Genomic Alterations in Melanocytic Tumors: A Review of Spitz Tumors, Blue Nevi, Deep Penetrating Melanocytomas and Pigmented Epithelioid Melanocytomas
Abstract
1. Introduction
2. Spitz Tumors
2.1. HRAS-Driven Spitz Tumors
2.2. ALK-Fused Spitz Tumors
2.3. ROS1-Fused Spitz Tumors
2.4. NTRK-Fused Spitz Tumors
2.5. MET-Fused Spitz Tumors
2.6. RET-Fused Spitz Tumors
2.7. BRAF-Fused Spitz Tumors
2.8. MAP3K8-Mutated Spitz Tumors
2.9. MAP2K1 Mutated Spitz Tumors
3. Blue Nevi
4. Deep Penetrating Melanocytoma (DPM)
5. Pigmented Epithelioid Melanocytoma (PEM)
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Engen-van Grunsven, A.C.; van Dijk, M.C.; Ruiter, D.J.; Klaasen, A.; Mooi, W.J.; Blokx, W.A. HRAS-mutated Spitz tumors: A subtype of Spitz tumors with distinct features. Am. J. Surg. Pathol. 2010, 34, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Bastian, B.C.; LeBoit, P.E.; Pinkel, D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am. J. Pathol. 2000, 157, 967–972. [Google Scholar] [CrossRef]
- Ishida, C.; Zubair, M.; Gupta, V. Molecular Genetics Testing. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Amin, S.M.; Haugh, A.M.; Lee, C.Y.; Zhang, B.; Bubley, J.A.; Merkel, E.A.; Verzì, A.E.; Gerami, P. A Comparison of Morphologic and Molecular Features of BRAF, ALK, and NTRK1 Fusion Spitzoid Neoplasms. Am. J. Surg. Pathol. 2017, 41, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Busam, K.J.; Kutzner, H.; Cerroni, L.; Wiesner, T. Clinical and pathologic findings of Spitz nevi and atypical Spitz tumors with ALK fusions. Am. J. Surg. Pathol. 2014, 38, 925–933. [Google Scholar] [CrossRef]
- Kiuru, M.; Jungbluth, A.; Kutzner, H.; Wiesner, T.; Busam, K.J. Spitz Tumors: Comparison of Histological Features in Relationship to Immunohistochemical Staining for ALK and NTRK1. Int. J. Surg. Pathol. 2016, 24, 200–206. [Google Scholar] [CrossRef]
- Kervarrec, T.; Pissaloux, D.; Tirode, F.; Samimi, M.; Jacquemus, J.; Castillo, C.; de la Fouchardière, A. Morphologic features in a series of 352 Spitz melanocytic proliferations help predict their oncogenic drivers. Virchows Arch. 2022, 480, 369–382. [Google Scholar] [CrossRef]
- Gerami, P.; Kim, D.; Compres, E.V.; Zhang, B.; Khan, A.U.; Sunshine, J.C.; Quan, V.L.; Busam, K. Clinical, morphologic, and genomic findings in ROS1 fusion Spitz neoplasms. Mod. Pathol. 2021, 34, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Peternel, S.; Mully, T.W.; North, J.P.; Pincus, L.B.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C.; Yeh, I. Spitz melanoma is a distinct subset of spitzoid melanoma. Mod. Pathol. 2020, 33, 1122–1134. [Google Scholar] [CrossRef]
- Yeh, I.; Busam, K.J.; McCalmont, T.H.; LeBoit, P.E.; Pissaloux, D.; Alberti, L.; de la Fouchardière, A.; Bastian, B.C. Filigree-like Rete Ridges, Lobulated Nests, Rosette-like Structures, and Exaggerated Maturation Characterize Spitz Tumors With NTRK1 Fusion. Am. J. Surg. Pathol. 2019, 43, 737–746. [Google Scholar] [CrossRef]
- de la Fouchardière, A.; Tee, M.K.; Peternel, S.; Valdebran, M.; Pissaloux, D.; Tirode, F.; Busam, K.J.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C.; et al. Fusion partners of NTRK3 affect subcellular localization of the fusion kinase and cytomorphology of melanocytes. Mod. Pathol. 2021, 34, 735–747. [Google Scholar] [CrossRef]
- Yeh, I.; Botton, T.; Talevich, E.; Shain, A.H.; Sparatta, A.J.; de la Fouchardiere, A.; Mully, T.W.; North, J.P.; Garrido, M.C.; Gagnon, A.; et al. Activating MET kinase rearrangements in melanoma and Spitz tumours. Nat. Commun. 2015, 6, 7174. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, T.; He, J.; Yelensky, R.; Esteve-Puig, R.; Botton, T.; Yeh, I.; Lipson, D.; Otto, G.; Brennan, K.; Murali, R.; et al. Kinase fusions are frequent in Spitz tumours and spitzoid melanomas. Nat. Commun. 2014, 5, 3116. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Compres, E.V.; Zhang, B.; Khan, A.U.; Sunshine, J.C.; Quan, V.L.; Gerami, P. A Series of RET Fusion Spitz Neoplasms with Plaque-Like Silhouette and Dyscohesive Nesting of Epithelioid Melanocytes. Am. J. Dermatopathol. 2021, 43, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Jung, M.; Kang, H.N.; Kim, H.; Park, C.W.; Kim, S.M.; Shin, S.J.; Kim, S.H.; Kim, S.G.; Kim, E.K.; et al. Oncogenic BRAF fusions in mucosal melanomas activate the MAPK pathway and are sensitive to MEK/PI3K inhibition or MEK/CDK4/6 inhibition. Oncogene 2017, 36, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Khan, A.U.; Compres, E.V.; Zhang, B.; Sunshine, J.C.; Quan, V.L.; Gerami, P. BRAF fusion Spitz neoplasms; clinical morphological, and genomic findings in six cases. J. Cutan. Pathol. 2020, 47, 1132–1142. [Google Scholar] [CrossRef]
- Perron, E.; Pissaloux, D.; Neub, A.; Hohl, D.; Tartar, M.D.; Mortier, L.; Alberti, L.; de la Fouchardiere, A. Unclassified sclerosing malignant melanomas with AKAP9-BRAF gene fusion: A report of two cases and review of BRAF fusions in melanocytic tumors. Virchows Arch. 2018, 472, 469–476. [Google Scholar] [CrossRef]
- Donati, M.; Kastnerova, L.; Ptakova, N.; Michal, M.; Kazakov, D.V. Polypoid Atypical Spitz Tumor with a Fibrosclerotic Stroma, CLIP2-BRAF Fusion, and Homozygous Loss of 9p21. Am. J. Dermatopathol. 2020, 42, 204–207. [Google Scholar] [CrossRef]
- Quan, V.L.; Zhang, B.; Zhang, Y.; Mohan, L.S.; Shi, K.; Wagner, A.; Kruse, L.; Taxter, T.; Beaubier, N.; White, K.; et al. Integrating Next-Generation Sequencing with Morphology Improves Prognostic and Biologic Classification of Spitz Neoplasms. J. Investig. Dermatol. 2020, 140, 1599–1608. [Google Scholar] [CrossRef]
- Houlier, A.; Pissaloux, D.; Masse, I.; Tirode, F.; Karanian, M.; Pincus, L.B.; McCalmont, T.H.; LeBoit, P.E.; Bastian, B.C.; Yeh, I.; et al. Melanocytic tumors with MAP3K8 fusions: Report of 33 cases with morphological-genetic correlations. Mod. Pathol. 2020, 33, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Quan, V.L.; Zhang, B.; Mohan, L.S.; Shi, K.; Isales, M.C.; Panah, E.; Taxter, T.J.; Beaubier, N.; White, K.; Gerami, P. Activating Structural Alterations in MAPK Genes Are Distinct Genetic Drivers in a Unique Subgroup of Spitzoid Neoplasms. Am. J. Surg. Pathol. 2019, 43, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.; Fan, L.; Pribnow, A.; Silkov, A.; Rice, S.V.; Lee, S.; Shao, Y.; Shaner, B.; Mulder, H.; Nakitandwe, J.; et al. Clinical genome sequencing uncovers potentially targetable truncations and fusions of MAP3K8 in spitzoid and other melanomas. Nat. Med. 2019, 25, 597–602. [Google Scholar] [CrossRef]
- Newman, S.; Pappo, A.; Raimondi, S.; Zhang, J.; Barnhill, R.; Bahrami, A. Pathologic Characteristics of Spitz Melanoma With MAP3K8 Fusion or Truncation in a Pediatric Cohort. Am. J. Surg. Pathol. 2019, 43, 1631–1637. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Kim, D.; Zhang, B.; Compres, E.V.; Khan, A.U.; Busam, K.J.; Gerami, P. Melanocytic Neoplasms with MAP2K1 in Frame Deletions and Spitz Morphology. Am. J. Dermatopathol. 2020, 42, 923–931. [Google Scholar] [CrossRef]
- Donati, M.; Nosek, D.; Waldenbäck, P.; Martinek, P.; Jonsson, B.A.; Galgonkova, P.; Hawawrehova, M.; Berouskova, P.; Kastnerova, L.; Persichetti, P.; et al. MAP2K1-Mutated Melanocytic Neoplasms With a SPARK-Like Morphology. Am. J. Dermatopathol. 2021, 43, 412–417. [Google Scholar] [CrossRef]
- Kerckhoffs, K.G.P.; Aallali, T.; Ambarus, C.A.; Sigurdsson, V.; Jansen, A.M.L.; Blokx, W.A.M. Expanding spectrum of “spitzoid” lesions: A small series of 4 cases with MAP2K1 mutations. Virchows Arch. 2021, 479, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; McCarthy, S.W.; Scolyer, R.A. Blue nevi and related lesions: A review highlighting atypical and newly described variants, distinguishing features and diagnostic pitfalls. Adv. Anat. Pathol. 2009, 16, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.A.; Ackerman, L.V. Cellular blue nevus: Clinicopathologic study of forty-five cases. Cancer 1968, 21, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Zembowicz, A. Blue Nevi and Related Tumors. Clin. Lab. Med. 2017, 37, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Seab, J.A., Jr.; Graham, J.H.; Helwig, E.B. Deep penetrating nevus. Am. J. Surg. Pathol. 1989, 13, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.; Morley-Quante, M.; Hempel, H.; McKee, P.H.; Calonje, E. Deep penetrating naevus: Clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology 2003, 43, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Luzar, B.; Calonje, E. Deep penetrating nevus: A review. Arch. Pathol. Lab. Med. 2011, 135, 321–326. [Google Scholar] [CrossRef]
- Mehregan, D.A.; Mehregan, A.H. Deep penetrating nevus. Arch. Dermatol. 1993, 129, 328–331. [Google Scholar] [CrossRef]
- Zembowicz, A.; Carney, J.A.; Mihm, M.C. Pigmented epithelioid melanocytoma: A low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am. J. Surg. Pathol. 2004, 28, 31–40. [Google Scholar] [CrossRef]
- Cohen, J.N.; Joseph, N.M.; North, J.P.; Onodera, C.; Zembowicz, A.; LeBoit, P.E. Genomic Analysis of Pigmented Epithelioid Melanocytomas Reveals Recurrent Alterations in PRKAR1A, and PRKCA Genes. Am. J. Surg. Pathol. 2017, 41, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Lee, S.; Wu, G.; Kerstetter, J.; Rahvar, M.; Li, X.; Easton, J.; Zhang, J.; Barnhill, R.L. Pigment-Synthesizing Melanocytic Neoplasm With Protein Kinase C Alpha (PRKCA) Fusion. JAMA Dermatol. 2016, 152, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Isales, M.C.; Mohan, L.S.; Quan, V.L.; Garfield, E.M.; Zhang, B.; Shi, K.; Arva, N.; Beaubier, N.; Yazdan, P.; White, K.; et al. Distinct Genomic Patterns in Pigmented Epithelioid Melanocytoma: A Molecular and Histologic Analysis of 16 Cases. Am. J. Surg. Pathol. 2019, 43, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.N.; Yeh, I.; Mully, T.W.; LeBoit, P.E.; McCalmont, T.H. Genomic and Clinicopathologic Characteristics of PRKAR1A-inactivated Melanomas: Toward Genetic Distinctions of Animal-type Melanoma/Pigment Synthesizing Melanoma. Am. J. Surg. Pathol. 2020, 44, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Benton, S.; Zhao, J.; Asadbeigi, S.; Kim, D.; Zhang, B.; Gerami, P. Pigmented Epithelioid Melanocytoma: Morphology and Molecular Drivers. Surg. Pathol. Clin. 2021, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.A.; Shah, N.; Danziger, N.; Montesion, M.; Sokol, E.S.; Pavlick, D.C.; Miller, V.A.; Ross, J.S.; Elvin, J.A.; Tse, J.Y. Clinical, histopathologic, and molecular profiles of PRKAR1A-inactivated melanocytic neoplasms. J. Am. Acad. Dermatol. 2021, 84, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.; Bahrami, A.; Bastian, B.C.; Busam, K.J.; Cerroni, L.; de la Fouchardiere, A.; Elder, D.E.; Gerami, P.; Lazova, R.; Schmidt, B.; et al. 2.-8.A. Malignant Spitz tumour (Spitz melanoma). In WHO Classification of Skin Tumours; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; World Health Organization: Geneva, Switzerland, 2018; Volume 4, pp. 108–110. [Google Scholar]
- Fernández-Medarde, A.; Santos, E. Ras in cancer and developmental diseases. Genes. Cancer 2011, 2, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Wang, D.; Saba, N.F.; Chen, Z.G. A Historic Perspective and Overview of H-Ras Structure, Oncogenicity, and Targeting. Mol. Cancer Ther. 2020, 19, 999–1007. [Google Scholar] [CrossRef]
- van Dijk, M.C.; Bernsen, M.R.; Ruiter, D.J. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am. J. Surg. Pathol. 2005, 29, 1145–1151. [Google Scholar] [CrossRef]
- Lezcano, C.M.; Yeh, I.; Eslamdoost, N.; Fang, Y.; LeBoit, P.E.; McCalmont, T.H.; Moy, A.P.; Zhang, Y.; Busam, K.J. Expanding the Spectrum of Microscopic and Cytogenetic Findings Associated With Spitz Tumors With 11p Gains. Am. J. Surg. Pathol. 2021, 45, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Porubsky, C.; Teer, J.K.; Zhang, Y.; Deschaine, M.; Sondak, V.K.; Messina, J.L. Genomic analysis of a case of agminated Spitz nevi and congenital-pattern nevi arising in extensive nevus spilus. J. Cutan. Pathol. 2018, 45, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Sarin, K.Y.; Sun, B.K.; Bangs, C.D.; Cherry, A.; Swetter, S.M.; Kim, J.; Khavari, P.A. Activating HRAS mutation in agminated Spitz nevi arising in a nevus spilus. JAMA Dermatol. 2013, 149, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Barreca, A.; Lasorsa, E.; Riera, L.; Machiorlatti, R.; Piva, R.; Ponzoni, M.; Kwee, I.; Bertoni, F.; Piccaluga, P.P.; Pileri, S.A.; et al. European T-Cell Lymphoma Study Group. Anaplastic lymphoma kinase in human cancer. J. Mol. Endocrinol. 2011, 47, R11–R23. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, B.; Palmer, R.H. The role of the ALK receptor in cancer biology. Ann. Oncol. 2016, 27 (Suppl. 3), iii4–iii15. [Google Scholar] [CrossRef] [PubMed]
- Zamo, A.; Chiarle, R.; Piva, R.; Howes, J.; Fan, Y.; Chilosi, M.; Levy, D.E.; Inghirami, G. Anaplastic lymphoma kinase (ALK) activates Stat3 and protects hematopoietic cells from cell death. Oncogene 2002, 21, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; de la Fouchardiere, A.; Pissaloux, D.; Mully, T.W.; Garrido, M.C.; Vemula, S.S.; Busam, K.J.; LeBoit, P.E.; McCalmont, T.H.; Bastian, B.C. Clinical, histopathologic, and genomic features of Spitz tumors with ALK fusions. Am. J. Surg. Pathol. 2015, 39, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Kastnerova, L.; Martinek, P.; Grossmann, P.; Steiner, P.; Vanecek, T.; Kyclova, J.; Ferak, I.; Zalud, R.; Slehobr, O.; Svajdler, P.; et al. A Clinicopathological Study of 29 Spitzoid Melanocytic Lesions With ALK Fusions, Including Novel Fusion Variants, Accompanied by Fluorescence In Situ Hybridization Analysis for Chromosomal Copy Number Changes, and Both TERT Promoter and Next-Generation Sequencing Mutation Analysis. Am. J. Dermatopathol. 2020, 42, 578–592. [Google Scholar] [CrossRef]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef]
- Donati, M.; Kastnerova, L.; Martinek, P.; Grossmann, P.; Sticová, E.; Hadravský, L.; Torday, T.; Kyclova, J.; Michal, M.; Kazakov, D.V. Spitz Tumors with ROS1 Fusions: A Clinicopathological Study of 6 Cases, Including FISH for Chromosomal Copy Number Alterations and Mutation Analysis Using Next-Generation Sequencing. Am. J. Dermatopathol. 2020, 42, 92–102. [Google Scholar] [CrossRef]
- Rubin, J.B.; Segal, R.A. Growth, survival and migration: The Trk to cancer. Cancer Treat. Res. 2003, 115, 1–18. [Google Scholar] [CrossRef]
- Wiesner, T.; Kutzner, H.; Cerroni, L.; Mihm MCJr Busam, K.J.; Murali, R. Genomic aberrations in spitzoid melanocytic tumours and their implications for diagnosis, prognosis and therapy. Pathology. 2016, 48, 113–131. [Google Scholar] [CrossRef]
- Wang, L.; Busam, K.J.; Benayed, R.; Cimera, R.; Wang, J.; Denley, R.; Rao, M.; Aryeequaye, R.; Mullaney, K.; Cao, L.; et al. Identification of NTRK3 Fusions in Childhood Melanocytic Neoplasms. J. Mol. Diagn. 2017, 19, 387–396. [Google Scholar] [CrossRef]
- Goto, K.; Pissaloux, D.; Tirode, F.; de la Fouchardière, A. Spitz nevus with a novel TFG-NTRK2 fusion: The first case report of NTRK2-rearranged Spitz/Reed nevus. J. Cutan. Pathol. 2021, 48, 1193–1196. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Tee, M.K.; Botton, T.; Shain, A.H.; Sparatta, A.J.; Gagnon, A.; Vemula, S.S.; Garrido, M.C.; Nakamaru, K.; Isoyama, T.; et al. NTRK3 kinase fusions in Spitz tumours. J. Pathol. 2016, 240, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cappellesso, R.; Nozzoli, F.; Zito Marino, F.; Simi, S.; Castiglione, F.; De Giorgi, V.; Cota, C.; Senetta, R.; Scognamiglio, G.; Anniciello, A.M.; et al. NTRK Gene Fusion Detection in Atypical Spitz Tumors. Int. J. Mol. Sci. 2021, 22, 12332. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.P.; Fletcher, C.D.M.; Hornick, J.L. Evaluation of pan-TRK immunohistochemistry in infantile fibrosarcoma, lipofibromatosis-like neural tumour and histological mimics. Histopathology 2018, 73, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Sierra, J.R.; Tsao, M.S. c-MET as a potential therapeutic target and biomarker in cancer. Ther. Adv. Med. Oncol. 2011, 3 (Suppl. 1), S21–S35. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sholl, L.M.; Zhang, B.; Merkel, E.A.; Amin, S.M.; Guitart, J.; Gerami, P. Atypical Spitzoid Neoplasms in Childhood: A Molecular and Outcome Study. Am. J. Dermatopathol. 2017, 39, 181–186. [Google Scholar] [CrossRef]
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Rev. Cancer 2014, 14, 173–186. [Google Scholar] [CrossRef]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167. [Google Scholar] [CrossRef]
- Wu, G.; Barnhill, R.L.; Lee, S.; Li, Y.; Shao, Y.; Easton, J.; Dalton, J.; Zhang, J.; Pappo, A.; Bahrami, A. The landscape of fusion transcripts in spitzoid melanoma and biologically indeterminate spitzoid tumors by RNA sequencing. Mod. Pathol. 2016, 29, 359–369. [Google Scholar] [CrossRef]
- Zarabi, S.K.; Azzato, E.M.; Tu, Z.J.; Ni, Y.; Billings, S.D.; Arbesman, J.; Funchain, P.; Gastman, B.; Farkas, D.H.; Ko, J.S. Targeted next generation sequencing (NGS) to classify melanocytic neoplasms. J. Cutan. Pathol. 2020, 47, 691–704. [Google Scholar] [CrossRef]
- Jarkowski A 3rd Khushalani, N.I. BRAF and beyond: Tailoring strategies for the individual melanoma patient. J. Carcinog. 2014, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Dankner, M.; Rose, A.A.N.; Rajkumar, S.; Siegel, P.M.; Watson, I.R. Classifying BRAF alterations in cancer: New rational therapeutic strategies for actionable mutations. Oncogene 2018, 37, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.E.; Lipson, D.; Stephens, P.J.; Otto, G.; Lehmann, B.D.; Lyle, P.L.; Vnencak-Jones, C.L.; Ross, J.S.; Pietenpol, J.A.; Sosman, J.A.; et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin. Cancer Res. 2013, 19, 6696–6702. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Matsumoto, M.L.; McKenzie, B.S.; Zarrin, A.A. TPL2 kinase action and control of inflammation. Pharmacol. Res. 2018, 129, 188–193. [Google Scholar] [CrossRef]
- Salmeron, A.; Ahmad, T.B.; Carlile, G.W.; Pappin, D.; Narsimhan, R.P.; Ley, S.C. Activation of MEK-1 and SEK-1 by Tpl-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996, 15, 817–826. [Google Scholar] [CrossRef]
- Hagemann, D.; Troppmair, J.; Rapp, U.R. Cot protooncoprotein activates the dual specificity kinases MEK-1 and SEK-1 and induces differentiation of PC12 cells. Oncogene 1999, 18, 1391–1400. [Google Scholar] [CrossRef]
- Ceci, J.D.; Patriotis, C.P.; Tsatsanis, C.; Makris, A.M.; Kovatch, R.; Swing, D.A.; Jenkins, N.A.; Tsichlis, P.N.; Copeland, N.G. Tpl-2 is an oncogenic kinase that is activated by carboxy-terminal truncation. Genes. Dev. 1997, 11, 688–700. [Google Scholar] [CrossRef]
- Gándara, M.L.; López, P.; Hernando, R.; Castaño, J.G.; Alemany, S. The COOH-terminal domain of wild-type Cot regulates its stability and kinase specific activity. Mol. Cell. Biol. 2003, 23, 7377–7390. [Google Scholar] [CrossRef]
- Bromberg-White, J.L.; Andersen, N.J.; Duesbery, N.S. MEK genomics in development and disease. Brief. Funct. Genom. 2012, 11, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Möller, I.; Murali, R.; Müller, H.; Wiesner, T.; Jackett, L.A.; Scholz, S.L.; Cosgarea, I.; van de Nes, J.A.; Sucker, A.; Hillen, U.; et al. Activating cysteinyl leukotriene receptor 2 (CYSLTR2) mutations in blue nevi. Mod. Pathol. 2017, 30, 350–356. [Google Scholar] [CrossRef]
- Goto, K.; Pissaloux, D.; Paindavoine, S.; Tirode, F.; de la Fouchardière, A. CYSLTR2-mutant Cutaneous Melanocytic Neoplasms Frequently Simulate “Pigmented Epithelioid Melanocytoma”, Expanding the Morphologic Spectrum of Blue Tumors: A Clinicopathologic Study of 7 Cases. Am. J. Surg. Pathol. 2019, 43, 1368–1376. [Google Scholar] [CrossRef]
- Urtatiz, O.; Van Raamsdonk, C.D. Gnaq and Gna11 in the Endothelin Signaling Pathway and Melanoma. Front. Genet. 2016, 7, 59. [Google Scholar] [CrossRef]
- O’Hayre, M.; Vázquez-Prado, J.; Kufareva, I.; Stawiski, E.W.; Handel, T.M.; Seshagiri, S.; Gutkind, J.S. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat. Rev. Cancer 2013, 13, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, E.; Zaremba, A.; Horn, S.; Ugurel, S.; Casalini, B.; Schlaak, M.; Hassel, J.C.; Herbst, R.; Utikal, J.S.; Weide, B.; et al. GNAQ and GNA11 mutant nonuveal melanoma: A subtype distinct from both cutaneous and uveal melanoma. Br. J. Dermatol. 2020, 183, 928–939. [Google Scholar] [CrossRef]
- Zembowicz, A.; Mihm, M.C. Dermal dendritic melanocytic proliferations: An update. Histopathology 2004, 45, 433–451. [Google Scholar] [CrossRef]
- Argenziano, G.; Zalaudek, I.; Ferrara, G.; Hofmann-Wellenhof, R.; Soyer, H.P. Proposal of a new classification system for melanocytic naevi. Br. J. Dermatol. 2007, 157, 217–227. [Google Scholar] [CrossRef]
- Temple-Camp, C.R.; Saxe, N.; King, H. Benign and malignant cellular blue nevus. A clinicopathological study of 30 cases. Am. J. Dermatopathol. 1988, 10, 289–296. [Google Scholar] [CrossRef]
- Yeh, I.; Lang, U.E.; Durieux, E.; Tee, M.K.; Jorapur, A.; Shain, A.H.; Haddad, V.; Pissaloux, D.; Chen, X.; Cerroni, L.; et al. Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi. Nat. Commun. 2017, 8, 644. [Google Scholar] [CrossRef]
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent Advances in Targeting CD8 T-Cell Immunity for More Effective Cancer Immunotherapy. Front. Immunol. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000, 11, 273–282. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- de la Fouchardière, A.; Caillot, C.; Jacquemus, J.; Durieux, E.; Houlier, A.; Haddad, V.; Pissaloux, D. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019, 474, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Berthon, A.S.; Szarek, E.; Stratakis, C.A. PRKACA: The catalytic subunit of protein kinase A and adrenocortical tumors. Front. Cell Dev. Biol. 2015, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Michie, A.M.; Nakagawa, R. The link between PKCalpha regulation and cellular transformation. Immunol. Lett. 2005, 96, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S. Protein kinase C alpha (PKC alpha): Regulation and biological function. J. Biochem. 2002, 132, 669–675. [Google Scholar] [CrossRef] [PubMed]
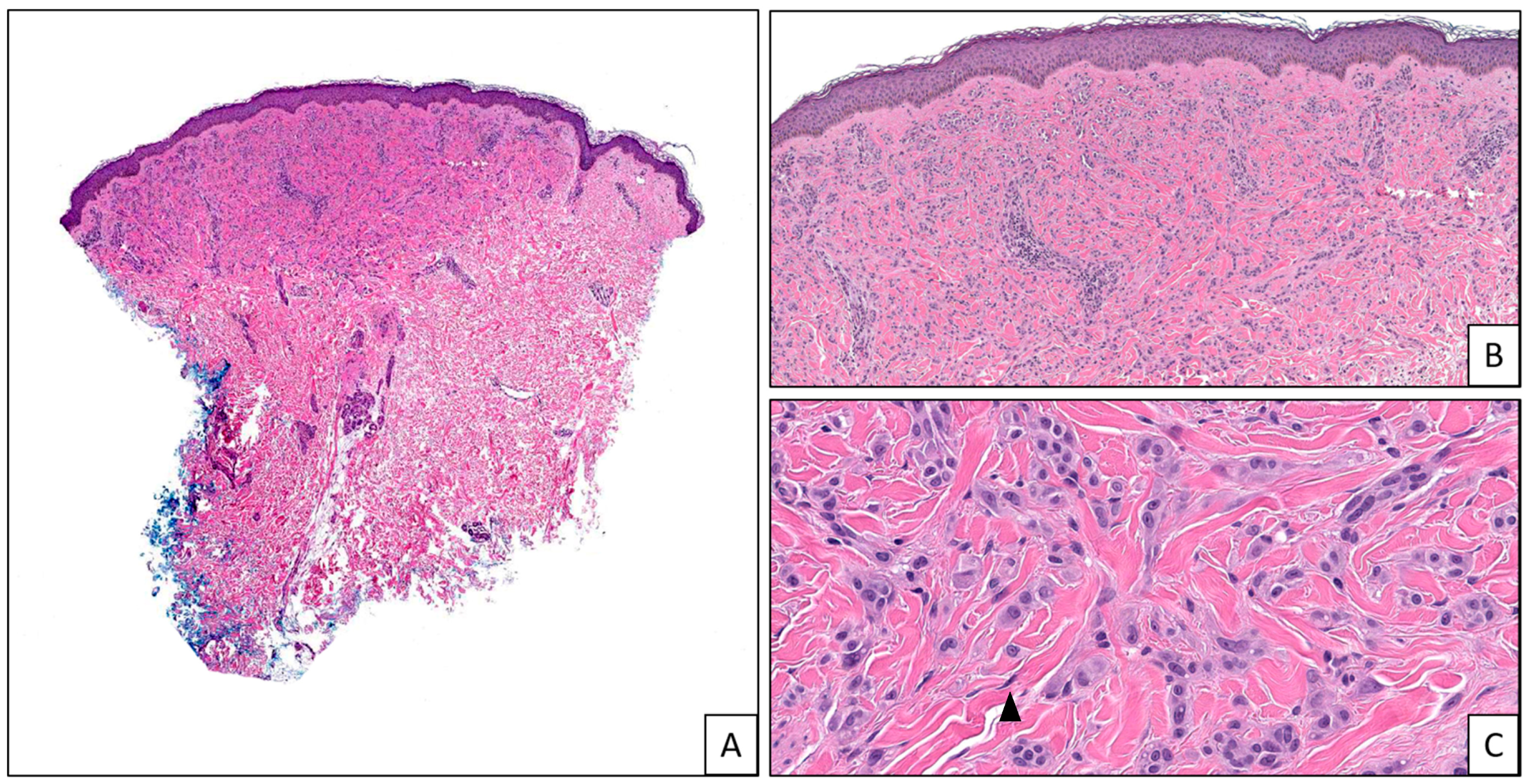
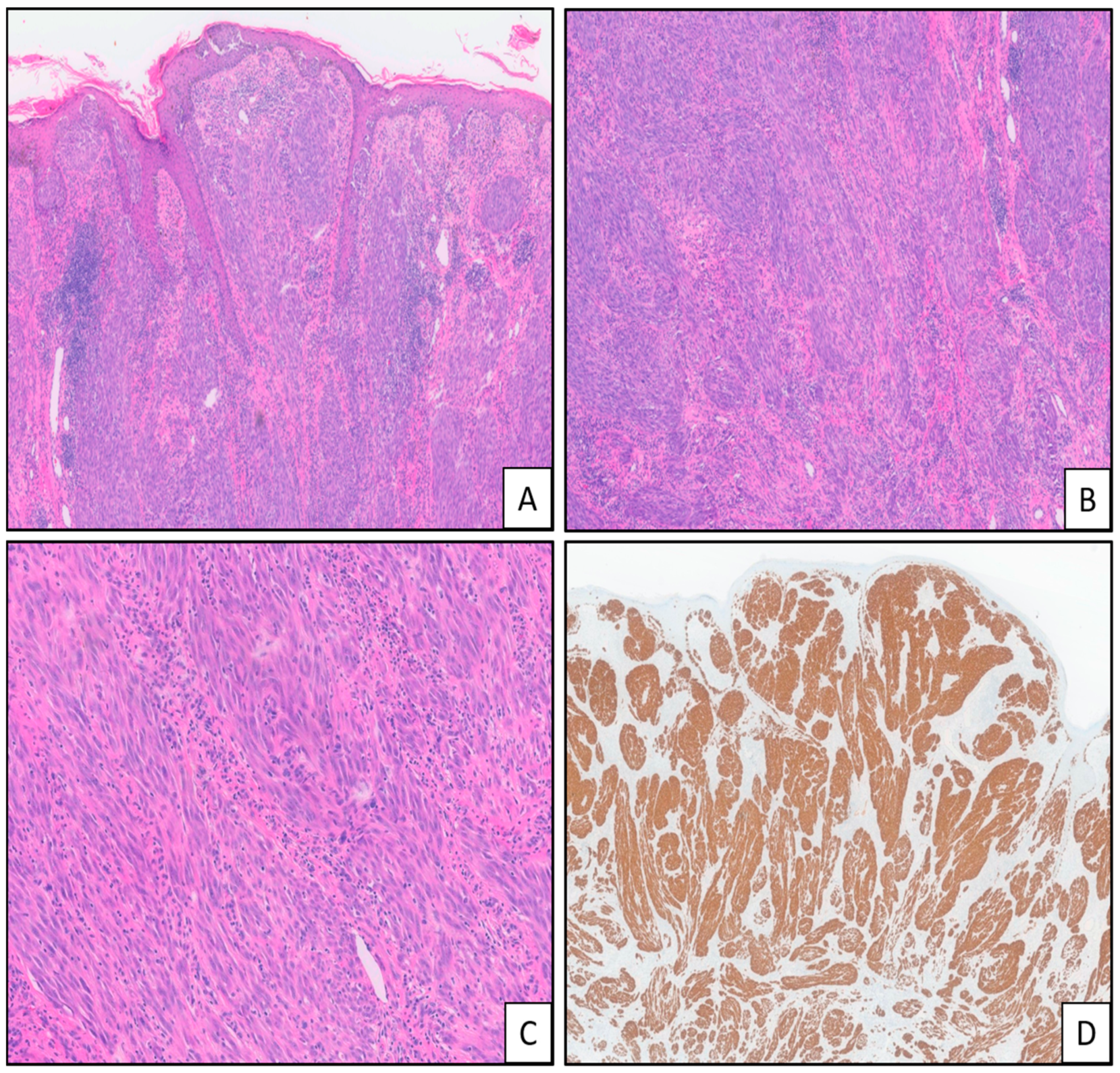
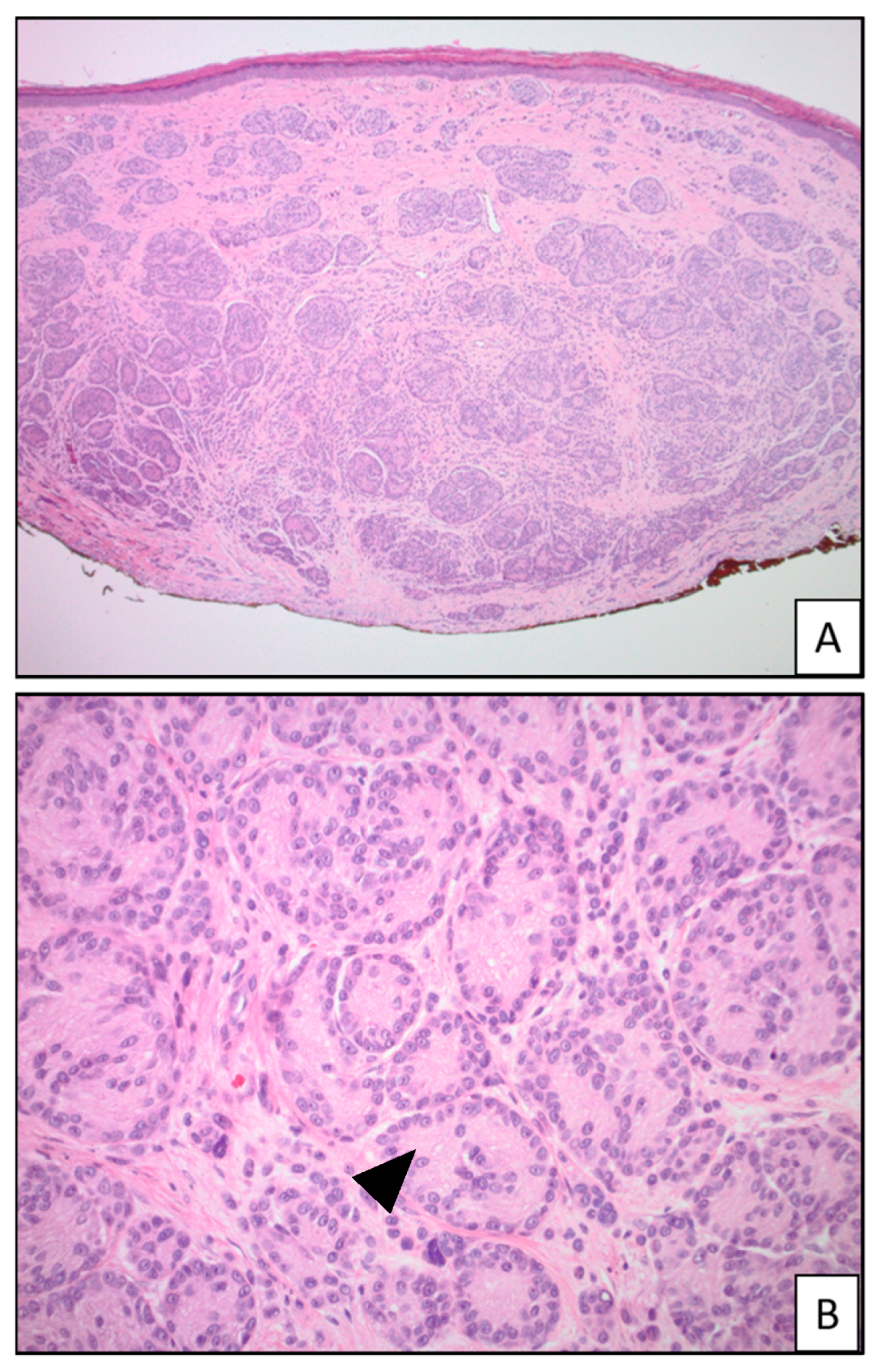
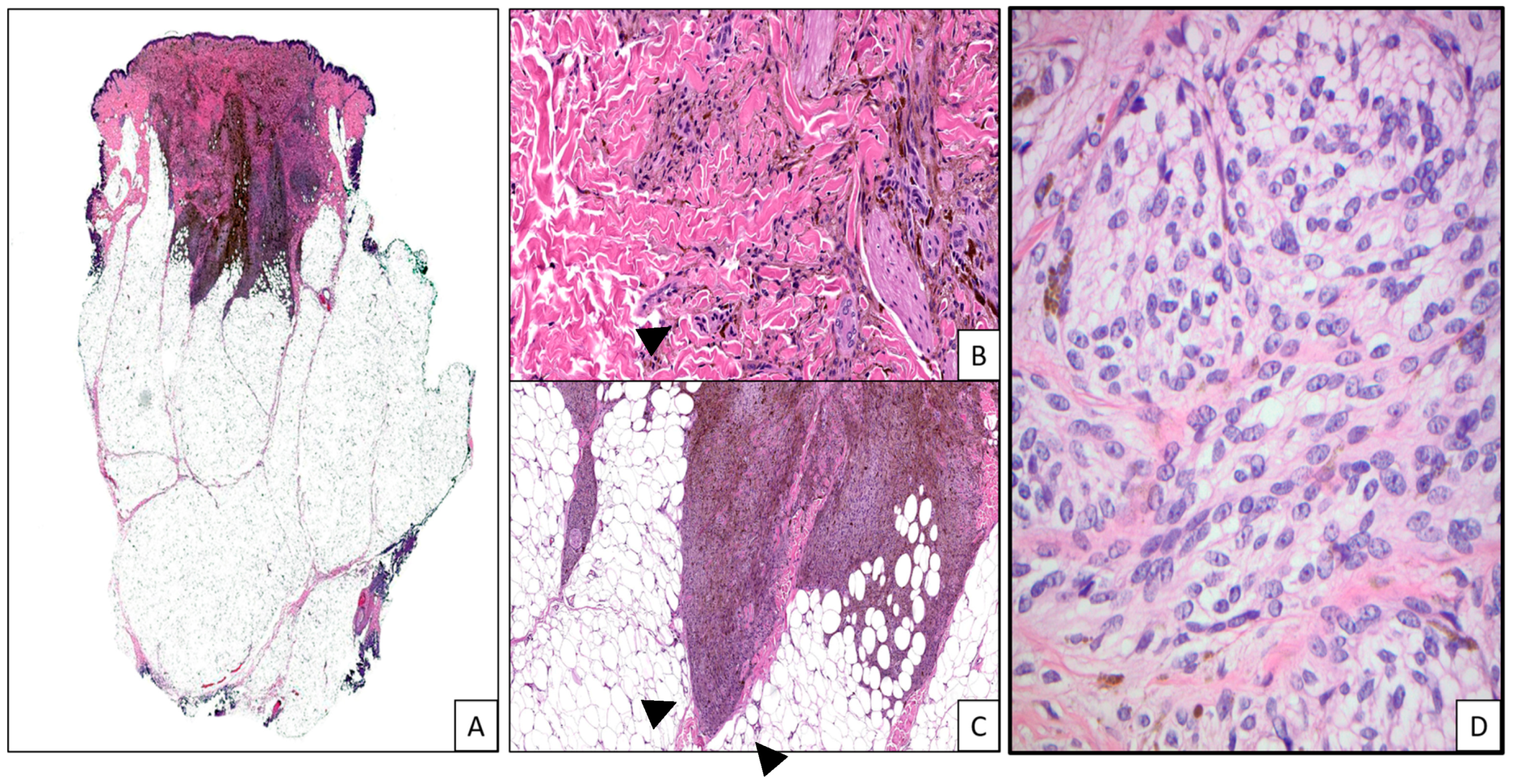


| Tumor Type | Genomic Alteration | Histologic Findings | Molecular Testing |
|---|---|---|---|
| Spitz tumors | HRAS mutations and/or 11p amplifications | Predominantly intradermal with desmoplastic stroma; large melanocytes [1,2] | NGS, FISH, SNP microarray, CGH [3] |
| ALK fusions | Plexiform architecture; non-pigmented melanocytes with pericellular clefts; can show cytologic atypia and increased mitotic activity [4,5,6,7] | NGS, FISH [3] | |
| ROS1 fusions | Prominent junctional melanocytic nests with trans-epidermal elimination and adnexal involvement; sometimes angiomatoid Spitz; can show cytologic atypia [8] | NGS, FISH [3] | |
| NTRK1 fusions | Thin elongated rete ridges; frequent Kamino bodies; rosette-like structures; extreme maturation; often associated with a lymphocytic infiltrate [4,6,9,10] | NGS, FISH [3] | |
| NTRK3::MYO5A fusions | Spindled melanocytes; fascicular to plexiform growth pattern; palisading and rosette-like structures [11] | NGS, FISH [3] | |
| NTRK3::ETV6 fusions | Epithelioid melanocytes with abundant eosinophilic cytoplasm and distinct cell borders; pleomorphic nuclei; large coalescing nests [7,11] | NGS, FISH [3] | |
| NTRK3::MYH9 fusions | Large epithelioid melanocytes; syncytial growth with central desmoplastic stroma and peripheral collagen trapping [11] | NGS, FISH [3] | |
| MET fusions | Large nests of intermediate to large epithelioid to spindled melanocytes; pericellular clefts; epidermal hyperplasia [12] | NGS, FISH [3] | |
| RET fusions | Large nests of small to intermediate-sized, monotonous epithelioid, discohesive melanocytes [13,14] | NGS, FISH [3] | |
| BRAF fusions | Superficial cellular sheet-like dermal component; less cellular base with prominent desmoplastic reaction; no maturation; large epithelioid melanocytes; can show severe cytologic atypia; increased mitotic figures [4,9,15,16,17,18] | NGS, FISH [3] | |
| MAP3K8 fusions and mutations | Asymmetric, nodular, or dome-shaped; epidermal hyperplasia; nested junctional component; no maturation; epithelioid melanocytes with large uniform nuclei and prominent nucleoli; multinucleated giant melanocytes; can show severe cytologic atypia; increased mitotic figures; ulceration [7,9,19,20,21,22,23] | NGS, FISH [3] | |
| MAP2K1 mutations | Wedge-shaped; overlapping features with deep penetrating and dysplastic nevi; plexiform growth pattern; tendency to converge around the adnexal structures and neurovascular bundles; melanin pigment accumulation in stroma and melanophages; poor maturation; large epithelioid melanocytes; can show severe cytologic atypia [21,24,25,26] | NGS [3] | |
| Blue nevi | GNAQ, GNA11 or CYSLTR2 mutations | Intradermal; spindled dendritic pigmented melanocytes and melanophages; sclerotic stroma; a cellular variant with distinct cellular fascicles and nests of plump spindled to oval, clear, or finely pigmented melanocytes; no maturation [27,28,29] | NGS [3] |
| Melanocytomas of the low cumulative sun damage (CSD) pathway: Deep penetrating melanocytoma | CTNNB1, APC, MAP2K1, BRAF mutations | Wedge-shaped; predominantly intradermal but can arise in a pre-existing nevus; can extend to the deep dermis and subcutis; fascicular and/or nested growth pattern; heavily pigmented; plump epithelioid to spindled cells; no maturation; can show cytologic atypia; occasional mitotic figures [30,31,32,33] | NGS [3] |
| Melanocytomas of the low cumulative sun damage (CSD) pathway: Pigmented epithelioid melanocytoma | PRKAR1A mutations or PRKCA fusions | Nodular or wedge-shaped; epidermal hyperplasia; predominantly intradermal; nested or solid growth pattern; heavily pigmented large multinucleated and small epithelioid, spindled, and dendritic melanocytes and melanophages; no maturation [34,35,36,37,38,39,40] | NGS, FISH [3] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saade, R.; Al-Rohil, R.N. Genomic Alterations in Melanocytic Tumors: A Review of Spitz Tumors, Blue Nevi, Deep Penetrating Melanocytomas and Pigmented Epithelioid Melanocytomas. Appl. Sci. 2024, 14, 1863. https://doi.org/10.3390/app14051863
Saade R, Al-Rohil RN. Genomic Alterations in Melanocytic Tumors: A Review of Spitz Tumors, Blue Nevi, Deep Penetrating Melanocytomas and Pigmented Epithelioid Melanocytomas. Applied Sciences. 2024; 14(5):1863. https://doi.org/10.3390/app14051863
Chicago/Turabian StyleSaade, Rayan, and Rami N. Al-Rohil. 2024. "Genomic Alterations in Melanocytic Tumors: A Review of Spitz Tumors, Blue Nevi, Deep Penetrating Melanocytomas and Pigmented Epithelioid Melanocytomas" Applied Sciences 14, no. 5: 1863. https://doi.org/10.3390/app14051863
APA StyleSaade, R., & Al-Rohil, R. N. (2024). Genomic Alterations in Melanocytic Tumors: A Review of Spitz Tumors, Blue Nevi, Deep Penetrating Melanocytomas and Pigmented Epithelioid Melanocytomas. Applied Sciences, 14(5), 1863. https://doi.org/10.3390/app14051863







