Abstract
Perinatal bisphenol A (BPA) exposure promotes the risk of cardiovascular diseases in adulthood. Currently, there is a dire need to develop new therapeutic strategies and options to treat the adverse fetal programming consequences of this exposure. The present study explored the protective effects of perinatal resveratrol (Rsv) administration on BPA exposure-induced adverse cardiovascular changes and hepatic steatosis in adult offspring mice. Pregnant apolipoprotein E-deficient mice were exposed to BPA in drinking water (1 μg/mL) or to both BPA (1 μg/mL) and Rsv (oral; 20 mg kg−1 day−1) during the gestation and lactation periods. Tissues from the heart, liver, left kidney, and brachiocephalic artery from adult offspring (20 weeks old) were processed for staining with H and E, Masson’s trichrome, and Verhoeff–van Gieson and subsequent histology analysis. In both female and male mice who received Rsv supplementation, the following changes were observed in the brachiocephalic arterial wall: (a) a reduction in the BPA exposure-induced increased thickness ratio of the tunica intima to tunica media from 1.3 ± 1.1 µm to 0.5 ± 0.37 µm (p = 0.027) and 0.72 ± 0.58 µm to 0.29 ± 0.25 µm (p = 0.038), respectively, (b) a reduction in the number of elastic lamina breaks (p < 0.05), and (c) the prevention of the BPA exposure-induced progression of atherosclerotic lesions. Further, it also reduced the BPA exposure-induced increased left ventricular thickness by 135 µm and 131 µm in female and male offspring, respectively. The BPA exposure-induced hepatic steatosis score was also significantly reduced with Rsv treatment in female offspring mice (p = 0.02). Renal cortical cytoplasmic vacuolation was identified in both BPA and/or Rsv-treated groups. Our findings suggest that Rsv could be a potential protective candidate against perinatal BPA exposure-induced cardiovascular changes and hepatic steatosis.
1. Introduction
Emerging evidence suggests that prenatal exposure to endocrine-disrupting chemicals (EDCs), whether of natural or synthetic origin, increases the susceptibility to cardiovascular diseases in adulthood [1]. Bisphenol A (BPA) is one of the ubiquitously manufactured EDCs, and is most widely used in various plastic-related consumer products. BPA is able to leach from consumer products and its contaminants are detected in water, food, and dust, etc. [2]. Its presence was also identified in biological solutions, including urine, placenta, breast milk, amniotic fluid, blood, tissues, and cord blood [3]. Although the United States Environmental Protection Agency (EPA) guidelines consider BPA exposure at a concentration of 50 μg/kg/day to be safe, some studies have shown various adverse health effects even at this concentration [4,5,6,7,8]. Humans are exposed to higher concentrations of BPA than the reference dose, Ref. [9] particularly industrial workers and children, where significant urinary BPA levels were noted [10]. The presence of BPA with higher concentrations than 11.2 ng/mL was associated with teratogenic effects in human embryos [11].
BPA could reach the fetus through the placenta and affect the development of various systems of the human body [12,13,14,15]. A recent study, in support of Barker’s hypothesis, has demonstrated that prenatal exposure to BPA triggers the onset of atherosclerosis in the adult progeny [16]. Furthermore, a few studies have investigated the direct impact of prenatal BPA exposure on cardiovascular structure and function in sheep, rodents, and monkeys. Perinatal BPA exposure significantly increased the ventricular wall thickness in the offspring of rodents [17,18,19]. Fetal hearts of BPA-exposed rats showed signs of fibrosis [20] and abnormal development by enhancing ferroptosis [21]. It has been demonstrated that prenatal BPA exposure affects fetal liver maturation [13], contributes to hepatic steatosis development [22,23], and alters nephrogenesis in adult offspring [24]. Epidemiological investigations have indicated that BPA exposure during critical periods of fetal development correlates with elevated blood pressure levels in children [25,26]. Various natural compounds have been investigated to ameliorate BPA exposure-induced adverse effects in various body systems [27]. However, the therapeutic potential of natural compounds against perinatal BPA exposure-induced cardiovascular alterations and hepatic steatosis is yet to be explored.
Rsv is a popular nutritional phytoalexin, commonly found in pistachios, red wine, dark chocolate, berry fruits, etc. Figure 1 illustrates the structures of Rsv and BPA. Potential health-beneficial effects of Rsv include its anti-obesogenic, anti-inflammatory, anti-atherosclerotic, antioxidant properties, anti-carcinogenic activity, platelet aggregation inhibition, and promotion of endothelial function, etc. [28]. The consumption of a reference dose of 5 g/day is considered to be safe [29]. Rsv is known to cross the placental barrier [30] and reduce fetal oxidative stress by improving fetal oxygen delivery and acting on fetal tissues to regulate the genes of the redox system [31]. In our previous study, Rsv exposure significantly prevented BPA exposure-induced atherosclerotic lesion formation in the offspring mice in adulthood [32]. The beneficial effects of Rsv treatment on cardiovascular health in adults have also been reported [33]. In this study, we sought to explore how perinatal Rsv exposure affects the adverse cardiovascular changes and hepatic steatosis induced by BPA in adult offspring.
Figure 1.
The chemical structures of resveratrol and bisphenol A.
2. Materials and Methods
2.1. Animals
ApoE−/− mice (C57BL/6 background; model # B6.129P2-Apoetm1Unc N11) were used, which were acquired from Taconic Biosciences, Inc. (Rensselaer, NY, USA). The study utilized 10-week-old mice, which were housed in polypropylene cages and maintained under standard laboratory conditions. These conditions included a temperature of 22 ± 2 °C, humidity of 60%, and a 12:12 h light and dark cycle. During the course of the experiment, animals were given a standard laboratory chow diet (Oman Flour Mills, Muscat, Oman). Institutional animal ethical committee approval was obtained prior to conducting the study (SQU/AEC/2016-17/12). International laws and guidelines were followed while performing animal experimental procedures.
2.2. Experimental Procedures and Treatments
After receiving the animals from Taconic Biosciences, they were allowed two weeks of acclimatization. For mating purposes, female and male ApoE−/− mice were kept overnight at a 2:1 ratio. Pregnancy was confirmed by the presence of a vaginal plug, and the pregnant mice were randomly divided into three groups: control (CN), BPA, and BPA and Rsv (n = 8 in each group). BPA (>99% purity, Sigma-Aldrich, St Louis, MO, USA) was e introduced in drinking water at a dose level of 1 μg/mL. To prepare BPA solution, BPA was dissolved in ethanol, and then diluted to achieve a final concentration of 0.1% (v/v). In the BPA and Rsv group, mice received an oral dose of 20 mg kg−1 day−1 [34,35] and BPA in their drinking water at the concentration of 1 μg/mL [36]. The drinking water bottle was changed once every two days. The Rsv (R5010, Sigma-Aldrich, St. Louis, MO, USA) was initially dissolved in ethanol and the resulting stock solution was stored in a refrigerator for future use. For the oral treatment, the Rsv dose was freshly prepared every day by diluting the stock to a final concentration of ethanol of 0.5% (v/v). The reference doses of BPA and Rsv were selected based on previous study findings [34,35,36]. The control group mice received the vehicle used, i.e., ethanol in drinking water (0.1%) and oral treatment (0.5%). The pregnant mice were exposed to BPA and Rsv throughout the pregnancy and lactation periods. Following weaning, the male and female offspring were separated from their mother and housed in separate cages. The offspring were allowed to grow until they reached 20 weeks of age. At the end of the 20th week, offspring mice (n = 30 male and n = 30 female) were weighed and asphyxiated by carbon dioxide inhalation. In each, following dissection of the chest cavity, the heart was explored. Perfusion was performed with ice-cold phosphate buffer saline through the apex of the heart. The organs, including the heart, liver, and left kidney, were removed, weighed, and subsequently used for histological analysis. The brachiocephalic artery was collected for histological analysis.
2.3. Histological Evaluation of Lesion Characteristics, Arterial Wall Thickness, and Elastic Preservation
The initial 2 mm length of the brachiocephalic artery was cut from the arch of the aorta carefully and fixed in 10% buffered formalin and then processed for paraffin embedding and sectioning. For the morphometric analysis, six sections (4 µm thick) from each mouse for all mice in the experimental groups were taken using a rotatory microtome. There was a 24 µm interval (6 sections apart) between adjacent sections. The sections (4 µm-thick) were processed for hematoxylin and eosin (H&E) and Verhoeff–van Gieson staining. Seven visual fields from each section were used for the morphometric analysis. H&E-stained slides were examined under the light microscope to evaluate the characteristics of atherosclerotic plaques and arterial wall thickness. Arterial wall thickness was measured as described previously [37]. The stained slides were scanned using a Ziss Axioskop 2 plus microscope equipped with AxioVision Software (R 4.8.2 SP1) to obtain 200× digital images. Using the Image J (1.51j8) program (National Institutes of Health), the thicknesses of the tunica intima and tunica media, respectively, were measured in the digital images, and their mean ratio was determined. The Verhoeff–van Gieson-stained slides were scanned for the presence of elastic breaks and quantified as described previously [38]. The elastin breaks were confirmed by the presence of an interruption in the elastin fiber along with the reappearance of continuous elastic fibers.
2.4. Histological Evaluation of LVW Thickness and Fibrosis
The heart specimens were cut transversely and fixed in 10% buffered formalin. They were then processed for paraffin embedding. Six paraffin sections (5 µm thick) with an interval of 30 µm between two adjacent sections of heart specimens were obtained and processed for H&E staining and Masson’s trichrome staining for light microscopic examination. Stained slides were scanned using the Ziss Axioskop 2 plus microscope equipped with AxioVision software (R 4.8.2 SP1) to obtain 50× digital images. These digital images were used to quantify the left ventricular wall (LVW) thickness with the Image J (1.51j8) program (National Institutes of Health), as described previously [18]. Briefly, 6 digital lines covering the entire thickness of the LVW were taken in a single stained section. The LVW thickness was quantified as the mean length of those six lines relative to the known length of a stage micrometer. The Masson’s trichrome-stained slides were scanned for the presence of blue-stained collagen in the sections.
2.5. Histopathology of the Liver and Kidney
The buffered formalin (10%)-fixed liver and kidney tissue samples were processed for paraffin embedding followed by sectioning. Six sections (5 µm thick) from each mouse for all mice in the experimental groups were taken for the morphometric analysis. There was a 30 µm interval (6 sections apart) between adjacent sections. Sections of tissues were subjected to H&E staining. Liver tissues were examined by light microscopy for the presence of steatosis and inflammation. Seven visual fields from each section were used for the morphometric analysis. In each mouse, micro- and macrosteatosis was recorded on a scale of zero to three (where 0 = 5%, 1 = 5–33%, 2 = 33–66%, and 3 = >66%) as described previously [37]. Kidney tissue slides were examined for the presence of histopathological changes in the renal corpuscles and renal tubules [24].
2.6. Statistical Analysis
The data analysis was performed with SPSS Statistics software for Windows (version 23.0, Professional) (IBM Corp., Armonk, NY, USA). The results were presented as mean ± standard error of the mean (M ± SEM). Differences between groups were assessed using one-way ANOVA followed by Tukey’s multiple comparisons post hoc test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Effect of Perinatal BPA or BPA and Rsv on Arterial Wall Thickness in Adult Offspring Mice
The effect of BPA or BPA and Rsv on brachiocephalic artery wall thickness was analyzed as an intima-to-media ratio. The intima-to-media ratio was significantly higher in BPA-exposed mice when compared to the CN group (p < 0.05). Rsv treatment significantly reduced the intima-to-media ratio in BPA-exposed female offspring mice from 1.3 ± 1.1 µm to 0.5 ± 0.37 µm (p = 0.027) and male offspring mice from 0.72 ± 0.58 µm to 0.29 ± 0.25 µm (p = 0.038) (Figure 2a). The morphological analysis revealed advanced atherosclerotic lesion characteristics in BPA-exposed mice. Treatment with Rsv prevented atherosclerosis lesion progression in the BPA-exposed mice (Figure 2b,c).
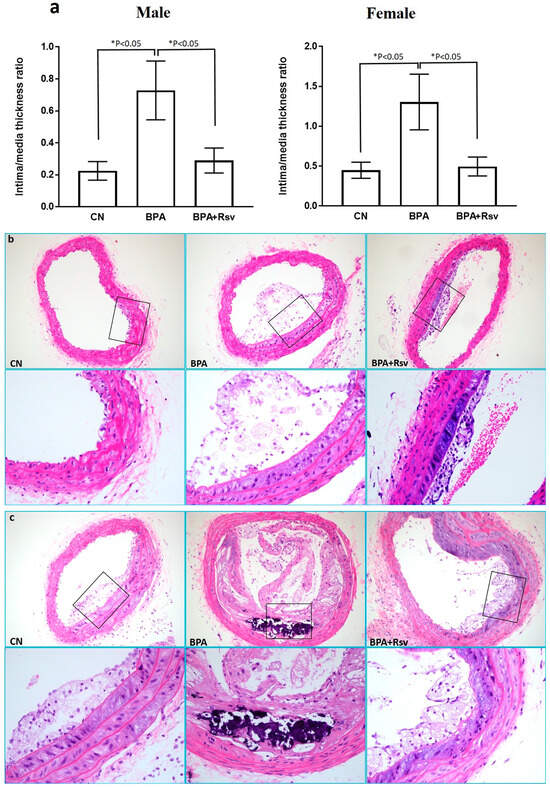
Figure 2.
(a–c): The morphometric analysis of brachiocephalic artery intima-to-media thickness ratio in the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups (a). The data are presented as the M ± SEM (n = 10), * p < 0.05. The statistical test one-way ANOVA and Tukey’s multiple comparisons post hoc test were used. Representative photomicrographs displaying different stages of atherosclerosis in the brachiocephalic artery in the male (b) and female (c) offspring mice. Note the control (CN) group showing initial stage of foam cells laid, thickening the tunica intima with focal acellular areas above the media, while BPA-treated group presents an advanced atherosclerotic lesion with damaged cells, cholesterol clefts, and calcium deposits. Treatment with Rsv in BPA and Rsv group showing the initial stage of atherosclerosis lesion formation. (H&E staining; magnification: 200×; enclosed window magnification: 400×).
3.2. Effect of Perinatal BPA or BPA and Rsv on Elastic Preservation in Adult Offspring Mice
The effect of BPA or BPA and Rsv on elastic preservation was analyzed as the number of elastic breaks in the tunica media. The morphometric analysis of the number of elastic lamina breaks revealed a significant increase in the BPA group (p < 0.05) when compared to the CN group. Rsv supplementation significantly reduced the number of elastic lamina breaks in the BPA-exposed male offspring mice from 4.7 ± 2.6 to 2.7 ± 1.2 (p = 0.04) and in female offspring mice, reduction occurred from 3.6 ± 1.8 to 1.9 ± 1.1 (p = 0.01) (Figure 3a–c).
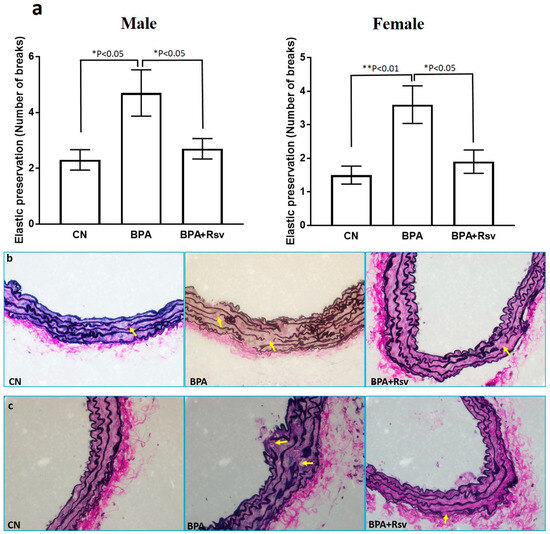
Figure 3.
(a–c): The morphometric analysis of the number of elastic lamina breaks enumerated in the brachiocephalic artery of three experimental groups (a). Significant differences in elastin preservation (number of breaks) between the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups. The data are presented as the M ± SEM (n = 10). The statistical test one-way ANOVA and Tukey’s multiple comparisons post hoc test were used, * p < 0.05, ** p < 0.01. Representative images of elastin stain showing the breaks in the elastic laminae in male (b) and female (c) offspring mice in the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups. (Verhoeff–van Gieson elastin staining; magnification: 400×). Yellow arrows indicate elastic lamina breaks.
3.3. Effect of Perinatal BPA or BPA and Rsv on Heart Weight, LVW Thickness, and Fibrosis in Adult Offspring Mice
The histo-morphometric analysis of cardiac muscle revealed a significant increase in the thickness of the LVW in the BPA-exposed group (male: p = 0.001; female: p = 0.0004) compared to the CN group. Rsv supplementation reduced the BPA exposure-induced increased left ventricular thickness in female (p = 0.001) and male (p = 0.002) offspring by 135 µm and 131 µm, respectively (Figure 4a–c). In addition, myocardial fibrosis was clearly evident in the BPA-exposed group. Treatment with Rsv significantly reduced the fibrosis in the BPA-exposed mice (Figure 5). Table 1 and Table 2 show the heart weight and body weight ratios (HW/BW) of the CN, BPA, and BPA and Rsv groups. There was a significantly increased HW/BW ratio for the BPA-exposed group when compared to the CN group (p = 0.02), while treatment with Rsv significantly reduced the HW/BW ratio (p = 0.46).
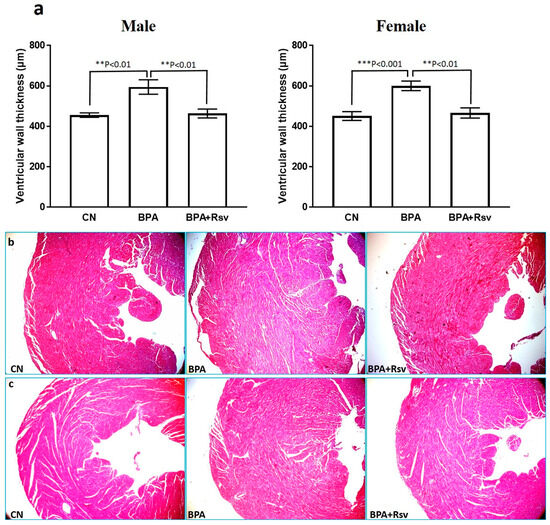
Figure 4.
(a–c): The morphometric analysis of the effect of Rsv on the BPA-induced left ventricular hypertrophy in the male and female offspring ApoE mice (a). The data are presented as M ± SEM (n = 10). The statistical test one-way ANOVA and Tukey’s multiple comparisons post hoc test were used, ** p < 0.01, *** p < 0.001. Representative light photomicrographs of male (b) and female (c) hearts from the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups. An increase in the left ventricular wall thickness was observed in the BPA group when compared to the CN group. Treatment with Rsv prevented the left ventricular hypertrophy in the BPA and Rsv group. (H&E staining; 400× magnification).
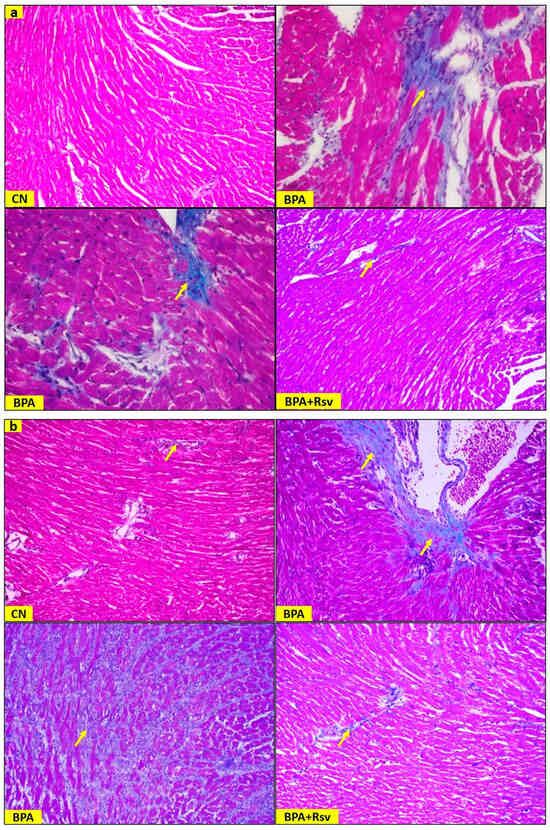
Figure 5.
(a,b): Representative light photomicrographs showing the fibrosis in cardiac muscles of male (a) and female (b) offspring mice from the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups. Note fibrosis (arrow) in the BPA-exposed group when compared to the CN group. Treatment with Rsv reduced the collagen deposition in the BPA-exposed group. (Masson’s trichrome staining; 400× magnification). Yellow arrows indicate fibrosis.

Table 1.
Effect of maternal BPA or BPA and Rsv on body weight and tissue weight in adult male offspring mice.

Table 2.
Effect of maternal BPA or BPA and Rsv on body weight and tissue weight in adult female offspring mice.
3.4. Effect of Perinatal BPA or BPA and Rsv on Hepatic Steatosis and Kidney Histopathology in Adult Offspring Mice
Table 1 and Table 2 show the liver weight and body weight ratio (LW/BW) and kidney weight and body weight ratio (KW/BW) of the CN, BPA, and BPA and Rsv groups. No significant differences in LW/BW and KW/BW among the experimental groups were observed. Light microscopy of the liver samples revealed the presence of steatosis in all three experimental groups. The combined micro- and macro-hepatic steatosis was significantly higher in the BPA-exposed female offspring mice (p = 0.008), while treatment with Rsv significantly reduced the steatosis in the BPA-exposed group (p = 0.02) (Figure 6). There were no significant changes observed in the hepatic steatosis scores in the male offspring (Figure 6). Detailed analysis of H&E-stained slides of the kidney tissue did not reveal any obvious pathological alterations in the renal corpuscles of the experimental groups. However, there was cytoplasmic vacuolation in the tubules of renal cortices, and it was observed in all three groups (Figure 7a,b).
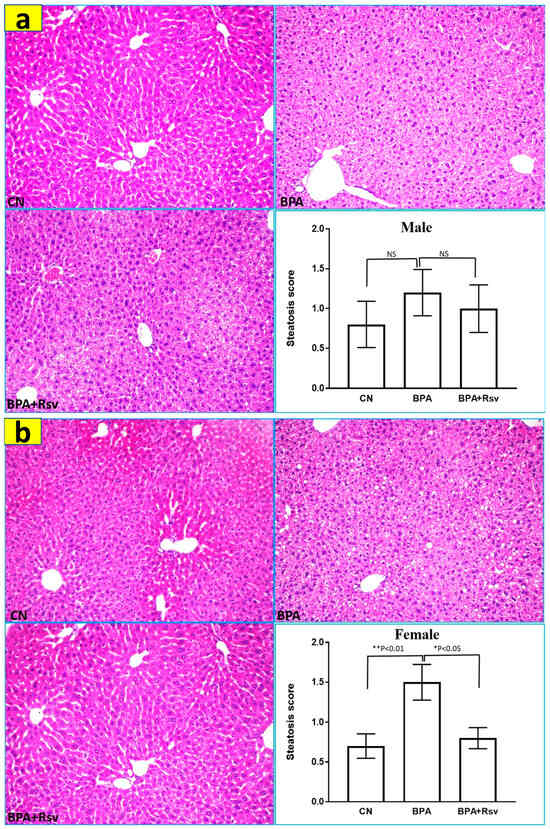
Figure 6.
(a,b): The morphometric analysis of the effect of Rsv on bisphenol A-induced hepatic steatosis in the male (a) and female (b) offspring ApoE mice. The combined micro- and macrohepatic steatosis was evaluated by severity scores from 0 to 3. The data are presented as the M ± SEM (n = 10). The statistical test one-way ANOVA and Tukey’s multiple comparisons post hoc test were used, * p < 0.05, ** p < 0.01. Representative light photomicrographs of liver tissue showing the differences in the amount of hepatic steatosis in the male and female offspring mice of experimental groups. (H&E staining; 200× magnification).
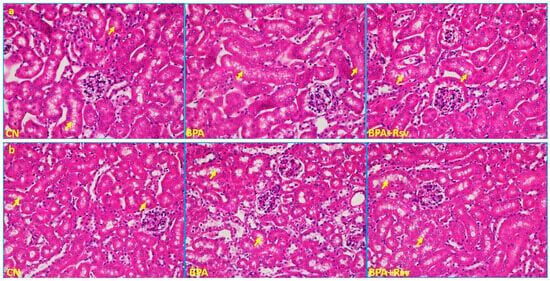
Figure 7.
(a,b): Representative light photomicrographs of kidney tissue showing the renal cortical cytoplasmic vacuolation in the tubules (arrow) in the control (CN), bisphenol A (BPA), and bisphenol A and resveratrol (BPA and Rsv) groups in male (a) and female (b) offspring. Note that these features were present in all three groups. (H&E staining; 200× magnification). Yellow arrows indicate cytoplasmic vacuolation.
4. Discussion
Our previous study findings indicate that perinatal exposure to BPA in ApoE mice results in the formation of atherosclerotic lesions in the aorta of adult offspring [32]. Similar findings were observed in a study of a PXR-Humanized mouse animal model [33]. In this study, we explored the lesion characteristics, arterial wall thickness, and elastic preservation of BPA-exposed offspring mice. Histo-morphometric analysis of the brachiocephalic artery revealed increased elastic breaks and intima-to-media thickness ratio in BPA-exposed mice. Indeed, elastin fibers are crucial for maintaining the structural integrity of blood vessels and regulating the proliferation and migration of smooth muscle cells [39]. During the development of atherosclerosis, the fragmentation and degradation of elastic laminae can occur, which enables SMC penetration into the intima layer. This process contributes to the formation of atheromatous plaques [40]. Increased disruption of elastin lamellae is typically observed in atherosclerosis progression [38,39]. Hence, the increased number of elastic breaks in BPA-exposed mice indicate its role in atherosclerosis progression. This is further evidenced by the increased intima-to-media thickness ratio and the presence of cholesterol clefts and calcium deposits in the intimal lesion in BPA-exposed mice.
Studies have explored the adverse effects of BPA exposure on fetal heart development and cardiac structure/function in adult offspring [17,18,20]. In an in vitro study, BPA exposure altered the normal function of the in vitro model of the fetal heart [20]. In a study by Patel et al., sex-specific effects on cardiac structure/function were observed in BPA-exposed adult male offspring mice [17]. The concentric remodeling of cardiac muscle and increased relative wall thickness were noted in male mice. Findings of the study also suggested that the altered structure is due to the direct action of BPA on the heart, as there was no pathological increase in blood pressure [17]. In a study by Belcher et al., prenatal BPA exposure significantly increased heart weight, LVW thickness, and perivascular and interstitial fibrosis in adult male offspring mice [18]. The possible mechanisms behind the BPA-induced adverse effects on heart development were studied. According to the study conducted by Chapalamadugu et al., maternal exposure to BPA was found to cause changes in the transcript expression profile in the fetal heart [41]. The same study findings revealed that the regulation of two genes (Myh6 and Adam12) in the left ventricle is shown to be related to cardiac hypertrophy. Another study revealed that developmental BPA exposure in pregnant rats alters miR-17-5p,-208-3p, and-210-3p expressions in fetal hearts [20]. In our study, the observed histo-morphometric changes in the heart support the previous in vitro and in vivo study findings that demonstrate the cardiotoxic effects of perinatal BPA exposure on adult offspring. However, contrary to most previous studies, we did not observe a sexually dimorphic effect of BPA on the rodent heart. These sex-specific differences could be due to different animal models used in these studies.
The worldwide reported pooled prevalence of non-alcoholic fatty liver disease (NAFLD) is 25.24%, with the highest prevalence rates in South American and Middle Eastern countries [42]. Current evidence suggests that reprogramming of the liver during the critical periods of gestation increases the risk of developing NAFLD [43], in addition to other factors, including obesity and a sedentary lifestyle. Experimental studies have reported BPA-induced hepatic steatosis in the liver without altering its weight [22,44,45]. In agreement with these studies, we observed sex-specific BPA-induced hepatic steatosis in adult female offspring. However, in a study by Rasdi et al., BPA exposure had no effect on fetal liver morphology [20]. The possible mechanisms behind prenatal BPA-induced hepatic steatosis include defective hepatic mitochondrial function and activated hepatic lipid metabolism [23,43], and also, importantly, the epigenetic regulation of novel hypomethylated sites in the Fas, Srebp-1c, and Nrf2 genes [22]. Hyperlipidemia is known to cause vacuolation in renal tubules [46]. In the present study, cytoplasmic vacuolation was evident in the tubules of the renal cortex in all the study groups. Nunez et al. observed male histological features in the renal structure of BPA-exposed female offspring [24]. In contrast, in the present study, we did not observe such changes in the kidneys of female offspring. The conflicting views with regard to BPA exposure on nephrogenesis could be attributed to the differences in experimental conditions. More studies are required to draw a conclusive result in this regard.
Perinatal Rsv supplementation significantly reduced heart weight, LVW thickness, and interstitial fibrosis and significantly prevented hepatic steatosis in the adult offspring. Further, Rsv-treated offspring mice had fewer elastin breaks and a reduced intima-to-media ratio in the BPA-exposed group, indicating the beneficial role of Rsv against atherosclerotic progression. Previously, Rsv supplementation during gestation was reported to prevent hypertension in the offspring [47]. Rsv has been shown to prevent cardiovascular diseases through Sirt1 and AMPK activation as well as promoting endogenous antioxidant enzymes [48]. Perinatal Rsv supplementation in rats inhibited hepatic lipogenesis in adult offspring by activating AMPK and upregulating Sirt 1 [49]. In another study, perinatal Rsv supplementation prevented high-fat diet-induced nonalcoholic fatty liver disease through the renin–angiotensin system [50]. The epigenetic modification or reprogramming of genes is known to be involved in the developmental or fetal origin of adult diseases. Dr Alan Wolffe defined epigenetics as the occurrence of heritable changes in gene expression without any alterations in the DNA sequence itself [51]. As per this definition, both DNA methylation and histone modifications are associated with epigenetic mechanisms [52,53]. Rsv is known to exhibit epigenetic regulation through its abilities to inhibit histone deacetylase [54] and DNA methyltransferase 1 [55,56]. Studies have proved that Rsv acts as an miRNA regulator in various disease-related therapies [57,58]. Based on these reported facts, we hypothesize that the beneficial effect of Rsv against BPA-induced cardiovascular changes in the adult offspring could be attributed to its epigenetic regulation and/or miRNA regulation. Molecular studies exploring the effects of perinatal Rsv exposure on epigenetic mechanisms are warranted.
The present study has the following limitation. The impact of the study would have been further enhanced by evaluating the epigenetic modifications and molecular mechanisms involved in the ameliorating effects of Rsv supplementation on prenatal BPA exposure-induced potentially pathological alterations in cardiovascular and liver tissues.
5. Conclusions
The results of the present study corroborate previous research showing that perinatal BPA exposure can cause cardiovascular abnormalities and sex-specific hepatic steatosis in the adult offspring. The supplementation of perinatal Rsv during gestation and early life prevents BPA exposure-induced adverse cardiovascular alterations and hepatic steatosis.
Author Contributions
Conceptualization, S.R.S., M.A.-M. and I.A.-H.; methodology, S.R.S., I.A.-H. and M.A.-M.; formal analysis, S.R.S. and I.A.-H.; investigation, S.R.S., N.A.-A., F.A.G. and I.A.-H.; data curation, S.R.S., N.A.-A. and F.A.G.; writing—original draft preparation, S.R.S. and M.A.-M.; writing—review and editing, I.A.-H., N.A.-A. and F.A.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sultan Qaboos University (Internal grant no. IG/MED/ANAT/18/01.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional animal ethical committee (SQU/AEC/2016-17/12).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of the present study are available from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
We acknowledge Taconic Biosciences, Inc. for providing ApoE−/− mice to conduct the experiments. The study findings were presented in 2nd Malaysian Anatomical Association Conference held in Malaysia from 1 November to 4 November 2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gutiérrez-Torres, D.S.; Barraza-Villarreal, A.; Hernandez-Cadena, L.; Escamilla-Nuñez, C.; Romieu, I. Prenatal Exposure to Endocrine Disruptors and Cardiometabolic Risk in Preschoolers: A Systematic Review Based on Cohort Studies. Ann. Glob. Health 2018, 84, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Khalili Sadrabad, E.; Hashemi, S.A.; Nadjarzadeh, A.; Askari, E.; Akrami Mohajeri, F.; Ramroudi, F. Bisphenol A release from food and beverage containers—A review. Food Sci. Nutr. 2023, 11, 3718–3728. [Google Scholar] [CrossRef]
- Wazir, U.; Mokbel, K. Bisphenol A: A Concise Review of Literature and a Discussion of Health and Regulatory Implications. In Vivo 2019, 33, 1421–1423. [Google Scholar] [CrossRef]
- Alonso-Magdalena, P.; Morimoto, S.; Ripoll, C.; Fuentes, E.; Nadal, A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ. Health Perspect. 2006, 114, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Mizuo, K.; Narita, M.; Miyagawa, K.; Narita, M.; Okuno, E.; Suzuki, T. Prenatal and neonatal exposure to bisphenol-A affects the morphine-induced rewarding effect and hyperlocomotion in mice. Neurosci. Lett. 2004, 356, 95–98. [Google Scholar] [CrossRef]
- Ryan, B.C.; Hotchkiss, A.K.; Crofton, K.M.; Gray, L.E., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 114, 133–148. [Google Scholar] [CrossRef]
- Tando, S.; Itoh, K.; Yaoi, T.; Ikeda, J.; Fujiwara, Y.; Fushiki, S. Effects of pre- and neonatal exposure to bisphenol A on murine brain development. Brain Dev. 2007, 29, 352–356. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Akingbemi, B.T.; Belcher, S.M.; Birnbaum, L.S.; Crain, D.A.; Eriksen, M.; Farabollini, F.; Guillette, L.J., Jr.; Hauser, R.; Heindel, J.J.; et al. Chapel Hill bisphenol A expert panel consensus statement: Integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod. Toxicol. 2007, 24, 131–138. [Google Scholar] [CrossRef]
- vom Saal, F.S.; Hughes, C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005, 113, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Seo, M.Y.; Choi, K.; Chang, Y.S.; Kim, S.H.; Park, M.J. Urinary bisphenol A concentrations and the risk of obesity in Korean adults. Sci. Rep. 2021, 11, 1603. [Google Scholar] [CrossRef]
- Pjanic, M. The role of polycarbonate monomer bisphenol-A in insulin resistance. PeerJ 2017, 5, e3809. [Google Scholar] [CrossRef]
- Casas, M.; Forns, J.; Martínez, D.; Avella-García, C.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Julvez, J.; Sunyer, J.; et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ. Res. 2015, 142, 671–679. [Google Scholar] [CrossRef]
- DeBenedictis, B.; Guan, H.; Yang, K. Prenatal Exposure to Bisphenol A Disrupts Mouse Fetal Liver Maturation in a Sex-Specific Manner. J. Cell. Biochem. 2016, 117, 344–350. [Google Scholar] [CrossRef]
- Hijazi, A.; Guan, H.; Cernea, M.; Yang, K. Prenatal exposure to bisphenol A disrupts mouse fetal lung development. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 4968–4977. [Google Scholar] [CrossRef]
- Moustafa, G.G.; Ahmed, A.A.M. Impact of prenatal and postnatal exposure to bisphenol A on female rats in a two generational study: Genotoxic and immunohistochemical implications. Toxicol. Rep. 2016, 3, 685–695. [Google Scholar] [CrossRef]
- Sui, Y.; Park, S.H.; Helsley, R.N.; Sunkara, M.; Gonzalez, F.J.; Morris, A.J.; Zhou, C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014, 3, e000492. [Google Scholar] [CrossRef]
- Patel, B.B.; Raad, M.; Sebag, I.A.; Chalifour, L.E. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2013, 133, 174–185. [Google Scholar] [CrossRef]
- Belcher, S.M.; Gear, R.B.; Kendig, E.L. Bisphenol A alters autonomic tone and extracellular matrix structure and induces sex-specific effects on cardiovascular function in male and female CD-1 mice. Endocrinology 2015, 156, 882–895. [Google Scholar] [CrossRef]
- MohanKumar, S.M.; Rajendran, T.D.; Vyas, A.K.; Hoang, V.; Asirvatham-Jeyaraj, N.; Veiga-Lopez, A.; Olivier, N.B.; Padmanabhan, V.; MohanKumar, P.S. Effects of prenatal bisphenol-A exposure and postnatal overfeeding on cardiovascular function in female sheep. J. Dev. Orig. Health Dis. 2017, 8, 65–74. [Google Scholar] [CrossRef]
- Rasdi, Z.; Kamaludin, R.; Ab Rahim, S.; Syed Ahmad Fuad, S.B.; Othman, M.H.D.; Siran, R.; Mohd Nor, N.S.; Abdul Hamid Hasani, N.; Sheikh Abdul Kadir, S.H. The impacts of intrauterine Bisphenol A exposure on pregnancy and expression of miRNAs related to heart development and diseases in animal model. Sci. Rep. 2020, 10, 5882. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Li, S.; Wang, W.; Wang, Y.; Yin, C. Exposure to Bisphenol A induces abnormal fetal heart development by promoting ferroptosis. Ecotoxicol. Environ. Saf. 2023, 255, 114753. [Google Scholar] [CrossRef]
- Shimpi, P.C.; More, V.R.; Paranjpe, M.; Donepudi, A.C.; Goodrich, J.M.; Dolinoy, D.C.; Rubin, B.; Slitt, A.L. Hepatic Lipid Accumulation and Nrf2 Expression following Perinatal and Peripubertal Exposure to Bisphenol A in a Mouse Model of Nonalcoholic Liver Disease. Environ. Health Perspect. 2017, 125, 087005. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xia, W.; Zhu, Y.; Li, X.; Wang, D.; Liu, J.; Chang, H.; Li, G.; Xu, B.; Chen, X.; et al. Mitochondrial dysfunction in early life resulted from perinatal bisphenol A exposure contributes to hepatic steatosis in rat offspring. Toxicol. Lett. 2014, 228, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, P.; Fernandez, T.; García-Arévalo, M.; Alonso-Magdalena, P.; Nadal, A.; Perillan, C.; Arguelles, J. Effects of bisphenol A treatment during pregnancy on kidney development in mice: A stereological and histopathological study. J. Dev. Orig. Health Dis. 2018, 9, 208–214. [Google Scholar] [CrossRef]
- Ouyang, F.; Zhang, G.H.; Du, K.; Shen, L.; Ma, R.; Wang, X.; Wang, X.; Zhang, J. Maternal prenatal urinary bisphenol A level and child cardio-metabolic risk factors: A prospective cohort study. Environ. Pollut. 2020, 265, 115008. [Google Scholar] [CrossRef]
- Bae, S.; Lim, Y.H.; Lee, Y.A.; Shin, C.H.; Oh, S.Y.; Hong, Y.C. Maternal Urinary Bisphenol A Concentration During Midterm Pregnancy and Children’s Blood Pressure at Age 4. Hypertension 2017, 69, 367–374. [Google Scholar] [CrossRef]
- Sirasanagandla, S.R.; Al-Huseini, I.; Sakr, H.; Moqadass, M.; Das, S.; Juliana, N.; Abu, I.F. Natural Products in Mitigation of Bisphenol A Toxicity: Future Therapeutic Use. Molecules 2022, 27, 5384. [Google Scholar] [CrossRef]
- Ni, C.; Ye, Q.; Mi, X.; Jiao, D.; Zhang, S.; Cheng, R.; Fang, Z.; Fang, M.; Ye, X. Resveratrol inhibits ferroptosis via activating NRF2/GPX4 pathway in mice with spinal cord injury. Microsc. Res. Tech. 2023, 86, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2007, 16, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, G.; Jiang, X.; Wu, G. Resveratrol ameliorates toxic effects of cadmium on placental development in mouse placenta and human trophoblast cells. Birth Defects Res. 2021, 113, 1470–1483. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Mohd Dollah, M.H.B.; Regnault, T.R.H.; Williams, M.T.; Morrison, J.L. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications-Are we ready for the clinic? Pharmacol. Res. 2019, 144, 264–278. [Google Scholar] [CrossRef]
- Sirasanagandla, S.R.; Al-Huseini, I.; Al Mushaiqri, M.; Al-Abri, N.; Al-Ghafri, F. Maternal resveratrol supplementation ameliorates bisphenol A-induced atherosclerotic lesions formation in adult offspring ApoE−/− mice. 3 Biotech 2022, 12, 36. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Dyck, J.R. Calorie restriction and resveratrol in cardiovascular health and disease. Biochim. Biophys. Acta 2011, 1812, 1477–1489. [Google Scholar] [CrossRef]
- Juan, M.E.; Vinardell, M.P.; Planas, J.M. The daily oral administration of high doses of trans-resveratrol to rats for 28 days is not harmful. J. Nutr. 2002, 132, 257–260. [Google Scholar] [CrossRef]
- Penumathsa, S.V.; Thirunavukkarasu, M.; Koneru, S.; Juhasz, B.; Zhan, L.; Pant, R.; Menon, V.P.; Otani, H.; Maulik, N. Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J. Mol. Cell. Cardiol. 2007, 42, 508–516. [Google Scholar] [CrossRef]
- Miyawaki, J.; Sakayama, K.; Kato, H.; Yamamoto, H.; Masuno, H. Perinatal and postnatal exposure to bisphenol a increases adipose tissue mass and serum cholesterol level in mice. J. Atheroscler. Thromb. 2007, 14, 245–252. [Google Scholar] [CrossRef]
- Smith, D.D.; Tan, X.; Raveendran, V.V.; Tawfik, O.; Stechschulte, D.J.; Dileepan, K.N. Mast cell deficiency attenuates progression of atherosclerosis and hepatic steatosis in apolipoprotein E-null mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H2612–H2621. [Google Scholar] [CrossRef]
- Suganuma, E.; Babaev, V.R.; Motojima, M.; Zuo, Y.; Ayabe, N.; Fogo, A.B.; Ichikawa, I.; Linton, M.F.; Fazio, S.; Kon, V. Angiotensin inhibition decreases progression of advanced atherosclerosis and stabilizes established atherosclerotic plaques. J. Am. Soc. Nephrol. JASN 2007, 18, 2311–2319. [Google Scholar] [CrossRef]
- Sukhova, G.K.; Zhang, Y.; Pan, J.H.; Wada, Y.; Yamamoto, T.; Naito, M.; Kodama, T.; Tsimikas, S.; Witztum, J.L.; Lu, M.L.; et al. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J. Clin. Investig. 2003, 111, 897–906. [Google Scholar] [CrossRef]
- De Meyer, G.R.; Bult, H. Mechanisms of neointima formation--lessons from experimental models. Vasc. Med. 1997, 2, 179–189. [Google Scholar] [CrossRef]
- Chapalamadugu, K.C.; Vandevoort, C.A.; Settles, M.L.; Robison, B.D.; Murdoch, G.K. Maternal bisphenol a exposure impacts the fetal heart transcriptome. PLoS ONE 2014, 9, e89096. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; De, A.; Chowdhury, A. Epidemiology of non-alcoholic and alcoholic fatty liver diseases. Transl. Gastroenterol. Hepatol. 2020, 5, 16. [Google Scholar] [CrossRef]
- Li, M.; Reynolds, C.M.; Segovia, S.A.; Gray, C.; Vickers, M.H. Developmental Programming of Nonalcoholic Fatty Liver Disease: The Effect of Early Life Nutrition on Susceptibility and Disease Severity in Later Life. BioMed Res. Int. 2015, 2015, 437107. [Google Scholar] [CrossRef]
- Wei, J.; Sun, X.; Chen, Y.; Li, Y.; Song, L.; Zhou, Z.; Xu, B.; Lin, Y.; Xu, S. Perinatal exposure to bisphenol A exacerbates nonalcoholic steatohepatitis-like phenotype in male rat offspring fed on a high-fat diet. J. Endocrinol. 2014, 222, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Marmugi, A.; Ducheix, S.; Lasserre, F.; Polizzi, A.; Paris, A.; Priymenko, N.; Bertrand-Michel, J.; Pineau, T.; Guillou, H.; Martin, P.G.; et al. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 2012, 55, 395–407. [Google Scholar] [CrossRef]
- Thomsen, J.L.; Kristensen, I.B.; Ottosen, P.D. The histological demonstration of lipids in the proximal renal tubules of patients with diabetic coma. Forensic Sci. Med. Pathol. 2006, 2, 249–252. [Google Scholar] [CrossRef]
- Care, A.S.; Sung, M.M.; Panahi, S.; Gragasin, F.S.; Dyck, J.R.; Davidge, S.T.; Bourque, S.L. Perinatal Resveratrol Supplementation to Spontaneously Hypertensive Rat Dams Mitigates the Development of Hypertension in Adult Offspring. Hypertension 2016, 67, 1038–1044. [Google Scholar] [CrossRef]
- Zordoky, B.N.; Robertson, I.M.; Dyck, J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta 2015, 1852, 1155–1177. [Google Scholar] [CrossRef]
- Tanaka, M.; Kita, T.; Yamasaki, S.; Kawahara, T.; Ueno, Y.; Yamada, M.; Mukai, Y.; Sato, S.; Kurasaki, M.; Saito, T. Maternal resveratrol intake during lactation attenuates hepatic triglyceride and fatty acid synthesis in adult male rat offspring. Biochem. Biophys. Rep. 2017, 9, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Tiao, M.M.; Lin, Y.J.; Yu, H.R.; Sheen, J.M.; Lin, I.C.; Lai, Y.J.; Tain, Y.L.; Huang, L.T.; Tsai, C.C. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis. 2018, 17, 178. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Matzke, M.A. Epigenetics: Regulation through repression. Science 1999, 286, 481–486. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Biel, M.; Wascholowski, V.; Giannis, A. Epigenetics—An epicenter of gene regulation: Histones and histone-modifying enzymes. Angew. Chem. (Int. Ed. Engl.) 2005, 44, 3186–3216. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Berger, A.; Böcker, A.; Busch, C.; Weiland, T.; Noor, S.; Leischner, C.; Schleicher, S.; Mayer, M.; Weiss, T.S.; et al. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS ONE 2013, 8, e73097. [Google Scholar] [CrossRef]
- Kala, R.; Tollefsbol, T.O. A Novel Combinatorial Epigenetic Therapy Using Resveratrol and Pterostilbene for Restoring Estrogen Receptor-α (ERα) Expression in ERα-Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0155057. [Google Scholar] [CrossRef]
- Gao, Y.; Tollefsbol, T.O. Combinational Proanthocyanidins and Resveratrol Synergistically Inhibit Human Breast Cancer Cells and Impact Epigenetic-Mediating Machinery. Int. J. Mol. Sci. 2018, 19, 2204. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, X.; Li, W.; Sang, H.; Qian, A.; Sun, L.; Li, X.; Li, C. Resveratrol Improves Endothelial Progenitor Cell Function through miR-138 by Targeting Focal Adhesion Kinase (FAK) and Promotes Thrombus Resolution In Vivo. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y. Resveratrol alleviates LPS-induced injury in human keratinocyte cell line HaCaT by up-regulation of miR-17. Biochem. Biophys. Res. Commun. 2018, 501, 106–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).