A Multistage Rigid-Affine-Deformable Network for Three-Dimensional Multimodal Medical Image Registration
Abstract
:1. Introduction
2. Background
3. Methods
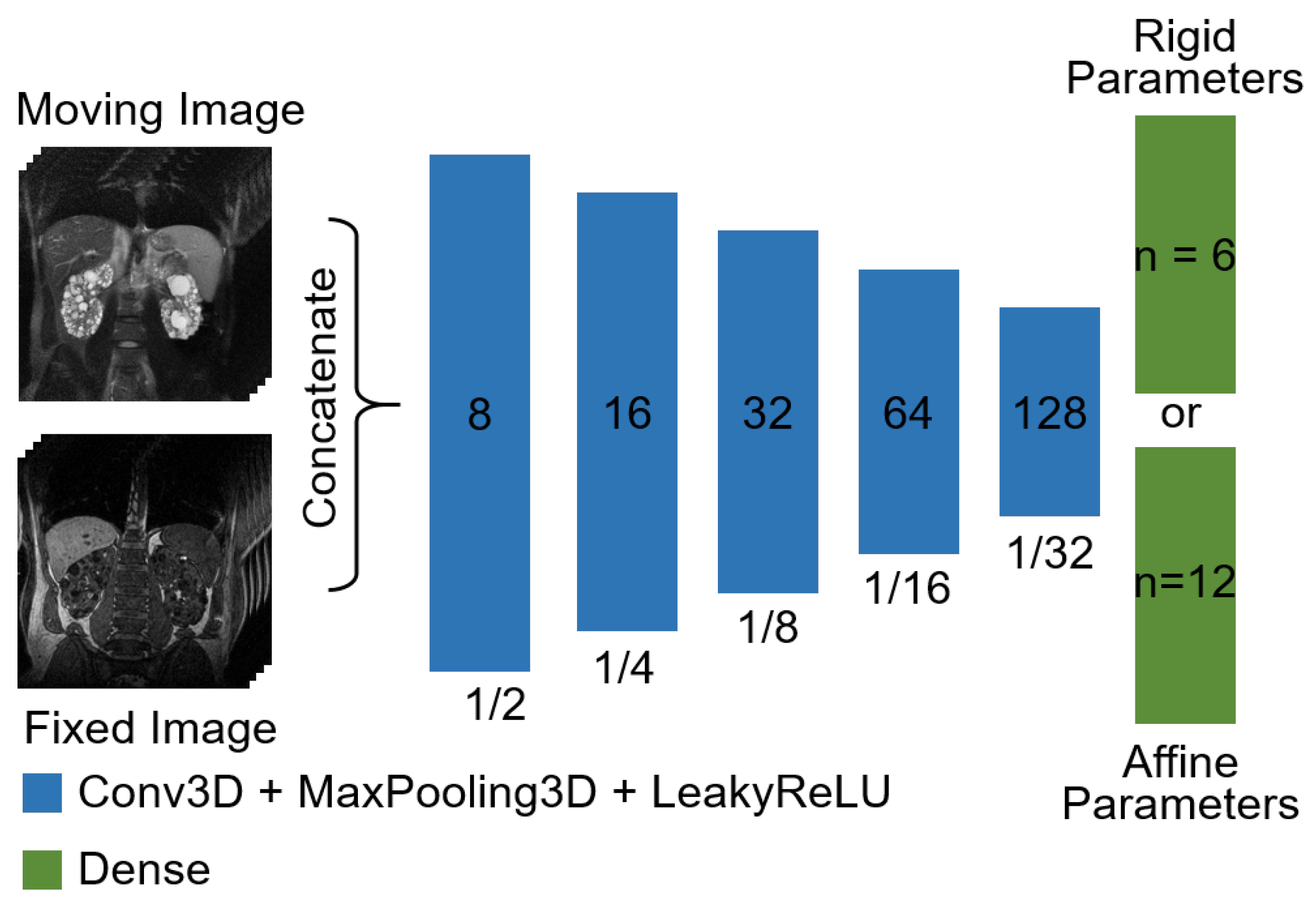
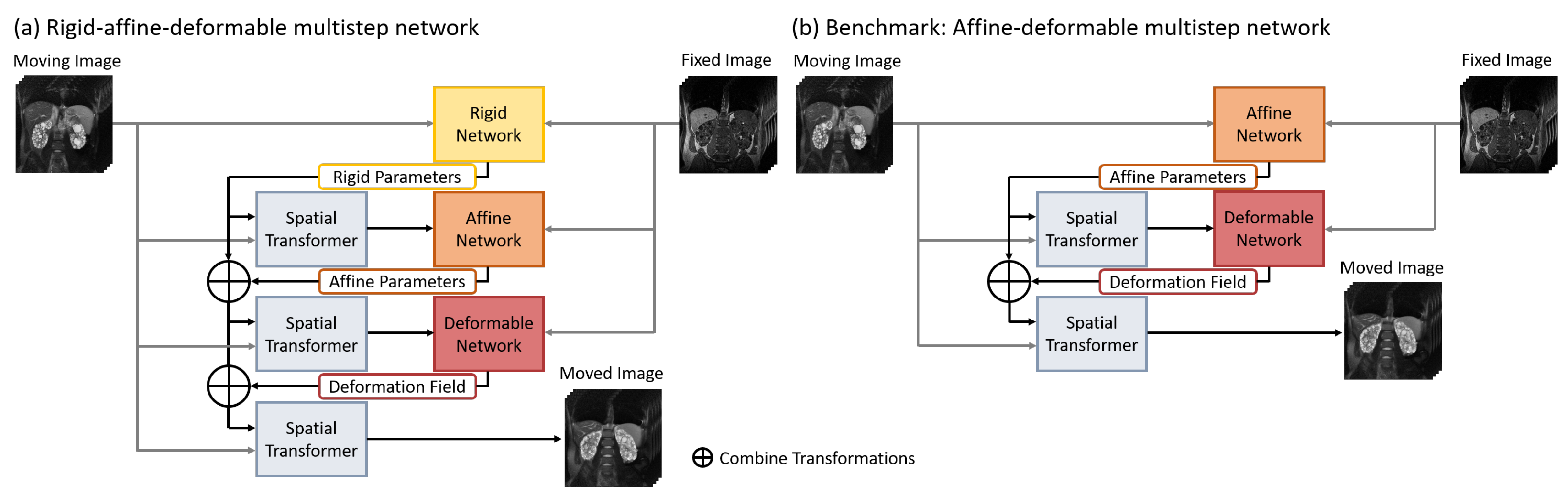
3.1. Network Architectures
3.2. Benchmark Neural Network
3.3. Training Setting for Neural Networks
3.4. Baseline Method
3.5. Datasets
3.5.1. NIDDK
3.5.2. External Kidney Dataset
3.5.3. M2OLIE
3.6. Evaluation Metrics
3.7. Experiments
3.7.1. Experiment I
3.7.2. Experiment II
3.7.3. Experiment III
4. Results
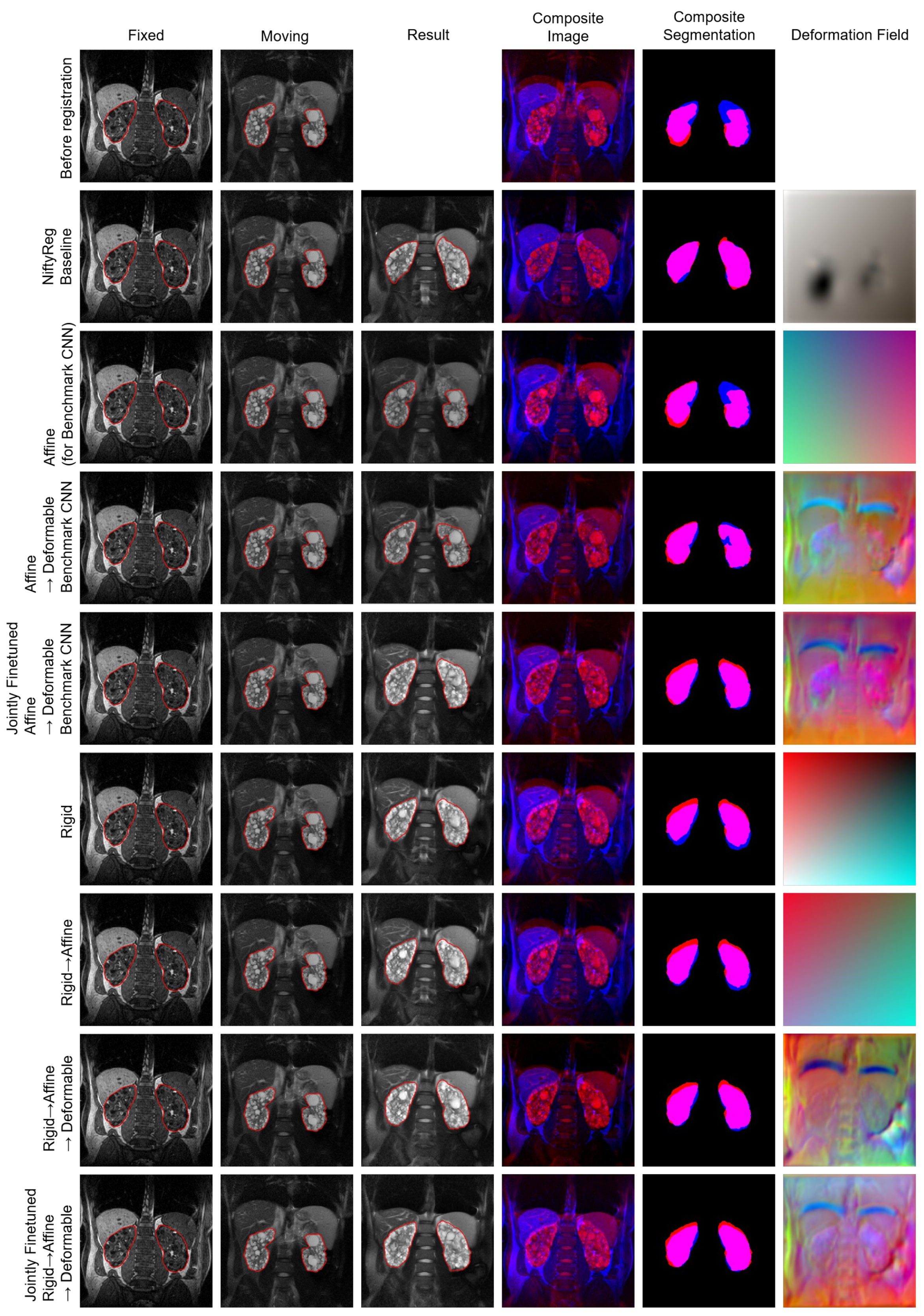
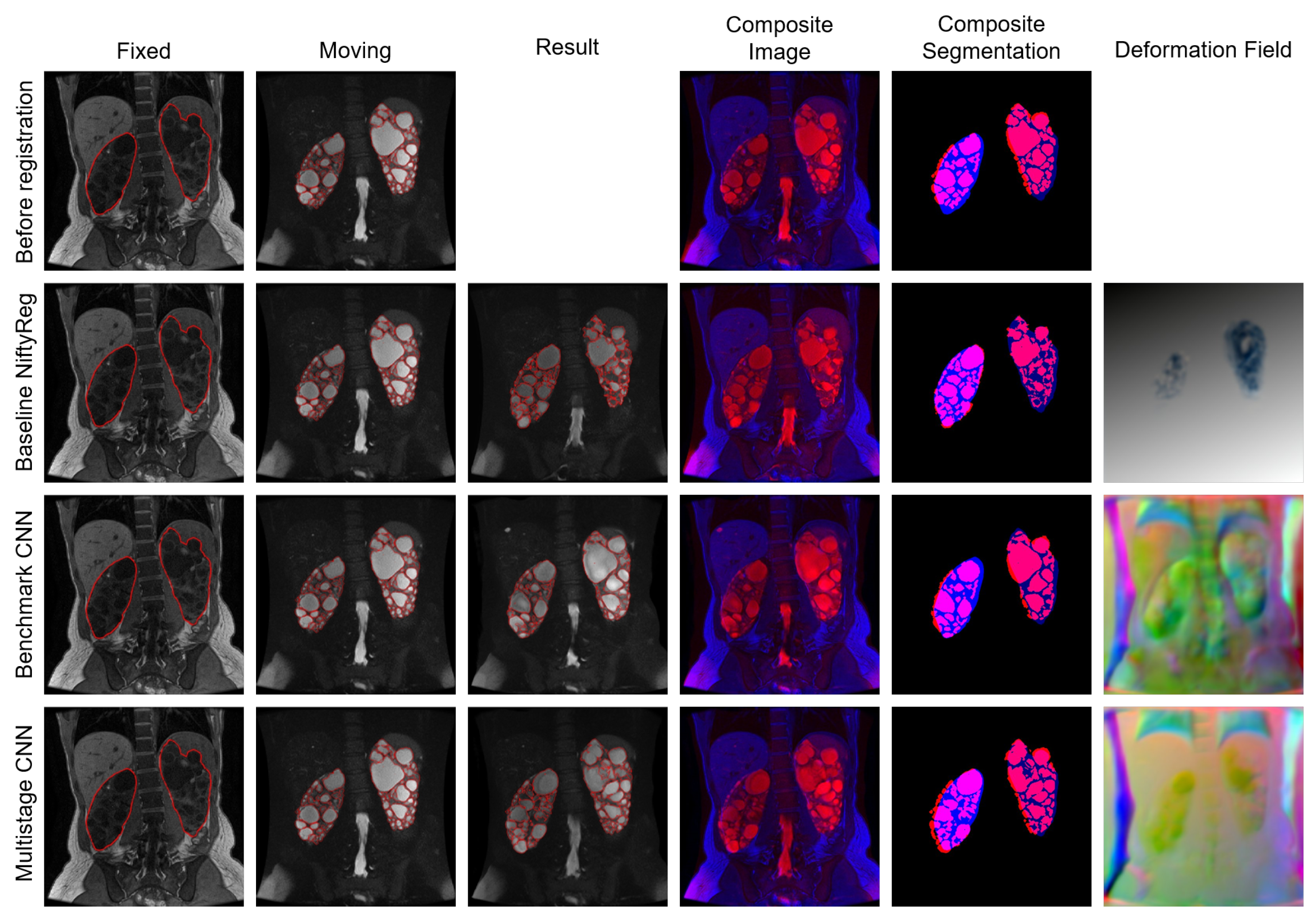
4.1. Experiment I
4.2. Experiment II
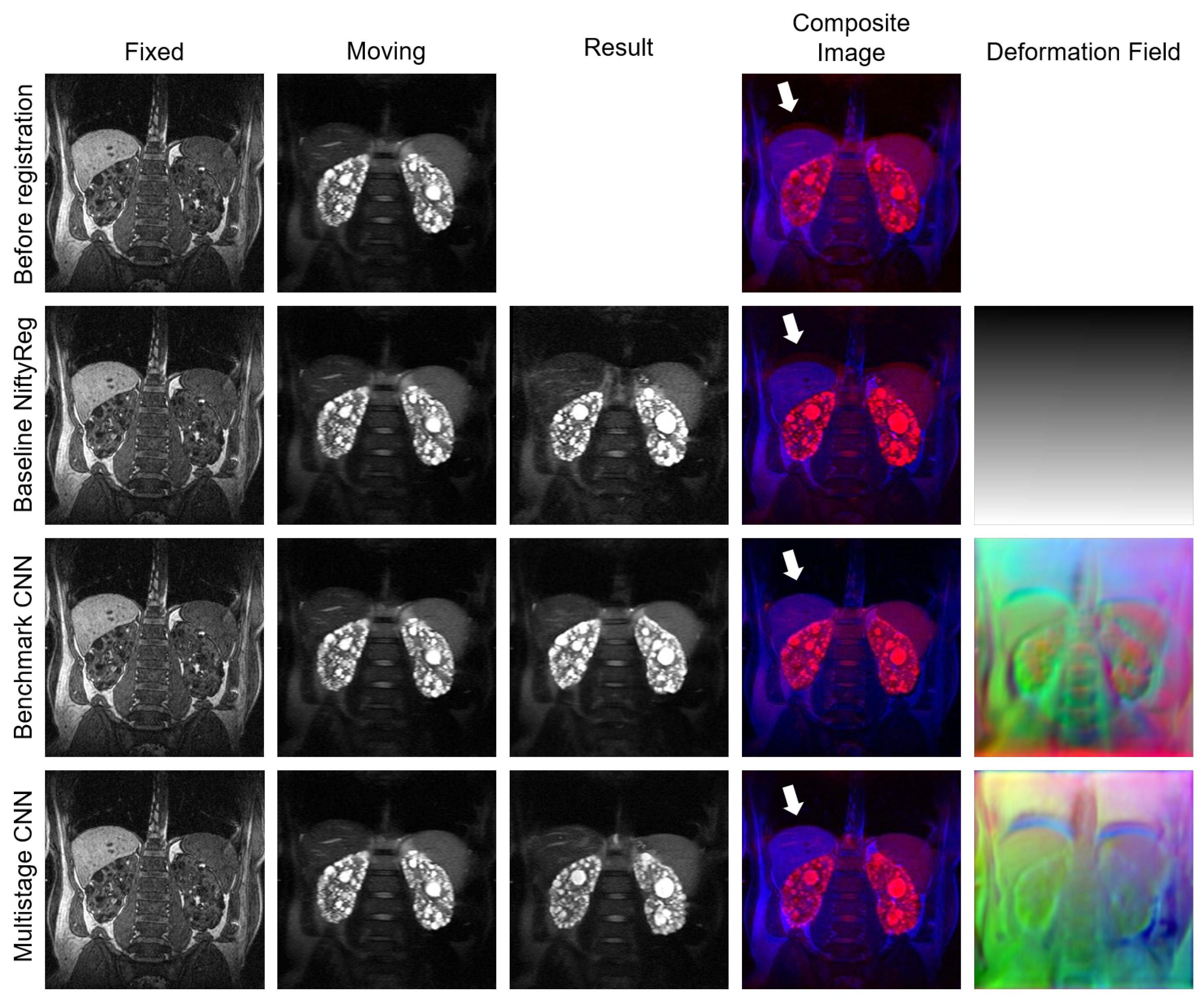
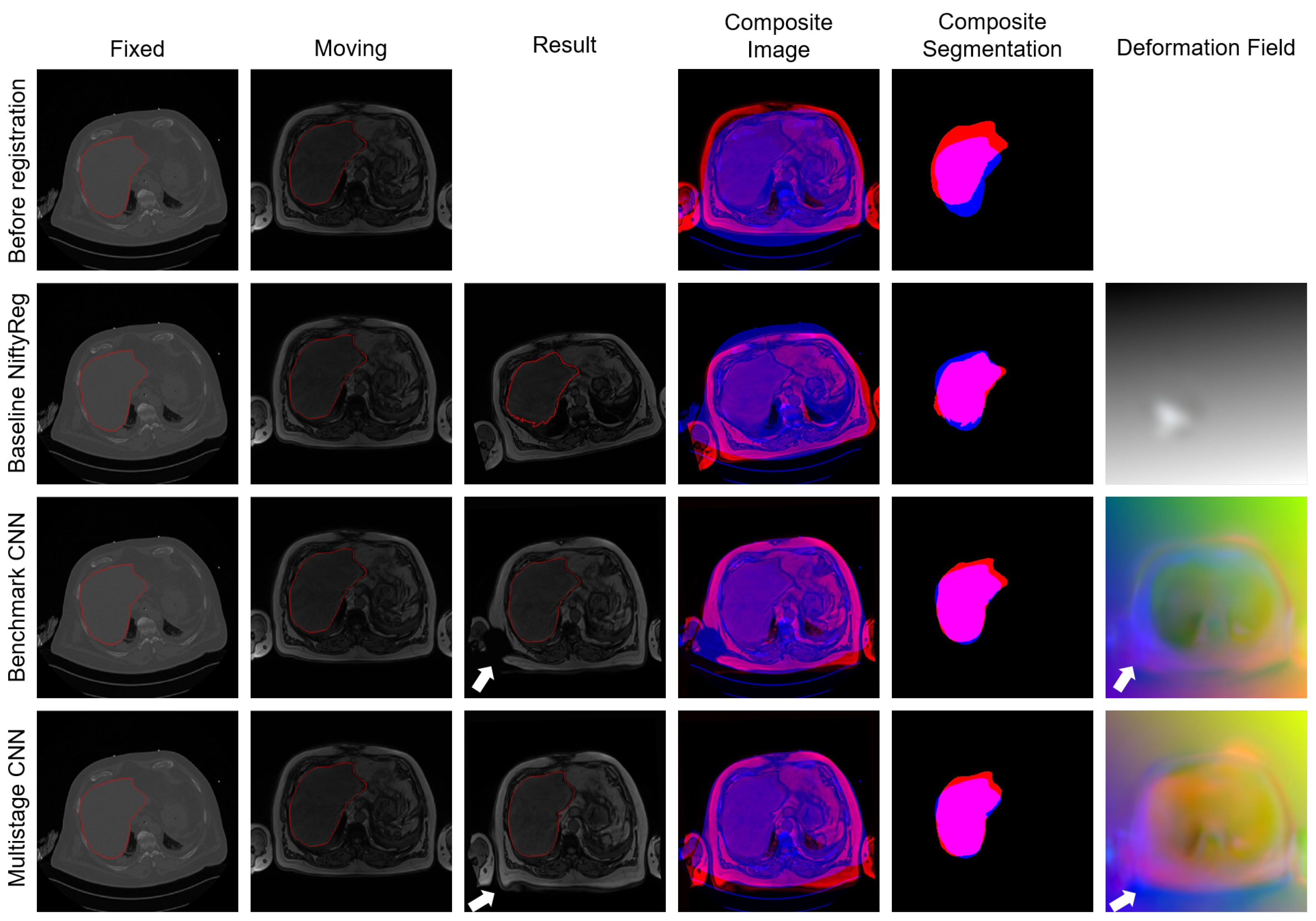
4.3. Experiment III
5. Discussion
5.1. Comparison to Published Multistage Registration Methods
5.2. Individual Networks
5.3. Individual Datasets
5.4. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Diaz-Pinto, A.; Ravikumar, N.; Frangi, A.F. Deep learning in medical image registration. Prog. Biomed. Eng. 2021, 3, 012003. [Google Scholar] [CrossRef]
- Fu, Y.; Lei, Y.; Wang, T.; Curran, W.J.; Liu, T.; Yang, X. Deep learning in medical image registration: A review. Phys. Med. Biol. 2020, 65, 20TR01. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Teng, X.; Liu, C.; Li, T.; Ren, G.; Yang, R.; Shen, D.; Cai, J. A review of deep learning-based three-dimensional medical image registration methods. Quant. Imaging Med. Surg. 2021, 11, 4895–4916. [Google Scholar] [CrossRef] [PubMed]
- Anderlik, A.; Munthe-Kaas, A.Z.; Oye, O.K.; Eikefjord, E.; Rorvik, J.; Ulvang, D.M.; Zöllner, F.G.; Lundervold, A. Quantitative assessment of kidney function using dynamic contrast enhanced MRI—Steps towards an integrated software prototype. In Proceedings of the 2009 6th International Symposium on Image and Signal Processing and Analysis, Salzburg, Austria, 16–18 September 2009; pp. 575–581. [Google Scholar] [CrossRef]
- Hodneland, E.; Keilegavlen, E.; Hanson, E.A.; Andersen, E.; Monssen, J.A.; Rørvik, J.; Leh, S.; Marti, H.P.; Lundervold, A.; Svarstad, E.; et al. In Vivo Detection of Chronic Kidney Disease Using Tissue Deformation Fields From Dynamic MR Imaging. IEEE Trans. Biomed. Eng. 2019, 66, 1779–1790. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, F.G.; Šerifović-Trbalić, A.; Kabelitz, G.; Kociński, M.; Materka, A.; Rogelj, P. Image registration in dynamic renal MRI—Current status and prospects. Magn. Reson. Mater. Phys. Biol. Med. 2019, 33, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Kline, T.L. Abdominal Imaging in ADPKD: Beyond Total Kidney Volume. J. Clin. Med. 2023, 12, 5133. [Google Scholar] [CrossRef] [PubMed]
- Riyahi, S.; Dev, H.; Blumenfeld, J.D.; Rennert, H.; Yin, X.; Attari, H.; Barash, I.; Chicos, I.; Bobb, W.; Donahue, S.; et al. Hemorrhagic Cysts and Other MR Biomarkers for Predicting Renal Dysfunction Progression in Autosomal Dominant Polycystic Kidney Disease. J. Magn. Reson. Imaging 2021, 53, 564–576. [Google Scholar] [CrossRef]
- Qiu, H.; Katz, A.W.; Milano, M.T. Oligometastases to the liver: Predicting outcomes based upon radiation sensitivity. J. Thorac. Dis. 2016, 8, E1384–E1386. [Google Scholar] [CrossRef]
- Ruers, T.; Coevorden, F.V.; Punt, C.J.A.; Pierie, J.P.E.N.; Borel-Rinkes, I.; Ledermann, J.A.; Poston, G.; Bechstein, W.; Lentz, M.A.; Mauer, M.; et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J. Natl. Cancer Inst. 2017, 109, djx015. [Google Scholar] [CrossRef]
- Bauer, D.F.; Rosenkranz, J.; Golla, A.K.; Tönnes, C.; Hermann, I.; Russ, T.; Kabelitz, G.; Rothfuss, A.J.; Schad, L.R.; Stallkamp, J.L.; et al. Development of an abdominal phantom for the validation of an oligometastatic disease diagnosis workflow. Med. Phys. 2022, 49, 4445–4454. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Guttag, J.; Dalca, A.V. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging 2019, 38, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Frey, E.C.; He, Y.; Segars, W.P.; Li, Y.; Du, Y. TransMorph: Transformer for unsupervised medical image registration. Med. Image Anal. 2022, 82, 102615. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Van Houtte, J.; Chen, Z.; Zheng, G. DeepASDM: A Deep Learning Framework for Affine and Deformable Image Registration Incorporating a Statistical Deformation Model. In Proceedings of the 2021 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Athens, Greece, 27–30 July 2021; pp. 1–4. [Google Scholar] [CrossRef]
- Gu, D.; Liu, G.; Tian, J.; Zhan, Q. Two-Stage Unsupervised Learning Method for Affine and Deformable Medical Image Registration. In Proceedings of the 2019 IEEE International Conference on Image Processing (ICIP), Taipei, Taiwan, 22–25 September 2019. [Google Scholar] [CrossRef]
- Hu, Y.; Modat, M.; Gibson, E.; Li, W.; Ghavami, N.; Bonmati, E.; Wang, G.; Bandula, S.; Moore, C.M.; Emberton, M.; et al. Weakly-supervised convolutional neural networks for multimodal image registration. Med. Image Anal. 2018, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yang, H.; Liu, X.; Li, C.; Zhang, I.; Wang, R.; Wang, S. A Coarse-to-Fine Deformable Transformation Framework for Unsupervised Multi-Contrast MR Image Registration with Dual Consistency Constraint. IEEE Trans. Med. Imaging 2021, 40, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, T.J.T. Deep Learning-Based Affine and Deformable 3D Medical Image Registration. Master’s Thesis, Aalto University, Espoo, Finland, 2021. [Google Scholar]
- Shao, W.; Banh, L.; Kunder, C.A.; Fan, R.E.; Soerensen, S.J.; Wang, J.B.; Teslovich, N.C.; Madhuripan, N.; Jawahar, A.; Ghanouni, P.; et al. ProsRegNet: A deep learning framework for registration of MRI and histopathology images of the prostate. Med. Image Anal. 2021, 68, 101919. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Han, X.; Xu, Z.; Niethammer, M. Networks for Joint Affine and Non-Parametric Image Registration. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 15–20 June 2019; pp. 4219–4228. [Google Scholar] [CrossRef]
- Tang, K.; Li, Z.; Tian, L.; Wang, L.; Zhu, Y. ADMIR–Affine and Deformable Medical Image Registration for Drug-Addicted Brain Images. IEEE Access 2020, 8, 70960–70968. [Google Scholar] [CrossRef]
- de Vos, B.D.; Berendsen, F.F.; Viergever, M.A.; Sokooti, H.; Staring, M.; Išgum, I. A deep learning framework for unsupervised affine and deformable image registration. Med. Image Anal. 2019, 52, 128–143. [Google Scholar] [CrossRef]
- Zeng, Q.; Fu, Y.; Tian, Z.; Lei, Y.; Zhang, Y.; Wang, T.; Mao, H.; Liu, T.; Curran, W.J.; Jani, A.B.; et al. Label-driven magnetic resonance imaging (MRI)-transrectal ultrasound (TRUS) registration using weakly supervised learning for MRI-guided prostate radiotherapy. Phys. Med. Biol. 2020, 65, 135002. [Google Scholar] [CrossRef]
- Zhao, S.; Dong, Y.; Chang, E.; Xu, Y. Recursive Cascaded Networks for Unsupervised Medical Image Registration. In Proceedings of the ICCV 2019, Seoul, Republic of Korea, 27 October–2 November 2019; Volume 2019, pp. 10600–10610. [Google Scholar] [CrossRef]
- Zhao, S.; Lau, T.; Luo, J.; Chang, E.I.C.; Xu, Y. Unsupervised 3D End-to-End Medical Image Registration With Volume Tweening Network. IEEE J. Biomed. Health Inform. 2020, 24, 1394–1404. [Google Scholar] [CrossRef]
- Zheng, Z.; Cao, W.; He, Z.; Luo, Y. Progressive anatomically constrained deep neural network for 3D deformable medical image registration. Neurocomputing 2021, 465, 417–427. [Google Scholar] [CrossRef]
- Zheng, Y.; Sui, X.; Jiang, Y.; Che, T.; Zhang, S.; Yang, J.; Li, H. SymReg-GAN: Symmetric Image Registration with Generative Adversarial Networks. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 5631–5646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cao, Y.; Qin, C.; Rao, Y.; Lin, D.; Dou, Q.; Ni, D.; Wang, Y. Joint affine and deformable three-dimensional networks for brain MRI registration. Med. Phys. 2021, 48, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, A.; Hertel, A.; Diehl, S.; Froelich, M.F.; Schoenberg, S.O.; Loges, S.; Boch, T.; Nowak, D.; Streuer, A.; Schad, L.R.; et al. A multistage registration of CT and biopsy CT images of lung tumors. In Proceedings of the 6th Conference on Image-Guided Interventions, Mannheim, Germany, 19–20 October 2023; pp. 17–18. [Google Scholar]
- Eiben, B.; Bertholet, J.; Menten, M.; Nill, S.; Oelfke, U.; McClelland, J. Consistent and invertible deformation vector fields for a breathing anthropomorphic phantom: A post-processing framework for the XCAT phantom. Phys. Med. Biol. 2020, 65, 165005. [Google Scholar] [CrossRef] [PubMed]
- Modat, M.; Cash, D.; Daga, P.; Winston, G.; Duncan, J.; Ourselin, S. Global image registration using a symmetric block-matching approach. J. Med. Imaging 2014, 1, 024003. [Google Scholar] [CrossRef] [PubMed]
- Modat, M.; Ridgway, G.R.; Taylor, Z.A.; Lehmann, M.; Barnes, J.; Hawkes, D.J.; Fox, N.C.; Ourselin, S. Fast free-form deformation using graphics processing units. Comput. Methods Programs Biomed. 2010, 98, 278–284. [Google Scholar] [CrossRef]
- Chumchob, N.; Chen, K. A robust affine image registration method. Int. J. Numer. Anal. Model. 2009, 6, 311–334. [Google Scholar]
- Waldkirch, B.I. Methods for Three-Dimensional Registration of Multimodal Abdominal Image Data. Ph.D. Thesis, Ruprecht Karl University of Heidelberg, Heidelberg, Germany, 2020. [Google Scholar]
- Marstal, K.; Berendsen, F.; Staring, M.; Klein, S. SimpleElastix: A User-Friendly, Multi-lingual Library for Medical Image Registration. In Proceedings of the 2016 IEEE Conference on Computer Vision and Pattern Recognition Workshops (CVPRW), Las Vegas, NV, USA, 26 June–1 July 2016; pp. 574–582. [Google Scholar] [CrossRef]
- Fluck, O.; Vetter, C.; Wein, W.; Kamen, A.; Preim, B.; Westermann, R. A survey of medical image registration on graphics hardware. Comput. Methods Programs Biomed. 2011, 104, e45–e57. [Google Scholar] [CrossRef]
- Strittmatter, A.; Schad, L.R.; Zöllner, F.G. Deep learning-based affine medical image registration for multimodal minimal-invasive image-guided interventions—A comparative study on generalizability. Z. Med. Phys. 2023, in press. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.R.; Guttag, J.V.; Dalca, A.V. An Unsupervised Learning Model for Deformable Medical Image Registration. In Proceedings of the 2018 IEEE/CVF Conference on Computer Vision and Pattern Recognition, Salt Lake City, UT, USA, 18–23 June 2018. [Google Scholar] [CrossRef]
- Jaderberg, M.; Simonyan, K.; Zisserman, A.; Kavukcuoglu, K. Spatial Transformer Networks. Adv. Neural Inf. Process. Syst. 2015, 28, 2017–2025. [Google Scholar]
- Li, L.; Jamieson, K.; DeSalvo, G.; Rostamizadeh, A.; Talwalkar, A. Hyperband: A Novel Bandit-Based Approach to Hyperparameter Optimization. J. Mach. Learn. Res. 2018, 18, 1–52. [Google Scholar]
- Abadi, M.; Agarwal, A.; Barham, P.; Brevdo, E.; Chen, Z.; Citro, C.; Corrado, G.S.; Davis, A.; Dean, J.; Devin, M.; et al. TensorFlow: Large-Scale Machine Learning on Heterogeneous Distributed Systems. arXiv 2016, arXiv:1603.04467. [Google Scholar]
- Chapman, A.B.; Guay-Woodford, L.M.; Grantham, J.J.; Torres, V.E.; Bae, K.T.; Baumgarten, D.A.; Kenney, P.J.; King, B.F.; Glockner, J.F.; Wetzel, L.H.; et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort1. Kidney Int. 2003, 64, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Tollens, F.; Caroli, A.; Nörenberg, D.; Zöllner, F.G. Automated prognosis of renal function decline in ADPKD patients using deep learning. Z. Med. Phys. 2023, in press. [CrossRef] [PubMed]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention—MICCAI 2015, Munich, Germany, 5–9 October 2015; Navab, N., Hornegger, J., Wells, W.M., Frangi, A.F., Eds.; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Soler, L.; Hostettler, A.; Agnus, V.; Charnoz, A.; Fasquel, J.B.; Moreau, J.; Osswald, A.B.; Bouhadjar, M.; Marescaux, J. 3D Image Reconstruction for Comparison of Algorithm Database: A Patient Specific Anatomical and Medical Image Database; Technical Report; IRCAD: Strasbourg, France, 2010. [Google Scholar]
- Kavur, A.E.; Selver, M.A.; Dicle, O.; Barış, M.; Gezer, N.S. CHAOS—Combined (CT-MR) Healthy Abdominal Organ Segmentation Challenge Data; Zenodo: Geneva, Switzerland, 2019. [Google Scholar] [CrossRef]
- German Cancer Research Center (DKFZ) Division of Medical Image Computing. Medical Imaging Interaction Toolkit (MITK). v2021.02. Available online: https://www.mitk.org/wiki/The_Medical_Imaging_Interaction_Toolkit_(MITK) (accessed on 15 June 2021).
- Solovyev, R.; Kalinin, A.A.; Gabruseva, T. 3D convolutional neural networks for stalled brain capillary detection. Comput. Biol. Med. 2022, 141, 105089. [Google Scholar] [CrossRef]
- Avants, B.; Epstein, C.; Grossman, M.; Gee, J. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008, 12, 26–41. [Google Scholar] [CrossRef]

| Dataset | Modality | Volumes | Volume Size | Resolution (mm) |
|---|---|---|---|---|
| First NIDDK dataset | T1-weighted MR scans | 100 | 256 × 256 × [30, 80] | 1.41 × 1.41 × 3.06 |
| T2-weighted MR scans | 100 | 256 × 256 × [12, 30] | 1.39 × 1.39 × 9.02 | |
| Second NIDDK dataset | T1-weighted MR scans | 100 | 256 × 256 × [30, 80] | 1.41 × 1.41 × 3.06 |
| T2-weighted MR scans | 250 | 256 × 256 × [10, 51] | 1.38 × 1.38 × 3.00 | |
| External kidney dataset | T1-weighted MR scans | 41 | 512 × 512 × [40, 66] | 0.74 × 0.74 × 4.00 |
| T2-weighted MR scans | 41 | 256 × 256 × [30, 70] | 1.46 × 1.46 × 4.02 | |
| M2OLIE dataset | CT scans | 47 | 512 × 512 × [25, 132] | 0.78 × 0.78 × 2.04 |
| T1-weighted MR scans | 73 | [190, 440] × [288, 640] × [52, 120] | 1.27 × 1.27 × 3.77 |
| Network | Trained Subnetwork | Learning Rate | Optimizer Function | -Parameter | |
|---|---|---|---|---|---|
| Benchmark | Affine | Affine | 3 × 10 | Adam | 0.8 |
| Affine-Deformable | Deformable | 2 × 10 | Adam | 0.7 | |
| Affine-Deformable Finetuned | All | 3 × 10 | Adam | 1 | |
| Proposed Multistage Network | Rigid | Rigid | 3 × 10 | Adam | 0.6 |
| Rigid-Affine | Affine | 5 × 10 | Adam | 0.7 | |
| Rigid-Affine-Deformable | Deformable | 5 × 10 | Adam | 1 | |
| Rigid-Affine-Deformable Finetuned | All | 3 × 10 | Adam | 0.8 |
| Parameter | Value |
|---|---|
| Translation (fraction of image size) | |
| Rotation (degrees) | |
| Scaling (factor) | |
| Deformation (parameter deformation_limits) |
| Network | DICE (%) | (%) | |
|---|---|---|---|
| Before Registration | 67.6 ± 18.1 | - | |
| Baseline NiftyReg | 70.9 ± 24.5 | 0.2 ± 0.6 | |
| Benchmark | Affine | 69.1 ± 16.5 | 0 ± 0 |
| Affine-Deformable | 76.4 ± 12.4 | 0.7 ± 0.8 | |
| Affine-Deformable Finetuned | 76.4 ± 12.7 | 0.7 ± 0.8 | |
| Proposed Multistage Network | Rigid | 68.7 ± 17.3 | 0 ± 0 |
| Rigid-Affine | 70.9 ± 14.2 | 0 ± 0 | |
| Rigid-Affine-Deformable | 76.7 ± 11.4 | 0.6 ± 0.7 | |
| Rigid-Affine-Deformable Finetuned | 76.7 ± 12.5 | 0.5 ± 0.7 | |
| Proposed Multistage Network | Benchmark | |||||
|---|---|---|---|---|---|---|
| Affine | Affine-Deformable | Affine-Deformable Finetuned | ||||
| DICE | Dice | Dice | ||||
| Rigid | 0.0784 | - | ||||
| Rigid-Affine | 0.0048 | - | ||||
| Rigid-Affine-Deformable | 0.3125 | <0.00001 | ||||
| Rigid-Affine-Deformable Finetuned | 0.43675 | <0.00001 | ||||
| Network | External Kidney Dataset without Augmentation | External Kidney Dataset with Augmentation | ||
|---|---|---|---|---|
| DICE (%) | (%) | DICE (%) | (%) | |
| Before Registration | 63.9 ± 11.3 | - | 45.1 ± 16.9 | - |
| Baseline NiftyReg | 67.3 ± 13.9 | 0.1 ± 0.2 | 59.5 ± 22.5 | 0.2 ± 1.0 |
| Benchmark | 62.4 ± 13.7 | 0.9 ± 0.4 | 62.5 ± 15.6 | 0.0 ± 0.0 |
| Proposed Multistage Network | 61.1 ± 14.0 | 1.1 ± 0.6 | 64.8 ± 16.2 | 0.0 ± 0.0 |
| Network | DICE (%) | (%) |
|---|---|---|
| Before Registration | 54.4 ± 19.9 | - |
| Baseline NiftyReg | 63.3 ± 25.5 | 0.0 ± 0.1 |
| Benchmark | 66.6 ± 24.0 | 0.1 ± 0.1 |
| Proposed Multistage Network | 68.1 ± 24.6 | 0.1 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strittmatter, A.; Caroli, A.; Zöllner, F.G. A Multistage Rigid-Affine-Deformable Network for Three-Dimensional Multimodal Medical Image Registration. Appl. Sci. 2023, 13, 13298. https://doi.org/10.3390/app132413298
Strittmatter A, Caroli A, Zöllner FG. A Multistage Rigid-Affine-Deformable Network for Three-Dimensional Multimodal Medical Image Registration. Applied Sciences. 2023; 13(24):13298. https://doi.org/10.3390/app132413298
Chicago/Turabian StyleStrittmatter, Anika, Anna Caroli, and Frank G. Zöllner. 2023. "A Multistage Rigid-Affine-Deformable Network for Three-Dimensional Multimodal Medical Image Registration" Applied Sciences 13, no. 24: 13298. https://doi.org/10.3390/app132413298
APA StyleStrittmatter, A., Caroli, A., & Zöllner, F. G. (2023). A Multistage Rigid-Affine-Deformable Network for Three-Dimensional Multimodal Medical Image Registration. Applied Sciences, 13(24), 13298. https://doi.org/10.3390/app132413298





