Abstract
The objective was to compare the strain of three calcium silicate cements using an optical system based on the 3D digital image correlation method (3D-DIC). Dentine disks from 30 upper premolars were sectioned transversely to obtain 2 mm-thick sections and enlarged with a 4 mm diameter bur. An additional 30 samples were made in Teflon molds (4 × 2 mm). Dentine discs and Teflon molds were divided into three groups with ten samples each and then filled with MTA+ (Cerkamed), Biodentine (Septodont) and Well-Root PT (Vericom). The strain was determined using the 3D-DIC method in two zones: the peripheral and central zones. Data were analyzed using ANOVA with Scheffe’s post hoc test, a paired t-test and Pearson correlation (α = 0.05). Analysis showed that there were significant differences in the values of deformation between all tested materials in both zones. Comparing the strain in both zones, there were significant differences between zones in the Biodentin and Well-Root PT group on dentine discs, and in the Biodentine and MTA group on Teflon discs. Comparing the strain measured on different disc types, the higher values were found on Teflon. All the examined calcium silicate-based cements showed deformation after initial setting. The highest strain was recorded for Biodentine and the lowest was for Well-Root PT.
1. Introduction
During endodontic treatment, as a result of the complex anatomy of the root canal system [1], procedural mistakes, such as perforation of the pulp chamber walls in coronal and intraradicular areas, can often occur [2,3]. With the interruption of the continuity of the dentine walls and the damage to cement tissue, contact is made with the periodontium. Frequently, the therapeutic outcome of these complications is unsuccessful and leads to the extraction of the tooth [4,5].
Therapeutic success depends on multiple factors: the localization of the damage, the size of the damage, the time elapsed since the occurrence of the perforation and the properties of the repairing material [6,7]. The recommendation is to close the defect as soon as possible to prevent bacterial contamination and the extrusion of infected dentin during treatment or filling material during retreatment [8,9].
Materials that are used for this purpose should provide firm sealing, lowering the probability of local inflammation, and also should promote periradicular tissue regeneration. Thanks to its positive properties such as its biocompatibility, bioactivity and the ability to achieve a good marginal seal in a moist environment, mineral trioxide aggregate (MTA) is one of the most frequently used materials for perforation repairment [10]. By mixing components of MTA, the result is a material of sandy consistency, difficult to manipulate, which has a long setting time and the potential for tooth discoloration [11]. The advancement in material properties in order to achieve higher clinical success has brought new formulations to the market. One of them, a material from a group of calcium silicate-based cements, is Biodentine (Septodont, Saint-Maur-des-Fosses, France). The main elements of this powder are tricalcium silicate, zirconium oxide and calcium carbonate. The liquid portion is a mixture of water, calcium chloride (setting accelerator) and a hydrosoluble polymer. The advancement in the basic components has brought a faster setting time with a low water to powder ratio [12], higher compression and flexural strength of the material [13] and extraordinary bioactivity [14]. Materials based on calcium silicate, which are a combination of powder and a liquid, such as MTA and Biodentine, belong to the hydraulic type of cement; materials that set by a hydration reaction [15]. Premixed calcium silicate-based cements supplied in a syringe in the form of a paste are also available. These cements need water from their surroundings for the process of hydration, because they are not mixed with water [16]. These premixed cements have a simplified application. One such cement is the radiopaque material based on calcium aluminosilicate, Well-Root PT (Vericom, Chuncheon-si, Korea). Studies have shown that this material possesses high antibacterial activity, forms phosphate crystals in phosphate-buffered saline (PBS) and has a solubility comparable to MTA and Biodentine [17]. There are no available studies in the literature on other physical or chemical properties of this cement, which would contribute to better application of this material in dentistry.
Materials placed in the defect zone are exposed to environmental influences (tissue fluid, blood and products of bacterial metabolism) and the effect of canal irrigants during endodontic treatment. The unfavorable effects of these factors cause the inability of the material to adequately set, the change in material properties (microhardness and bond strength) [18,19,20] and changes in the surface chemical structure [21]. Good marginal adaptation [22] and the ability to withstand the dislodging forces, which lead to marginal gap formation [23] and bacterial leakage [24], are necessary properties of materials for perforation repair. Different degrees of dimensional changes lead to formation of gaps and voids of different volume [25].
Changes in material dimensions have been measured in previous studies using a linear variable transducer [26] and scanning electron microscopy (SEM) [27]. Recent research has uses micro-computed tomography (μCT) to analyze dimensional stability, porosity and estimate the size and character of pores present in the material [28]. Although this method is accurate and reliable, it takes more time, especially if the change is observed during a certain time interval [29]. A three-dimensional (3D) optical system based on the digital image correlation (DIC) method is a new method that has been used for the evaluation of strain on the surface of the material. It is a noninvasive, noncontact optical method, based on digital image correlation for deformation analysis of local fields. The DIC method has been widely used from testing process equipment and defining new geometries of test samples to developing dental models for experimental research [30,31,32,33]. Application of this method in dentistry has proven to be very useful in analysis in the bone denture complex [34].
The aim of this research was to determine, analyze and compare the strain of three calcium silicate-based materials: MTA+ (Cerkamed Company, Stalowa Wola, Poland), Biodentine (Septodont, Saint-Maur-des-Fosses, France) and Well-Root PT (Vericom, Chuncheon-si, Korea) during setting time, using an optical system based on the 3D digital image correlation method (3D-DIC).
2. Materials and Methods
Determination of the strain fields that occurred during the setting time of the tested cements was performed using the 3D optical system Aramis 2M (GOM, Braunschweig, Germany). The measuring system recorded the image of the tested object with the help of two high-resolution cameras (1600 × 1200 pixel resolution, 1µm displacement sensitivity and 12 Hz maximum frame rate) that provide images for 3D measuring (Figure 1). Automatic measurements can be performed thanks to a previous system calibration. Calibration was performed by using the calibration panel according to the volume of the measured object or according to the dimensions of the specimen surface. By adjusting the frequency and time intervals of recording, the resulting series of images were analyzed for determining displacement, which was used to calculate the surface strain of the object.
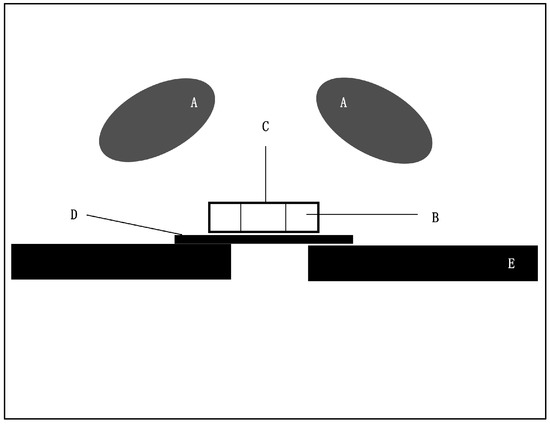
Figure 1.
Scheme of the experimental set-up. (A) Cameras, (B) Dentin/Teflon mold, (C) Sample, (D) Glass plate and (E) Sample holder.
In this research, 30 intact upper premolars, extracted due to orthodontic reasons, were used. Teeth were cleaned of debris, and kept in 0.02% chloramine solution for one month prior to the experiment. The use of extracted teeth in this research was approved by the Ethical Board of the Faculty of Dentistry in Pančevo (reference number 1139/2-2022). Dentine disks were sectioned transversely from the middle third of the tooth crowns, in order to obtain 2 mm-thick sections. In each section, the central space was enlarged with a 4 mm diameter cylindrical diamond bur to obtain standardized cavities within each disc. Dentine discs were divided into three groups with ten samples each, and then filled with MTA+ (Cerkamed Company, Stalowa Wola, Poland), Biodentine (Septodont, Saint-Maur-des-Fosses, France) or Well-Root PT (Vericom, Gangwon-Do, Korea). Materials were prepared according to the manufacturer’s instructions. An additional ten samples of each cement were made within ring-like Teflon molds of 4 mm diameter and 2 mm height. The samples were placed on a wet cotton gauze. The upper surface of each specimen was sprayed with a thin layer of matte white and matte black spray and dried within 10–20 s (Figure 2). In this way, a field of contrast was created, which allowed the specialized software Aramis 6.2.0 (GOM, Braunschweig, Germany) to more easily track the surface points. The software automatically defined the reference coordinate system and recorded reference points on the surface by assigning coordinates to them. Each change was compared with the previous pictures in the sequence by using the digital image correlation method (DIC). Collecting digital images began immediately upon the placement of materials and continued automatically every five seconds. Three sections were observed on each specimen. The first section was circular, positioned on a peripheral segment of each sample (SO). The remaining two sections were linear, positioned perpendicularly to each other and they measured the central deformation (SS). The sections were obtained according to the locations of the largest measured deformations in the selected regions. Software analysis of the images was used to determine the von Mises strain (%). The period of imaging for each material was chosen in accordance with the manufacturer’s predetermined setting time. Biodentine offers an initial setting time of 12 min. The setting time of Well-Root PT is within ten minutes. MTA+ cures for six to ten minutes after mixing with water.

Figure 2.
Sprayed samples.
Results obtained in this experiment were analyzed using the IBM SPSS Statistics 25 program (IBM, Armonk, NY, USA). Nested (hierarchic) ANOVA was used for testing the differences between the means of the three cements. The Scheffe post hoc test was used after significant F-statistic determination in ANOVA, to find out which pairs of means were significantly different. In order to look for significant differences between means of SS and SO, the paired t-test was used. Pearson correlation analysis was used to determine if there was a linear relationship between the values of deformation in both measured zones for each material group. For testing the differences between the deformation of different disk types (dentin/Teflon) in both zones of each material group, analysis of variance (ANOVA) was used.
3. Results
Figure 3, Figure 4 and Figure 5 shows the representative images of the von Mises strain across the surface of each material of dentine disc.
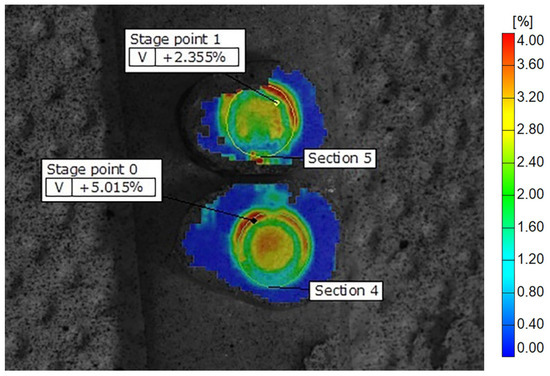
Figure 3.
Display of Biodentine deformation fields across the samples on dentine discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0 and 1.

Figure 4.
Display of MTA deformation fields across the samples on dentine discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0, 1 and 2.
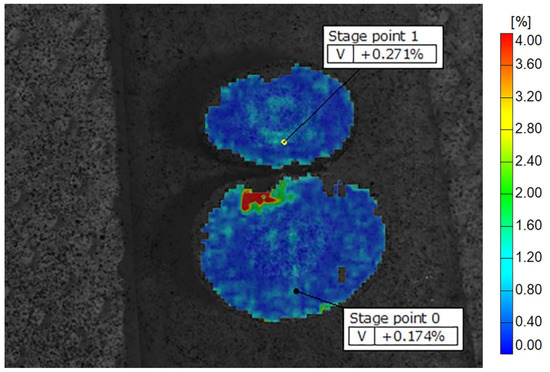
Figure 5.
Display of Well-Root PT deformation fields across the samples on dentine discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0 and 1.
Comparing the results measured on dentine discs, according to nested ANOVA, significant differences were found between all three materials, both centrally (SS) (F = 19.90; P < 0.0001) and peripherally (SO) (F = 18.69; P < 0.0001), as illustrated in Table 1.

Table 1.
Nested ANOVA analysis of significant differences between the materials and dentine discs in the central (SS) and peripheral (SO) zones.
The mean values of the von Mises strain of different materials on dentine discs are shown in Figure 6. The highest values of strain were measured in the Biodentine group in both zones. The lowest values of the von Mises strain were measured in the Well-Root PT group in both zones. The Scheffe post hoc test showed that there were significant differences in the values of deformation between all tested materials in the central zone, as well as in the peripheral zone (Figure 6).
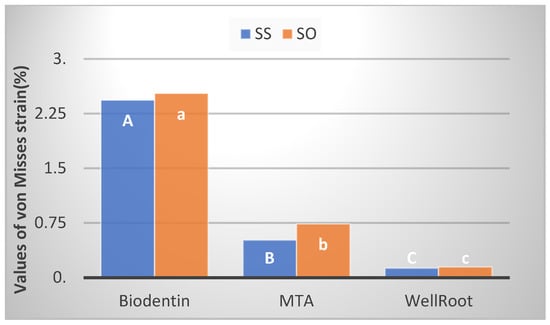
Figure 6.
Bar chart shows mean values of von Mises strain in both zones, central (SS) and peripheral (SO), measured on dentine discs. Significant differences in the central zone are marked with different upper-case letters and those in the peripheral zone are marked with different lower-case letters.
Figure 7, Figure 8 and Figure 9 are representative images of the von Mises strain across the surface of each material on Teflon discs.
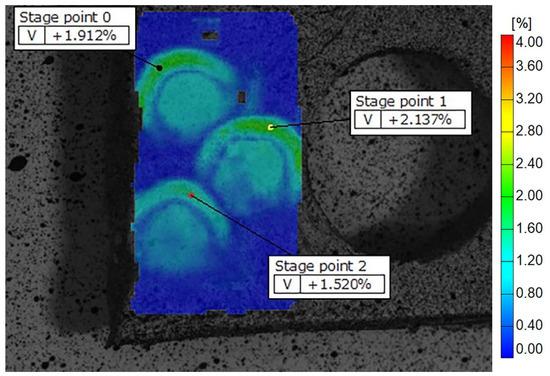
Figure 7.
Display of Biodentine deformation fields across the samples on Teflon discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0, 1 and 2.
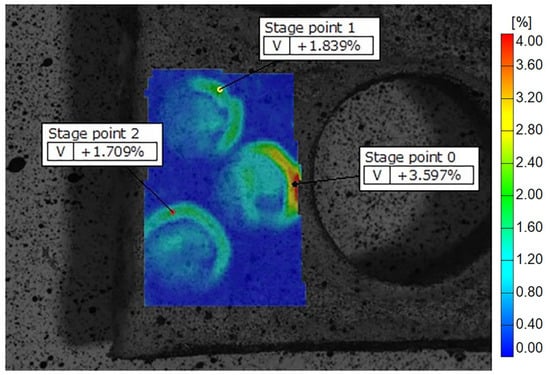
Figure 8.
Display of MTA deformation fields across the samples on Teflon discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0, 1 and 2.
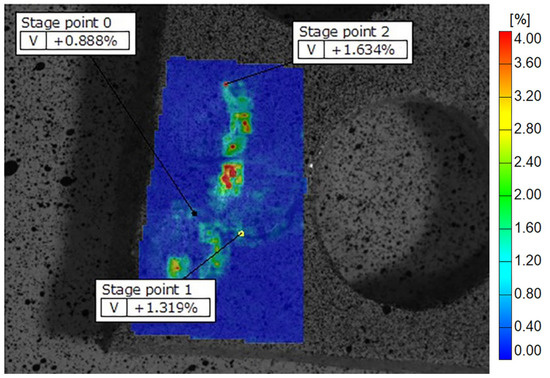
Figure 9.
Display of Well Root PT deformation fields across the samples on Teflon discs, showing peripheral zones with higher and central zones with lower strain values. The color scale indicates different strain values, with the highest marked in red and orange. Some of the recorded reference points are shown as stage points 0, 1 and 2.
The nested ANOVA showed that there were significant differences between tested materials on the Teflon discs in the central zone (SS) (F = 13.96; P < 0.0001), as well as in the peripheral (SO) (F = 8.35, P < 0.0001), as illustrated in Table 2.

Table 2.
Nested ANOVA analysis of significant differences between the materials and Teflon discs in the central (SS) and peripheral (SO) zones.
The mean strain values measured on the Teflon discs are shown in Figure 10. The highest value of strain was within the MTA group in the peripheral zone, while the lowest value was in the Well-Root PT group in the central zone. According to the Scheffe post hoc test, there was a significant difference between all three materials in both measured zones (SS and SO) (Figure 10).

Figure 10.
Bar chart shows mean values of von Mises strain in both zones, central (SS) and peripheral (SO), measured on Teflon discs. Significant differences in the central zone are marked with different upper-case letters and those in the peripheral zone are marked with different lower-case letters.
Comparing the strain of central and peripheral sections within all groups of materials, higher strain values were measured peripherally. The paired T-test revealed significant differences between zones in Biodentin and Well-Root PT group on dentine discs, and in Biodentine and MTA group on Teflon discs (Table 3).

Table 3.
Results of paired T-test for differences between two measures zones (SS and SO) for three materials in dentin and Teflon.
Comparing the strain measured on different disc types (dentine/Teflon), ANOVA revealed significant differences in both zones in each material group. In MTA and Well-Root PT groups, higher values were found with Teflon, whilst in the Biodentine group, higher values of strain were measured with dentine discs within both measured zones (Table 4).

Table 4.
ANOVA analysis of differences between two different disc types (dentine and Teflon) for each material group (Biodentin, MTA and Well-Root PT).
Significant positive correlations between the deformation in the central and peripheral zones (SS and SO) were found in each of the used materials and on both disc types. For all the materials and both disc types, the correlation was very high. The values of Pearson’s correlation coefficient were higher than 0.8, except for in the case of Well-Root PT–Teflon, whose value was smaller (r = 0.4) (Table 5).

Table 5.
Pearson’s correlation coefficient between mean values of central and peripheral zones (SS and SO) in three groups of materials and on both disc types.
4. Discussion
In this research, a 3D optical system based on the digital image correlation method was used for the evaluation of dimensional changes on the surface of a material during setting time. Strain is the quantity that is used to reflect deformation. Von Mises strain was chosen as it represents an index combining the principal strain at any given point at which strain occurring on the X-, Y- and Z-axis will cause failure [35]. This system is able to read a broad spectrum of micro-changes on the tested surfaces, it is simple to perform and it can be repeated. Following the full-field strain measurement, a 3D image is made of the whole field of deformation and the zones of greatest changes are clearly visible [36].
The time period during which the dimensional changes are analyzed is the setting time specified by the manufacturer’s instructions. The setting time is defined as the length of time for a material to transition from a fluid state into a hardened state [37]. The goal of this research was to record dimensional changes during and immediately after setting. Considering that the microhardness [38] and bond strength to dentin [39] of MTA and Biodentin are similar, and that, according to the micro-CT findings in [40], MTA and Biodentin show a comparable result in terms of their internal adaptation on the dentin surface, then the degree of dimensional stability of materials could indicate the reliability of the adherent sealing of the chosen material. During the setting time, dimensional changes and deformation of the material occur [41], on which canal irrigation agents could have a negative impact [42].
In this research, the results showed a significant difference in deformation between all tested materials in the central and peripheral sections of the dentine discs. The highest deformation was measured in the Biodentine group and the lowest deformation was in the Well-Root PT group in both zones.
Previous research on the dimensional changes of calcium silicate materials showed that particle size has an effect on the expansion of cements during the setting time. Well-Root PT has the smallest particle size (5 μm to 30 μm) and therefore less dimensional changes are expected [43]. White MTA also has a small particle size (73% of it is in the range of 6–10 μm), and therefore has less hygroscopic expansion during the setting of the material [44,45,46]. Material expansion is related to water uptake [46,47]. It does not occur in cements immersed in oil [46]. Additionally, 90% of specimens of dry MTA powder inside root canals had hardened simply by the absorption of water through the root surface within 72 h [48].
The lower values of von Mises strain of Well-Root PT could be explained by the fact that the material is premixed, injectable and its putty consistency significantly improves its handling characteristics. As a result of the better manipulation ability, a better adaptation of the material to the walls of the perforation cavity can be expected, which will have an effect on the sealing ability of the material. Well-Root PT material is based on tricalcium silicate and it includes zirconium oxide, calcium aluminosilicate and a thickening agent. The presence of aluminosilicate in the powder provides a more rapid setting compared to calcium silicate. There are few published data related to the properties of this material, but there are several studies concerning Well-Root ST sealer from the same manufacturer and based on the same basic components. Well-Root ST demonstrated less microleakage in comparison with other canal sealers. The size of the cement particles ranged from 5 to 30 μm and it does not shrink upon setting [43,49]. Well-Root PT showed alkaline pH, biomineralization properties and had comparable solubility in comparison to Biodentine and MTA [17]. The contact angle indicates the capability of the material to wet the dentin surface or the ability to achieve good adhesion. Research has shown that contact angles, investigated after 10 s, for Biodentin and Well-Root PT were 15° and 9°, respectively [17]. That implies that the bond of Well-Root to dentin could be more favorable than the bond created between Biodentine and dentin.
On the other hand, scanning electron micrographs of the hydrated cements, Biodentine and MTA, showed that Biodentine has more pronounced hydration kinetics. Concerning Biodentine, the particles of tricalcium silicate are smaller than those in MTA, it has a larger specific surface area and a significantly denser and finer consistency is achieved by intermixing the hydration products [50]; therefore, dimensional changes should be less. The highest strain measured in this study was for Biodentine, which could be a result of adding CaCl2. This is added in order to accelerate the setting reaction, but its presence increased the osmotic potential and the material expansion. An additional contributing factor to this result could be a more pronounced advancement in the hydration reaction, during which there was not enough moisture [48,51,52]. By placing a wet gauze underneath the sample, researchers tried to replicate real conditions during the closure of the perforations. In previous research, Camilleri et al. illustrated that Biodentine, even though it has low porosity, showed a tendency towards creating cracks within the material, as well as at the interface with the dentin wall in dry conditions [53]. These results are in agreement with research performed by Askel et al.; MTA did not show any dimensional changes, while Biodentine demonstrated shrinkage and crack formation in dry conditions [54]. According to these authors, in wet conditions, ProRoot MTA expanded by 0.41% and 0.17% on the 3rd and 28th day, respectively, while Biodentine expanded by 0.85% and 1.44%. The more pronounced expansion of Biodentine is attributed to the higher concentration of liberated Ca2+ ions and the accumulation of hydroxyapatite on the surface of the material. When observing the peripheral section, the greatest deformation was recorded in Biodentin samples, which could be caused by the hydrostatic tension due to shrinkage, which separated the material from the surface of dentin walls [53].
Within each group of materials, the difference in the strain between the central and peripheral zone was evaluated. A significant difference was shown between the samples of Biodentine and Well-Root PT on the dentine discs, as well as between Biodentin and MTA on the Teflon discs. Higher values of deformation were measured in the peripheral zones in all tested materials. Similar results were obtained by other authors concerning Biodentine on Teflon, with the highest deformation in the peripheral zone [55]. Worse results peripherally could be explained by the dynamic changes in the material interface zone with dentin or Teflon. A Pearson correlation analysis showed that a positive correlation between deformation of the central and peripheral zones is present in all groups, which could point to the homogenous structure of the cements. The lack of phosphates, as well as lack of a period of dimensional change examination in this research, limits the bioactivity effects of calcium silicate cements on sealing ability. In the examined moment, all the materials adhere to the walls macromechanically thanks to the micro-roughened surface of the dentin samples, the presence of moisture within dentinal tubules, along with the moist base on which the samples were placed. Investigations using micro-CT for evaluating void structures of MTA and Biodentin showed the presence of smaller voids evenly distributed throughout the specimen. Large voids with a radius of >35 μm were concentrated in the upper part of the specimen and at the interface [56]. The interface of the material with the dentin wall is of large clinical significance, and research has shown that precisely that kind of connection is the weak point in the material.
Results of this study showed that a significant difference exists between the values of deformation on Teflon and dentine discs, and that the values of deformation are higher on Teflon discs in samples of MTA and Well-Root PT. Teflon is a material that has a low surface free energy (SFE) and high values of contact angle (116.986° ± 5.02 for water). This makes it difficult to bond to, and therefore it can be characterized as a hydrophobic material [57]. On the other hand, with the advancement in hydration reactions, the hydration products will fill the gaps between the unhydrated cement grains [58], which contributes to a better adaptation of the material to dentin. The release of Ca2+ ions during and after the setting time in an environment rich in phosphates leads to the formation of hydroxyapatite crystals, the formation of which strengthens the bond between the material and the surface of tooth tissue by forming tag-like structures within dentinal tubules [59]. MTA constantly induces the initial precipitation of amorphous calcium phosphate after setting, resulting in the formation of a tag-like structure at the interface between the cement and the canal wall [56]. We are not able to claim that this degree of deformation and dimensional change has clinical significance, additional research will be needed to make that conclusion. The limit of this study is that the deformation fields were observed on the surface of the sample.
5. Conclusions
Within the limits of this study, all the examined calcium silicate-based cements showed dimensional change and deformation during the setting time. After the clinical application of immediate perforation repair, this deformation, especially in the peripheral section, could contribute to compromising marginal sealing. The highest strain was recorded in the Biodentine group and the lowest was in the Well-Root PT group.
Author Contributions
Conceptualization, A.N. and I.M.; Methodology, A.N. and I.M.; Software, M.M. and I.T.; Formal Analysis, M.M., I.T. and A.N.; Investigation, A.N., M.L. and D.P.; Resources, A.N. and I.M.; Data curation, A.N.; Writing-original draft, A.N. and I.M.; Writing-review & editing, D.P., V.K. and I.M.; Visualization, M.L. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Faculty of Dentistry in Pančevo (reference number 1139/2-2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reda, R.; Zanza, A.; Bhandi, S.; Biase, A.; Testarelli, L.; Miccoli, G. Surgical-anatomical evaluation of mandibular premolars by CBCT among the Italian population. Dent. Med. Probl. 2022, 59, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ingle, J.I. A standardized endodontic technique utilizing newly designed instruments and filling materials. Oral Surg. Oral Med. Oral Pathol. 1961, 14, 83–91. [Google Scholar] [CrossRef]
- Pinheiro, L.S.; Kopper, P.M.P.; Quintana, R.M.; Scarparo, R.K.; Grecca, F.S. Does MTA provide a more favourable histological response than other materials in the repair of furcal perforations? A systematic review. Int. Endod. J. 2021, 54, 2195–2218. [Google Scholar] [CrossRef] [PubMed]
- Sarao, S.K.; Berlin-Broner, Y.; Levin, L. Occurrence and risk factors of dental root perforations: A systematic review. Int. Dent. J. 2020, 71, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Mancino, D.; Meyer, F.; Haikel, Y. Improved single visit management of old infected iatrogenic root perforations using Biodentine®®. G. Ital. Di Endod. 2018, 32, 17–24. [Google Scholar]
- Estrela, C.; Decurcio, D.A.; Rossi-Fedele, G.; Silva, J.A.; Guedes, O.A.; Borges, Á.H. Root perforations: A review of diagnosis, prognosis and materials. Braz. Oral Res. 2018, 32 (Suppl. 1), e73. [Google Scholar] [CrossRef]
- Askerbeyli Örs, S.; Aksel, H.; Küçükkaya Eren, S.; Serper, A. Effect of perforation size and furcal lesion on stress distribution in mandibular molars: A finite element analysis. Int. Endod. J. 2019, 52, 377–384. [Google Scholar] [CrossRef]
- PalatyŃska-Ulatowska, A.; BuŁa, K.; Klimek, L. Influence of sodium hypochlorite and ultrasounds on surface features and chemical composition of Biodentine tricalcium silicate-based material. Dent. Mater. J. 2020, 39, 587–592. [Google Scholar] [CrossRef]
- Guneser, M.B.; Akbulut, M.B.; Eldeniz, A.U. Effect of various endodontic irrigants on the push-out bond strength of biodentine and conventional root perforation repair materials. J. Endod. 2013, 39, 380–384. [Google Scholar] [CrossRef]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Parirokh, M.; Torabinejad, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part I: Vital pulp therapy. Int. Endod. J. 2018, 51, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Grech, L.; Mallia, B.; Camilleri, J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent. Mater. 2013, 29, e20–e28. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, H.; Dhillon, J.S.; Batra, M.; Saini, M. MTA versus biodentine: Review of literature with a comparative analysis. J. Clin. Diagn. Res. 2017, 11, ZG01–ZG05. [Google Scholar] [CrossRef] [PubMed]
- Malkondu, Ö.; Karapinar Kazandağ, M.; Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. Biomed. Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef]
- Kot, K.; Kucharski, Ł.; Marek, E.; Safranow, K.; Lipski, M. Alkalizing Properties of Six Calcium-Silicate Endodontic Biomaterials. Mater. (Basel) 2022, 15, 6482. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Classification of Hydraulic Cements Used in Dentistry. Front. Dent. Med. 2020, 1, 9. [Google Scholar] [CrossRef]
- Ashi, T.; Mancino, D.; Hardan, L.; Bourgi, R.; Zghal, J.; Macaluso, V.; Al-Ashkar, S.; Alkhouri, S.; Haikel, Y.; Kharouf, N. Physicochemical and Antibacterial Properties of Bioactive Retrograde Filling Materials. Bioengineering 2022, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Ballal, V.; Marques, J.N.; Campos, C.N.; Lima, C.O.; Simão, R.A.; Prado, M. Effects of chelating agent and acids on Biodentine. Aust. Dent. J. 2018, 63, 170–176. [Google Scholar] [CrossRef]
- Rahimi, S.; Ghasemi, N.; Shahi, S.; Lotfi, M.; Froughreyhani, M.; Milani, A.S.; Bahari, M. Effect of blood contamination on the retention characteristics of two endodontic biomaterials in simulated furcation perforations. J. Endod. 2013, 39, 697–700. [Google Scholar] [CrossRef]
- Rebolloso de Barrio, E.; Gancedo-Caravia, L.; García-Barbero, E.; Pérez-Higueras, J.J. Effect of exposure to root canal irrigants on the push-out bond strength of calcium silicate-based cements. Clin. Oral Investig. 2021, 25, 3267–3274. [Google Scholar] [CrossRef]
- Elnaghy, A.M. Influence of acidic environment on properties of biodentine and white mineral trioxide aggregate: A comparative study. J. Endod. 2014, 40, 953–957. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, L.Z.; Bajkin, B.V. Scanning electron microscopy analysis of marginal adaptation of mineral trioxide aggregate, tricalcium silicate cement, and dental amalgam as a root end filling materials. Microsc. Res. Tech. 2021, 84, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Amoroso-Silva, P.A.; Marciano, M.A.; Guimarães, B.M.; Duarte, M.A.; Sanson, A.F.; Moraes, I.G. Apical adaptation, sealing ability and push-out bond strength of five root-end filling materials. Braz. Oral Res. 2014, 28, S1806–S83242014000100252. [Google Scholar] [CrossRef] [PubMed]
- Refaei, P.; Jahromi, M.Z.; Moughari, A.A.K. Comparison of the microleakage of mineral trioxide aggregate, calcium-enriched mixture cement, and Biodentine orthograde apical plug. Dent. Res. J. 2020, 17, 66–72. [Google Scholar]
- Toia, C.C.; Teixeira, F.B.; Cucco, C.; Valera, M.C.; Cavalcanti, B.N. Volumetric Evaluation of Voids and Gaps of Different Calcium-Silicate Based Materials Used in Furcal Perforations: A Micro-CT Study. Dent. J. 2022, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Mallia, B. Evaluation of the dimensional changes of mineral trioxide aggregate sealer. Int. Endod. J. 2011, 44, 416–424. [Google Scholar] [CrossRef]
- Zanza, A.; Reda, R.; Vannettelli, E.; Donfrancesco, O.; Relucenti, M.; Bhandi, S.; Patil, S.; Mehta, D.; Krithikadatta, J.; Testarelli, L. The influence of Thermomechanical Compaction on the Marginal Adaptation of 4 Different Hydraulic Sealers: A Comparative Ex Vivo Study. J. Compos. Sci. 2023, 7, 10. [Google Scholar] [CrossRef]
- Torres, F.F.E.; Guerreiro-Tanomaru, J.M.; Bosso-Martelo, R.; Espir, C.G.; Camilleri, J.; Tanomaru-Filho, M. Solubility, Porosity, Dimensional and Volumetric Change of Endodontic Sealers. Braz. Dent. J. 2019, 30, 368–373. [Google Scholar] [CrossRef]
- Küçükkaya Eren, S.; Aksel, H.; Askerbeyli Örs, S.; Serper, A.; Koçak, Y.; Ocak, M.; Çelik, H.H. Obturation quality of calcium silicate-based cements placed with different techniques in teeth with perforating internal root resorption: A micro-computed tomographic study. Clin. Oral Investig. 2019, 23, 805–811. [Google Scholar] [CrossRef]
- Mitrović, N.; Milošević, M.; Momčilović, N.; Petrović, A.; Mišković, Ž.; Sedmak, A.; Popović, P. Local strain and stress analysis of globe valve housing subjected to external axial loading. Key Eng. Mater. 2013, 586, 214–217. [Google Scholar] [CrossRef]
- Travica, M.; Mitrovic, N.; Petrovic, A.; Trajkovic, I.; Milosevic, M.; Sedmak, A.; Berto, F. Experimental Evaluation of Hoop Stress–Strain State of 3D-Printed Pipe Ring Tensile Specimens. Metals 2022, 12, 1560. [Google Scholar] [CrossRef]
- Trajković, I.; Milošević, M.; Travica, M.; Rakin, M.; Mladenović, G.; Kudrjavceva, L.J.; Medjo, B. Novel method for measurement of pipeline materials fracture resistance-examination on selective laser sintered cylindrical specimens. Sci. Sinter. 2022, 54, 373–386. [Google Scholar] [CrossRef]
- Tanasić, I.; Tihaček-Sojić, L.J.; Mitrović, N.; Milić Lemić, A.; Vukadinović, M.; Marković, A.; Milošević, M. An attempt to create a standardized (reference) model for experimental investigations on implant’s sample. Measurement 2015, 72, 37–42. [Google Scholar] [CrossRef]
- Tanasić, I.; Tihaček-Sojić, L.J.; Milić Lemić, A.; Djurić, M.; Mitrović, N.; Milošević, M.; Sedmak, A. Optical aspect of deformation analysis in the bone-denature complex. Coll. Antropol. 2012, 36, 173–178. [Google Scholar]
- Lezaja, M.; Veljovic, D.; Manojlovic, D.; Milosevic, M.; Mitrovic, N.; Janackovic, D.; Miletic, V. Bond strength of restorative materials to hydroxyapatite inserts and dimensional changes of insert-containing restorations during polymerization. Dent. Mater. 2015, 31, 171–181. [Google Scholar] [CrossRef]
- Mitrović, A.; Antonović, D.; Tanasić, I.; Mitrović, N.; Bakić, G.; Popović, D.; Milošević, M. 3D Digital Image Correlation Analysis of the Shrinkage Strain in Four Dual Cure Composite Cements. Biomed. Res. Int. 2019, 2019, 2041348. [Google Scholar] [CrossRef]
- Ya, S.; Bin, P.; Yan, Y.; Ma, J.; Markus, H. What do different tests tell about the mechanical and biological properties of bioceramic materials? Endod. Topics. 2015, 32, 47–85. [Google Scholar] [CrossRef]
- Bansal, K.; Jain, A.; Aggarwal, N.; Jain, A.; Biodentine, V.S. MTA: A comparitive analysis. Int. J. Oral Health Dent. 2020, 6, 201–208. [Google Scholar] [CrossRef]
- Stefaneli Marques, J.H.; Silva-Sousa, Y.T.C.; Rached-Júnior, F.J.A.; Macedo, L.M.D.; Mazzi-Chaves, J.F.; Camilleri, J.; Sousa-Neto, M.D. Push-out bond strength of different tricalcium silicate-based filling materials to root dentin. Braz. Oral Res. 2018, 32, e18. [Google Scholar] [CrossRef]
- Al Tuwirqi, A.A.; El Ashiry, E.A.; Alzahrani, A.Y.; Bamashmous, N.; Bakhsh, T.A. Tomographic Evaluation of the Internal Adaptation for Recent Calcium Silicate-Based Pulp Capping Materials in Primary Teeth. Biomed. Res. Int. 2021, 2021, 5523145. [Google Scholar] [CrossRef]
- Formosa, L.M.; Mallia, B.; Camilleri, J. The effect of curing conditions on the physical properties of tricalcium silicate cement for use as a dental biomaterial. Int. Endod. J. 2012, 45, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, M.O.; Nagas, E.; Sahin, C.; Dagli, F.; Cehreli, Z.C. Effects of different irrigation regimens on the sealing properties of repaired furcal perforations. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e91–e95. [Google Scholar] [CrossRef]
- Reszka, P.; Nowicka, A.; Lipski, M.; Dura, W.; Droździk, A.; Woźniak, K. A Comparative Chemical Study of Calcium Silicate-Containing and Epoxy Resin-Based Root Canal Sealers. Biomed. Res. Int. 2016, 2016, 9808432. [Google Scholar] [CrossRef] [PubMed]
- Storm, B.; Eichmiller, F.C.; Tordik, P.A.; Goodell, G.G. Setting expansion of gray and white mineral trioxide aggregate and Portland cement. J. Endod. 2008, 34, 80–82. [Google Scholar] [CrossRef]
- Komabayashi, T.; Spångberg, L.S. Comparative analysis of the particle size and shape of commercially available mineral trioxide aggregates and Portland cement: A study with a flow particle image analyzer. J. Endod. 2008, 34, 94–98. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Iacono, F.; Agee, K.; Siboni, F.; Tay, F.; Pashley, D.H.; Prati, C. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and ProRoot MTA. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; Alshali, R.Z.; Silikas, N. The effect of desiccation on water sorption, solubility and hygroscopic volumetric expansion of dentine replacement materials. Dent. Mater. 2018, 34, e205–e213. [Google Scholar] [CrossRef] [PubMed]
- Darvell, B.W.; Wu, R.C. “MTA”-an Hydraulic Silicate Cement: Review update and setting reaction. Dent. Mater. 2011, 27, 407–422. [Google Scholar] [CrossRef]
- Kelmendi, T.; Koçani, F.; Kurti, A.; Kamberi, B.; Kamberi, A. Comparison of Sealing Abilities Among Zinc Oxide Eugenol Root-Canal Filling Cement, Antibacterial Bioceramic Paste, and Epoxy Resin, using Enterococcus faecalis as a Microbial Tracer. Med. Sci. Monit. Basic Res. 2022, 28, e936319. [Google Scholar] [CrossRef]
- Camilleri, J.; Sorrentino, F.; Damidot, D. Investigation of the hydration and bioactivity of radiopacified tricalcium silicate cement, Biodentine and MTA Angelus. Dent. Mater. 2013, 29, 580–593. [Google Scholar] [CrossRef]
- Sheethal Dsouza, T.; Shetty, A.; Dsouza, N. Evaluation of pH, Calcium Ion Release, and Dimensional Stability of an Experimental Silver Nanoparticle-Incorporated Calcium Silicate-Based Cement. Bioinorg. Chem. Appl. 2021, 2021, 3919543. [Google Scholar] [CrossRef] [PubMed]
- Akinci, L.; Simsek, N.; Aydinbelge, H.A. Physical properties of MTA, BioAggregate and Biodentine in simulated conditions: A micro-CT analysis. Dent. Mater. J. 2020, 39, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Grech, L.; Galea, K.; Keir, D.; Fenech, M.; Formosa, L.; Damidot, D.; Mallia, B. Porosity and root dentine to material interface assessment of calcium silicate-based root-end filling materials. Clin. Oral Investig. 2014, 18, 1437–1446. [Google Scholar] [CrossRef]
- Aksel, H.; Küçükkaya Eren, S.; Askerbeyli Õrs, S.; Karaismailoğlu, E. Surface and vertical dimensional changes of mineral trioxide aggregate and biodentine in different environmental conditions. J. Appl. Oral Sci. 2018, 27, e20180093. [Google Scholar] [CrossRef]
- Savić-Stanković, T.V. Analysis of Tricalcium Silicate (Biodentin) as a Dentin Substitute under Composite Restorations in Posteriorteeth (Doctoral dissertation). 2014. Available online: https://nardus.mpn.gov.rs/handle/123456789/5329 (accessed on 25 May 2016).
- Chung, S.Y.; Kim, Y.H.; Chae, Y.K.; Jo, S.S.; Choi, S.C.; Nam, O.H. Void characteristics and tortuosity of calcium silicate-based cements for regenerative endodontics: A micro-computed tomography analysis. BMC Oral Health 2021, 21, 565. [Google Scholar] [CrossRef]
- Lu, H.Y.; Hou, J.; Takahashi, Y.; Tamura, Y.; Kasugai, S.; Kuroda, S.; Nakata, H. Periodontal Pathogen Adhesion, Cytotoxicity, and Surface Free Energy of Different Materials for an Implant Prosthesis Screw Access Hole. Medicina 2022, 58, 329. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Hydration mechanisms of mineral trioxide aggregate. Int. Endod. J. 2007, 40, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Nosrat, A.; Fouad, A.F. Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J. Dent. 2015, 43, 241–247. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).