Abstract
(1) Background: Foodborne illnesses are recognized as a significant threat to public health and the economy in both developed and developing nations. The safety of foods containing microorganisms has consequently become a major worldwide concern. One of the most frequent causes of food deterioration in the world is microbial contamination. (2) Methods: Yogurt containers that were bought commercially in Riyadh, Saudi Arabia during their validity period contained four different species of bacteria and one type of fungus. Using nutritional agar and Czapek-Dox agar medium, the bacteria and fungi were isolated. The isolates of the fungi and bacteria were identified using a scanning microscope. The isolates were further identified and classified for molecular evolutionary analyses using the 16S rRNA and ITS sequences from the bacteria and fungi, respectively, in conjunction with the universal primers 27F, 1492R, ITS1F, and ITS4R. (3) Results: A total of 131 separate strains were identified from 12 yogurt samples based on their phenotypic characteristics. In total, 79 isolates (60.3%) consisted of Serratia marcescens, Bacillus subtilis, and Mucor circinelloides, with 52 isolates (39.7%) being Bacillus cereus. While the cells of Bacillus and Serratia are shaped like rods, the sporangia of Mucor are large, round, and black. Each strain was identified by its accession number, which were as follows: MK590996.1: B. cereus; MK591144.1: B. subtilis; MK591002.1 and MK591014.1: S. marcescens; and MK559692.1: M. circinelloides. The maximum identification was found to be between 98.64 and 100% when BLAST was used to compare the sequences to the NCBI GeneBank database. (4) Conclusions: Genus and species identification was performed using the similarity score values. Yogurt products containing high concentrations (39.7%) of Bacillus cereus isolates carry a significant risk of health hazards due to the potential for spreading pathogenic bacteria to humans.
1. Introduction
Foodborne diseases have been known to be a major public health and economic risk in both rich and poor countries. As a result, the safety of microbial foods has emerged as a major global concern. Microbial contamination is among the most common causes of food degradation worldwide [1]. Food contamination by bacteria can happen at any time as in the industry. Contamination refers to any change that produces unsafe food. During the preservation of most dairy products, various factors might affect their microbiological safety and quality [2]. Between the final manufacturing phase and their use, ready-to-eat foods are not modified [3].
Milk and dairy products are vital components of human nutrition and play a significant role in many people’s diets around the world because they include protein, vitamins, and minerals that are essential for the health of people of all ages and genders [1]. Dairy products preserve milk’s nutritional content while also making it more attractive to consumers [4]. Fresh milk’s microbiome is complex, and it changes depending on a variety of factors, including cleanliness, seasonality, animals, and others [5]. Denaturation happens when microbes break down milk’s carbohydrates, proteins, and lipids, producing a harmful output [4]. Milk produced from healthy cows can be considered bacteria-free, but agricultural and dairy environments can be sources of contamination, especially during milking and in cheese production. In particular, B. cereus was previously known to be responsible for the spoilage of raw milk [6]. Moreover, a brief risk assessment of B. cereus in the Netherlands predicted that almost 7% of pasteurized milk is characterized by an amount of this pathogen greater than 5 log cfu∙mL−1 [7]. Although vegetative B. cereus cells cannot survive pasteurization, their spores are resistant to heat treatments, highlighting their viability in pasteurized milk. Bacillus cereus has also been found in some dairy products, with their frequency varying from 2 to 52%, depending on the type [8,9].
Bacteria that cause disease, like Escherichia coli, Escherichia faecalis, and Bacillus cereus, can grow in yogurt. These bacteria can enter milk and its derivatives through certain internal and external factors found during the processing of dairy products, such as poor line hygiene. Contaminated equipment in manufacturing, the air flow in production rooms, and the personal hygiene of workers are not guaranteed [10]. Industrial processes have improved the quality, storage, transportation, and marketing of products. Microbiological parameters, especially coliform counts in general, Escherichia coli and Enterococcus bacteria, are commonly used to verify these conditions [11]. Acidity and temperature standards are also followed to assess the yogurt’s ability to preserve its state. Statistics show variations from 0.6 to 1.5 g of lactic acid per 100 g of product, and storage temperatures in dairy markets and industries should not be higher than 100 °C [12].
The presence of foodborne pathogens and microorganisms in yogurt, which include bacteria and fungi, is determined not only by the animal’s health, but also via the conditions of its manufacture, storage facilities, and the technologies used [13]. Spore-forming bacteria are a special problem that can penetrate milk production steps because they have the potential to resist abiotic stresses in the spore phase [14,15]. All the same, De Jonghe [2] discovered that Bacillus cereus was not involved in food contamination, while cellular tests confirmed the creation and efficiency of Bacillus circulans, Bacillus lentus, and Bacillus subtilis at high temperatures [16,17]. B. cereus is found in milk products with varying levels of pollution in Egypt and India [18,19]. Serratia spp. can build colonies on abiotic surfaces [20] and create heat-resistant enzymes, allowing them to degrade milk at various stages of production [21,22]. It is really difficult to prevent microbial infections of milk during the preparation of different dairy foods; consequently, the microbiological content of foods is an important factor in establishing their acceptability from a safety standpoint [23]. As a result, it is recommended that pathogens in milk and dairy products be detected using microbiological, immunological, and molecular approaches to achieve human food safety.
Despite advancements in current preparations, fungal spoiling remains a problem for dairy producers, and novel (bio) conservation strategies, such as the utilization of protective cultures, have been developed recently [24]. Types of food might be physically, chemically, or microbially damaged when it comes to their expiration. The principal agents of microbiological degradation include parasites, bacteria, and/or fungi [24]. Due to the diversity of fungus species, food processors suffer significant damage as a result of fungal deterioration. Furthermore, fungal degradation is thought to account for between 5% and 10% of global food output [25,26]. Fungal infections of dairy products can happen at any time throughout the supply chain, from dairy farms to dairy production sites to customers’ homes. Rhizopus is the only species in which mucoralean fungi that cause mucormycosis occur more frequently than in Mucor species [27,28]. M. circinelloides was linked to a nutrition disease outbreak following the consumption of infected yogurt, according to a US FDA investigation. Snyder et al. [29] reported that storage temperature and natamycin dose interact to cause M. circinelloides degradation.
Yogurt is among the world’s most popular milk products [30]. Even though yeasts and molds are the major pollutants in yogurt, bacterial infection by spore-forming resistant bacteria during commercial thermal processing can cause its value to decline [31]. The aim of our study is to isolate and identify the bacterial and fungal strains in fresh yogurts in Saudi Arabia by using SEM, 16S rRNA, and ITS.
2. Materials and Methods
2.1. Bacterial and Fungal Isolation
Twelve samples of the same brand of yogurt commercially purchased within their validity period were gathered from Riyadh supermarket in Saudi Arabia and sent to a lab for analysis. For microbiological isolation, the direct inoculation method was chosen. Fungi were isolated using potato dextrose agar, Sabouraud dextrose agar, and Czapek-Dox agar (Oxoid Ltd, Basingstoke, England, UK), all of which were supplemented with chloramphenicol. Nutrient agar (Oxoid, UK) was utilized for bacterial isolation. Two types of culture methods were used: (1) the dilution plate method and (2) the plate method [32]. Before being examined, the dishes were cultured at 28 °C for seven days for fungi and three days for bacteria. Following incubation, each plate was examined again to obtain purified fungus cultures through serial inoculation.
The fungal morphology was examined macroscopically by observing colony features (color, shape, size, and hyphae) and microscopically by a compound microscope equipped with a digital camera and a lactophenol cotton blue-stained (LCB) slide mounted with a small portion of the mycelium. Gram staining was used to examine bacteria macroscopically.
The samples were examined using SEM (scanning electron microscopy) (JEOL 7500FA JEOL, Peabody, MA, USA). The specimens were fixed for 6 h at 4 °C with 2.5% glutaraldehyde; dehydrated in a series of 25, 50, 75, and 100% ethanol (10 min each); and dehydrated in a centrifuge tube at 30 °C. After that, the samples were mounted on aluminum sticks, covered with gold, and observed using SEM at a 10 kV accelerating voltage to determine the samples’ color and shape.
2.2. DNA Extraction, PCR Amplification, and Purification
To further identify the bacterial and fungal isolates, ITS and 16S rRNA genes were amplified via polymerase chain reaction (PCR) testing and sequenced as follows: after being freshly grown, a little bit of each isolate was taken and dissolved in autoclaved distilled water in 2 mL sterile Eppendorf tubes, which were then heated for 15 min at 100 °C. Genomic DNA was extracted from fungal and bacterial isolates using the InstaGeneTM Matrix Genomic DNA Kit (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer’s instructions. These lifeless bacteria and fungi were transported to the SolGent Company in Daejeon, Republic of Korea for DNA isolation using SPINeasy DNA Kit for Tissue & Bacteria (MP Biomedicals Korea, Seoul, Republic of Korea)
To use isolated genomic DNA as a template, PCR amplification of bacterial 16S rRNA and fungal ITS from yogurt specimens was carried out. For bacteria, the primers were 27F and 1492R (5’AGAGTTTGATCMTGGCTCAG3’ and 5’TACGGYTACCTTGTTACGACTT3’, respectively), while for fungi, they were ITS1F and ITS4R (5’TCCGTAGGTGAACCTGCGG3’ and 5’TCCTCCGCTTATTGATATGC3’, respectively) [33]. The PCR reaction mixture was prepared using the following steps: 2 µL of 10x Taq PCR Buffer, 1.6 µL of 2.5 mM dNTP mixture, 1.0 µL of F and R primers (10 pmol/µL), 0.2 µL of KOMA Taq (2.5 U/µL), and 2 µL of DNA template (20 ng/µL) were combined, and using distilled water of HPLC grade, the reaction volume was adjusted to 20 µL. Under the following circumstances, amplification was performed in a thermal cycler: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 0.5 min, annealing at 55 °C for 2 min, and extension at 68 °C for 1.5 min. This was followed by a final polymerization extension at 68 °C for 10 min. Using 1% agarose gel electrophoresis, amplicon was verified. The Montage PCR Cleanup Kit (Millipore Sigma, Burlington, MA, USA) was used to purify the amplifiers.
2.3. DNA Sequencing
The amplification primers and BigDyeTM Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) were used to sequence the purified PCR products. Macrogen, Inc. used the 3730xl DNA Analyzer automated DNA sequencing system (Applied Biosystems) (Seoul, Republic of Korea) to sequence amplified products.
Sequence analysis: The obtained sequences were edited with Geneious Prime software Version 2020.1.2 [34]. Forward and reverse sequences were combined to form consensus sequences. The sequences were aligned using the National Center for Biotechnology Information’s (NCBI) Basic Local Alignment Search Tool (BLAST) with the GenBank database of available nucleotide sequences.
Phylogenetic analyses: The evolutionary history was inferred by using the maximum likelihood method and Tamura–Nei model [35]. The tree with the highest log likelihood (−5755.83) is shown. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura–Nei model and then selecting the topology with superior log likelihood value. This analysis involved 6 nucleotide sequences. Codon positions included were 1st + 2nd + 3rd + Noncoding. There were a total of 1106 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [36].
Data availability: This study’s data are presented here. The isolates’ ITS and 16S rDNA gene sequences have been deposited in the NCBI’s GenBank. The fungal strain has accession number MK559692.1, while the bacterial strains have numbers MK590996.1, MK591144.1, MK591002.1, and MK591014.1.
2.4. Statistical Analysis
The data were analyzed using mean ± standard deviation based on samples in PAST 3.2 program [37]. The outcomes were presented in the form of numbers and percentages.
3. Results
3.1. Phenotypic Characteristics of Bacterial and Fungal Isolates
Twelve (12) fresh commercial yogurt specimens were obtained from a Riyadh supermarket. The bacteria were isolated from the nutrient agar medium (NA) following 3 days of incubation at 28 °C. The fungi were recovered from the Czapek-Dox agar which had been cultivated for up to a week at 28 °C. The percentage of frequency of the isolates in the yogurt samples was as follows: B. cereus 52 (39.7%), M. circinelloides 30 (22.9%), B. subtilis 26 (19.8%), and S. marcescens 23 (17.6%) (Table 1). The p-value of the test is 0.01641, which is less than the significance level alpha = 0.05. We can conclude that the isolation number and mean are significantly correlated with a correlation coefficient of −0.9447834 and a p-value of 0.01641.

Table 1.
The above output displays the frequency and cumulative frequency of each (a) isolation number, and (b) mean of bacterial and fungal isolates.
The SEM images of the bacterial and fungal samples revealed that the investigated strains differed in size and shape (Figure 1; A–C). B. cereus, B. subtilis, and S. marcescens were Bacillaceae and Yersiniaceae family members with rod-shaped cells, with most of the cells arranged in pairs or chains. The isolates’ colonies had a convex form. Bacillus colonies were often whitish, while Serratia colonies were pale red. Cells could be found individually, in pairs, in chains, or in clusters. M. circinelloides belonged to the Mucoraceae family and had a morphology characterized by big black sporangia with a mean area of 100%, a 0.202 µm mean circular shape, and a mean solidity of 0.488 µm (Figure 1; D, and Table 2). There is a significant difference between the sample medians (p = 0.003305).
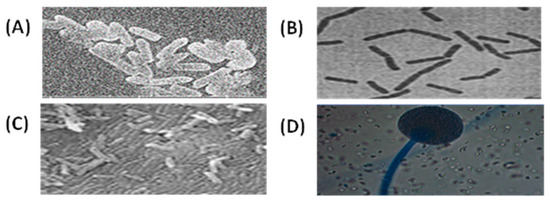
Figure 1.
SEM images for (A) Bacillus cereus, (B) Bacillus subtilis, (C) Serratia marcescens, and (D) Mucor circinelloides detected using SEM at a magnification of 10.00. The scale bar represents 500 μm.

Table 2.
The above output displays the frequency and cumulative frequency of each characteristic for Mucor circinelloides isolates.
3.2. Molecular Identification of Bacterial and Fungal Isolates
The isolation of genomic DNA was carried out to obtain pure DNA from the bacterial and fungal isolates. The extracted DNA molecules were used as templates for the PCR amplification of the 16S rRNA and ITS genes using the universal primers 27F, 1492R, ITS1F, and ITS4R, respectively, at an annealing temperature of 55 °C. The amplified products of the bacterial isolates were verified using 1.5% agarose gel stained with ethidium bromide, which revealed a fragment of 1088 bp for the B. cereus strain B-191, 1111 bp for B. subtilis, 1099 bp for the S. marcescens strain B-192, and 1106 bp for the S. marcescens strain B-193 when 16S rRNA was used. The M. circinelloides amplification was 600 bp when 18S rRNA was used as the ITS region. The sequence of isolates was submitted to the NCBI. The accession numbers for the bacterial sequence isolates were as follows: MK590996.1—B. cereus strain B-191, MK591144.1—B. subtilis strain B-195, MK591002.1—S. marcescens strain B-192, and MK591014.1—S. marcescens strain B-193 (Table 3). The accession numbers for fungal isolates were as follows: MK559692.1—M. circinelloides isolate (AUMC 10367), identified by internal transcribed spacer 1, partial sequence; the 5.8S ribosomal RNA gene and internal transcribed spacer 2, complete sequence; and the large subunit ribosomal RNA gene, partial sequence. Sequences from the GenBank nucleotide database were compared to the ones produced. A phylogenetic tree with a 98–100% similarity is used to identify the species. The MEGA 11 program was used to perform the phylogenetic analysis and sequence alignment.

Table 3.
The molecular identification of isolated bacteria and fungi is shown.
The phylogenetic relation between the B. cereus strain B-191, B. subtilis strain B-195, S. marcescens strains B-192 and B-193, and M. circinelloides, as well as members of all other Bacillus, Serratia, and Mucor species are shown in Figure 2, Figure 3, Figure 4 and Figure 5. The bootstrap values (n = 1000) are shown at the branches. The numbers adjacent to each branch in a tree provide a percentage measure of support for that branch, with 1000 being the maximum support. The ‘bootstrapping’ approach was performed, and the outcome yielded a high value, indicating that there is significant proof and that the sequences to the side of the branch were grouped exclusively with respect to one another.

Figure 2.
Phylogenic tree of Bacillus cereus strain B-191 (MK590996.1) isolated from yogurt and known bacterial relatives (using the neighbor-joining method) with MK305199.1, MG706123.1, MG430181.1, LC189367.1, and LC189362.1.

Figure 3.
Phylogenic tree of Bacillus subtilis strain B-195 (MK591144.1) isolated from yogurt and known bacterial relatives (using the neighbor-joining method) with KU206485.1, KC311340.1, MT114510.1, MW467894.1, and MN330082.1.

Figure 4.
Phylogenic tree of Serratia marcescens strain B-192 (MK591002.1) and Serratia marcescens strain B-193 (MK591014.1) isolated from yogurt and known bacterial relatives (using the neighbor-joining method) with OK067431.1, KR817904.1, GU212864.1, KF891398.1, and MH669313.1.
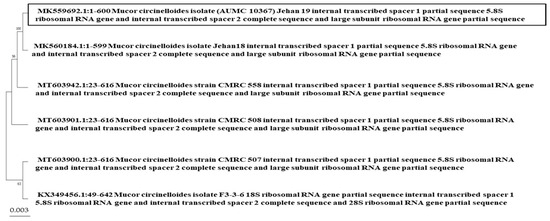
Figure 5.
Phylogenic tree of Mucor circinelloides (AUMC 10367) (MK559692.1) isolated from yogurt and known fungal relatives (using the neighbor-joining method) with MK560184.1, MT603942.1, MT603901.1, MT603900.1, and KX349456.1.
Among the nucleotide sequences accessible in the NCBI database, B. cereus (MK590996.1) has a strong resemblance to B. cereus (MK305199.1), Bacillus sp. (MG706123.1), Bacillus sp. (MG430181.1), B. cereus (LC189367.1), and B. cereus (LC189362.1) (Figure 2). Bacillus subtilis (MK591144.1) has a high similarity to B. subtilis (KU206485.1) (99.10%), (KC311340.1) (99.02%), B. subtilis subsp. subtilis (MT114510.1) (99.01%), B. subtilis (MW467894.1) (99.01%), and (MN330082.1) (99.01%) (Figure 3). S. marcescens (MK591002.1) has a high resemblance to S. marcescens (MK591014.1) and Serratia sp. (OK067431.1) (98.82%), S. marcescens (KR817904.1) (98.73%), S. marcescens (GU212864.1), Serratia sp. (KF891398.1), and S. marcescens (MH669313.1) (Figure 4). M. circinelloides (MK559692.1) shared a high degree of similarity (100%) with M. circinelloides (MK560184.1) and (99.49%) with M. circinelloides (MT603942.1), M. circinelloides (MT603901.1), M. circinelloides (MT603900.1), and M. circinelloides (KX349456.1) (Figure 5).
4. Discussion
4.1. Phenotypic Characteristics of Bacterial and Fungal Isolates
Microbial contamination is one of the most important causes of food spoilage worldwide [1]. The bacterial contamination of food can occur at any stage of the industrial process. When it comes to food expiration, a portion of a food could be physically, chemically, or microbially destroyed. Parasites, bacteria, and/or fungi are the main agents of microbiological deterioration [24]. Spore-forming bacteria are a unique concern among the microorganisms that might enter the milk supply chain on farms or through milk production lines because they can tolerate abiotic stressors while still in the spore phase [14,15]. We isolated four bacterial strains and one fungal strain from 12 commercially available fresh yogurts. The first family was Bacillaeae for B. cereus and B. subtilis, and the second was Yersiniaceae for S. marcescens. The highest frequencies of the isolates were as follows: 39.7% for B. cereus, 22.9% for M. circinelloides, 19.8% for B. subtilis, and 17.6% for S. marcescens. Bacillus cereus was recorded as having the highest frequency of the isolates. Bacillus and Serratia were characterized as rod-shaped. According to Oyeleke [38], the frequency of occurrence of bacterial contaminants in Bacillus sp. was 70%. Out of 120 samples (milk, yogurt, and milk contact surfaces), 80 bacteria from nine different species were isolated; the percentage of S. aureus contamination was 17 (21.3%) [39]. In Nigeria, Taiwo [40] noted eleven bacterial isolates were found in yogurt; Lactobacillus and Bacillus spp. made up 16% of the total microbial load; Corynebacterium, Klebsiela, Staphylococcus, and Pseudomonas spp. accounted for 8%; Proteus, Micrococcus, Shigella, Listeria, and Streptococcus spp. accounted for 4%; and Mucor spp. (22%), Geotrichum spp. (17%), Montospora spp. (11%), and Aspergillus, Rhizopus, and Fusanrium spp. made up 6%. In 200 samples of both balady and brand-name yogurt, 27 isolates (13.5%) of E. Coli, 11 isolates of E. faecalis, and 8 isolates of B. cereus were identified, along with 37 molds (19 Aspergillus niger, 5 Aspergillus fumigatus, 5 Mucor spp., 3 Aspergillus flavus, 3 Penicillium spp., and 2 Rhizopus spp.) and 43 yeasts (23 C. albicans, 12 Rhodotorula spp., and 8 C. tropicalis) [41].
In Egypt and India, B. cereus was discovered in milk products with various amounts of contamination [18,19]. Serratia spp. can invade abiotic surfaces and produce heat-resistant enzymes, causing them to degrade milk at various stages of production [20,21,22]. S. liquefaciens was found in 27.78% of the positive samples tested [42]. According to a report [42], S marcescens had the highest isolation percentage (57.14% out of 21 positive samples). Serratia organisms have been linked to human infections such as septicemia, pulmonary and urinary tract infections, and urinary tract abscesses [20].
The third family was Mucoraceae for M. circinelloides. The morphology of M. circinelloides is characterized by big black sporangia with a mean area of 100%, a 0.202 µm mean circular shape, and a mean solidity of 0.488 µm. Numerous Mucor species are of high relevance to the field of biotechnology due to their ability to generate proteolytic enzymes and bioactive components [43,44]. Overall, the growth temperature affects the physiological properties of fungi. Mucor spp., in particular, can cause human infections [45]. Food processors suffer severe harm as a result of fungal deterioration due to the large spectrum of fungal species. According to a US FDA probe, M. circinelloides was related to a nutrition disease outbreak after infected yogurt was consumed. Storage temperature and natamycin interact to produce M. circinelloides yogurt deterioration, according to Snyder et al. [29]. Meanwhile, El-Shinawy et al. [46] found no fungal growth in yogurt samples. There are previous reports of isolated and recognized yeast and mold species from yogurt samples [47,48]. The microbial quality of yogurt affects its quality and acceptability. The possibility of microbial contamination due to unhygienic conditions raises the risk of serious consequences for consumer health [13].
A food’s shelf life is the amount of time it can be stored without losing its safety, nutritional value, or sensory qualities, making it suitable for human consumption [49]. The product will lose some of its desirable qualities while being stored. Food can be harmed by oxygen, water, light, and harmful microorganisms. It can produce toxins as well as off-flavors and odors. Assessing a food’s shelf life is simple; one observes how its colors and odors change. The quantity of microorganisms (bacteria and fungi) and acid produced during starter fermentation determine how long a food can be stored. Pasteurization does not kill all microorganisms. Other bacteria, in addition to a starter, may contribute to a reduction in pH levels [50]. The viability of the microorganisms during storage is influenced by several variables, including acidity, pH, hydrogen peroxide [51], storage temperature, and oxygen content. Microorganism viability will be decreased by unfavorable environmental conditions and poor nutrition [50]. This study revealed that Bacillus cereus exhibited the highest frequency of the isolates tested, resulting in bacteria that persist in pasteurized milk and shorten its shelf life.
4.2. Molecular Identification of Bacterial and Fungal Isolates
Our molecular analysis of both the fungal and bacterial isolates using ITS and 16S rRNA sequences revealed that the four isolates belonged to one fungal genus, Mucor, and two bacterial genera, Bacillus and Serratia, respectively. In M. circinelloides, a partial sequence consisting of internal transcribed spacers 1 and 2, the large subunit ribosomal RNA gene, and 5.8S ribosomal RNA was found. For a wide range of fungi, the ITS area is one of the indicators with the greatest chance of identification [52]. Several fungal researchers have reported the ITS region as an appropriate fungal barcode [53,54,55].
The nucleotide sequences of the bacterial and fungal species were matched to those in the GenBank database (NCBI), and a phylogenetic tree was built in MEGA 11 using the neighbor-joining method [36]. In NCBI, there was significant clustering and a high degree of similarity with the associated identified species. Bacillus cereus was 99.63% identified; B. subtilis was 99.01 to 99.10% identified; S. marcescens was 98.64% identical to 98.82% of the nucleotide sequences in the NCBI GenBank, and M. circinelloides was 99.49% to 100% identified. The nucleotide sequences that were determined for the five microorganisms helped in the confirmation of their identities. We obtained a sufficient definition of the species within the genera Bacillus, Serratia, and Mucor and our results agreed with those of [56], who proposed describing the classification of the species in the genus Diaporthe based on morphological, cultural, and DNA sequences.
The similarity of the strains to linked identified species suggests that these fungi have not been subjected to varied environmental conditions that would otherwise generate increased genetic variation, which is generally referred to as coordinated evolution [57]. A BLAST search indicated that certain isolates from various places were 100% identifiable, such as in M. circinelloides (MK559692.1) and M. circinelloides (MK560184.1). Teymori et al. [58] found that 23% of yogurt samples were contaminated with harmful microorganisms. To remove and limit the potential for contamination, it is necessary to identify critical limits with automated control systems [58].
5. Conclusions
SEM revealed that the Bacillus and Serratia cells were rod-shaped, whereas the Mucor cells were rounded and circular-shaped. B. cereus isolates were found at a high frequency (39.7%) in the yogurt products, causing them to degrade the yogurt at various stages of its production. We used the 16S rRNA and ITS sequences to describe bacterial and fungal isolates from yogurt containers purchased commercially during their validity period from a supermarket in Riyadh, Saudi Arabia. They were found to be B. cereus strain B-191, B. subtilis strain B-195, S. marcescens strain B-192, S. marcescens strain B-193 (Accession No: MK590996.1, MK591144.1, MK591002.1, and MK591014.1), and M. circinelloides isolates (Accession No: MK559692.1). Thus, the genotyping method based on the 16S rRNA and ITS gene sequences is straightforward and effective in identifying strains. The nucleotide sequences that were determined for the five microorganisms helped in the confirmation of their identities. The importance of such information will be significant for understanding microbial habitat ecology, and it will be simple to develop special protocols to address such microbes. Our research is the first to look specifically at fresh yogurt contaminants in KSA by isolating Bacillus, Serratia, and Mucor, which had been previously unrecorded.
Author Contributions
Conceptualization.; methodology; software.; validation; writing—review and editing and formal analysis, J.S.A.-b.; investigation; resources.; data curation, O.A.A. and A.I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Princess Nourah Bint Abdulrahman University Researchers Supporting Project (number PNURSP2022R180), Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pal, M. Spoilage of Dairy Products Due to Fungi. Beverage Food World 2014, 41, 37–38. [Google Scholar]
- De Jonghe, V.; Coorevits, A.; De Block, J.; Van Coillie, E.; Grijspeerdt, K.; Herman, L.; De Vos, P.; Heyndrickx, M. Toxinogenic and Spoilage Potential of Aerobic Spore-Formers Isolated from Raw Milk. Int. J. Food Microbiol. 2010, 136, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, J.; Dobranić, V.; Filipović, I.; Zdolec, N. Microbiological Quality of Soft, Semi-Hard and Hard Cheeses during the Shelf-Life. Maced. Vet. Rev. 2016, 39, 59–64. [Google Scholar] [CrossRef]
- Das, S.; Hasan, G.A.; Parveen, S. Evaluation of Microbial Load and Quality of Milk & Milk Based Dairy Products. Octa J. Biosci. 2015, 3, 1–4. [Google Scholar]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The Complex Microbiota of Raw Milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Bartoszewicz, M.; Hansen, B.M.; Swiecicka, I. The Members of the Bacillus Cereus Group Are Commonly Present Contaminants of Fresh and Heat-Treated Milk. Food Microbiol. 2008, 25, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Notermans, S.; Dufrenne, J.; Teunis, P.; Beumer, R.; Te Giffel, M.; Weem, P.P. A Risk Assessment Study ofBacillus Cereuspresent in Pasteurized Milk. Food Microbiol. 1997, 14, 143–151. [Google Scholar] [CrossRef]
- Svensson, B.; Monthan, A.; Shaheen, R.; Andersson, M.A.; Salkinoja-Salonen, M.; Christiansson, A. Occurrence of Emetic Toxin Producing Bacillus Cereus in the Dairy Production Chain. Int. Dairy J. 2006, 16, 740–749. [Google Scholar] [CrossRef]
- Spanu, C.; Scarano, C.; Spanu, V.; Pala, C.; Casti, D.; Lamon, S.; Cossu, F.; Ibba, M.; Nieddu, G.; De Santis, E.P. Occurrence and Behavior of Bacillus Cereus in Naturally Contaminated Ricotta Salata Cheese during Refrigerated Storage. Food Microbiol. 2016, 58, 135–138. [Google Scholar] [CrossRef]
- Muehlhoff, E.; Bennett, A. Milk and Dairy Products in Human Nutrition; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Tamime, A.Y.; Tamine, A.Y.; Robinson, R.K.; Robinson, R.K. Tamime and Robinson’s Yoghurt Science and Technology, 3rd ed.; Taylor & Francis: Abingdon, UK, 2007; ISBN 978-1-4200-4453-9. [Google Scholar]
- Rodrigues, L.A.; Ortolani, M.B.T.; Nero, L.A. Microbiological Quality of Yoghurt Commercialized in Viçosa, Minas Gerais, Brazil. Afr. J. Microbiol. Res. 2010, 4, 210–213. [Google Scholar]
- Pal, M.; Tefera, M.; Tasew, A.; Jergefa, T.; Deressa, A. Hygienic and Microbial Quality of Yoghurt. Beverage Food World 2015, 42, 25–31. [Google Scholar]
- Postollec, F.; Mathot, A.-G.; Bernard, M.; Divanac’h, M.-L.; Pavan, S.; Sohier, D. Tracking Spore-Forming Bacteria in Food: From Natural Biodiversity to Selection by Processes. Int. J. Food Microbiol. 2012, 158, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vos, P.; Garrity, G.; Jones, D.; Krieg, N.R.; Ludwig, W.; Rainey, F.A.; Schleifer, K.-H.; Whitman, W.B. Bergey’s Manual of Systematic Bacteriology: Volume 3: The Firmicutes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011; ISBN 978-0-387-68489-5. [Google Scholar]
- Beattie, S.H.; Williams, A.G. Detection of Toxigenic Strains of Bacillus Cereus and Other Bacillus Spp. with an Improved Cytotoxicity Assay. Lett. Appl. Microbiol. 1999, 28, 221–225. [Google Scholar] [CrossRef] [PubMed]
- From, C.; Pukall, R.; Schumann, P.; Hormazábal, V.; Granum, P.E. Toxin-Producing Ability among Bacillus Spp. Outside the Bacillus Cereus Group. Appl. Environ. Microbiol. 2005, 71, 1178–1183. [Google Scholar] [CrossRef]
- Hassan, G.M.; Al-Ashmawy, M.A.M.; Meshref, A.M.S.; Afify, S.I. Studies on Enterotoxigenic Bacillus Cereus in Raw Milk and Some Dairy Products. J. Food Saf. 2010, 30, 569–583. [Google Scholar] [CrossRef]
- Kumari, S.; Sarkar, P.K. Prevalence and Characterization of Bacillus Cereus Group from Various Marketed Dairy Products in India. Dairy Sci. Technol. 2014, 94, 483–497. [Google Scholar] [CrossRef]
- Mahlen, S.D. Serratia Infections: From Military Experiments to Current Practice. Clin. Microbiol. Rev. 2011, 24, 755–791. [Google Scholar] [CrossRef]
- Cleto, S.; Matos, S.; Kluskens, L.; Vieira, M.J. Characterization of Contaminants from a Sanitized Milk Processing Plant. PLoS ONE 2012, 7, e40189. [Google Scholar] [CrossRef]
- Decimo, M.; Morandi, S.; Silvetti, T.; Brasca, M. Characterization of Gram-Negative Psychrotrophic Bacteria Isolated from Italian Bulk Tank Milk. J. Food Sci. 2014, 79, M2081–M2090. [Google Scholar]
- Singh, V.; Kaushal, S.; Tyagi, A.; Sharma, P. Screening of Bacteria Responsible for the Spoilage of Milk. J. Chem. Pharm. Res. 2011, 3, 348–350. [Google Scholar]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and Control of Spoilage Fungi in Dairy Products: An Update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef]
- in’t Veld, J.H.H. Microbial and Biochemical Spoilage of Foods: An Overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.I.; Hocking, A.D. Spoilage of Stored, Processed and Preserved Foods. In Fungi and Food Spoilage; Pitt, J.I., Hocking, A.D., Eds.; Springer: Boston, MA, USA, 2009; pp. 401–421. ISBN 978-0-387-92207-2. [Google Scholar]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Aguado, J.M.; Bonatti, H.; Forrest, G.; Gupta, K.L.; Safdar, N.; John, G.T.; Pursell, K.J.; Muñoz, P.; Patel, R. Zygomycosis in Solid Organ Transplant Recipients: A Prospective, Matched Case-Control Study to Assess Risks for Disease and Outcome. J. Infect. Dis. 2009, 200, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.B.; Churey, J.J.; Worobo, R.W. Characterization and Control of Mucor Circinelloides Spoilage in Yogurt. Int. J. Food Microbiol. 2016, 228, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.; Jung, S.; Lamsal, B. Food Processing: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- MacBean, R.D. Packaging and the Shelf Life of Yogurt. In Food Packaging and Shelf Life; Taylor and Francis Group, LLC: Boca Raton, FL, USA, 2010; pp. 143–156. [Google Scholar]
- Waksman, S.A. A Method for Counting the Number of Fungi in the Soil. J. Bacteriol. 1922, 7, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Dong, H.; Zhang, G.; Yu, B.; Chapman, L.R.; Fields, M.W. Microbial Diversity in Water and Sediment of Lake Chaka, an Athalassohaline Lake in Northwestern China. Appl. Environ. Microbiol. 2006, 72, 3832–3845. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Oyeleke, S.B. Microbial Assessment of Some Commercially Prepared Yoghurt Retailed in Minna, Niger State. Afr. J. Microbiol. Res. 2009, 3, 245–248. [Google Scholar]
- Asafo-Agyei, K.O.; Samant, H. Hepatocellular Carcinoma; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Taiwo, O.S.; Afolabi, R.O.; Oranusi, S.U.; Owolabi, J.B.; Oloyede, A.R.; Isibor, P.O.; Omonigbehin, E.A.; Popoola, J.O.; Obafemi, Y.D.; Ejoh, S.A. Microbiological Assessment of Commercial Yogurt Sold in Ota Metropolis, Ogun State, Nigeria. IOP Conf. Ser. Earth Environ. Sci. 2018, 210, 012019. [Google Scholar] [CrossRef]
- Fetouh, M.; Ibrahim, E.; ElBarbary, H.; Maarouf, A. Isolation and Genotypic Identification of Some Spoilage and Pathogenic Microbes from Yogurt. Benha Vet. Med. J. 2022, 43, 123–128. [Google Scholar] [CrossRef]
- Sobeih, A.M.; AL-Hawary, I.; Khalifa, E.; Ebied, N. Prevalence of Enterobacteriaceae in Raw Milk and Some Dairy Products. Kafrelsheikh Vet. Med. J. 2020, 18, 9–13. [Google Scholar] [CrossRef]
- Simões, K.; Dietrich, S.; Hahn, M.G.; Braga, M.R. Purification and Characterization of a Phytoalexin Elicitor from Spores of the Saprobe Mucor Ramosissimus. Braz. J. Bot. 2005, 28, 735–744. [Google Scholar] [CrossRef]
- Souza, P.M.d.; Bittencourt, M.L.d.A.; Caprara, C.C.; Freitas, M.d.; Almeida, R.P.C.d.; Silveira, D.; Fonseca, Y.M.; Ferreira Filho, E.X.; Pessoa Junior, A.; Magalhães, P.O. A Biotechnology Perspective of Fungal Proteases. Braz. J. Microbiol. 2015, 46, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Jeon, Y.J.; Mun, H.Y.; Goh, J.; Chung, N.; Lee, H.B. Isolation and Characterization of Four Unrecorded Mucor Species in Korea. Mycobiology 2020, 48, 29–36. [Google Scholar] [CrossRef]
- El-Shinawy, S.; El-Kholy, A.; Meshref, A.; Sharkawy, S. Mycological Evaluation of Milk and Some Milk Products in Beni-Suef City. Assiut Vet. Med. J. 2018, 64, 117–122. [Google Scholar]
- Keta, J.N.; Hadi, S.; Aliero, A.A.; Keta, M.N.; Hamisu, A. Evaluation of Fungi Species from Commercial Yoghurt in Birnin Kebbi, Kebbi State Nigeria. Equity J. Sci. Technol. 2020, 6, 72. [Google Scholar]
- Dalia, A.; Flourage, M.; El-Toukhy, E.I. Fungal Contamination of Some Local Dairy Products and Extent Production of Aflatoxins. Life Sci. J. 2020, 17, 7–13. [Google Scholar]
- Jedermann, R.; Nicometo, M.; Uysal, I.; Lang, W. Reducing Food Losses by Intelligent Food Logistics. Phil. Trans. R. Soc. A 2014, 372, 20130302. [Google Scholar] [CrossRef] [PubMed]
- Priadi, G.; Setiyoningrum, F.; Afiati, F. The Shelf Life of Yogurt Starter and Its Derivatives Based on the Microbiological, Physical and Sensory Aspects. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012014. [Google Scholar] [CrossRef]
- Lankaputhra, W.E.V.; Shah, N.P.; Britz, M.L. Survival of Bifidobacteria during Refrigerated Storage in the Presence of Acid and Hydrogen Peroxide. Milchwissenschaft 1996, 51, 65–69. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Consortium, F.B.; List, F.B.C.A.; Bolchacova, E. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Region as a Universal DNA Barcode Marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Dentinger, B.T.; Didukh, M.Y.; Moncalvo, J.-M. Comparing COI and ITS as DNA Barcode Markers for Mushrooms and Allies (Agaricomycotina). PLoS ONE 2011, 6, e25081. [Google Scholar] [CrossRef]
- Kelly, L.J.; Hollingsworth, P.M.; Coppins, B.J.; Ellis, C.J.; Harrold, P.; Tosh, J.; Yahr, R. DNA Barcoding of Lichenized Fungi Demonstrates High Identification Success in a Floristic Context. New Phytol. 2011, 191, 288–300. [Google Scholar] [CrossRef]
- Seena, S.; Pascoal, C.; Marvanová, L.; Cássio, F. DNA Barcoding of Fungi: A Case Study Using ITS Sequences for Identifying Aquatic Hyphomycete Species. Fungal Divers. 2010, 44, 77–87. [Google Scholar] [CrossRef]
- Gomes, R.R.; Glienke, C.; Videira, S.I.R.; Lombard, L.; Groenewald, J.Z.; Crous, P.W. Diaporthe: A Genus of Endophytic, Saprobic and Plant Pathogenic Fungi. Persoonia Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar] [CrossRef]
- Ganley, A.R.; Kobayashi, T. Highly Efficient Concerted Evolution in the Ribosomal DNA Repeats: Total rDNA Repeat Variation Revealed by Whole-Genome Shotgun Sequence Data. Genome Res. 2007, 17, 184–191. [Google Scholar] [CrossRef]
- Teymori, R.; Ghazanfarirad, N.; Dehghan, K.; Kheyri, A.; Hajigholizadeh, G.; Kazemi-Ghoshchi, B.; Bahmani, M. Monitoring Microbial Quality of Commercial Dairy Products in West Azerbaijan Province, Northwest of Iran. Asian Pac. J. Trop. Dis. 2014, 4, S824–S829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).