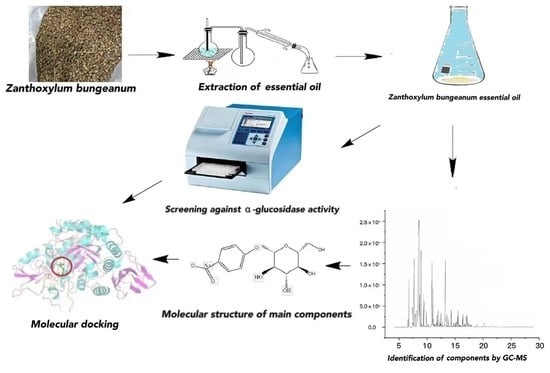
Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Materials
2.2. Chemicals and Solutions
2.3. Extraction of Zanthoxylum bungeanum Essential Oil
2.4. Screening of the Plant Essential Oils against α-Glucosidase Activity
| Group | PBS | Sample | Enzyme | PNPG | Na2CO3 |
|---|---|---|---|---|---|
| Blank | 80 | 0 | 10 | 10 | 100 |
| Background | 80 | 10 | 0 | 10 | 100 |
| Acarbose | 70 | 10 | 10 | 10 | 100 |
| Sample | 70 | 10 | 10 | 10 | 100 |
2.5. Component Identification of Zanthoxylum bungeanum Essential Oil
2.6. Molecular Docking between the Main Components of Zanthoxylum bungeanum Essential Oil and α-Glucosidase
3. Results and Discussion
3.1. Extraction Yield of Zanthoxylum bungeanum Essential Oil
3.2. Screening for Anti-α-Glucosidase Activity of Plant Essential Oil
3.3. Identification of Components of Zanthoxylum bungeanum Essential Oil
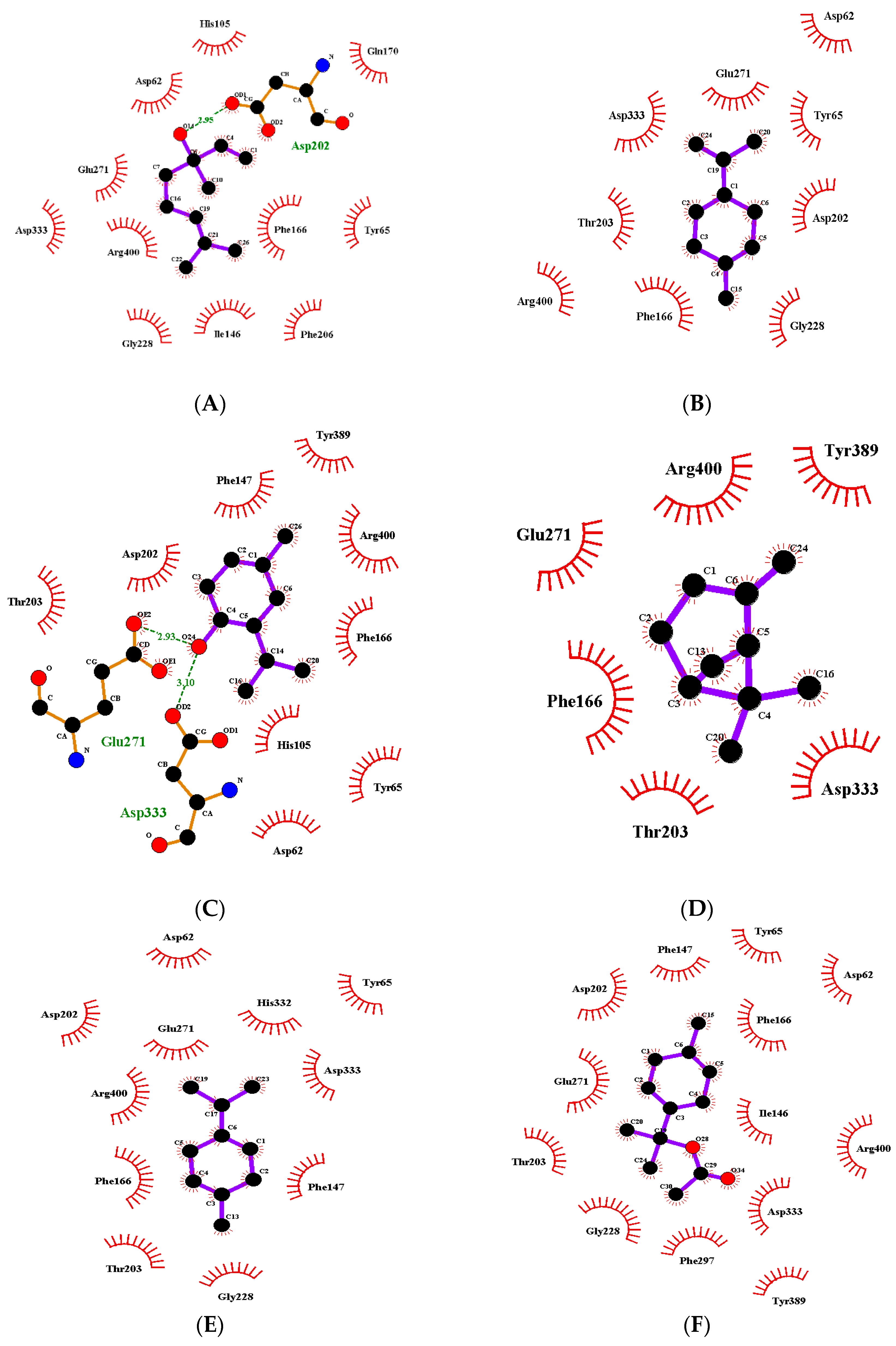
3.4. Results of Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviation
| Biological, Chemical, and Microbiological | |
| DM | Diabetes mellitus |
| DES | Deep eutectic solvents |
| PNPG | 4-nitrophenyl-α-D-glucopyranoside |
| DMSO | Dimethyl sulfoxide |
| PEG2000 | Polyethylene glycol |
| PBS | Phosphate buffer solution |
| Glu | Glutamic acid |
| Asp | Aspartic acid |
| Arg | Arginine |
| Instrumental techniques and organization | |
| HPLC-ESI-MS | High-performance liquid chromatography–electrospray ionization–mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| CAS | American Chemical Society |
References
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Y.; Wen, Q.; Feng, Y.; Tan, T. Comprehensive chemical and metabolic profiling of anti-hyperglycemic active fraction from Clerodendranthi Spicati Herba. J. Sep. Sci. 2021, 44, 1805–1814. [Google Scholar] [CrossRef] [PubMed]
- Alssema, M.; Ruijgrok, C.; Blaak, E.E.; Egli, L.; Dussort, P.; Vinoy, S.; Dekker, J.M.; Denise Robertson, M. Effects of alpha-glucosidase-inhibiting drugs on acute postprandial glucose and insulin responses: A systematic review and meta-analysis. Nutr. Diabetes 2021, 11, 11. [Google Scholar] [CrossRef]
- Khwaja, N.U.D.; Arunagirinathan, G. Efficacy and Cardiovascular Safety of Alpha Glucosidase Inhibitors. Curr. Drug Saf. 2021, 16, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Jinli, Q.; Yan, Z.; Pei, L.; Liangchuan, G.; Junwei, H. Polyphenol Composition and Antioxidant and Hypoglycemic Activities in Wild Blue Honeysuckle Fruit. Food Sci. 2021, 42, 47–55. [Google Scholar] [CrossRef]
- Xiong, S.L.; Yue, L.M.; Lim, G.T.; Yang, J.M.; Lee, J.; Park, Y.D. Inhibitory effect of raspberry ketone on α-glucosidase: Docking simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2018, 113, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.H.; Choi, S.W. Isolation, Identification, and Quantification of Tyrosinase and α-Glucosidase Inhibitors from UVC-Irradiated Mulberry (Morus alba L.) Leaves. Prev. Nutr. Food Sci. 2019, 24, 84–94. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, C.; Zhang, B.; Huang, Q. The inhibitory effects of flavonoids on α-amylase and α-glucosidase. Crit. Rev. Food Sci. Nutr. 2020, 60, 695–708. [Google Scholar] [CrossRef]
- Ajebli, M.; Khan, H.; Eddouks, M. Natural Alkaloids and Diabetes Mellitus: A Review. Endocr. Metab. Immune Disord.-Drug Targets 2021, 21, 111–130. [Google Scholar] [CrossRef]
- Lv, Q.Q.; Cao, J.J.; Liu, R.; Chen, H.Q. Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem. 2021, 341, 128218. [Google Scholar] [CrossRef]
- Kyei-Barffour, I.; Kwarkoh, R.K.B.; Arthur, O.D.; Akwetey, S.A.; Acheampong, D.O.; Aboagye, B.; Brah, A.S.; Amponsah, I.K.; Adokoh, C.K. Alkaloidal extract from Zanthoxylum zanthoxyloides stimulates insulin secretion in normoglycemic and nicotinamide/streptozotocin-induced diabetic rats. Heliyon 2021, 7, e07452. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.B.; Lei, Y.T.; Zhang, Q.; Li, Y.C.; Chen, W.J.; Liu, D.Y. Encapsulation of Zanthoxylum bungeanum essential oil to enhance flavor stability and inhibit lipid oxidation of Chinese-style sausage. J. Sci. Food Agric. 2022, 102, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.; Yang, Z.; Shi, J.; Zhao, L.; Battino, M.; Xiao, J.; Deng, X.; Wu, Y.; Wang, C.; et al. Interactions between Phenols and Alkylamides of Sichuan Pepper (Zanthoxylum Genus) in α-Glucosidase Inhibition: A Structural Mechanism Analysis. J. Agric. Food Chem. 2021, 69, 5583–5598. [Google Scholar] [CrossRef]
- Khalid, K.A.; Cai, W.M.; Ahmed, A.M.A. Effect of Harvesting Treatments and Distillation Methods on the Essential Oil of Lemon Balm and Apple Geranium Plants. J. Essent. Oil Bear. Plants 2009, 12, 120–130. [Google Scholar] [CrossRef]
- Huang, C.X.; Chen, X.N.; Wei, C.; Wang, H.W.; Gao, H. Deep Eutectic Solvents as Active Pharmaceutical Ingredient Delivery Systems in the Treatment of Metabolic Related Diseases. Front. Pharmacol. 2021, 12, 794939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Wang, P.; Zheng, W.; Yu, G.W.; Li, Z.G.; She, Y.B.; Lee, M. Three-stage microwave extraction of cumin (Cuminum cyminum L.) Seed essential oil with natural deep eutectic solvents. Ind. Crops Prod. 2019, 140, 111660. [Google Scholar] [CrossRef]
- Tang, B.; Zhang, H.; Row, K.H. Application of deep eutectic solvents in the extraction and separation of target compounds from various samples. J. Sep. Sci. 2015, 38, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Della Corte, D. Using molecular docking and molecular dynamics to investigate protein-ligand interactions. Mod. Phys. Lett. B 2021, 35, 2130002. [Google Scholar] [CrossRef]
- Kavitha, R.; Karunagaran, S.; Chandrabose, S.S.; Lee, K.W.; Meganathan, C. Pharmacophore modeling, virtual screening, molecular docking studies and density functional theory approaches to identify novel ketohexokinase (KHK) inhibitors. Biosystems 2015, 138, 39–52. [Google Scholar] [CrossRef]
- Izadpanah, E.; Riahi, S.; Abbasi-Radmoghaddam, Z.; Gharaghani, S.; Mohammadi-Khanaposhtanai, M. A simple and robust model to predict the inhibitory activity of alpha-glucosidase inhibitors through combined QSAR modeling and molecular docking techniques. Mol. Divers. 2021, 25, 1811–1825. [Google Scholar] [CrossRef]
- Sakkiah, S.; Thangapandian, S.; Lee, K.W. Pharmacophore modeling, molecular docking, and molecular dynamics simulation approaches for identifying new lead compounds for inhibiting aldose reductase 2. J. Mol. Model. 2012, 18, 3267–3282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, S.; Zhao, Y.; Wang, J.; Yu, Z. Insights into the Hydrogen Bond Interactions in Deep Eutectic Solvents Composed of Choline Chloride and Polyols. ACS Sustain. Chem. Eng. 2019, 7, 7760–7767. [Google Scholar] [CrossRef]
- Sabiu, S.; O’Neill, F.H.; Ashafa, A.O.T. Kinetics of α-amylase and α-glucosidase inhibitory potential of Zea mays Linnaeus (Poaceae), Stigma maydis aqueous extract: An in vitro assessment. J. Ethnopharmacol. 2016, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, Y.; Zhou, L.; Shi, X.; Guo, Z.; Wang, M.; Jiang, W. Chemical composition and antifungal activity of the fruit oil of Zanthoxylum bungeanum Maxim.(Rutaceae) from China. J. Essent. Oil Res. 2009, 21, 174–178. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Bhandari, A.K.; Singh, P.; Sundriyal, R.C. Chemical Constituents and Biological Activities of the Genus Zanthoxylum: A review. Afr. J. Pure Appl. Chem. 2011, 5, 412–416. [Google Scholar]
- Zhu, R.; Zhong, K.; Zeng, W.; He, X.; Gu, X.; Zhao, Z.; Gao, H. Essential oil composition and antibacterial activity of Zanthoxylum bungeanum. Afr. J. Microbiol. Res. 2011, 5, 4631–4637. [Google Scholar]
- Chen, I.-S.; Chen, T.-L.; Chang, Y.-L.; Teng, C.-M.; Lin, W.-Y. Chemical Constituents and Biological Activities of the Fruit of Zanthoxylum integrifoliolum. J. Nat. Prod. 1999, 62, 833–837. [Google Scholar] [CrossRef]
- Yang, X. Aroma Constituents and Alkylamides of Red and Green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Promyos, N.; Temviriyanukul, P.; Suttisansanee, U. Investigation of Anthocyanidins and Anthocyanins for Targeting alpha-Glucosidase in Diabetes Mellitus. Prev. Nutr. Food Sci. 2020, 25, 263–271. [Google Scholar] [CrossRef]



| Essential Oil | Inhibition Rate% | Essential Oil | Inhibition Rate% |
|---|---|---|---|
| Litsea cubeba | 4.66 ± 3.27 | Artemisia argyi | 6.96 ± 2.75 |
| Magnolia liliflora | 9.86 ± 4.82 | Nepeta cataria | 3.92 ± 1.05 |
| Olibanum | 9.00 ± 4.01 | Ligusticum chuanxiong | 10.49 ± 6.01 |
| Houttuynia cordata | 9.58 ± 6.47 | Forsythia suspensa | 2.51 ± 0.58 |
| Centella asiatica | 6.33 ± 2.74 | Acorus tatarinowii | 3.21 ± 0.76 |
| Panax quinquefolius | 9.98 ± 1.15 | Syringa oblata | 4.41 ± 1.56 |
| Schisandra chinensis | 6.99 ± 2.35 | Cuminum cyminum | 10.79 ± 0.53 |
| Zanthoxylum bungeanum | 57.10 ± 0.30 | Citrus reticulata | 2.97 ± 0.39 |
| Angelica sinensis | 5.56 ± 1.27 | Atractylodes lancea | −0.07 ± 0.70 |
| Pogostemon cablin | 12.33 ± 3.25 | Panax ginseng | 9.37 ± 2.25 |
| Blank | 0.90 ± 0.71 | Acarbose 40 μg/mg | 88.97 ± 2.58 |
| PEG2000 | 6.81 ± 1.99 | Acarbose 80 μg/mg | 88.80 ± 0.41 |
| No. | Component | Relative Content (%) | CAS | Molecular Formula | Retention Time (Min) |
|---|---|---|---|---|---|
| 1 | (-)-terpinen-4-ol | 13.13 | 20126-76-5 | C10H18O | 10.893 |
| 2 | (-)-β-pinene | 11.17 | 18172-67-3 | C10H16 | 7.708 |
| 3 | γ-terpinene | 9.45 | 99-85-4 | C10H16 | 8.927 |
| 4 | terpinyl acetate | 9.36 | 80-26-2 | C12H20O2 | 13.276 |
| 5 | α-terpineol | 5.40 | 98-55-5 | C10H18O | 11.063 |
| Component | Binding Energies (kcal/mol) | Binding Energies (KJ/mol) |
|---|---|---|
| Linalool | −108.63 | −25.95 |
| Limonene | −106.86 | −25.53 |
| (-)-terpinen-4-ol | −120.89 | −28.88 |
| (-)-β-pinene | −106.91 | −25.54 |
| γ-terpinene | −110.38 | −26.37 |
| terpineol acetate | −119.13 | −28.46 |
| α-terpineol | −115.66 | −27.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Hu, W.; Cheng, W.; Zhang, S.; Zou, R. Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking. Appl. Sci. 2023, 13, 2627. https://doi.org/10.3390/app13042627
Liang S, Hu W, Cheng W, Zhang S, Zou R. Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking. Applied Sciences. 2023; 13(4):2627. https://doi.org/10.3390/app13042627
Chicago/Turabian StyleLiang, Shaoqi, Wei Hu, Wensi Cheng, Sheng Zhang, and Ruisi Zou. 2023. "Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking" Applied Sciences 13, no. 4: 2627. https://doi.org/10.3390/app13042627
APA StyleLiang, S., Hu, W., Cheng, W., Zhang, S., & Zou, R. (2023). Zanthoxylum bungeanum Essential Oil: Extraction and Component Analysis for α-Glucosidase Inhibitory Activity and the Underlying Mechanism Based on Molecular Docking. Applied Sciences, 13(4), 2627. https://doi.org/10.3390/app13042627