Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents
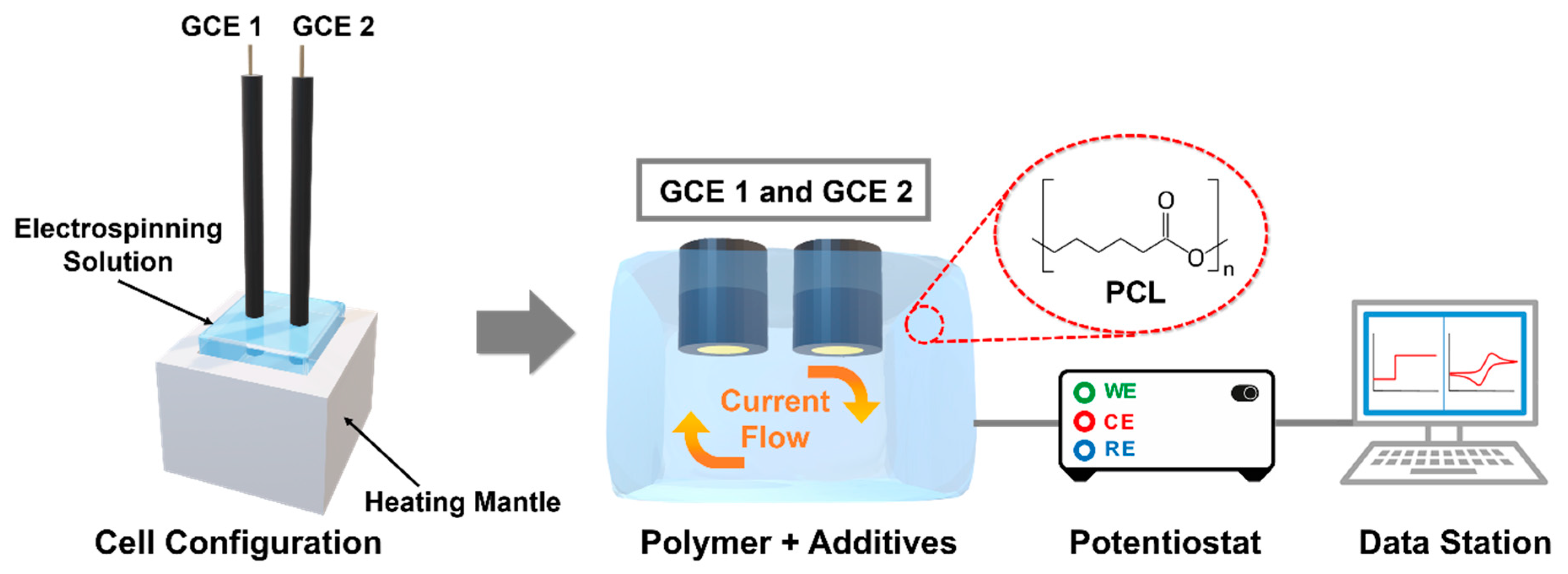
2.2. Experimental Setup for Measuring Conductance
2.3. Measurement of Viscosity
3. Results and Discussion
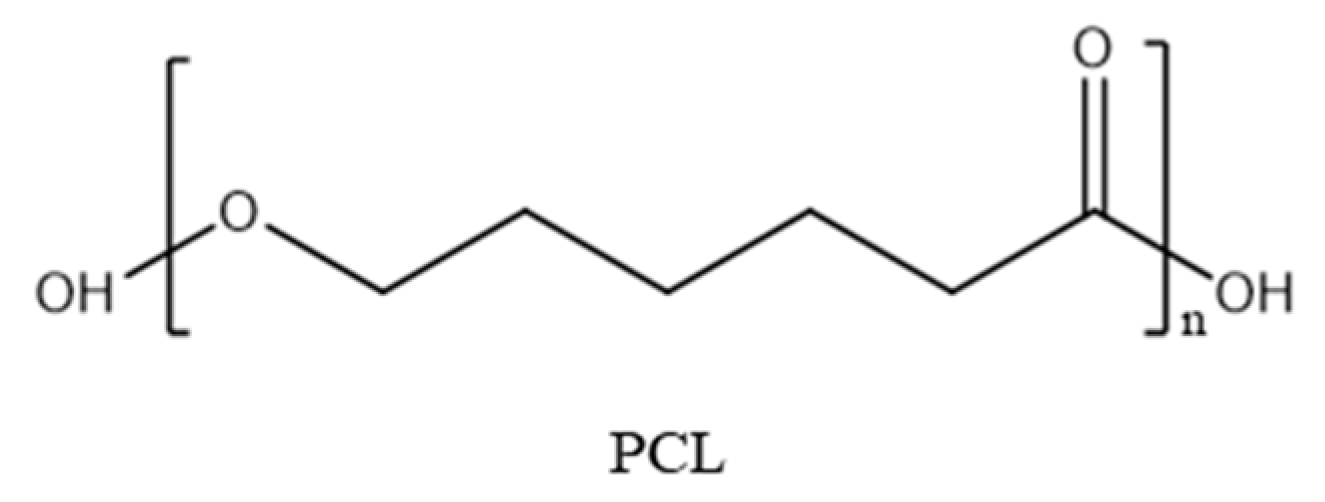
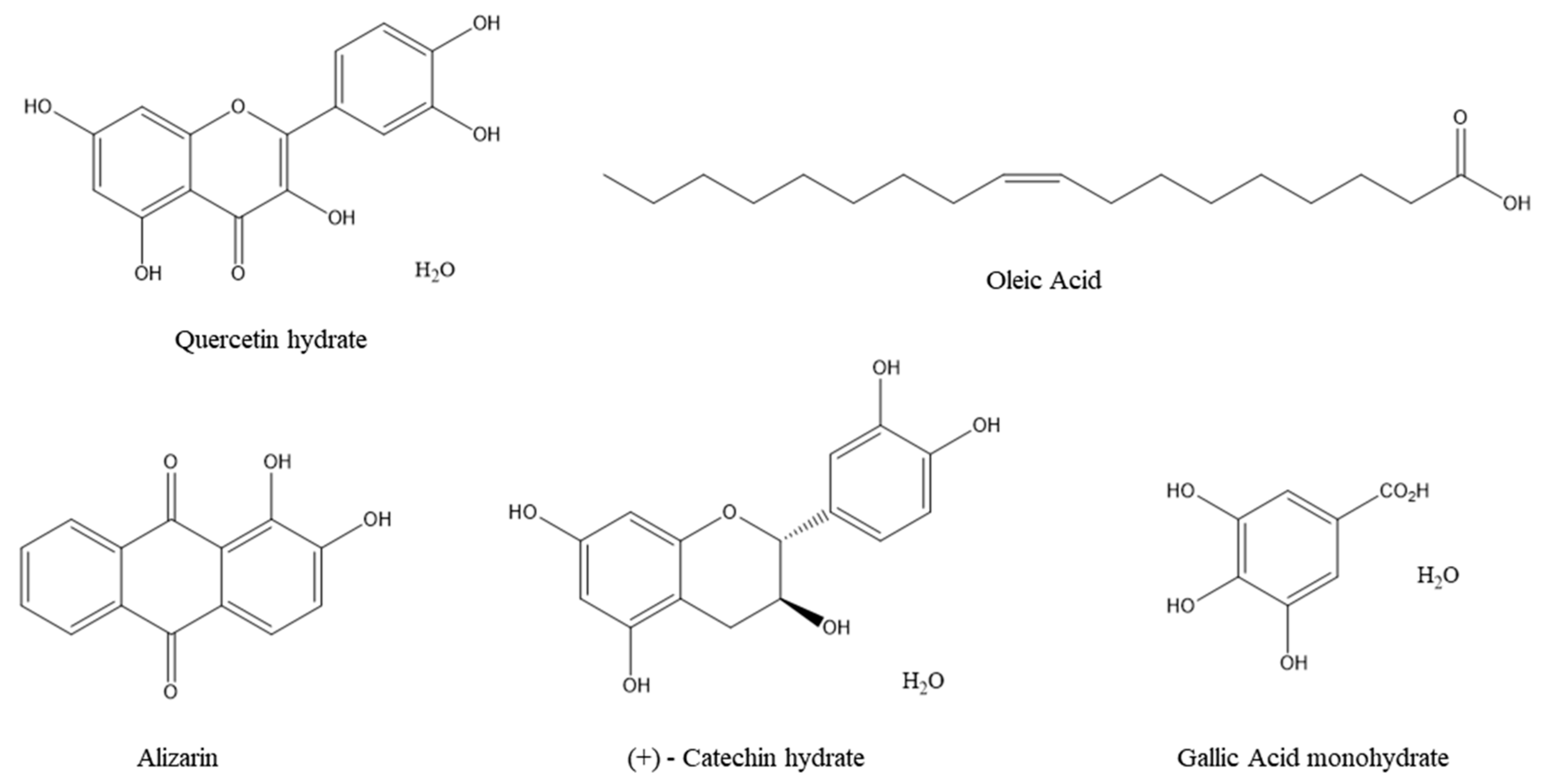
3.1. Characteristics of Additives
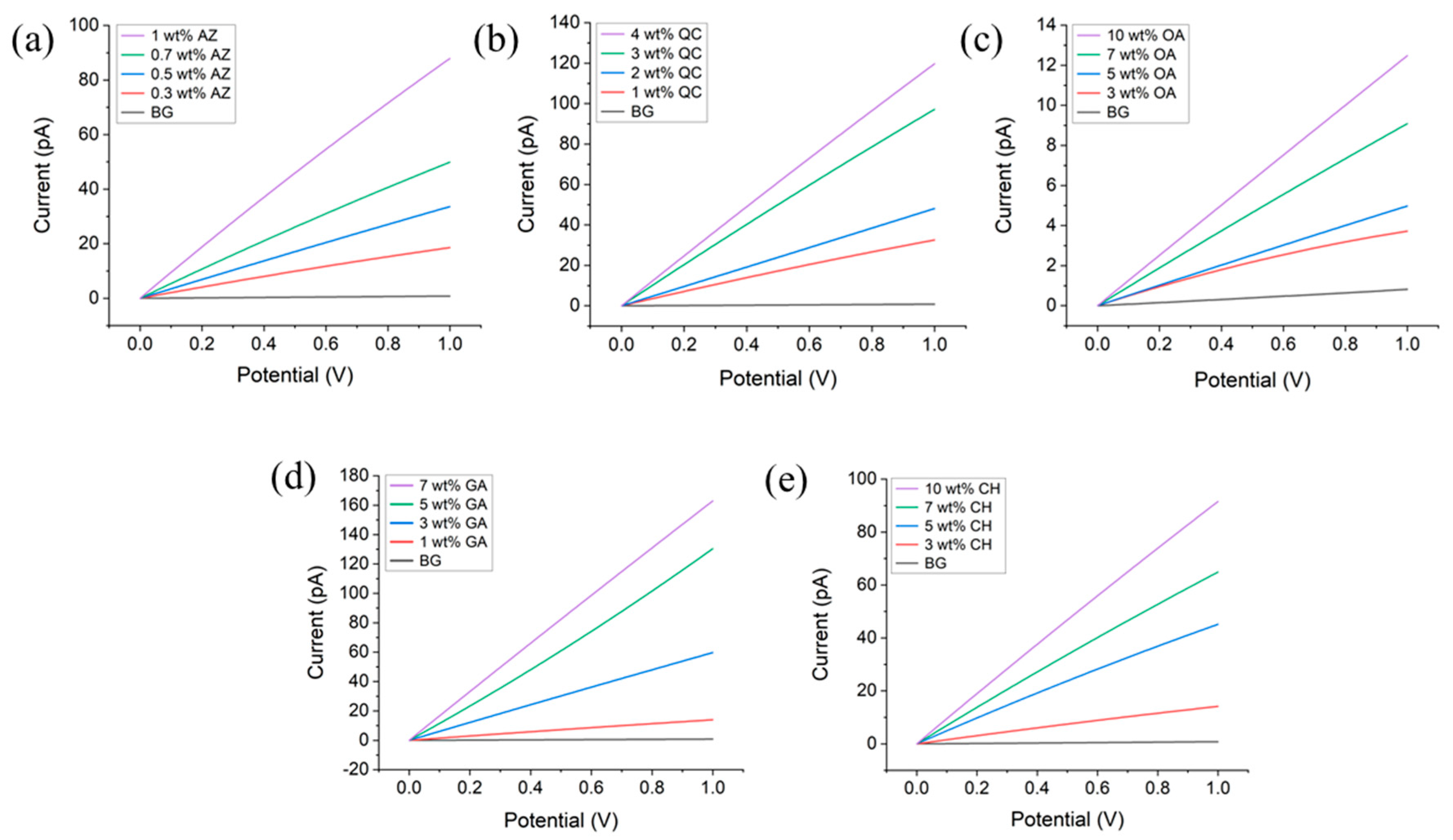
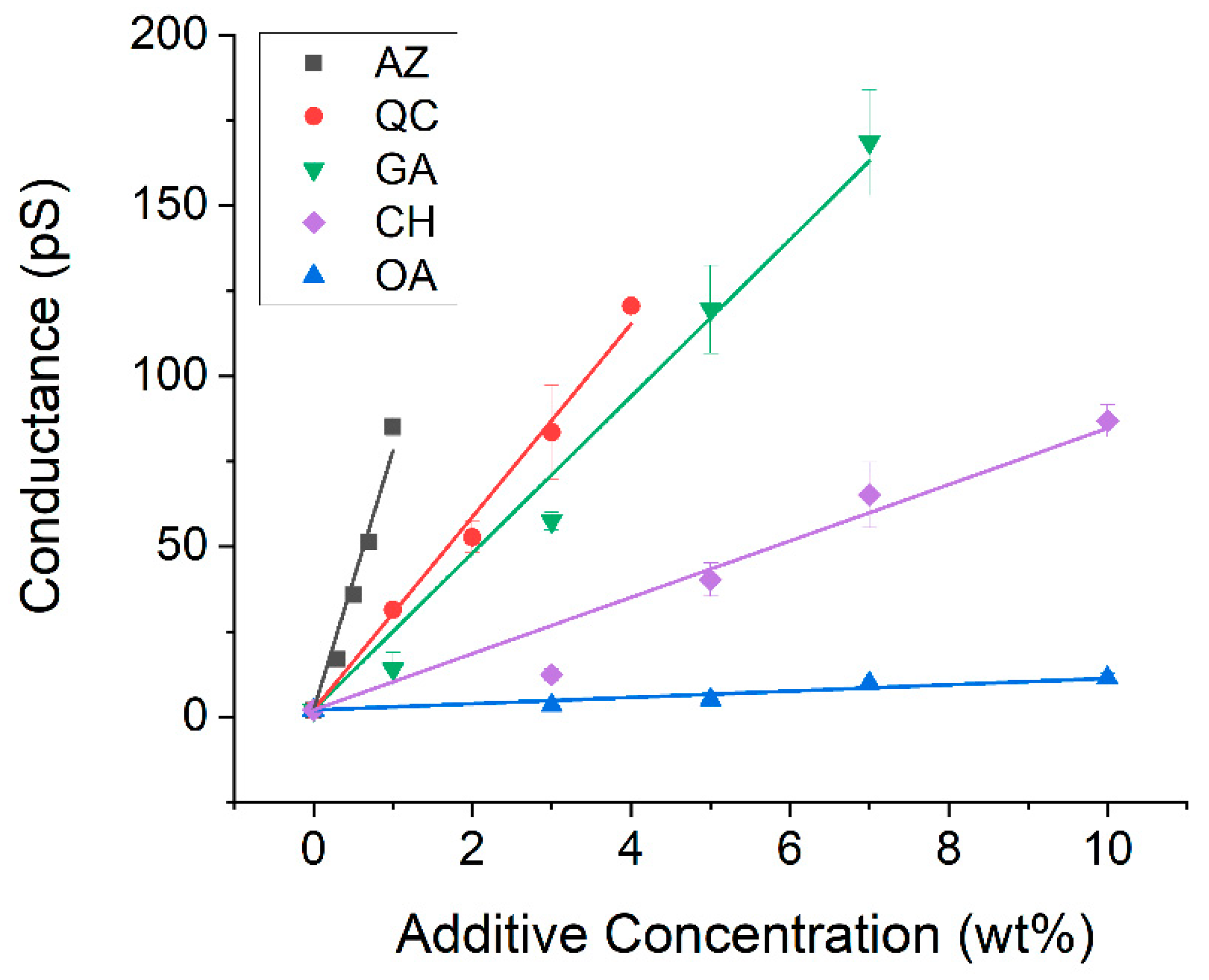
3.2. Improvement of Electrical Conductivity by Additives
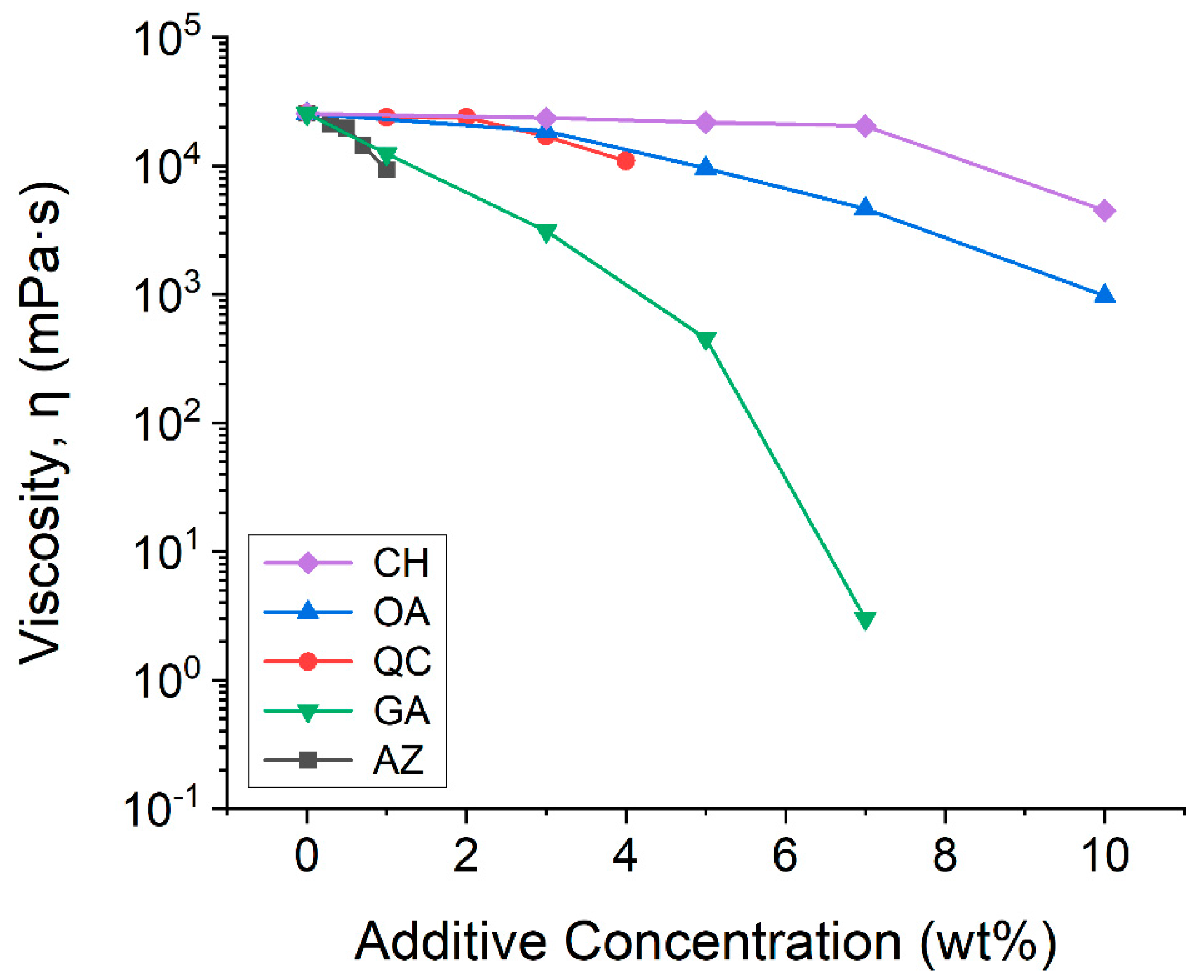
3.3. Effect of Additives on Viscosity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lam, C.X.; Teoh, S.H.; Hutmacher, D.W. Comparison of the degradation of polycaprolactone and polycaprolactone–(β-tricalcium phosphate) scaffolds in alkaline medium. Polym. Int. 2007, 56, 718–728. [Google Scholar] [CrossRef]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.S.; Fazliah, M.N.S.N.; Mislia, O.; Syazana, A.N.; Azrul, Z.M. Development of bioactive glass-poly-ε-caprolactone polymer composite film for soft tissue regeneration. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020. [Google Scholar]
- Lo, H.-Y.; Kuo, H.-T.; Huang, Y.-Y. Application of Polycaprolactone as an Anti-Adhesion Biomaterial Film. Artif. Organs 2010, 34, 648–653. [Google Scholar] [CrossRef]
- Shi, R.; Xue, J.; Wang, H.; Wang, R.; Gong, M.; Chen, D.; Zhang, L.; Tian, W. Fabrication and evaluation of a homogeneous electrospun PCL–gelatin hybrid membrane as an anti-adhesion barrier for craniectomy. J. Mater. Chem. B 2015, 3, 4063–4073. [Google Scholar] [CrossRef] [PubMed]
- Labet, M.; Thielemans, W. Synthesis of polycaprolactone: A review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bae, J.E.; Shin, M.J. Controlled release of curcumin from coaxial electrospun nanofiber mats. Bull. Korean Chem. Soc. 2022, 43, 984–989. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2017, 29, 863–893. [Google Scholar] [CrossRef]
- Krunal, Y.E.P. Polycaprolactone Market by Form (Pellet, Microsphere, and Nanosphere), Manufacturing Method (Ring Opening Polymerization and Polycondesation of Carboxylic Acid), and Application (Coating & Thermoplastic Polyurethane, Healthcare, and Others): Global Opportunity Analysis and Industry Forecast, 2019–2026 (Report No. A06096). Allied Market Research. 2020. Available online: https://www.alliedmarketresearch.com/polycaprolactone-market-A06096.html (accessed on 28 January 2023).
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Du, J.; Shintay, S.; Zhang, X. Diameter control of electrospun polyacrylonitrile/iron acetylacetonate ultrafine nanofibers. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1611–1618. [Google Scholar] [CrossRef]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 87, 4531–4547. [Google Scholar] [CrossRef]
- Lin, T.; Wang, H.; Wang, H.; Wang, X. The charge effect of cationic surfactants on the elimination of fibre beads in the electrospinning of polystyrene. Nanotechnology 2004, 15, 1375–1381. [Google Scholar] [CrossRef]
- Nayak, R.; Kyratzis, I.L.; Truong, Y.B.; Padhye, R.; Arnold, L. Melt-electrospinning of polypropylene with conductive additives. J. Mater. Sci. 2012, 47, 6387–6396. [Google Scholar] [CrossRef]
- Luo, C.; Stride, E.; Edirisinghe, M. Mapping the Influence of Solubility and Dielectric Constant on Electrospinning Polycaprolactone Solutions. Macromolecules 2012, 45, 4669–4680. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibres by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Ekren, N.; Suleymanoglu, M.; Erdem-Kuruca, S.; Lin, C.-C.; Bulbul, E.; Erdol, M.N.; Oktar, F.N.; Terzi, U.K.; Kilic, O.; et al. Novel electrospun polycaprolactone/graphene oxide/Fe3O4 nanocomposites for biomedical applications. Colloids Surfaces B Biointerfaces 2018, 172, 718–727. [Google Scholar] [CrossRef]
- Nadim, A.; Khorasani, S.N.; Kharaziha, M.; Davoodi, S.M. Design and characterization of dexamethasone-loaded poly (glycerol sebacate)-poly caprolactone/gelatin scaffold by coaxial electro spinning for soft tissue engineering. Mater. Sci. Eng. C 2017, 78, 47–58. [Google Scholar] [CrossRef]
- Goreninskii, S.; Danilenko, N.; Bolbasov, E.; Evtina, A.; Buldakov, M.; Cherdyntseva, N.; Opitz, J.; Filimonov, V.; Tverdokhlebov, S. Enhanced properties of poly(ε-caprolactone)/polyvinylpyrrolidone electrospun scaffolds fabricated using 1,1,1,3,3, 3-hexafluoro-2-propanol. J. Appl. Polym. Sci. 2021, 138, app50535. [Google Scholar] [CrossRef]
- Kulpreechanan, N.; Bunaprasert, T.; Rangkupan, R. Electrospinning of Polycaprolactone in Dichloromethane/Dimethylformamide Solvent System. Adv. Mater. Res. 2014, 849, 337–342. [Google Scholar] [CrossRef]
- Zhang, S.; Campagne, C.; Salaün, F. Influence of Solvent Selection in the Electrospraying Process of Polycaprolactone. Appl. Sci. 2019, 9, 402. [Google Scholar] [CrossRef]
- He, Y.; Wildman, R.; Tuck, C.; Christie, S.; Edmondson, S. An Investigation of the Behavior of Solvent based Polycaprolactone ink for Material Jetting. Sci. Rep. 2016, 6, 20852. [Google Scholar] [CrossRef] [PubMed]
- Teske, M.; Arbeiter, D.; Schober, K.; Eickner, T.; Grabow, N. Systematic analysis about residual chloroform removal from PCL films. Curr. Dir. Biomed. Eng. 2018, 4, 567–569. [Google Scholar] [CrossRef]
- Sinha, V.R.; Trehan, A. Development, Characterization, and Evaluation of Ketorolac Tromethamine-Loaded Biodegradable Microspheres as a Depot System for Parenteral Delivery. Drug Deliv. 2008, 15, 365–372. [Google Scholar] [CrossRef]
- Piyasin, P.; Yensano, R.; Pinitsoontorn, S. Size-Controllable Melt-Electrospun Polycaprolactone (PCL) Fibers with a Sodium Chloride Additive. Polymers 2019, 11, 1768. [Google Scholar] [CrossRef]
- Zhang, L.-H.; Duan, X.-P.; Yan, X.; Yu, M.; Ning, X.; Zhao, Y.; Long, Y.-Z. Recent advances in melt electrospinning. RSC Adv. 2016, 6, 53400–53414. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Wendorff, J.H. Nanostructured fibers via electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Garg, K.; Bowlin, G.L. Electrospinning jets and nanofibrous structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef]
- Deng, R.; Liu, Y.; Ding, Y.; Xie, P.; Luo, L.; Yang, W. Melt electrospinning of low-density polyethylene having a low-melt flow index. J. Appl. Polym. Sci. 2009, 114, 166–175. [Google Scholar] [CrossRef]
- Hardick, O.; Stevens, B.; Bracewell, D.G. Nanofibre fabrication in a temperature and humidity controlled environment for improved fibre consistency. J. Mater. Sci. 2011, 46, 3890–3898. [Google Scholar] [CrossRef]
- Narayanan, G. Electrospinning of Poly (ϵ-Caprolactone) Fibers Functionalized with Cyclodextrins and Their Inclusion Complexes; North Carolina State University: Raleigh, NC, USA, 2014. [Google Scholar]
- Zhou, H. Electrospun Fibers from Both Solution and Melt: Processing, Structure and Property; The American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Ramakrishna, S. An introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005. [Google Scholar]
- Lyons, J.M. Melt-Electrospinning of Thermoplastic Polymers: An Experimental and Theoretical Analysis; Drexel University: Philadelphia, PA, USA, 2004. [Google Scholar]
- Nayak, R.; Padhye, R.; Kyratzis, I.L.; Truong, Y.B.; Arnold, L. Effect of viscosity and electrical conductivity on the morphology and fiber diameter in melt electrospinning of polypropylene. Text. Res. J. 2013, 83, 606–617. [Google Scholar] [CrossRef]
- Bachs-Herrera, A.; Yousefzade, O.; del Valle, L.; Puiggali, J. Melt Electrospinning of Polymers: Blends, Nanocomposites, Additives and Applications. Appl. Sci. 2021, 11, 1808. [Google Scholar] [CrossRef]
- Reddy, C.V.S.; Sharma, A.; Rao, V.N. Effect of plasticizer on electrical conductivity and cell parameters of PVP+ PVA+ KClO3 blend polymer electrolyte system. J. Power Sources 2002, 111, 357–360. [Google Scholar] [CrossRef]
- Malakhov, S.N.; Belousov, S.I.; Orekhov, A.S.; Chvalun, S.N. Electrospinning of Nonwoven Fabrics from Polypropylene Melt with Additions of Stearates of Divalent Metals. Fibre Chem. 2018, 50, 27–32. [Google Scholar] [CrossRef]
- Lyons, J.; Li, C.; Ko, F. Melt-electrospinning part I: Processing parameters and geometric properties. Polymer 2004, 45, 7597–7603. [Google Scholar] [CrossRef]
- Larrondo, L.; St. John Manley, R. Electrostatic fiber spinning from polymer melts. I. Experimental observations on fiber formation and properties. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 909–920. [Google Scholar] [CrossRef]
- Roth, M.; Pfaendner, R.; Simon, D. Molecular Weight Modification of Thermoplastic Polymers. U.S. Patent No. 6,949,594, 27 September 2005. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Park, S.; Park, K.; Kim, B.-K. Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. Appl. Sci. 2023, 13, 1844. https://doi.org/10.3390/app13031844
Kim JW, Park S, Park K, Kim B-K. Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. Applied Sciences. 2023; 13(3):1844. https://doi.org/10.3390/app13031844
Chicago/Turabian StyleKim, Jee Woo, Seongho Park, Kyungsoon Park, and Byung-Kwon Kim. 2023. "Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning" Applied Sciences 13, no. 3: 1844. https://doi.org/10.3390/app13031844
APA StyleKim, J. W., Park, S., Park, K., & Kim, B.-K. (2023). Non-Toxic Natural Additives to Improve the Electrical Conductivity and Viscosity of Polycaprolactone for Melt Electrospinning. Applied Sciences, 13(3), 1844. https://doi.org/10.3390/app13031844






