Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review
Abstract
1. Introduction
2. Qualitative Methods
2.1. Estimation of the Carbonation Degree Based on pH Indicator
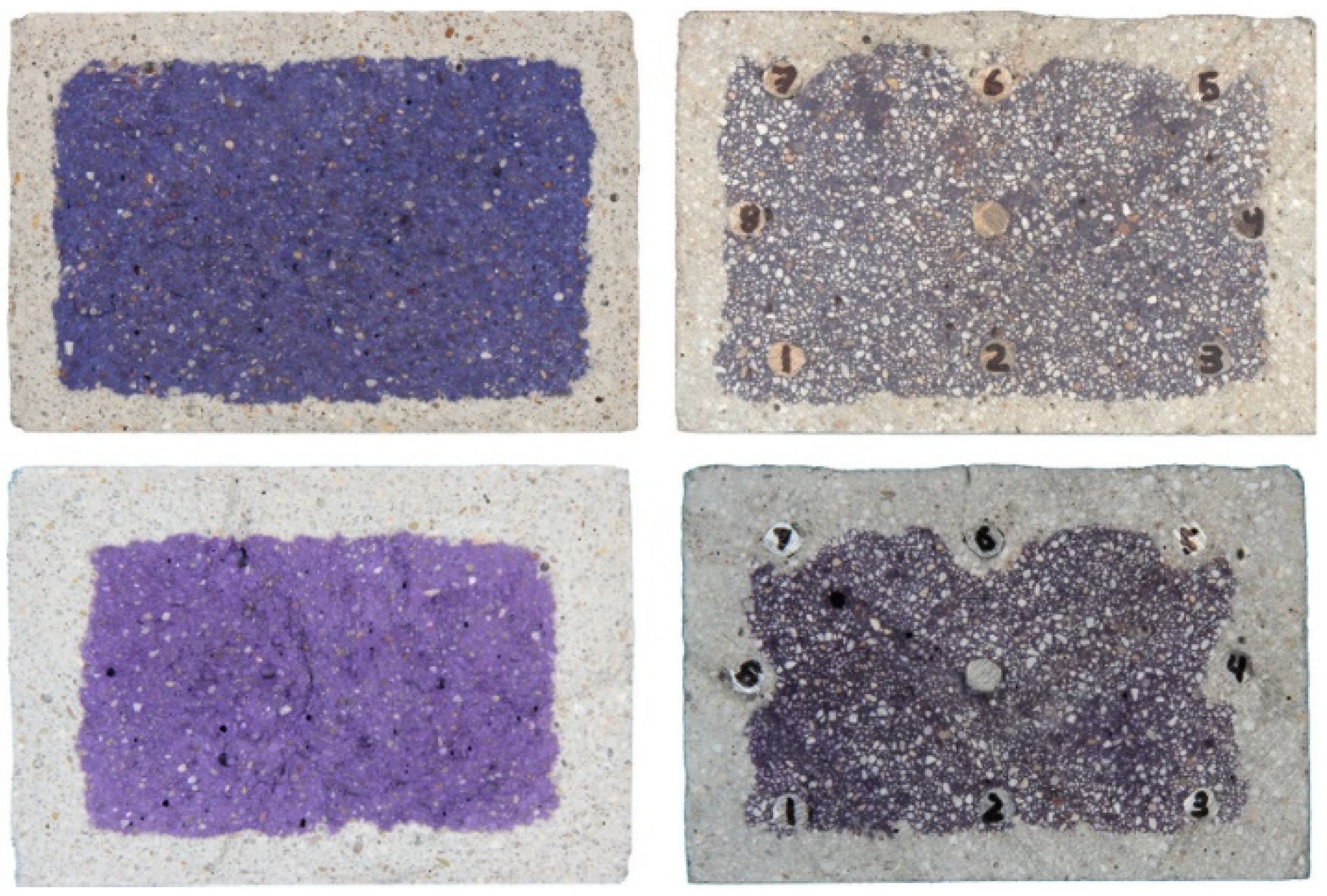
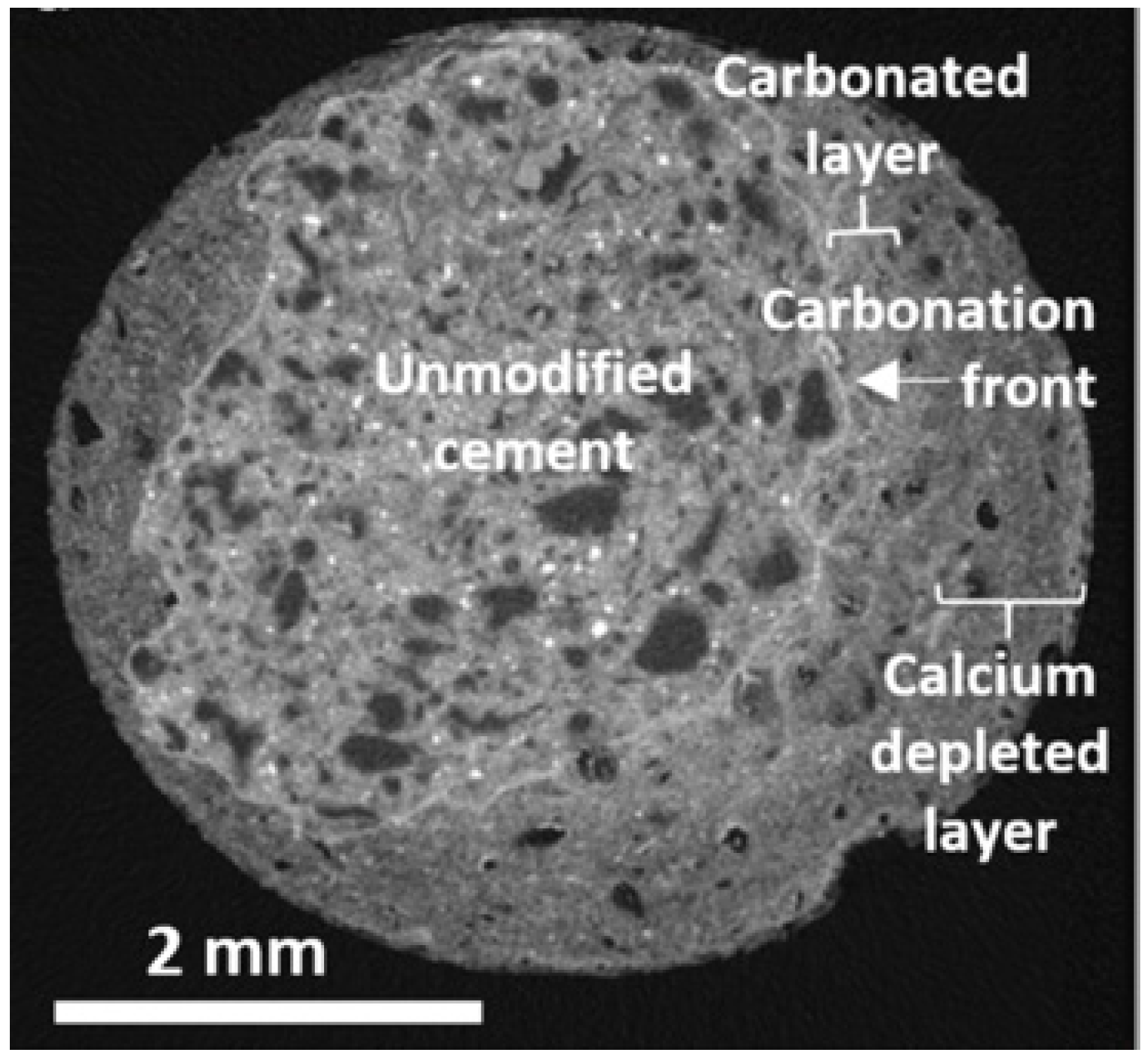
2.2. Image Analysis
2.3. Phase Analysis
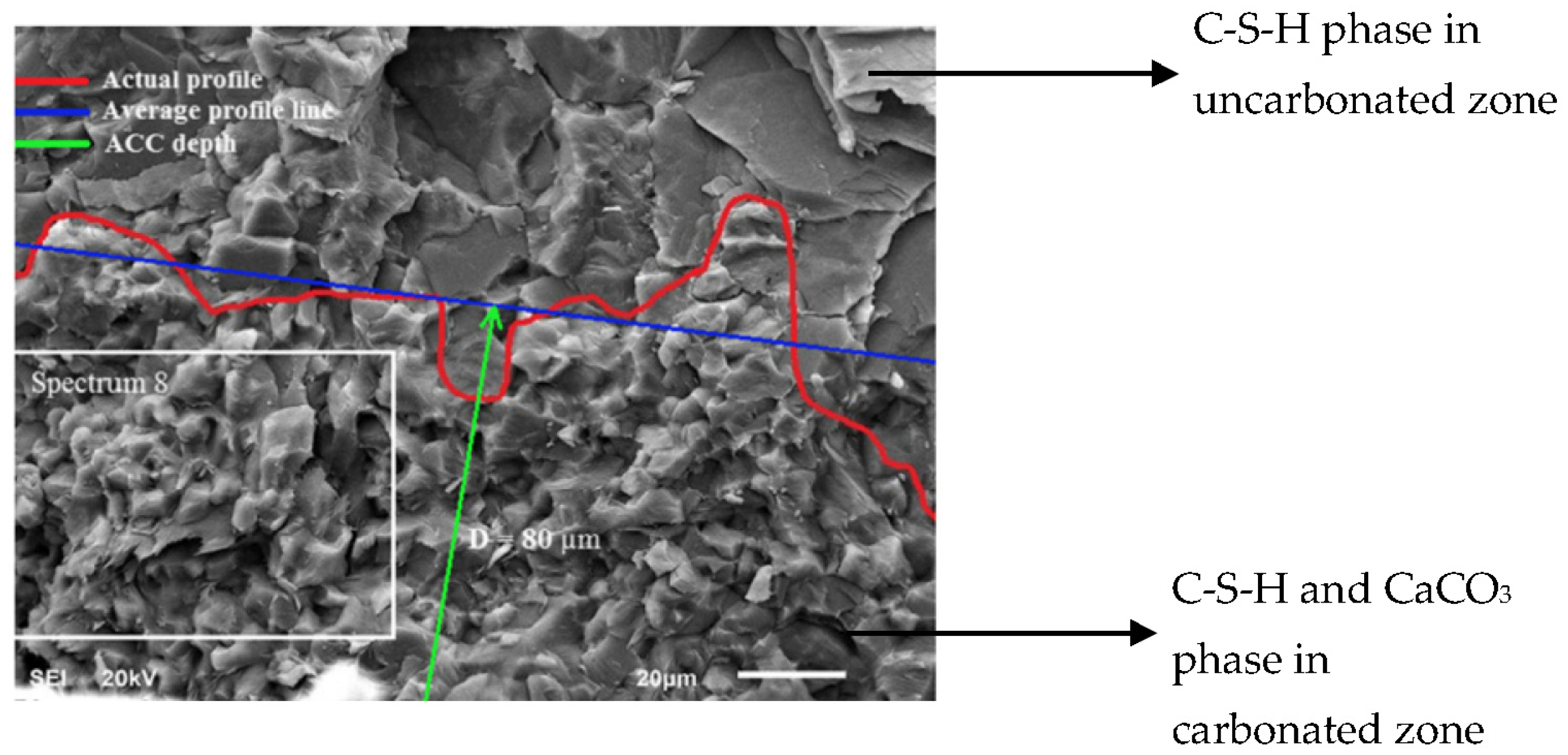
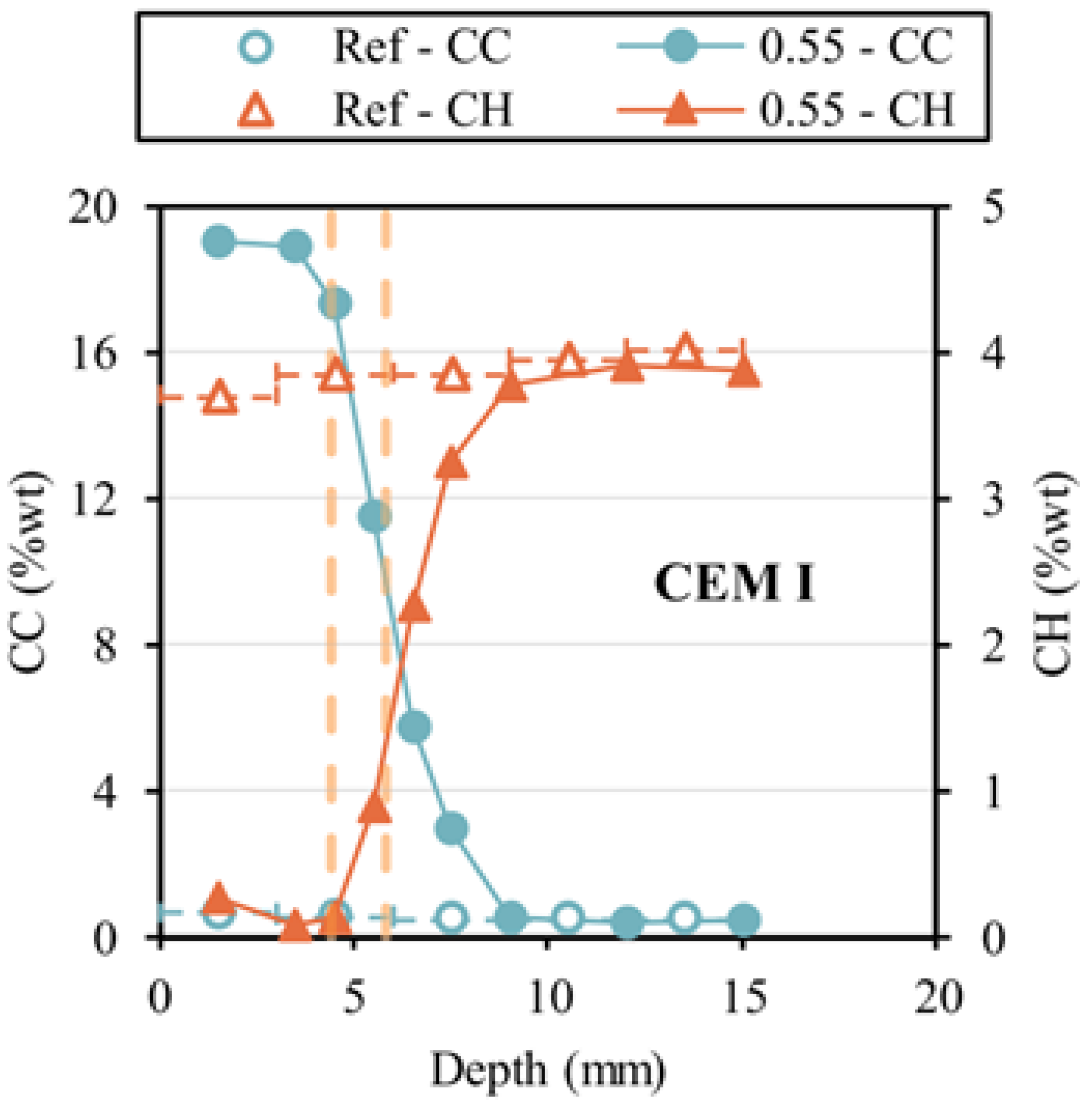
2.3.1. Thermogravimetric Analysis (TGA)
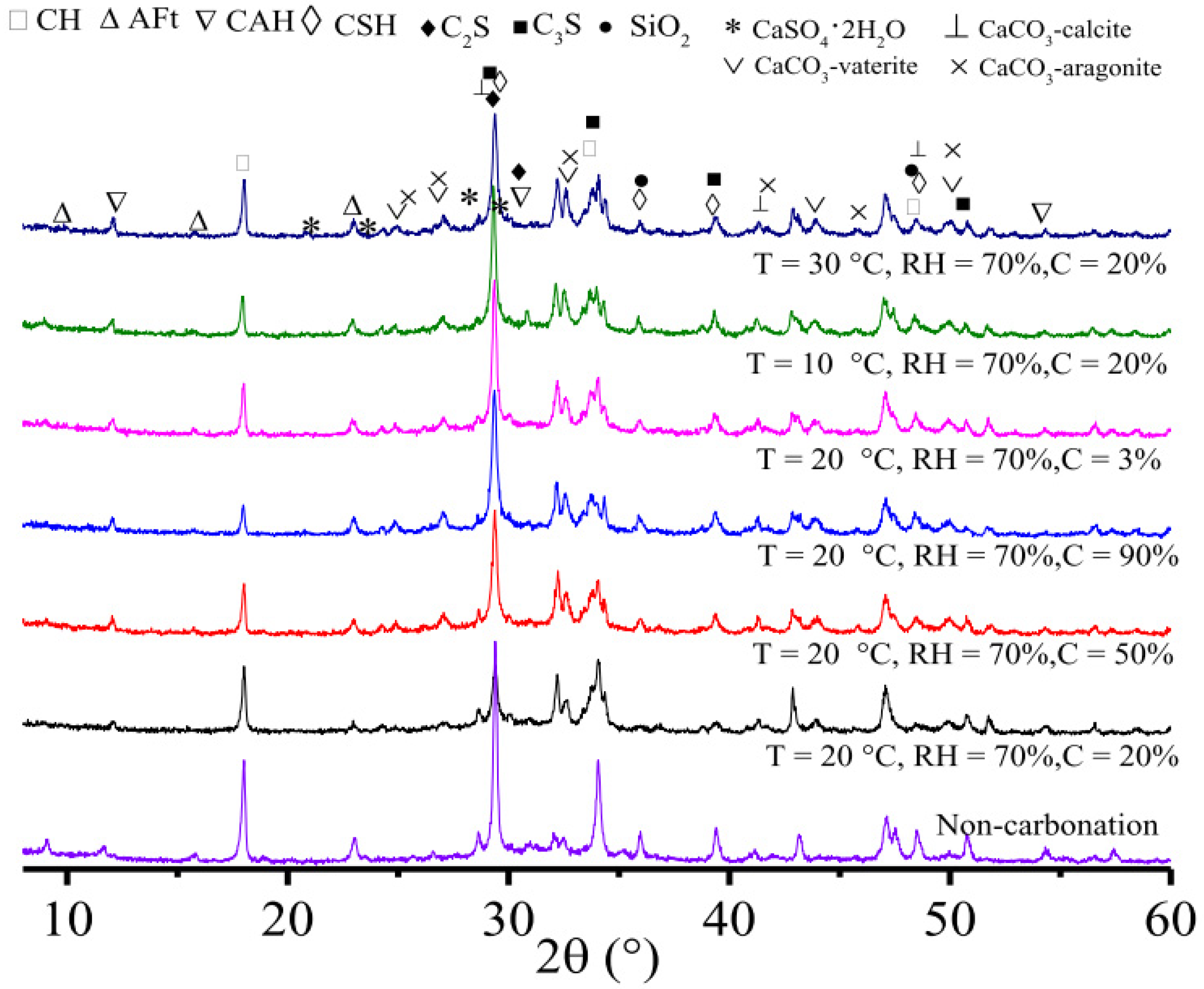
2.3.2. X-ray Diffraction (XRD)
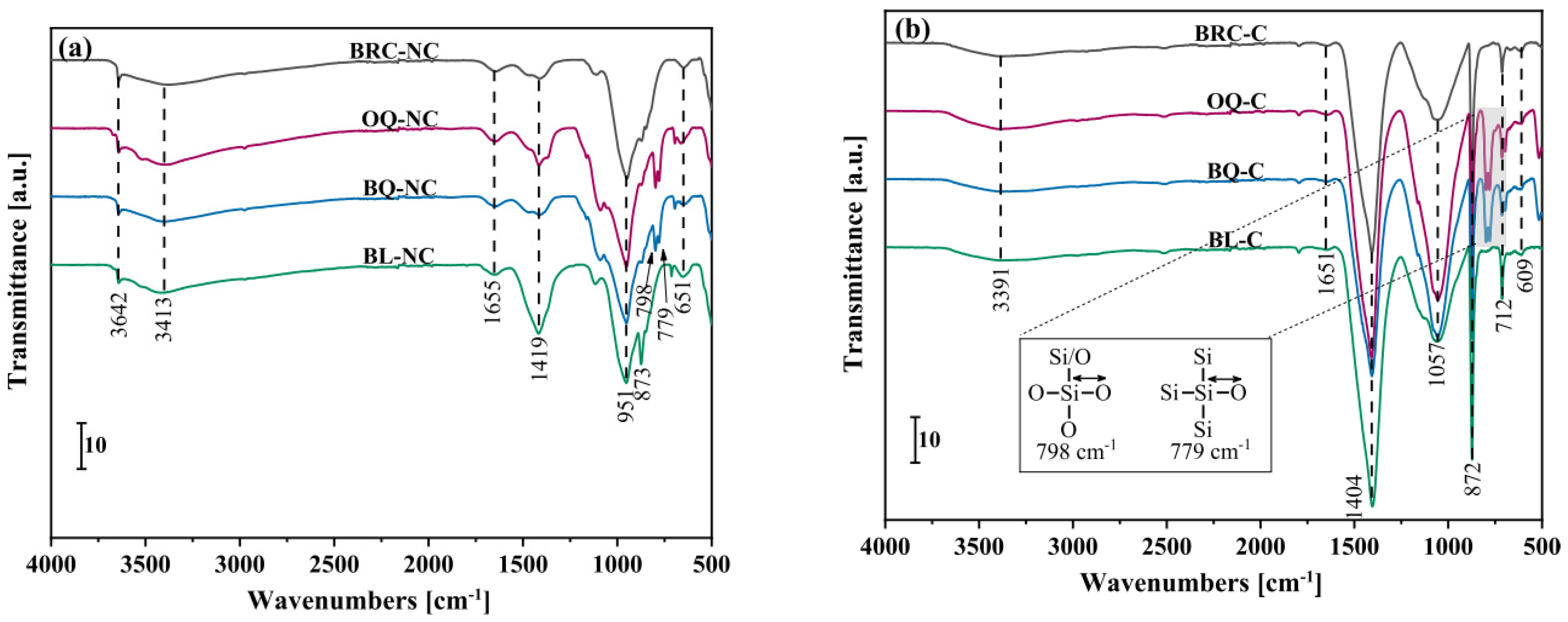
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
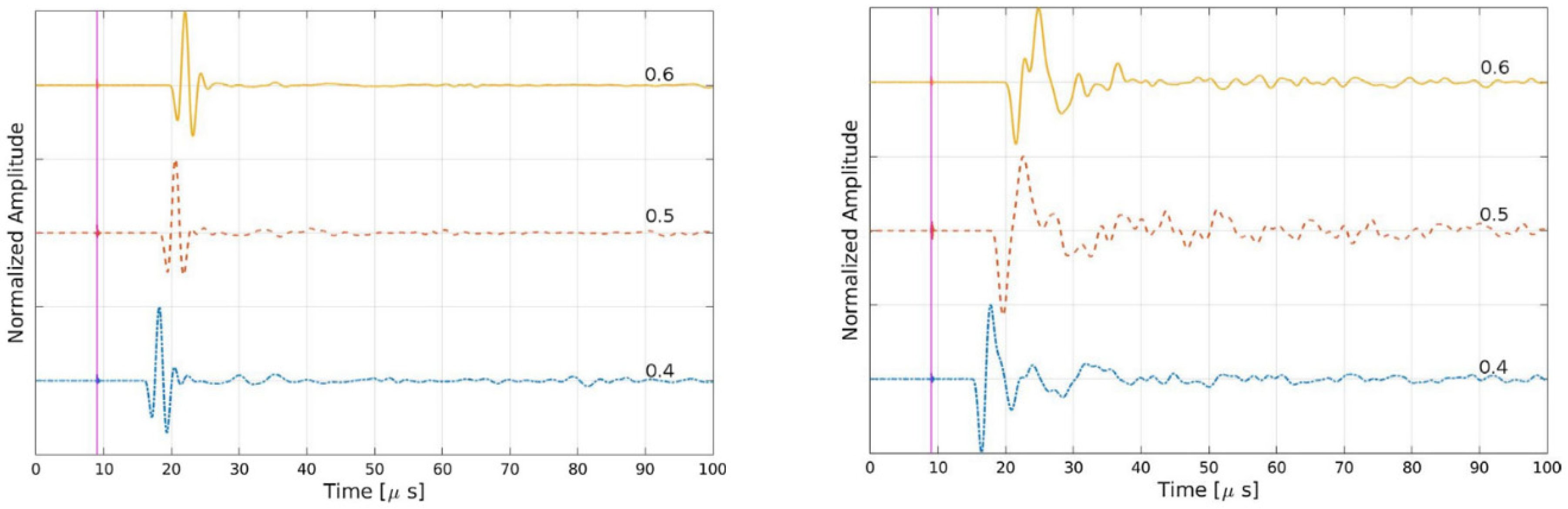
2.3.4. Ultrasonic Phase Velocity Technique
3. Quantitative Methods
3.1. Phenolphthalein Indicator
3.2. CO2 Uptake
4. Conclusions
- -
- Using suitable indicators is highly recommended as a straightforward and simple approach. Even if quantitative methods have been applied, pH indicators were also used to detect the carbonated zones. All the pH measurement methods gave similar findings; thus, the authors suggest the easiest and cheapest pH measurement method: phenolphthalein. However, its applicability in evaluating CO2 diffusion capacity is problematic in the case of partial carbonation owing to its potentially limited detection of phase change.
- -
- pH indicator could be used widely, but the combination with other techniques needs to be performed. Non-destructive methods by means of embedded fiber optic sensors could offer an adequate and accurate estimation of long-term pH monitoring of reinforced concrete structures in different conditions.
- -
- If the problem of high cost could be overlooked, image analysis presented a significant advantage in exploring crystal habit, morphology and size of the core of the sample, with high accuracy in the qualitative carbonation measurement.
- -
- Phase analysis seems to be an accurate method when phase measurements are performed by using XRD, TGA, FTIR and ultrasonic phase velocity technique. The margin of error of these measurements has very limited impact on the result and does not need to be familiar for carbonation determination (less than ±5%).
- -
- A new method, the fiber optic system, has been developed recently to control the change in internal chemistry of a concrete structure. All phase analysis techniques such as XRD, TGA, FTIR and gamma-0densimetry are carried out in the laboratory with very small-scale specimens and require little destruction to the structure in order to obtain samplings. However, fiber optic systems overcome most these drawbacks and obtain non-destructive, continuous and real-time data in actual time conditions for concrete structures. This method could be considered as a promising application in the field of controlling deterioration in concrete structures.
- -
- For the determination of the uptake of CO2, it is sufficient to measure weight loss at a temperature between 500 and 1000 °C with regard to TGA method. The CaO content should be considered because it relates to the carbonation of hydrates. The use of CO2 uptake content to determine the carbonation degree of concrete requires that the carbonation zones are defined in advance.
- -
- The gamma-densimetry method should be considered for use at laboratory scale due to the high accuracy in measurement of the CO2 penetrated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Possan, E.; Thomaz, W.A.; Aleandri, G.A.; Felix, E.F.; dos Santos, A.C.P. CO2 uptake potential due to concrete carbonation: A case study. Case Stud. Constr. Mater. 2017, 6, 147–161. [Google Scholar] [CrossRef]
- Li, L.; Xuan, D.; Sojobi, A.O.; Liu, S.; Sun, C. Efficiencies of carbonation and nano silica treatment methods in enhancing the performance of recycled aggregate concrete. Constr. Build. Mater. 2021, 308, 125080. [Google Scholar] [CrossRef]
- Bui, H.; Boutouil, M.; Levacher, D.; Sebaibi, N. Evaluation of the influence of accelerated carbonation on the microstructure and mechanical characteristics of coconut fibre-reinforced cementitious matrix. J. Build. Eng. 2021, 39, 102269. [Google Scholar] [CrossRef]
- Gattuso, J.P.; Magnan, A.; Billé, R.; Cheung, W.W.L.; Howes, E.L.; Joos, F.; Allemand, D.; Bopp, L.; Cooley, S.R.; Eakin, C.M.; et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science 2015, 349, 6243. [Google Scholar] [CrossRef]
- Yoon, I.S.; Çopuroǧlu, O.; Park, K.B. Effect of global climatic change on carbonation progress of concrete. Atmos. Environ. 2007, 41, 7274–7285. [Google Scholar] [CrossRef]
- De Weerdt, K.; Ben Haha, M.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation curing versus steam curing for precast concrete production. J. Mater. Civ. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Tang, J.; Wu, J.; Zou, Z.; Yue, A.; Mueller, A. Influence of axial loading and carbonation age on the carbonation resistance of recycled aggregate concrete. Constr. Build. Mater. 2018, 173, 707–717. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of steel in concrete, Swedish Cement and Concrete. Stockholm ISB 1977, 655–661. [Google Scholar] [CrossRef]
- Shi, X.; Yao, Y.; Wang, L.; Zhang, C.; Ahmad, I. A modified numerical model for predicting carbonation depth of concrete with stress damage. Constr. Build. Mater. 2021, 304, 124389. [Google Scholar] [CrossRef]
- Zhang, K.; Xiao, J. Prediction model of carbonation depth for recycled aggregate concrete. Cem. Concr. Compos. 2018, 88, 86–99. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Ghantous, R.M.; Poyet, S.; L’Hostis, V.; Tran, N.C.; François, R. Effect of crack openings on carbonation-induced corrosion. Cem. Concr. Res. 2017, 95, 257–269. [Google Scholar] [CrossRef]
- Belda Revert, A.; De Weerdt, K.; Hornbostel, K.; Geiker, M.R. Carbonation-induced corrosion: Investigation of the corrosion onset. Constr. Build. Mater. 2018, 162, 847–856. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Shen, Q.; Pan, G.; Bao, B. A method for calculating the carbonation degree of calcium–silicate–hydrate. Adv. Cem. Res. 2018, 30, 427–436. [Google Scholar] [CrossRef]
- Shah, V.; Bishnoi, S. Carbonation resistance of cements containing supplementary cementitious materials and its relation to various parameters of concrete. Constr. Build. Mater. 2018, 178, 219–232. [Google Scholar] [CrossRef]
- Seo, J.; Kim, S.; Park, S.; Bae, S.J.; Lee, H.K. Microstructural evolution and carbonation behavior of lime-slag binary binders. Cem. Concr. Compos. 2021, 119, 104000. [Google Scholar] [CrossRef]
- Qiu, Q. A state-of-the-art review on the carbonation process in cementitious materials: Fundamentals and characterization techniques. Constr. Build. Mater. 2020, 247, 118503. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Carbonation of calcium sulfoaluminate mortars. Cem. Concr. Compos. 2017, 80, 123–134. [Google Scholar] [CrossRef]
- RILEM Committee CPC-18. Measurement of hardened concrete carbonation depth. Mater. Struct. 1988, 18, 453–455. [Google Scholar]
- Lee, H.J.; Kim, D.G.; Lee, J.H.; Cho, M.S. A study for carbonation degree on concrete using a phenolphthalein indicator and Fourier-Transform Infrared Spectroscopy. Int. J. Civ. Environ. Struct. Constr. Archit. Eng. 2012, 6, 95–101. [Google Scholar] [CrossRef]
- Li, Z.; Li, S. Effects of wetting and drying on alkalinity and strength of fly ash/slag-activated materials. Constr. Build. Mater. 2020, 254, 119069. [Google Scholar] [CrossRef]
- Chang, C.F.; Chen, J.W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- De Weerdt, K.; Plusquellec, G.; Revert, A.B.; Geiker, M.R.; Lothenbach, B. Effect of carbonation on the pore solution of mortar. Cem. Concr. Res. 2019, 118, 38–56. [Google Scholar] [CrossRef]
- Steiner, S.; Lothenbach, B.; Proske, T.; Borgschulte, A.; Winnefeld, F. Effect of relative humidity on the carbonation rate of portlandite, calcium silicate hydrates and ettringite. Cem. Concr. Res. 2020, 135, 106116. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A.; Candeias, A. Effects of natural and accelerated carbonation on the properties of lime-based materials. J. CO2 Util. 2021, 49, 101552. [Google Scholar] [CrossRef]
- Wang, J.; Su, H.; Du, J. Influence of coupled effects between flexural tensile stress and carbonation time on the carbonation depth of concrete. Constr. Build. Mater. 2018, 190, 439–451. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Mejía de Gutiérrez, R.; van Deventer, J.S.J. Accelerated carbonation testing of alkali-activated slag/metakaolin blended concretes: Effect of exposure conditions. Mater. Struct. Constr. 2014, 48, 653–669. [Google Scholar] [CrossRef]
- Seo, J.; Kim, S.; Park, S.; Yoon, H.N.; Lee, H.K. Carbonation of calcium sulfoaluminate cement blended with blast furnace slag. Cem. Concr. Compos. 2021, 118, 103918. [Google Scholar] [CrossRef]
- Bao, H.; Yu, M.; Chi, Y.; Liu, Y.; Ye, J. Performance evaluation of steel-polypropylene hybrid fiber reinforced concrete under supercritical carbonation. J. Build. Eng. 2021, 43, 103159. [Google Scholar] [CrossRef]
- Bao, H.; Yu, M.; Liu, Y.; Ye, J. Experimental and statistical study on the irregularity of carbonation depth of cement mortar under supercritical condition. Constr. Build. Mater. 2018, 174, 47–59. [Google Scholar] [CrossRef]
- Martín-Del-Río, J.J.; Alejandre, F.J.; Márquez, G.; Blasco, F.J. An argument for using alizarine yellow R and indigo carmine to determine in situ the degree of alkalinity in reinforced concrete. Constr. Build. Mater. 2013, 40, 426–429. [Google Scholar] [CrossRef]
- Rimmelé, G.; Barlet-Gouédard, V.; Porcherie, O.; Goffé, B.; Brunet, F. Heterogeneous porosity distribution in Portland cement exposed to CO2-rich fluids. Cem. Concr. Res. 2008, 38, 1038–1048. [Google Scholar] [CrossRef]
- Gawel, K.; Wenner, S.; Edvardsen, L. Effect of carbonation on bulk resistivity of cement/carbon nanofiber composites. Constr. Build. Mater. 2021, 305, 124794. [Google Scholar] [CrossRef]
- Herrera, R.; Kinrade, S.D.; Catalan, L.J.J. A comparison of methods for determining carbonation depth in fly ash-blended cement mortars. ACI Mater. J. 2015, 112, 287–294. [Google Scholar] [CrossRef]
- Bui, N.K.; Satomi, T.; Takahashi, H. Influence of industrial by-products and waste paper sludge ash on properties of recycled aggregate concrete. J. Clean. Prod. 2019, 214, 403–418. [Google Scholar] [CrossRef]
- Li, L.; Nam, J.; Hartt, W.H. Ex situ leaching measurement of concrete alkalinity. Cem. Concr. Res. 2005, 35, 277–283. [Google Scholar] [CrossRef]
- Plusquellec, G.; Geiker, M.R.; Lindgård, J.; Duchesne, J.; Fournier, B.; De Weerdt, K. Determination of the pH and the free alkali metal content in the pore solution of concrete: Review and experimental comparison. Cem. Concr. Res. 2017, 96, 13–26. [Google Scholar] [CrossRef]
- Safavi, A.; Bagheri, M. Novel optical pH sensor for high and low pH values. Sens. Actuators B Chem. 2003, 90, 143–150. [Google Scholar] [CrossRef]
- Basheer, M.P.A.; Grattan, K.T.V.; Sun, T.; Long, A.E.; McPolin, D.; Xie, W. Fiber optic chemical sensor systems for monitoring pH changes in concrete. In Advanced Environmental, Chemical, and Biological Sensing Technologies II; SPIE: Bellingham, WA, USA; Volume 5586. [CrossRef]
- Fan, L.; Bao, Y. Review of fiber optic sensors for corrosion monitoring in reinforced concrete. Cem. Concr. Compos. 2021, 120, 104029. [Google Scholar] [CrossRef]
- McPolin, D.O.; Basheer, P.A.M.; Grattan, K.T.V.; Long, A.E.; Sun, T.; Xie, W. Preliminary development and evaluation of fibre optic chemical sensor. J. Mater. Civ. Eng. 2011, 23, 1200–1210. [Google Scholar] [CrossRef]
- Blumentritt, M.; Melhorn, K.; Flachsbarth, J.; Kroener, M.; Kowalsky, W.; Johannes, H.H. A novel fabrication method of fiber-optical planar transmission sensors for monitoring pH in concrete structures. Sens. Actuators B Chem. 2008, 131, 504–508. [Google Scholar] [CrossRef]
- Tariq, A.; Baydoun, J.; Remy, C.; Ghasemi, R.; Lefevre, J.P.; Mongin, C.; Dauzères, A.; Leray, I. Fluorescent molecular probe based optical fiber sensor dedicated to pH measurement of concrete. Sens. Actuators B Chem. 2021, 327, 128906. [Google Scholar] [CrossRef]
- Rémy, C.; Allain, C.; Leray, I. Synthesis and photophysical properties of extended π conjugated naphthalimides. Photochem. Photobiol. Sci. 2017, 16, 539–546. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Venugopala, T.; Chen, S.; Sun, T.; Grattan, K.T.V.; Taylor, S.E.; Basheer, P.A.M.; Long, A.E. Fluorescence based fibre optic pH sensor for the pH 10-13 range suitable for corrosion monitoring in concrete structures. Sens. Actuators B Chem. 2014, 191, 498–507. [Google Scholar] [CrossRef]
- Behnood, A.; Van Tittelboom, K.; De Belie, N. Methods for measuring pH in concrete: A review. Constr. Build. Mater. 2016, 105, 176–188. [Google Scholar] [CrossRef]
- Haněa, K.; Koronthályová, O.; Matiašovský, P. The carbonation of autoclaved aerated concrete. Cem. Concr. Res. 1997, 27, 589–599. [Google Scholar] [CrossRef]
- Bao, H.; Yu, M.; Xu, L.; Saafi, M.; Ye, J. Experimental study and multi-physics modelling of concrete under supercritical carbonation. Constr. Build. Mater. 2019, 227, 116680. [Google Scholar] [CrossRef]
- Hubler, M.H.; Wendner, R.; Bažant, Z.P. Comprehensive database for concrete creep and shrinkage: Analysis and recommendations for testing and recording. ACI Mater. J. 2015, 112, 547–558. [Google Scholar] [CrossRef]
- Il Choi, J.; Lee, Y.; Kim, Y.Y.; Lee, B.Y. Image-processing technique to detect carbonation regions of concrete sprayed with a phenolphthalein solution. Constr. Build. Mater. 2017, 154, 451–461. [Google Scholar] [CrossRef]
- Damasceno Costa, A.R.; Pereira Gonçalves, J. Accelerated carbonation of ternary cements containing waste materials. Constr. Build. Mater. 2021, 302, 124159. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Cao, Z.; Guo, M.; Zheng, J. Effect of carbonated coarse recycled concrete aggregate on the properties and microstructure of recycled concrete. J. Clean. Prod. 2019, 233, 421–428. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the carbonation mechanism of CH and C-S-H in terms of kinetics, microstructure changes and moisture properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C-S-H and C-A-S-H samples studied by 13C, 27Al and 29Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Monkman, S.; Kenward, P.A.; Dipple, G.; MacDonald, M.; Raudsepp, M. Activation of cement hydration with carbon dioxide. J. Sustain. Cem. Mater. 2018, 7, 160–181. [Google Scholar] [CrossRef]
- Ahmad, S.; Assaggaf, R.A.; Maslehuddin, M.; Al-Amoudi, O.S.B.; Adekunle, S.K.; Ali, S.I. Effects of carbonation pressure and duration on strength evolution of concrete subjected to accelerated carbonation curing. Constr. Build. Mater. 2017, 136, 565–573. [Google Scholar] [CrossRef]
- Drouet, E.; Poyet, S.; Le Bescop, P.; Torrenti, M.J.; Bourbon, X. Carbonation of hardened cement pastes: Influence of temperature. Cem. Concr. Res. 2019, 115, 445–459. [Google Scholar] [CrossRef]
- Sáez del Bosque, I.F.; Van den Heede, P.; De Belie, N.; Sánchez de Rojas, M.I.; Medina, C. Carbonation of concrete with construction and demolition waste based recycled aggregates and cement with recycled content. Constr. Build. Mater. 2020, 234, 117336. [Google Scholar] [CrossRef]
- Santos, S.F.; Schmidt, R.; Almeida, A.E.F.S.; Tonoli, G.H.D.; Savastano, H. Supercritical carbonation treatment on extruded fibre—Cement reinforced with vegetable fibres. Cem. Concr. Compos. 2015, 56, 84–94. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Zeng, W.; Poon, C.S. Carbonation treatment of recycled concrete aggregate: Effect on transport properties and steel corrosion of recycled aggregate concrete. Cem. Concr. Compos. 2019, 104, 103360. [Google Scholar] [CrossRef]
- Yahui, Y.; Xu, G.; Bin, T. Carbonation characteristics of cement-based materials under the uniform distribution of pore water. Constr. Build. Mater. 2020, 275, 121450. [Google Scholar] [CrossRef]
- Dheilly, R.M.; Tudo, J.; Sebaibi, Y.; Quéneudec, M. Influence of storage conditions on the carbonation of powdered Ca(OH)2. Constr. Build. Mater. 2002, 16, 155–161. [Google Scholar] [CrossRef]
- Galan, I.; Glasser, F.P.; Baza, D.; Andrade, C. Assessment of the protective effect of carbonation on portlandite crystals. Cem. Concr. Res. 2015, 74, 68–77. [Google Scholar] [CrossRef]
- Bui, H.; Sebaibi, N.; Boutouil, M.; Levacher, D. Determination and review of physical and mechanical properties of raw and treated coconut fibers for their recycling in construction materials. Fibers 2020, 8, 37–55. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Microstructure changes of waste hydrated cement paste induced by accelerated carbonation. Constr. Build. Mater. 2015, 76, 360–365. [Google Scholar] [CrossRef]
- Chen, T.; Bai, M.; Gao, X. Carbonation curing of cement mortars incorporating carbonated fly ash for performance improvement and CO2 sequestration. J. CO2 Util. 2021, 51, 101633. [Google Scholar] [CrossRef]
- Malheiro, R.; Camões, A.; Meira, G.; Amorim, M.T. Influence of chloride contamination on carbonation of cement-based materials. Constr. Build. Mater. 2021, 296, 123756. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, P.; Yu, Z. Effects of environmental factors on concrete carbonation depth and compressive strength. Materials 2018, 11, 2167. [Google Scholar] [CrossRef] [PubMed]
- Justnes, H.; Skocek, J.; Østnor, T.A.; Engelsen, C.J.; Skjølsvold, O. Microstructural changes of hydrated cement blended with fly ash upon carbonation. Cem. Concr. Res. 2020, 137, 106192. [Google Scholar] [CrossRef]
- von Greve-Dierfeld, S.; Lothenbach, B.; Vollpracht, A.; Wu, B.; Huet, B.; Andrade, C.; Medina, C.; Thiel, C.; Gruyaert, E.; Vanoutrive, H.; et al. Understanding the carbonation of concrete with supplementary cementitious materials: A critical review by RILEM TC 281-CCC. Mater. Struct. 2020, 53, 136. [Google Scholar] [CrossRef]
- Cultrone, G.; Sebastián, E.; Huertas, M.O. Forced and natural carbonation of lime-based mortars with and without additives: Mineralogical and textural changes. Cem. Concr. Res. 2005, 35, 2278–2289. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, J. Effect of different hydration time on carbonation degree and strength of steel slag specimens containing zeolite. Materials 2020, 13, 3898. [Google Scholar] [CrossRef]
- Lin, R.-S.; Wang, X.-Y.; Yi-Han. Effects of cement types and addition of quartz and limestone on the normal and carbonation curing of cement paste. Constr. Build. Mater. 2021, 305, 124799. [Google Scholar] [CrossRef]
- Trtnik, G.; Turk, G.; Kavčič, F.; Bosiljkov, V.B. Possibilities of using the ultrasonic wave transmission method to estimate initial setting time of cement paste. Cem. Concr. Res. 2008, 38, 1336–1342. [Google Scholar] [CrossRef]
- Ye, G.; Lura, P.; Van Breugel, K.; Fraaij, A.L.A. Study on the development of the microstructure in cement-based materials by means of numerical simulation and ultrasonic pulse velocity measurement. Cem. Concr. Compos. 2004, 26, 491–497. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.Y.; Kurtis, K.E.; Jacobs, L.J.; Le Pape, Y.; Guimaraes, M. Quantitative evaluation of carbonation in concrete using nonlinear ultrasound. Mater. Struct. Constr. 2016, 49, 399–409. [Google Scholar] [CrossRef]
- Villarreal, A.; Cosmes-López, M.; León-Martínez, F.M.; Castellanos, F.; Solis-Najera, S.E.; Medina, L. Ultrasonic phase velocity of carbonated cement paste probes. Appl. Acoust. 2019, 154, 129–134. [Google Scholar] [CrossRef]
- Liu, S.; Shen, P.; Xuan, D.; Li, L.; Sojobi, A.; Zhan, B.; Poon, C.S. A comparison of liquid-solid and gas-solid accelerated carbonation for enhancement of recycled concrete aggregate. Cem. Concr. Compos. 2021, 118, 103988. [Google Scholar] [CrossRef]
- Chi, J.M.; Huang, R.; Yang, C.C. Effects of carbonation on mechanical properties and durability of concrete using accelerated testing method. J. Mar. Sci. Technol. 2002, 10, 14–20. [Google Scholar] [CrossRef]
- Andrade, C. Evaluation of the degree of carbonation of concretes in three environments. Constr. Build. Mater. 2020, 230, 116804. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Mi, R.; Pan, G.; Li, Y.; Kuang, T. Carbonation degree evaluation of recycled aggregate concrete using carbonation zone widths. J. CO2 Util. 2021, 43, 101366. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, Y.; Wei, H. Carbonation-cementation of recycled hardened cement paste powder. Constr. Build. Mater. 2018, 192, 224–232. [Google Scholar] [CrossRef]
- Andersson, R.; Stripple, H.; Gustafsson, T.; Ljungkrantz, C. Carbonation as a method to improve climate performance for cement-based material. Cem. Concr. Res. 2019, 124, 105819. [Google Scholar] [CrossRef]
- Henrique, G.; Tonoli, D.; Fernando, G.; Alexandre, C.; Savastano, H. Influence of the initial moisture content on the carbonation degree and performance of fiber-cement composites. Constr. Build. Mater. 2019, 215, 22–29. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J.; He, Z. Microstructure of cement paste subject to early carbonation curing. Cem. Concr. Res. 2012, 42, 186–193. [Google Scholar] [CrossRef]
- Asano, S.; Kamatani, Y.; Inoue, Y. On the carbonation of calcium silicate compounds. J. Ceram. Assoc. Jpn. 1971, 79, 303–311. [Google Scholar] [CrossRef]
- Matsushita, F.; Aono, Y.; Shibata, S. Carbonation degree of autoclaved aerated concrete. Cem. Concr. Res. 2000, 30, 1741–1745. [Google Scholar] [CrossRef]
- Li, J.; Yu, Q.; Huang, H.; Yin, S. Effects of Ca/Si ratio, aluminum and magnesium on the carbonation behavior of calcium silicate hydrate. Materials 2019, 12, 1268. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, X.; Chen, T. Influence of mineral admixtures on carbonation curing of cement paste. Constr. Build. Mater. 2019, 212, 653–662. [Google Scholar] [CrossRef]
- Phung, Q.T.; Maes, N.; Jacques, D.; Bruneel, E.; Van Driessche, I.; Ye, G.; De Schutter, G. Effect of limestone fillers on microstructure and permeability due to carbonation of cement pastes under controlled CO2 pressure conditions. Constr. Build. Mater. 2015, 82, 376–390. [Google Scholar] [CrossRef]
- Xian, X.; Shao, Y. Microstructure of cement paste subject to ambient pressure carbonation curing. Constr. Build. Mater. 2021, 296, 123652. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M.; Platret, G. Measurement methods of carbonation profiles in concrete: Thermogravimetry, chemical analysis and gammadensimetry. Cem. Concr. Res. 2007, 37, 1182–1192. [Google Scholar] [CrossRef]
- Villain, G.; Thiery, M. Gammadensimetry: A method to determine drying and carbonation profiles in concrete. NDT E Int. 2006, 39, 328–337. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Komar Kawatra, S.; Eisele, T.C.; Sutter, L.L. Carbon dioxide sequestration in cement kiln dust through mineral carbonation. Environ. Sci. Technol. 2009, 43, 1986–1992. [Google Scholar] [CrossRef]
- Dapkus, G.; Stankevicius, V. Cellular concrete carbonation: Effect on moisture absorption of building envelopes. Batim. Int. Build. Res. Pract. 1985, 13, 184–187. [Google Scholar] [CrossRef]
- Iwasaki, M.; Tada, S. Carbonation of aerated concrete. In Proceedings of the 1985 Beijing International Symposium on Cement and Concrete, Beijing, China, 14–17 May 1985; Volume 3, pp. 414–423. [Google Scholar]
- Andrade, C.; Sanjuán, M.Á. Updating carbon storage capacity of Spanish cements. Sustainability 2018, 10, 4806. [Google Scholar] [CrossRef]
- Song, B.; Shi, C.; Hu, X.; Ouyang, K.; Ding, Y.; Ke, G. Effect of early CO2 curing on the chloride transport and binding behaviors of fly ash-blended Portland cement. Constr. Build. Mater. 2021, 288, 123113. [Google Scholar] [CrossRef]
- Tu, Z.; Shi, C.; Farzadnia, N. Effect of limestone powder content on the early-age properties of CO2-cured concrete. J. Mater. Civ. Eng. 2018, 30, 04018164. [Google Scholar] [CrossRef]

| Indicator | pH Value Range | Carbonation Degree | Ref. |
|---|---|---|---|
| Phenolphthalein | 8.3–9.5 | Completed carbonation | [23] |
| Thymolphthalein | 9.3–10.5 | ||
| Alizarin yellow R | 10.2–12.2 | ||
| Tropaeolin O | 11.1–12.7 | ||
| Phenolphthalein | <10.5 | Completed carbonation | [24] |
| 10.5–11.5 | Partial carbonation | ||
| Phenolphthalein | <7.5 | 100% | [25] |
| 7.5 < pH < 9 | 50–100% | ||
| 9 < pH < 11.5 | 0–50% | ||
| >11.5 | 0% | ||
| Thymolphthalein | 9–10.5 | Completed carbonation | [26] |
| Phenolphthalein | 8.2–9.8 | ||
| Phenolphthalein | <7 | Completed carbonation | [8] |
| Phenolphthalein | <8.2 | Completed carbonation | [27,28] |
| Equation of the Carbonation Degree | Feature | Ref. |
|---|---|---|
| [16] | |
| [84,88] | |
| [87] | |
| [89,92,97,98,99] | |
| [97] | |
| [100] | |
| [101,102] |
| Methods | Concept | Advantages | Drawbacks |
|---|---|---|---|
| |||
| pH indicator | Evaluation the change in pH level | Simple and effective. Inexpensive. | Difficult to detect partial carbonated area. Outcome is partly depended on the experience and skill of examining staff. Destructive test |
| Image analysis | Characterization of carbonation based on image analysis. | Uncomplicate to determine the carbonation front width. Allow to study deeply into the newly transformed phases of the carbonated sample. Rapid measurement. | High cost. Restrictions in depth exploration of the interior of structure. |
| Phase analysis | |||
| TGA | Investigation the weigh loss of profile ground powder of sample at the temperature range 450–900 °C. | Accurate measurement of chemical constituent at the different carbonated level. | Problems associated with the sampling (small mass). Suitable for only laboratory scale. |
| XRD | Observation the intensity, the disappearance and generation of the diffraction peaks created by carbonation reaction. | ||
| FTIR | Passing infrared light across the sample to observe relative intensity of the absorption bandwidths associated with the creation of chemical compositions during carbonation process. | Detect the main phases of typical carbonation reaction products. Therefore, partial carbonated area is easy to detect. Rapid measurement, time-saving. | Suitable for only laboratory scale. |
| Ultrasonic phase velocity | Detecting the change in the frequency of ultrasonic wave interacted with surface of sample. | Non-destructive test. Sensitivity to microstructure changes. | It is difficult to investigate the inner area of concrete. |
| |||
| phenolphthalein | Degree of carbonation was evaluated by calculating the rate of the carbonated areas and the total fresh broken area of sample analyzed. | Simple and fast. | The data is not high enough to accurate quantitation. |
| CO2 uptake | Accurate estimation of CO2 absorption content | Able to accurate evaluation of the carbonation degree in accordance with the carbonation time. | Complicated task due to the absence of a definite calculation method. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bui, H.; Delattre, F.; Levacher, D. Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review. Appl. Sci. 2023, 13, 2533. https://doi.org/10.3390/app13042533
Bui H, Delattre F, Levacher D. Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review. Applied Sciences. 2023; 13(4):2533. https://doi.org/10.3390/app13042533
Chicago/Turabian StyleBui, Huyen, Francois Delattre, and Daniel Levacher. 2023. "Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review" Applied Sciences 13, no. 4: 2533. https://doi.org/10.3390/app13042533
APA StyleBui, H., Delattre, F., & Levacher, D. (2023). Experimental Methods to Evaluate the Carbonation Degree in Concrete—State of the Art Review. Applied Sciences, 13(4), 2533. https://doi.org/10.3390/app13042533







