Abstract
Aim: To analyze and compare the effectiveness of two antibacterial gels in the treatment of mucositis. Methods: After signing the informed consent, in accordance with the Helsinki Declaration of 1975, revised in 2000, 21 patients were included in the study and divided by randomization into two groups. At the baseline, the modified bleeding index (mBI) and plaque index (PI) values were measured, and an oral hygiene and implant disinfection session was carried out. The session was repeated at 7, 15, and 30 days and after 2 and 3 months from the baseline. Two products were used—a bioadhesive gel in the test group and a 1% chlorhexidine gel in the control group. Results: Due to three dropouts, the final sample was composed of 18 patients. An improvement in periodontal indices was observed, similar to mBI and PI values, in both groups. There were no complications except for the appearance of pigmentations in the control group. Conclusions: The antibacterial power and effectiveness of the two gels are comparable. Considering the small sample size of the study cohort, further studies are needed to validate the results obtained from this pilot study.
1. Introduction
Implant-supported rehabilitation, over time, can attain a high rate of success [1]. However, it has been documented that pathologies of soft and hard tissues that surround implants can emerge. Peri-implant health is characterized by the absence of erythema, absence of bleeding on probing, and no signs of swelling and/or suppuration. Maintaining peri-implant health is a fundamental prerequisite for ensuring the duration of prosthetic rehabilitation over time [2,3,4]. Peri-implantitis is a pathological condition related to plaque that affects the tissues surrounding the implant. Sites affected by peri-implantitis have evident signs of inflammation, bleeding from the peri-implant sulcus and/or suppuration, and increased depth of probing associated with bone loss visible at the radiographic level [5]. The etiopathogenesis of peri-implantitis is well known and is related to the bacterial biofilm that colonizes the peri-implant environment and the soft tissue/implant interface [6], going through factors related to the patient (systemic diseases, smoking, plaque control) and host–parasite balance [7].
In the initial stage, the peri-implant lesion is defined as mucositis, which is an inflammation limited to the peri-implant soft tissues characterized by bleeding at the probing and clinical signs of inflammation [5]. This condition determines the impairment of the mucous implant seal, but it is not associated with bone resorption around the implant [8]. This lesion is reversible but, if left untreated, it can evolve into peri-implantitis. It is therefore essential to treat these lesions early to limit the progression of the disease.
One of the most documented methods for the treatment of mucositis is non-surgical therapy, which involves the mechanical breakdown of bacterial biofilm, the removal of the irritant factors present inside the peri-implant sulcus, and the decontamination of the implant surface using antibacterial solutions [2,9,10,11].
This therapy must be combined with proper oral hygiene at home and a program of periodic professional checks in order to verify the qualitative status of the marginal seal [12].
In the literature, many useful active ingredients have been suggested for the disinfection of implants, including chlorhexidine at different concentrations and formulations and different antibiotics with topical or systemic administration [13].
Chlorhexidine has small contraindications if used for a fairly long period of time, such as those required for the treatment of mucositis. Among the most frequent are the appearance pigmentation on the tongue, dental surfaces, and prostheses and a marked alteration of taste [14]. It would therefore be necessary for a treatment to contain active ingredients that the patient can use even for medium–long periods without contraindications.
The objective of this study is to evaluate the healing of peri-implant tissues affected by mucositis with a non-surgical treatment protocol comparing two different methods. In the control group a gel containing 1% chlorhexidine was used, whose antiseptic power has now been validated with numerous scientific trials [14,15], while in the test group a bio-adhesive gel with natural active ingredients was used.
The test gel contains antibacterial substances, Cetylpyridinium chloride and essential oils of Manuka and Melaleuca and re-epithelial substances, including the same essential oils; hyaluronic acid at different molecular weights, including molecule oligomers; and a PVP/hydrogen peroxide complex. It also has a marked bio-adhesion thanks to a complex original system of natural gums and resins (Ca/Na PVM-Ma copolymer). In addition, in its interior there are soothing substances, such as allantoin, bisabolol, and vitamin E.
Cetylpyridinium chloride is a cationic quaternary ammonium compound [16]; its effectiveness has been demonstrated in the control of gingival plaque and in case of gingivitis [17,18,19].
The null hypothesis for this study is that there is no difference for mBI and PI between the two processing protocols used.
2. Materials and Methods
2.1. Patients Recruiting
The study took place at the implant-prosthesis unit of the Santi Paolo and Carlo Hospital in Milan.
The main condition for entering the protocol screening was that patients had at least one rehabilitated implant in the oral cavity affected by mucositis.
Inclusion criteria of this RCT were:
- -
- Patients aged over 18 years;
- -
- At least one dental implant with mucositis (clinical signs of inflammation with bleeding at probing and probing depth > 4 mm);
- -
- No exclusion criteria present;
- -
- Signing of informed consent.
Patients who refused to enter the study and those who had the following conditions were excluded from the study:
- -
- Suppuration;
- -
- RX evidence of bone lesion with a depth > of 2 mm;
- -
- Peri-implantitis;
- -
- Physical or mental disabilities that affect correct domiciliary oral hygiene operations;
- -
- Abuse of alcohol or drugs;
- -
- Conditions or circumstances that prevent study participation completion or interfere with the analysis of study results.
All patients were informed about the clinical trial and were fully aware of the procedures in place. The study was carried out in accordance with the 1975 Helsinki Declaration, revised in 2000. Patients were not subject to additional costs for participation in the study and were not paid. Patients who did not consent to participation in the study continued treatment according to normal protocols. The study was approved by the Ethics Committee of the University of Milan (Minute n. 90/22).
2.2. Sample Dimension
The first treatment gives a total resolution of peri-implant bleeding (value 0), while in the second treatment there is evident bleeding (value 1), with a standard deviation for each group of 0.5, considering alpha = 0.05 and power = 0.95; with Student’s t-test with two tails for the differences of two group’s averages, which required eight patients each. Considering the dropout rate of the study was 10%, the two groups needed a minimum consistency of 10 cases each.
2.3. Initial Preparation and Randomization
The patients included in the study were treated as follows: at the first appointment, the plaque index (PI) and the modified bleeding index (mBI) of each patient were assessed, and then they underwent an initial session of professional scaling. After 15 days the patients underwent a second visit (baseline) during which each patient was assigned randomly to the test group or control group using a computer-generated number (www.calculator.net, accessed on 1 January 2023) with a range of 1–100. An even number indicated participation in the control group and an odd number in the test group.
2.4. Test and Control Treatment
Patients in the control group underwent a professional oral hygiene session. The disinfection of the implants affected by mucositis was performed through the passage of cup and spongy floss with 1% chlorhexidine gel (Corsodyl gel®, GlaxoSmithKline Consumer Healthcare S.r.l., Baranzate, Italy) and washing with a solution of 0,2% of Chlorhexidine (Corsodyl mouthwash®, GlaxoSmithKline Consumer Healthcare S.r.l., Baranzate, Milan, Italy) diffused in the peri-implant groove with disposable syringe and blunt needle. The patient was told not to rinse their mouth for at least 30 min.
Patients in the test group, after first rinsing with Chlorhexidine 0,2%, were subjected to a session of professional oral hygiene with disinfection of the implants affected by mucositis through cup and spongy floss with a gel made of Cetylpyridinium chloride, triclosan and essential oils (Hobagel Plus®, Hobama SRL, Milan, Italy) and the insertion of the same gel into the peri-implant groove with disposable blunt needle syringe. Again, the patient was told not to rinse their mouth for at least 30 min. Patients were blinded to group assignment.
2.5. Control and Follow-Up Visits
Patients from both groups were recalled after 7, 15, and 30 days and 2 and 3 months from the first treatment. During these control appointments, the mBI and PI indexes were recorded, and photographs were taken to verify and document changes in the inflammatory state of the implant sites. Then, in the same session, the test/control procedure was performed again according to the assignment group.
At each appointment the patients were strongly motivated and instructed regarding the correct operation of oral hygiene at home. Both test patients and control patients were provided with gel in anonymous packages and were required to continue using it at home twice a day, every 12 h.
2.6. Outcomes
The objective of the study was to demonstrate that the two treatments were similar in terms of plaque control and bleeding during treatment. To demonstrate this, clinical measurements of plaque and bleeding were performed at the beginning of treatment, at 7, 15, and 30 days and after 2 and 3 months from the first effective treatment session (baseline).
2.7. Measurements
The plaque index (PI) [20] evaluates the amount of plaque present.
It can have a value from 0 to 3; the maximum value was assigned for each implant affected by mucositis and subsequently the average for the individual patient was calculated. The modified bleeding index (mBI) [21] was detected by passing the probe along the gum line. It ranged from 0 to 3; the maximum value was assigned for each implant and subsequently the average for the individual patient was calculated (Table 1).

Table 1.
Peri-implant indices considered in the study.
2.8. Methodological Aspects
All clinical procedures were performed by a single, properly trained operator. The reference unit was the patient and not the individual implant. Therefore, for each patient, even in the presence of several implants, only one value per parameter was indicated. The data were collected in tables and the difference between the last session and the baseline was determined. The Mann–Whitney U-test and Fisher’s exact test were used to assess whether there were statistically significant differences between the two groups. The significance threshold was set at p < 0.05.
3. Results
A total of 21 patients with mucositis were included in this randomized trial. All patients (12 women and 9 men with an average age of 64 years) agreed to participate in the study and were recruited, resulting in a total number of 94 implants. After randomization, 11 patients were assigned to the test group and 10 patients to the control group. Three patients (one in the test group and two in the control group) left the study after the third visit (after 1 month) due to personal problems that did not allow them to continue therapy and did not show adverse events. For this reason, they were excluded from the study and the results, due to the inconstancy to follow-up program. The final number of patients analyzed was eighteen—ten in the test group and eight in the control group. Figure 1 presents the CONSORT flow chart of the clinical trial detailing the study process.
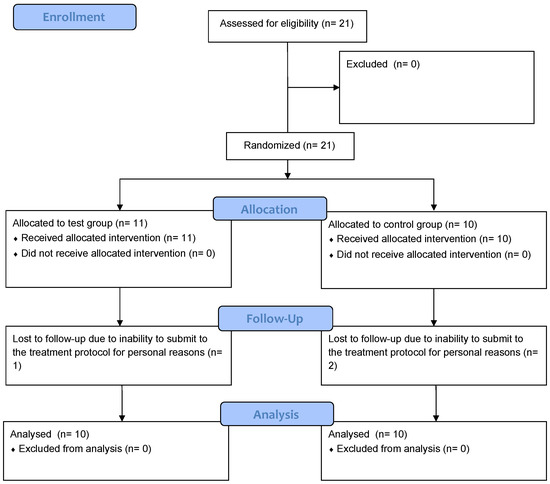
Figure 1.
CONSORT flow chart of the clinical trial.
In the test group, five patient were smokers; thus, 50% of them presented an adjunctive risk factor. From a prosthetic point of view, five patients had single crowns, three patients had single crowns in one arch and fixed total prostheses on implants in the other arch, and two patients only had a fixed total prosthesis on implants, resulting in a total of 53 implants. The control group presented two smoking patients; regarding prosthetic rehabilitation type, the distribution was as follows: three patients had a total removable implant-retainer prosthesis and a total fixed prosthesis on implants, one patient had only one total removable implant prosthesis, another patient had only one fixed total prosthesis on implants, two patients had single crowns, and one patient had both a total removable implant-holding prosthesis and single crowns, resulting in a total of 43 implants. Table 2 and Table 3 and Figure 2 and Figure 3 illustrate the results of the study.

Table 2.
Summary of prosthesis data.

Table 3.
Results.
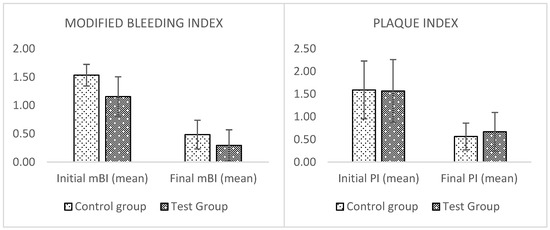
Figure 2.
Graphs of the results. No significant difference was found in the two groups.
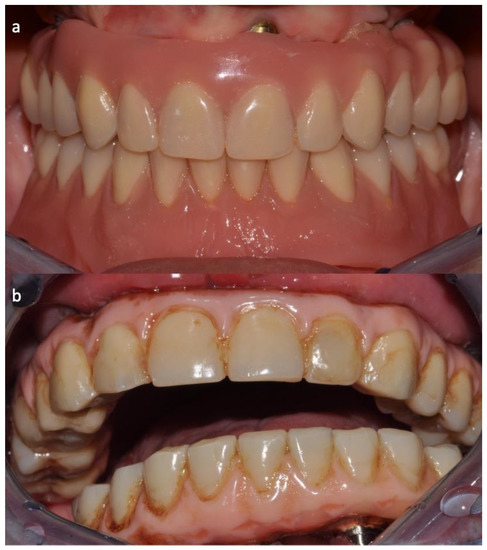
Figure 3.
Clinical appearance after 3 months. The onset of chlorhexidine-related stains is evident. (a) Experimental group; (b) control group.
Both groups show an improvement in the two analyzed periodontal indices. Regarding the bleeding index (mBI), in the test group, there was an improvement ranging from a minimum of 42% in a patient to a maximum of 100% in three patients with single crowns. The control group had a minimum percentage of 36% and a maximum of 88%. The plaque index improved in both groups, with a minimum reduction of 0% to a maximum of 100% in a patient for the test group. The control group demonstrated a minimum percentage of 43% and a maximum of 100% in a patient. The average rate of improvement for the values of mBI and PI of the two groups at the end of experimental treatment was also calculated.
Higher values of mBI were found in the test group with a reduction of 71% compared to 68% in the control group (p = 0.56) (Table 3). A similar reduction in PI values of 64% was found in the control group compared to 58% in the test group (p = 0.60). However, there are no statistically significant differences between both indices.
At the end of the three months of treatment, the implants that no longer showed mucositis and bleeding was present in 36 out of 53 patients (67%) in the test group and 28 out of 43 patients (65%) in the control group.
Figure 2 presents the results comparing the initial data and those after three months of treatment: MBI and PI improved in both groups, but the differences of these improvements between the two groups were not statistically significant. Therefore, it could be assumed that the experimental gel and standard therapy with CHX have similar effects, referring to the prefigured endpoint of managing the peri-implant mucositis problem.
There were no complications in either of the two clinical groups. In the control group, the undesirable appearance of pigmentation on crowns and bars due to the constant use of CHX over a prolonged time should be reported; however, this problem was not found in the test group (Figure 2).
4. Discussion
An increase in implant rehabilitation has revealed an emerging number of peri-implant pathologies [22]. The prevalence of these conditions reach a range of 32–54% [23,24], and a possible natural evolution of the mucositis condition may be peri-implantitis affection [25].
Currently, the strategies implemented for the management of peri-implantitis are derived from the knowledge and treatment standards used for periodontitis. Recent studies show that there is no consensus on the most suitable and effective treatment for the cure of peri-implantitis; however, the results are less encouraging than those obtained from periodontology through natural elements. Research has evaluated various therapeutic opportunities: the detachment of a full thickness flap and subsequent debridement seems more predictable than debridement procedures alone. On the other hand, the local use of antibiotic and antiseptic molecules also seem to lead to better clinical results compared to the non-surgical debridement procedure alone.
Since mucositis represents the onset of peri-implant pathology, it therefore represents the crucial stage in which to intervene to avoid the manifestation of more serious signs and symptoms that could significantly compromise the success and survival of the implant [26,27,28].
Although mucositis definition is complex, bleeding during probing, depending on probing depth, is a predictable marker for health or pathological status [22,23]. According to previous definitions, the modified bleeding index (mBI) was used as an evaluation parameter in the present randomized clinical study.
Plaque presence is an adjunctive etiological factor for the beginning of mucositis and a risk factor for the permanence of this condition [29]; so, in addition to mBI index, the plaque index (PI) was utilized as a clinical evaluation parameter. Moreover, smoking, surface roughness, residual cement, the dimension of the keratinized tissue, the time of implant in function, and diabetes are adjunctive risk factors, but the significance is not already conclusive [22,29] and no definitive treatment for the condition has been found [22].
Some RCTs reported the superior outcomes of clinical parameters in favor of rinsing with an essential oil [30], the use of irrigation with CHX [31], or use of a triclosan-containing toothpaste [32].
A multicenter randomized clinical trial by Matchei et al. evaluated the efficacy of an adjunctive treatment to debridement in patients affected by peri-implantitis using chlorhexidine chips (chlorhexidine gluconate 2.5 mg chips). Patients were on strict plaque control regimen for 24 weeks and gingival index was evaluated; results presented a significant reduction in implant pocket depths (IPD) compared to the control group [33].
On the other hand, other RCTs failed to report a superiority of different protocols, such as brushing with adjunctive CHX gel [34] and the adjunctive use of CHX rinsing or gel application. There is emerging evidence regarding patient control of the inflammation level, namely that the lack of annual supportive therapy in patients diagnosed with peri-implant mucositis was associated with increased risk for the conversion of mucositis in peri-implantitis [22]. The literature provides a study evaluating the efficacy of patient-administered treatment with domiciliary oral hygiene procedures [35], and another focused on the efficacy of professionally administered measures [36] for plaque control in patients with peri-implant mucositis; these studies taken together show that a well administered home care program is a suitable solution for the maintenance of the implants affected by this pathology. According to the consensus report of the VIII European Workshop [37], three major groups of treatment were identified, i.e., mechanical plaque removal by means of manual or powered toothbrushes, chemical plaque control by means of adjunctive delivery of antimicrobials, and triclosan-containing toothpastes or gel. Nevertheless, a variety of control treatments had been used for comparison, indicating that there is a lack of an accepted gold standard of care [22]. Chemical plaque controls tested either through oral rinses or a dentifrice had limited adjunctive effect. Patient-administered mechanical plaque control alone (with manual or powered toothbrush) can be considered the current standard of care [22]. In well-designed clinical randomized trials, therapeutic measures demonstrating efficacy should be evaluated in field studies. The comparison of clinical parameters should be based on changing the relationship between time and treatment rather than absolute values [22].
The study shows that the results obtained are similar for both test and control groups, in the sense that it was not possible to find significant differences between the two groups in terms of PI and mBI. Chlorhexidine is a powerful broad-spectrum antiseptic, effective both as Gram-positive and Gram-negative. Its bactericidal properties have long been recognized by numerous studies in the literature [13,38].
The dicationic nature of CHX molecule permits the bacteriostatic and germicide effect of the product and a long lasting presence on the tooth surface; however, this chemical treatment has a side effect of tooth pigmentation [39]. This side effect, could potentially have a negative impact on patients’ compliance [40]. So, the research of a new class of molecules potentially used as CHX, but with no side effects, could be a strategy for fighting against peri-implant mucositis.
The test group gel is based on the principle that the combination of several bactericidal or bacteriostatic substances, such as Cetylpyridinium chloride, triclosan, and some essential oils, multiplies the disintegration effect of the biofilm.
The efficacy and safety of Cetylpyridinium chloride was previously evaluated in in vitro and in vivo studies. Cetylpyridinium chloride has been shown to reduce bacterial load on titanium surfaces in vitro while reducing undesired side effects [19]. In an in vivo study, Cetylpyridinium chloride in combination with chlorhexidine was effective in reducing peri-implant mucositis [41].
Another 2019 study by Carinci et al. highlighted the potential of chlorhexidine as an antibacterial molecule for covering the internal chamber of the implant, developing a procedure and a strategy to reduce the risk of onset of mucositis and peri-implantitis. The results of this study show good soft tissue healing and an absence of tissue inflammation demonstrated by PCR analysis. Furthermore, the coating is also able to influence the quality of the microbiota [42].
The results of the present study show that the reduction of bleeding at probing is not different for the two groups; the test group has a percentage reduction of 71% and the control group has a reduction of 68%. These results can be attributed to the great bactericidal power of both substances. Another important feature of chlorhexidine is the high thickness and therefore the ability to adhere to dental surfaces, thus ensuring its action for a long time while also inhibiting the formation of bacterial plaque. The gel used in the test group containing the mixed salt of Na/Ca of the copolymer methyl-vinylether/polyvinyl-pyrylidone/Na carboxymethylcellulose has an increased ability to adhere over time to the tissues of the teeth and other mucous membranes of the oral cavity. However, in the light of these results, it could be hypothesized that the inhibiting power against bacterial plaque is slightly different from that of chlorhexidine, which is slightly higher (65% PI reduction for the control group and 60% for the test group). The use of the test group gel does not appear to cause pigmentation on crowns or bars, as it was found in the group that used chlorhexidine.
From the results obtained both products have been shown to reduce the amount of plaque and bacterial load and aid in a reduction in bleeding during probing; in more than half the cases contributing to the healing of mucositis (67% of the healed implants in the test group and 65% of the healed implants in the control group). However, it is crucial to emphasize how early diagnosis, close checks with repeated professional hygiene sessions, and the carrying out of correct oral hygiene at home are an integral and indispensable part of the success of the therapy. It should also be noted that this protocol did not resolve all the cases of diagnosticated mucositis.
A limitation of this RCT study is that it has a small sample size. This was a pilot study and further studies on larger scale samples are needed to find the correct protocol for mucositis management.
5. Conclusions
Results show how the antibacterial power and effectiveness of experimental gel in the treatment of mucositis is comparable to that of chlorhexidine gel 1%, which could be assumed as gold standard treatment. This gel from the test group could be utilized in the medium-term treatment of peri-implant mucositis as a valid alternative to standard treatment, avoiding pigmentations and other complications associated with the use of high-concentration chlorhexidine. It is important to point out that close controls and extreme attention to domiciliary oral hygiene are, however, indispensable for the treatment of mucositis, regardless of the product used, so an important focus on patient instruction and motivation is necessary to obtain the correct compliance. Further studies are necessary to validate the results obtained from this pilot RCT study. It should be noted that this working protocol has not enabled the solving of all cases of treated mucositis, so no elective treatment can be taken from this study. The treatment of peri-implant mucositis remains a complex challenge and it is necessary to develop treatments that obtain even higher success rates.
Author Contributions
Conceptualization, S.S. and S.M.L.; methodology, S.S. and S.M.L.; formal analysis, S.M.L.; investigation, G.P. and E.R.; resources, R.R.y.B.; data curation, B.M.; writing—original draft preparation, S.S., G.P., E.R. and B.M.; writing—review and editing, D.D.M., C.T., R.R.y.B. and S.M.L.; visualization, S.M.L.; supervision, R.R.y.B. and S.S.; project administration, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the University of Milan (protocol code 90/22).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available in the integral version upon request of the interested parties.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rizzo, S.; Zampetti, P.; Rodriguez, Y.; Baena, R.; Svanosio, D.; Lupi, S.M. Retrospective Analysis of 521 Endosseous Implants Placed under Antibiotic Prophylaxis and Review of Literature. Minerva Stomatol. 2010, 59, 75–88. [Google Scholar]
- Lupi, S.M.; Redoglia, L.; Rodriguez, Y.; Baena, A.; Garbelli, G.; Rodriguez, Y.; Baena, R. Detection of Peri-Implant Inflammation by the Use of a Matrix Metalloproteinase-8 Chair-Side Test. Minerva Stomatol. 2019, 68, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.; Granati, M.; Butera, A.; Collesano, V.; Rodriguez, Y.; Baena, R. Air-Abrasive Debridement with Glycine Powder versus Manual Debridement and Chlorhexidine Administration for the Maintenance of Peri-Implant Health Status: A Six-Month Randomized Clinical Trial. Int. J. Dent. Hyg. 2017, 15, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Lupi, S.; Zaffe, D.; Baena, R.R.Y.; Rizzo, S.; Botticelli, A. Cytopathological and Chemico-Physical Analyses of Smears of Mucosa Surrounding Oral Piercing. Oral Dis. 2010, 16, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Margutti, E.; Ghisolfi, M.; Chiapasco, M.; Vogel, G. Long-Term Survival and Success of Oral Implants in the Treatment of Full and Partial Arches: A 7-Year Prospective Study with the ITI Dental Implant System. Int. J. Oral Maxillofac. Implants 2004, 19, 247–259. [Google Scholar]
- Tonetti, M.S.; Schmid, J. Pathogenesis of Implant Failures. Periodontol. 2000 1994, 4, 127–138. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.; Lekholm, U.; Thomsen, P. Differential Diagnosis and Treatment Strategies for Biologic Complications and Failing Oral Implants: A Review of the Literature. Int. J. Oral Maxillofac. Implants 1999, 14, 473–490. [Google Scholar]
- Máximo, M.B.; de Mendonça, A.C.; Renata Santos, V.; Figueiredo, L.C.; Feres, M.; Duarte, P.M. Short-Term Clinical and Microbiological Evaluations of Peri-Implant Diseases before and after Mechanical Anti-Infective Therapies. Clin. Oral Implants Res. 2009, 20, 99–108. [Google Scholar] [CrossRef]
- Thöne-Mühling, M.; Swierkot, K.; Nonnenmacher, C.; Mutters, R.; Flores-de-Jacoby, L.; Mengel, R. Comparison of Two Full-Mouth Approaches in the Treatment of Peri-Implant Mucositis: A Pilot Study. Clin. Oral Implants Res. 2010, 21, 504–512. [Google Scholar] [CrossRef]
- Trejo, P.M.; Bonaventura, G.; Weng, D.; Caffesse, R.G.; Bragger, U.; Lang, N.P. Effect of Mechanical and Antiseptic Therapy on Peri-Implant Mucositis: An Experimental Study in Monkeys. Clin. Oral Implants Res. 2006, 17, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Isidor, F.; Lang, N.P.; Karring, T. Consensus Report of Session IV. In Proceedings of the First European Workshop on Periodontology; Quintessence Publishing: London, UK, 2014; pp. 365–369. ISBN 9781119130536. [Google Scholar]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Venugopal, A.; Marya, A.; Scribante, A. Evaluation of Adjuvant Systems in Non-Surgical Peri-Implant Treatment: A Literature Review. Healthcare 2022, 10, 886. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.; Hase, J.; Grassi, M.; Hämmerle, C.; Weigel, C.; Kelty, E.; Frutig, F. Plaque Formation and Gingivitis after Supervised Mouthrinsing with 0.2% Delmopinol Hydrochloride, 0.2% Chlorhexidine Digluconate and Placebo for 6 Months. Oral Dis. 2008, 4, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ouhayoun, J.-P. Penetrating the Plaque Biofilm: Impact of Essential Oil Mouthwash. J. Clin. Periodontol. 2003, 30, 10–12. [Google Scholar] [CrossRef]
- Mandel, I.D. Chemotherapeutic Agents for Controlling Plaque and Gingivitis. J. Clin. Periodontol. 1988, 15, 488–498. [Google Scholar] [CrossRef]
- Costa, X.; Laguna, E.; Herrera, D.; Serrano, J.; Alonso, B.; Sanz, M. Efficacy of a New Mouth Rinse Formulation Based on 0.07% Cetylpyridinium Chloride in the Control of Plaque and Gingivitis: A 6-Month Randomized Clinical Trial. J. Clin. Periodontol. 2013, 40, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Mor-Reinoso, C.; Pascual, A.; Nart, J.; Quirynen, M. Inhibition of de Novo Plaque Growth by a New 0.03 % Chlorhexidine Mouth Rinse Formulation Applying a Non-Brushing Model: A Randomized, Double Blind Clinical Trial. Clin. Oral Investig. 2016, 20, 1459–1467. [Google Scholar] [CrossRef]
- Becker, K.; Brunello, G.; Scotti, L.; Drescher, D.; John, G. Efficacy of 0.05% Chlorhexidine and 0.05% Cetylpyridinium Chloride Mouthwash to Eliminate Living Bacteria on In Situ Collected Biofilms: An In Vitro Study. Antibiotics 2021, 10, 730. [Google Scholar] [CrossRef]
- Silness, J.; Löe, H. Periodontal Disease in Pregnancy II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- Mombelli, A.; Marxer, M.; Gaberthüel, T.; Grander, U.; Lang, N.P. The Microbiota of Osseointegrated Implants in Patients with a History of Periodontal Disease. J. Clin. Periodontol. 1995, 22, 124–130. [Google Scholar] [CrossRef]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary Prevention of Peri-Implantitis: Managing Peri-Implant Mucositis. J. Clin. Periodontol. 2015, 42, S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, B.C.D.V.; Montenegro, S.C.L.; Dantas, P.M.C.; Pascoal, A.L.D.B.; Lima, K.C.; Calderon, P.D.S. Frequency of Peri-Implant Diseases and Associated Factors. Clin. Oral Implants Res. 2017, 28, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.M.; Ferreira, S.D.; Silva, G.L.M.; Costa, J.E. Peri-Implant Disease in Subjects with and without Preventive Maintenance: A 5-Year Follow-Up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Treatment of Peri-Implantitis: What Interventions Are Effective? A Cochrane Systematic Review. Eur. J. Oral Implantol. 2012, 5, S21–S41. [Google Scholar] [PubMed]
- Berglundh, T.; Wennström, J.L.; Lindhe, J. Long-Term Outcome of Surgical Treatment of Peri-Implantitis. A 2-11-Year Retrospective Study. Clin. Oral Implants Res. 2018, 29, 404–410. [Google Scholar] [CrossRef]
- Büchter, A.; Meyer, U.; Kruse-Lösler, B.; Joos, U.; Kleinheinz, J. Sustained Release of Doxycycline for the Treatment of Peri-Implantitis: Randomised Controlled Trial. Br. J. Oral Maxillofac. Surg. 2004, 42, 439–444. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Risk Indicators for Peri-Implant Mucositis: A Systematic Literature Review. J. Clin. Periodontol. 2015, 42, S172–S186. [Google Scholar] [CrossRef]
- Golub, L.M.; Sorsa, T.; Lee, H.-M.; Ciancio, S.; Sorbi, D.; Ramamurthy, N.S.; Gruber, B.; Salo, T.; Konttinen, Y.T. Doxycycline Inhibits Neutrophil (PMN)-Type Matrix Metalloproteinases in Human Adult Periodontitis Gingiva. J. Clin. Periodontol. 2005, 22, 100–109. [Google Scholar] [CrossRef]
- Felo, A.; Shibly, O.; Ciancio, S.G.; Lauciello, F.R.; Ho, A. Effects of Subgingival Chlorhexidine Irrigation on Peri-Implant Maintenance. Am. J. Dent. 1997, 10, 107–110. [Google Scholar]
- Peres Pimentel, S.; Vieira Ribeiro, F.; Correa Casarin, R.; Ribeiro Cirano, F.; Haguihara Luchesi, V.; Gallego Arias Pecorari, V.; Zaffalon Casati, M. Triclosan-Containing Fluoride Toothpaste on Clinical Parameters and Osteo-Inflammatory Mediators When Applied in a Stent during Experimental Peri-Implant Mucositis in Smokers. Clin. Oral Implants Res. 2019, 30, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated Delivery of Chlorhexidine Chips for the Treatment of Peri-implantitis: A Multicenter, Randomized, Comparative Clinical Trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Botticelli, D.; Mombelli, A.; Faddy, M.; Lang, N.P. Anti-Infective Treatment of Peri-Implant Mucositis: A Randomised Controlled Clinical Trial. Clin. Oral Implants Res. 2011, 22, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Ramseier, C.A. Efficacy of Patient-Administered Mechanical and/or Chemical Plaque Control Protocols in the Management of Peri-Implant Mucositis. A Systematic Review. J. Clin. Periodontol. 2015, 42, S187–S201. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of Professionally Administered Plaque Removal with or without Adjunctive Measures for the Treatment of Peri-Implant Mucositis. A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2015, 42, S202–S213. [Google Scholar] [CrossRef]
- Sanz, M.; Chapple, I.L. Clinical Research on Peri-Implant Diseases: Consensus Report of Working Group 4. J. Clin. Periodontol. 2012, 39, 202–206. [Google Scholar] [CrossRef]
- Pałka, Ł.; Nowakowska-Toporowska, A.; Dalewski, B. Is Chlorhexidine in Dentistry an Ally or a Foe? A Narrative Review. Healthcare 2022, 10, 764. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is It Still the Gold Standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef]
- Slot, D.; Berchier, C.; Addy, M.; Van der Velden, U.; Van der Weijden, G. The Efficacy of Chlorhexidine Dentifrice or Gel on Plaque, Clinical Parameters of Gingival Inflammation and Tooth Discoloration: A Systematic Review. Int. J. Dent. Hyg. 2014, 12, 25–35. [Google Scholar] [CrossRef]
- Pulcini, A.; Bollaín, J.; Sanz-Sánchez, I.; Figuero, E.; Alonso, B.; Sanz, M.; Herrera, D. Clinical Effects of the Adjunctive Use of a 0.03% Chlorhexidine and 0.05% Cetylpyridinium Chloride Mouth Rinse in the Management of Peri-Implant Diseases: A Randomized Clinical Trial. J. Clin. Periodontol. 2019, 46, 342–353. [Google Scholar] [CrossRef]
- Carinci, F.; Lauritano, D.; Bignozzi, C.A.; Pazzi, D.; Candotto, V.; Santos de Oliveira, P.; Scarano, A. A New Strategy Against Peri-Implantitis: Antibacterial Internal Coating. Int. J. Mol. Sci. 2019, 20, 3897. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).