Abstract
Extracellular electron transfer (EET) is a biological mechanism that plays a crucial role in various bioelectrochemical systems (BESs) and has substantial implications for renewable energy production. By utilizing the metabolic capacities of exoelectrogens, BESs offer a viable and environmentally friendly approach to electricity generation and chemical production; however, the diminished effectiveness of EET remains a hindrance to their optimal application in practical contexts. This paper examines the various strategies that have the potential to be employed to enhance the efficiency of EET systems and explores the potential for the integration of BESs technology with contemporary technologies, resulting in the development of an enhanced and sustainable system. It also examines how quorum sensing, electrode modifications, electron shuttles, and mediators can aid in improving EET performance. Many technological innovations, such as additive manufacturing, the science of nanotechnology, the technique of genetic engineering, computational intelligence, and other combinations of technologies that can be used to augment the efficacy of BESs are also discussed. Our findings will help readers understand how BESs, though an evolving technology, can play an important role in addressing our environmental concerns. Technical constraints are identified, and future directions in the field of EET are suggested.
1. Introduction
Microorganisms have devised specific processes to transmit electrons to minerals that are located near the surface of the cell, and this process is termed extracellular electron transfer (EET) [1]. The microbial EET process serves as a source of energy for the growth and maintenance of microorganisms. These microorganisms capable of conducting the EET process are referred to as electroactive microorganisms (EAMs) [2]. EAMs are now used for many applications in many environmental biotechnologies such as energy recovery and environmental remediation. For bioelectrochemical systems like microbial electrolysis cells (MECs) and microbial fuel cells (MFCs), the power production, fuel generation rates, and overall energy recovery from organic matter are all improved by increasing EET efficiency [3]. In addition to the generation of bioelectricity, the biotransformation of valuable chemicals, and the bioremediation of environmentally hazardous pollutants, EAMs are now useful for biosensing and even biocomputing [4]. EET enhancement is important in studying and harnessing microbe–metal interactions [5,6,7,8,9], but the complexity of electron transfer mechanisms, the wide range of microbes in question, the influence of environmental factors, and the scaling-up of EET processes are among the key challenges that researchers and engineers face in this field [9,10]. Overcoming these challenges will pave the way for the development of innovative applications for harnessing the potential of EET.
There are now several techniques used in environmental biotechnology to improve EET. To improve the EET capabilities of EAMs like Shewanella or Geobacter species, genetic engineering approaches can be used. This includes adding exogenous electron transfer mediators or overexpressing crucial EET genes or pathways [3]. Biofilm growth can also improve EET between microorganisms and electrodes. Optimizing growth conditions, surface modification, and biofilm engineering approaches are methods to improve the formation of biofilm [11,12]. Another method of improving EET is to optimize the material of the electrode. EET can be enhanced by modifying the composition and surface features of the electrodes [13,14,15]. This includes enhancing the contact between microbes and electrodes by employing conductive materials, such as carbon nanotubes or conductive polymers [2]. Redox mediators like riboflavin, neutral red, or anthraquinone derivatives can improve EET by promoting electron transport between microorganisms and solid surfaces or electron acceptors [16,17].
Electrochemical techniques are essential in studying the behavior of microorganisms for various purposes. One popular electrochemical method for examining the redox behavior of electroactive species in a system is cyclic voltammetry (CV). CV is used in bioelectrochemical systems to describe the electrochemical activity of redox-active mediators and microorganisms involved in EET. CV offers insights into the kinetics and thermodynamics of EET processes by assisting in determining the redox potentials of electron-donor and electron-acceptor compounds. Chronocoulometry (CC) is an additional electrochemical method utilized to quantify the overall electric charge that traversed an electrochemical cell within a designated time interval. This method can be performed by imparting a constant potential to the working electrode and measuring the resulting current as a function of time. The term “chronocoulometry” is derived from the words “coulometry”, which quantifies the amount of electricity (charge) exchanged within a system, and “chrono”, which denotes time. Pyocyanin (PYO) is an identified redox mediator molecule produced by Pseudomonas aeruginosa. The electrochemical technique utilizing CV and CC was employed in a recent study [18], to facilitate the analysis of the adsorption process of PYO onto carbon-based electrodes using glassy carbon. By utilizing these electrochemical methods in conjunction with FT-IR analysis, the authors deduced that P. aeruginosa NEJ07R establishes electronic communication with the electrode via PYO adsorption on the carbon surface. The research endeavors to enhance the engagement between redox mediators, microorganisms, and electrodes. This, in turn, may contribute to the advancement of microbial electrochemical technologies that address ecological challenges including, but not limited to, waste valorization, wastewater treatment, and renewable energy generation.
Gaining a comprehensive understanding of the electrochemical characteristics exhibited by bio-anodes is crucial to enhancing the overall efficiency and effectiveness of bioelectrochemical systems. Electrochemical impedance spectroscopy (EIS) is frequently employed for the comprehensive investigation of these characteristics. In a study conducted by Heijne et al. [19], the authors aimed to measure the properties of bio-anodes, specifically biofilm capacitance, charge transfer, biofilm resistance, and diffusion resistance. To ensure accurate measurements without the influence of electrode capacitance, the researchers utilized Fluorinated Tin Oxide (FTO) as the electrode material for cultivating the electroactive biofilm. FTO has favorable characteristics as an electrode material in the electrochemical investigation of bio-anodes. This is primarily due to its exceptional stability and significantly lower capacitance, which typically ranges in the tens of microfarads per square centimeter (μF cm−2), in contrast to conventional carbon electrodes, such as graphite plates, that possess a capacitance of approximately 1 millifarad per square centimeter (~1 mF cm−2). The researchers conducted a study to observe the formation of an electroactive biofilm on FTO (by the utilization of EIS and polarization experiments). The findings of the study demonstrate that the capacitance of the biofilm exhibited a significant rise from an initial value of 2 μF cm−2 to a final value of 450 μF cm−2 as the biofilm developed. This observation suggests a correlation between the capacitance and both the current and the total charge generated. The findings of the study additionally indicate that biofilm capacitance can serve as an indicator of the quantity of active biomass present in bioelectrochemical systems.
Scanning electrochemical microscopy (SECM) is an additional method that enables the visualization of electrochemical phenomena at the microscale. In the field of bioelectrochemical systems, this technique can be employed to see and analyze the spatial distribution of electroactive species as well as the level of microbial activity on electrode surfaces. SECM offers valuable insights into the electrochemical activity of biofilms, enabling a deeper understanding of the variability in electron transport mechanisms across microbial communities. The technique has also demonstrated its utility in various applications, including the evaluation of membrane permeability, neurotransmitter levels, and intracellular parameters [20].
Furthermore, the utilization of potentiostatic and galvanostatic operation is an electrochemical technique wherein the potential or current, accordingly, is regulated within an electrochemical system. Within the field of bioelectrochemical systems, potentiostatic and galvanostatic techniques are employed to manipulate the electrochemical conditions, modulate the rate of electron transfer, and examine the reaction of microorganisms to varying applied potentials or currents [21,22]. These electrochemical techniques play an important role in the characterization, optimization, and understanding of the intricate mechanisms associated with extracellular electron transfer in bioelectrochemical systems. Their contributions are crucial in the advancement of microbial electrochemistry and the pragmatic implementation of these systems in the fields of energy generation, wastewater treatment, and environmental monitoring.
Over time, substantial advancements have been achieved in the understanding of the mechanisms underlying microbial EET. Engineering techniques for enhancing the interaction between EAMs and electrodes can present encouraging opportunities for enhancing the EET efficiency of EAM. In light of recent research, this review gives a brief overview of the mechanism of EET in microorganisms, and then the different ways the performance of EET can be improved ranging from the use of quorum sensing, electrode modifications, electron shuttles, and mediators, to the use of many technological innovations, such as additive manufacturing, the science of nanotechnology, the technique of genetic engineering, computational intelligence, and other combinations of technologies. Finally, suggestions for prospective techniques that would enhance the effectiveness of EET are discussed.
2. Mechanism of Extracellular Electron Transport in Microorganisms
2.1. Direct Electron Transfer (DET)
Microorganisms can carry out EET in different ways as depicted in Figure 1. For DET, electrons can move from the bacteria to electrodes independent of external mediators. This method necessitates the proximity of microorganisms to the outer electron recipient and entails the transfer of electrons via outer membrane proteins known as c-type cytochromes [23,24]. The limited distance of electron transfer in DET is known to be a contributing factor to the improved EET process. The transfer of electrons through the cytochrome pathway has been extensively studied in Geobacter sulfurreducens. This has been achieved through the use of mutant strains, wherein the gene responsible for encoding cytochrome c proteins has been either overexpressed or deleted [25].
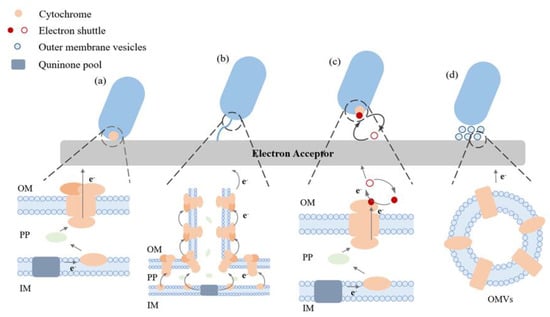
Figure 1.
Elucidating the extracellular electron transport pathways that are commonly observed in electroactive microorganisms. (a) DET facilitated by cytochrome proteins, (b) the process of DET facilitated through nanowires, (c) the mode of IET facilitated by electron shuttles, (d) the method of indirect electron transfer facilitated through exterior vesicles of the membrane (OMVs). Outer membrane (OM), interior membrane (IM), and periplasm (PP).
The DET process is not exhibited by all microorganisms; hence, there has been a focus on investigating an alternative mechanism that is comparable but less comprehended, referred to as microbial nanowires. This mechanism has been occasionally referred to as a variant of DET. Nanowires are cellular membrane protrusions that bear a resemblance to pili. Due to their relatively elevated conductivity, it is postulated that they facilitate the conduction of electrons. Consequently, this confers an advantage to microorganisms by enabling them to inhabit a location that is distanced from the metal oxide or electrode. Additionally, this could facilitate inter-species electron transfer within a biofilm [23].
2.2. Indirect Electron Transfer (IET)
In IET, microorganisms can generate and secrete diminutive molecules that can undergo redox cycling, thereby acting as an ES (Figure 1). They function as the final electron acceptor and, upon reduction, are capable of transferring electrons to iron oxides, subsequently undergoing reoxidation. This type of shuttle offers a means for an indirect reduction process. The repetitive cycling of a solitary shuttle molecule can significantly influence the turnover rate of the terminal oxidant, such as iron, within a specific situation. Compounds such as humic compounds, quinones, phenazines, and molecules containing thiols have the potential to fulfil this function [26,27,28,29]. Electron shuttles can be classified into two distinct categories, specifically exogenous and endogenous, depending on their presence in the environment or the inherent ability of the cell to produce them. Thionine is the predominant exogenous mediator utilized, while other compounds such as quinine, phenoxazine, phenazine, and phenothiazine have also been employed in this capacity. On the other hand, endogenous mediators are released from cells in a reduced state and undergo oxidation to enable electron transfer at the electrode. The mediators under consideration in this category consist of flavin compounds produced by S. oneidensis MR-1 and 2-amino-3-carboxy-1,4-naphthoquinone, which is excreted by S. putrefaciens [24].
2.3. Factors Affecting EET Efficiency
EET is subject to the influence of multiple factors, encompassing temperature, pH levels, the existence of electron acceptors and donors, redox potential, as well as the properties of the electrode. Temperature affects the rate of chemical reactions and the availability of electron transfer [30]. High temperatures can enhance EET efficiency by increasing the rate of enzymatic reactions involved in electron transfer. The pH level is an essential determinant that affects both the activity of enzymes involved in EET and the proton gradient across cellular membranes. Enzymes involved in extracellular electron transfer often exhibit pH-dependent activity, and an increase in pH can disrupt the proton gradient and impede EET efficiency [31]. Electron acceptors and donors are also critical factors in EET efficiency. Oxygen is a highly favorable electron recipient because of its strong reduction potential and abundance in many natural environments [32]. In environments with a limited availability of electron acceptors, microorganisms may employ alternative strategies for EET. Organic substances like sugars, fatty acids, alcohols, and hydrogen gas are typical electron donors [33]. Redox potential is another crucial factor in EET processes. Microorganisms can utilize various electron acceptors with different redox potentials, such as solid metal oxides and metals like gold and platinum. The redox potential of these metal oxides influences their ability to accept electrons from microbial donors [34]. Electrode properties also play a significant role in EET efficiency. Conductive materials like graphite, carbon nanotubes, and metals like gold and platinum are often employed as electrodes because they possess strong electrical conductivity [35,36]. Biochar, a representative carbon-based material, has also been shown to serve as an ES to mediate the DIET process of Methanosarcina and Geobacter [37]. Thus, temperature, pH level, the presence of electron acceptors and donors, redox potential, and the characteristics of the electrodes are a few factors that generally affect the efficiency of EET.
3. Strategies for Enhancing EET in Environmental Biotechnology
3.1. Genetic Engineering Approaches
- (a)
- Overexpression of key EET genes
Overexpression of specific EET genes can have a major impact on the efficiency and effectiveness of EET processes. Microorganisms can improve their ability to transmit electrons across cell membranes and interact with external electron acceptors or donors by boosting the expression levels of essential genes [34]. This strategy has been extensively studied and applied in various environmental biotechnology applications. MtrC, which encodes a c-type cytochrome protein found in Shewanella species, is one example of a critical gene involved in EET. The upregulation of cytochrome c, specifically CymA, in the bacterium S. oneidensis MR-1, has resulted in an enhancement of both bioelectricity production and cell proliferation within a microbial fuel cell [38]. To enhance the expression of cytochrome c and optimize electron transition, Su et al. [39] employed error-prone PCR to introduce random mutations into ccmH. Subsequently, they investigated the resulting increment in cytochrome synthesis. The findings of the study demonstrated that the introduction of the mutant ccmH gene significantly augmented the efficacy of the genetic circuit responsible for regulating EET, resulting in a notable 77% rise in current. The electron transfer process acquired by the metabolism of cells from the interior membrane protein, which is the CymA, to terminal periplasmic reductases is of significant importance in the chain of electron transport in S. oneidensis. When the CymA undergoes mutation, there was an observed reduction in the current generation by around 80%. On the other hand, MFCs that were inoculated with genetically modified strains that overexpress CymA demonstrated an increased maximum power output of 0.13 mW and a higher specific growth rate of 0.087 h−1. In comparison, the wild-type MFCs had an optimal energy output of 0.11 mW and an increase of 0.043 h−1 [38]. Enhancing EET can also be achieved by strategically improving the network of periplasmic cytochromes. A diverse array of c-type cytochromes is spatially scattered inside the periplasm, collectively establishing an intricate network for electron transport. To simplify the pathway of electrons in the periplasm and mitigate the possibility of the competitive response of MR-1, a study conducted gene knockouts of the periplasmic cytochrome genes, which are nrfA, ccpA, napB, and napA, and subsequently introduced the expression of cytochromes cctA at the respective knockout locations. According to Delgado et al. [40], the findings of the study indicated a positive correlation between the copy number of cctA and the EET rate. Moreover, it was observed that the current exhibited a 23% rise in response to this relationship. Hence, the simplification of the periplasmic cytochrome network has the potential to modify the extracellular redox potential and expedite the process of electron transfer.
The overexpression of these key EET genes can be achieved through various genetic engineering techniques, such as plasmid-based gene expression systems or genome editing tools like CRISPR-Cas9 [41]. By manipulating the expression levels of these genes, it can be possible to fine-tune the EET capabilities of microorganisms and optimize their performance in environmental biotechnology applications.
- (b)
- Exogenous EET
Exogenous EET refers to the introduction of external electron transfer mediators or genetically engineered microorganisms into an environment to enhance electron transfer capabilities. This process can be introduced through various means, such as the addition of redox-active compounds or the genetic modification of microorganisms to express specific EET-related proteins. The introduction of exogenous EET can have several benefits in environmental biotechnology. Firstly, it can enhance the overall efficiency of electron transfer processes. By introducing external electron transfer mediators, such as quinones or conductive polymers, the rate and extent of electron transfer can be significantly improved [41]. This enhancement may result in enhanced power production in microbial fuel cells or faster breakdown rates in bioremediation applications. Secondly, exogenous EET pathways can broaden the range of bacteria that can execute EET. Only a few microbes in natural habitats have innate EET capabilities. By introducing exogenous EET, it becomes possible to engineer non-electrogenic microorganisms to participate in EET processes [32].
Research carried out in 1993 was the pioneering study to make use of artificial soluble redox mediators as a strategy for harnessing the antioxidant potential of viable bacteria [42]. Methylene blue, a redox dye created by German chemist Heinrich Caro in 1876 [43], was one of the first mediators to be employed. According to Arup [44], Neisser and Wechsberg made the initial suggestion that methylene blue could be a valuable tool for determining the bacterial load in milk in 1900. A recent study by Liu et al. [45] employed methylene blue (MB) as a transitory intermediate to augment EET between exoelectrogenic anaerobic granular sludge (EGS) and electrodes. The study revealed that the optimum electricity of MB-EGS (8.88 mA) exhibited a statistically substantial rise compared to that of EGS alone (1.34 mA). Currently, in addition to methylene blue, other mediators such as thionine, neutral red, and 2,6-dichlorophenol [46] are also being employed.
3.2. Biofilm Formation and Optimization
- (a)
- Biofilm matrix engineering
The spatial arrangement of microbial cells and their relationship within the surrounding environment can be impacted by the manipulation of the biofilm matrix composition and structure. This, in turn, can improve the accessibility of substrates and facilitate efficient electron transfer. The manipulation of EPS, which are a significant component of the biofilm matrix, is one strategy in biofilm matrix engineering. EPS components are influential in determining the physical properties of the matrix and are significant in facilitating electron transfer mechanisms [47]. By manipulating EPS composition, it is possible to engineer biofilms with enhanced capacity to transfer electrons. For example, increasing the production of conductive polymers within the EPS can promote DET between microbial cells and solid surfaces or electrodes. Conductive polymers, such as extracellular electron shuttles or conductive nanomaterials, can serve as conduits for long-range electron transfer within the biofilm. Polypyrrole is an example of a conductive polymer and it has been used and coated with Shewanella oneidensis MR-1 as an anode in MFCs, Ref. [48] the research on this shows that the polypyrrole-coated MR-1/carbon cloth anode attained a power density of 147.9 mW/cm2 and was 14.1-fold greater than that of the original strain. Furthermore, in the case of Shewanella, it was discovered that the optimum energy output is obtained once the biofilm reaches a thickness ranging from 5 to 7 micrometers [49]. Additionally, studies have demonstrated that the implementation of controlled micro and nano porosity configurations increases current density. Another strategy in biofilm matrix engineering is to engineer biofilms with optimized spatial organization to improve mass transport and substrate availability. Materials engineering is another technique for increasing the thickness and conductivity of biofilms through the use of conductive substances [48,50] and electrode functionalization [51,52].
- (b)
- Quorum sensing manipulation for biofilm development
The process of quorum sensing (QS) serves as a means of communication between bacterial cells, enabling them to detect and respond to autoinducers to modulate their activities and effectively adapt to environmental fluctuations [53]. The signaling molecules involved in quorum sensing (QS) exhibit variations between Gram-positive and Gram-negative bacteria [54]. These molecules can broadly be classified into three categories, as described in [55]. (i) LuxI/R-type signaling systems: these are a class of signaling systems that are characterized by the presence of LuxI and LuxR proteins. N-acyl-homoserine lactones (AHLs) are involved in quorum sensing in the majority of bacteria species that are Gram-negative. AHLs are produced by the enzyme LuxI and are released through a specific transport mechanism following intracellular accumulation. Their concentration reaches a specific level before they engage with LuxR, subsequently initiating the activation of downstream genes. (ii) Small-molecule peptide-based signaling molecules: Gram-positive bacteria mostly utilize oligopeptide-based quorum sensing (QS) mechanisms, wherein modified small-molecule peptides, namely anti-inducing peptides (AIPs), serve as the messenger molecules. (iii) LuxS/AI-2 type receptor molecules: these molecules primarily function to facilitate signal transmission between two distinct genera. In general, quorum sensing (QS) systems have a significant impact on cellular motility, chemotaxis, and the production of biofilms.
Research has provided empirical evidence regarding the effects of QS on BESs. According to the findings of Venkataraman et al. [56], the activity of quorum sensing (QS) was found to have an impact on the electrical generation of Pseudomonas aeruginosa through the regulation of phenazine synthesis. Based on this observation, various researchers have endeavored to enhance existing output using genetic modification of the quorum sensing (QS) system. In their study, Yong et al. [57] conducted an experiment where they upregulated the rhl QS cassette of Pseudomonas aeruginosa. This resulted in an increase of around 1.6-fold of the optimal power generated compared to the wild-type. One study also incorporated an artificial PqsE gene into a 2-heptyl-3,4-dihydroxyquinoline QS system negative mutant of P. aeruginosa and this genetic modification resulted in a significant five-fold enhancement in the maximum current density compared to the original strain [58]. Another study confirmed that external QS signals can stimulate QS and subsequently improve the electrical performance of MFCs that was established using the extremophile Halanaerobium praevalens [59]. A study by Chen et al. [60] which investigated the impact of AHLs on the behavior of mixed-culture electroactive bacteria (EABs), found that both intrinsic and extrinsic acylhomoserine lactones functioned as regulators enhancing the electrochemical activities of EAMs [60]. The utilization of synthetic biology techniques allows for the design and fabrication of synthetic QS systems. These engineered systems can be tailored to specific environmental biotechnology applications, enabling precise control over biofilm development and electron transfer processes. QS circuits in Escherichia coli have also been successfully engineered to enhance current generation in MFCs [61].
Enhancing electron transfer in environmental biotechnology through quorum sensing manipulation offers several advantages. Firstly, it can improve the efficiency and performance of microbial processes by promoting biofilm formation, which provides a stable and protected environment for microbial communities. Biofilms offer an increased area of contact that promotes the adhesion of microorganisms and facilitates the exchange of electrons between cells and electrodes or other electron acceptors/donors. Secondly, quorum sensing manipulation can enable the development of biofilms with desired characteristics, such as increased thickness or specific microbial compositions. This control over biofilm properties allows for the optimization of electron transfer rates and overall process performance. Lastly, manipulating quorum sensing pathways can also help mitigate issues associated with biofouling. The term “biofouling” pertains to the undesired buildup of microorganisms on various surfaces, leading to potential disruptions in electron transfer mechanisms. By understanding and manipulating quorum sensing mechanisms, it is possible to prevent or control biofouling by promoting the growth of desirable biofilms while inhibiting the growth of undesirable ones. It is imperative to highlight the substantial distinctions between QS signaling molecules and electron shuttling molecules. Electron shuttles serve as redox mediators, facilitating the transfer of electrons between bacteria and electrodes. On the other hand, QS signals possess the capability to regulate the production of electron shuttling molecules but cannot function as redox shuttles themselves.
3.3. Electron Shuttles and Mediators
- (a)
- Use of natural or synthetic compounds as electron shuttles
One class of electron shuttles commonly used in environmental biotechnology is quinones. Quinones are naturally occurring compounds that possess redox-active properties. They can undergo reversible two-electron transfers, wherein they can accept electrons from microbial cells and subsequently donate them to electrodes or other acceptors. Quinones can be found in various environmental matrices, including soils, sediments, and wastewater. Examples of natural quinones are humic substances [30]. In addition to natural quinones, synthetic quinones have also been developed for use as electron shuttles. These synthetic compounds are often designed to have specific redox potentials and electron transfer kinetics, allowing for tailored electron transfer processes. For example, anthraquinone derivatives such as 2,6-anthraquinone disulfonate and 9,10-anthraquinone-2,7-disulfonate have been widely used as synthetic electron shuttles. These compounds have shown significant promise in enhancing the efficiency of microbial fuel cells by facilitating electron transfer and decreasing the overpotential required for electron transfer. The possibility of humic substances functioning as a terminal electron acceptor in the respiration by microbes has been explored by Lovley et al. [62]. Additionally, their research also investigated the ability of humic substances to act as electron carriers between microorganisms that reduce Fe (III) and Fe (III) oxides that are insoluble. The findings indicated that the utilization of Fe (III) oxide with low crystallinity as the electron acceptor for the proliferation of G. metallireducens was significantly enhanced by the introduction of a minimal concentration of 100 μM of the humic analogue, specifically anthraquinone-2,6-disulfonate. Therefore, the incorporation of humic substances can be employed to augment the facilitation of electron transfer between microorganisms that reduce Fe (III) and Fe (III) oxides. This approach holds potential as a viable technique to promote the restoration of soils and sediments that have been contaminated with organic or metallic pollutants [63]. Similarly, the impact of 14 ESs on EET in Shewanella oneidensis MR-1 was investigated by Xu et al. [64]. The results revealed that anthraquinone-2-sulfonate (AQS) exhibited the most significant effects, as it resulted in the highest cathodic current density, total charge production, and formation of reduction products. Thus, it can be inferred that the presence of AQS, functioning as electron shuttles, significantly contributed to enhancing the efficiency of EET between the electrode and microorganisms.
The phenazines, which serve as the major mediators in Pseudomonas aeruginosa, have a vital function in promoting effective EET. The gene known as phzM, which encodes for methyltransferase, has been found to have the ability to augment the synthesis of PYO. Overexpression of this gene in Pseudomonas aeruginosa has been observed to lead to a 1.6-times boost in the production of PYO, and a four-time increment in the optimal energy production [65]. Feng et al. [66] incorporated synthesis-related genes associated with phenazine-1-carboxylic acid (PCA) from Pseudomonas aeruginosa into Escherichia coli. The endogenous PCA led to an improvement in EET efficiency. This enhancement resulted in an optimal power generation of 249 mA/m2 for MFCs, which was found to be twice as high as that observed in the original strain. Consequently, the incorporation of exogenous electron shuttles into non-electroactive microorganisms has been shown to significantly enhance electron exchange efficiency and enhance the performance of MFCs. Furthermore, the interactions between electron shuttles and microorganisms or other components of the system must be thoroughly studied to understand their impact on overall performance. Further research and development in this field will likely lead to the discovery of new and improved electron shuttles, enabling even greater advancements in environmental biotechnology.
- (b)
- The use of conductive nanowires
E-pili or conductive nanowires serve as effective pathways for long-distance electron transfer, facilitating intercellular aggregation and the production of EABs. Advancements in protein design have facilitated the simplification of the fabrication process for synthetic nanowires. A study was conducted to provide a regulatory framework for the fabrication of very stable nanowires. This was achieved by developing protein templates through the utilization of specifically designed linker proteins. Chen et al. [67] employed γPFD (a helical protein produced by the microorganism Methanocaldococcus jannaschi), as a nanomaterial scaffold to facilitate the localization and alignment of cytochromes, resulting in the construction of a nanowire structure within G. sulfurreducens. This methodology not only contributes to the existing conventional techniques for constructing nanowires but also enables the manipulation of nanowire structures, hence broadening the potential applications of natural bioelectrical materials. In recent years, there has been a lot of focus on promoting the synthesis and assembly of e-pili in addition to changing their structure to increase their electrical conductivity. In a study, the nanowire-related genes omcS and omcB in S. oneidensis were modified using genome editing (iEditing), the modified strain was expressed and the growth was assessed. The data showed an increase in cytochrome content and an improvement in EET efficiency, as evidenced by a peak power generation of 310 mA/m2 in the modified strain compared to wild-type [68]. Furthermore, the application of peptides in modifying pilin monomers can enhance the adherence of fimbriae, facilitate the development of electrically conductive nanowire structures known as pilis, and ultimately enhance the possible utilization of biomaterials. To enhance the adaptability of the e-pili surface for improved sensing capabilities or enhanced compatibility with different substances, a study has devised a strategy involving the integration of a concise peptide tag at the carboxyl-terminal of pilA [69]. This modification facilitated the formation of a conductive nanowire structure that exhibited targeted adhesion properties while preserving its ability to conduct electricity. The gene segments that have been produced consist of pilA-6His and pilA-HA. These fragments gave rise to conductive pili that possess a “His-tag” and a peptide “HA-tag”. These tags are located on the external surface of the pili and can bind to metals and antibodies that are specific to them. This approach exhibits considerable promise for application in the development of innovative materials and products. Although there has been significant research conducted on the characteristics and functionalization of conducting nanowires for almost two decades, the number of innovative electronic devices utilizing these nanowires remains extremely limited. The complexity and time-consuming nature of producing conductive nanowires, along with the early stage of their integration into circuits, account for this phenomenon. Hence, it is imperative to employ a straightforward bottom-up methodology that encompasses dependable nanowire biogenesis and enables the regulated production of nanowire electronics.
3.4. Electrode Modifications and Design
Numerous measures have been implemented to augment the efficacy of EET by optimizing electrode materials, operating parameters, and components of MFCs. Additionally, the introduction of metal ions, including Cu2+, Cd2+ and Ca2+ has been explored as a potential strategy [25]. Surface functionalization of electrode materials can enhance EET by increasing surface area, facilitating electron transfer between microbes and electrodes, and enhancing electrical conductivity [70]. This can be achieved through the utilization of molecules that are redox-active or electron shuttles. The utilization of carbon nanomaterials, including graphene, carbon nanotubes, and their composites with non-carbon materials, has been investigated as a means of reducing charge transfer resistance. A positive correlation exists between the geometric surface area of an electrode and its potential to accommodate a microbial colony, which in turn may lead to increased power output (Figure 2a) [71]. However, materials such as felt, veils, or foam electrodes have micro and nano-size geometries, which include the presence of pores. According to existing research, it has been demonstrated that current generation output is dependent upon both the area of the electrode and on pore size [49]. The selection of the type and shape of material to use for the electrodes in MFCs varies according to the volume of the MFC. Commonly used electrode materials are made from carbon. The fabrication of microscale electrodes involves the application or deposition of a thin layer of metal or carbon conductive material to achieve a significant increase in surface area. The utilization of a carbon veil as an electrode material in larger-scale electrodes offers several advantages. Firstly, it provides an ample number of tiny pores that facilitate excellent porosity, allowing for the effective transfer of nutrients by advective transport via perfusion. Secondly, the thread continuity of the carbon veil leads to lower resistance compared to carbon felt or other carbon materials with discontinuous strands [71] (Figure 2b).
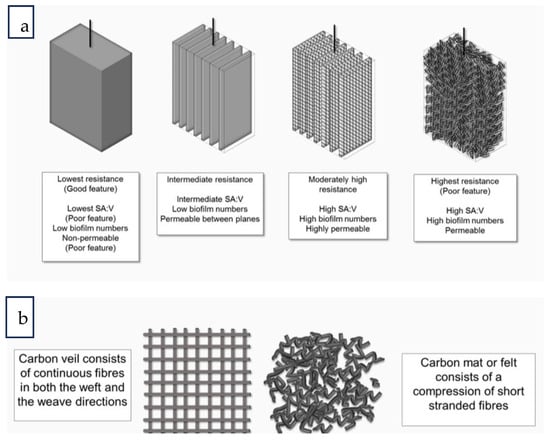
Figure 2.
The structure of MFC carbon electrodes: (a) ordinary electrodes made from carbon with identical geometric macro size, and (b) a comparison between carbon veil and carbon felt (or mat) electrodes. V denotes the ratio of surface area to volume [71].
Additionally, conductive polymers or nanomaterials can improve electrode conductivity and promote direct contact between microorganisms and electrode surfaces. It has also been established that nanostructured electrodes can also improve conductivity, allowing faster and more efficient electron transfer along the electrode surface [72]. These electrodes offer unique properties that promote specific interactions with microorganisms or biomolecules, further enhancing their affinity towards target species involved in EET processes. Nanostructured electrodes have shown promise in applications like MFCs and biosensors, where they enhance sensitivity and selectivity by providing a larger surface area for biomolecular recognition and improving electron transfer efficiency.
3.5. External Electricity or Electric Field
EET can be enhanced through the supply of external resistance. The rate of transfer of electrons amongst microorganisms and the electrode or electron acceptor can be regulated by adding external resistance to the circuit of a bioelectrochemical system. The resistance value affects the kinetics of EET by controlling the flow of electrons through the circuit. While reduced resistance enables faster transfer rates, higher resistance delays the transfer of electrons [73]. In bioelectrochemical systems, external resistance enables the optimization of energy harvesting. It is possible to balance the electron transfer rate with the system’s aims for power output or energy recovery by altering the resistance. This optimization guarantees the effective use of available electron donors, maximizing the capacity for energy production or storage [74].
Several studies have been conducted to examine the effect of external resistance on EET. One study employed a highly varied mixed culture MFC [75] to investigate the effects of different resistance loads on biofilm formation and power output. The researchers noticed that a low resistance load resulted in the formation of thinner biofilms, accompanied by increased current production. Conversely, electrodes subjected to high external resistance loads exhibited the formation of thicker biofilms, but with lower power output. The influence of biofilm permeability and external resistance on MFC current production, microorganism growth, and the transfer of electrons from the redox mediator to the anode was investigated by Cai et al. [76]. Their findings show that lower external resistances resulted in improved MFC performance. MFCs with lower external resistance produced more extracellular mediators in addition to building biofilm with more exoelectrogens. Similarly, it was discovered that a larger external electrical load may severely restrict the flow of electrons between the electrodes [73] and is thus generally unfavorable for MFC power generation. The influence of external resistance on cellular performance was also investigated by Rossi and Logan [77], and it was discovered that modifying the external resistance during the acclimation of MFCs can significantly affect the performance of the anode, and this impact is observed in terms of charge transfer, diffusion, and the overall resistance of the anode. In the majority of cases, a load below 1 kΩ was identified as the most appropriate choice of external resistance [78]. Therefore it was suggested that future investigations aiming to optimize system performance in terms of power production should consider experimenting within this range of load values [78].
3.6. Enhancing EET by Photo
The EET process in bioelectrochemical systems can be enhanced by the use of light, particularly photosynthesis and photovoltaic activities. Photosynthetic bacteria can transmit electrons directly to the electrode surface, enabling effective EET. Advancements in electron transfer phenomena have resulted in the development of photoelectrochemical systems with microbial characteristics and solar energy support. Nevertheless, the limited current density observed in photo-bioelectrochemical cells (PBEC) imposes constraints on their progress and real-world implementation. These studies [79,80] have shown that the introduction of OmcS from Geobacter into genetically altered Synechococcus elongatus PCC7942 led to an increased capacity for EET. This enhancement in EET capacity led to a substantial rise in photocurrent generation at the anode of the PBEC system, about nine-fold greater than the control group. This methodology did not merely enhance the generation of photocurrent in cyanobacteria but additionally, it expedited the advancement of photosynthetic MFC technology. Solar light has been demonstrated to stimulate non-phototrophic bacteria’s metabolic and development activities through the photocatalytic activity of semiconducting minerals [81] and bacteria that do not rely on photosynthesis are capable of converting carbon dioxide into acetate by employing cadmium sulfide nanoparticles in the presence of light [82].
Hematite, a prevalent mineral found in natural environments, has been employed as a photoanode. Analysis of the methods by which semiconducting minerals and microorganisms transport electrons when exposed to solar radiation has advanced in current research. Photo generation is the process by which semiconductor minerals produce electron and hole pairs when exposed to light. The extracellular electron transport pathway of microorganisms may be impacted by this event. Crystalline Fe (III) oxide is known to be excited by light and is frequently used as an electron acceptor in the natural environment by extracellular electron transfer microbial systems.
The potential of visible light excitation for outer membrane c-type cytochromes in dissimilatory metal-reducing bacteria was investigated by Zhang et al. [83] and the impact of excited c-type cytochromes on the EET process between bacteria and semi-conductive electron acceptors using a TiO2-coated electrode was demonstrated. According to the study, the EET rate to TiO2 was observed to exhibit a significant increase of more than eight-fold upon exposure to visible light. Similarly, the interaction between a hematite photoelectrode and P. aeruginosa PAO1 when subjected to visible light irradiation was studied by Ren et al. [84] as depicted in Figure 3.
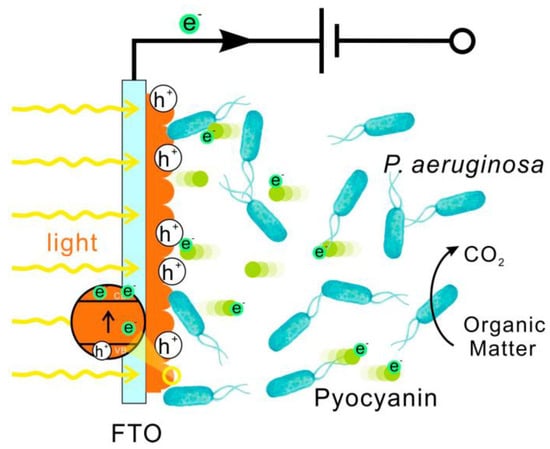
Figure 3.
An illustration of the electron transfer mechanism between hematite and PAO1 when exposed to visible light [84].
According to this study, the photocurrent density at the hematite electrode was 240% higher in the presence of light. This showed that light enhanced the transfer of electrons from hematite to PAO1, and also suggests that the microorganism Pseudomonas aeruginosa PAO1 also played a crucial role in this process. Likewise, the extent to which visible light affected electron transport between birnessite photoanodes and Pseudomonas aeruginosa PAO1 was investigated by Ren et al. [85]. The photocurrent density of the electrochemical system consisting of light, birnessite, and PAO1 was 279.57 μA/cm2 under circumstances of light illumination and positive bias. This number was 322% and 170% higher than the photocurrent densities reported in the abiotic control and dead culture, respectively. These data suggest that birnessite and Pseudomonas have a photo-enhanced electrochemical interaction and the study exhibited a rapid light-induced EET process between the bacterium Pseudomonas aeruginosa PAO1 and the semiconducting material birnessite. The findings of the study also contributed to the enhancement of our comprehension regarding the correlation between the EET exhibited by microorganisms and the photocatalytic properties of semiconducting minerals within their natural habitat. Also, the findings have the potential to pave the way for the future creation of bioelectronic devices that harness solar energy.
Furthermore, the marine euphotic zone, focusing on semiconducting minerals and microbial communities was also investigated by Liu et al. [86]. They found that semiconducting minerals can interact with electroactive microorganisms, enhancing electron transfer. However, current research on the interplay between light, semiconducting minerals, and microbes is limited, mainly focusing on bacteria and solar irradiation. Further research is needed to understand the electron transfer phenomenon between microorganisms and mineral photocatalysis.
3.7. Enhancing EET by Magnetism
The application of magnetism can serve to augment the effectiveness of EET. Magnetic nanoparticles, such as magnetite (Fe3O4) or maghemite (γ-Fe2O3), can function as electron mediators in EET processes. Numerous scholars have employed the implementation of a magnetic field to enhance the efficiency of EET between the EABbeing applied and the surface of the electron acceptor.
The possibility of magnetic effects as a cost-effective technique for increasing the performance of MFCs using a magnetic field with an intensity of 100 mT was investigated by Velasquez-Orta et al. [87]. The absence of air in the MFC system resulted in a more significant loss in potential. The use of a magnet may be advantageous in instances when air supply is not feasible or when there is a need to reduce costs associated with air supply. A magnetic field with 100 mT intensity was used by Tong et al. [88] to reduce the startup time of a one-chamber MFC supplied with mixed wastewater from six days to two days.
Likewise, an enhancement in the performance of MECs through the utilization of magnetite nanoparticles (Figure 4) was reported by Wang et al. [89].
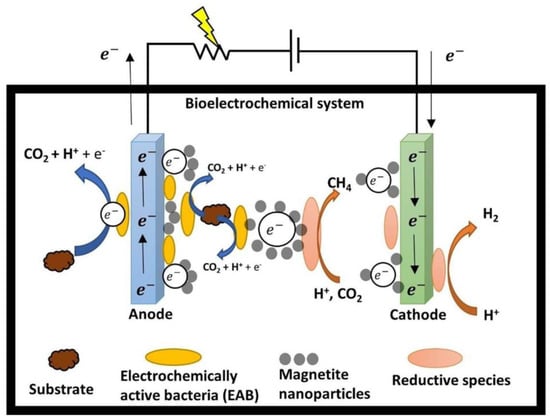
Figure 4.
Conceptual depiction of an electron transfer pathway established by magnetite nanoparticles, facilitating the ease of electron movement between microorganisms and electrodes [90].
The observation suggests that magnetite nanoparticles possess the ability to function as electron conductors, allowing for the flow of electrons from the surface of the anode to facilitate reduction reactions over significant distances. The performance of three different configurations of MFCs inoculated with a pure culture of Shewanella oneidensis was evaluated by Li et al. [91]. The findings of the study demonstrated that all three configurations exhibited a substantial voltage output when subjected to magnetic fields. The observed increase in efficiency can be attributed to an elevation in the mediator’s discharge and the catalytic activity of S. oneidensis. This led to a more rapid transfer of electrons and a reduction in the loss of activation energy. It was also found that the utilization of a magnetic field has the potential to diminish the resistance encountered during electron transport to the anode [92]. Consequently, this reduces the internal resistance of MFCs, ultimately resulting in an improvement in their overall performance. Among the three different intensities of magnetic fields (MF) examined by Yin et al. [92] only the 200 mT intensity demonstrated an outstanding ability to enhance electricity generation. As a result, insufficient or excessive levels of intensity can lead to outcomes that are inconsistent with the intended objectives. It was found that the application of an appropriate static magnetic field can augment the extracellular electron transfer mechanism in microorganisms, resulting in an elevation of the maximum voltage output [93].
Excessive field strength may impede microbial growth and result in unfavorable consequences. The optimal magnetic field strength range that promotes the proliferation of EAMs and consequently amplifies the power generation of MFCs remains undetermined and requires additional investigation. Therefore, it is imperative to conduct a multi-omic approach in investigating the molecular regulation of genes and proteins related to EET to comprehend the mechanism behind the stimulation of EET by the magnetic field [94].
The effects of different magnetic field intensities on both attractive and repulsive fields were investigated by Tao and Zhou [95] and the findings demonstrated that the utilization of magnetic fields with intensities of 50 and 100 mT led to an augmentation in electricity generation and a faster initiation of the process, particularly when employing the 50-mT magnetic field intensity. Conversely, the overall performance was adversely affected when the magnetic field intensity was increased to 200 mT. The resultant findings indicated that consistent with prior research, the operation of MFCs is negatively impacted by MFs at elevated intensities. The appropriate selection of MF intensity may have a gradual impact on the electrochemical performance of BESs. Nonetheless, such an effect must be maintained over an extended duration. To assess the impact, four distinct intensities of MF specifically, 20, 120, 220, and 360 mT were investigated by Zhao et al. [93]. The findings of the study demonstrated a notable enhancement in electricity generation and overall efficiency across various levels of intensity, with the most optimal performance observed at an intensity of 220 mT. An in-depth analysis of biofilm development also unveiled a noticeable disparity in the adhesion of microorganisms onto the carbon-felt surface used. Their findings illustrated that the presence of biofilm is significantly reduced at 360 mT compared to the control and bare carbon felt. This suggests that elevated intensities may have a detrimental effect on the formation of biofilm.
The utilization of an external magnetic field and an applied potential in MES exhibited a mutually beneficial effect in stimulating EPS secretion and promoting the proliferation of EAB on the surface of the electrode. Microalgae was employed in a biocathode as a means to address the issue of membrane resistance resulting from catholyte accumulation [96]. From the study, the utilization of a magnetic field with an intensity of 200 mT resulted in a greater biomass concentration and led to enhanced cell performance through expedited oxygen reduction reactions. The aforementioned observation suggests that the utilization of MF exhibits a considerable capacity and is appropriate for augmenting bioelectrochemical reactions.
3.8. Integrated Strategies for Enhancing EET
Presently, there is a growing interest among researchers in investigating the integration of different modalities to enhance EET and improve the performance of MES. Here we present the various contemporary technologies that can be integrated with BESs to enhance its efficiency.
- Additive manufacturing (AM): AM, commonly referred to as 3D printing, is a rapidly advancing technological innovation that has made significant contributions to MFC technology in recent times.A study by Calignano et al. [97] (Figure 5) explored the limits of AM technology in the creation of a powering device that is entirely based on AM. The study focused on utilizing low-density and open porosities to accommodate microorganisms, as well as developing systems that are easily fueled continually and operate securely. A maximum energy recovery of approximately 3 kWh m−3 per day was achieved, which is capable of supplying power to sensing devices and low-energy electronic gadgets. This facilitates data conveyance and analysis from remote and challenging situations. AM facilitates the design for the assembly of MFCs, thereby mitigating mistakes as a result of human intervention and expediting the installation procedure. In a study by Zawadzki et al. [98], AM techniques were also employed to construct an MFC with the incorporation of an exchangeable membrane slot. This enabled them to examine the effects of different separator membranes. The utilization of AM in the production of tango membranes has demonstrated promising prospects for membrane manufacturing and power-generating capabilities. However, it is worth noting that the power output achieved (0.92 μW) by this method was relatively low in comparison to the conventional cation exchange membrane technology (11.39 μW). Similarly, Slate [99] investigated enhancing the power outputs of MFCs with the integration of graphene into electrodes fabricated using 3D printing technology. The electrodes underwent an evaluation to assess their electrical efficiency, chemistry, and appearance, thereby showcasing the prospective application of AM in the production of MFCs. Furthermore, AM technology, such as inkjet printing, was used in a study by Sawa et al. [100]. The research showcased the practicability of employing a readily available commercial inkjet printer for the production of a thin-film paper-based biophotovoltaic cell. This cell comprised a layer of cyanobacterial cells positioned above a conducting surface composed of carbon nanotubes. The researchers discovered that the printed cyanobacteria can produce a continuous electrical current for a duration exceeding 100 h. This phenomenon occurs both in the absence of light, functioning as a “solar bio-battery”, and in the presence of light, functioning as a “bio-solar-panel”. These findings suggest its possible use in low-power devices. The utilization of the inkjet printing technique facilitated precise positioning of the cyanobacteria onto the anode surface, hence facilitating the proximity of the printed cathode. The printing technique was optimized to achieve ideal biofilm layer thicknesses to maximize power generation. The aforementioned authors were pioneers in employing an inkjet printer to print photosynthetic microorganisms, specifically cyanobacteria, onto a bio-photovoltaic (BPV) cell. Consequently, AM offers the potential to utilize diverse materials and 3D printing techniques to develop a comprehensive MFC tailored to different applications. This helps to expedite the optimization process of individual MFC components, leading to enhanced effectiveness and ease in reactor design and consequently, the system will experience functional improvements. AM has the potential to significantly impact the field of biological power generation devices due to its advantages such as decreased lead time, enhanced design flexibility, cost-effectiveness, and expedited prototyping capabilities. In addition, AM offers a shorter route from the research laboratory to large-scale production. Ultimately, the advancements offered by AM can be effectively implemented in several other bioelectronic systems, including biological sensors, implantable biomedical devices, and biocomputing systems [101].
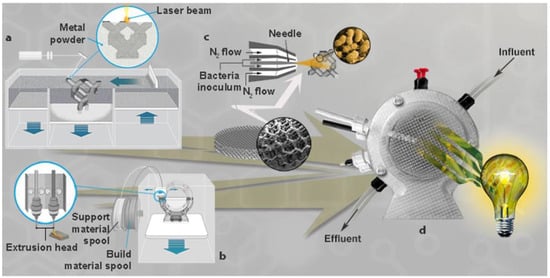 Figure 5. Three distinct additive manufacturing processes are employed in the fabrication process of revolutionary microbial fuel cells (MFCs). (a) In Selective Laser Melting (SLM) technology the 3D object which is to be fabricated is divided into layers of a predetermined thickness of 30 μm and thereafter, transferred to the SLM machine. The process begins with the application of a layer of powdered material onto the building platform and the powders are fully melted along the designated path corresponding to the initial section of the component. The substrate platform thereafter undergoes a reduction in layer thickness along the z-axis, followed by the application of a new coating, and this iterative process continues until the whole part is complete. (b) Fused Deposition Modelling (FDM) technology operates by sequentially constructing parts in a layered manner (bottom-to-up approach), wherein a thermoplastic filament is heated and extruded. The thermoplastic material is subjected to heat within the 3D printer, causing it to transition into a partially liquid state. Subsequently, this semi-liquid material is deposited along the extrusion path in the form of ultra fine beads. (c) The bacteria is then sprayed using an Anest-Iwata spray gun. An airbrush connected to nitrogen gas is used to directly spray five milliliters of inoculum, or approximately 10% of the anodic solution, onto the 3D anode surface of the Al structure. The inoculum is then preserved at 4 °C in a liquid medium until the MFC assembly. (d) MFC assembly [97].
Figure 5. Three distinct additive manufacturing processes are employed in the fabrication process of revolutionary microbial fuel cells (MFCs). (a) In Selective Laser Melting (SLM) technology the 3D object which is to be fabricated is divided into layers of a predetermined thickness of 30 μm and thereafter, transferred to the SLM machine. The process begins with the application of a layer of powdered material onto the building platform and the powders are fully melted along the designated path corresponding to the initial section of the component. The substrate platform thereafter undergoes a reduction in layer thickness along the z-axis, followed by the application of a new coating, and this iterative process continues until the whole part is complete. (b) Fused Deposition Modelling (FDM) technology operates by sequentially constructing parts in a layered manner (bottom-to-up approach), wherein a thermoplastic filament is heated and extruded. The thermoplastic material is subjected to heat within the 3D printer, causing it to transition into a partially liquid state. Subsequently, this semi-liquid material is deposited along the extrusion path in the form of ultra fine beads. (c) The bacteria is then sprayed using an Anest-Iwata spray gun. An airbrush connected to nitrogen gas is used to directly spray five milliliters of inoculum, or approximately 10% of the anodic solution, onto the 3D anode surface of the Al structure. The inoculum is then preserved at 4 °C in a liquid medium until the MFC assembly. (d) MFC assembly [97]. - Artificial Intelligence (AI) and Machine Learning: Numerous research investigations have been undertaken to examine the performance of mathematical models in the field of artificial intelligence (AI). These studies include the works of [102,103,104,105]. A study by de Ramon-Fernande et al. [106] employed an artificial neural network (ANN) computational model to investigate the relationship between power output and flow rate in a ceramic MFC that was supplied with human excreta, specifically urine. The training, comparison, precision forecasting, and time to convergence assessment were performed using three algorithms: the quasi-Newton technique, the Levenberg–Marquardt algorithm, and the conjugate gradient method, all of which are of third order. The Levenberg–Marquardt algorithm had superior performance in comparison with alternative models in terms of precision, with an R-value of 95%. Additionally, it exhibited faster convergence, with a time of 7.8 s. The findings of the study demonstrate that the use of an ANN computational model is both helpful and accurate for predicting energy requirements in ceramic MFCs when subjected to varying flow rates. Additionally, the feasibility of adopting this model for energy forecasting in ceramic MFCs is established. Lesnik and Liu [107] also implemented the utilization of artificial neural network (ANN) models to forecast the biofilm communities and reactor performance of MFCs. The research observed that ANN models that integrated biotic interactions demonstrated superior accuracy in predicting reactor performance outcomes compared to models that did not incorporate such interactions. This finding suggests that data mining and machine-learning techniques can effectively forecast the behavior of microbial communities and the performance of bioelectrochemical systems. In another study by Zhang et al. [108], a four-input ANN model was effectively utilized to accurately forecast the levels of glycerol, 1,3-PDO, and biomass. The proposed model exhibits applicability not only in the realm of software for online measurement of glycerol and 1,3-PDO during the industrialization process but also offers valuable insights for other fermentation processes. In BESs, substrate prediction can also be carried out with AI-based techniques. In a study by Cai et al. [109], six machine learning algorithms were trained and it was discovered that feed substrates could be predicted using information about the microbial community. These algorithms included logistic regression multiclass (GLMNET), random forest (RF), the scalable tree boosting system (XGBOOST), the neural network (NNET), k-nearest neighbor (KNN), and the support vector machine with radial kernel (SVM). The NNET method demonstrated the highest accuracy (93 ± 6%), allowing for the distinction between various feed substrates [110]. Similarly, a study by Leropoulos et al. [111] applied automation by utilizing a robotic platform known as EcoBot. This platform was developed using artificial intelligence techniques derived from artificial evolution. The concept aimed to leverage the use of robots in facilitating evolutionary processes to create functional ecosystems that establish symbiotic relationships between living organisms and artificial life chemistries. This results in an enhancement of energy generation in MFCs. The conversion of a robot platform, derived from an open-source 3D printer, enables its utilization for liquid management and real-time monitoring of experimental outcomes. The outcomes derived from these illustrative instances demonstrate that artificial intelligence has the potential to enhance and evaluate the performance of MFCs. Furthermore, genetic programming (GP) is an additional technique that makes it possible to optimize the AI model’s structure by minimizing errors through the use of algorithms like cross-validation and the Kennard–Stone algorithm [110]. To incorporate novel components into bacterial cells, one can use the de novo protein design technique. The assembly of peptides into protein structure is connected to the design architecture of de novo and the bundling of α-helices into metal-binding proteins is one way to accomplish this. Short gene fragments are integrated into the genes of E. coli in bacterial surface display systems and LamB, OmpA, Lpp-OmpA, and PhoE coli OM proteins coordinate protein integration into the outer membrane cells [112]. Similarly, genetically modifying E. coli was used to facilitate EET by introducing a biosynthetic pathway for Pseudomonas phenazine-1-carboxylic acid [113]. The findings of the study demonstrated that the implementation of this electrochemical system resulted in a notable augmentation in the synthesis of phenazine-1-carboxylic acid, consequently improving the EET efficiency of MFCs. Another versatile algorithm for flexible diagnosis and control is fuzzy logic (FL). This technique is capable of observing data and forecasting system outputs logically. FL is frequently used as an intermediate step to streamline data processing and lessen the negative impact on model accuracy [114].
- Hybrid Systems: The hybrid technique is another system which integrates two treatment systems, resulting in the enhanced performance efficiency of the overall system. It was noted that the implementation of a constructed wetland (CW)-MFC containing activated carbon granules and manganese ore can enhance the biochemical processes involved in N-transformations in the absence of oxygen [115].
The plant–microbial fuel cell (Plant–MFC) is also an emerging technology that can effectively harness solar energy to generate bioelectricity through the symbiotic link between plants and microorganisms in the rhizosphere region. Table 1 shows the different plants that have been utilized in Plant–MFCs and the maximum power density obtained in the different scenarios.

Table 1.
Power density obtained in different plant–microbial fuel cells.
Additionally, the efficacy of EET can be augmented through the concurrent integration of mediator material reinforcement and light amplification. Research has demonstrated that Shewanella xiamenensis is capable of internalizing carbon dots at the nanoscale level [127]. Carbon dots have been found to not only augment the electrical conductivity of cells but also stimulate the secretion of a significant quantity of flavin-like ESs, thereby facilitating the process of EET [128]. In addition, internalized carbon dots can augment the process of EET under illumination by enabling light-enhancing mechanisms that promote electron transfer to cellular metabolism [129]. Certain investigations have combined the biological anode and photoanode to increase the power generation of the photocoupled system [130]. This has been achieved by creating a hybrid photoelectrochemical and microbial electrochemical system that harnesses both solar and biological energy. The cathode reaction barrier can be effectively reduced by the activation of the biocathode reaction through the amalgamation of visible light as an energy source. The biocathode can use the difference in voltage between the anode and the cathode to make it easier to separate photogenerated hole/electron pairs [131]. The study conducted by [132], involved the design and testing of an air-cathode MFC-adsorption hybrid system, which utilized an earthen pot. The purpose of the system was to achieve simultaneous wastewater treatment and energy recovery. The design exhibited notable attributes including a low internal resistance of 29.3 Ω, making it cost-effective andthus resulting in efficient wastewater treatment and power generation. It achieved a power output of 55 mW/m3 while maintaining an average current of 2.13 ± 0.4 mA. A comparison was made between the performance of the MFC-adsorption hybrid system and the standalone adsorption system. The results indicated that the integrated system exhibited significant removal of contaminants.
The field of synthetic biology has also been seen to integrate biology with engineering to advance the efficient EET of electroactive cells. Recent advancements in synthetic biology techniques include expanding the range of feedstocks, enhancing intracellular electron generation, improving conductive cytochrome systems, stimulating the production and release of ESs, and creating conductive biofilms [61]. Numerous research endeavors have integrated synthetic biology with mediator material augmentation, light amplification, and other modalities to facilitate EET. Proteorhodopsin has been utilized to augment the efficacy of substrate absorption, specifically lactate, in S. oneidensis [133]. The study found that upon illumination, there was an acceleration in the consumption of lactate, and this led to a 250% increase in current production when compared to the wild strain of MR-1.
In addition, the efficacy of Osmium-based polymers has been substantiated in promoting direct extracellular electron transfer (EET) in organisms that do not possess the intrinsic capability to engage in such processes. This accomplishment which has been documented by Myers et al. [134] is accomplished by altering the surfaces of electrodes and integrating redox-active osmium components into the polymer’s framework. To elucidate this phenomenon, the Gram-positive bacterium Enterococcus faecalis was used by Pankratova et al. [135] to examine its electrochemical interaction with electrodes. The findings of the study revealed that the experiments employing the redox polymer demonstrated the highest current density. Conversely, the control experiments conducted without the redox polymer did not manifest any discernible current response. The increasing interest in hybrid systems originates from the numerous issues encountered with standalone MFC systems. The development of a hybrid MFC system has become possible due to technological advancements and the combination of diverse technological innovations. A hybrid MFC system can be constructed through the incorporation of biological, chemical, and physical elements to meet the requirements of various applications, including power production, wastewater management, separation of chemicals, and desalination.
In conclusion, the integration of BESs with other contemporary technologies offers a multitude of benefits including enhanced energy efficiency, improved operational performance, cost savings, and sustainability.
4. Challenges and Future Directions
Challenges associated with the diminished effectiveness of EET in practical contexts include the following:
- Diversity of microorganisms: The effectiveness of electrochemically active microbial communities in BESs can be impacted by their diverse features [136]. The coexistence of diverse species can lead to competition for resources and electron donors/acceptors, impacting the efficiency of EET. Syntrophic interactions between different species can also affect EET efficacy. Changes in microbial diversity resulting from variations in environmental circumstances or disturbances, such as pH, temperature, and oxygen levels, can induce modifications in EET. The structure and composition of biofilms on electrode surfaces can also be influenced by microbial diversity. Different microbial species use different methods for electron transfer, and the effectiveness and kinetics of EET can be influenced by the presence of different microbial species that possess unique electron transfer mechanisms. A diverse microbial community can also display a broad spectrum of redox potentials, potentially causing inefficiencies if there is a lack of alignment between the electron donor and acceptor potentials.
- Scaling up: While laboratory-scale studies have demonstrated the potential of BESs for various applications, scaling up these systems for practical implementation poses significant challenges [137], including the design and arrangement of electrodes, achieving uniform current distribution, and integrating nanostructured materials like carbon nanotubes or graphene into larger systems. Ensuring biofilm stability and uniformity across larger electrode surfaces is crucial for efficient performance. Additionally, expanding EET operations requires supplementary energy inputs, ensuring equilibrium between input and output, and considering the financial implications of implementing the technology. Adherence to environmental and safety regulations is also essential for real-world implementation, as the size of systems increases.
- Substrate availability and selectivity: The efficacy of EET processes is contingent upon the presence of appropriate electron acceptors or donors within the surrounding environment [138]. Furthermore, the selection and accessibility of acceptable substrates for bioelectrochemical systems may be constrained. Numerous systems are dependent on intricate organic substrates or wastewater, the accessibility and economic viability of which may not be generally prevalent. In the context of bioremediation applications, the utilization of EET by microorganisms to break down pollutants relies heavily on the presence of electron acceptors, specifically metal oxides or electrodes, which play a vital role in supporting and maintaining microbial activity. The presence of a limited amount of substrate can result in partial degradation of pollutants, hence diminishing the overall efficiency of remediation processes. Likewise, within MFCs, the process of microbial oxidation of organic materials to produce electricity via EET can be hindered by inadequate substrate availability, resulting in diminished power generation and compromised overall MFC efficiency. Additionally, there is the possibility of substrate competition, when microorganisms exhibit a preference for other pathways instead of EET, resulting in a decrease in the efficiency of electron transfer.
- Biofouling and system stability: The occurrence of biofouling results in the development of insulating layers on the surfaces of electrodes [139], and this can impede electron transmission between microbes and electrodes. Consequently, this results in instability in bioelectrochemical systems due to variations in biofilm thickness, microbial composition, and detachment–reattachment dynamics. Biofouling can also deteriorate electrode materials, necessitating frequent replacements. Effective management and prevention of biofouling often require increased maintenance efforts, which can be costly and result in logistical complexities in the practical implementation of BESs.
- Integration with renewable energy systems: Renewable energy sources, like solar or wind power, can be integrated with bioelectrochemical systems to improve their overall sustainability and energy efficiency [140]. Nevertheless, there are obstacles to overcome concerning power management, system integration, and the optimization of the synergy between the various components. Numerous forms of renewable energy, including wind and solar, are intermittent by nature. The intermittent nature of this power supply may result in variations in its accessibility, which may have an impact on the reliability and consistency of EET operations. It can be difficult to deal with the intermittent character of renewable energy sources with the continuous operation necessary for specific EET applications. An additional obstacle that may arise is the efficacy of transforming renewable energy into electrical power, which is subsequently employed in EET processes. It is essential to maximize overall efficiency by minimizing losses at each stage of the system and optimizing energy conversion processes. Unanticipated obstacles, such as fluctuations in microbial community dynamics or severe weather conditions, may affect the dependability and stability of the integrated system. Therefore, it is crucial that the stability and resilience of long-term practical implementation of integrated EET and renewable energy systems can be guaranteed.
The abovementioned factors can contribute to the complexity of EET making it challenging to fully adopt in practical contexts. However, viable solutions to the challenges include:
- Leverage advanced molecular techniques for microbial community analysis and engineering: Advanced molecular techniques, including metagenomics, metatranscriptomics, and metaproteomics, offer the opportunity to thoroughly analyze the composition, functional capabilities, and activity of microbial communities in various ecological settings [141]. Through the implementation of these techniques, it becomes feasible to discern crucial microbial participants engaged in EET and attain a deeper understanding of their metabolic pathways, processes of electron transfer, and interconnections within intricate ecological systems. In addition, the utilization of sophisticated molecular techniques enables precise modification of microbial communities to enhance the efficiency of EET. This may encompass the engineering of crucial EET-related genes in dominant or electrochemically active microorganisms, including the creation of synthetic microbial communities with predetermined functions in electron transfer mechanisms. The utilization of molecular tools enables the development of customized strategies to address the adverse effects of microbial diversity on EET applications.
- Development of advanced electrode materials and reactor designs: It is possible to create electrodes that possess improved conductivity and surface characteristics that enable efficient long-distance electron transmission in larger-scale systems. Furthermore, the use of innovative reactor designs that enhance mass transport and promote biofilm development [142] can play a significant role in addressing the obstacles related to the scaling up of EET processes.
- Utilizing a wide range of substrates: Various microbes possess unique metabolic capacities. The provision of varied substrates will promote the proliferation of a heterogeneous microbial community, characterized by distinct enzymatic activities and pathways involved in EET. The adaptability of these entities enables them to adjust to varying circumstances and guarantees uninterrupted electron transport, even in situations when there is a scarcity of particular substrates. This phenomenon may also serve to bolster the stability and structure of biofilms, as a well-organized biofilm can improve the effectiveness of EET mechanisms by creating a conducive environment for microbial adhesion and electron transfer.
- Managing and controlling biofouling: The modification of surfaces involved in EET systems has the potential to mitigate the occurrence of biofouling. One such approach is the utilization of electrode materials possessing anti-fouling capabilities [139]. As biofouling is frequently driven by biofilm production, techniques aiming at stimulating the formation of conductive biofilms through the selection of electrode materials that enable the growth of conductive biofilms can be beneficial. Furthermore, efforts for controlling biofilm thickness and composition will aid in maintaining a balance between the advantages of biofilm-mediated electron transfer and the possible disadvantages of excessive biofilm growth.
- Introduction of energy storage and hybrid systems and smart grid technologies: Energy storage technologies, such as batteries and pumped hydro storage [140], can efficiently store surplus energy created during periods of high production and subsequently release it during periods of low output. By incorporating these storage devices with sustainable energy sources, the variations in power availability can be alleviated, hence offering a more uniform and reliable power provision for EET operations. Moreover, the integration of various renewable energy sources or the fusion of renewable energy with conventional power sources gives rise to hybrid systems. This strategy effectively broadens the range of energy sources utilized and reduces the impact of intermittent energy supply. Furthermore, the integration of smart grid technologies facilitates enhanced control and allocation of electrical power resources. These systems possess the potential to effectively manage the equilibrium between supply and demand by dynamically adjusting to variations in the availability of renewable energy sources.
BESs have garnered considerable interest in recent years as a developing technology capable of mitigating a range of environmental issues. This technology possesses the capacity to fundamentally transform how we react to environmental concerns, encompassing waste management, energy generation, resource retrieval, and pollution mitigation. Within the domain of wastewater treatment, BESs assume a pivotal and transformational function. Conventional treatment methodologies frequently entail substantial energy use, as such BESs provide a viable and sustainable solution by utilizing the energy generated through microbial metabolisms to facilitate electrochemical reactions, hence promoting energy efficiency and cost-effectiveness in the treatment of wastewater. BESs play a significant role in the retrieval of valuable resources from waste streams. Through the utilization of the metabolic processes of microbes, BESs facilitate the harnessing of energy in the form of electricity, as well as the conversion of organic substances into valuable outputs like methane or hydrogen. This is consistent with the ideas of a circular economy, wherein waste is transformed into a valuable resource. In the global pursuit of carbon emissions reduction, BESs present a viable and sustainable pathway. They enable the processing of organic waste while minimizing dependence on energy sources derived from fossil fuels. The decrease in the carbon footprint holds significant importance within the framework of addressing climate change and transitioning towards more environmentally friendly energy alternatives. BESs also play a significant role in the field of bioremediation by augmenting the process of pollutant breakdown. The microorganisms present in BESs have a high level of efficacy in the degradation of pollutants found in soil or water, hence providing a sustainable and environmentally conscious approach to remediation purposes. These findings have significant implications for the mitigation of problems such as soil pollution, oil spills, and several other types of environmental contamination. BESs also have the potential to function as effective platforms for the monitoring of water quality. The feasibility of real-time monitoring of water parameters is enhanced by the integration of microbial sensors into electrochemical systems. This is crucial for early detection of contaminants and ensuring the safe use of water resources. BESs play a crucial role in facilitating the advancement of integrating renewable energy sources and one example involves the integration of BES technology with solar panels or wind turbines, resulting in hybrid systems that effectively utilize both microbiological and conventional renewable energy sources to ensure continuous power output.
Although the potential of this technology is evident and ongoing research in this domain is advancing at a rapid pace, significant strides need to be made before this technology can be implemented effectively. Frequent and current reviews about these subjects will be important to enhance comprehension and facilitate swift progress in the field of technology. Continued monitoring and assessment of the feasibility of various process configurations should be conducted by continued life cycle assessment (LCA) and technoeconomic studies. Despite being viewed as a technology that is still in the process of development, MFCs are expected to have a significant impact in addressing our environmental concerns.
Author Contributions
Conceptualization, Y.X. and O.O.H.; validation, Y.X., O.O.H. and B.Z.; investigation, Y.X. and B.Z.; writing—original draft preparation, O.O.H.; writing—review and editing, Y.X., O.O.H. and B.Z.; visualization, Y.X., O.O.H. and B.Z.; supervision, Y.X.; funding acquisition, Y.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Natural Science Foundation of Fujian for Distinguished Young Scholars (2021J06036), the National Natural Science Foundation of China (22276183), the Youth Innovation Foundation of Xiamen (3502Z20206089), and the Youth Innovation Promotion Association, Chinese Academy of Sciences (Y2022082).
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Spietz, R.L.; Payne, D.; Szilagyi, R.; Boyd, E.S. Reductive biomining of pyrite by methanogens. Trends Microbiol. 2022, 30, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Naaz, T.; Kumar, A.; Vempaty, A.; Singhal, N.; Pandit, S.; Gautam, P.; Jung, S.P. Recent advances in biological approaches towards anode biofilm engineering for improvement of extracellular electron transfer in microbial fuel cells. Environ. Eng. Res. 2023, 28, 220666. [Google Scholar] [CrossRef]
- Wang, Y.X.; Hou, N.; Liu, X.L.; Mu, Y. Advances in interfacial engineering for enhanced microbial extracellular electron transfer. Bioresour. Technol. 2022, 345, 126562. [Google Scholar] [CrossRef]
- Zou, L.; Huang, Y.-H.; Long, Z.-E.; Qiao, Y. On-going applications of Shewanella species in microbial electrochemical system for bioenergy, bioremediation and biosensing. World J. Microbiol. Biotechnol. 2019, 35, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Patil, S.A. Strategies for improving the electroactivity and specific metabolic functionality of microorganisms for various microbial electrochemical technologies. Biotechnol. Adv. 2020, 39, 107468. [Google Scholar] [CrossRef]
- Sibi, G. Environmental Biotechnology: Fundamentals to Modern Techniques; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Tucci, M.; Viggi, C.C.; Núnez, A.E.; Schievano, A.; Rabaey, K.; Aulenta, F. Empowering electroactive microorganisms for soil remediation: Challenges in the bioelectrochemical removal of petroleum hydrocarbons. Chem. Eng. J. 2021, 419, 130008. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Sun, J.; Zhang, L.; Jiao, J.; Wang, Q.; Liu, S. Nanomaterials Facilitating Microbial Extracellular Electron Transfer at Interfaces. Adv. Mater. 2021, 33, 2004051. [Google Scholar] [CrossRef]
- Zhao, J.; Li, F.; Cao, Y.; Zhang, X.; Chen, T.; Song, H.; Wang, Z. Microbial extracellular electron transfer and strategies for engineering electroactive microorganisms. Biotechnol. Adv. 2020, 53, 107682. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, R.J. Bidirectional extracellular electron transfers of electrode-biofilm: Mechanism and application. Bioresour. Technol. 2019, 271, 439–448. [Google Scholar] [CrossRef]
- Wen, L.; Huang, L.; Wang, Y.; Yuan, Y.; Zhou, L. Facet-engineered hematite boosts microbial electrogenesis by synergy of promoting electroactive biofilm formation and extracellular electron transfer. Sci. Total Environ. 2022, 819, 153154. [Google Scholar] [CrossRef]
- You, Z.; Li, J.; Wang, Y.; Wu, D.; Li, F.; Song, H. Advances in mechanisms and engineering of electroactive biofilms. Biotechnol. Adv. 2023, 66, 108170. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, H.; Zhang, J.; Qin, H. A short review of graphene in the microbial electrosynthesis of biochemicals from carbon dioxide. RSC Adv. 2022, 12, 22770–22782. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Tawalbeh, M.; Javed, R.M.N.; Al-Othman, A.; Almomani, F. The novel advancements of nanomaterials in biofuel cells with a focus on electrodes’ applications. Fuel 2022, 322, 124237. [Google Scholar] [CrossRef]
- Gemünde, A.; Lai, B.; Pause, L.; Krömer, J.; Holtmann, D. Redox mediators in microbial electrochemical systems. ChemElectroChem 2022, 9, e202200216. [Google Scholar] [CrossRef]
- Wang, X.; Chen, T.; Gao, C.; Xie, Y.; Zhang, A. Use of extracellular polymeric substances as natural redox mediators to enhance denitrification performance by accelerating electron transfer and carbon source metabolism. Bioresour. Technol. 2022, 345, 126522. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, J.A.; Bacame-Valenzuela, F.J.; Manríquez, J.; Castañeda-Zaldívar, F.; Reyes-Vidal, Y. Electrochemical analysis of extracellular electron transfer process of Pseudomonas aeruginosa NEJ07R using pyocyanin on a carbon electrode. J. Environ. Chem. Eng. 2023, 11, 110708. [Google Scholar] [CrossRef]
- Heijne, A.t.; Liu, D.; Sulonen, M.; Sleutels, T.; Fabregat-Santiago, F. Quantification of bio-anode capacitance in bioelectrochemical systems using Electrochemical Impedance Spectroscopy. J. Power Sources 2018, 400, 533–538. [Google Scholar] [CrossRef]
- Zhou, Y.; Takahashi, Y.; Fukuma, T.; Matsue, T. Scanning electrochemical microscopy for biosurface imaging. Curr. Opin. Electrochem. 2021, 29, 100739. [Google Scholar] [CrossRef]
- Fatima, S.; Aarti, T.; Sridhar, S. Overview of wastewater treatment approaches related to the microbial electrochemical system. In Advanced Nanomaterials and Nanocomposites for Bioelectrochemical Systems; Elsevier: Amsterdam, The Netherlands, 2023; pp. 57–80. [Google Scholar] [CrossRef]
- Zeppilli, M.; Cristiani, L.; Dell’Armi, E.; Villano, M. Potentiostatic vs galvanostatic operation of a Microbial Electrolysis Cell for ammonium recovery and biogas upgrading. Biochem. Eng. J. 2021, 167, 107886. [Google Scholar] [CrossRef]
- Doyle, L.; Marsili, E. The dynamics and characterization of electroactive biofilms. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 524–528. [Google Scholar]
- Matsena, M.T.; Nkhalambayausi Chirwa, E.M. Advances in microbial fuel cell technology for zero carbon emission energy generation from waste. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 321–358. [Google Scholar] [CrossRef]
- Zhou, J. Establishing Bisphenol a Degradation and Enhancing Microbial Fuel Cell Performance by Biofilm Optimization of Shewanella Oneidensis MR1. Ph.D. Thesis, Illinois Institute of Technology, Chicago, IL, USA, 2023. [Google Scholar]
- Hernandez, M.E.; Newman, D.K. Extracellular electron transfer. Cell. Mol. Life Sci. 2001, 58, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Kato, S. Biotechnological Aspects of Microbial Extracellular Electron Transfer. Microbes Environ. 2015, 30, 133–139. [Google Scholar] [CrossRef]
- Tavker, N.; Kumar, N. Bioelectrochemical systems: Understanding the basics and overcoming the challenges. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–98. [Google Scholar]
- Verma, M.; Singh, V.; Mishra, V. Moving towards the enhancement of extracellular electron transfer in electrogens. World J. Microbiol. Biotechnol. 2023, 39, 130. [Google Scholar] [CrossRef] [PubMed]
- Franza, T.; Gaudu, P. Quinones: More than electron shuttles. Res. Microbiol. 2022, 173, 103953. [Google Scholar] [CrossRef] [PubMed]
- Lusk, B.G.; Peraza, I.; Albal, G.; Marcus, A.K.; Popat, S.C.; Torres, C.I. pH Dependency in Anode Biofilms of Thermincola ferriacetica Suggests a Proton-Dependent Electrochemical Response. J. Am. Chem. Soc. 2018, 140, 5527–5534. [Google Scholar] [CrossRef] [PubMed]
- Kracke, F.; Vassilev, I.; Kromer, J.O. Microbial electron transport and energy conservation—The foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.S.; Kim, T.; Pandit, S.; Song, Y.E.; Afsharian, Y.P.; Rahimnejad, M.; Kim, J.R.; Oh, S.E. Overview of electroactive microorganisms and electron transfer mechanisms in microbial electrochemistry. Bioresour. Technol. 2022, 347, 126579. [Google Scholar] [CrossRef]
- Levar, C.E.; Hoffman, C.L.; Dunshee, A.J.; Toner, B.M.; Bond, D.R. Redox potential as a master variable controlling pathways of metal reduction by Geobacter sulfurreducens. ISME J. 2017, 11, 741–752. [Google Scholar] [CrossRef]
- Liu, F.; Rotaru, A.-E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Markovaite, B.; Chen, S.; Nevin, K.P.; Lovley, D.R. Direct interspecies electron transfer between Geobacter metallireducens and Methanosarcina barkeri. Appl. Environ. Microbiol. 2014, 80, 4599–4605. [Google Scholar] [CrossRef]
- Zhang, K.; Deng, Y.; Liu, Z.; Feng, Y.; Hu, C.; Wang, Z. Biochar Facilitated Direct Interspecies Electron Transfer in Anaerobic Digestion to Alleviate Antibiotics Inhibition and Enhance Methanogenesis: A Review. Int. J. Environ. Res. Public Health 2023, 20, 2296. [Google Scholar] [CrossRef] [PubMed]
- Vellingiri, A.; Song, Y.E.; Munussami, G.; Kim, C.; Park, C.; Jeon, B.H.; Lee, S.G.; Kim, J.R. Overexpression of c-type cytochrome, CymA in Shewanella oneidensis MR-1 for enhanced bioelectricity generation and cell growth in a microbial fuel cell. J. Chem. Technol. Biotechnol. 2019, 94, 2115–2122. [Google Scholar] [CrossRef]
- Su, L.; Fukushima, T.; Prior, A.; Baruch, M.; Zajdel, T.J.; Ajo-Franklin, C.M. Modifying cytochrome c maturation can increase the bioelectronic performance of engineered Escherichia coli. ACS Synth. Biol. 2019, 9, 115–124. [Google Scholar] [CrossRef]
- Delgado, V.P.; Paquete, C.M.; Sturm, G.; Gescher, J. Improvement of the electron transfer rate in Shewanella oneidensis MR-1 using a tailored periplasmic protein composition. Bioelectrochemistry 2019, 129, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, Z.; Unver, T.; Zhang, B. CRISPR/Cas: A powerful tool for gene function study and crop improvement. J. Adv. Res. 2021, 29, 207–221. [Google Scholar] [CrossRef]
- Allen, R.M.; Bennetto, H.P. Microbial fuel-cells: Electricity production from carbohydrates. Appl. Biochem. Biotechnol. 1993, 39, 27–40. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Hasan, M.; Petroianu, G.A. Cellular and molecular actions of methylene blue in the nervous system. Med. Res. Rev. 2011, 31, 93–117. [Google Scholar] [CrossRef]
- Arup, P. The reductase test for milk. Analyst 1918, 43, 20–31. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Li, Z.; Zhang, Y.; Burns, K.; Zhao, N. Extracellular electron transfer in electroactive anaerobic granular sludge mediated by the phenothiazine derivative. J. Power Sources 2022, 527, 231212. [Google Scholar] [CrossRef]
- Babanova, S.; Hubenova, Y.; Mitov, M. Influence of artificial mediators on yeast-based fuel cell performance. J. Biosci. Bioeng. 2011, 112, 379–387. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, E.; Zhang, J.; Dai, Y.; Yang, Z.; Christensen, H.E.M.; Ulstrup, J.; Zhao, F. Extracellular polymeric substances are transient media for microbial extracellular electron transfer. Sci. Adv. 2017, 3, e1700623. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Wu, Y.; Lin, Z.Q.; Xie, J.; Tan, C.H.; Loo, J.S.C.; Cao, B.; Zhang, J.R.; Zhu, J.J.; Zhang, Q. Living and Conducting: Coating Individual Bacterial Cells with In Situ Formed Polypyrrole. Angew. Chem. Int. Ed. Engl. 2017, 56, 10516–10520. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Hashimoto, K.; Watanabe, K. Microbial interspecies electron transfer via electric currents through conductive minerals. Proc. Natl. Acad. Sci. 2012, 109, 10042–10046. [Google Scholar] [CrossRef] [PubMed]
- Luckarift, H.R.; Sizemore, S.R.; Roy, J.; Lau, C.; Gupta, G.; Atanassov, P.; Johnson, G.R. Standardized microbial fuel cell anodes of silica-immobilized Shewanella oneidensis. Chem. Commun. 2010, 46, 6048–6050. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Fu, B.; Liu, F.; He, K.; Yang, H.; Ma, J.; Wang, H.; Zhang, X.; Liang, P.; Huang, X. Construction of innovative 3D-weaved carbon mesh anode network to boost electron transfer and microbial activity in bioelectrochemical system. Water Res. 2020, 172, 115493. [Google Scholar] [CrossRef]
- Zou, L.; Wu, X.; Huang, Y.; Ni, H.; Long, Z.E. Promoting Shewanella Bidirectional Extracellular Electron Transfer for Bioelectrocatalysis by Electropolymerized Riboflavin Interface on Carbon Electrode. Front. Microbiol. 2018, 9, 3293. [Google Scholar] [CrossRef]
- Bareia, T.; Pollak, S.; Eldar, A. Self-sensing in Bacillus subtilis quorum-sensing systems. Nat. Microbiol. 2018, 3, 83–89. [Google Scholar] [CrossRef]
- Reading, N.C.; Sperandio, V. Quorum sensing: The many languages of bacteria. FEMS Microbiol. Lett. 2006, 254, 1–11. [Google Scholar] [CrossRef]
- Hawver, L.A.; Jung, S.A.; Ng, W.-L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016, 40, 738–752. [Google Scholar] [CrossRef]
- Venkataraman, A.; Rosenbaum, M.; Arends, J.B.; Halitschke, R.; Angenent, L.T. Quorum sensing regulates electric current generation of Pseudomonas aeruginosa PA14 in bioelectrochemical systems. Electrochem. Commun. 2010, 12, 459–462. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Yu, Y.-Y.; Li, C.-M.; Zhong, J.-J.; Song, H. Bioelectricity enhancement via overexpression of quorum sensing system in Pseudomonas aeruginosa-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 30, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.B.; Chua, S.-L.; Cao, B.; Seviour, T.; Nesatyy, V.J.; Marsili, E.; Kjelleberg, S.; Givskov, M.; Tolker-Nielsen, T.; Song, H. Engineering PQS biosynthesis pathway for enhancement of bioelectricity production in Pseudomonas aeruginosa microbial fuel cells. PLoS ONE 2013, 8, e63129. [Google Scholar] [CrossRef] [PubMed]
- Monzon, O.; Yang, Y.; Li, Q.; Alvarez, P.J. Quorum sensing autoinducers enhance biofilm formation and power production in a hypersaline microbial fuel cell. Biochem. Eng. J. 2016, 109, 222–227. [Google Scholar] [CrossRef]
- Chen, S.; Jing, X.; Tang, J.; Fang, Y.; Zhou, S. Quorum sensing signals enhance the electrochemical activity and energy recovery of mixed-culture electroactive biofilms. Biosens. Bioelectron. 2017, 97, 369–376. [Google Scholar] [CrossRef]
- Li, F.; Wang, L.; Liu, C.; Wu, D.; Song, H. Engineering exoelectrogens by synthetic biology strategies. Curr. Opin. Electrochem. 2018, 10, 37–45. [Google Scholar] [CrossRef]
- Lovley, D.R.; Fraga, J.L.; Blunt-Harris, E.L.; Hayes, L.A.; Phillips, E.J.P.; Coates, J.D. Humic Substances as a Mediator for Microbially Catalyzed Metal Reduction. Acta Hydrochim. Hydrobiol. 1998, 26, 152–157. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E. Electromicrobiology: The ecophysiology of phylogenetically diverse electroactive microorganisms. Nat. Rev. Microbiol. 2022, 20, 5–19. [Google Scholar] [CrossRef]
- Xu, N.; Wang, T.L.; Li, W.J.; Wang, Y.; Chen, J.J.; Liu, J. Tuning Redox Potential of Anthraquinone-2-Sulfonate (AQS) by Chemical Modification to Facilitate Electron Transfer From Electrodes in Shewanella oneidensis. Front. Bioeng. Biotechnol. 2021, 9, 705414. [Google Scholar] [CrossRef]
- Yong, X.-Y.; Shi, D.-Y.; Chen, Y.-L.; Jiao, F.; Lin, X.; Zhou, J.; Wang, S.-Y.; Yong, Y.-C.; Sun, Y.-M.; OuYang, P.-K. Enhancement of bioelectricity generation by manipulation of the electron shuttles synthesis pathway in microbial fuel cells. Bioresour. Technol. 2014, 152, 220–224. [Google Scholar] [CrossRef]
- Feng, J.; Qian, Y.; Wang, Z.; Wang, X.; Xu, S.; Chen, K.; Ouyang, P. Enhancing the performance of Escherichia coli-inoculated microbial fuel cells by introduction of the phenazine-1-carboxylic acid pathway. J. Biotechnol. 2018, 275, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.X.; Ing, N.L.; Wang, F.; Xu, D.; Sloan, N.B.; Lam, N.T.; Winter, D.L.; Egelman, E.H.; Hochbaum, A.I.; Clark, D.S. Structural determination of a filamentous chaperone to fabricate electronically conductive metalloprotein nanowires. ACS Nano 2020, 14, 6559–6569. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Tang, Q.; Li, Y.; Li, F.H.; Wu, J.H.; Li, W.W.; Yu, H.Q. Rapid and highly efficient genomic engineering with a novel iEditing device for programming versatile extracellular electron transfer of electroactive bacteria. Environ. Microbiol. 2021, 23, 1238–1255. [Google Scholar] [CrossRef] [PubMed]
- Ueki, T.; Walker, D.J.; Woodard, T.L.; Nevin, K.P.; Nonnenmann, S.S.; Lovley, D.R. An Escherichia coli chassis for production of electrically conductive protein nanowires. ACS Synth. Biol. 2020, 9, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, J.A.; Lopez, C.; Babanova, S.; Santoro, C.; Artyushkova, K.; Ista, L.; Schuler, A.J.; Atanassov, P. Surface modification for enhanced biofilm formation and electron transport in shewanella anodes. J. Electrochem. Soc. 2015, 162, H597. [Google Scholar] [CrossRef][Green Version]
- Greenman, J.; Gajda, I.; You, J.; Mendis, B.A.; Obata, O.; Pasternak, G.; Ieropoulos, I. Microbial fuel cells and their electrified biofilms. Biofilm 2021, 3, 100057. [Google Scholar] [CrossRef]
- Zhao, C.E.; Gai, P.; Song, R.; Chen, Y.; Zhang, J.; Zhu, J.J. Nanostructured material-based biofuel cells: Recent advances and future prospects. Chem. Soc. Rev. 2017, 46, 1545–1564. [Google Scholar] [CrossRef]
- Jung, S.; Regan, J.M. Influence of external resistance on electrogenesis, methanogenesis, and anode prokaryotic communities in microbial fuel cells. Appl. Environ. Microbiol. 2011, 77, 564–571. [Google Scholar] [CrossRef]
- Caizan-Juanarena, L.; Borsje, C.; Sleutels, T.; Yntema, D.; Santoro, C.; Ieropoulos, I.; Soavi, F.; Ter Heijne, A. Combination of bioelectrochemical systems and electrochemical capacitors: Principles, analysis and opportunities. Biotechnol. Adv. 2020, 39, 107456. [Google Scholar] [CrossRef]
- Pasternak, G.; Greenman, J.; Ieropoulos, I. Dynamic evolution of anodic biofilm when maturing under different external resistive loads in microbial fuel cells. Electrochemical perspective. J. Power Sources 2018, 400, 392–401. [Google Scholar] [CrossRef]
- Cai, W.-F.; Geng, J.-F.; Pu, K.-B.; Ma, Q.; Jing, D.-W.; Wang, Y.-H.; Chen, Q.-Y.; Liu, H. Investigation of a two-dimensional model on microbial fuel cell with different biofilm porosities and external resistances. Chem. Eng. J. 2018, 333, 572–582. [Google Scholar] [CrossRef]
- Rossi, R.; Logan, B.E. Impact of external resistance acclimation on charge transfer and diffusion resistance in bench-scale microbial fuel cells. Bioresour. Technol. 2020, 318, 123921. [Google Scholar] [CrossRef]
- Koók, L.; Nemestóthy, N.; Bélafi-Bakó, K.; Bakonyi, P. The influential role of external electrical load in microbial fuel cells and related improvement strategies: A review. Bioelectrochemistry 2021, 140, 107749. [Google Scholar] [CrossRef]
- Dong, F.; Lee, Y.S.; Gaffney, E.M.; Liou, W.; Minteer, S.D. Engineering cyanobacterium with transmembrane electron transfer ability for bioelectrochemical nitrogen fixation. ACS Catal. 2021, 11, 13169–13179. [Google Scholar] [CrossRef]
- Sekar, N.; Jain, R.; Yan, Y.; Ramasamy, R.P. Enhanced photo-bioelectrochemical energy conversion by genetically engineered cyanobacteria. Biotechnol. Bioeng. 2016, 113, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Li, Y.; Jin, S.D.; Wang, X.; Wu, X.L.; Zeng, C.; Li, Y.; Ding, H.; Hao, R.-X.; Lv, M.; et al. Growth of non-phototrophic microorganisms using solar energy through mineral photocatalysis. Nat. Commun. 2012, 3, 768. [Google Scholar] [CrossRef]
- Cestellos-Blanco, S.; Zhang, H.; Kim, J.M.; Shen, Y.-X.; Yang, P. Photosynthetic semiconductor biohybrids for solar-driven biocatalysis. Nat. Catal. 2020, 3, 245–255. [Google Scholar] [CrossRef]
- Zhang, B.; Cheng, H.-Y.; Wang, A. Extracellular electron transfer through visible light induced excited-state outer membrane C-type cytochromes of Geobacter sulfurreducens. Bioelectrochemistry 2021, 138, 107683. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Y.; Sun, M.; Li, Y.; Lu, A.; Ding, H. Visible light enhanced extracellular electron transfer between a hematite photoanode and Pseudomonas aeruginosa. Minerals 2017, 7, 230. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Y.; Ding, Y.; Lu, A.; Li, Y.; Wang, C.; Ding, H. Enhancing extracellular electron transfer between Pseudomonas aeruginosa PAO1 and light driven semiconducting birnessite. Bioelectrochemistry 2018, 123, 233–240. [Google Scholar] [CrossRef]
- Liu, J.; Sun, Y.; Lu, A.; Liu, Y.; Ren, G.; Li, Y.; Li, Y.; Ding, H. Extracellular electron transfer of electrochemically active bacteria community promoted by semiconducting minerals with photo-response in marine euphotic zone. Geomicrobiol. J. 2021, 38, 329–339. [Google Scholar] [CrossRef]
- Velasquez-Orta, S.B.; Head, I.M.; Curtis, T.P.; Scott, K.; Lloyd, J.R.; von Canstein, H. The effect of flavin electron shuttles in microbial fuel cells current production. Appl. Microbiol. Biotechnol. 2010, 85, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.H.; Yu, H.Q.; Li, W.W.; Wang, Y.K.; Sun, M.; Liu, X.W.; Sheng, G.P. Application of a weak magnetic field to improve microbial fuel cell performance. Ecotoxicology 2015, 24, 2175–2180. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ye, L.; Jin, J.; Chen, H.; Xu, X.; Zhu, L. Magnetite nanoparticles enhance the performance of a combined bioelectrode-UASB reactor for reductive transformation of 2,4-dichloronitrobenzene. Sci. Rep. 2017, 7, 10319. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayyahi, R.B.; Park, S.-G.; Jadhav, D.A.; Hussien, M.; Mohamed, H.O.; Castaño, P.; Al-Qaradawi, S.Y.; Chae, K.-J. Unraveling the influence of magnetic field on microbial and electrogenic activities in bioelectrochemical systems: A comprehensive review. Fuel 2023, 331, 125889. [Google Scholar] [CrossRef]
- Li, W.W.; Sheng, G.P.; Liu, X.W.; Cai, P.J.; Sun, M.; Xiao, X.; Wang, Y.K.; Tong, Z.H.; Dong, F.; Yu, H.Q. Impact of a static magnetic field on the electricity production of Shewanella-inoculated microbial fuel cells. Biosens. Bioelectron. 2011, 26, 3987–3992. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, G.; Tong, Y.; Liu, Y.; Zhang, L. Electricity production and electrochemical impedance modeling of microbial fuel cells under static magnetic field. J. Power Sources 2013, 237, 58–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Ren, Y.; Wang, X. Effect of static magnetic field on the performances of and anode biofilms in microbial fuel cells. RSC Adv. 2016, 6, 82301–82308. [Google Scholar] [CrossRef]
- Zhou, H.; Mei, X.; Liu, B.; Xie, G.; Xing, D. Magnet anode enhances extracellular electron transfer and enrichment of exoelectrogenic bacteria in bioelectrochemical systems. Biotechnol. Biofuels 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Tao, Q.; Zhou, S. Effect of static magnetic field on electricity production and wastewater treatment in microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 9879–9887. [Google Scholar] [CrossRef]
- Chu, F.-J.; Sie, C.-Y.; Wan, T.-J.; Liu, S.-H.; Pai, T.-Y.; Kao, P.-M. Effects of magnetic fields on electricity generation in a photosynthetic ceramic microbial fuel cell. Int. J. Hydrogen Energy 2021, 46, 11411–11418. [Google Scholar] [CrossRef]
- Calignano, F.; Tommasi, T.; Manfredi, D.; Chiolerio, A. Additive manufacturing of a microbial fuel cell—A detailed study. Sci. Rep. 2015, 5, 17373. [Google Scholar] [CrossRef]
- Zawadzki, D.; Pędziwiatr, P.; Michalska, K. A novel microbial fuel cell with exchangable membrane-application of additive manufacturing technology for device fabrication. In Acta Innovations; Centrum Badań i Innowacji Pro-Akademia: Konstantynów Łódzki, Poland, 2018; pp. 20–31. [Google Scholar]
- Slate, A.J. Optimisation of a Pseudomonas aeruginosa Microbial Fuel Cell Coupled with Additive Manufacturing of Graphene Electrodes to Enhance Power Outputs. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2019. [Google Scholar]
- Sawa, M.; Fantuzzi, A.; Bombelli, P.; Howe, C.J.; Hellgardt, K.; Nixon, P.J. Electricity generation from digitally printed cyanobacteria. Nat. Commun. 2017, 8, 1327. [Google Scholar] [CrossRef]
- Strack, G. Additive manufacturing approaches for biological power generation. Curr. Opin. Electrochem. 2019, 17, 167–173. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, E.; Kim, S.; Jung, S. Membrane fluidity sensoring microbial fuel cell. Bioelectrochemistry 2003, 59, 121–127. [Google Scholar] [CrossRef]
- Picioreanu, C.; Katuri, K.P.; Van Loosdrecht, M.C.; Head, I.M.; Scott, K. Modelling microbial fuel cells with suspended cells and added electron transfer mediator. J. Appl. Electrochem. 2010, 40, 151–162. [Google Scholar] [CrossRef]
- Sousa, R., Jr.; Gonzalez, E.R. Mathematical modeling of polymer electrolyte fuel cells. J. Power Sources 2005, 147, 32–45. [Google Scholar] [CrossRef]
- Yao, K.; Karan, K.; McAuley, K.; Oosthuizen, P.; Peppley, B.; Xie, T. A review of mathematical models for hydrogen and direct methanol polymer electrolyte membrane fuel cells. Fuel Cells 2004, 4, 3–29. [Google Scholar] [CrossRef]
- de Ramón-Fernández, A.; Salar-García, M.J.; Fernández, D.R.; Greenman, J.; Ieropoulos, I. Evaluation of artificial neural network algorithms for predicting the effect of the urine flow rate on the power performance of microbial fuel cells. Energy 2020, 213, 118806. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, K.L.; Liu, H. Predicting microbial fuel cell biofilm communities and bioreactor performance using artificial neural networks. Environ. Sci. Technol. 2017, 51, 10881–10892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Zhu, K.Y.; Zhuang, X.Y.; Liao, L.X.; Huang, S.Y.; Yao, C.Y.; Fang, B.S. A robust soft sensor to monitor 1, 3-propanediol fermentation process by Clostridium butyricum based on artificial neural network. Biotechnol. Bioeng. 2020, 117, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Lesnik, K.L.; Wade, M.J.; Heidrich, E.S.; Wang, Y.; Liu, H. Incorporating microbial community data with machine learning techniques to predict feed substrates in microbial fuel cells. Biosens. Bioelectron. 2019, 133, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, D.; Dang, Y.; Sun, D.; Li, P. Application of artificial intelligence-based methods in bioelectrochemical systems: Recent progress and future perspectives. J. Environ. Manag. 2023, 344, 118502. [Google Scholar] [CrossRef] [PubMed]
- Ieropoulos, I.; Melhuish, C.; Greenman, J.; Horsfield, I. EcoBot-II: An artificial agent with a natural metabolism. Int. J. Adv. Robot. Syst. 2005, 2, 31. [Google Scholar] [CrossRef]
- Dwivedi, K.A.; Huang, S.-J.; Wang, C.-T. Integration of various technology-based approaches for enhancing the performance of microbial fuel cell technology: A review. Chemosphere 2022, 287, 132248. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lu, Q.; Li, K.; Xu, S.; Wang, X.; Chen, K.; Ouyang, P. Construction of an Electron Transfer Mediator Pathway for Bioelectrosynthesis by Escherichia coli. Front. Bioeng. Biotechnol. 2020, 8, 590667. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.K.M.; Hussain, M.A.; Wahab, A.K.A. Fuzzy logic controller implementation on a microbial electrolysis cell for biohydrogen production and storage. Chin. J. Chem. Eng. 2021, 40, 149–159. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Cao, X.; Xu, Z.; Huang, W.; Wang, Y.; Ge, X. Integration of manganese ores with activated carbon granules into CW-MFC to trigger anoxic electron transfer and removal of ammonia nitrogen. J. Clean. Prod. 2022, 334, 130202. [Google Scholar] [CrossRef]
- Strik, D.P.; Hamelers, H.; Snel, J.F.; Buisman, C.J. Green electricity production with living plants and bacteria in a fuel cell. Int. J. Energy Res. 2008, 32, 870–876. [Google Scholar] [CrossRef]
- Schamphelaire, L.D.; Bossche, L.V.d.; Dang, H.S.; Höfte, M.; Boon, N.; Rabaey, K.; Verstraete, W. Microbial fuel cells generating electricity from rhizodeposits of rice plants. Environ. Sci. Technol. 2008, 42, 3053–3058. [Google Scholar] [CrossRef]
- Timmers, R.A.; Strik, D.P.; Hamelers, H.V.; Buisman, C.J. Long-term performance of a plant microbial fuel cell with Spartina anglica. Appl. Microbiol. Biotechnol. 2010, 86, 973–981. [Google Scholar] [CrossRef]
- Helder, M.; Strik, D.; Hamelers, H.; Kuhn, A.; Blok, C.; Buisman, C. Concurrent bio-electricity and biomass production in three Plant-Microbial Fuel Cells using Spartina anglica, Arundinella anomala and Arundo donax. Bioresour. Technol. 2010, 101, 3541–3547. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Mohanakrishna, G.; Chiranjeevi, P. Sustainable power generation from floating macrophytes based ecological microenvironment through embedded fuel cells along with simultaneous wastewater treatment. Bioresour. Technol. 2011, 102, 7036–7042. [Google Scholar] [CrossRef] [PubMed]
- Chiranjeevi, P.; Mohanakrishna, G.; Mohan, S.V. Rhizosphere mediated electrogenesis with the function of anode placement for harnessing bioenergy through CO2 sequestration. Bioresour. Technol. 2012, 124, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xing, D.; Ren, Z.J. Microbial community structure accompanied with electricity production in a constructed wetland plant microbial fuel cell. Bioresour. Technol. 2015, 195, 115–121. [Google Scholar] [CrossRef]
- Klaisongkram, N.; Holasut, K. Electricity Generation of Plant Microbial Fuel Cell (PMFC) using Cyperus Involucratus R. Eng. Appl. Sci. Res. 2015, 42, 117–124. [Google Scholar]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.; Strik, D.P. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energy 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Oon, Y.-L.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Oon, Y.-S.; Lehl, H.K.; Thung, W.-E. Hybrid system up-flow constructed wetland integrated with microbial fuel cell for simultaneous wastewater treatment and electricity generation. Bioresour. Technol. 2015, 186, 270–275. [Google Scholar] [CrossRef]
- Ueoka, N.; Sese, N.; Sue, M.; Kouzuma, A.; Watanabe, K. Sizes of anode and cathode affect electricity generation in rice paddy-field microbial fuel cells. J. Sustain. Bioenergy Syst. 2016, 6, 10. [Google Scholar] [CrossRef]
- Li, J.; Han, H.; Chang, Y.; Wang, B. The material-microorganism interface in microbial hybrid electrocatalysis systems. Nanoscale 2023, 15, 6009–6024. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, C.; Li, Z.; Liu, J.; Xiao, X.; Li, D.; Chen, C.; Yu, M.; Feng, Y. Accelerating the extracellular electron transfer of Shewanella oneidensis MR-1 by carbon dots: The role of carbon dots concentration. Electrochim. Acta 2022, 421, 140490. [Google Scholar] [CrossRef]
- Liu, S.; Yi, X.; Wu, X.; Li, Q.; Wang, Y. Internalized Carbon Dots for Enhanced Extracellular Electron Transfer in the Dark and Light. Small 2020, 16, e2004194. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhou, X.; Qu, Y.; Liu, J.; Feng, Y.; Ren, N. Enhanced electricity generation and pollutant degradation by hybrid photoelectrochemical and microbial fuel cells. Energy Technol. 2017, 5, 402–405. [Google Scholar] [CrossRef]
- Tong, Y.; Wei, J.; Mo, R.; Ma, H.; Ai, F. Photocatalytic Microbial Fuel Cells and Performance Applications: A Review. Front. Chem. 2022, 10, 953434. [Google Scholar] [CrossRef] [PubMed]
- Tee, P.-F.; Abdullah, M.O.; Tan, I.A.W.; Amin, M.A.M.; Nolasco-Hipolito, C.; Bujang, K. Performance evaluation of a hybrid system for efficient palm oil mill effluent treatment via an air-cathode, tubular upflow microbial fuel cell coupled with a granular activated carbon adsorption. Bioresour. Technol. 2016, 216, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.T.; Baron, D.B.; Naranjo, B.; Bond, D.R.; Schmidt-Dannert, C.; Gralnick, J.A. Enhancement of survival and electricity production in an engineered bacterium by light-driven proton pumping. Appl. Environ. Microbiol. 2010, 76, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Myers, B.; Hill, P.; Rawson, F.; Kovács, K. Enhancing Microbial Electron Transfer Through Synthetic Biology and Biohybrid Approaches: Part II: Combining approaches for clean energy. Johns. Matthey Technol. Rev. 2022, 66, 455–465. [Google Scholar] [CrossRef]
- Pankratova, G.; Hasan, K.; Leech, D.; Hederstedt, L.; Gorton, L. Electrochemical wiring of the Gram-positive bacterium Enterococcus faecalis with osmium redox polymer modified electrodes. Electrochem. Commun. 2017, 75, 56–59. [Google Scholar] [CrossRef]
- Widder, S.; Allen, R.J.; Pfeiffer, T.; Curtis, T.P.; Wiuf, C.; Sloan, W.T.; Cordero, O.X.; Brown, S.P.; Momeni, B.; Shou, W.; et al. Challenges in microbial ecology: Building predictive understanding of community function and dynamics. ISME J. 2016, 10, 2557–2568. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Savla, N.; Pandit, S.; Gupta, P.K.; Mathuriya, A.S.; Lahiri, D.; Jadhav, D.A.; Rai, A.K.; KanuPriya; Ray, R.R. Microbial fuel cell united with other existing technologies for enhanced power generation and efficient wastewater treatment. Appl. Sci. 2021, 11, 10777. [Google Scholar] [CrossRef]
- Vassilev, I.; Averesch, N.J.H.; Ledezma, P.; Kokko, M. Anodic electro-fermentation: Empowering anaerobic production processes via anodic respiration. Biotechnol. Adv. 2021, 48, 107728. [Google Scholar] [CrossRef]
- Pasternak, G.; de Rosset, A.; Tyszkiewicz, N.; Widera, B.; Greenman, J.; Ieropoulos, I. Prevention and removal of membrane and separator biofouling in bioelectrochemical systems: A comprehensive review. iScience 2022, 25, 104510. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ajo-Franklin, C.M. Reaching full potential: Bioelectrochemical systems for storing renewable energy in chemical bonds. Curr. Opin. Biotechnol. 2019, 57, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Zhi, W.; Ge, Z.; He, Z.; Zhang, H. Methods for understanding microbial community structures and functions in microbial fuel cells: A review. Bioresour. Technol. 2014, 171, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Flimban, S.G.; Ismail, I.M.; Kim, T.; Oh, S.-E. Overview of recent advancements in the microbial fuel cell from fundamentals to applications: Design, major elements, and scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).