Abstract
Salt-affected soil reclamation provides opportunities for crop production and carbon sequestration. In arid regions such as Pakistan, limited studies have been reported involving soil reclamation and crop production under wheat–maize rotation, but no study has reported predictions on long-term carbon sequestration in reclaimed soils for the treatments used in this study. Thus, a field-scale fallow period and crop production experiment was conducted for wheat–maize rotation on salt-affected soils in Pakistan for 3 years to check the effectiveness of organic amendments for reclamation of the salt-affected soils, carbon sequestration and food grain production. Treatments used were the control (with no additional amendments to reduce salinity), gypsum alone and gypsum in combination with different organic amendments (poultry manure, green manure, and farmyard manure). The treatment with gypsum in combination with farmyard manure was most effective at increasing soil carbon (+169% over the three-year period of the trial). The maximum wheat yield was also recorded in year 3 with gypsum in combination with farmyard manure (51%), while the effect of green manure combined with gypsum also showed a significant increase in maize yield in year 3 (49%). Long-term simulations suggested that the treatments would all have a significant impact on carbon sequestration, with soil C increasing at a steady rate from 0.53% in the control to 0.86% with gypsum alone, 1.25% with added poultry manure, 1.69% with green manure and 2.29% with farmyard manure. It is concluded that food crops can be produced from freshly reclaimed salt-affected soils, and this can have added long-term benefits of carbon sequestration and climate change mitigation.
1. Introduction
Salinization is a major threat to soil and is widespread in over 100 countries of the world. Climate change is also triggering more soils to become saline through increased evaporation of irrigation water associated with water shortages and increased soil temperatures [1,2]. Globally, an area of 9.54 × 108 has been declared as salt affected [3]. Of the area of land that is fit for arable production, 10% has deteriorated due to salinity or sodicity. Along with the existing salt-affected area, an additional 1–2% of the productive soils of the world are becoming salt affected every year [4].
The problem of salinization is of particular concern because the global population has a growing requirement for food; in 2020, 8.11 × 108 people were food insecure. The global population of 7.4 × 109 in 2016 is projected to increase to 9.7 × 109 by 2050, with almost all increases occurring in developing countries. Therefore, to avoid food insecurity, global food production should be increased up to 70% by 2050 [5]. Reducing the risks of food insecurity associated with both climate change and the increasing population is a major challenge to be addressed [6,7]. Reclaiming soil affected by salinity will play a major role in meeting this challenge.
Soils affected by salinity can be reclaimed by the addition of gypsum (CaSO4. 2H2O). This replaces Na+ in the cation exchange sites with an easily accessible supply of Ca2+. The Na2SO4 formed by the exchange is easily removed from the soil profile, either by downward leaching or by absorption by halophytic grasses [8]. Unlike in the EU, where reduction in coal-burning power plants is likely to result in future shortages of gypsum [9], gypsum in Pakistan is mined and readily available at less than a dollar per 50 kg bag of gypsum, with no prospects of future shortages [10].
Soils affected by salinity can also be reclaimed by the addition of different organic amendments, which also improves crop production by increasing the organic matter content, available nutrients and biological activity of the soil [11,12,13,14,15]. A productive soil requires a sufficient concentration of organic matter [16], but salt-affected soils usually contain less than 1% organic matter and, thus, require extra organic inputs [17]. Carbon (C) sequestration in soils also helps to mitigate climate change [18,19], and the increased soil organic matter (SOM) content can enhance soil fertility, nutrient availability and water holding capacity [20,21].
As Pakistan lies in a semi-arid zone, it suffers from large-scale soil salinization. Of the irrigated area, 25% is affected by salinity (6 × 106 ha), which is equivalent to 3.9% of the total salt-affected soils worldwide [22,23]. Reclaiming salt-affected soils could provide a significant contribution to reducing food insecurity in Pakistan [24]. Many papers have reported the use of organic and inorganic amendments, and their combinations, for the reclamation of saline soils (e.g., [25,26,27,28,29]). Other work has assessed the use of reclaimed soils for wheat and rice crop production (e.g., [27,28,30,31,32,33]). In Pakistan, previous studies have demonstrated the potential of combined gypsum and organic amendments to reclaim salt-affected soils and crop production [27,30]. However, only limited work has been executed on the potential for the reclamation of salt-affected soils to increase long-term C sequestration in the soil [23]. Moreover, limited work is reported on the use of the RothC model for the prediction of carbon sequestration after the reclamation of salt-affected soils during wheat–maize rotation in a semi-arid region, such as Pakistan. This is an important omission because while applying gypsum could be of immediate benefit to short-term food security combining the treatment with organic amendments could increase long-term C sequestration, thus improving the water holding capacity and resilience of crop production to climate change. Therefore, the research reported in this paper assesses the impacts of treating soils with gypsum in combination with different organic amendments, focusing on changes in crop production and the properties of marginally salt-affected soils, using simulation modelling to assess the longer-term impacts on SOM and C sequestration.
2. Materials and Methods
2.1. Site Description
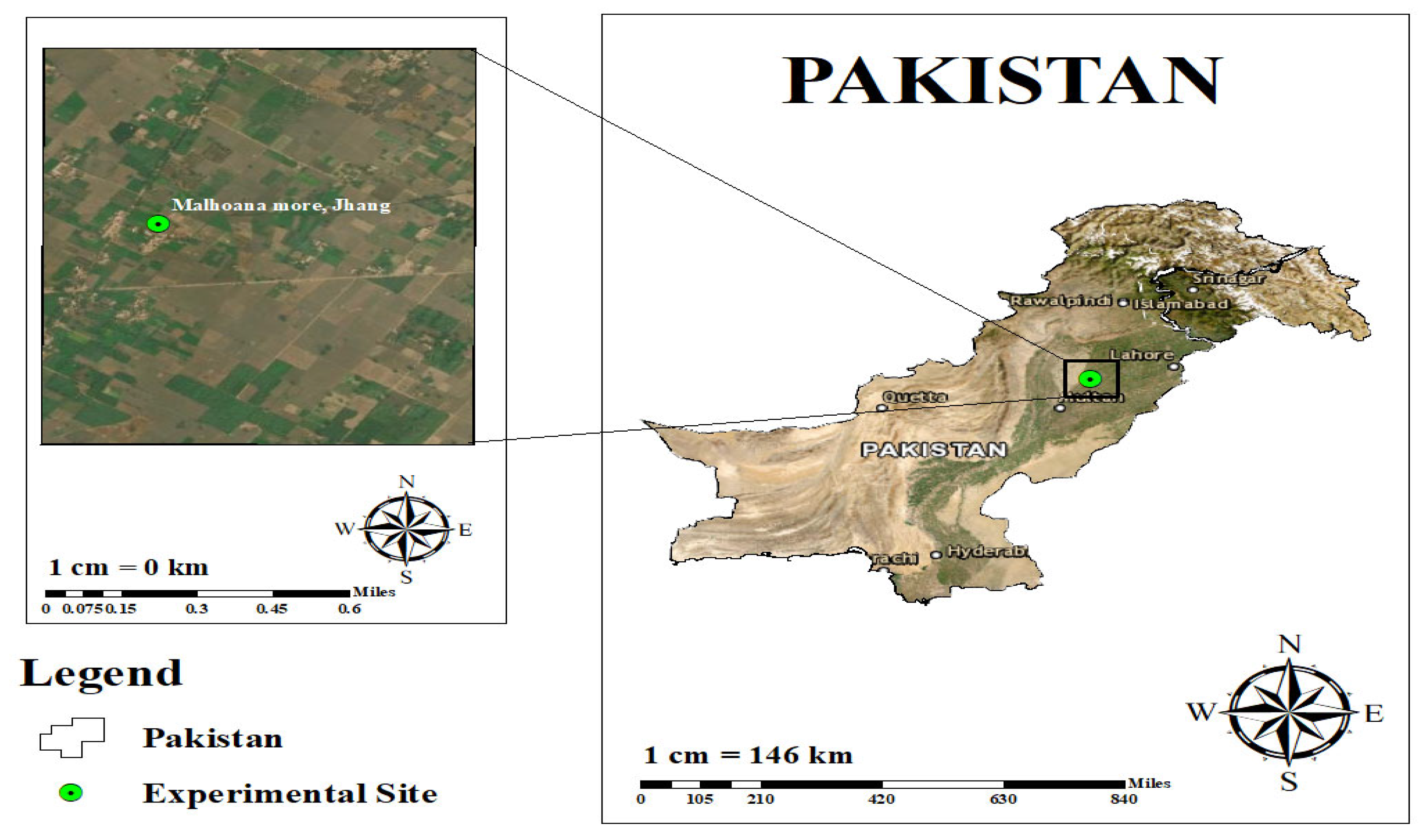
The field trials were conducted in District Jhang, situated on the east bank of the river Chenab of the Punjab, Pakistan (Figure 1). This is a semi-arid, northern plain region, which is widely irrigated and dominated by sandy clay loam soils. The experiment was carried-out in a pre-selected marginally salt-affected area located between 31.0844° N and 72.1332° E.
Figure 1.
Study area.
2.2. Treatments and Experimental Setup
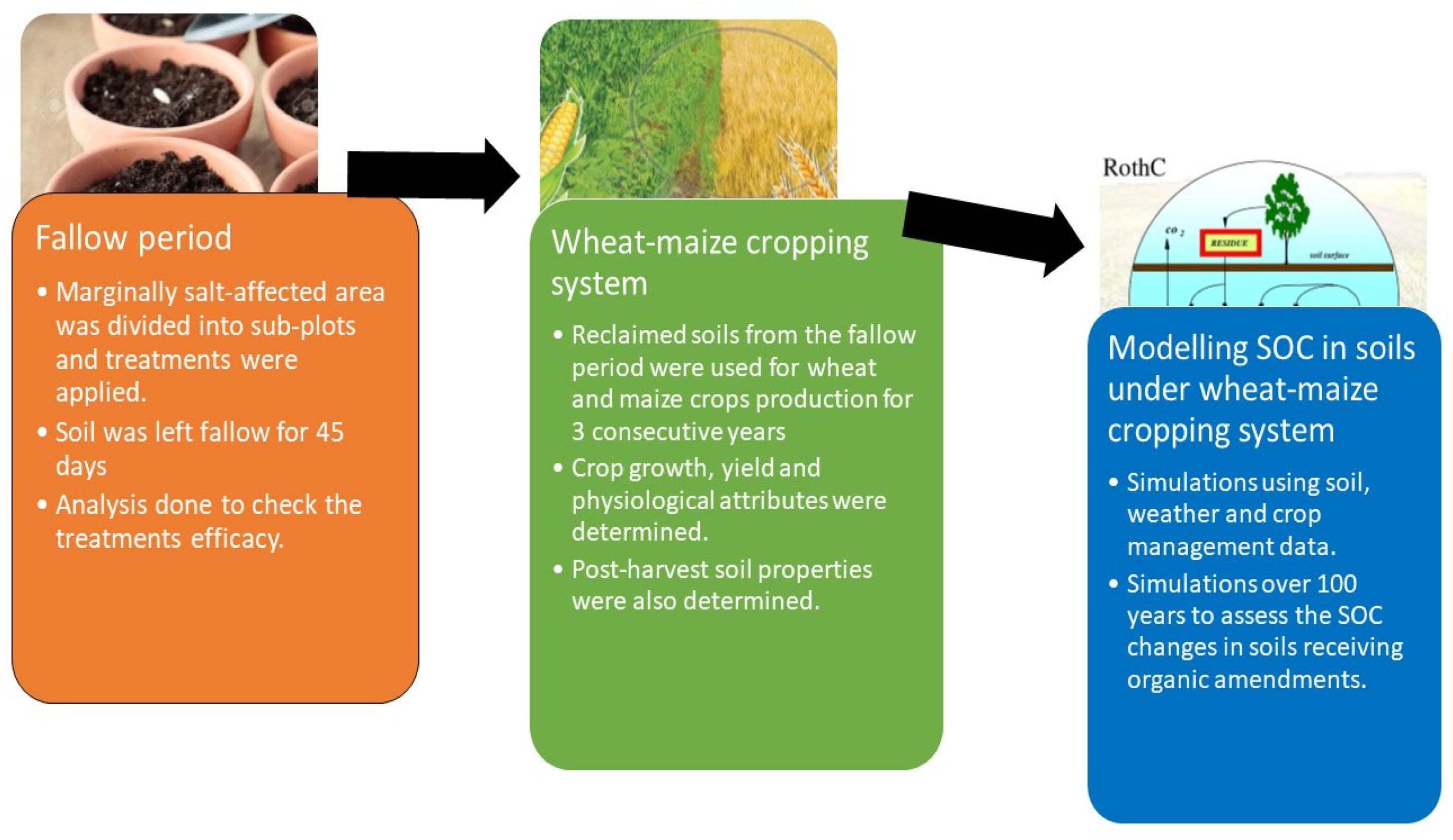
The field study involved an initial fallow period followed by crop production for the next three years. A detailed description and flow chart of the experiment is provided in Figure 2. The total area of the field was 26 m2 and was divided into 1.5 × 1.5 m2 plots. Each plot was separated from other plots using 60 cm high and 20 cm wide ridges. Five treatments were included in the experiment: the control with no applications, treatment with a 100% soil gypsum requirement (SGR) (G100), and 3 other treatments receiving 50% SGR (5 t ha−1) combined with 12 t ha−1 farmyard manure (FYM + G50), poultry manure (PM + G50) and green manure (GM + G50). The properties of organic amendments are presented in Supplementary Table S1. The amendments were applied evenly and mixed into the soil manually before the experiment started, and then the plots were covered and left to fallow. The purpose of the fallow period was to monitor the impact of the selected treatments on the salt-affected soil area without complicating the impact of the growing crop. This was performed over the first 45 days. During the fallow period, all of the sub-plots were regularly irrigated to maintain the moisture levels of the soil at field capacity. The meteorological variation during this experiment is presented in Supplementary Figure S1. Soil samples were taken using random sampling by auger to a depth of 30 cm. Randomly positioned soil samples were collected and homogenized to make a composite sample before and after the 45-day fallow period.
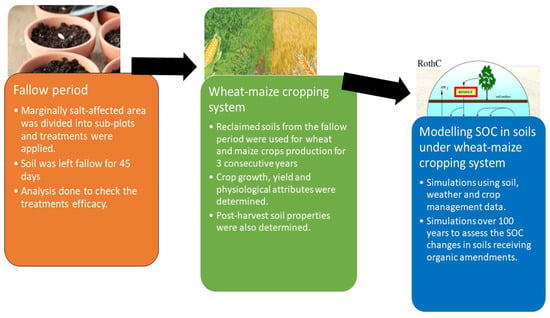
Figure 2.
Overview of the study. Note: SOC = soil organic carbon.
2.3. Crop Husbandry
After a fallow period of 45 days, the seeds of the wheat crop were sown in all of the plots. A wheat–maize rotation was followed for three years (2017–2020). Wheat (Iqbal-2000) was grown in the rabi season (December to May), while maize (Pioneer-1543) was the kharif crop (July to October) (Supplementary Table S2). Standard irrigation and fertilizer application practices were used throughout the study period (Supplementary Table S2). Urea, di-ammonium phosphate and sulphate of potash were applied as synthetic N, P and K fertilizers at the rate of 79:57:62 for wheat and 135:63:50 kg ha−1 for maize. Fertilizer was evenly broadcast. The trialed treatments (i.e., gypsum, FYM, PM and GM) were randomly allocated to plots and applied throughout the study period.
2.4. Plant and Soil Analysis
Physiological plant parameters (Photosynthetic rate and stomatal conductance) were measured during the growing season using an Infrared Gas Analyzer (IRGA, LCA-4, Analytical Development, Hoddesdon, UK). Chlorophyl content was determined using a SPAD-502 meter (Minolta, Osaka, Japan), using the procedure prescribed by Saqib, et al. [34]. All of the physiological parameters were taken during heading stage 10.5. Soils were sampled immediately after each harvest. Plant sampling was performed at maturity. The samples were washed first with tap water, followed by 1% (v/v) HCl water, and then finally with distilled water. The collected samples were air-dried under the shade for 24 h, followed by drying in an oven at 65 ± 2 °C until a constant weight was achieved. After oven drying, the grain yield and the dry weights of the roots, shoots and leaves were recorded.
Saturated soil pastes were prepared to measure the pH using a calibrated pH meter (Hanna HI-83141). The soil water was extracted from the prepared soil paste using a negative pressure extraction pump for further analysis. The electrical conductivity (EC) of the water extracted from the saturated soil paste was determined using a conductivity meter (Lovibond SensoDirect con200) after calculating the cell constant for the conductivity meter. The soil total nitrogen (N) was determined using the Kjeldahl apparatus [35]. Available phosphorus (P) was extracted following the Olsen method devised by Terry, et al. [36]. Potassium (K+) was analyzed using the method described by Norman [37]. The Walkley-Black method was used to determine the SOM content [38]. The cation exchange capacity (CEC) of the sampled soil was determined using the method described by Estefan [39]. The sodium adsorption ratio (SAR) was calculated with the equation below:
where is the sodium adsorption ratio (mmol dm−3)1/2), is the concentration of sodium ions (mmolc dm−3) and
is concentration of calcium plus magnesium (mmolc dm−3).
2.5. Biological Parameters
2.5.1. Microbial Biomass Carbon
The microbial biomass C was measured by using the fumigation–incubation technique with a total of 10 g of soil [40]. Chloroform was used to fumigate one sample for 24 h while not fumigating the control. Following the fumigation period, the sample was shaken with 25 mL of 0.5 M K2SO4 for 30 min to extract the salt-extractable SOC from both soil sub-samples. The extracts were filtered using qualitative filter paper (30–50 m), and the Multi N/C3100, manufactured by Analytik Jena AG in Jena, Germany, was used to measure the results. The difference in extractable C between unfumigated and fumigated soils, corrected by an extractability correction factor of 0.45, was used to estimate the microbial biomass C concentrations.
2.5.2. Soil Respiration
Soil respiration was measured using the method described by Anderson [41] in which CO2 was trapped with 1 M NaOH and back titrated against 0.1 M HCl. For this, 1 cm3 of trapped solution, 6–7 drops of BaCl2 solution and 2–3 drops of phenolphthalein indicator were added and then back titrated. The blank sample (1 M NaOH) was also titrated similarly against the 0.1 M HCl, and respiration rate was determined using the following equation:
where the soil respiration (1), is the volume (cm3) of NaOH used for CO2 trapping, is the volume (cm3) of NaOH used for titration, is blank sample reading (cm3), is the volume (cm3) of HCl used for titration, and 2 is the conversion factor. 12 is the molecular weight of C, while 0.1 is the molarity of HCl used for titration. As 1 mL 0.1 M HCl is equivalent to 2 mg CO2 emitted, it is used as conversion factor.
2.5.3. Dehydrogenase Activity
Dehydrogenase activity is a sign of microbial activity in the soil. It was determined following the method of Casida Jr, et al. [42]. It was measured in units of g of 2,3,5-triphenyl formazan (TPF) per g of oven-dry soil per hour. 6 g of soil, 1 cm3 of 3% TPF aqueous solution, 120 mg of CaCO3 and 2–4 cm3 of deionized water were added in 50 cm3 glass flasks. The minimal amount of loose liquid was kept at the soil’s surface by swirling the flasks once they were snugly shut. Soil samples were incubated for a total of 20 h at 30 °C. Using a UV-visible spectrophotometer (ThermoFisher Scientific, Oxford, UK), the TPF product was extracted with 94% ethanol for 60 min at 20 °C in the dark.
2.6. Source of Experimental Materials
The varieties used in the experiment were Iqbal-2000 and Pioneer-1543 for wheat and maize, respectively, and were procured from the Ayub Agricultural Research Institute (AARI), Faisalabad, Pakistan. Fresh farmyard manure, poultry manure and green manure were purchased from livestock, poultry and agricultural farms near the study area, and their oven-dry weights were measured and used to determine application rate in the field. Double-distilled water and analytical grade chemicals and solvents from the Merck company (Darmstadt, Germany) were used for the analysis.
2.7. Simulation of Potential Carbon Sequestration in Soils
A modified version of the RothC model by Powlson, et al. [43] was used to simulate the potential C sequestration in the three-year field study at Jhang. This model describes soil organic C as a series of pools with different decomposition rate constants: fresh plant material composed of decomposable plant material (DPM) and resistant plant material, decomposing soil organic matter composed of humified organic matter (HUM) and actively decomposing microbial biomass, and inert organic matter that does not decompose. The rate of decomposition from each of these pools is adjusted in the model according to the environmental conditions, accounting for moisture, temperature, pH, salinity and crop cover. The model used here differed from the standard version of RothC in that it included a rate modifier for salinity (no units). This was determined according to the equation provided by Setia, et al. [44];
where is the measured EC in a 1:5 soil/water suspension (dS m−1).
2.7.1. Initialization of Soil Organic Matter Pools and Plant Inputs in the Control
The model was initialized to first obtain the soil C pools at the start of the experiment. In order to initialize the model, it was assumed that the arable land used at the site had remained unchanged for at least 3 decades before the start of our study. Following the approach of Smith, et al. [45], the model was initialized into a steady state using the ratio of the soil C measured at the start of the field trial to the value simulated after a long-term run. This ratio was used as a multiplier to iteratively adjust the plant inputs until measured and simulated values of soil C matched. This provided an estimate of the plant inputs and pool sizes at the start of the trial.
Because the early phases of the experiment had changed the management of the land from the steady-state conditions, plant inputs in the control treatment, (t ha−1), were then further adjusted by an iterative fitting procedure to give the lowest variation between the simulated and the measured values of soil C in the control treatment. This was quantified as the root mean squared error RMSE (%)Smith [46].
2.7.2. Plant Inputs in the Treatments
Having initialized the model to a steady state and having determined the plant inputs for the control and the treatments, . (t ha−1) was obtained by following the method used by [45]. This approach adjusted the plant inputs in proportion to the yields measured in the treatment and the control as:
where is the plant inputs estimated in the control (t ha−1), is the yield in the current season (t ha−1) and is the yield used in the steady-state run (t ha−1).
2.7.3. Organic Manure Inputs in the Treatments
The parameters used to describe the organic manures applied in the field trials were obtained from pot experiments using the same manures but at different sites (Dijkot and Uchkera; Farooqi et al., in prep.). The C percentages and dry matter contents of the manures were directly measured and used to quantify the amounts of C applied in the manures. The decomposability of the C added in the manures was specified using the ratio of DPM to HUM reported by Smith, et al. [47] as 31.45 for poultry manure and green manure and 1 for the farmyard manure. The accuracy of these generic DPM:HUM ratios for the specific manures used in this trial was evaluated using data from the pot experiments at Dijkot and Uchkera. If the simulations did not provide a good fit to the experimental data (good fit arbitrarily set to RMSE < 15% and R2 > 0.8), the DPM:HUM ratio was adjusted to provide an improved fit. The values for the DPM:HUM ratios optimized for the manures used in this trial were then evaluated against a further pot experiment at the trial site (Jhang; Farooqi et al. in prep.).
2.7.4. Model Evaluation
The level of uncertainty expected in long-term forward runs of the model was established by evaluating against the data from the treatments at Jhang. Ideally, long-term trials should be used to assess the uncertainty in the long-term soil C turnover processes, but these trials do not yet exist. Therefore, the uncertainty was determined using this three-year trial. The simulations therefore provide an estimate of uncertainty in C sequestration, assuming the soil and climate conditions, and the plant inputs remain unchanged from the three-year period of the study. In both the pot and field trials, the accuracy of the simulations of the measured values was quantified as previously executed by Smith, et al. [48]. The extent of association between simulations and measurements was quantified as the square of the regression coefficient (R2). The uncertainty was determined as the RMSE (%).
2.7.5. Simulations of Long-Term Changes in Soil Organic Carbon
The model was run using repeated weather data from 2012 to 2020 to assess the potential impacts of the applied treatments on C sequestration. Simulations were continued until a new steady state was achieved in order to provide an estimate of potential C sequestration in the soil. Note that this neglects any impacts of changing climates, soil conditions or crop productivity, which will be considered in future work.
2.8. Economic Analysis
The analysis was performed to compare the total cost of production until the harvesting and sale of the grain yield and plant biomass. Both the net profit and benefit–cost ratio (BCR) was used to show the profitability of the crops. The procedure by the CIMMYT Economics Program, et al. [49] was used for economic analysis in which total permanent cost remained fixed for all of the treatments, and this cost included the cost of seed, fertilizer, irrigation, plant protection and harvesting. Net benefits were calculated by subtracting the total cost from gross income per treatment. The total input costs (variable and fixed costs) for all of the treatments were calculated after determining their prices. Net benefits for each treatment were noted by subtracting the total variable cost from total benefits. The BCR was calculated by dividing the net profit by total costs.
2.9. Statistical Analysis
The data regarding all of the soil and plant related parameters were subject to statistical analysis. The analysis of variance (ANOVA) in completely a randomized design under single-factor factorial composition was used, and all treatment means were compared using the least significant different (LSD) test in the Minitab 17.1.0 software (Minitab Ltd., Coventry, UK).
3. Results
3.1. Physical, Chemical and Biological Properties
3.1.1. Pre-Analysis of Soils
The physical and chemical characteristics and elemental compositions of the experimental field topsoils (0–30 cm depth) are given in Table 1. The soil texture in the study area was designated as sandy clay loam. The soil appeared to be marginally saline–sodic (Table 1).

Table 1.
Properties of the soil before the experiment. Rounded-off values after ± represent standard errors (n = 3). Note: EC = electrical conductivity, SAR = sodium adsorption ratio, CEC = cation exchange capacity, MBC = microbial biomass carbon, SR = soil respiration, DHA = dehydrogenase activity.
3.1.2. Temporal Changes in the Soil’s Physical, Chemical and Biological Properties (Fallow Period)
All measured soil characteristics changed significantly over the fallow period (p < 0.001) in all treatments (Table 2), with the maximum change observed by day 45 of the fallow period. The soil pH, EC and SAR all decreased following the treatment. The highest decline was in FYM + G50 for pH (a 10% fall from (9.50 ± 0.01) to (8.66 ± 0.02)) and SAR (a 62% fall from (42.3 ± 0.4) to (16 ± 1) (mmol L−1)1/2). The highest decline in EC was in GM + G50 (a 13% fall from (5.38 ± 0.01) to (4.70 ± 0.01) dS m−1). By contrast, the CEC, TN, SOM, MBC, SR and DHA all increased following treatment. The highest increase in chemical characteristics was in PM + G50: for CEC, this was a 10.24% increase from (7.42 ± 0.01) to (8.18 ± 0.03) cmolc kg−1, a 220% increase from (0.025 ± 0.009) to (0.81 ± 0.01) g kg−1 for TN, and a 122% increase from (0.31 ± 0.01) to (0.71 ± 0.01)% for SOM. The highest increase in biological characteristics was in GM + G50: for MBC, this was a 225% increase from (57.0 ± 0.0) to (185.0 ± 0.3) mg kg−1, a 225% increase from (16.0 ± 0.0) to (52.0 ± 0.7) mmol m−2 s−1 for SR, and a 185% increase in 2,3,5-triphenyl formazan from (178 ± 2) to (507 ± 10) μg g−1 h−1 for DHA.

Table 2.
Variation in the soil’s physico-chemical properties after different treatments in the fallow period. Rounded-off values after ± represents standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
3.2. The Seed Germination, Physiology and Productivity of the Wheat–Maize Cropping System
3.2.1. Seed Germination and Plant Height
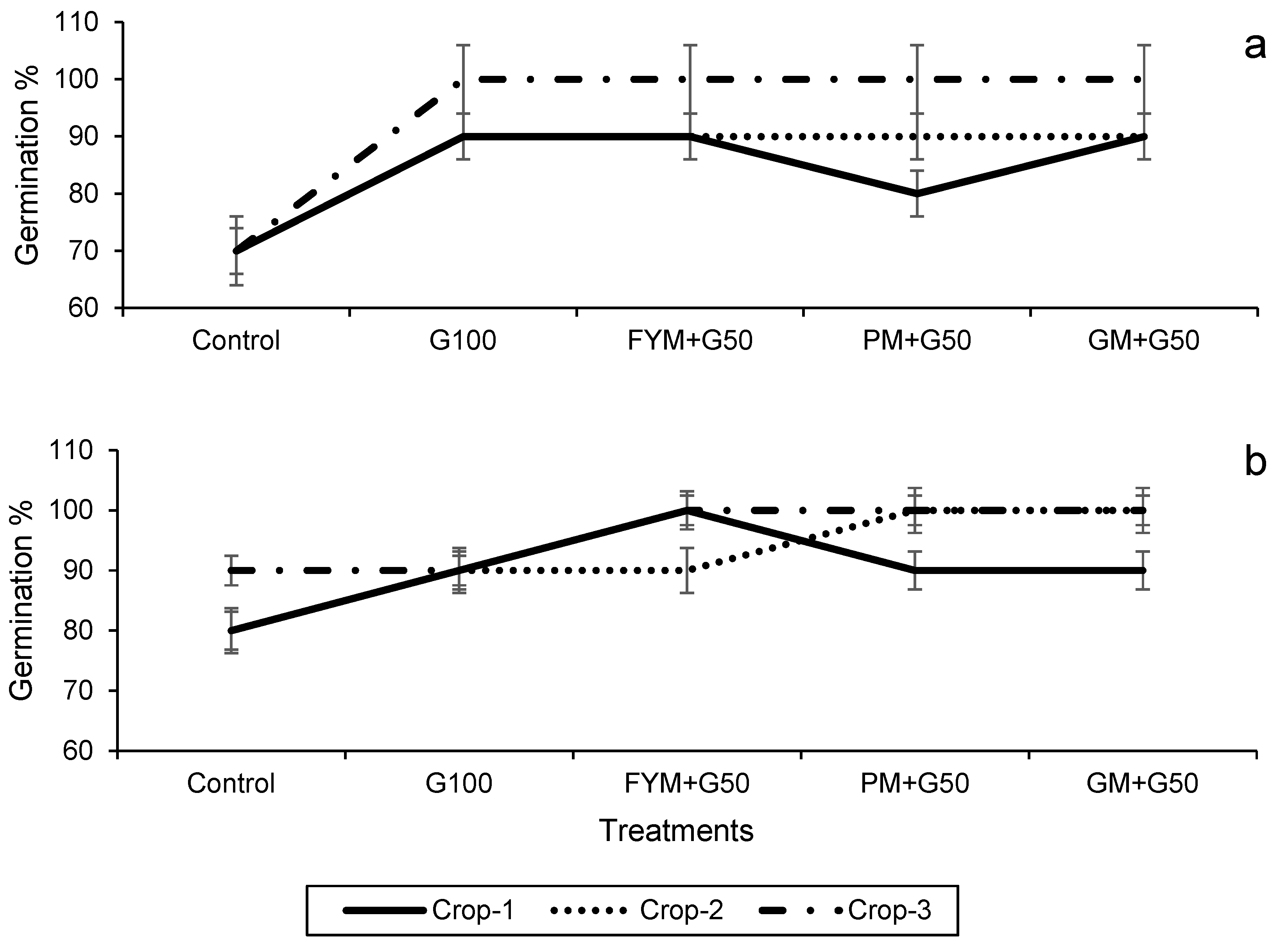
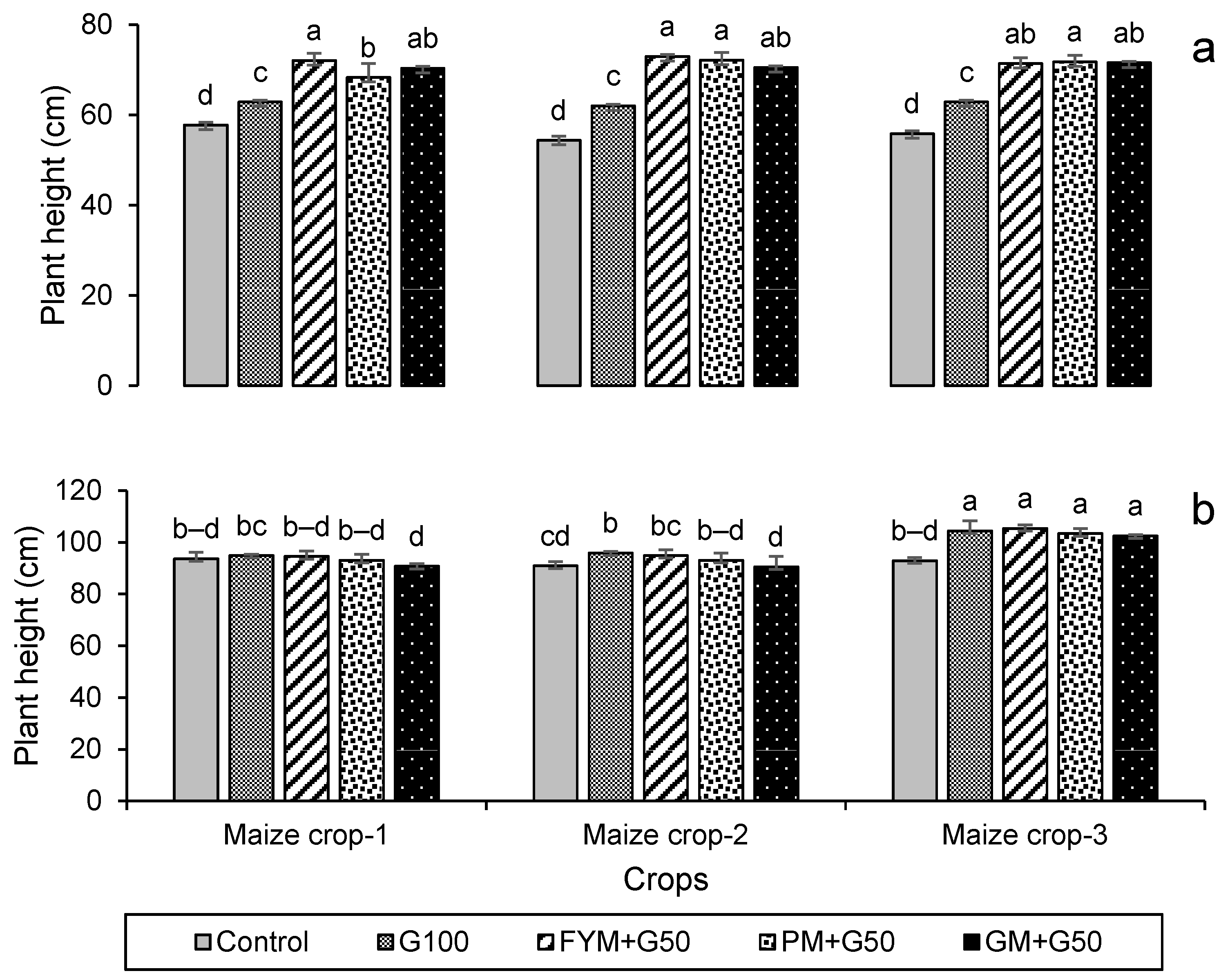
The data on seed germination after 7 days of sowing and the plant height at harvest are presented in Figure 3. In both the wheat and maize crops, treatments significantly (p < 0.05) affected seed germination and plant height but note this may in part be due to the increased rate of N application in the treatments. In the wheat, in year 3 100% germination was exhibited in all of the treatments except the control (Figure 3a), and also in the maize in all treatments except the control and G100 (Figure 3b). Plant height also showed a significant increase with the amended treatments compared to the control (p < 0.05). For wheat, the maximum plant height (73.0 ± 0.5 cm) was recorded in treatment FYM + G50 in year 2, with a 37% increase over the control (Figure 4a), while for maize it was in year 3 (105 ± 1.30 cm), with 13% increase over control (Figure 4b).
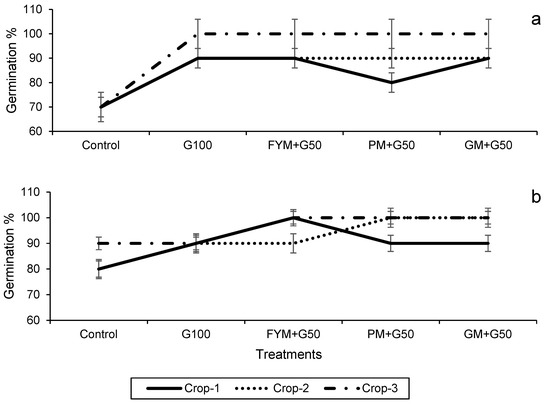
Figure 3.
Impacts of different treatments on seed germination of (a) wheat and (b) maize. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
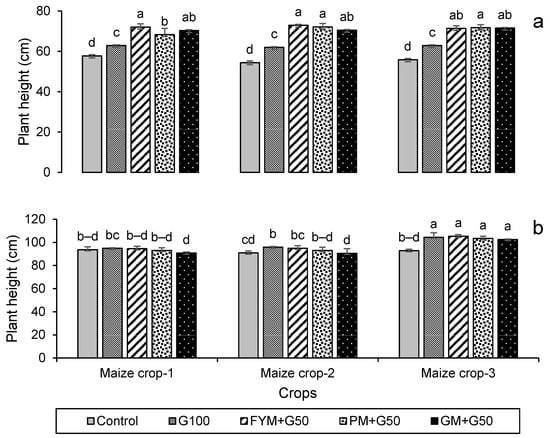
Figure 4.
Impacts of different treatments on (a) wheat and (b) maize plant height. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
3.2.2. Crop Growth and Yield Attributes
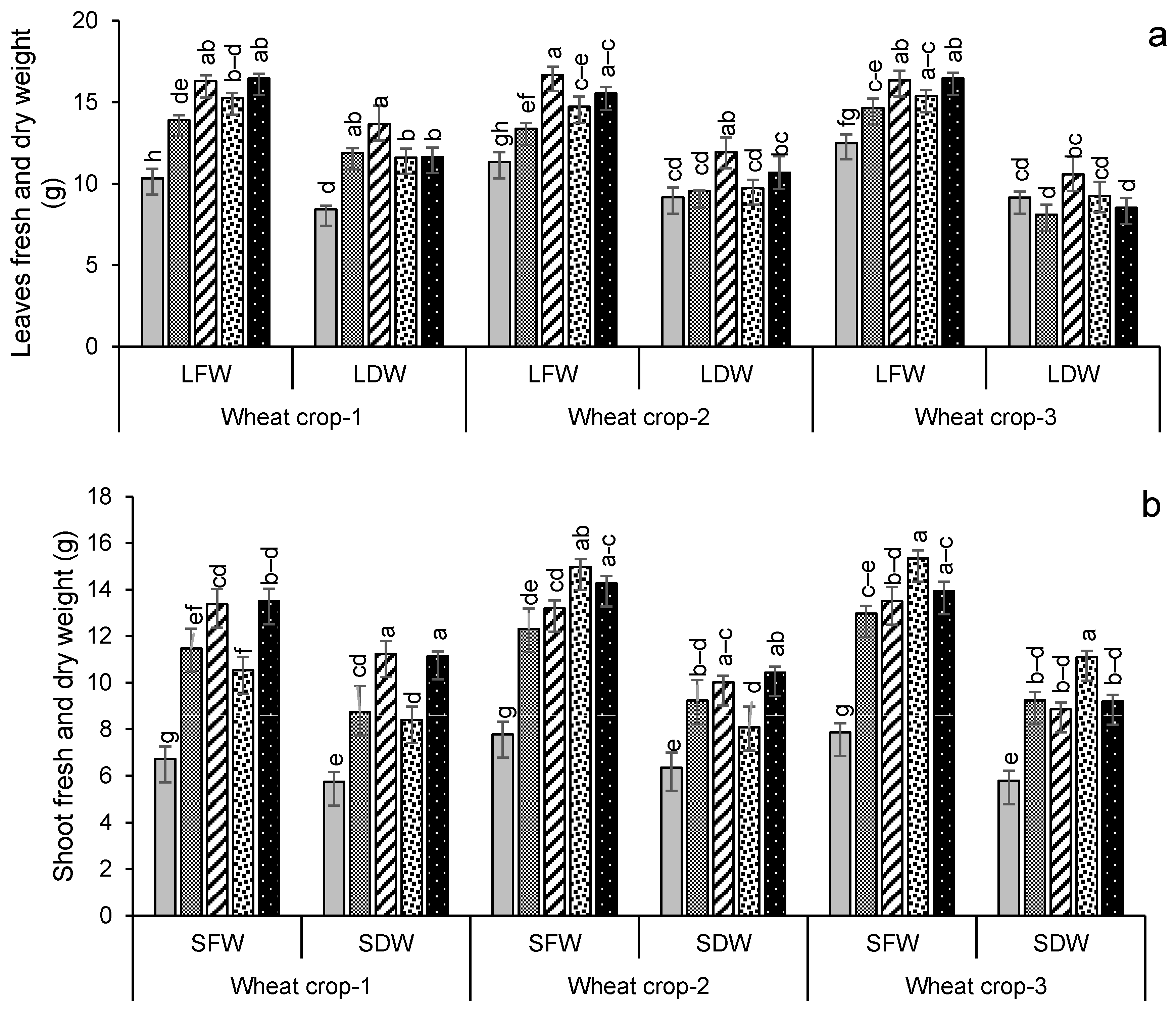
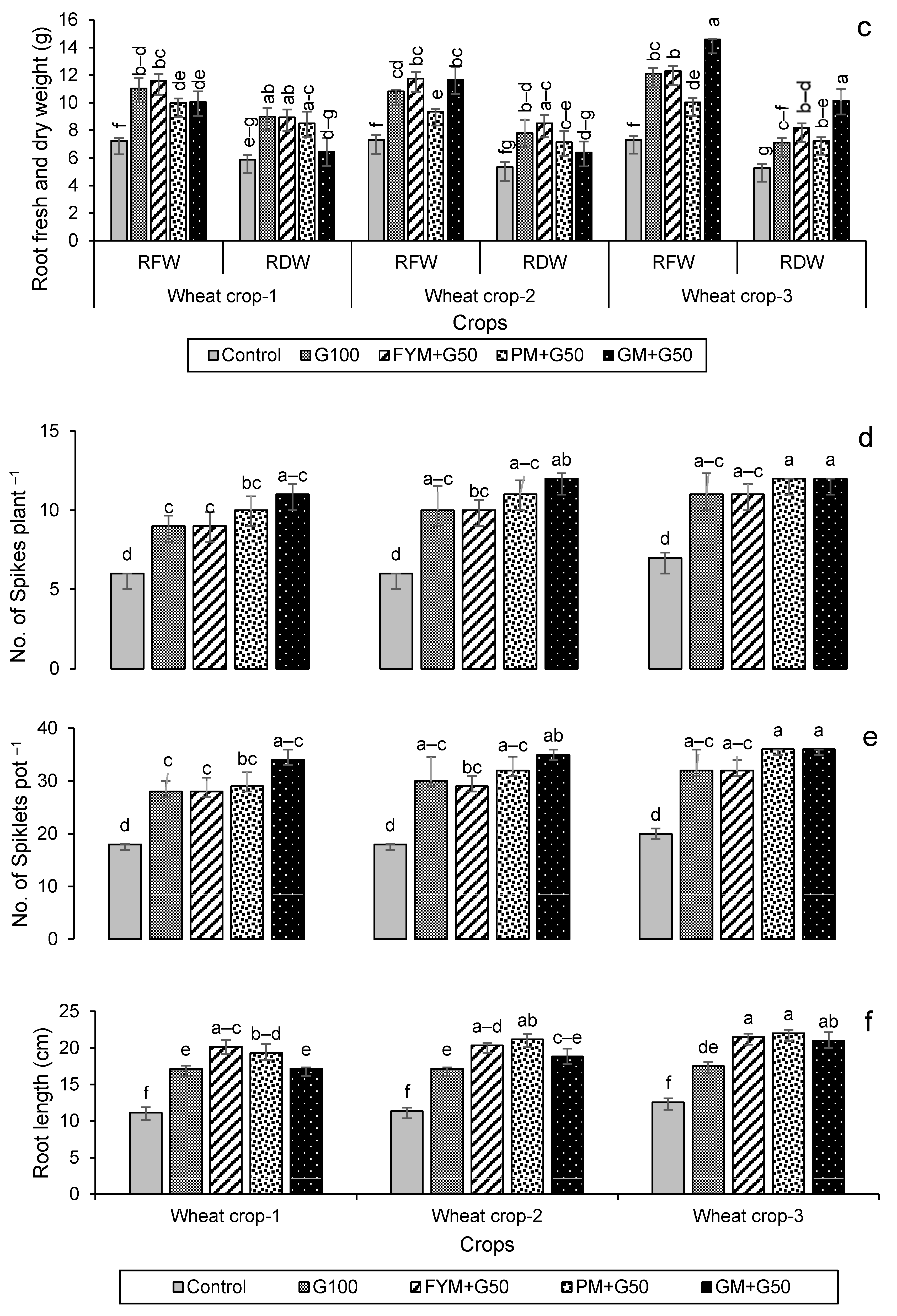
Growth and yield parameters for wheat and maize are given in Figure 5. For wheat, their plant biomass production was significantly (p < 0.05) increased in the later years compared to the first year of amendment (Figure 5a–c). Leaf dry weight increased most in year 1 for treatment FYM + G50, increasing by 62% over the control. By contrast, the highest shoot and root dry weights were found in the year 3 wheat crop with PM + G50 and GM + G50 (91% and 92% more compared to their respective controls). The maximum number of spikes (12) was recorded for wheat in PM + G50 and GM + G50 in year 3, which was a 71% increase over the control (Figure 5d). Similarly, a significant (p < 0.005) increase in the number of spikelets (36) was observed in year 3 with an 80% increase over the control (Figure 5e). Root lengths significantly increased (p < 0.05) over the controls in all of the treatments, increasing by 71% in FYM + G50 and 75% in PM + G50 (Figure 5f).
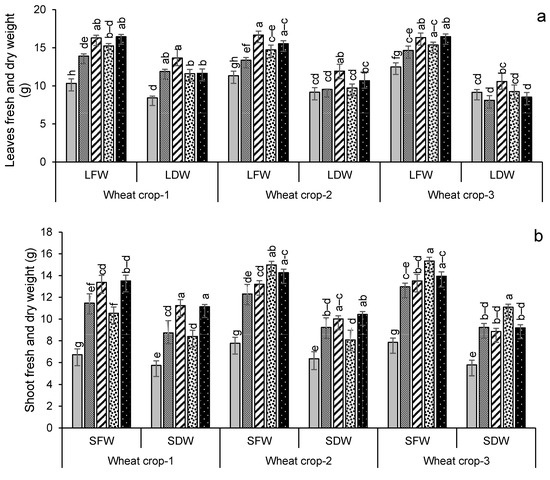
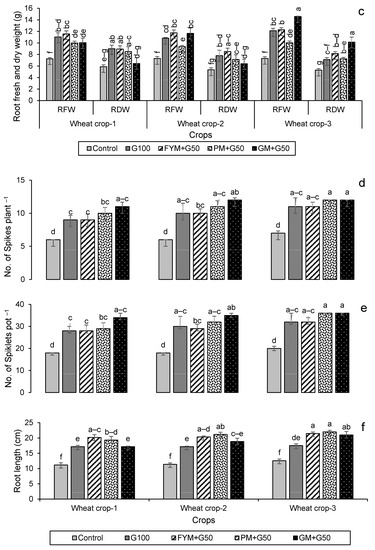
Figure 5.
Impacts of different treatments on (a) leaves, (b) shoots and (c) root fresh and dry weights, (d) no. of spikes, (e) no. of spikelets and (f) root length of wheat. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
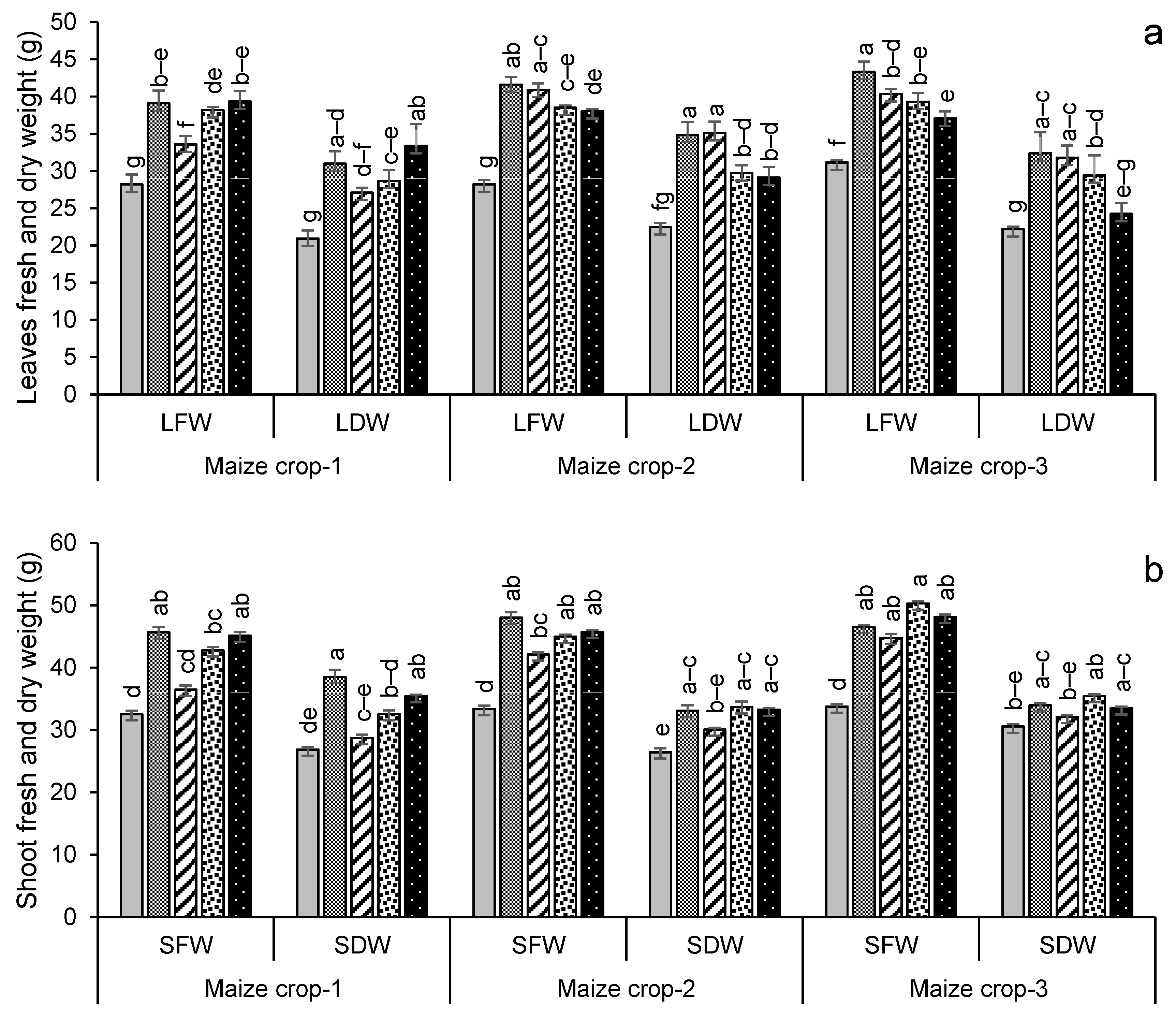
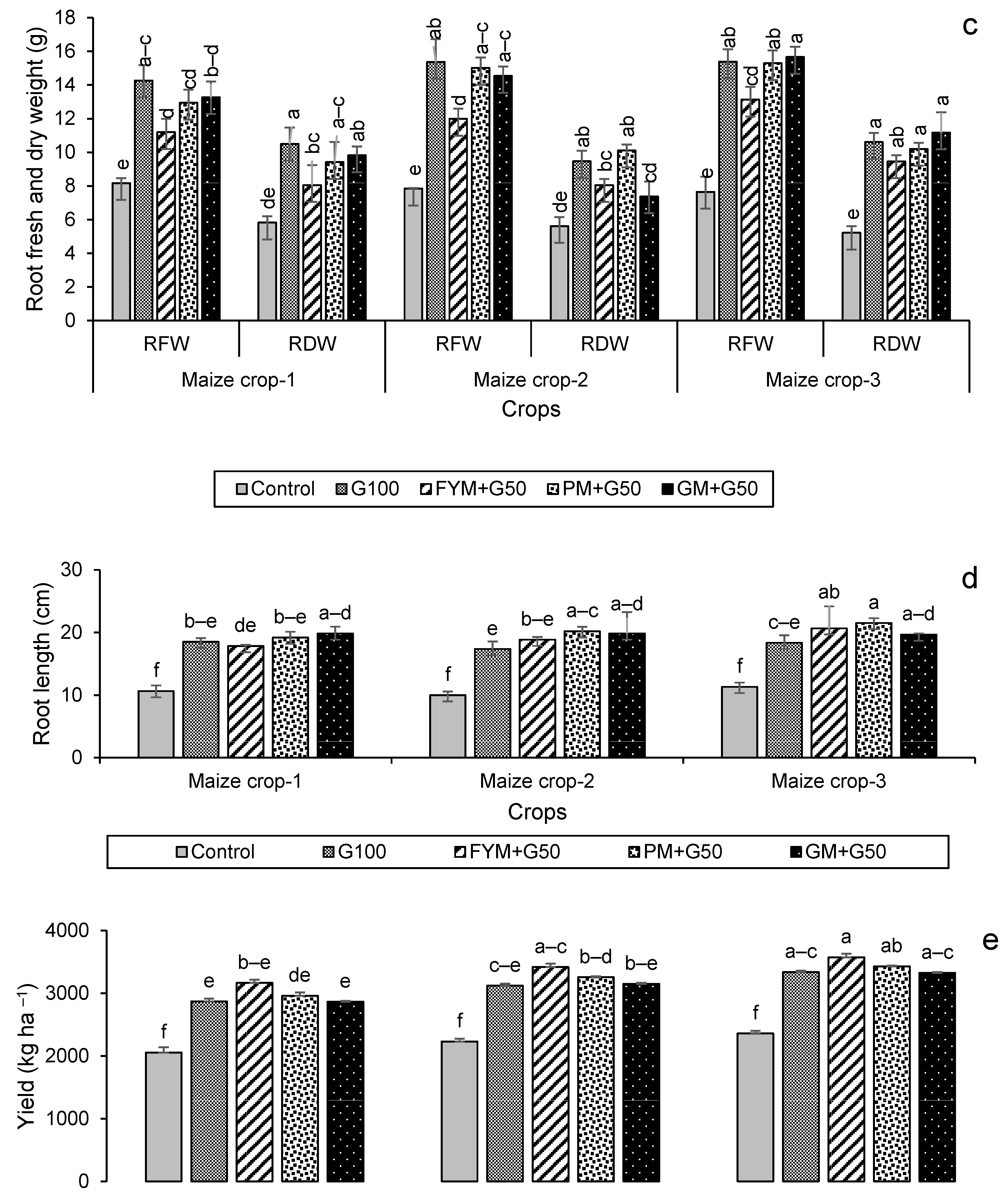
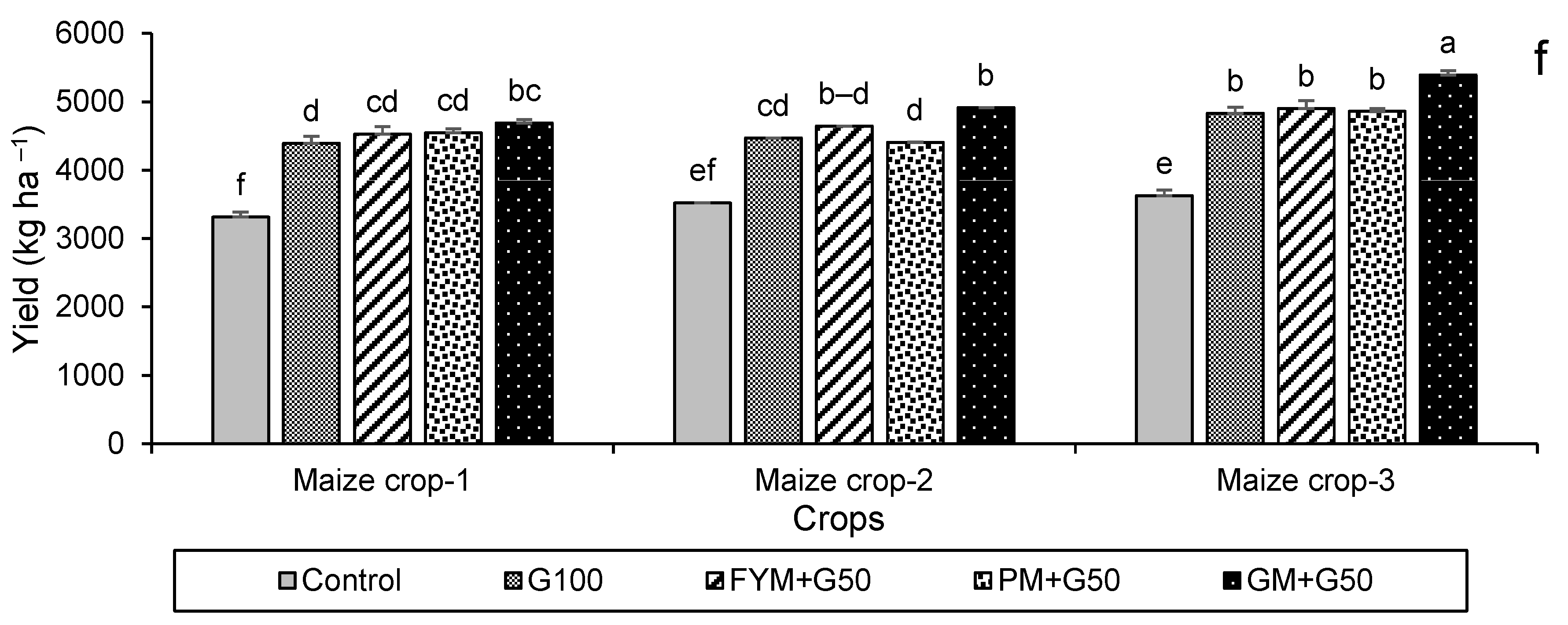
For maize, the highest leaf dry weight was in year 2 in the treatment FYM + G50, with a 56% increase over the control (Figure 6a), while shoot and root dry weights were highest in year 2 for FYM + G50 (a 49% increase over the control) and in year 3 for GM + G50 (a 114% increase over the control) (Figure 6b,c). In the maize crop, the maximum increase in root length (+90%) was recorded in the treatment PM + G50 in year 3 (Figure 6d). The wheat and maize crop yields showed a significant variation with treatment and year (Figure 7). The maximum wheat yield in the year 3 treatment FYM + G50 showed a 51% increase over the control (Figure 6a), while the highest maize yield was a 49% increase in GM + G50 in year 3 (Figure 6b).
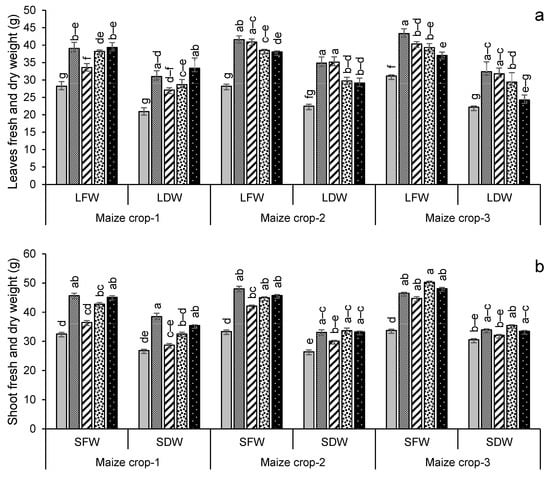
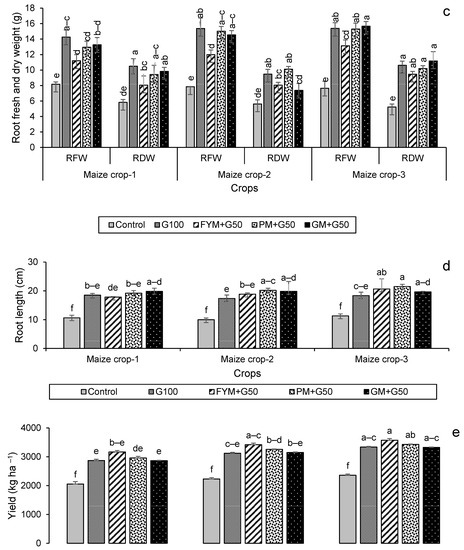
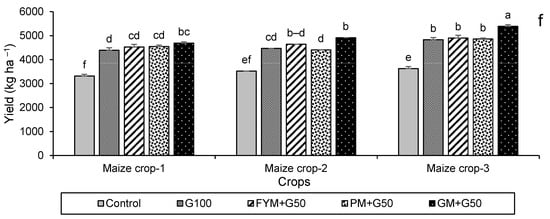
Figure 6.
Impacts of different treatments on (a) leaf, (b) shoot, and (c) root fresh and dry weights, (d) root length of maize, (e) yield of wheat and (f) yield of maize. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
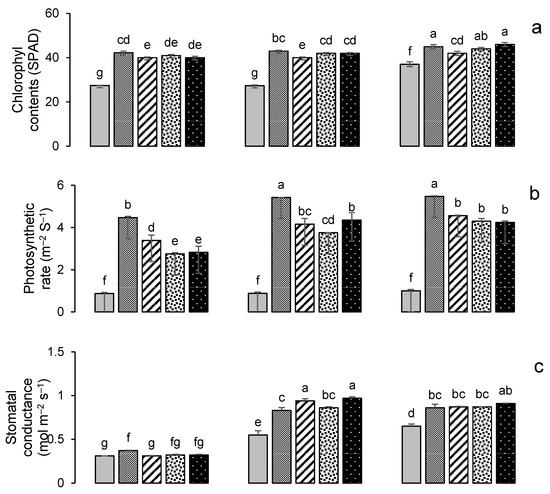
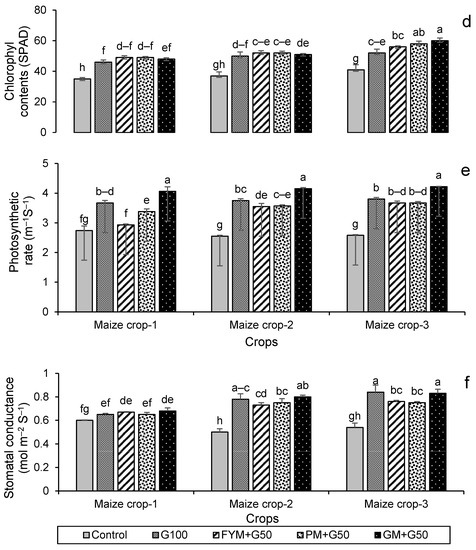
Figure 7.
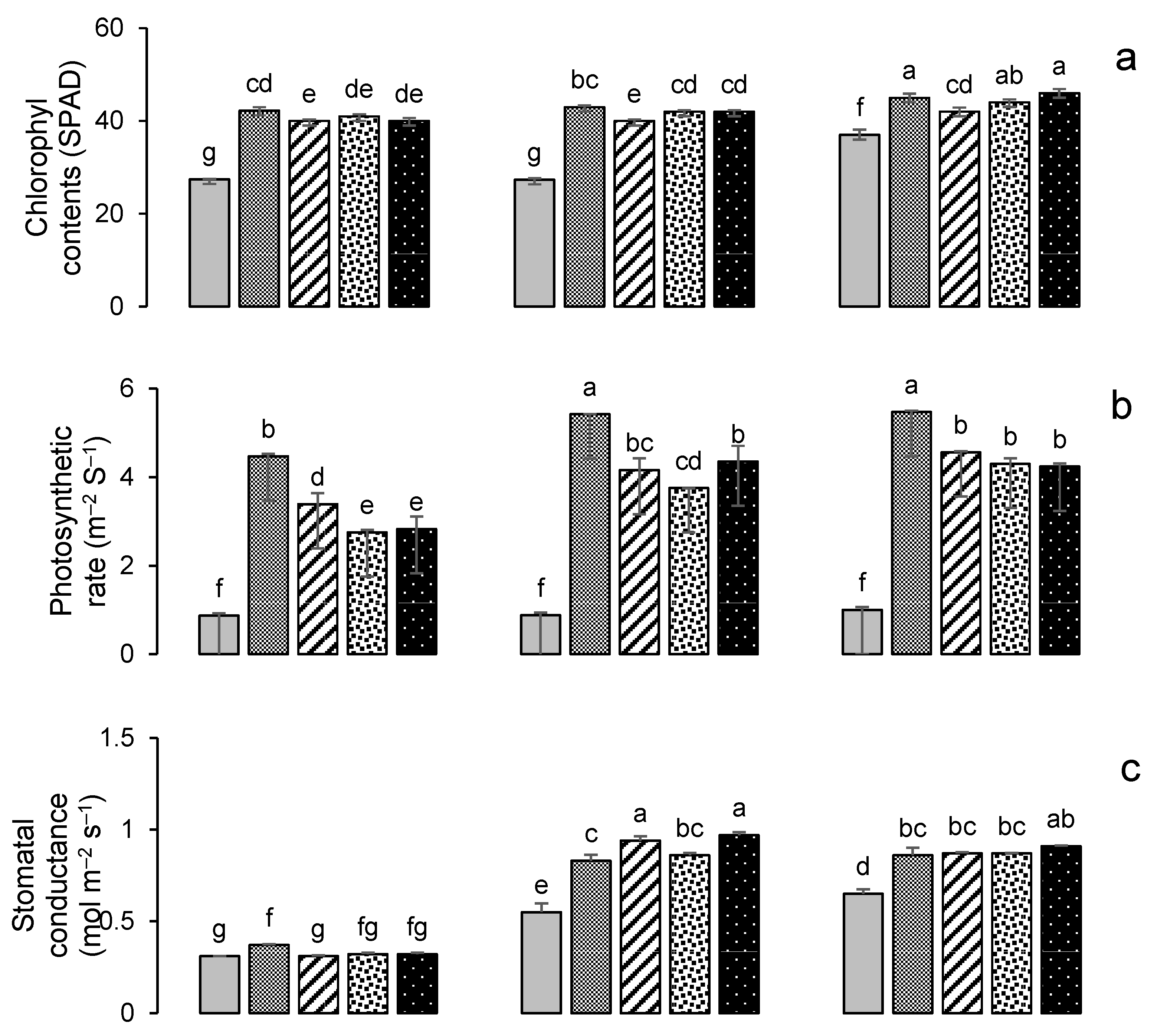
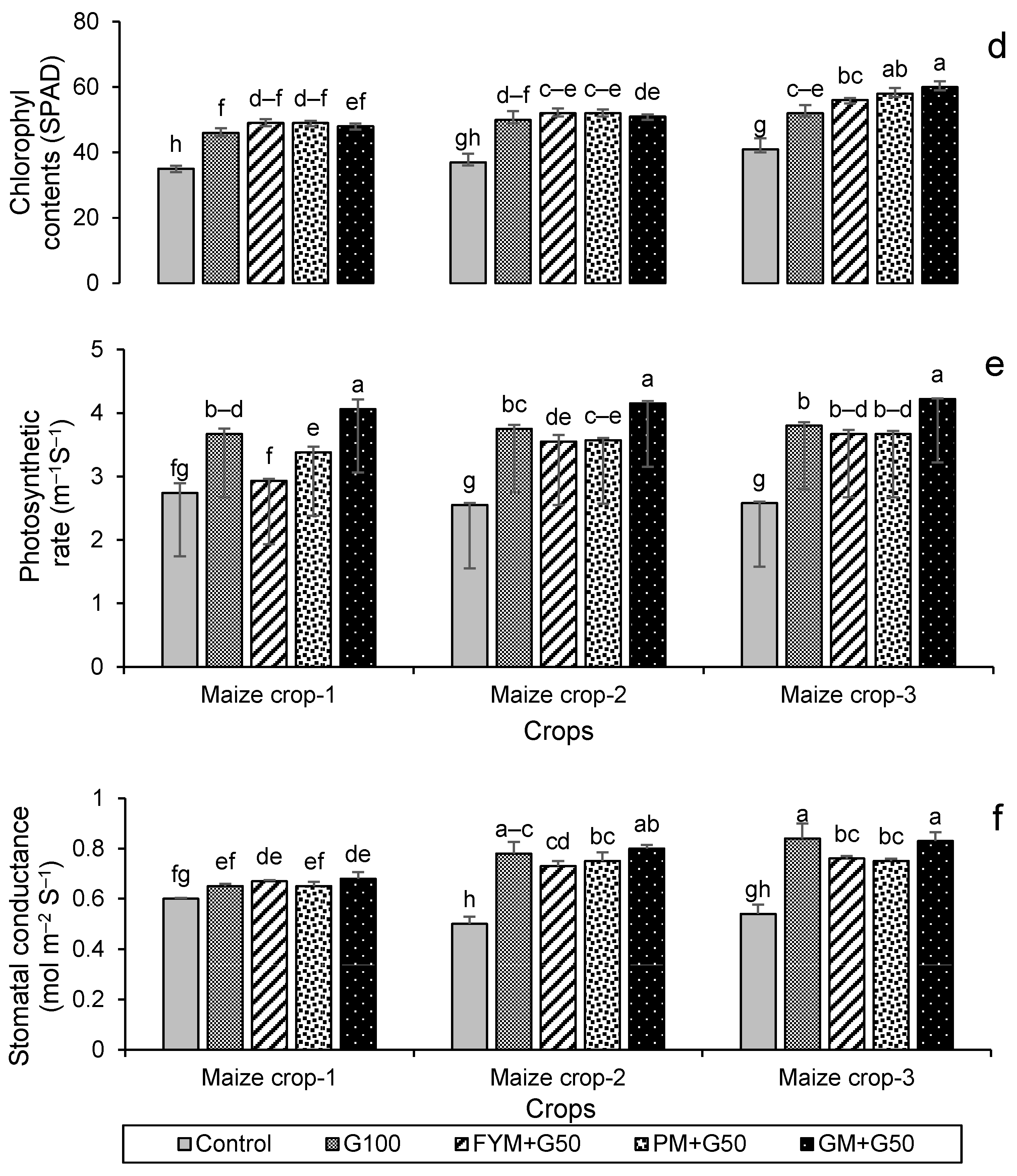
Impacts of different treatments on (a) chlorophyl contents, (b) photosynthetic rate, (c) stomatal conductance of wheat, (d) chlorophyl contents, (e) photosynthetic rate, and (f) stomatal conductance of maize. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
3.2.3. Crop Physiological Attributes
All treatments resulted in a significant (p < 0.001) increase in the chlorophyll contents (SPAD value) of both wheat and maize (Figure 7). The highest chlorophyll contents were recorded in year 3; this was for treatment GM + G50 (Figure 7a) in wheat, a 24% increase over the control, while in maize it was for treatment PM + G50, with a 46% increase over the control (Figure 7d). The maximum photosynthetic rates were also recorded in year 3; for wheat, this was 5.47 μmol m−2 s−1 in the G100 treatment (Figure 7b), and for maize, this was 4.22 μmol m−2 s−1 in the GM + G50 treatment (Figure 7e). Maximum stomatal conductance was recorded in year 2 for wheat (0.97 mmol m−2 s−1 in GM + G50) (Figure 7c) and in year 3 for maize (0.84 mmol m−2 s−1 in G100) (Figure 7f).
3.3. Soil Organic Carbon
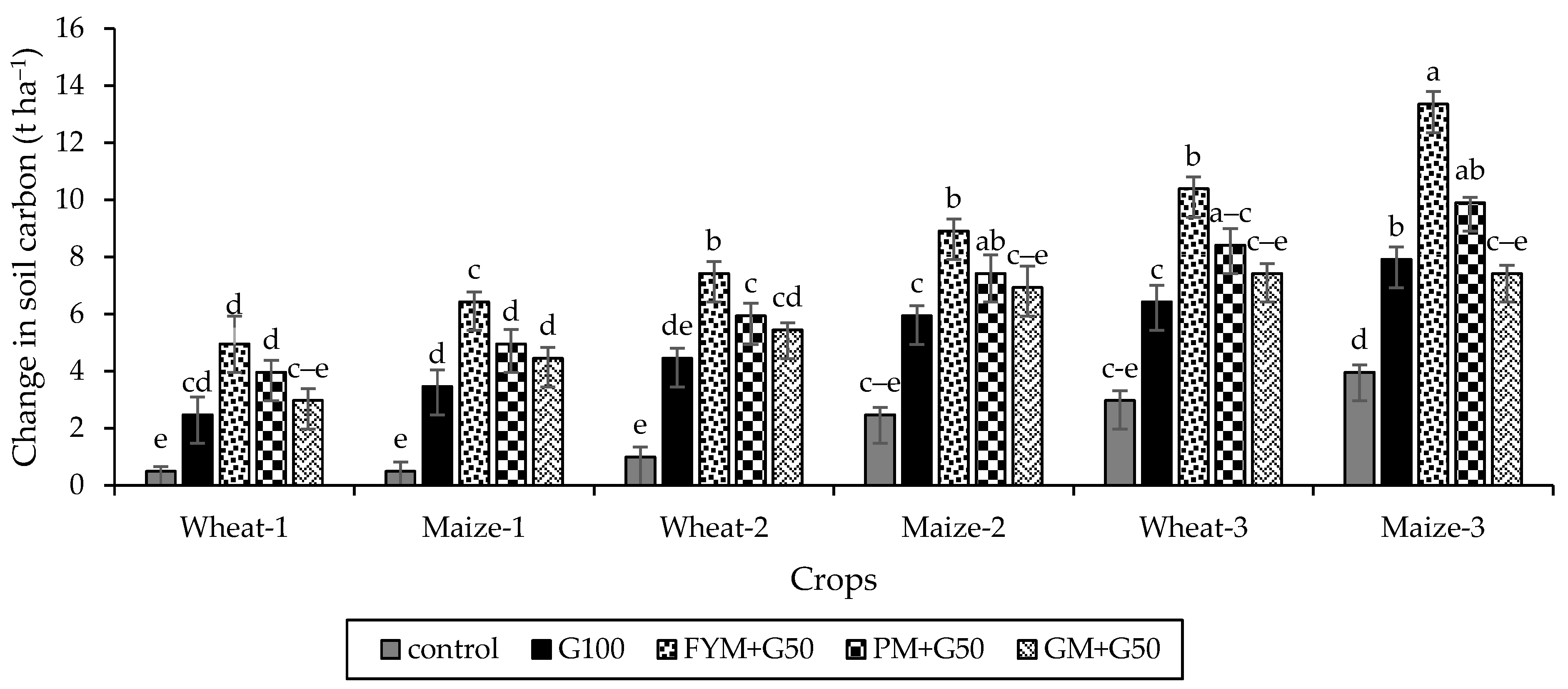
The soil C significantly increased (p < 0.05) under all treatments relative to the control (Figure 8). After 3 years, the maximum increase in soil C was recorded with FYM + G50 (13.36 t ha−1) compared to control (3.96 t ha−1). Overall, the soil C increased in the order control < GM + G50 < G100 < PM + G50 < FYM + G50.
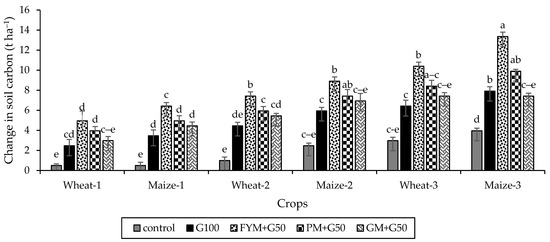
Figure 8.
Carbon sequestration in freshly reclaimed marginally salt-affected soils during the wheat–maize crops rotation system. Error bars represent the standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
3.4. Post-Harvest Soil Analysis
Post-harvest soil properties are presented in Table 3. There were significant changes between the treatments when compared to the respective controls. The soil’s EC was reduced by 24%, the pH by 14% and the SAR by 72%. By contrast, the CEC increased by 17%, the TN by 660% and the SOM by 281%, respectively. Changes in soil properties are presented in Table 3.

Table 3.
Post-harvest soil analysis. Note: EC = electrical conductivity, SAR = sodium adsorption ratio, OM = organic matter, CEC = cation exchange capacity, TN = total nitrogen, MBC = microbial biomass carbon, SR = soil respiration, DHA = dehydrogenase activity. Rounded-off values after ± represents standard error (n = 3). Different letters indicate statistical difference (LSD, p < 0.05).
3.5. Economic Analysis of the Crop Production
An economic analysis of the value of the different treatments to the farmer is presented in Table 4. The analysis aimed to compare the total cost of production up to the point of harvesting and sale of the grain and plant biomass. The net profit and the benefit–cost ratio was used to show the profitability of the different treatments. The combined treatment with farmyard manure and gypsum (FYM + G50) was the most profitable treatment for wheat, with a PKR 97,165 (USD 433) profit and a benefit–cost ratio of 2.32 in year 3. For maize, the most profitable treatment was green manure and gypsum (GM + G50), with a profit of PKR 60,969 (USD 272) and a benefit–cost ratio of 1.83 in year 3.

Table 4.
Cost–benefit analysis (USD ha−1) during reclamation and crop production in study area during wheat–maize rotation.
3.6. Simulation of Short-Term Changes in Soil Carbon
3.6.1. Derivation of Organic Waste Parameters Using Soils from Pot Experiments at Dijkot and Uchkera
The weather data and soil properties used in the simulation of the pot experiment soils are given in Supplementary Figure S2 and Supplementary Table S3, respectively (columns Dijkot and Uchkera). The crop management data is shown in Supplementary Table S2, and the crop yields are shown in Supplementary Table S4. The weather data from the pot study site in the experimental area of the University of Agriculture Faisalabad (Supplementary Figure S2d) was used in the forward run, as this was the location of the pots during the trial. The values of soil C, measured in the control and used to adjust plant inputs, are shown in Supplementary Table S8. The soil C measurements for the treatments used to evaluate the model are also given in the same table. The organic manure parameters used in the simulation are presented in Table 5. The evaluation of the simulations of soil organic C values using these organic manure parameters is presented in Table 6. These simulations matched the measured values to within 10.7% for all treatments (Table 5). Therefore, the organic waste parameters were used at the Jhang site unchanged.

Table 5.
Organic manure parameters used in the simulations. Note: DPM = decomposable plant material; HUM = humus (recalcitrant plant material).

Table 6.
Evaluation of organic manure parameters in pot experiments using soils from Dijkot and Uchkera. Note: FYM = farmyard manure, PM = poultry manure, GM = green manure (all applied at 12 t ha−1); G100 = 100% soil gypsum requirement; G50 = 50% soil gypsum requirement.
3.6.2. Evaluation of Organic Waste Parameters Using Pot and Field Measurements at an Independent Site, Jhang
As for the previous simulations, the data used to run and evaluate the pot trial are given in Supplementary Figures S2 and S3, and also in Tables S3–S5. The weather data used to simulate the field trial is shown in Supplementary Figure S3. The model simulations showed a good fit and a strong correlation to the measurements from both the pot experiments and field trials, with an uncertainty, expressed as RMSE, of less than 10% and a correlation R2 over 0.99 in all treatments (Table 7). Therefore, the model can be used to assess the impact of treatments on C sequestration over the long term, with a confidence of ±10%.

Table 7.
Evaluation of simulations of the impact of gypsum and organic manures on soil carbon in pot and field experiments at Jhang. Note: FYM = farmyard manure, PM = poultry manure, GM = green manure (all applied at 12 t ha−1); G100 = 100% soil gypsum requirement; G50 = 50% soil gypsum requirement.
3.6.3. Simulation of Long-Term Carbon Sequestration
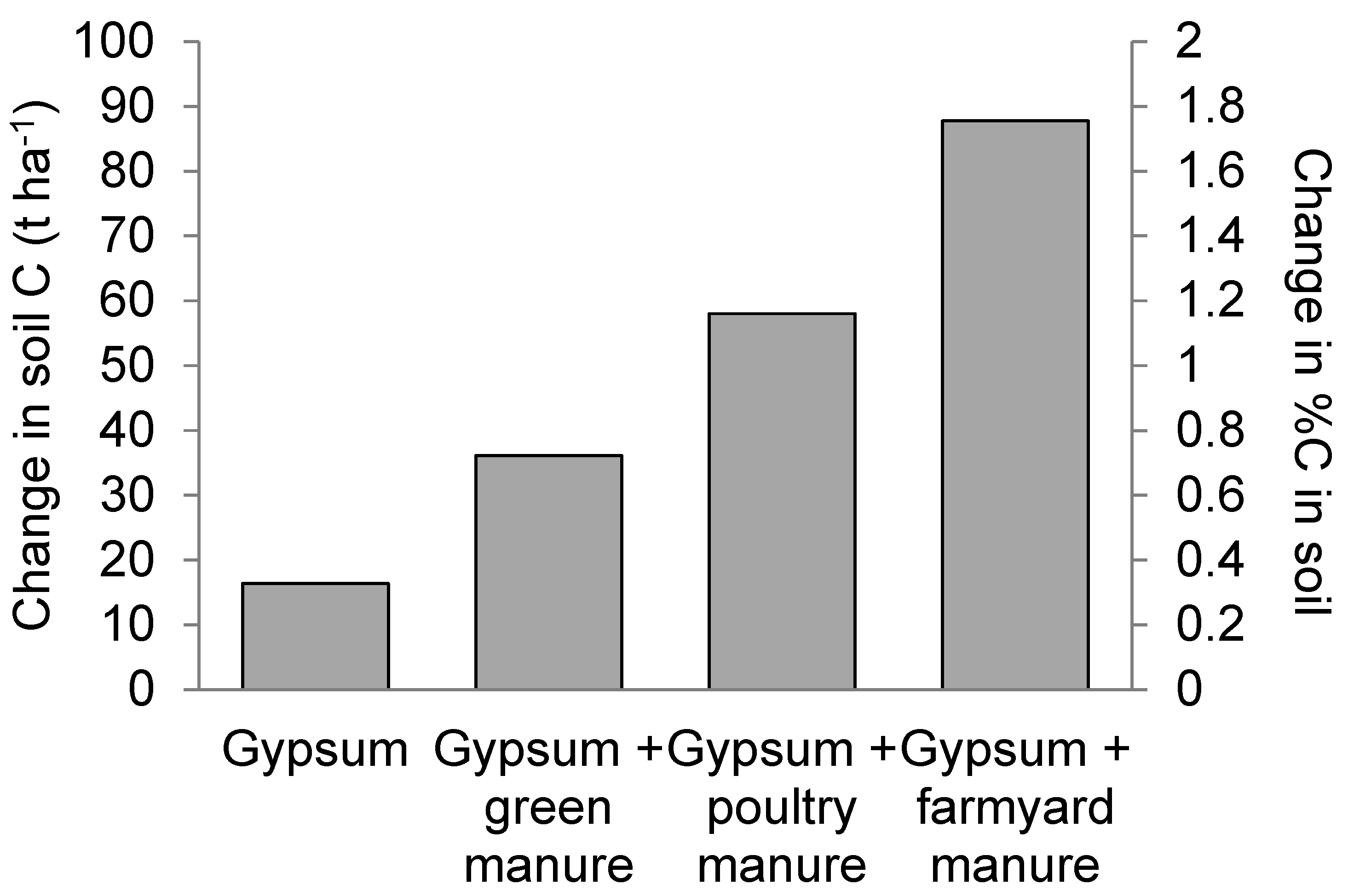
The potential C sequestration in different treatments is shown in Figure 9. The soil organic C content in all studies reached a stable level within 100 years of the same treatment application. Treatment with FYM (FYM + G50) showed the highest potential for C sequestration, increasing (88 ± 12) t ha−1 (324%) above the control.
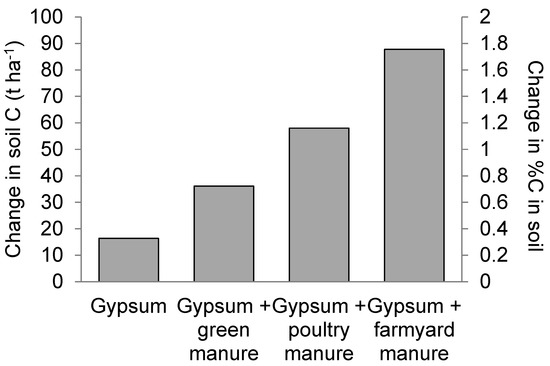
Figure 9.
Simulated potential carbon (C) sequestration assuming continued application of the treatments used in the field study (left axis) change in C stock to a depth of 30 cm; (right axis) change in %C in the soil.
4. Discussion
4.1. Impacts of Different Treatments on Soil Properties
It has been widely reported that amending salinity-prone soil with organic matter is an effective strategy for improving soil health. Organic amendments are easy to procure and reported by many authors to be effective in the restoration of marginally saline soils [50,51]. The leaching of salts from salt-affected soils requires the replacement of Na+ with divalent cations, such as Ca2+, which can be provided by amendments of gypsum [11]. Adding organic matter to the soil provides more exchange sites to capture Ca2+ ions, which can be held by a strong organo-mineral Ca2+ bridges rather than single bonds to Na+ ions.
In our study, we found that the addition of gypsum and three other types of organic amendments to the marginally salt-affected soil reduced the pH in all cases (Table 2). This is a benefit in salt-affected soils that tend to have a pH that is above the optimum range for crop growth. In other studies, soil pH was also shown to be reduced by the application of organic amendments [52,53]. It is likely that the addition of organic amendments causes a decrease in the soil pH by increasing microbial activity, the formation of organic acids and subsequent dissolution of salts that are then leached down the soil horizons. A decrease in the pH of salt-affected soils may also be due to the higher quantity of basic-cationic compounds in the added organic amendments. This was shown in the study by Chen, et al. [54], who found that the addition of compost, biochar and peat all decreased the soil pH.
The addition of organic amendments helps to bind soil particles into aggregates, mainly by the deprotonation of humic and fulvic acids. The addition of poultry manure to saline–sodic soils has been demonstrated to enhance both the CEC and the quantity of exchangeable K+ ions in the soil; exchangeable K+ competes with Na+ ions and limits its entry into the soil environment [11]. The addition of green manure has been shown to increase microbial decomposition and respiration, and to reduce the soil pH, increasing the solubility of CaCO3 due to the increased CO2 partial pressure in the soil [55,56]. Organic manures have also been observed to stimulate enzymatic activity. When gypsum and organic amendments are applied in combination, they reduce SAR, pH and soil particle dispersion [57].
The concentration of soluble cations also varied depending on the kind of organic amendment used in the experiment. Applying farmyard manure, poultry manure and green manure had less significant impacts on the Ca2+ levels in the soil than applications of gypsum. Applying gypsum restored soils by replacing Na+ with Ca2+ on the soil exchange sites [58]. When organic amendments were also added, they triggered organic acids production, which, in turn, raised the Ca2+ concentration by dissolving calcium carbonate (CaCO3). Addition of Ca2+ ions has been shown to decrease SAR and EC, increase CEC, and improve soil structure [59,60]. The release of native Ca+ and silicates acts to exchange Na+ with silicon (Si) and Ca+ on the exchange sites, resulting in leaching of Na+ out of the soil [61,62].
Total soil N concentrations were significantly increased after the application of farmyard manure, poultry manure and green manure because these manures are naturally rich in N [63]. This increased the soil microbial activity, which may have also improved N use efficiency and activated the free-living soil micro-organisms responsible for N fixation [64]. Rapid decomposition of organic amendments resulted in the production of mineral N, which is of immediate benefit to crop production [65].
The SOM is critical to the establishment and functioning of agricultural ecosystems. In this study, the addition of organic amendments greatly boosted the SOM content; this finding is also supported by other studies (e.g., [16,52,66]). The increase in SOM was dependent on the source and type of organic amendment applied. Farmyard manure and poultry manure had the greatest impact on SOM because of the greater quantity of easily accessible nutrients, boosting crop production and plant inputs. The addition of poultry manure has been observed to increase available N, due to its low in C:N ratio and rapid decomposition when applied to the soils [67]. Furthermore, organic amendments reduces the soil bulk density, and improves soil porosity, the water infiltration/percolation rate and the soil aggregate stability. The composition and diversity of soil microbial communities may be altered as well as stimulated by manure-based amendments [50].
Application of the organic amendments had a direct and positive impact on the soil microbial activity. Significant changes were observed when organic amendments were applied in combination with gypsum. All treatments greatly boosted soil MBC, SR and DHA compared to the controls. The increase in SOM could be explained by the formation of organic horizons with the addition of organic manures [68]. Moreover, organic amendments have the potential to increase soil organism biomass and to benefit plant health by decomposing organic matter, cycling nutrients and improving the structure of the soil [69,70]. The decomposition and transformation of organic matter and nutrients in organically modified soils are driven by an increase in MBC as well as an increase in soil enzyme activity [71,72]. The primary reason for the increase in DHA is likely to be the increased supply of organic C in stressed saline soils [73]. Singh, et al. [74] reported a 101% increase in DHA with the addition of municipal solid waste compost and gypsum, while Garcia-Gil et al. [75] reported a 730% increase in DHA with addition of municipal solid waste compost, mainly attributed to the metabolic state of the soil biodiversity. The addition and fast degradation of organic amendments can provide nitrogenous substrates to the soil [76], which boost soil microbiota and enzyme production. By contrast, the low osmotic potential of saline soils causes a reduction in the microbial community by killing them through microbial cell lysis [77]. This is reflected in the concentration of dehydrogenase being inversely proportional to the salinity in the soils [74,78].
4.2. Impacts of Soil Restoration on Plant Growth, Physiology and Yield
In this study, growth and yield were significantly improved by the application of gypsum, both alone and in combination with organic amendments; similar improvements were reported by Edrisi, et al. [79]. All treatments showed an increase in germination, plant height, dry matter content and yield when compared to the respective control. Badar, et al. [80] observed that soil salinity can decrease crop growth, metabolism, and the quality of the produce, with salinity causing a decrease in root length of up to 36%, which significantly increased (up to 80%) when organic wastes were applied; similar findings were reported by Rop, et al. [81] and Bah, et al. [82]. Other authors observed that addition of gypsum and organic amendments compensated for deficiencies in both soil micro- and macronutrients during and after soil reclamation [66]. Ahmad, et al. [83] observed differences in nutrient absorption under different treatments that greatly influenced growth and yield potential. Ahmad, et al. [84] observed that compost applications improved all growth parameters measured, including crop yield. Many previous studies have shown that, with the application of organic wastes, soil nutrients and nutrient use efficiency are increased by providing the slow release of nutrients to plants [85]. Increases in crop yield with organic amendments were also reported by [33]. The addition of organic fertilizers increases N and P availability and enhances soil microbial activity [86], thus, contributing to improved plant nutrition and enhancing the root system of the crop [87].
The fresh and dry weight of the crops also showed a significant increase with the gypsum and organic amendments. A similar increase in dry matter production with the application of gypsum was reported by Crusciol, et al. [88]; they suggested that gypsum increases the soil water and nutrient use efficiency, which ultimately increases the growth and yield of plants. It has been also reported that a 48.4% increase occurred in the plant biomass in a wheat crop after the application of rapeseed meal in saline soils in China [53]. Similarly, efficient use of all available resources may be further improved by the low and consistent supply of nutrients and increased water absorption associated with the applied organic manures [89].
Plant physiology was also significantly improved through the application of gypsum and organic amendments. The crops were able to absorb a higher quantity of light, resulting in a significantly greater amount of vegetative development in all treatments compared to the control. This could be explained by the addition of N in the amendments, which is an integral constituent of the chlorophyl [90,91]. As the chlorophyl content increased, the rate of photosynthesis also increased. The maximum increase recorded was 47% higher than the control treatment, as previous similar results were reported by [92]. Stomatal conductance also increased compared to the control, suggesting that the plant is better supplied with water associated with improved soil structure and water holding capacity. The higher stomatal conductance could also be attributed to the input of potassium in the manure, which is the key element in stomatal regulation [93]. Improved stomatal regulation will result in more efficient use of water resources by the plant.
4.3. The Impacts of Organic Amendments and Crop Rotation on Soil Carbon Storage
After three years of treatment with organic additions, the soil organic C content in these saline soils had significantly increased. The greatest change in the soil organic C was in treatment FYM + G50, which increased from 8.90 to 22.30 t ha−1, a 151% increase in soil organic C. The high level of C accumulation suggests that loss of organic matter is reduced in the treatments by the improved soil structure, reduced erosion and reduced dispersion of soil particles due to improved aggregation in the reclaimed soils [94,95]. The deficiency in organic matter is compensated for by the addition of organic amendments, which remove salt as well as adding the organic matter and nutrients that are necessary for crop growth and improvement of soil structure, stability and aggregation [96,97,98]. Organic amendments have been shown to increase soil organic C accumulation by many authors (e.g., [99,100]). The results obtained here are similar to the results obtained by Banger, et al. [101], who reported a 56% increase in soil organic C during the application of farmyard manure at the rate of 10 t ha−1 in Karnataka, India, while Kukal and Benbi [102] reported a 55% increase in soil organic C after 3 years of crop cultivation in India after the application of farmyard manure at the rate of 20 t ha−1 during maize–wheat cropping systems. Ghosh, et al. [103] reported a 26% increase in soil organic C after the application of farmyard manure and inorganic fertilizers in rice and wheat cropping systems in India. The large increase in soil organic C in our experiment was due to the continued application over three years of the recommended doses of synthetic fertilizers, plus harvested crop residue incorporation, in a soil that was previously un-managed and salt affected. The increase in soil organic C stocks in reclaimed/salt-affected soils could be explained by two theories: hierarchal aggregation and macroaggregate turnover [51]. According to the hierarchal aggregation theory, the addition of organic amendments causes small soil particles to form clusters, microaggregates and macroaggregates, which are then bound together through coagulation or cementation. The macroaggregate turnover theory suggests that macroaggregates start to form around the perimeter of added organic amendments, which then fragment and repeatedly reform to stabilize the organic matter by macroaggregation. The addition of organic amendments in our study is likely to have increased the formation of organic soil colloids, which promoted the formation of organo-mineral complexes and lead to the formation of aggregates. Decomposition of the organic amendments also increased the availability of nutrients.
4.4. Simulations of Soil Carbon Sequestration in Freshly Reclaimed Marginally Salt-Affected Soils under Wheat–Maize Cropping System
Our simulations predicted that the application of organic amendments in freshly reclaimed salt-affected soils would greatly increase the soil organic C stocks over the control. There were differences in the sequestration of soil organic C between treatments, due to differences in the properties of the organic amendment. Significant long-term increases in soil organic C have been observed with the application of organic fertilizers, such as farmyard manure, poultry manure and green manures [103,104,105,106]. Gypsum application improves the physico-chemical condition of the soil (bulk density, aggregation and water holding capacity) by reducing the concentration of Na+ ions, and improving the potential of the soil to retain organic C [32]. The improved soil properties played a significant role in enhancing the organic C sequestration in the reclaimed soils [107].
5. Conclusions
The application of gypsum together, with organic amendments, improved soil health, crop yield, soil organic C content and soil properties. Gypsum in combination with farmyard manure performed the best for improving soil health, with a 13%, 10% and 62% reduction in soil EC, pH and SAR, and a 10%, 220% and 122% increase in CEC, total nitrogen and organic matter. An increase of 225% for MBC and SR and a 185% increase for DHA was recorded during the fallow period. Yield increases of 51% and 49% were recorded in wheat and maize yields with FYM + G50 and GM + G50 treatments. Post-harvest soil analysis indicated a 24%, 14% and 72% decrease in soil EC, pH and SAR. The soil carbon storage increased up to 13.36 t ha−1 with FYM + G50. These results suggest that farmers in both arid and semi-arid regions with marginally saline–sodic soils could reclaim their soils through the application of organic manures in combination with gypsum, with farmyard manure being the most effective treatment. Applying gypsum and manures to saline soils improves the soil structure, soil health and productivity, and, thus, could greatly contribute to reducing food insecurity and sequestering C to mitigate climate change. Modelling and simulations were performed using historical climatic conditions assuming no change in future climate. Further studies could consider different climatic scenarios and determine the potential interaction of the treatments with the changing climate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13031436/s1.
Author Contributions
Conceptualization, M.S. (Muhammad Sabir) and Z.U.R.F.; methodology, M.S. (Muhammad Sabir) and J.S.; software, J.S., H.R.A.; validation, J.S.; formal analysis, Z.U.R.F.; investigation, Z.U.R.F.; resources, Z.U.R.F., J.S.; data curation, Z.U.R.F.; writing—original draft preparation, Z.U.R.F.; writing—review and editing, J.S., M.S. (Muhammad Shahbaz), M.S. (Muhammad Sabir), J.S.; visualization, Z.U.R.F.; supervision, M.S. (Muhammad Sabir) and J.S.; project administration, M.S. (Muhammad Sabir); funding acquisition, Z.U.R.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Education Commission (HEC) of Pakistan, grant number 518-75399-2PS5-005, and the APC was funded through a bench fee from the International Research Support Initiative Program (IRSIP) through the HEC, award number IRSIP-45-BMS-75.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All of the data is presented in the manuscript or available as Supplementary Data attached with the manuscript.
Acknowledgments
The primary author wants to acknowledge the Modelling Group, Institute of Biological and Environmental Sciences, University of Aberdeen, UK for providing all of the facilities required in the drafting of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Jat, H.S.; Sharma, P.C.; Bolan, N.S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J. Environ. Manag. 2021, 280, 111736. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 43–53. [Google Scholar]
- Hossain, M.S. Present scenario of global salt affected soils, its management and importance of salinity research. Int. Res. J. Biol. Sci. 2019, 1, 1–3. [Google Scholar]
- Smyth, S.J.; Phillips, P.W.; Kerr, W.A. EU failing FAO challenge to improve global food security. Trends Biotechnol. 2016, 34, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Singh, A.K.; Singh, Y.P. Bioremediation of salt affected soils: An Indian perspective; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Haile, M.G.; Wossen, T.; Tesfaye, K.; von Braun, J. Impact of climate change, weather extremes, and price risk on global food supply. Econ. Disasters Clim. Chang. 2017, 1, 55–75. [Google Scholar] [CrossRef]
- Abate, S.; Belayneh, M.; Ahmed, F. Reclamation and amelioration of saline-sodic soil using gypsum and halophytic grasses: Case of Golina-Addisalem irrigation scheme, Raya Kobo Valley, Ethiopia. Cogent Food Agric. 2021, 7, 1859847. [Google Scholar] [CrossRef]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Khan, A.; Faisal, S.; Shafique, M.; Khan, S.; Bacha, A.S. ASTER-based remote sensing investigation of gypsum in the Kohat Plateau, north Pakistan. Carbonates Evaporites 2020, 35, 3. [Google Scholar] [CrossRef]
- Amini, S.; Ghadiri, H.; Chen, C.; Marschner, P. Salt-affected soils, reclamation, carbon dynamics, and biochar: A review. J. Soils Sediments 2016, 16, 939–953. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, N.; Wu, L.; Xu, M.; Bingham, I.J.; Li, Z. Effects of enhancing soil organic carbon sequestration in the topsoil by fertilization on crop productivity and stability: Evidence from long-term experiments with wheat-maize cropping systems in China. Sci. Total Environ. 2016, 562, 247–259. [Google Scholar] [CrossRef]
- Bright, M.B.; Diedhiou, I.; Bayala, R.; Assigbetse, K.; Chapuis-Lardy, L.; Ndour, Y.; Dick, R.P. Long-term Piliostigma reticulatum intercropping in the Sahel: Crop productivity, carbon sequestration, nutrient cycling, and soil quality. Agric. Ecosyst. Environ. 2017, 242, 9–22. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar]
- Rashid, M.F.; Aziz, T.; Maqsood, M.A.; Farooq, M. Soil phosphorus fractions and their transformation in normal and salt affected soils as affected by organic amendments. Pak. J. Agric. Sci. 2019, 56, 301–312. [Google Scholar]
- Oldfield, E.E.; Wood, S.A.; Bradford, M.A. Direct effects of soil organic matter on productivity mirror those observed with organic amendments. Plant Soil 2018, 423, 363–373. [Google Scholar] [CrossRef]
- Wood, S.A.; Tirfessa, D.; Baudron, F. Soil organic matter underlies crop nutritional quality and productivity in smallholder agriculture. Agric. Ecosyst. Environ. 2018, 266, 100–108. [Google Scholar] [CrossRef]
- Leifeld, J.; Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 2018, 9, 1071. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Sabir, M.; Zia-Ur-Rehman, M.; Hussain, M.M. Mitigation of climate change through carbon sequestration in agricultural soils. In Climate Change and Agroforestry Systems; Apple Academic Press: Burlington, ON, USA, 2020; pp. 87–118. [Google Scholar]
- del Mar Montiel-Rozas, M.; Panettieri, M.; Madejón, P.; Madejón, E. Carbon sequestration in restored soils by applying organic amendments. Land Degrad. Dev. 2016, 27, 620–629. [Google Scholar] [CrossRef]
- Haque, M.M.; Biswas, J.; Maniruzaman, M.; Akhter, S.; Kabir, M. Carbon sequestration in paddy soil as influenced by organic and inorganic amendments. Carbon Manag. 2020, 11, 231–239. [Google Scholar] [CrossRef]
- Qureshi, A.S.; McCornick, P.G.; Qadir, M.; Aslam, Z. Managing salinity and waterlogging in the Indus Basin of Pakistan. Agric. Water Manag. 2008, 95, 1–10. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.M.; Akanda, R.; Stille, L.; Singh, R.K.; Awan, A.R.; Mahmood, K.; Faaij, A.P. Biomass production in agroforestry and forestry systems on salt-affected soils in South Asia: Exploration of the GHG balance and economic performance of three case studies. J. Environ. Manag. 2013, 127, 324–334. [Google Scholar] [CrossRef]
- Qadir, M.; Schubert, S.; Oster, J.D.; Sposito, G.; Minhas, P.S.; Cheraghi, S.A.; Murtaza, G.; Mirzabaev, A.; Saqib, M. High-magnesium waters and soils: Emerging environmental and food security constraints. Sci. Total Environ. 2018, 642, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, G.; Murtaza, B.; Kahlon, U.Z.; Yaseen, M. A Comparative study of different amendments on amelioration of saline-sodic soils irrigated with water having different EC: SAR ratios. Commun. Soil Sci. Plant Anal. 2017, 48, 2630–2641. [Google Scholar] [CrossRef]
- Ghafoor, A.; Murtaza, G.; Rehman, M.; Sabir, M. Reclamation and salt leaching efficiency for tile drained saline-sodic soil using marginal quality water for irrigating rice and wheat crops. Land Degrad. Dev. 2012, 23, 1–9. [Google Scholar] [CrossRef]
- Murtaza, G.; Ghafoor, A.; Owens, G.; Qadir, M.; Kahlon, U. Environmental and economic benefits of saline-sodic soil reclamation using low-quality water and soil amendments in conjunction with a rice–wheat cropping system. J. Agron. Crop Sci. 2009, 195, 124–136. [Google Scholar] [CrossRef]
- Ghafoor, A.; Murtaza, G.; Ahmad, B.; Boers, T.M. Evaluation of amelioration treatments and economic aspects of using saline–sodic water for rice and wheat production on salt-affected soils under arid land conditions. Irrig. Drain. 2008, 57, 424–434. [Google Scholar] [CrossRef]
- Zaman, G.; Murtaza, B.; Imran, M.; Shahid, M.; Shah, G.M.; Amjad, M.; Naeem, M.A.; Mubeen, M.; Murtaza, G. Utilization of bio-municipal solid waste improves saline-sodic soils and crop productivity in rice-wheat. Compost. Sci. Util. 2020, 28, 16–27. [Google Scholar] [CrossRef]
- Murtaza, G.; Murtaza, B.; Usman, H.M.; Ghafoor, A. Amelioration of Saline-sodic Soil using Gypsum and Low Quality Water in Following Sorghum-berseem Crop Rotation. Int. J. Agric. Biol. 2013, 15, 640–648. [Google Scholar]
- Garcia-Franco, N.; Wiesmeier, M.; Hurtarte, L.C.C.; Fella, F.; Martínez-Mena, M.; Almagro, M.; Martínez, E.G.; Kögel-Knabner, I. Pruning residues incorporation and reduced tillage improve soil organic matter stabilization and structure of salt-affected soils in a semi-arid Citrus tree orchard. Soil Tillage Res. 2021, 213, 105129. [Google Scholar] [CrossRef]
- Basak, N.; Sheoran, P.; Sharma, R.; Yadav, R.K.; Singh, R.K.; Kumar, S.; Krishnamurthy, T.; Sharma, P.C. Gypsum and pressmud amelioration improve soil organic carbon storage and stability in sodic agroecosystems. Land Degrad. Dev. 2021, 32, 4430–4444. [Google Scholar] [CrossRef]
- Soni, P.G.; Rai, A.K.; Basak, N.; Kumar, P.; Sundha, P. Productivity and profitability of sorghum-wheat cropping system in saline soils as influenced by conservation agriculture practices. Range Manag. Agrofor. 2021, 42, 277–285. [Google Scholar]
- Saqib, Z.A.; Akhtar, J.; Haq, M.; Ahmad, I. Salt induced changes in leaf phenology of wheat plants are regulated by accumulation and distribution pattern of Na ion. Pak. J. Agric. Sci 2012, 49, 141–148. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total nitrogen analysis of soil and plant tissues. J. Assoc. Off. Anal. Chem. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Terry, R.E.; Nelson, S.D.; Carr, J.; Parnell, J.; Hardin, P.J.; Jackson, M.W.; Houston, S.D. Quantitative phosphorus measurement: A field test procedure for archaeological site analysis at Piedras Negras, Guatemala. Geoarchaeology 2000, 15, 151–166. [Google Scholar] [CrossRef]
- Norman, A. Methods of Soil Analysis: Part 2-Chemical and Microbiological Properties; ASA SSSA: Madison, WI, USA, 1965. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Estefan, G. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region; International Center for Agricultural Research in the Dry Areas: Beirut, Lebanon, 2013. [Google Scholar]
- Vance, E.; Brookes, P.; Jenkinson, D. An Extraction Method for Measuring Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Anderson, J.P. Soil respiration. Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1983; Volume 9, pp. 831–871. [Google Scholar]
- Casida, L., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Powlson, D.S.; Smith, P.; Smith, J.U. Evaluation of Soil Organic Matter Models: Using Existing Long-Term Datasets; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 38. [Google Scholar]
- Setia, R.; Smith, P.; Marschner, P.; Baldock, J.; Chittleborough, D.; Smith, J. Introducing a decomposition rate modifier in the Rothamsted carbon model to predict soil organic carbon stocks in saline soils. Environ. Sci. Technol. 2011, 45, 6396–6403. [Google Scholar] [CrossRef]
- Smith, J.; Smith, P.; Wattenbach, M.; Zaehle, S.; Hiederer, R.; Jones, R.J.; Montanarella, L.; Rounsevell, M.D.; Reginster, I.; Ewert, F. Projected changes in mineral soil carbon of European croplands and grasslands, 1990–2080. Glob. Chang. Biol. 2005, 11, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.H. Using primary productivity as an index of coastal eutrophication: The units of measurement matter. J. Plankton Res. 2007, 29, 1–6. [Google Scholar] [CrossRef]
- Smith, J.; Abegaz, A.; Matthews, R.B.; Subedi, M.; Orskov, E.R.; Tumwesige, V.; Smith, P. What is the potential for biogas digesters to improve soil carbon sequestration in Sub-Saharan Africa? Comparison with other uses of organic residues. Biomass Bioenergy 2014, 70, 73–86. [Google Scholar] [CrossRef]
- Smith, J.; Smith, P.; Addiscott, T. Quantitative methods to evaluate and compare soil organic matter (SOM) models. In Evaluation of Soil Organic Matter Models; Springer: Berlin/Heidelberg, Germany, 1996; pp. 181–199. [Google Scholar]
- Program, C.E.; Maize, I.; Center, W.I. From Agronomic Data to Farmer Recommendations: An Economics Training Manual; CIMMYT: Texcoco, Mexico, 1988. [Google Scholar]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Tsang, D.C. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Singh, K.; Pankaj, U.; Verma, S.K.; Verma, R.K.; Patra, D. Effect of organic amendments and microbial application on sodic soil properties and growth of an aromatic crop. Ecol. Eng. 2017, 102, 127–136. [Google Scholar] [CrossRef]
- Yang, L.; Bian, X.; Yang, R.; Zhou, C.; Tang, B. Assessment of organic amendments for improving coastal saline soil. Land Degrad. Dev. 2018, 29, 3204–3211. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef]
- Liang, Y.; Yang, Y.; Yang, C.; Shen, Q.; Zhou, J.; Yang, L. Soil enzymatic activity and growth of rice and barley as influenced by organic manure in an anthropogenic soil. Geoderma 2003, 115, 149–160. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Nikolic, M.; Peng, Y.; Chen, W.; Jiang, Y. Organic manure stimulates biological activity and barley growth in soil subject to secondary salinization. Soil Biol. Biochem. 2005, 37, 1185–1195. [Google Scholar] [CrossRef]
- Vance, W.; Tisdall, J.; McKenzie, B. Residual effects of surface applications of organic matter and calcium salts on the subsoil of a red-brown earth. Aust. J. Exp. Agric. 1998, 38, 595–600. [Google Scholar] [CrossRef]
- Abdul Qadir, A.; Murtaza, G.; Zia-ur-Rehman, M.; Waraich, E.A. Application of Gypsum or Sulfuric Acid Improves Physiological Traits and Nutritional Status of Rice in Calcareous Saline-Sodic Soils. J. Soil Sci. Plant Nutr. 2022, 22, 1846–1858. [Google Scholar] [CrossRef]
- David, R.; Dimitrios, P. Diffusion and cation exchange during the reclamation of saline-structured soils. Geoderma 2002, 107, 271–279. [Google Scholar] [CrossRef]
- Qadir, A.A.; Murtaza, G.; Zia-ur-Rehman, M.; Waraich, E.A. Effect of chemical reclamation on the physiological and chemical response of rice grown in varying salinity and sodicity conditions. Int. J. Agric. Biol. 2021, 26, 97–104. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Murtaza, G.; Bibi, S.; Sabir, M.; Owens, G.; Saifullah; Ahmad, I.; Zeeshan, N. Immobilization of cadmium in soil-plant system through soil and foliar applied silicon. Int. J. Phytoremediation 2022, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yang, Y.; Peng, H.; Zhang, L.; Zhou, Y.; Zhang, J.; Du, C.; Liu, J.; Lin, X.; Wang, N.; et al. Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef] [PubMed]
- Hijbeek, R.; Ten Berge, H.; Whitmore, A.; Barkusky, D.; Schröder, J.; Van Ittersum, M. Nitrogen fertiliser replacement values for organic amendments appear to increase with N application rates. Nutr. Cycl. Agroecosystems 2018, 110, 105–115. [Google Scholar] [CrossRef]
- Yang, J.; Gao, W.; Ren, S. Long-term effects of combined application of chemical nitrogen with organic materials on crop yields, soil organic carbon and total nitrogen in fluvo-aquic soil. Soil Tillage Res. 2015, 151, 67–74. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The influence of organic amendment source on carbon and nitrogen mineralization in different soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Leogrande, R.; Vitti, C. Use of organic amendments to reclaim saline and sodic soils: A review. Arid. Land Res. Manag. 2019, 33, 1–21. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Olaniran, A.F.; Adenusi, T.T.; Aremu, C.; Ejue, W.S.; Iranloye, Y.M.; Gbadamosi, A.; Olayanju, A. Effects of cow dung and wood biochars and green manure on soil fertility and tiger nut (Cyperus esculentus L.) performance on a savanna Alfisol. Sci. Rep. 2020, 10, 21021. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Zhu, M.; Zhang, J.; Li, P.; Dai, X.; Xu, Y.; Liu, L. Evolution of soil properties following reclamation in coastal areas: A review. Geoderma 2014, 226, 130–139. [Google Scholar] [CrossRef]
- Pereg, L.; Morugán-Coronado, A.; McMillan, M.; García-Orenes, F. Restoration of nitrogen cycling community in grapevine soil by a decade of organic fertilization. Soil Tillage Res. 2018, 179, 11–19. [Google Scholar] [CrossRef]
- Jing, Y.; Zhang, Y.; Han, I.; Wang, P.; Mei, Q.; Huang, Y. Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 2020, 10, 8837. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gao, J.; Bai, L.; Wang, Y.; Huang, J.; Kumar, M.; Zeng, X. Influence of green manure and rice straw management on soil organic carbon, enzyme activities, and rice yield in red paddy soil. Soil Tillage Res. 2019, 195, 104428. [Google Scholar] [CrossRef]
- Deng, L.-N.; Feng, G.-N.; Gao, Y.; Shen, Y.-X.; Li, H.-S.; Gu, Y.; Luan, H.-Y. Phytochemical constituents and antioxidant enzyme activity profiles of different barley (Hordeum vulgare L.) cultivars at different developmental stages. Agronomy 2019, 10, 37. [Google Scholar] [CrossRef]
- Rao, D.; Pathak, H. Ameliorative influence of organic matter on biological activity of salt-affected soils. Arid. Land Res. Manag. 1996, 10, 311–319. [Google Scholar] [CrossRef]
- Singh, P.; Choudhary, O.; Mavi, M. Irrigation-induced salinization effects on soil chemical and biological properties under cotton-wheat rotation on loamy sand soil in northwest India. J. Indian Soc. Soil Sci. 2018, 66, 386–391. [Google Scholar] [CrossRef]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef]
- Frankenberger, W., Jr.; Bingham, F. Influence of salinity on soil enzyme activities. Soil Sci. Soc. Am. J. 1982, 46, 1173–1177. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Sikora, S.; Huić Babić, K.; Filipović, V.; Gerke, H.H.; Romić, D. Response of soil dehydrogenase activity to salinity and cadmium species. J. Soil Sci. Plant Nutr. 2020, 20, 530–536. [Google Scholar] [CrossRef]
- Edrisi, S.A.; Tripathi, V.; Chaturvedi, R.K.; Dubey, D.K.; Patel, G.; Abhilash, P.C. Saline Soil Reclamation Index as an efficient tool for assessing restoration progress of saline land. Land Degrad. Dev. 2021, 32, 123–138. [Google Scholar] [CrossRef]
- Badar, R.; Batool, B.; Ansari, A.; Mustafa, S.; Ajmal, A.; Perveen, S. Amelioration of salt affected soils for cowpea growth by application of organic amendments. J. Pharmacogn. Phytochem. 2015, 3, 87–90. [Google Scholar]
- Rop, K.; Karuku, G.N.; Mbui, D.; Njomo, N.; Michira, I. Evaluating the effects of formulated nano-NPK slow release fertilizer composite on the performance and yield of maize, kale and capsicum. Ann. Agric. Sci. 2019, 64, 9–19. [Google Scholar] [CrossRef]
- Bah, H.; Zhou, M.; Kizito, S.; Xiao, R.; Raza, S.T.; Dong, Z.; Zhu, B. Carbon balance under organic amendments in the wheat-maize cropping systems of sloppy upland soil. Sustainability 2020, 12, 2747. [Google Scholar] [CrossRef]
- Ahmad, G.; Ansar, M.; Kaleem, S.; Nabi, G.; Hussain, M. Performance of early maturing oats (Avena sativa L.) cultivars for yield and quality. J. Agric. Res. 2008, 46, 341–346. [Google Scholar]
- Ahmad, R.; Shahzad, S.M.; Khalid, A.; Arshad, M.; Mahmood, M.H. Growth and yield response of wheat (Triticum aestivum L.) and maize (Zea mays L.) to nitrogen and L-tryptophan enriched compost. Pak. J. Bot. 2007, 39, 541. [Google Scholar]
- Gómez, C.; Green, D.R. Small unmanned airborne systems to support oil and gas pipeline monitoring and mapping. Arab. J. Geosci. 2017, 10, 202. [Google Scholar] [CrossRef]
- Gharbi, M.; Soualmia, A.; Dartus, D.; Masbernat, L. Comparison of 1D and 2D hydraulic models for floods simulation on the Medjerda Riverin Tunisia. J. Mater. Environ. Sci 2016, 7, 3017–3026. [Google Scholar]
- Alcívar, M.; Zurita-Silva, A.; Sandoval, M.; Muñoz, C.; Schoebitz, M. Reclamation of saline–sodic soils with combined amendments: Impact on quinoa performance and biological soil quality. Sustainability 2018, 10, 3083. [Google Scholar] [CrossRef]
- Crusciol, C.A.; Marques, R.R.; Carmeis Filho, A.C.; Soratto, R.P.; Costa, C.H.; Ferrari Neto, J.; Castro, G.S.; Pariz, C.M.; Castilhos, A.M.; Franzluebbers, A.J. Lime and gypsum combination improves crop and forage yields and estimated meat production and revenue in a variable charge tropical soil. Nutr. Cycl. Agroecosyst. 2019, 115, 347–372. [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Shen, H.; Wang, X.; Zhu, M.; Ge, Y.; Sun, L. Effects of soil reclamation on the oat cultivation in the newly reclaimed coastal land, eastern China. Ecol. Eng. 2019, 129, 115–122. [Google Scholar] [CrossRef]
- Abid, M.; Batool, T.; Siddique, G.; Ali, S.; Binyamin, R.; Shahid, M.J.; Rizwan, M.; Alsahli, A.A.; Alyemeni, M.N. Integrated nutrient management enhances soil quality and crop productivity in maize-based cropping system. Sustainability 2020, 12, 10214. [Google Scholar] [CrossRef]
- Racero, F.J.; Bueno, S.; Gallego, M.D. Predicting students’ behavioral intention to use open source software: A combined view of the technology acceptance model and self-determination theory. Appl. Sci. 2020, 10, 2711. [Google Scholar] [CrossRef]
- Azhar, M.; ur Rehman, M.Z.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; ul Haq, M.A.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Papadakis, I.; Papaioannou, A.; Chatzissavvidis, C.; Giannakoula, A. Comparative study effects between manure application and a controlled-release fertilizer on the growth, nutrient uptake, photosystem II activity and photosynthetic rate of Olea europaea L. (cv.‘Koroneiki’). Sci. Hortic. 2020, 264, 109176. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Mohanty, M.; Sinha, N.K.; Somasundaram, J.; McDermid, S.S.; Patra, A.K.; Singh, M.; Dwivedi, A.; Reddy, K.S.; Rao, C.S.; Prabhakar, M. Soil carbon sequestration potential in a Vertisol in central India-results from a 43-year long-term experiment and APSIM modeling. Agric. Syst. 2020, 184, 102906. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Lal, R.; James, R. Crop management effects on soil carbon sequestration on selected farmers’ fields in northeastern Ohio. Soil Tillage Res. 2005, 81, 265–276. [Google Scholar] [CrossRef]
- Li, Z.; Schneider, R.L.; Morreale, S.J.; Xie, Y.; Li, C.; Li, J. Woody organic amendments for retaining soil water, improving soil properties and enhancing plant growth in desertified soils of Ningxia, China. Geoderma 2018, 310, 143–152. [Google Scholar] [CrossRef]
- Yupeng, W.; Yufei, L.; Zhang, Y.; Yanmeng, B.; Zhenjun, S. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar]
- Chatterjee, S.; Bandyopadhyay, K.; Pradhan, S.; Singh, R.; Datta, S. Effects of irrigation, crop residue mulch and nitrogen management in maize (Zea mays L.) on soil carbon pools in a sandy loam soil of Indo-gangetic plain region. Catena 2018, 165, 207–216. [Google Scholar] [CrossRef]
- Yao, Z.; Zhang, D.; Liu, N.; Yao, P.; Zhao, N.; Li, Y.; Zhang, S.; Zhai, B.; Huang, D.; Wang, Z. Dynamics and sequestration potential of soil organic carbon and total nitrogen stocks of leguminous green manure-based cropping systems on the Loess Plateau of China. Soil Tillage Res. 2019, 191, 108–116. [Google Scholar] [CrossRef]
- Banger, K.; Kukal, S.; Toor, G.; Sudhir, K.; Hanumanthraju, T. Impact of long-term additions of chemical fertilizers and farm yard manure on carbon and nitrogen sequestration under rice-cowpea cropping system in semi-arid tropics. Plant Soil 2009, 318, 27–35. [Google Scholar] [CrossRef]
- Kukal, S.; Benbi, D. Soil organic carbon sequestration in relation to organic and inorganic fertilization in rice–wheat and maize–wheat systems. Soil Tillage Res. 2009, 102, 87–92. [Google Scholar] [CrossRef]
- Ghosh, S.; Wilson, B.; Ghoshal, S.; Senapati, N.; Mandal, B. Organic amendments influence soil quality and carbon sequestration in the Indo-Gangetic plains of India. Agric. Ecosyst. Environ. 2012, 156, 134–141. [Google Scholar] [CrossRef]
- Maltas, A.; Kebli, H.; Oberholzer, H.R.; Weisskopf, P.; Sinaj, S. The effects of organic and mineral fertilizers on carbon sequestration, soil properties, and crop yields from a long-term field experiment under a Swiss conventional farming system. Land Degrad. Dev. 2018, 29, 926–938. [Google Scholar] [CrossRef]
- Alam, M.; Rahman, M.; Biswas, J.C.; Akhter, S.; Maniruzzaman, M.; Choudhury, A.K.; Jahan, M.; Miah, M.; Uddin, M.; Sen, R. Nitrogen transformation and carbon sequestration in wetland paddy field of Bangladesh. Paddy Water Environ. 2019, 17, 677–688. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, P.; Zhao, N.; Cao, W.; Zhang, S.; Li, Y.; Huang, D.; Zhai, B.; Wang, Z.; Gao, Y. Building up the soil carbon pool via the cultivation of green manure crops in the Loess Plateau of China. Geoderma 2019, 337, 425–433. [Google Scholar] [CrossRef]
- Walia, M.K.; Dick, W.A. Selected soil physical properties and aggregate-associated carbon and nitrogen as influenced by gypsum, crop residue, and glucose. Geoderma 2018, 320, 67–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).