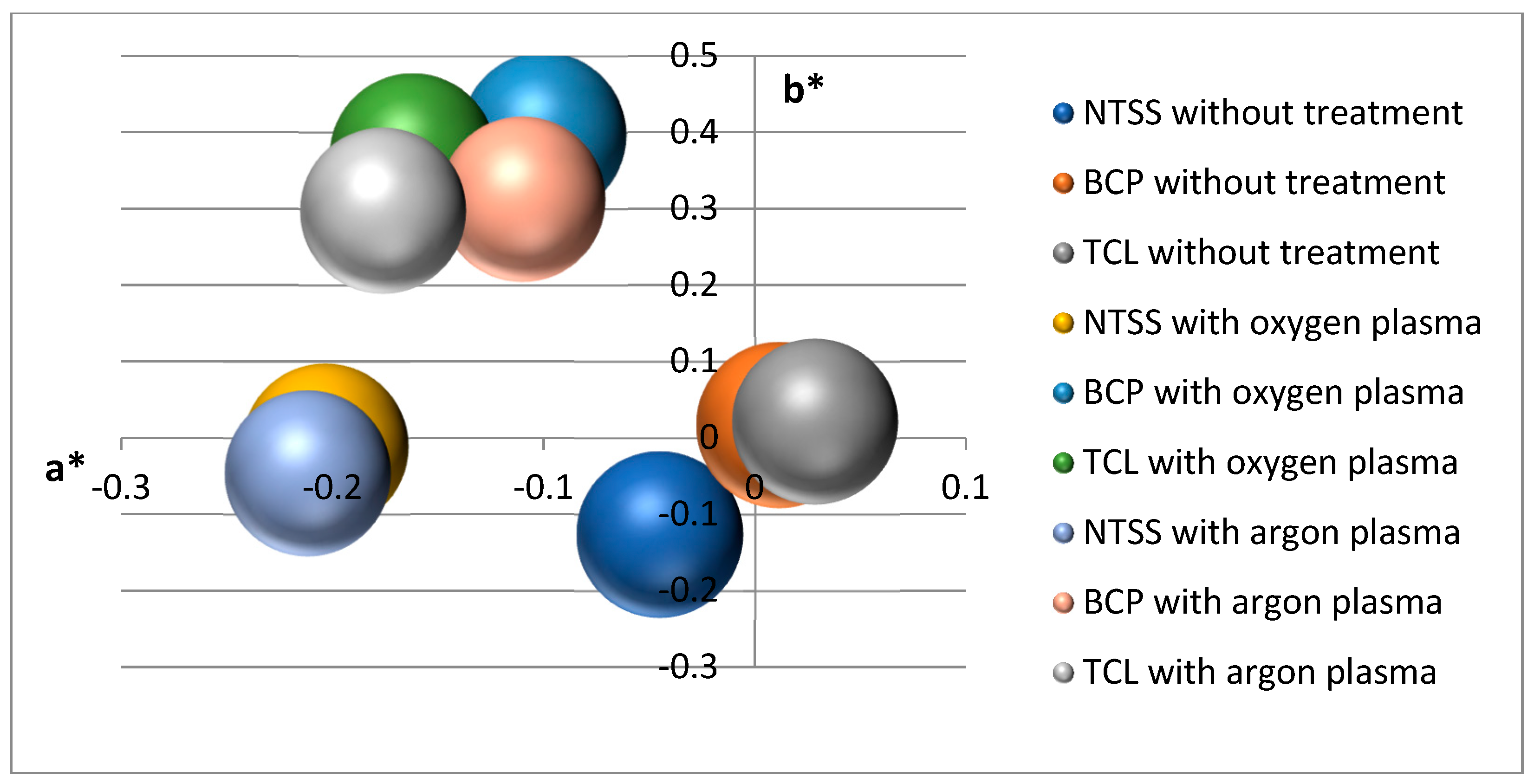

| Film Name | Nativia BOPLA NTSS | EarthFirst PLA BCP | EarthFirst PLA TCL |
|---|---|---|---|
| Abbreviation used | NTSS | BCP | TCL |
| Thickness (μm) | 20 | 50 | 50 |
| Surface free energy (mJ/m2) | 37 | 38 | 38 |
| Gloss (GU) | 80 (45°) | 125 (60°) | 115 (60°) |
| Haze (%) | 1.5 | 7.0 | 3.0 |
| Tensile strength MD/TD (N/mm2) | 105/205 | 55/55 | 98/182 |
| After Treatment | After 1 Day | After 7 Days | After 14 Days | After 30 Days | After 60 Days | |
|---|---|---|---|---|---|---|
| water contact angle [°] | ||||||
| NTSS with oxygen plasma | 38.3 ± 0.4 | 38.8 ± 2.4 | 41.8 ± 0.7 | 56.0 ± 1.1 | 51.3 ± 2.0 | 48.7 ± 0. 6 |
| BCP with oxygen plasma | 39.6 ± 0.8 | 42.6 ± 0.6 | 43.4 ± 0.8 | 64.8 ± 1.7 | 54.4 ± 2.4 | 58.0 ± 2.2 |
| TCL with oxygen plasma | 37.3 ± 1.7 | 42.3 ± 0.4 | 43.1 ± 1.5 | 63.3 ± 1.2 | 53.2 ± 3.1 | 56.7 ± 2.5 |
| NTSS with argon plasma | 37.6 ± 3.0 | 42.5 ± 2.5 | 45.3 ± 1.7 | 42.7 ± 0. 9 | 46.5 ± 0.8 | 48.2 ± 0. 9 |
| BCP with argon plasma | 43.4 ± 1.4 | 47.7 ± 2.3 | 48.2 ± 0.9 | 48.5 ± 2.3 | 51.5 ± 1.7 | 53.4 ± 1.9 |
| TCL with argon plasma | 44.2 ± 1.5 | 46.1 ± 1.1 | 48.2 ± 0.7 | 49.1 ± 1.0 | 48.8 ± 0.5 | 55.5 ± 2.9 |
| diiodomethane contact angle [°] | ||||||
| NTSS with oxygen plasma | 39.8 ± 0.9 | 40.7 ± 1.6 | 40.3 ± 0.1 | 42.7 ± 2.4 | 37.9 ± 1.9 | 44.4 ± 1.3 |
| BCP with oxygen plasma | 39.9 ± 1.3 | 40.2 ± 0.9 | 41.6 ± 0.9 | 42.6 ± 0.6 | 40.7 ± 0.6 | 42.2 ± 0.6 |
| TCL with oxygen plasma | 34.4 ± 4.5 | 39.9 ± 1.3 | 42.6 ± 0.5 | 40.6 ± 0.9 | 41.8 ± 0.4 | 43.8 ± 1.1 |
| NTSS with argon plasma | 37.6 ± 3.7 | 37.2 ± 4.9 | 41.4 ± 1.2 | 40.7 ± 0.9 | 41.6 ± 0.6 | 43.0 ± 0.5 |
| BCP with argon plasma | 39.0 ± 0.9 | 40.5 ± 2.6 | 39.3 ± 0.7 | 39.6 ± 1.3 | 41.8 ± 0.5 | 42.2 ± 0.5 |
| TCL with argon plasma | 34.8 ± 4.3 | 37.9 ± 2.2 | 41.5 ± 0.5 | 39.6 ± 0.7 | 41.3 ± 0.6 | 44.9 ± 0.2 |
| ethylene glycol contact angle [°] | ||||||
| NTSS with oxygen plasma | 14.7 ± 1.0 | 15.5 ± 0.5 | 16.0 ± 1.0 | 34.5 ± 0.4 | 23.8 ± 2.8 | 25.2 ± 1.3 |
| BCP with oxygen plasma | 9.6 ± 2.3 | 11.1 ± 1.3 | 14.2 ± 1.1 | 32.2 ± 1.5 | 23.5 ± 1.6 | 29.4 ± 1.5 |
| TCL with oxygen plasma | 11.9 ± 2.1 | 13.7 ± 1.3 | 17.7 ± 1.4 | 33.9 ± 1.6 | 23.6 ± 2.6 | 33.8 ± 1.6 |
| NTSS with argon plasma | 13.4 ± 2.7 | 10.5 ± 2.0 | 12.5 ± 1.5 | 18.1 ± 1.8 | 22.7 ± 2.3 | 20.8 ± 1.8 |
| BCP with argon plasma | 16.6 ± 3.0 | 16.3 ± 3.2 | 19.4 ± 1.0 | 21.3 ± 2.2 | 24.5 ± 2.4 | 26.2 ± 1.0 |
| TCL with argon plasma | 11.3 ± 2.9 | 13.9 ± 2.1 | 22.3 ± 1.3 | 23.0 ± 1.5 | 27.2 ± 0.8 | 31.5 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izdebska-Podsiadły, J.; Trokowska, P.; Dörsam, E. Aging of Polylactide Films Exposed to Plasma—Hydrophobic Recovery and Selected Application Properties. Appl. Sci. 2023, 13, 2751. https://doi.org/10.3390/app13052751
Izdebska-Podsiadły J, Trokowska P, Dörsam E. Aging of Polylactide Films Exposed to Plasma—Hydrophobic Recovery and Selected Application Properties. Applied Sciences. 2023; 13(5):2751. https://doi.org/10.3390/app13052751
Chicago/Turabian StyleIzdebska-Podsiadły, Joanna, Paula Trokowska, and Edgar Dörsam. 2023. "Aging of Polylactide Films Exposed to Plasma—Hydrophobic Recovery and Selected Application Properties" Applied Sciences 13, no. 5: 2751. https://doi.org/10.3390/app13052751
APA StyleIzdebska-Podsiadły, J., Trokowska, P., & Dörsam, E. (2023). Aging of Polylactide Films Exposed to Plasma—Hydrophobic Recovery and Selected Application Properties. Applied Sciences, 13(5), 2751. https://doi.org/10.3390/app13052751









