Abstract
Zanthoxylum bungeanum essential oil was extracted using a low-eutectic solvent (choline chloride and 1,3-butanediol) and steam distillation. The results showed that the yield (9.36 and 10.00%) did not differ significantly between the two methods. The α-glucosidase inhibitory activity of essential oils from 20 species was screened by the PNPG (4-p-nitrophenyl-a-d-glucopyranosylase) colorimetric method, and the results showed that the inhibition rate of Zanthoxylum bungeanum essential oil reached 57.1%, far more than those of the other essential oils tested. The main components and contents of Zanthoxylum bungeanum essential oil were terpineol-4-ol (13.13%), (-)-β-pinene (11.17%), γ-terpinene (9.45%), terpinyl acetate (9.36%), and α-terpineol (5.40%), which were identified by GC-MS (gas chromatography–mass spectrometry). According to the literature, linalool and limonene are also high-content components. These seven components were docked with the α-glucosidase molecule. It was found that the binding energies between terpineol-4-ol, (-)-β-pinene, γ-terpinene, terpinyl acetate, α-terpineol, linalool, limonene, and α-glucosidase were −28.88, −25.54, −26.37, −28.46, −27.63, −25.95, and −25.53 (KJ/mol), respectively, similar to those of anthocyanins. From the research results, the following conclusions can be drawn: the extraction rate of Zanthoxylum bungeanum essential oil can be increased with steam distillation. Of the many plant essential oils, Zanthoxylum bungeanum essential oil shows excellent anti-α-glucosidase activity, suggesting that it has potential for application in hypoglycemic drugs. The strong binding ability of the monoterpene-based chemical components with the α-glucosidase molecule revealed the chemical mechanism of the inhibitory effect of Zanthoxylum bungeanum essential oil on α-glucosidase. The above conclusions provide a theoretical basis for the utilization of Zanthoxylum bungeanum essential oil in hypoglycemic drugs and further expand the applications of small-molecule chemical components.
1. Introduction
Diabetes mellitus (DM) is closely related to blood glucose, and blood glucose regulation is closely related to α-glucosidase activity [1]. An important research direction for hypoglycemic drug research is to screen α-glucosidase inhibitory active components [2]. Voglibose, miglitol, and acarbose are α-glucosidase inhibitor drugs that have been successfully marketed for the treatment of type Ⅱ DM [3]. However, these drugs can also cause side effects such as diarrhea, abdominal distention, and flatulence [4]. In recent years, it has been reported that the α-glucosidase inhibitory activity effects of wild blue honeysuckle fruit [5], raspberry [6], and mulberry [7] are remarkable, making them potential targets for α-glucosidase inhibitors. Several α-glucosidase inhibitory active components from natural products have been reported, such as flavonoids [8], alkaloids [9], polysaccharides [10], and so on, which have become research topics due to their milder side effects compared with voglibose, miglitol, and acarbose.
Zanthoxylum bungeanum is a deciduous tree, and its fruit is an important food condiment for humans. The aroma of Zanthoxylum bungeanum fruit is due to the high amount of essential oil. It has been reported that Zanthoxylum bungeanum essential oil has hypoglycemic [11] and antioxidant [12] activities. Phenols and alkylamides (ethanol extract) have been identified by HPLC-ESI-MS and are considered the main components underlying α-glucosidase inhibition [13]. However, the contribution of the volatile components (essential oil) of Zanthoxylum bungeanum to α-glucosidase inhibition has not been determined.
Steam distillation is the most common extraction method for plant essential oils [14]. In recent years, deep eutectic solvent (DES) has also been used to improve the extraction yield of plant essential oils [15]. DES can dissolve the plant fiber structure so that the active ingredients can be more easily released from cells [16]. DES, a new ionic liquid analog discovered by Abbott, is a green, environmentally friendly, and efficient extraction solvent [17]. It is a low-eutectic mixture composed of two or three components, containing a certain proportion of hydrogen bond donors and receptors.
Ligand molecules and receptor molecules are simulated and connected by molecule docking technology so as to find the best binding mode between them, as well as to determine the active sites and reasonable conformation [18]. Nowadays, molecular docking is a powerful tool that is used to reveal the mechanism of biological activity [19,20,21].
The present study aims to provide a comparison of the inhibitory activity of Zanthoxylum bungeanum essential oil and other plant essential oils against α-glucosidase. In addition, the study aims to determine how the components of Zanthoxylum bungeanum essential oil induce their inhibitory action against α-glucosidase, thus, contributing findings that may be beneficial to the utilization of Zanthoxylum bungeanum as a health food.
2. Materials and Methods
2.1. Collection of Materials
In this assay, Zanthoxylum bungeanum was collected from Mianyang city, Sichuan Province, China, and named Jiu-Ye-Qing. Zanthoxylum bungeanum was collected and dried, and impurities were removed according to the requirements of Chinese Pharmacopoeia 2020. The other 19 species of plant essential oils were provided by Nuoze Biotechnology Co., Ltd., Yiyang city, Hunan Province, China.
2.2. Chemicals and Solutions
Choline chloride was purchased from Asiatic Biotechnology Co., Ltd., Shandong Province, China and 1,3-butanediol was purchased from Zhonghe Shengtai Chemical Co., Ltd., Tianjin, China. Acarbose, PNPG (4-nitrophenyl-α-d-glucopyranoside) and α-glucosidase were purchased from Yuanye Biological Co., Ltd., Shanghai, China. DMSO (dimethyl sulfoxide), PEG2000 (polyethylene glycol), and potassium dihydrogen phosphate were conventional analytical grade, and we prepared a PBS solution (phosphate buffer solution) with pH = 6.8.
2.3. Extraction of Zanthoxylum bungeanum Essential Oil
In this paper, the Zanthoxylum bungeanum essential oil was extracted by a low-eutectic solvent and compared with extraction using steam distillation [22]. The Zanthoxylum bungeanum fruits were crushed with a pulverizer and sieved with a 60-mesh sieve. Unscreened and sieved particles were weighed in the proportion of 1:2. During the actual experiment, unscreened particles (7 g) and sieved powder (14 g) were collected each time. The low-eutectic solvent was prepared by choline chloride and 1,3-butanediol in the molar ratio of 1:3. The sample was placed in a round-bottom flask, and then 50% low-eutectic solvent (200 mL) was added. After the volatile oil extraction device was installed, it started heating for distillation extraction for 1.0 h at 100 °C; the essential oil was collected, and the yield was recorded. Furthermore, the same weight of sample was completely distilled with water.
The yield of Zanthoxylum bungeanum essential oil was calculated according to the following formula:
where x is the yield of Zanthoxylum bungeanum essential oil/%; a is the volume of Zanthoxylum bungeanum essential oil/mL; and m is the mass of the Zanthoxylum bungeanum sample /g.
2.4. Screening of the Plant Essential Oils against α-Glucosidase Activity
The anti-α-glucosidase activity of the plant essential oils was measured according to the method described by Sabiu et al. [23] with slight modifications. PEG2000 (50 mg) was placed in a 5.0 mL volumetric flask, dissolved with 50 °C deionized water, and used to prepare the PEG solution (10.0 mg/mL). PEG solution (1.0 mL) was placed in a 5.0 mL volumetric flask by pipette, and 5.0 mg plant essential oil was added. Then, deionized water was added to the flask to obtain the test sample solution. The various solutions required for the enzyme reaction are shown in Table 1, in detail. The absorbance of the test solution was recorded by a microplate reader (Multiskan GO, Thermo Fisher Scientific, Waltham, MA, USA). On the microplate, the sample solution (including acarbose solution), α-glucosidase solution, and PBS solution were added according to Table 1, incubated at 37 °C for 10 min, and then the PNPG solution was added to carry out chromogenic reaction at 37 °C for 10 min. The reaction was terminated after adding sodium carbonate solution (1.0 mol/L) at 100 μL. The absorbance of the test solution was measured at 405 nm. Parallel detections were carried out 3 times, and the average value was taken. The inhibition rate was calculated as follows:
A0 is the absorption value of the blank group, A1 is the absorption value of the background group, and A2 is the absorption value of the sample group.

Table 1.
Sample solution addition (μL) of each group.
Table 1.
Sample solution addition (μL) of each group.
| Group | PBS | Sample | Enzyme | PNPG | Na2CO3 |
|---|---|---|---|---|---|
| Blank | 80 | 0 | 10 | 10 | 100 |
| Background | 80 | 10 | 0 | 10 | 100 |
| Acarbose | 70 | 10 | 10 | 10 | 100 |
| Sample | 70 | 10 | 10 | 10 | 100 |
2.5. Component Identification of Zanthoxylum bungeanum Essential Oil
The components of the Zanthoxylum bungeanum essential oil were qualitatively and quantitatively analyzed by gas chromatography–mass spectrometry (GC7890A-MSD5975C, Agilent Technologies, Santa Clara, CA, USA). The chromatographic column was an HP-5MS ultra insert (30 m × 250 μm × 0.25 μm), and the injection volume was 1 μL. The programmed temperature parameters were as follows: the initial temperature was 60 °C, held for 2 min and then increased to 280 °C at a rate of 10 °C/min within 5 min. The temperatures of the injection port, auxiliary heater, and ion source were 220, 280, and 230 °C, respectively. The carrier gas was high-purity helium, and the flow rate was 1.0 mL/min (split ratio was 50:1). The ionization mode was EI, and the scanning quality range was m/z: 35–900. The identification of the components was based on retrieval and matching by the mass spectrometry database of the GC-MS instrument, combined with literature reports and CAS (American Chemical Society, Washington, DC, USA) data. The relative content of each component was calculated according to the peak area normalization method.
2.6. Molecular Docking between the Main Components of Zanthoxylum bungeanum Essential Oil and α-Glucosidase
According to Section 2.5, the main components of the Zanthoxylum bungeanum essential oil were ligands, which were used to build the model using the Rdkit program. The lowest energy conformations of the molecules were obtained by energy minimization modules of the program. As ligands, the molecules were added as atomic charges and allocated atomic types. All flexible bonds were rotatable by default.
The α-glucosidase molecule was the receptor of this docking. The crystal structure of the α-glucosidase (PDB ID: 3wy1) was obtained from the protein database (http://www.rcsb.org/pdb, accessed on 15 January 2020). All water molecules and original ligands in the α-glucosidase molecule were deleted, polar hydrogen atoms were added, and Gasteiger charges were calculated by Autodock tools 1.5.6 software (Center for Computational Structural Biology, La Jolla, CA, USA).
The active sites of the molecular docking and protein active pocket were determined according to the coordinates of the original ligand in the crystal protein complex, and molecular docking were carried out by Autodock Vina 1.1.2. The ligand was set to be flexible, and the receptor was set to be rigid. The gridbox location of the active center was set to x = −9.088, y = −16.231, z = 21.156. The size was set to 6.0 and the spaces were set to 1. The search accuracy was set to 32, num modes were 5, and other parameters were default values.
3. Results and Discussion
3.1. Extraction Yield of Zanthoxylum bungeanum Essential Oil
The extraction yield of Zanthoxylum bungeanum essential oil by DES was 9.36 ± 0.99%, while the yield by steam extraction was 10.00 ± 0.83% (n = 3). According to t-test analysis, p > 0.05, there was no significant difference in the extraction yield of Zanthoxylum bungeanum essential oil extracted by steam distillation and the low-eutectic solvent.
The output of the Zanthoxylum bungeanum essential oil under different extraction times is shown in Figure 1. It can be seen that although the output of Zanthoxylum bungeanum essential oil extracted by the low-eutectic solvent was higher than that of steam distillation in the early stage of extraction, the output tended to be the same after 30 min. Therefore, the low-eutectic solvent did not significantly improve the yield of Zanthoxylum bungeanum essential oil.
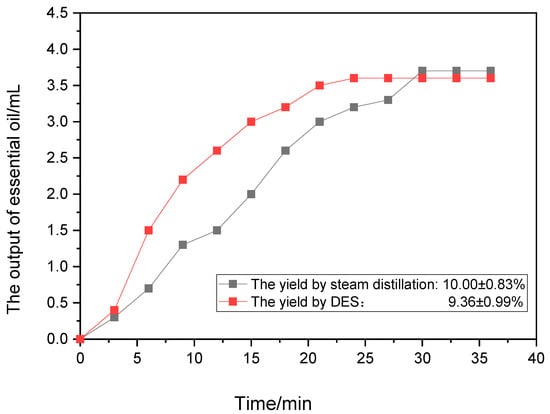
Figure 1.
The output of Zanthoxylum bungeanum essential oil.
Since there was no significant difference between the extraction of Zanthoxylum bungeanum essential oil with the low-eutectic solvent and steam distillation, steam distillation is the best way to obtain essential oil. The following experiments were used to extract Zanthoxylum bungeanum essential oil via steam distillation.
3.2. Screening for Anti-α-Glucosidase Activity of Plant Essential Oil
The inhibitory effect of essential oils from 20 plant species against glucosidase is shown in Table 2. The results of the blank group, positive control group (acarbose 40 μg/mg, 80 μg/mg), and negative control group (PEG2000) are also shown in Table 2.

Table 2.
The inhibition of α-glucosidase due to the activity of plant essential oils (x ± s).
In the positive control group, acarbose solutions of different concentrations exhibited a high inhibition rate, while the PEG2000 solution, as the negative control group, did not exhibit an obvious inhibitory effect against α-glucosidase. The above results show that the experimental method and operation were accurate and reliable. The inhibition rate of α-glucosidase due to the activity of Zanthoxylum bungeanum essential oil was 57.10%, while that of the other essential oils did not exceed 20.0%. It has been reported that although 26 varieties of Zanthoxylum pericarp extracts and their main inhibitory ingredients against α-glucosidase have been measured [13], they are not essential oils and are different in composition, which is a considerable difference from this study.
3.3. Identification of Components of Zanthoxylum bungeanum Essential Oil
The total ion flow diagram of Zanthoxylum bungeanum essential oil obtained by GC-MS is shown in Figure 2.
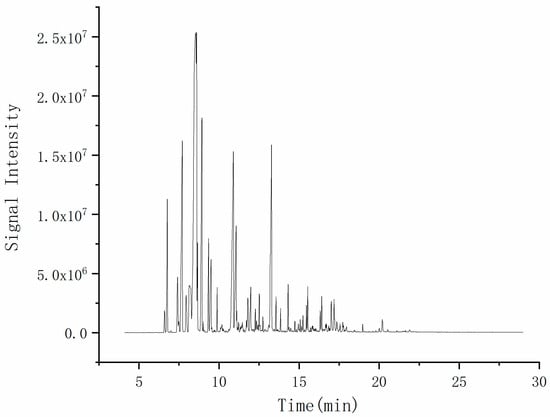
Figure 2.
Total ion flow diagram of Zanthoxylum bungeanum essential oil.
The names of the components of Zanthoxylum bungeanum essential oil and their relative contents are shown in Table 3.

Table 3.
Main components and contents of Zanthoxylum bungeanum essential oil.
The top five components were (-)-terpinen-4-ol (13.13%), (-)-β-pinene (11.17%), γ-terpinene (9.45%), terpinyl acetate (9.36%), and α-terpineol (5.40%). According to the literature, the main components of Shaanxi red Zanthoxylum bungeanum essential oil were (-)-terpinen-4-ol with a content of 19.7% [24], β-pinene with a content of 14.31% [25], limonene with a content of 21% [26], terpinyl acetate with a content of 5.40% [27], α-terpineol with a content of 5.83% [28], and linalool with a content of 29% [29]. It was shown that the components of Zanthoxylum bungeanum essential oil identified in this paper were basically the same as those in the literature except for limonene and linalool. Although linalool and limonene were not detected in this study, they are still important components of Zanthoxylum bungeanum essential oil according to the literature. Therefore, the molecular structures of seven components ((-)-terpinen-4-ol, (-)-β-pinene, γ-terpinene, terpinyl acetate, α-terpineol, linalool, limonene) were used for molecular docking in the subsequent molecular docking in this paper.
3.4. Results of Molecular Docking
The molecular docking binding energies between linalool, limonene, (-)-terpinen-4-ol, (-)-β-pinene, γ-terpinene, terpineol acetate, α-terpineol and α-glucosidase were −25.95, −25.53, −28.88, −25.54, −26.37, −28.46, and −27.63 KJ/mol, shown in Table 4, respectively. A three-dimensional diagram of molecular docking can be found in the Supplementary Materials.

Table 4.
Molecular docking binding energies.
The absolute value of the binding energy reflects the strength of the intermolecular force. The binding energies (absolute value) between the seven main components in Zanthoxylum bungeanum essential oil and α-glucosidase were greater than 20 KJ/mol, similar to those between anthocyanins and α-glucosidase (−29.85 KJ/mol) [29]. Anthocyanin-rich foods have been shown to reduce the risk of diabetes [29]. The binding energies between these components in Zanthoxylum bungeanum essential oil and α-glucosidase are close to those of anthocyanins, providing strong evidence to expound the mechanism of α-glucosidase inhibitory activity for Zanthoxylum bungeanum essential oil.
Two-dimensional diagrams of the interaction between the ligand and receptor are shown in Figure 3A–G. The polar amino acids of α-glucosidase were mainly linked with the components of Zanthoxylum bungeanum essential oil. Therefore, the interaction force was mainly the hydrophilic action of polar amino acid. Furthermore, hydrogen bonding existed between (-)-terpinen-4-ol and amino acid residues Glu271 (glutamic acid) and Asp333 (aspartic acid), with bond lengths of 2.93 Å and 3.10 Å, respectively; between linalool and amino acid residue Asp202, with a bond length of 2.95 Å; and between α-terpineol and amino acid residues Arg400 (Arginine) and Asp62, with bond lengths of 2.95 Å and 2.96 Å, respectively.
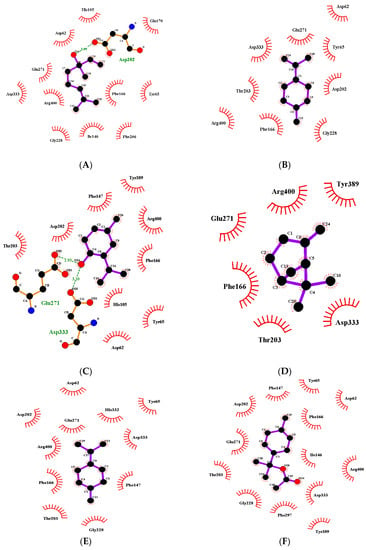
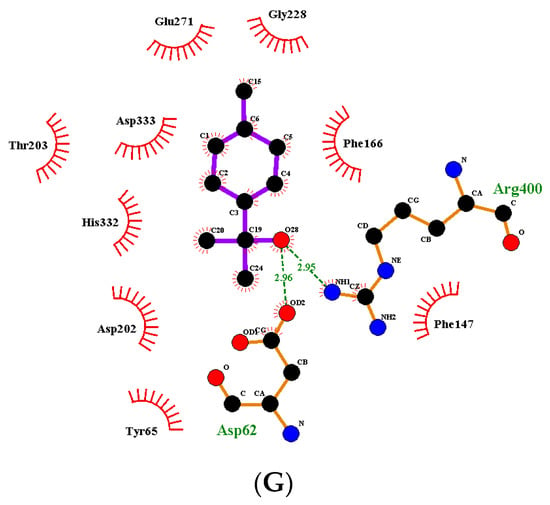
Figure 3.
(A) Two-dimensional diagram of the interaction between α-glucosidase and linalool. (B) Two-dimensional diagram of the interaction between α-glucosidase and limonene. (C) Two-dimensional diagram of the interaction between α-glucosidase and (-)-terpinene-4-ol. (D) Two-dimensional diagram of the interaction between α-glucosidase and -β-pinene. (E) Two-dimensional diagram of the interaction between α-glucosidase and terpinene. (F) Two-dimensional diagram of the interaction between α-glucosidase and terpineol acetate. (G) Two-dimensional diagram of the interaction between α-glucosidase and α-terpineol.
4. Conclusions
In this paper, the yield of Zanthoxylum bungeanum essential oil extracted by steam distillation and a low-eutectic solvent-assisted extraction was compared, and there was no significant difference. Therefore, steam distillation can be used as the method to extract Zanthoxylum bungeanum essential oil. Of the 20 plant essential oils, Zanthoxylum bungeanum essential oil showed excellent anti-α-glucosidase activity, suggesting that it has potential application in hypoglycemic drugs. The main components of Zanthoxylum bungeanum essential oil are terpineol-4-ol, (-)-β-pinene, γ-terpinene, terpinyl acetate, α-terpineol, linalool, and limonene. The strong binding ability of these chemical components with the α-glucosidase molecule by molecular docking revealed the chemical mechanism of Zanthoxylum bungeanum essential oil against α-glucosidase. It was confirmed that the main components in Zanthoxylum bungeanum essential oil can closely combine with α-glucosidase to inhibit the decomposition of starch polysaccharides. The results provide a theoretical basis for Zanthoxylum bungeanum essential oil in the field of preventing diabetes and further expand the application of small molecules.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13042627/s1, Figure S1: Molecular docking pattern of α-glucosidase and linalool; Figure S2: Molecular docking pattern of α-glucosidase and limonene; Figure S3: Molecular docking pattern of α-glucosidase and terpineol; Figure S4: Molecular docking pattern of α-glucosidase and -β-pinene; Figure S5: Molecular docking pattern of α-glucosidase and terpinene; Figure S6: Molecular docking pattern of α-glucosidase and terpineol acetate; Figure S7: Molecular docking pattern of α-glucosidase and α-terpineol.
Author Contributions
Conceptualization, S.Z.; methodology, S.L.; validation, W.H.; writing—original draft preparation, S.L.; writing—review and editing, W.C.; investigation, R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [Changsha Municipal Natural Science Foundation] grant number [Grant no. kq2202327], [Innovation and entrepreneurship training program for Chinese College Students] grant number [grant number S202110538078S].
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
| Biological, Chemical, and Microbiological | |
| DM | Diabetes mellitus |
| DES | Deep eutectic solvents |
| PNPG | 4-nitrophenyl-α-D-glucopyranoside |
| DMSO | Dimethyl sulfoxide |
| PEG2000 | Polyethylene glycol |
| PBS | Phosphate buffer solution |
| Glu | Glutamic acid |
| Asp | Aspartic acid |
| Arg | Arginine |
| Instrumental techniques and organization | |
| HPLC-ESI-MS | High-performance liquid chromatography–electrospray ionization–mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| CAS | American Chemical Society |
References
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Y.; Wen, Q.; Feng, Y.; Tan, T. Comprehensive chemical and metabolic profiling of anti-hyperglycemic active fraction from Clerodendranthi Spicati Herba. J. Sep. Sci. 2021, 44, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Alssema, M.; Ruijgrok, C.; Blaak, E.E.; Egli, L.; Dussort, P.; Vinoy, S.; Dekker, J.M.; Denise Robertson, M. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: A systematic review and meta-analysis. Nutr. Diabetes 2021, 11, 11. [Google Scholar] [CrossRef]
- Khwaja, N.U.D.; Arunagirinathan, G. Efficacy and Cardiovascular Safety of Alpha Glucosidase Inhibitors. Curr. Drug Saf. 2021, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jinli, Q.; Yan, Z.; Pei, L.; Liangchuan, G.; Junwei, H. Polyphenol Composition and Antioxidant and Hypoglycemic Activities in Wild Blue Honeysuckle Fruit. Food Sci. 2021, 42, 47–55. [Google Scholar] [CrossRef]
- Xiong, S.L.; Yue, L.M.; Lim, G.T.; Yang, J.M.; Lee, J.; Park, Y.D. Inhibitory effect of raspberry ketone on α-glucosidase: Docking simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2018, 113, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.H.; Choi, S.W. Isolation, Identification, and Quantification of Tyrosinase and α-Glucosidase Inhibitors from UVC-Irradiated Mulberry (Morus alba L.) Leaves. Prev. Nutr. Food Sci. 2019, 24, 84–94. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Kyei-Barffour, I.; Kwarkoh, R.K.B.; Arthur, O.D.; Akwetey, S.A.; Acheampong, D.O.; Aboagye, B.; Brah, A.S.; Amponsah, I.K.; Adokoh, C.K. Alkaloidal extract from Zanthoxylum zanthoxyloides stimulates insulin secretion in normoglycemic and nicotinamide/streptozotocin-induced diabetic rats. Heliyon 2021, 7, e07452. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.B.; Lei, Y.T.; Zhang, Q.; Li, Y.C.; Chen, W.J.; Liu, D.Y. Encapsulation of Zanthoxylum bungeanum essential oil to enhance flavor stability and inhibit lipid oxidation of Chinese-style sausage. J. Sci. Food Agric. 2022, 102, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Yang, Z.; Shi, J.; Zhao, L.; Battino, M.; Xiao, J.; Deng, X.; Wu, Y.; Wang, C.; et al. Interactions between Phenols and Alkylamides of Sichuan Pepper (Zanthoxylum Genus) in α-Glucosidase Inhibition: A Structural Mechanism Analysis. J. Agric. Food Chem. 2021, 69, 5583–5598. [Google Scholar] [CrossRef]
- Khalid, K.A.; Cai, W.M.; Ahmed, A.M.A. Effect of Harvesting Treatments and Distillation Methods on the Essential Oil of Lemon Balm and Apple Geranium Plants. J. Essent. Oil Bear. Plants 2009, 12, 120–130. [Google Scholar] [CrossRef]
- Huang, C.X.; Chen, X.N.; Wei, C.; Wang, H.W.; Gao, H. Deep Eutectic Solvents as Active Pharmaceutical Ingredient Delivery Systems in the Treatment of Metabolic Related Diseases. Front. Pharmacol. 2021, 12, 794939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Wang, P.; Zheng, W.; Yu, G.W.; Li, Z.G.; She, Y.B.; Lee, M. Three-stage microwave extraction of cumin (Cuminum cyminum L.) Seed essential oil with natural deep eutectic solvents. Ind. Crops Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Della Corte, D. Using molecular docking and molecular dynamics to investigate protein-ligand interactions. Mod. Phys. Lett. B 2021, 35, 2130002. [Google Scholar] [CrossRef]
- Kavitha, R.; Karunagaran, S.; Chandrabose, S.S.; Lee, K.W.; Meganathan, C. Pharmacophore modeling, virtual screening, molecular docking studies and density functional theory approaches to identify novel ketohexokinase (KHK) inhibitors. Biosystems 2015, 138, 39–52. [Google Scholar] [CrossRef]
- Izadpanah, E.; Riahi, S.; Abbasi-Radmoghaddam, Z.; Gharaghani, S.; Mohammadi-Khanaposhtanai, M. A simple and robust model to predict the inhibitory activity of alpha-glucosidase inhibitors through combined QSAR modeling and molecular docking techniques. Mol. Divers. 2021, 25, 1811–1825. [Google Scholar] [CrossRef]
- Sakkiah, S.; Thangapandian, S.; Lee, K.W. Pharmacophore modeling, molecular docking, and molecular dynamics simulation approaches for identifying new lead compounds for inhibiting aldose reductase 2. J. Mol. Model. 2012, 18, 3267–3282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Sabiu, S.; O’Neill, F.H.; Ashafa, A.O.T. Kinetics of α-amylase and α-glucosidase inhibitory potential of Zea mays Linnaeus (Poaceae), Stigma maydis aqueous extract: An in vitro assessment. J. Ethnopharmacol. 2016, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang, M.; Jiang, W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum Maxim.(Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical Constituents and Biological Activities of the Genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Zhu, R.; Zhong, K.; Zeng, W.; He, X.; Gu, X.; Zhao, Z.; Gao, H. Essential oil composition and antibacterial activity of Zanthoxylum bungeanum. Afr. J. Microbiol. Res. 2011, 5, 4631–4637. [Google Scholar]
- Chen, I.-S.; Chen, T.-L.; Chang, Y.-L.; Teng, C.-M.; Lin, W.-Y. Chemical Constituents and Biological Activities of the Fruit of Zanthoxylum integrifoliolum. J. Nat. Prod. 1999, 62, 833–837. [Google Scholar] [CrossRef]
- Yang, X. Aroma Constituents and Alkylamides of Red and Green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of Anthocyanidins and Anthocyanins for Targeting alpha-Glucosidase in Diabetes Mellitus. Prev. Nutr. Food Sci. 2020, 25, 263–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).