Abstract
This study undertook the mineralogical and chemical analysis of anglesite (PbSO4), cerussite (PbCO3), gypsum (CaSO4∙2H2O), langite (Cu4(SO4)(OH)6∙2H2O), malachite (Cu2CO3(OH)2), and posnjakite (Cu4(SO4)(OH)6·H2O) detected for the first time in the abandoned Mastricarro Barite Mine (Catanzaro, Calabria, southern Italy). Geological conditions make this one of the few well-preserved, partly accessible mines in the Calabria region. Numerous mineralogical species, some beautifully crystallized, have been discovered among the alteration products of the sulfides present within the barite veins. The newly identified crystals occur as secondary minerals in the oxidized portions of deposits originally containing lead and copper sulfides; they are widely distributed and are almost always detected as microcrystals. The anglesite, cerussite, gypsum, langite, malachite, and posnjakite crystals were identified and characterized using optical microscopy (OM), micro-Raman spectroscopy (µR), and scanning electron microscopy, combined with energy dispersive spectrometry (SEM/EDS). The new mineral occurrences can be useful for scientific and didactic purposes; further, for langite, malachite, and posnjakite crystals, new Raman bands, which have not been mentioned before in the literature, were identified.
1. Introduction
Minerals are essential for sustaining the economic development of our society, and the increase in the world population in recent years has sparked a renewed interest in mineral exploration. In 1964, the Southern Mining Industries (joint stock company) of Naples explored an area in the Molino Mastricarro locality, along the Fiumarella stream, northwest of Catanzaro (Calabria, Southern Italy), to assess the existing outcrops for mineral exploitation. Geological investigations led to the discovery of a 1 to 3 m-thick deposit consisting of barite veins running mainly along the porphyry–granite contact [1]. Secondary minerals with low concentrations of fluorite, galena, pyrite, chalcopyrite, and sphalerite were also found [1,2,3,4]. Once prospecting ended in 1967, the “Mastricarro Barite Mine” mining concession was issued to the Southern Mining Industry company: barite exploitation began in 1968–1969, with an average production of about 80,000 tons per year [1]. Extraction initially took place using the “cut in direction” method, which proved unsafe for processing and resulted in medium productivity due to the geological setting of the barite veins; the subsequent adoption of the “room and pillar” method greatly improved both productivity and safety in the workplace. The extracted mineral, with a good degree of whiteness, was used prevalently in the paint industry and, secondarily, as an additive in oil drilling [2]. In 1980, the Southern Mining Industry renounced the concession because the exploitation of the area was no longer economically viable. At the end of the exploitation activities, work was carried out to ensure the safety of the site, including the construction of concrete diaphragm walls to stabilize the entrances and prevent access to the tunnels [2].
In the Mastricarro Barite Mine, minerals such as connellite, linarite, and wulfenite were recently detected, in addition to primary minerals such as barite and galena [5,6]. This makes the Mastricarro Barite Mine particularly interesting not only for its naturalistic value, but also for its economic potential. In recent years, the European Union has assigned great importance to securing mineral resources for industry, as there are some 30 million jobs depending on the availability of raw minerals that are in great demand for strategic sectors such as renewable energy. Note that barite is present in the EU’s list of critical raw minerals [7], and EU policies and the UNESCO strongly encourage its identification, characterization, and subsequent mining. Currently, most of the mines and quarries in the Calabria region are dismissed and testify to a grand monument of the Calabrian industrial archaeology. Nowadays, the study of the Mastricarro Barite Mine represents a great challenge for the use of available mineral resources. Therefore, the detailed knowledge of minerals from the Mastricarro Barite Mine may also tackle the urgency of mineral availability and supply.
This study aims to fully characterize new minerals detected for the first time in the Mastricarro Barite Mine: anglesite, cerussite, gypsum, langite, malachite, and posnjakite. Furthermore, the aim of this study is to explore in detail the mineral characteristics by multi-analytical investigations to understand the relationships between the mineralogical and physicochemical properties.
Samples were identified and characterized through optical microscopy (OM), micro-Raman spectroscopy (µ-R), and scanning electron microscopy, combined with energy dispersive spectrometry (SEM/EDS). In particular, micro-Raman spectroscopy allowed us to identify and characterize minerals through rapid, non-destructive, and economic analyses, as demonstrated by its successful use in numerous studies [8,9,10,11,12].
New Raman bands were identified for langite, malachite, and posnjakite. The study provides indispensable mineralogical information for the correct exploitation and use of anglesite, cerussite, gypsum, langite, malachite, and posnjakite, in agreement with literature data [13,14,15,16,17,18,19,20]. The findings suggest that the Mastricarro Barite mine is a site of economic interest. Carbonate and sulphate class minerals represent a quite valuable set of materials in view of their relevance from a scientific and prospecting perspective and in line with European Union policies and UNESCO purposes, which encourage the identification, characterization, and mining of raw minerals.
2. Geological Setting
The Mastricarro Barite Mine (38°55′19.19″ N; 16°34′25.71″ E) lies west of the city of Catanzaro (Calabria, southern Italy), in the Molino Mastricarro locality along the Fiumarella stream (Figure 1).
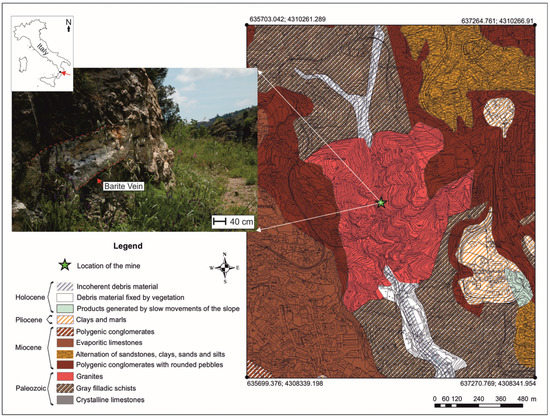
Figure 1.
Location of the Mastricarro Barite Mine and geological map of the area (after [5,6]). The red arrow indicates the barite vein in which the anglesite, cerussite, gypsum, langite, malachite, and posnjakite crystals were found.
A small strip of the Stilo Unit crops out in the area [21]. Mainly present in the southeaster and southern reaches of the Calabria Peloritani Orogen, it is also present as small strips north of the Catanzaro Line, within a series of WNW-ESE-aligned klippen extending from Amantea (Tyrrhenian Calabria) to Catanzaro (Ionian Calabria). The Stilo Unit is made up of metamorphic rocks (phyllites, schists, and gneisses) intruded by late Hercynian plutonic rocks; only its southernmost outcrops consist dominantly of metamorphic rocks.
The rocks in the area of the Fiumarella Stream are mostly granodiorites and microgranodiorites, with subordinate aplites and porphyroid rocks, all of crustal origin and Hercynian age [22,23], which intruded the phyllites and the gneisses, overlain by carbonates and sandstones [24]. Barite veins are found in both the diorites and porphyrites, whereas no evidence of mineralization was observed in the phyllites. This suggests that barite formed in igneous rock fractures, finding a barrier in the metamorphic rocks [2]. Early studies by Vighi [3] found that the magmatic rocks are intersected by multiple fracture systems, the first of which was affected by the deposition of barite and by the subsequent mineralization of Cu-Pb sulfide. Studies on barite fluid inclusions have established that mineralization occurred at a minimum temperature of 210 °C and at a minimum depth of 201 m below the paleo-watertable [2].
The Molino Mastricarro barite deposits seem to have affinity with epithermal “Precious Metal”-type veins or even with “Kuroko”-type veins [2]. The barite veins are generally sub-horizontal, with a thickness of up to 3 m, and have often been dislocated by subsequent intense fracturing.
3. Materials and Methods
Samples were collected from the rocky walls near the mining area and from an exploratory tunnel dug on the orographic right of the Fiumarella Stream. The minerals mainly represent weathered phases of galena, pyrite, and chalcopyrite in the cores of the barite veins of the Mastricarro Barite Mine. They were initially studied under an optical stereo microscope (Askania, GSZ 2T, Germany, fitted with a digital camera Fuji X-E2, Japan) at a variable magnification of 4–40×. Images of the minerals were generated using the “focus stacking” technique. The method consists of taking enough equally spaced photographs to cover the entire area while maintaining focus. This is important since the portion of the object that remains in focus decreases as the shooting magnification increases. Some one hundred photos were taken of each mineral. The images were merged using Affinity Photo software and processed to obtain the best possible framing and remove artifacts.
Crystals were subsequently studied by SEM/EDS and micro-Raman spectrometry without pretreatment. Semi-quantitative element analysis of the minerals was performed using an Environmental Scanning Electron Microscope FEI QUANTA 200 (FEI, Hillsboro, OR, USA) equipped with an X-ray EDS suite comprising an EDAX-GENESIS4000 (EDAX, Tokyo, Japan) Si/Li crystal detector. Working conditions and detector constants were as follows: voltage 20 kV and tilt angle 0°. For SEM analysis, each sample was coated with graphite after being fixed on SEM stub using double-sided conductive adhesive tape. Microanalysis (EDS) was achieved by the standardless quantification using ZAF correction method.
Micro-Raman analyses were performed using a Thermo Fisher DXR Raman microscope (Waltham, MA, USA) equipped with OMNICxi Raman Imaging software 1.0, an objective of 10×, a grating of 900 ln/mm (full width at half maximum, FWHM), and an electron-multiplying charge-coupled device (EMCCD). The 532 nm line (solid state laser) was used at an incident power output ranging from 1.8 to 7 mW.
All measurements (OM, SEM/EDS, and µ-R) were done at the Department of Biology, Ecology, and Earth Sciences of the University of Calabria.
4. Results and Discussion
4.1. Anglesite
Anglesite is a lead sulfate (PbSO4) which takes its name from the Island of Anglesey in the Irish Sea (Wales, UK), where it was first identified in 1783. Considerable crystals come from Sardinia (Italy), Scotland, Australia, and Mexico. The pure mineral is colourless, but it can take on shades of yellow to green or blue, although the latter are very rare.
Anglesite in the Mastricarro Mine is an oxidation product of galena [1,2,3,4]. In terms of its crystal structure, anglesite is comprised of SO4 tetrahedra with Pb2+ in pseudo-eight- to twelve-fold co-ordination with the oxygen ions [25]. In the study area, it usually has an orthorhombic euhedral habit, at times elongated, and is colourless with a vitreous or adamantine luster (Figure 2). In some areas, it has streaks along the main axis of elongation (Figure 2). Colourless crystals of anglesite measure up to 2 mm in length (Figure 2) and they occasionally show autoepitaxial growth parallel to the c axis (Figure 2). Under SEM, all anglesite crystals appear to be euhedral and were often found nestled in cavities lined with galena (Figure 3).

Figure 2.
Optical image of well-developed colourless anglesite crystals from the Mastricarro Barite Mine (Catanzaro, Southern Italy).
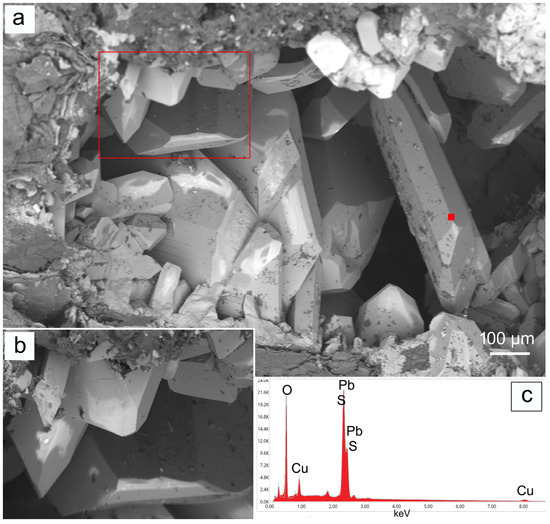
Figure 3.
(a,b) SEM image with magnification of anglesite from the Mastricarro Barite Mine (Catanzaro, Southern Italy) and corresponding EDS spot analysis (c) acquired in the point indicated by red filled square.
The semi-quantitative EDS/SEM chemical analysis revealed a PbO content of 71.8 wt% and a SO3 content of 22.6 wt%, in agreement with the existing literature data [26,27]. Moreover, copper impurities (about 5 wt%) were detected on the crystal surfaces (Figure 3).
The Raman spectrum of anglesite (Figure 4) reveals the typical bands at 1156, 1060, 978, 641, 609, 451, 439, 135, 100, and 60 cm−1 (Table 1). The relatively huge unit cell gives ascent to a somewhat complex Raman vibrational spectrum (Figure 4). In particular, we have the v1 (symmetric stretch of the (SO4)−2 tetrahedra) mode at 978 cm−1, the v2 (tetrahedral symmetric bend) mode with two components at 439 and 451 cm−1, the v3 (tetrahedral antisymmetric stretch) mode with two components at 1156 and 1160 cm−1, and the v4 (tetrahedral antisymmetric bend) mode at 641 cm−1 and 609 cm−1 [28–31, RRUFF ID R050408].
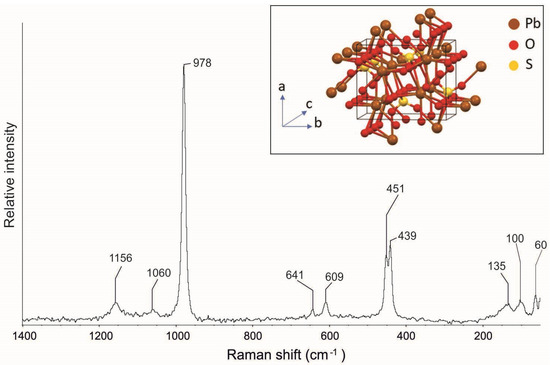
Figure 4.
Raman spectrum of anglesite from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the anglesite [25].

Table 1.
Micro-Raman band wavenumber/cm−1 for anglesite, cerussite, gypsum, langite, malachite, and posnjakite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy). The list shows observed Raman bands previously reported in the literature [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43] and in the RRUFF database [39], as well as those here identified for the first time.
4.2. Cerussite
Cerussite is a lead carbonate (PbCO3). Its name derives from the Latin “cerussa”, which means “white lead”. It has been employed since ancient times as a white pigment for paints and was used as a cosmetic between 1500 and 1600, only for its toxicity to later be discovered. Deposits are found in Sardinia (Italy), Australia, and Tsumeb (Namibia, Africa).
Lately, the rising demand for lead metal has generated new processes to extract lead from cerussite (PbCO3) or from other oxidation products of lead ores such as anglesite (PbSO4). Cerussite is isostructural with an aragonite-type structure in which (CO3)2– groups (slightly planar) along c point alternately to the +b and −b directions, while layers of nine-co-ordinated Pb2+ are parallel to (001), and the atoms occur approximately in the positions of hexagonal close packing [44].
Among the alteration minerals found in the Mastricarro Barite Mine, cerussite, an oxidation product of galena, is certainly the most widespread [1,2,3,4]. Cerussite occurs as perfectly formed crystals with a squat prismatic to elongated prismatic habit and often shows star-shaped twinning. A bipyramidal habit has occasionally been observed. The colour ranges from transparent to white to grey, and the luster is usually adamantine, although opaque crystals are often observed. The crystals, all measuring up to 2 × 1 mm in size, show three different morphologies: well-formed prismatic crystals (Figure 5a), V-twinned crystals (Figure 5b), or straw aggregates. These last two morphologies are similar to those of the cerussite samples found at Tsumeb (Namibia, Africa).

Figure 5.
Optical images of cerussite from the Mastricarro Barite Mine (Catanzaro, southern Italy): (a) aggregates of prismatic colourless cerussite crystals; (b) V-twinned cerussite.
Under SEM, the cerussite crystals appeared to lack a regular morphology, and showed the formation of block aggregates (Figure 6). An EDS semi-quantitative analysis performed on the cerussite crystal (Figure 6) highlighted a chemical composition made of 80.2 wt% PbO and 17.5 wt% CO2, in accordance with data from the literature [27,33].
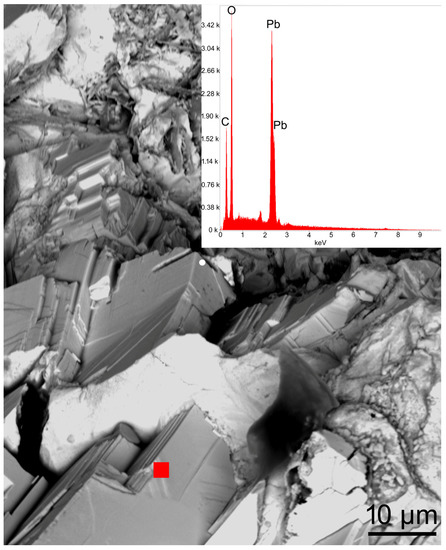
Figure 6.
SEM image and corresponding EDS spot analysis (red filled square) of cerussite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy). The cleavage planes are clearly visible.
In the Raman spectrum shown in Figure 7, the Raman active ν1 (carbonate symmetric stretching) and ν2 (carbonate symmetric bending) modes are, respectively, observed at 1051 and 837 cm–1. In addition, the spectrum highlighted the other typical cerussite Raman bands at 231, 177, 118, 105, 72, and 58 cm−1 (Figure 7, Table 1) in agreement with the data reported in the literature [28, 33, 34; RRUFF ID R060017].
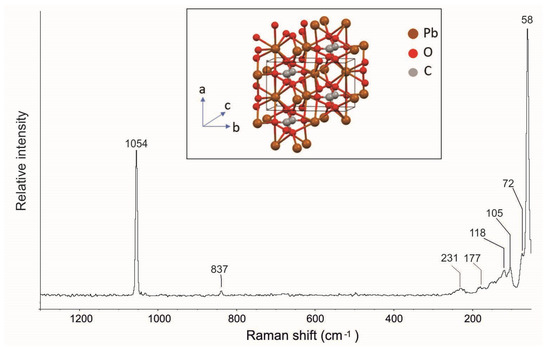
Figure 7.
Raman spectrum of cerussite from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the cerussite [44].
4.3. Gypsum
Gypsum is a calcium dihydrate sulfate (CaSO4∙2H2O). One of the oldest known minerals, it has found very widespread use in cultural heritage, construction, medicine, and industry. Gypsum deposits are visible in numerous localities, especially in Germany, France, Austria, and Italy. The mineral is usually found in sedimentary evaporite deposits, but it can also form as a product of sulfide oxidation. The structure of gypsum is based upon a pair of adjacent layers parallel to (010) which contain Ca ions and tetrahedral SO4 ions. Between successive pairs of layers, the water molecules are located in such a way that they are hydrogen-bonded to oxygens of SO4 groups [45]. Each Ca ion is co-ordinated by six oxygens of sulphate groups and by two water molecules [30].
In the Mastricarro Barite Mine, it is a by-product of pyrite oxidation, which gives rise to single crystals and, more often, to aggregates of elongated monoclinic crystals (Figure 8a). Usually colourless, sometimes tending towards white, the crystals have a glassy sheen and longitudinal streaks (Figure 8b and Figure 9). The largest crystal measures 3 mm in length, the smallest a few microns.

Figure 8.
Optical images of gypsum crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy): (a) aggregates of prismatic euhedral monoclinic crystals; (b) magnification of the rectangle outlined in (a) showing the parallel intergrowth.
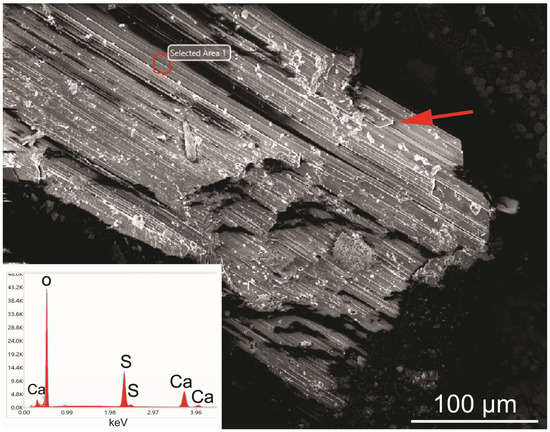
Figure 9.
SEM image and corresponding EDS spot analysis (empty red square) of a gypsum crystal from the Mastricarro Barite Mine (Catanzaro, southern Italy). The red arrow indicates the monoclinic character of the gypsum crystals.
The EDS semi-quantitative chemical analysis (Figure 9) revealed that gypsum crystals contain about 38 wt% CaO and about 59 wt% SO3. The data highlight a shift from the stoichiometric composition of gypsum, with a SO3/CaO ratio of 1.55 instead of 1.60, which is the theoretical value. This shift is due to the inability of the EDS/SEM analysis to detect the water content (generally about 21 wt%). Gypsum exhibits lamellar morphologies characterized by parallel intergrowth in which the monoclinic symmetry is clearly observed (Figure 9).
In the Raman spectrum shown in Figure 10, it is possible to observe the typical gypsum bands at 1134, 1008, 668, 619, 493, 415, 210, and 178 cm−1 (Table 1). In particular, the highest band at 1008 cm−1 corresponds to the sulfate symmetric stretching mode (v1), while the bands of medium intensity, assigned to the ν2 sulfate symmetric bend mode, were registered at 415 and 493 cm−1. The band at 1134 cm−1 is linked to the sulfate antisymmetric stretching mode (ν3) while the bands at 619 and 668 cm−1 correspond to the sulfate antisymmetric bending mode (ν4) [28, 30, 35; RRUFF ID R0400299].
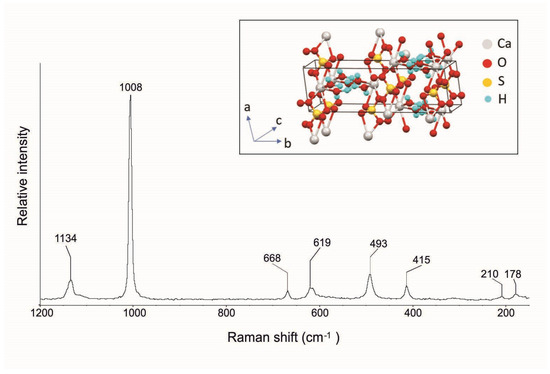
Figure 10.
Raman spectrum of gypsum crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the gypsum [45].
4.4. Langite
Langite is a hydrated copper sulfate (Cu4(SO4)(OH)6∙2H2O) that forms through the oxidation of sulphides in copper deposits. It owes its name to Victor von Lang, a Vienna University physicist and crystallographer who discovered it in 1864. Cornwall (England, UK) is the type locality for langite. It normally occurs as small blue, sometimes greenish-blue, crystals or patinas with a vitreous luster. The crystal structure of langite consists of double layers of close-packed OH- ions with Cu2+ ions in octahedral co-ordination. Among the double layers, there are sulphate groups (SO4) linked to one side of the layers by corner sharing. The SO4 tetrahedra are linked to one another by the hydrogen bonds of H2O molecules and are also connected to the Cu-OH layers by H+ bonds [40,46].
Often in association with other minerals such as posnjakite, it is present in the Mastricarro Barite Mine as a chalcopyrite alteration product. Langite is present as perfectly formed light blue, elongated prismatic crystals up to 1 mm in length; twinned crystals are sometimes arranged in a fan pattern (Figure 11).

Figure 11.
Optical image showing light blue monoclinic langite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy) arranged in a radial pattern.
As for its chemical composition, major elements include Cu and S (Figure 12), with a CuO/SO3 ratio equal to 3.5. Again, this value slightly deviates from the stoichiometric chemical composition of langite; the lower value (1.90) is due to the water content (19 wt%), which is not detected by EDS/SEM analysis. Langite crystals under SEM show euhedral shapes, in which the monoclinic symmetry is clearly evident (Figure 12).

Figure 12.
SEM image and corresponding EDS spot analysis (red filled square) of monoclinic langite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy).
Raman spectroscopy confirms the presence of this mineral. Figure 13 shows the langite Raman spectrum where, in agreement with the spectra reported in the literature [36–40; RUFF ID R060090], it is possible to observe its typical bands at 1153, 1096, 970, 612, 506, 488, 433, 240, and 181 cm−1 (Table 1). In particular, the following are visible: the (SO4)2- symmetric stretching mode (v1) at 970 cm−1, the antisymmetric stretching mode (v3) at 1096 and 1153 cm−1, the symmetric bending mode (v2) at 506, 488, and 433 cm−1, and the antisymmetric bending (v4) band at 612 cm−1. The Raman spectrum of the low-wavenumber region shows bands at 240 and 181 cm−1, which are characteristic of the basic copper sulphates [36]. Furthermore, Raman spectroscopy highlighted new bands at 357, 290, 265, 160, 126, and 92 cm−1, which have not been mentioned before in the literature.
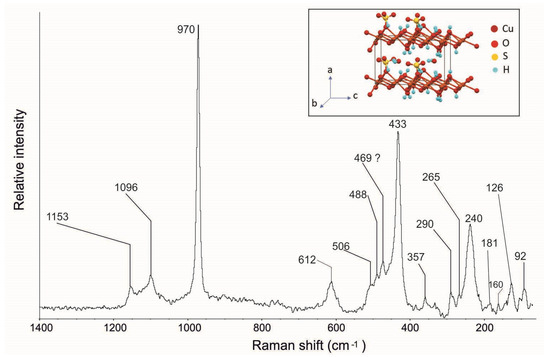
Figure 13.
Raman spectrum of langite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the langite [40,46].
4.5. Malachite
Malachite is a copper carbonate hydroxide (Cu2CO3(OH)2) occurring as a secondary mineral in the oxidized zones of copper ore deposits. The essential features of the malachite structure are based on Cu2O6 dimers bonded to CO3 groups [47]. Its name comes from the Greek “malache”, which means “mallow”, an herb with green leaves. Since antiquity, malachite was used as a green pigment and as an ornamental stone as well as a primary source of copper [35,41,42]. Malachite deposits are found in Africa, Russia, Australia, and United States. The malachite identified in the Mastricarro Barite Mine is a chalcopyrite alteration product with an intense green color and vitreous-silky luster that often occurs on irregular pale white aggregates of cerussite crystals (Figure 14a). It forms dome-shaped aggregates of needle-like crystals up to 0.5 mm in size. A double-rosette aggregate morphology was also observed (Figure 14b). This morphology is very similar to that found in Morocco and at the Tsumeb Mine in Namibia.

Figure 14.
Optical images showing malachite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy): (a) white acicular tufts of cerussite and dome-shaped aggregates of green needle-like malachite crystals; (b) double rosette aggregate of green needle-like malachite crystals.
A qualitative EDS/SEM analysis of single crystals (Figure 15) revealed that the chemical composition of malachite is generally close to the ideal one, with 74.7 wt% CuO and 23.9 wt% CO2. Samples also contain minor impurities of Si, Mg, and Ca at the surface of the crystals as EDS detected (Figure 15). Malachite often grows on pseudo-circular holes and exhibits a lamellar habit (Figure 15).
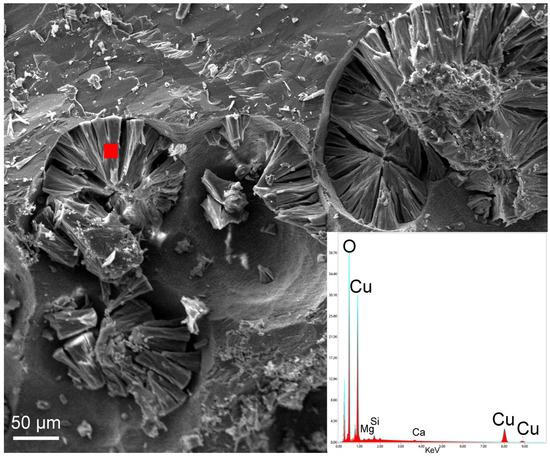
Figure 15.
SEM image and corresponding EDS spot analysis (red filled square) of malachite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy).
The Raman spectrum of malachite contains modes of three separate vibrational groups: OH, CO3, and Cu–O [43]. In Figure 16 are visible the typical malachite bands at 1493, 1366, 1099, 1064, 718, 536, 510, 434, 353, 275, 221, 180, and 154 cm−1 (Table 1), in agreement with spectra reported in the literature [28,35,39,43]. In particular, the CO3 symmetric stretching mode (v1) is observed at 1099 and 1064 cm−1, the v3 mode at 1493 cm−1, and the v4 mode at 718 cm−1; the OH stretching modes are visible at 536, 434, 275, and 221 cm−1, while for the Cu ion, there is a band at 353 cm−1. Moreover, in this case, new Raman bands at 120, 111, 79, and 65 cm−1 (Table 1), that have not been previously described in the literature, were highlighted.
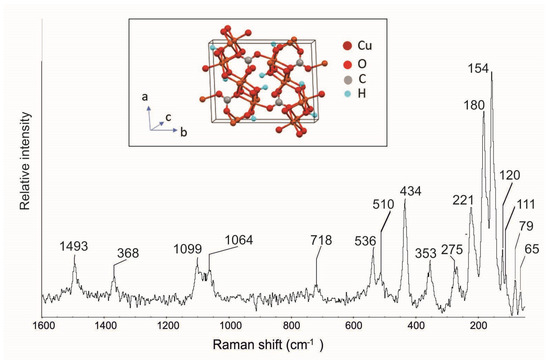
Figure 16.
Raman spectrum of malachite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the malachite [47].
4.6. Posnjakite
Posnjakite is a basic hydrated copper sulphate Cu4(SO4)(OH)6·(H2O). A secondary mineral which forms through the oxidation of copper sulphide deposits, it can be considered an alteration product of chalcopyrite. It was named after Eugene Waldemar Posnjak, a geochemist who worked at the Geophysical Laboratory (Washington, USA) and investigated the CuO-SO3-H2O system. Numerous deposits are found in the Karaganda Region (Kazakhstan), often used as a pigment [40,41,42]. The posnjakite structure consists of sheets of copper-distorted octahedra, parallel to (001), with sulfate groups connected to one side of the pseudo-octahedral sheet by corner sharing. The resultant composite octahedral–tetrahedral sheets are assured by hydrogen bonds [40]. Posnjakite normally occurs as small tabular crystals frequently associated with langite [40]. Elongated prismatic crystals can be found in the Mastricarro Barite Mine (Figure 17a and Figure 18): these sometimes form sub-globular aggregates (Figure 17b), which grow by oxidation of the chalcopyrite.

Figure 17.
Optical images showing posnjakite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy): (a) hundreds of tiny pale-green elongated prismatic crystals forming sprays of posnjakite; (b) spherical aggregates of elongated tabular posnjakite crystals.
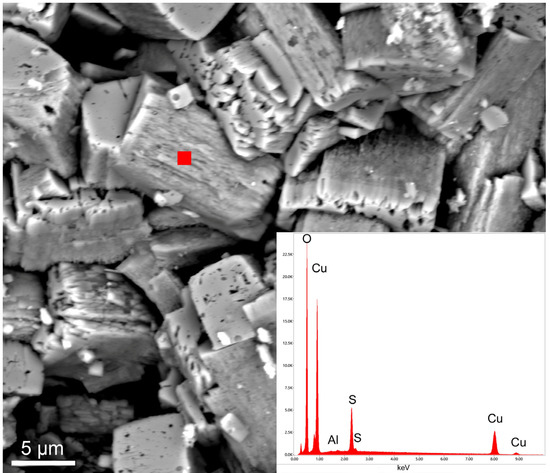
Figure 18.
SEM image and corresponding EDS spot analysis (red filled square) of spongy-looking posnjakite crystals from the Mastricarro Barite Mine (Catanzaro, southern Italy).
Posnjakite is usually pale green with a vitreous luster and individual crystals are rarely greater than 0.5 mm long. The chemical composition of the crystals, which appear spongy under the SEM (Figure 18), reveals a CuO/SO3 ratio of 3.93, which is very close to the theoretical stoichiometric ratio (3.97); furthermore, a low amount of Al was present as an impurity (Figure 18).
The Raman spectrum of the posnjakite crystals shows considerable complexity (Figure 19—Table 1), in agreement with the data reported in the literature [36,37; RRUFF ID R070316], with its typical bands at 1126, 1098, 1076, 972, 914, 731, 621, 612, 597, 506, 482, 422, 320, 242, 197, and 141 cm−1 (Table 1). In particular, it is possible to observe the (SO4)2- symmetric stretching mode (v1) at 972 cm−1 and the antisymmetric stretching mode (v3) at 1126, 1098, and 1076 cm−1. More complex is the bending region that shows bands at 511, 482, 447, 422, 386, and 363 cm−1 for the symmetric bending mode (v2), and bands at 621, 6012, and 597 cm−1 for the antisymmetric bending mode (v4).
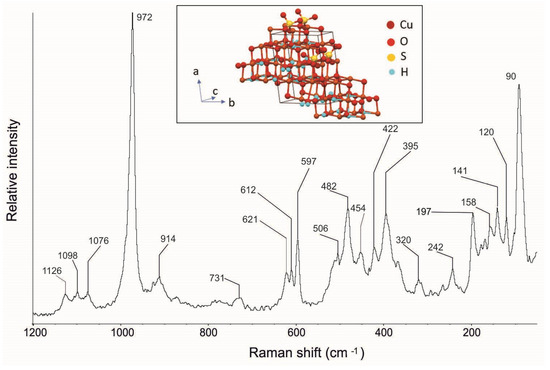
Figure 19.
Raman spectra of posnjakite from the Mastricarro Barite Mine (Catanzaro, southern Italy). In the insert is the structure of the posnjakite [40].
As with langite and malachite, in this case as well, Raman spectroscopy detected further bands at 454, 395, 158, and 90 cm−1 (Table 1), which have not been highlighted previously in the literature.
5. Conclusions
Anglesite, cerussite, gypsum, langite, malachite, and posnjakite crystals have been detected for the first time in the abandoned Mastricarro Barite Mine, located northwest of Catanzaro (Calabria, southern Italy). These minerals, here representing alteration products of pyrite, chalcopyrite, and galena, were characterized for both local and global comparisons. Data reported in this work can be used by researchers to compare the composition and morphological features of other anglesite, cerussite, gypsum, langite, malachite, and posnjakite crystals in other parts of the world and/or in similar geological environments. Due to their outstanding characteristics, these minerals or bearing elements (i.e., Pb) are used in several industrial applications. It is worth mentioning that lead can be extracted from cerussite and anglesite [48,49] and that gypsum is used in various industrial sectors such as cement, paper, and rubber. It should be emphasized that most green pigments are based on copper compounds such as langite, posnijakite, and malachite [41,42]. In addition, these basic copper sulphates are of interest for their presence in many environmental situations such as copper pipe corrosion, the restoration of brass and bronze objects and of frescoes, and the leaching from waste mineral dumps [36].
In the case of langite, malachite, and posnjakite, Raman spectroscopy allowed us to identify new Raman bands that have never been reported before in the literature. This is an important contribution to the expansion of existing Raman databases, which can support both mineralogical and geological research. The observation of additional bands may be attributed to a number of factors, including element impurities, local stress in the crystals, and crystal orientation effects.
This study suggests that the Mastricarro Barite mine could be a site of economic interest. Carbonate and sulphate minerals represent a quite valuable set of materials in view of their relevance in ore prospecting and economic applications. In recent years, the European Union has encouraged the identification, characterization, and the mining of raw minerals that are in great demand for strategic sectors. Mineralogical data from this work is indispensable for the correct exploitation and use of these minerals.
The study and the characterization of these minerals are important not only from a scientific and economic standpoint, but also for the beauty of the specimens, which may be put on display or sold to collectors.
All these aspects highlight the scientific, economic, and naturalistic importance of the Mastricarro Barite Mine. The findings from this study may also be used to promote the Fiumarella Stream locality in accordance with the Convention on the Protection of the World Cultural and Natural Heritage adopted by the UNESCO in 1972. Finally, in our opinion, our multi-analytical study succeeded in highlighting the intrinsic properties of important valuable minerals. In conclusion, in the context of the mineral resources efficiency policy of Europe, minerals from Mastricarro Barite Mine (Calabria, southern Italy) may play a strategic role not only in the local economy, but also in the societal progress, as well as in monitoring available minerals and planning their sustainable use.
Author Contributions
Conceptualization, A.B.; methodology, A.B, L.D. and D.M.; formal analysis, A.B. and D.M.; investigation, A.B., L.D. and D.M.; resources, A.B. and D.M.; writing—original draft preparation, A.B., L.D., D.M. and R.D.L.; writing—review and editing, A.B., L.D., D.M. and R.D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
Not applicable.
Acknowledgments
A.B. is particularly grateful to E. Barrese (University of Calabria, Italy) for his constructive suggestions. A.B. thanks N. Godbert for his support in the chemical structures and thanks 4 anonymous reviewers for their suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Procopio, F.; La Pietra, T.; Marabini, S.; Muto, F.; Palumbo, R.; Pellegrino, A.M. La miniera di barite Molino Mastricarro: Un geosito per la città di Catanzaro. ISPRAM Ambiente E Soc. 2011, 3, 157–170. [Google Scholar]
- Buchanan, L.J.; De Vivo, B.; Kramer, A.K.; Lima, A. Fluid inclusion study of the Fiumerella Barite Deposit (Catanzaro–S. Italy). Miner Deposita. 1981, 16, 215–226. [Google Scholar] [CrossRef]
- Vighi, L. Studio di un’area mineralizzata a baritina e solfuri vari in Calabria. Rice Sci. 1948, 10, 1339–1344. [Google Scholar]
- Dattola, L. La miniera di barite del Torrente Fiumarella presso Catanzaro: I minerali. Riv. Mineral. Ital. 1996, 20, 289–292. [Google Scholar]
- Bloise, A.; Dattola, L.; Allegretta, I.; Terzano, R.; Taranto, M.; Miriello, D. First evidence of wulfenite in Calabria Region (Southern Italy). Data Br. 2018, 19, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Bloise, A.; Dattola, L.; Allegretta, I.; Terzano, R.; Miriello, D. Linarite and connellite dataset from Calabria region (Southern Italy): First evidence. Data Br. 2019, 27, 104597. [Google Scholar] [CrossRef]
- CRM List 2020. Available online: https://rmis.jrc.ec.europa.eu/?page=crm-list-2020-e294f6 (accessed on 21 December 2022).
- Miriello, D.; Bloise, A.; Crisci, G.M.; De Luca, R.; De Nigris, B.; Martellone, A.; Osanna, M.; Pace, R.; Pecci, A.; Ruggieri, N. Non-destructive multi-analytical approach to study the pigments of wall painting fragments reused in mortars from the archaeological site of Pompeii (Italy). Minerals 2018, 8, 134. [Google Scholar] [CrossRef]
- Bloise, A.; Abd El Salam, S.; De Luca, R.; Crisci, G.M.; Miriello, D. Flux growth and characterization of cuprorivaite: The influence of temperature, flux, and silica source. Appl. Phys. 2016, 122, 650. [Google Scholar] [CrossRef]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal Behaviour of Mineral Fibres. In Mineral Fibres: Crystal Chemistry, Chemical–Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; pp. 215–252. [Google Scholar]
- Padyar, F.; Rahgoshay, M.; Tarantola, A.; Caumon, M.C.; Pourmoafi, S.M. High fH2 _ fS2 Conditions Associated with Sphalerite in Latala Epithermal Base and Precious Metal Deposit, Central Iran: Implications for the Composition and Genesis Conditions of Sphalerite. Int. J. Earth Sci. 2020, 31, 523–535. [Google Scholar] [CrossRef]
- Sundararajan, M.; Rejith, R.G.; Renjith, R.A.; Peer Mohamed, A.; Gayathri, G.S.; Resmi, A.N.; Jinesh, K.B.; Loveson, V.J. Raman XPS spectroscopic investigation of heavy mineral sands along Indian coast. Geo-Mar. Lett. 2021, 41, 22. [Google Scholar] [CrossRef]
- Aikawa, K.; Ito, M.; Kusano, A.; Jeon, S.; Park, I.; Hiroyoshi, N. Development of a Sustainable Process for Complex Sulfide Ores Containing Anglesite: Effect of Anglesite on Sphalerite Floatability, Enhanced Depression of Sphalerite by Extracting Anglesite, and Recovery of Extracted Pb2+ as Zero-Valent Pb by Cementation Using Zero-Valent Fe. Minerals 2022, 12, 723. [Google Scholar]
- Elizondo-Álvarez, M.A.; Uribe-Salas, A.; Bello-Teodoro, S. Comparative study of the interaction mechanisms between galena, cerussite and anglesite with benzohydroxamate and octanohydroxamate in aqueous solution. Min. Eng. 2022, 176, 107355. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Ning, J.L.; Xie, H.; Lv, J.F.; Hu, P.J.; Pang, J. Study on surface modification of cerussite by thermochemical processing with pyrite. Physicochem. Probl. Miner. Process. 2021, 57, 156–167. [Google Scholar] [CrossRef]
- Catalano, M.; Bloise, A.; Miriello, D.; Apollaro, C.; Critelli, T.; Muto, F.; Cazzanelli, E.; Barrese, E. The mineralogical study of the grotta inferiore di sant’angelo (southern italy). J. Cave Karst Stud. 2014, 76, 51–61. [Google Scholar] [CrossRef]
- Pierre, C.; Rouchy, J.M. Origin of the gypsum veins associated with the diagenetic dolomite nodules from the Neogene Marls of South-East Spain. Chem. Geol. 2020, 542, 119597. [Google Scholar] [CrossRef]
- Stanimirova, T.; Ivanova, K. Transformation of ktenasite-type minerals to langite, posnjakite, and brochantite under water treatment. C. R. Acad. Bulg. Sci. 2019, 72, 768–776. [Google Scholar]
- Kettanah, Y.A. Copper mineralization and alterations in Gercus Basalt within the Gercus Formation, northern Iraq. Ore Geol. Rev. 2019, 111, 102974. [Google Scholar] [CrossRef]
- Mills, S.; Aishima, J.; Aragao, D.; Caradoc-Davies, T.T.; Cowieson, N.; Gee, C.L.; Ericsson, D.; Harrop, S.; Panjikar, S.; Smith, K.M.L.; et al. Crystal structure of posnjakite formed in the first crystal water-cooling line of the ANSTO Melbourne Australian Synchrotron MX1 Double Crystal Monochromator. Acta Crystallogr. 2020, 76, 1136–1138. [Google Scholar] [CrossRef]
- Amodio-Morelli, L.; Bonardi, G.; Colonna, V.; Dietrich, D.; Giunta, G.; Ippolito, F.; Liguori, V.; Lorenzoni, S.; Paglionico, A.; Perrone, V.; et al. L’arco calabro-peloritano nell’orogene appenninico-maghrebide. Mem. Soc. Geol. Ital. 1976, 17, 160Antonioli. [Google Scholar]
- Borsi, S.; Merlin, H.O.; Lorenzoni, S.; Paglionico, A.; Lorenzoni-Zanettin, E. Stilo Unit and “Dioritic-Kinzingitic” Unit. in Le Serre (Calabria, Italy). Geological, petrological, geochronological characters. Boll. Soc. Geol. Ital. 1976, 95, 219–244. [Google Scholar]
- Crisci, G.M.; Leoni, R.; Mazzuoli, L.; Moresi, M.; Paglionico, A. Petrological and geochemical data on two intrusive stocks of the “Serre” (Calabria, Southern Italy). N. Jb. Miner. Abb. 1980, 138, 274–291. [Google Scholar]
- Bonardi, G.; Giunta, G.; Perrone, V.; Russo, M.; Zuppetta, A.; Ciampo, G. Osservazioni sull’evoluzione dell’arco Calabro-Peloritano nel Miocene Inferiore: La Formazione di Stilo-Capo d’Orlando. Boll. Soc. Geol. Ital. 1980, 99, 365–393. [Google Scholar]
- Miyake, M.; Minato, I.; Morikawa, H.; Iwai, S. Crystal structures and sulphate force constants of barite, celestite, and anglesite. Am. Min. 1978, 63, 506–510. [Google Scholar]
- Dove, P.M.; Czank, C.A. Crystal chemical controls on the dissolution kinetics of the isostructural sulfates: Celestite, anglesite, and barite. Geochim. Cosmochim. Acta 1995, 59, 1907–1915. [Google Scholar] [CrossRef]
- Elizondo-Álvarez, M.A.; Uribe-Salas, A.; Nava-Alonso, F. Flotation studies of galena (PbS), cerussite (PbCO3) and anglesite (PbSO4) with hydroxamic acids as collectors. Min. Eng. 2020, 155, 106456. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J.H. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta A Mol. Biomol. 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Bouchard, M.; Smith, D.C. Catalogue of 45 reference Raman spectra of minerals concerning research in art history or archaeology, especially on corroded metals and coloured glass. Spectrochim. Acta A Mol. Biomol. 2003, 59, 2247–2266. [Google Scholar] [CrossRef] [PubMed]
- Jehlička, J.; Vítek, P.; Edwards, H.G.M.; Hargreaves, M.D.; Čapounc, T. Fast detection of sulphate minerals (gypsum, anglesite, baryte) by a portable Raman spectrometer. J. Raman Spectrosc. 2009, 40, 1082–1086. [Google Scholar] [CrossRef]
- Sawchuk, K.; O’Bannon, E.F.; Vennari, C.; Kavner, A.; Knittle, E.; Williams, Q. An infrared and Raman spectroscopic study of PbSO4-anglesite at high pressures. Phys. Chem. Mine. 2019, 46, 623–637. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Jiao, X. A novel insight of the effect of sodium chloride on the sulfidization flotation of cerussite. Powder Technol. 2019, 344, 103–107. [Google Scholar] [CrossRef]
- Martens, W.N.; Rintoul, L.; Llew, J.T.; Kloprogge, R.L. Frost, Single crystal raman spectroscopy of cerussite. Am. Min. 2004, 89, 352–358. [Google Scholar] [CrossRef]
- Farsang, S.; Widmer, R.N.; Redfern, S.A. High-pressure and high-temperature vibrational properties and anharmonicity of carbonate minerals up to 6 GPa and 500 degrees C by Raman spectroscopy. Am. Min. 2021, 106, 581–598. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker version 3.0, a handy set for conservation scientists: A free online Raman spectra database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Martens, W.; Frost, R.L.; Kloprogge, J.T.; Williams, P.A. Raman spectroscopic study of the basic copper sulphates-implications for copper corrosion and ‘bronze disease’. J. Raman Spectrosc. 2003, 34, 145–151. [Google Scholar] [CrossRef]
- Hayez, V.; Guillaume, J.; Hubin, A.; Terryn, H. Micro-Raman spectroscopy for the study of corrosion products on copper alloys: Setting up of a reference database and studying works of art. J. Raman Spectrosc. 2004, 35, 732–738. [Google Scholar] [CrossRef]
- Coccato, A.; Bersani, D.; Coudray, A.; Sanyova, J.; Moens, L.; Vandenabeele, P. Raman spectroscopy of green minerals and reaction products with an application in Cultural Heritage research. J. Raman Spectrosc. 2016, 47, 1429–1443. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The RRUFF Project. In Highlights in Mineralogical Crystallography, Armbruster, T., Danisi, R.M., Eds.; Walter de Gruyter: Berlin, Germany, 2015; pp. 1–30. Available online: https://rruff.info/about/downloads/HMC1-30.pdf) (accessed on 21 December 2022).
- Mellini, M.; Merlino, S. Posnjakite: 2∞[Cu4(OH)6(H2O)O] octahedral sheets in its structure. Z. Kristallogr. Cryst. Mater. 1979, 149, 249–258. [Google Scholar] [CrossRef]
- Melo, M.J.; Otero, V.; Vitorino, T.; Araújo, R.; Muralha, V.S.; Lemos, A.; Picollo, M. A spectroscopic study of brazilwood paints in medieval books of hours. App. Spectr. 2014, 68, 434–443. [Google Scholar] [CrossRef]
- Zaffino, C.; Guglielmi, V.; Faraone, S.; Vinaccia, A.; Bruni, S. Exploiting external reflection FTIR spectroscopy for the in-situ identification of pigments and binders in illuminated manuscripts. Brochantite and posnjakite as a case study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 136, 1076–1085. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.N.; Rintoul, L.; Mahmutagic, E.; Kloprogge, J.T. Raman spectroscopic study of azurite and malachite at 298 and 77 K. J. Raman Spectrosc. 2002, 33, 252–259. [Google Scholar] [CrossRef]
- Antao, S.M.; Hassan, I. The orthorhombic structure of CaCO3, SrCO3, PbCO3 and BaCO3: Linear structural trends. Canad. Mineral. 2009, 47, 1245–1255. [Google Scholar] [CrossRef]
- Schofield, P.F.; Knight, K.S.; Stretton, I.C. Thermal expansion of gypsum investigated by neutron powder diffraction. Am. Min. 1996, 81, 847–851. [Google Scholar] [CrossRef]
- Gentsch, M.; Weber, K. Structure of langite, Cu4 [(OH)6|SO4]. 2H2O. Acta Crystallogr. C Struct. 1984, 40, 1309–1311. [Google Scholar] [CrossRef]
- Süsse, P.J.A.C. Verfeinerung der kristallstruktur des malachits, Cu2(OH)2CO3. Acta Crystallogr. 1967, 22, 146–151. [Google Scholar] [CrossRef]
- Wu, Z.; Dreisinger, D.B.; Urch, H.; Fassbender, S. Fundamental study of lead recovery from cerussite concentrate with methanesulfonic acid (MSA). Hydrometallurgy 2014, 142, 23–35. [Google Scholar] [CrossRef]
- Ciszewski, M.; Drzazga, M.; Kowalik, P.; Orda, S.; Hawełek, Ł. Lead electrodeposition from aliphatic polyamines solutions. SN Appl. Sci. 2022, 4, 130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).