Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks
Abstract
1. Introduction
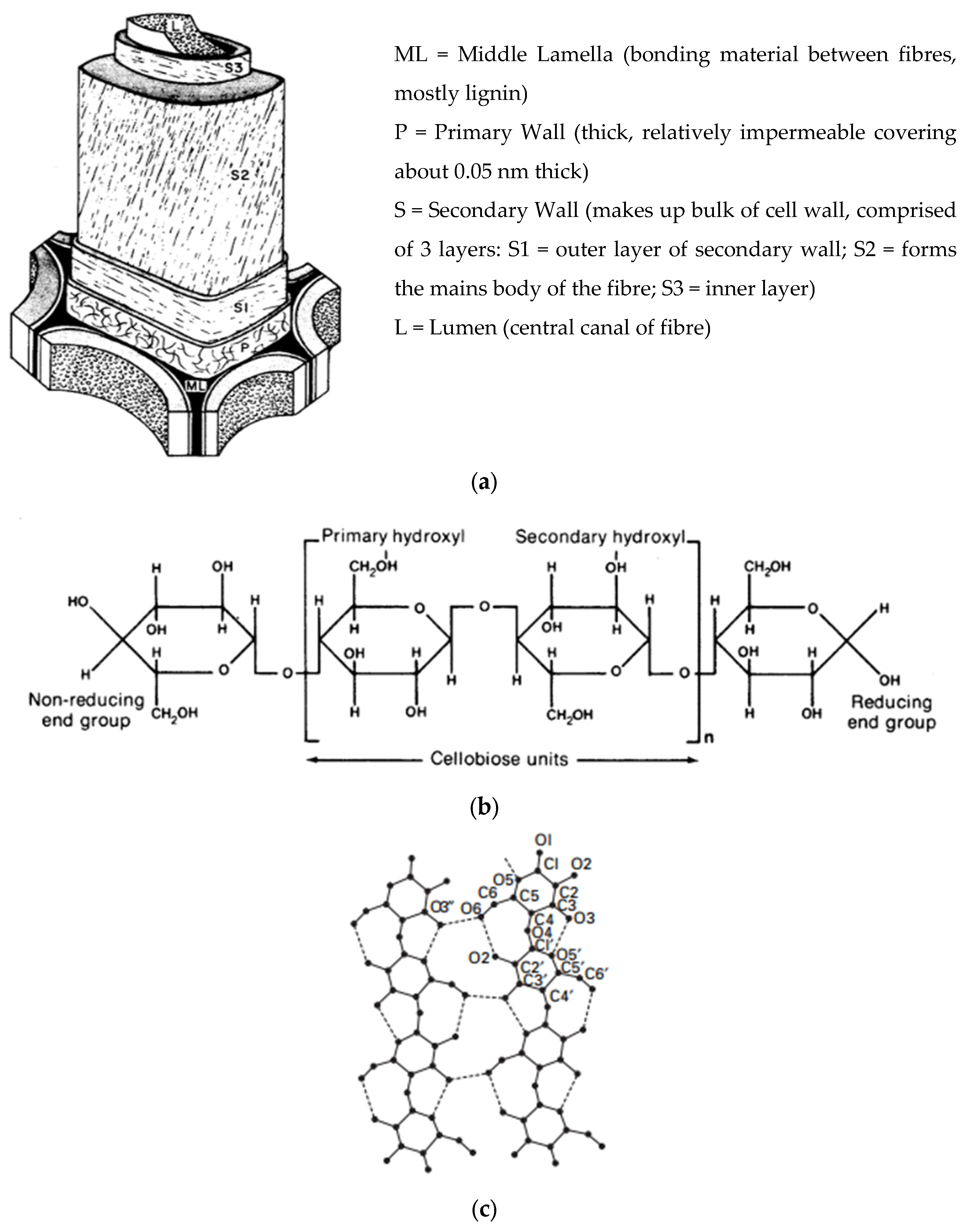
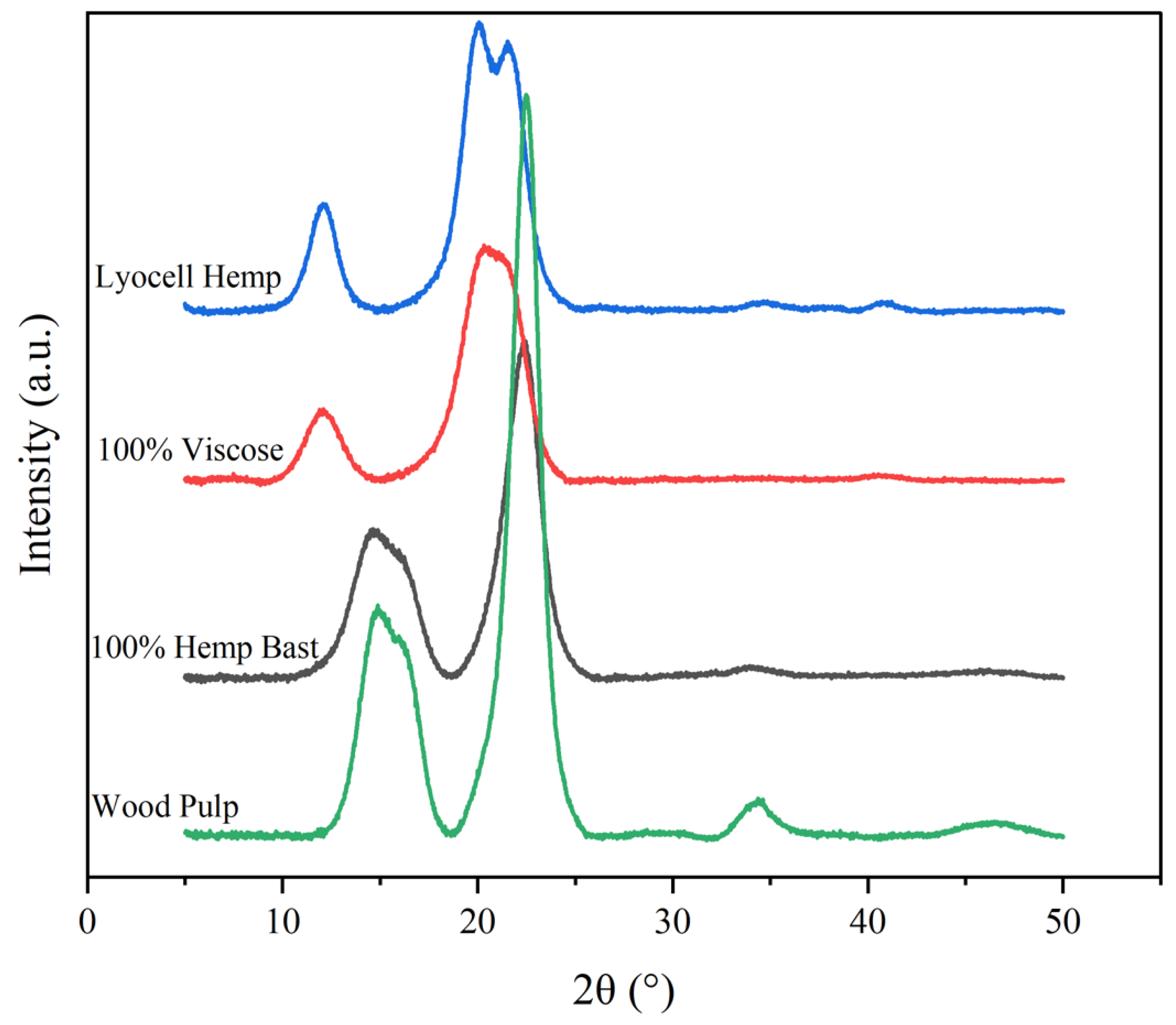
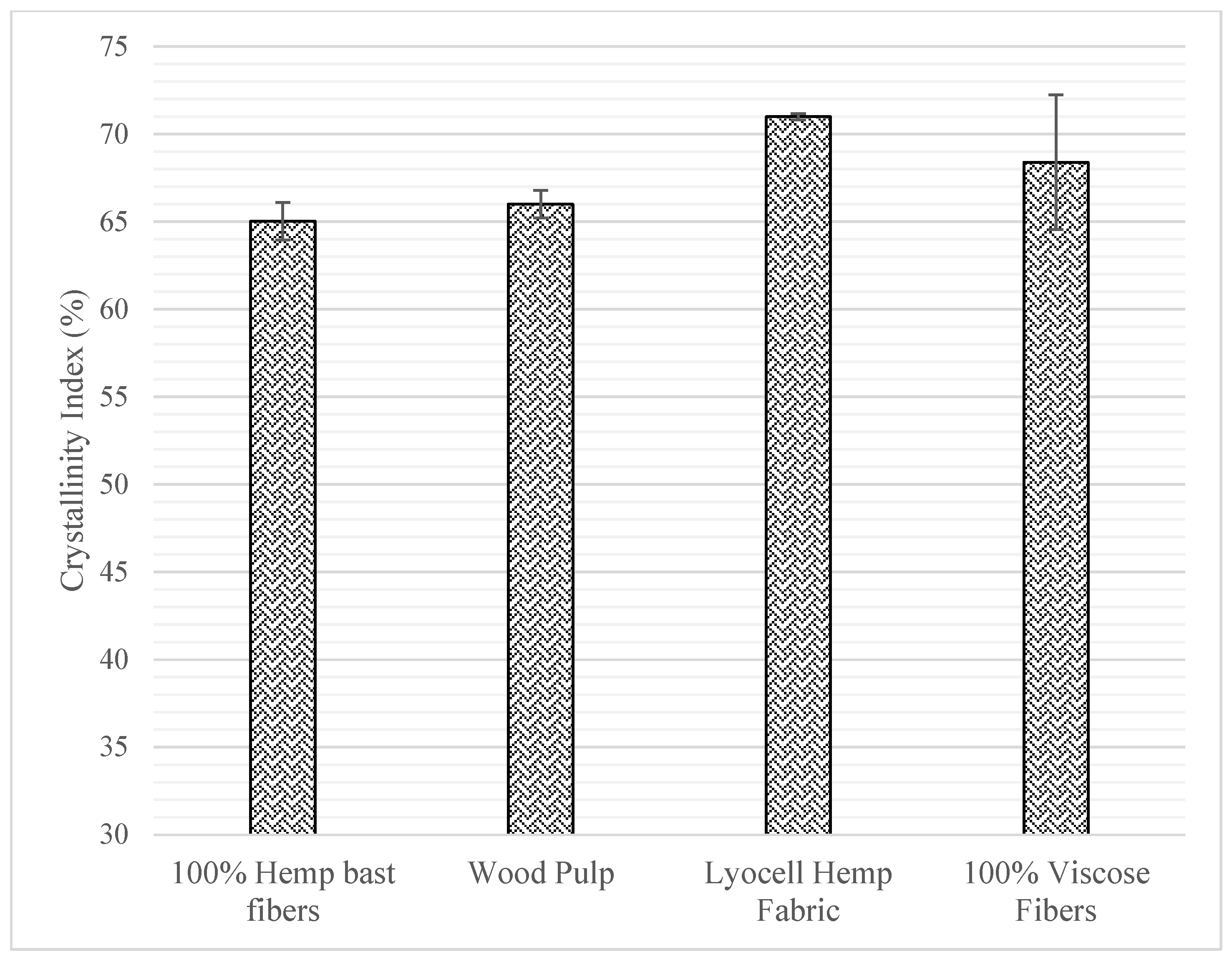
2. Cellulose Morphology
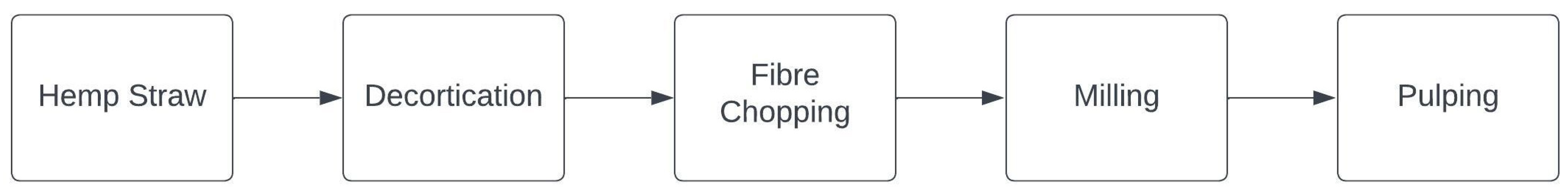
3. L-MMCF Cellulose Feedstock Sources
4. L-MMCF Manufacturing Process
- Preparation of feedstock, including characterisation (i.e., carbohydrate, extractive, ash, metal, sulphur, moisture content).
- Chemical pulp preparation of feedstock (acid sulphite or prehydrolysis Kraft), including characterisation (i.e., carbohydrate, extractive, ash, metal, sulphur, moisture content, intrinsic viscosity (DP), and kappa number).
- Removal of contaminants through washing, chelation, and bleaching.
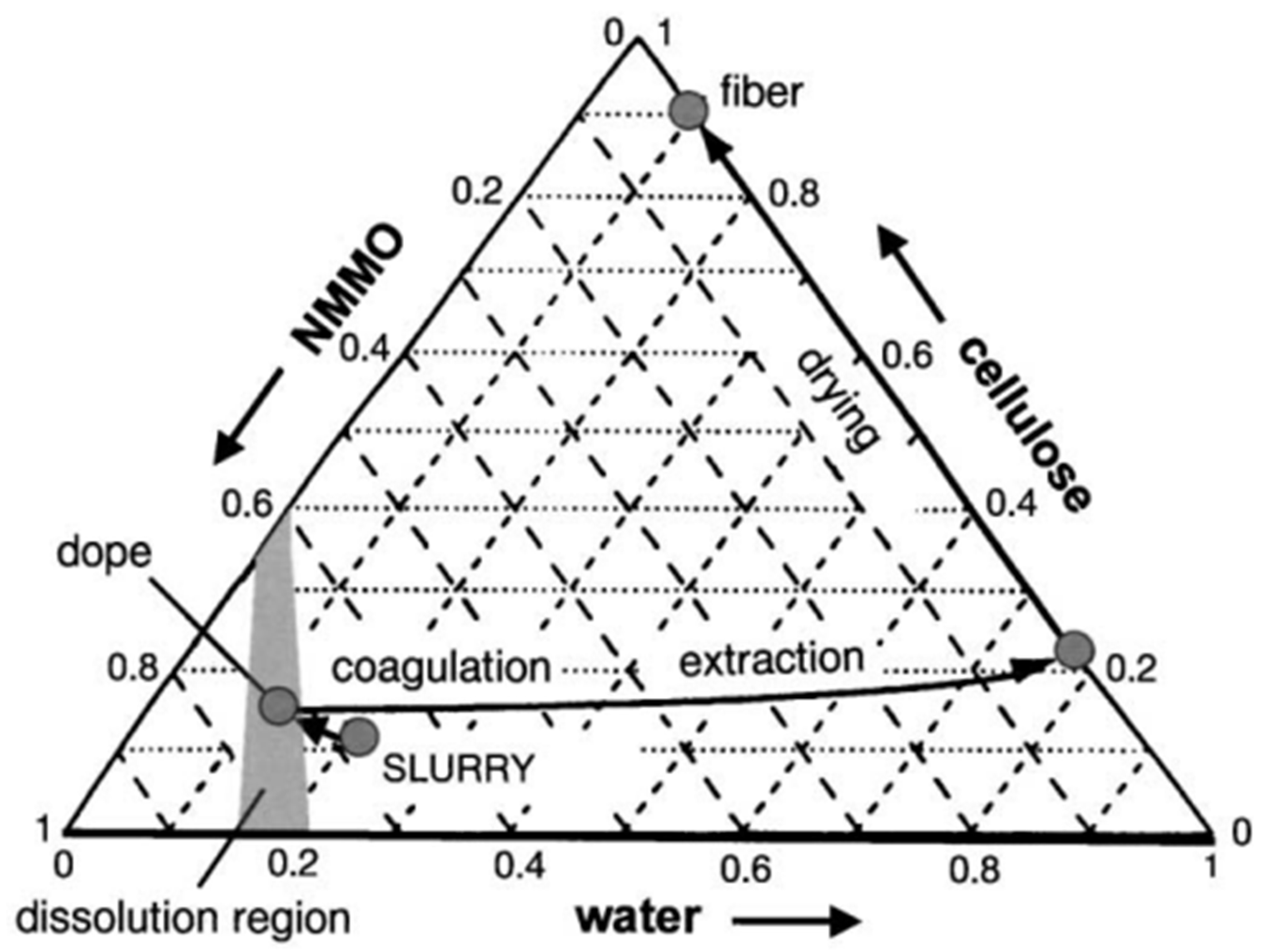
- Dissolution of pulp in n-methyl-morpholine-n-oxide (NMMO, specific to L-MMCF).
- Fibre spinning/extrusion, drawing, washing, drying, and winding.
4.1. Preparation of Feedstock
- Carbohydrate content:
- Alpha-, beta-, and gamma-cellulose in pulp: Alpha cellulose in undegraded pulp has a high molecular weight; beta cellulose is soluble in NaOH (S18), but can be precipitated when neutralised, and gamma cellulose remains soluble in the NaOH solution, even after neutralisation, and consists primarily of hemicellulose [73,74]. Both hemicellulose (gamma) and amorphous cellulose (beta cellulose, which may be the result of cellulose deterioration during the pulping process), are removed prior to solvent dissolution.
- Alpha cellulose content: The L-MMCF spinning requires a very high alpha-cellulose content of more than 90% for L-MMCF fibre properties, such as tensile strength [17,75]. As shown in Table 2, plant biomass has a much lower alpha cellulose content, requiring additional processing steps to acquire the higher percentage.
- Hemicellulose: Gamma, or hemicellulose, should be less than 5% of the total cellulose content, based on dry weight to ensure a higher quality of pulp [76].
- Extractives: solvent and hot water-soluble components (e.g., fats, waxes, and terpenes), tannins, and inorganic salts. Extractive concentration can increase with the age of the feedstock (i.e., the older the plant, the more extractives are present) [77]. Extractives can lead to reduced strength and yellowing or discolouration in refined pulps [37,77]. Generally, extractives do not survive the Kraft pulping process, though they do increase chemical consumption in cooking and can cause foaming issues in the pulping process, both of which are undesirable. Extractives content can be reduced by “seasoning” the chips in a pile for several months before feeding them into the mill, which is equivalent of hemp stalk retting. Retting is a process that breaks down the pectin (an extractive) in hemp stalks, which reduces cooking chemical requirements during the Kraft pulping process.
- Ash content (inorganics): “The ash content of the sample may consist of: (1) various residues from chemicals used in its manufacture, (2) metallic matter from piping and machinery, (3) mineral matter in the pulp from which the paper was made, and (4) filling, coating, pigmenting and/or other added materials” (p. 1) [39]. The measurement of the ash content helps determine inorganic contaminants, with a focus on heavy metal content. As discussed in Section 4.3, heavy metal content needs to be measured methodically throughout the pulping, dope preparation, and spinning process to reduce the risk of exothermic reactions.
- Metallic ion content, including heavy metals, alkaline, and earth alkaline, needs to be determined separately from ash content via inductively-coupled plasma optical emission spectroscopy (ICP-OES) [60,65,78,79]. Concentration of metal ions, particularly transition metals with multiple oxidation states, above certain limits can result in runaway exothermic reactions or degradation of cellulose pulp yield.
- Sulphur content: High sulphur content in feedstock, as well as content introduced to Kraft pulps via sodium sulphide (Na2S) in the cooking liquor, can lead to yellowing, reduced strength, and evolution of toxic gases during the pulping and regenerated cellulose spinning process [80]. Sulphur must be rinsed out after PHK pulping.
- Moisture content during all stages of L-MMCF manufacturing needs to be carefully monitored as this directly affects the proper processing of the dissolved pulp and dissolution of the pulp. Evaporation of moisture is needed to obtain the required ratio of NMMO:water:cellulose in the final L-MMCF dope prior to spinning.
4.2. Pulping Process
4.3. Chelation and Bleaching
4.4. Pulp Dissolution
4.5. Fibre Spinning
5. Challenges and Opportunities with Alternative Feedstocks for L-MMCF
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ingildeev, D.; Effenberger, F.; Bredereck, K.; Hermanutz, F. Comparison of Direct Solvents for Regenerated Cellulosic Fibers via the Lyocell Process and by Means of Ionic Liquids. J. Appl. Polym. Sci. 2013, 128, 4141–4150. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, C.; Duan, C.; Hu, H.; Li, H.; Li, J.; Liu, Y.; Ma, X.; Stavik, J.; Ni, Y. Regenerated Cellulose by the Lyocell Process, a Brief Review of the Process and Properties. BioResources 2018, 13, 4577–4592. [Google Scholar] [CrossRef]
- White, P. 4—Lyocell: The Production Process and Market Development. In Regenerated Cellulose Fibres; Woodings, C., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2001; pp. 62–87. ISBN 978-1-85573-459-3. [Google Scholar]
- Perepelkin, K.E. Lyocell Fibres Based on Direct Dissolution of Cellulose in N-Methylmorpholine N-Oxide: Development and Prospects. Fibre Chem. 2007, 39, 163–172. [Google Scholar] [CrossRef]
- Bredereck, K.; Hermanutz, F. Man–Made Cellulosics. Rev. Prog. Color. Relat. Top. 2005, 35, 59–75. [Google Scholar] [CrossRef]
- Haemmerle, F.M. The Cellulose Gap (the Future of Cellulose Fibres). Lenzing. Berichte 2011, 89, 12–21. [Google Scholar]
- Lawson, L.; Degenstein, L.M.; Bates, B.; Chute, W.; King, D.; Dolez, P.I. Cellulose Textiles from Hemp Biomass: Opportunities and Challenges. Sustainability 2022, 14, 15337. [Google Scholar] [CrossRef]
- Kim, D.B.; Pak, J.J.; Jo, S.M.; Lee, W.S. Dry Jet-Wet Spinning of Cellulose/N-Methylmorpholine N-Oxide Hydrate Solutions and Physical Properties of Lyocell Fibers. Text. Res. J. 2005, 75, 331–341. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 608826. [Google Scholar] [CrossRef]
- Mboowa, D. A Review of the Traditional Pulping Methods and the Recent Improvements in the Pulping Processes. In Biomass Conversion and Biorefinery; Spring: Berlin/Heidelberg, Germany, 2021. [Google Scholar] [CrossRef]
- Parajuli, P.; Acharya, S.; Rumi, S.S.; Hossain, M.T.; Abidi, N. 4—Regenerated Cellulose in Textiles: Rayon, Lyocell, Modal and Other Fibres. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; The Textile Institute Book Series; Woodhead Publishing: Cambridge, UK, 2021; pp. 87–110. ISBN 978-0-12-821483-1. [Google Scholar]
- Textile Exchange. Preferred Fiber and Materials Market Report 2022. Available online: https://textileexchange.org/knowledge-center/reports/preferred-fiber-and-materials/ (accessed on 5 November 2022).
- The Wye Group. Endangered Forests: Priority High Conservation Value Forests For Protection Guidance For Corporate Commitments. In Proceedings of the Forest Leadership Forum: Collaborative Pathways to Responsible Trade, Cobb Galleria, Atlanta, GA, USA, 25 April 2002. [Google Scholar]
- Canopy. Ancient Forest Friendly Defined. Available online: https://canopyplanet.org/solutions/ancient-forest-friendly/ancient-forest-friendly-defined/ (accessed on 20 November 2023).
- Rosenau, T.; Potthast, A.; Sixta, H.; Kosma, P. The Chemistry of Side Reactions and Byproduct Formation in the System NMMO/Cellulose (Lyocell Process). Prog. Polym. Sci. 2001, 26, 1763–1837. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, Y.; Chen, X.; Liu, W. A Review on Raw Materials, Commercial Production and Properties of Lyocell Fiber. J. Bioresour. Bioprod. 2020, 5, 16–25. [Google Scholar] [CrossRef]
- Balkissoon, S.; Andrew, J.; Sithole, B. Dissolving Wood Pulp Production: A Review. In Biomass Conversion and Biorefinery; Spring: Berlin/Heidelberg, Germany, 2022. [Google Scholar] [CrossRef]
- Chen, C.; Duan, C.; Li, J.; Liu, Y.; Ma, X.; Zheng, L.; Stavik, J.; Ni, Y. Cellulose (Dissolving Pulp) Manufacturing Processes and Properties: A Mini-Review. BioResources 2016, 11, 5553–5564. [Google Scholar] [CrossRef]
- Seisl, S.; Hengstmann, R. Manmade Cellulosic Fibers (MMCF)—A Historical Introduction and Existing Solutions to a More Sustainable Production. In Sustainable Textile and Fashion Value Chains: Drivers, Concepts, Theories and Solutions; Matthes, A., Beyer, K., Cebulla, H., Arnold, M.G., Schumann, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–22. ISBN 978-3-030-22018-1. [Google Scholar]
- Fink, H.-P.; Weigel, P.; Purz, H.J.; Ganster, J. Structure Formation of Regenerated Cellulose Materials from NMMO-Solutions. Prog. Polym. Sci. 2001, 26, 1473–1524. [Google Scholar] [CrossRef]
- Chavan, R.; Patra, A. Development and Processing of Lyocell. Indian J. Fibre Text. Res. 2004, 29, 483–492. [Google Scholar]
- Collier, B.J.; Dever, M.; Petrovan, S.; Collier, J.R.; Li, Z.; Wei, X. Rheology of Lyocell Solutions from Different Cellulose Sources. J. Polym. Environ. 2000, 8, 151–154. [Google Scholar] [CrossRef]
- Costa, M.; Costa, S.A.; Pahl, R.; Mazzola, P.G.; Marcicano, J.P.P.; Pessoa, A., Jr. Textile Fiber Produced from Sugarcane Bagasse Cellulose: An Agro-Industrial Residue. Int. J. Text. Fash. Technol. IJTFT 2013, 3, 15–28. [Google Scholar]
- Yang, G.; Zhou, Y.; Zhang, H.; Wang, S.; Yao, X.; Shao, H. Preparation and Characterization of Dissolving Pulp and Lyocell Fibers from Corncob. Cellulose 2023, 30, 4841–4853. [Google Scholar] [CrossRef]
- Paulitz, J.; Sigmund, I.; Kosan, B.; Meister, F. Lyocell Fibers for Textile Processing Derived from Organically Grown Hemp. Procedia Eng. 2017, 200, 260–268. [Google Scholar] [CrossRef]
- Thümmler, K.; Fischer, J.; Fischer, S.; Kosan, B.; Meister, F. LyohempTM Fibres from Hemp Shive Dissolving Pulp. Lenzing. Berichte 2022, 97, 7. [Google Scholar]
- Janjic, S.; Kostic, M.; Skundric, P. Direct Hemp Cellulose Dissolution in N-Methylmorpoline-N-Oxide. J. Nat. Fibers 2007, 4, 23–36. [Google Scholar] [CrossRef]
- Gröndahl, J.; Karisalmi, K.; Vapaavuori, J. Micro- and Nanocelluloses from Non-Wood Waste Sources; Processes and Use in Industrial Applications. Soft Matter 2021, 17, 9842–9858. [Google Scholar] [CrossRef]
- Tencel Lenzing Collaborates with Orange Fiber as Part of New TENCELTM Limited Edition Initiative. Available online: https://www.tencel.com/b2b/news-and-events/lenzing-collaborates-with-orange-fiber-as-part-of-new-tencel-limited-edition-initiative (accessed on 16 September 2022).
- Makarov, I.S.; Golova, L.K.; Smyslov, A.G.; Vinogradov, M.I.; Palchikova, E.E.; Legkov, S.A. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers 2022, 10, 45. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Egorov, Y.E.; Kulichikhin, V.G.; Mikhailov, Y.M. New Hydrated Cellulose Fiber Based on Flax Cellulose. Russ. J. Gen. Chem. 2021, 91, 1807–1815. [Google Scholar] [CrossRef]
- Dever, M.; Collier, B.J.; Petrovan, S.; Collier, J.R. Lyocell Solutions from Alternative Cellulose Sources. Cloth. Text. Res. J. 2003, 21, 167–173. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Liu, T.; Liu, T.; Zhang, J.; Liu, H. Eco-Friendly Post-Consumer Cotton Waste Recycling for Regenerated Cellulose Fibers. Carbohydr. Polym. 2019, 206, 141–148. [Google Scholar] [CrossRef]
- Davis, R. REFIBRATM: Sustainable Lyocell Production. Available online: https://www.textileworld.com/textile-world/2020/02/refibra-sustainable-lyocell-production/ (accessed on 5 October 2023).
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper: Raw Material and Pulp Making, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, ISBN 978-0-12-814240-0. [Google Scholar]
- Sixta, H. (Ed.) Handbook of Pulp, 1st ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; ISBN 978-3-527-61988-7. [Google Scholar]
- Smook, G.A. Handbook for Pulp & Paper Technologists, 4th ed.; Tappi Press: Atlanta, GA, USA, 2016; ISBN 978-1-5231-3074-0. [Google Scholar]
- TAPPI. TAPPI Test Method 211 Om-22: Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525°. 2022. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T211.aspx (accessed on 23 November 2023).
- Morton, W.E.; Hearle, J.W.S. Physical Properties of Textile Fibres, 4th ed.; Woodhead publishing in textiles: No. 68; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-84569-442-5. [Google Scholar]
- Manian, A.P.; Cordin, M.; Pham, T. Extraction of Cellulose Fibers from Flax and Hemp: A Review. Cellulose 2021, 28, 8275–8294. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of Cotton Fabric Scouring by FT-IR ATR Spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper: Paper and Board Making, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, ISBN 978-0-12-814239-4. [Google Scholar]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Jamaluddin, J.; Awasthi, M.K.; Sarsaiya, S. A Review on Systematic Study of Cellulose. J. Appl. Nat. Sci. 2010, 2, 330–343. [Google Scholar] [CrossRef]
- Kroon-Batenburg, L.M.J.; Kroon, J. The Crystal and Molecular Structures of Cellulose I and II. Glycoconj. J. 1997, 14, 677–690. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Rheology of Lyocell Solutions from Different Cellulosic Sources and Development of Regenerated Cellulosic Microfibers. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2003. [Google Scholar]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; French, A.D.; Pal, L.; Lucia, L.A. Comparison and Assessment of Methods for Cellulose Crystallinity Determination. Chem. Soc. Rev. 2023, 52, 6417–6446. [Google Scholar] [CrossRef]
- Chen, X.; Burger, C.; Fang, D.; Ruan, D.; Zhang, L.; Hsiao, B.S.; Chu, B. X-ray Studies of Regenerated Cellulose Fibers Wet Spun from Cotton Linter Pulp in NaOH/Thiourea Aqueous Solutions. Polymer 2006, 47, 2839–2848. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Olmos, G.V.; Taleb, M.C.; Felissia, F.E.; Ehman, N.V.; Peresin, M.S.; Area, M.C.; Maximino, M.G. Dissolving Pulp from Eucalyptus Sawdust for Regenerated Cellulose Products. Cellulose 2022, 29, 4645–4659. [Google Scholar] [CrossRef]
- Marquardt, S. Growing Hemp for the Future: A Global Fiber Guide; Textile Exchange: Lamesa, TX, USA, 2023. [Google Scholar]
- Blake, A.W.; Marcus, S.E.; Copeland, J.E.; Blackburn, R.S.; Knox, J.P. In Situ Analysis of Cell Wall Polymers Associated with Phloem Fibre Cells in Stems of Hemp, Cannabis Sativa L. Planta 2008, 228, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rosell, J.A. Bark in Woody Plants: Understanding the Diversity of a Multifunctional Structure. Integr. Comp. Biol. 2019, 59, 535–547. [Google Scholar] [CrossRef]
- Hiebert, H. Harvesting Fibers. Hand Papermaking. 2021. Available online: https://www.handpapermaking.org/post/harvesting-fibers (accessed on 5 October 2023).
- Leoni, M.; Musio, S.; Croci, M.; Tang, K.; Magagnini, G.M.; Thouminot, C.; Müssig, J.; Amaducci, S. The Effect of Agronomic Management of Hemp (Cannabis Sativa L.) on Stem Processing and Fibre Quality. Ind. Crops Prod. 2022, 188, 115520. [Google Scholar] [CrossRef]
- Ontario Ministry of Agriculture, Food and Rural Affairs Factsheet #22-019: Growing Industrial Hemp in Ontario. Available online: http://www.ontario.ca/page/growing-industrial-hemp-ontario (accessed on 6 October 2023).
- Small, E.; Pocock, T.; Cavers, P.B. The Biology of Canadian Weeds. 119. Cannabis Sativa L. Can. J. Plant Sci. 2003, 83, 217–237. [Google Scholar] [CrossRef]
- Thomsen, A.B.; Rasmussen, S.; Bohn, V.; Nielsen, K.V.; Thygesen, A. Hemp Raw Materials: The Effect of Cultivar, Growth Conditions and Pretreatment on the Chemical Composition of the Fibres; Risoe-R No 1507EN; Risø DTU-National Laboratory for Sustainable Energy: Roskilde, Denmark, 2005; Volume 31. [Google Scholar]
- Gandolfi, S.; Ottolina, G.; Riva, S.; Fantoni, G.P.; Patel, I. Complete Chemical Analysis of Carmagnola Hemp Hurds and Structural Features of Its Components. BioResources 2013, 8, 2641–2656. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Rodríguez, I.M.; del Río, J.C. Chemical Characterization of Lignin and Lipid Fractions in Industrial Hemp Bast Fibers Used for Manufacturing High-Quality Paper Pulps. J. Agric. Food Chem. 2006, 54, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kongklieng, P.; Ibaraki, A. Regenerated Cellulose Materials. In Encyclopedia of Materials: Plastics and Polymers; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2022; pp. 541–554. ISBN 978-0-12-823291-0. [Google Scholar]
- Duan, C.; Li, J.; Ma, X.; Chen, C.; Liu, Y.; Stavik, J.; Ni, Y. Comparison of Acid Sulfite (AS)- and Prehydrolysis Kraft (PHK)-Based Dissolving Pulps. Cellulose 2015, 22, 4017–4026. [Google Scholar] [CrossRef]
- Rusch, F.; Wastowski, A.D.; de Lira, T.S.; Moreira, K.C.C.S.R.; de Moraes Lúcio, D. Description of the Component Properties of Species of Bamboo: A Review. Biomass Convers. Biorefinery 2023, 13, 2487–2495. [Google Scholar] [CrossRef]
- Tandy, E. Reactivity Increasement of Prehydrolysis Kraft Pulp from Acacia crassicarpa and Eucalyptus Hybrids. Master’s Thesis, The Royal Institute of Technology, School of Engineering Sciences in Chemistry, Biotechnology and Health, Stockholm, Sweden, 2022. [Google Scholar]
- Meister, F.; Kosan, B. A Tool Box for Characterization of Pulps and Cellulose Dopes in Lyocell Technology. Nord. Pulp Pap. Res. J. 2015, 30, 112–120. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A Comprehensive Review on the Framework to Valorise Lignocellulosic Biomass as Biorefinery Feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent Updates on Different Methods of Pretreatment of Lignocellulosic Feedstocks: A Review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Qiu, J.; Song, J.; Wang, J.; Liu, M.; Wo, Q.; Jiang, L.; Yang, T. Properties and Application of Kraft Pulp Prepared from Waste Bamboo Powder. BioResources 2022, 17, 6262–6276. [Google Scholar] [CrossRef]
- Sczostak, A. Cotton Linters: An Alternative Cellulosic Raw Material. Macromol. Symp. 2009, 280, 45–53. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, H.; Zhao, L.; Huang, M.; Tian, D.; Deng, S.; Hu, J.; Zhang, X.; Shen, F. Fabrication of Regenerated Cellulose Fibers Using Phosphoric Acid with Hydrogen Peroxide Treated Wheat Straw in a DMAc/LiCl Solvent System. Cellulose 2023, 30, 6187–6201. [Google Scholar] [CrossRef]
- Wei, W.; Tian, Z.; Ji, X.; Wang, Q.; Chen, J.; Zhang, G.; Lucia, L.A. Understanding the Effect of Severity Factor of Prehydrolysis on Dissolving Pulp Production Using Prehydrolysis Kraft Pulping and Elemental Chlorine-Free Bleaching Sequence. BioResources 2020, 15, 4323–4336. [Google Scholar] [CrossRef]
- Walls, C. Kraft Pulp Mill Wood Chips. Available online: https://iem.ca/pdf/resources/Kraft%20Pulp%20Mill%20Wood%20Chips.pdf (accessed on 12 October 2023).
- TAPPI. Tappi Test Method T203 CM-22: Alpha-, Beta- and Gamma-Cellulose in Pulp, Test Method T 203.2022. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T203.aspx (accessed on 5 October 2023).
- Launer, H.F. Simplified Volumetric Determination of Alpha, Beta, and Gamma Cellulose in Pulps and Papers. J. Res. Natl. Bur. Stand. 1937, 18, 333–342. [Google Scholar] [CrossRef]
- Kosan, B.; Meister, F.; Sigmund, I.; Paulitz, J. Innovative Dissolving Pulps for Application in Cellulose MMF Production. Lenzing. Berichte 2019, 95, 9–14. [Google Scholar]
- Kim, C.H.; Lee, J.; Treasure, T.; Skotty, J.; Floyd, T.; Kelley, S.S.; Park, S. Alkaline Extraction and Characterization of Residual Hemicellulose in Dissolving Pulp. Cellulose 2019, 26, 1323–1333. [Google Scholar] [CrossRef]
- Lehr, M.; Miltner, M.; Friedl, A. Removal of Wood Extractives as Pulp (Pre-)Treatment: A Technological Review. SN Appl. Sci. 2021, 3, 886. [Google Scholar] [CrossRef]
- Thomas, R.J. Measuring Heavy Metal Contaminants in Cannabis and Hemp; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-00-019339-8. [Google Scholar]
- Goto, T.; Zaccaron, S.; Hettegger, H.; Bischof, R.H.; Fackler, K.; Potthast, A.; Rosenau, T. Evaluating Chelating Agents and Their Effects on Cellulosic Pulps during P-Stage Bleaching. Part 1: Analytical Method Development. Cellulose 2023, 30, 3887–3900. [Google Scholar] [CrossRef]
- Gevert, B.S.; Lohmander, S.F. Influence of Sulfur Compounds, Manganese, and Magnesium on Oxygen Bleaching of Kraft Pulp, TAPPI JOU. TAPPI J. 1997, 80, 263–268. [Google Scholar]
- TAPPI. TAPPI Test Method TAPPI/ANSI T 236 Om-22: Kappa Number of Pulp. 2022. Available online: https://webstore.ansi.org/standards/tappi/ansitappi236om22#:~:text=ANSI%2FTAPPI%20T%20236%20om-22%20Kappa%20number%20of%20pulp,and%20semibleached%20pulps%20obtained%20in%20yields%20under%2060%25 (accessed on 5 October 2023).
- Dou, X.; Tang, Y. The Influence of Cold Caustic Extraction on the Purity, Accessibility and Reactivity of Dissolving-Grade Pulp. ChemistrySelect 2017, 2, 11462–11468. [Google Scholar] [CrossRef]
- Harding, S.E. Intrinsic Viscosity. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1123–1129. ISBN 978-3-642-16712-6. [Google Scholar]
- Uddin, M.N.; Nayeem, J.; Islam, M.S.; Jahan, M.S. Rapid Determination Method of Dissolving Pulp Properties by Spectroscopic Data and Chemometrics. Biomass Convers. Biorefinery 2019, 9, 585–592. [Google Scholar] [CrossRef]
- Utomo, N.W.; Nazari, B.; Parisi, D.; Colby, R.H. Determination of Intrinsic Viscosity of Native Cellulose Solutions in Ionic Liquids. J. Rheol. 2020, 64, 1063–1073. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwak, S.-Y.; Song, Y.; Kim, H. Physical State of Cellulose in BmimCl: Dependence of Molar Mass on Viscoelasticity and Sol-Gel Transition. Phys. Chem. Chem. Phys. 2016, 18, 1460–1469. [Google Scholar] [CrossRef]
- Zaccaron, S.; Ahn, K.; Henniges, U.; Potthast, A.; Rosenau, T. An Improved, Less Erroneous Protocol for the Classical “Cuen”, “Cuoxam” or “Cadoxen” Viscosity Measurements of Pulps. Cellulose 2022, 29, 3733–3744. [Google Scholar] [CrossRef]
- Burkhardt, S. Does the Kappa Number Method Accurately Reflect Lignin Content in Nonwood Pulps? TAPPI J. 2018, 17, 611–617. [Google Scholar] [CrossRef]
- Saukkonen, E.; Kautto, J.; Rauvanto, I.; Backfolk, K. Characteristics of Prehydrolysis-Kraft Pulp Fibers from Scots Pine. Holzforschung 2012, 66, 801–808. [Google Scholar] [CrossRef]
- Li, G.; Fu, S.; Zhou, A.; Zhan, H. Improved Cellulose Yield in the Production of Dissolving Pulp from Bamboo Using Acetic Acid in Prehydrolysis. BioResources 2014, 10, 877–886. [Google Scholar] [CrossRef]
- Koistinen, A.; Phiri, J.; Kesari, K.; Vuorinen, T.; Maloney, T. Effect of Pulp Prehydrolysis Conditions on Dissolution and Regenerated Cellulose Pore Structure. Cellulose 2023, 30, 2827–2840. [Google Scholar] [CrossRef]
- Schild, G.; Sixta, H. Sulfur-Free Dissolving Pulps and Their Application for Viscose and Lyocell. Cellulose 2011, 18, 1113–1128. [Google Scholar] [CrossRef]
- Loureiro, P.E.G.; Cadete, S.M.S.; Tokin, R.; Evtuguin, D.V.; Lund, H.; Johansen, K.S. Enzymatic Fibre Modification During Production of Dissolving Wood Pulp for Regenerated Cellulosic Materials. Front. Plant Sci. 2021, 12, 717776. [Google Scholar] [CrossRef]
- Martino, D.C.; Student, D.; Colodette, J.L.; Jardim, J.M.; Student, M.; Chandra, R.P.; Saddler, J.N. Hot Water Pretreatment to Enhance the Production of a Eucalypt Dissolving Pulp. In Proceedings of the 7 th International Colloquium on Eucalyptus Pulp, Vitória, Espirito Santo, Brazil, 26 May 2015; p. 6. [Google Scholar]
- Laivins, G.V.; Scallan, A.M. The Mechanism of Hornification of Wood Pulps. In Products of Papermaking: Transactions of the Tenth Fundamental Research Symposium Held at Oxford, September 1993; PIRA International: Leatherhead, UK, 1993; pp. 1235–1260. [Google Scholar]
- Fernandes Diniz, J.M.B.; Gil, M.H.; Castro, J.A.A.M. Hornification—Its Origin and Interpretation in Wood Pulps. Wood Sci. Technol. 2004, 37, 489–494. [Google Scholar] [CrossRef]
- Kosan, B.; Michels, C.; Meister, F. Dissolution and Forming of Cellulose with Ionic Liquids. Cellulose 2008, 15, 59–66. [Google Scholar] [CrossRef]
- Sayyed, A.J.; Deshmukh, N.A.; Pinjari, D.V. A Critical Review of Manufacturing Processes Used in Regenerated Cellulosic Fibres: Viscose, Cellulose Acetate, Cuprammonium, LiCl/DMAc, Ionic Liquids, and NMMO Based Lyocell. Cellulose 2019, 26, 2913–2940. [Google Scholar] [CrossRef]
- Zhang, J.; Tominaga, K.; Yamagishi, N.; Gotoh, Y. Comparison of Regenerated Cellulose Fibers Spun from Ionic Liquid Solutions with Lyocell Fiber. J. Fiber Sci. Technol. 2020, 76, 257–266. [Google Scholar] [CrossRef]
- Rosenau, T.; French, A.D. N-Methylmorpholine-N-Oxide (NMMO): Hazards in Practice and Pitfalls in Theory. Cellulose 2021, 28, 5985–5990. [Google Scholar] [CrossRef]
- Jadhav, S.; Lidhure, A.; Thakre, S.; Ganvir, V. Modified Lyocell Process to Improve Dissolution of Cellulosic Pulp and Pulp Blends in NMMO Solvent. Cellulose 2021, 28, 973–990. [Google Scholar] [CrossRef]
- Prasakis, J.; Sain, M.; Daneault, C. Metal Management Improves Peroxide Bleaching of TMP. Tappi J. 1996, 79, 161–166. [Google Scholar]
- Lindholm-Lehto, P. Biosorption of Heavy Metals by Lignocellulosic Biomass and Chemical Analysis. BioResources 2019, 14, 4952–4995. [Google Scholar] [CrossRef]
- Abdu, N.; Abdullahi, A.A.; Abdulkadir, A. Heavy Metals and Soil Microbes. Environ. Chem. Lett. 2017, 15, 65–84. [Google Scholar] [CrossRef]
- Government of Alberta. Agriculture and Forestry. Growing Hemp in Alberta—Growing Hemp in Alberta—Open Government. Available online: https://open.alberta.ca/dataset/growing-hemp-in-alberta/resource/9babce43-b6f6-422c-85cd-283ea1a56147 (accessed on 14 September 2022).
- Pinto, I.S.S.; Ascenso, O.S.; Barros, M.T.; Soares, H.M.V.M. Pre-Treatment of the Paper Pulp in the Bleaching Process Using Biodegradable Chelating Agents. Int. J. Environ. Sci. Technol. 2015, 12, 975–982. [Google Scholar] [CrossRef][Green Version]
- Pinto, I.S.S.; Neto, I.F.F.; Soares, H.M.V.M. Biodegradable Chelating Agents for Industrial, Domestic, and Agricultural Applications—A Review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef] [PubMed]
- Virtapohja, J.S. The Use and Determination of Chelating Agents (EDTA & DTPA) in TCF Bleaching. In Proceedings of the International Pulp Bleaching Conference, Washington, DC, USA, 14–18 April 1996; Volume 2, pp. 537–539. [Google Scholar]
- Sharma, K. Chelating Agents: Properties, Types, Applications. Available online: https://thechemistrynotes.com/chelating-agents-properties-applications/ (accessed on 18 October 2023).
- Isaza Ferro, E.; Ruuttunen, K.; Perrin, J.; Vuorinen, T. Sustainable Bleaching of Eucalyptus Sp. Kraft Pulp with Hypochlorous Acid, Ozone and Hydrogen Peroxide. Ind. Crops Prod. 2021, 172, 114004. [Google Scholar] [CrossRef]
- Pulp & Paper Canada Pulping & Bleaching. Available online: https://www.pulpandpapercanada.com/pulping-bleaching-1000143592/ (accessed on 3 October 2023).
- Assis, T.D.; Perrin, J.; Kirkman, A.; Lachenal, D.; Jameel, H.; Phillips, R.; Gonzalez, R. Techno-Economic Analysis of ECF Bleaching and TCF Bleaching for a Bleached Eucalyptus Kraft Pulp Mill. TAPPI J. 2017, 16, 583–594. [Google Scholar] [CrossRef]
- Santos, R.B.; Hart, P.W. Brownstock Washing: A Review of the Literature. TAPPI J. 2014, 13, 9–19. [Google Scholar] [CrossRef]
- Melikhov, I.; Bacher, M.; Hosoya, T.; Hettegger, H.; Potthast, A.; Rosenau, T. On the Chemical Fate of Propyl Gallate as Stabilizer in Lyocell Spinning Dopes. Cellulose 2023, 30, 5373–5390. [Google Scholar] [CrossRef]
- Röder, T.; Morgenstern, B. The Influence of Activation on the Solution State of Cellulose Dissolved in N-Methylmorpholine-N-Oxide-Monohydrate. Polymer 1999, 40, 4143–4147. [Google Scholar] [CrossRef]
- Rosenau, T.; Potthast, A.; Hettegger, H.; Bacher, M.; Opietnik, M.; Röder, T.; Adorjan, I. On the Role of N-Methylmorpholine-N-Oxide (NMMO) in the Generation of Elemental Transition Metal Precipitates in Cellulosic Materials. Cellulose 2021, 28, 10143–10161. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Y.; Zhang, H.; Shao, H. Influences of Stabilizers on Lyocell Spinning Dope and Fiber Properties. Polym. Test. 2021, 99, 107228. [Google Scholar] [CrossRef]
- Borbély, É. Lyocell, The New Generation of Regenerated Cellulose. Acta Polytech. Hung. 2008, 5, 8. [Google Scholar]
- Moriam, K.; Sawada, D.; Nieminen, K.; Hummel, M.; Ma, Y.; Rissanen, M.; Sixta, H. Towards Regenerated Cellulose Fibers with High Toughness. Cellulose 2021, 28, 9547–9566. [Google Scholar] [CrossRef]
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The Environmental Price of Fast Fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- EEA’s European Topic Centre on Waste and Materials in a Green Economy. Plastic in Textiles: Towards a Circular Economy for Synthetic Textiles in Europe—European Environment Agency. Available online: https://www.eea.europa.eu/themes/waste/resource-efficiency/plastic-in-textiles-towards-a (accessed on 11 October 2022).
- Weber, S.; Weber, O.; Habib, K.; Dias, G.m. Textile Waste in Ontario, Canada: Opportunities for Reuse and Recycling. Resour. Conserv. Recycl. 2023, 190, 106835. [Google Scholar] [CrossRef]
- Business Wire. Decreasing Per Capita Arable Land and Increasing Demand for Food Are Key Drivers in the Global Soil Treatment Market. Available online: https://www.businesswire.com/news/home/20200916005541/en/Decreasing-Per-Capita-Arable-Land-and-Increasing-Demand-for-Food-are-Key-Drivers-in-the-Global-Soil-Treatment-Market---ResearchAndMarkets.com (accessed on 22 October 2023).
- Zhang, X.; Cai, X. Climate Change Impacts on Global Agricultural Land Availability. Environ. Res. Lett. 2011, 6, 014014. [Google Scholar] [CrossRef]
- Tarr, D.; Trushin, E. Did the Desire for Cotton Self-Sufficiency Lead to the Aral Sea Environmental Disaster? A Case Study on Trade and the Environment; The World Bank: Washington, DC, USA, 2004. [Google Scholar]
- Loodin, N. Aral Sea: An Environmental Disaster in Twentieth Century in Central Asia. Model. Earth Syst. Environ. 2020, 6, 2495–2503. [Google Scholar] [CrossRef]
- NASA. The Aral Sea, before the Streams Ran Dry. Available online: https://earthobservatory.nasa.gov/images/77193/the-aral-sea-before-the-streams-ran-dry (accessed on 22 October 2023).
- Kalayci, E.; Avinc, O.; Yavas, A. An Alternative Fiber Source in Sustainable Textile and Fashion Design: Cellulosic Akund Fibers. In Novel Sustainable Raw Material Alternatives for the Textiles and Fashion Industry; Muthu, S.S., Ed.; Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Springer Nature Switzerland: Cham, Switzerland, 2023; pp. 185–198. ISBN 978-3-031-37323-7. [Google Scholar]
- Missouri Historic Costume and Textile Collection Flora and Fashion: Seed Fibers: Cotton, Coir, Kapok, Milkweed. Available online: https://mhctc.missouri.edu/exhibitions/origins-dress-and-textiles-series-2018-2019/flora-and-fashion/seed-fibers-cotton-coir-kapok-milkweed/ (accessed on 22 October 2023).
- Nair, G.R.; Rho, D.; Raghavan, G.S.V. Application of Electro-Technologies in Processing of Flax Fiber. Fibers 2013, 1, 21–35. [Google Scholar] [CrossRef]
- Sun, Y.; Li, D.; Yu, Y.; Chen, J.; Fan, W. Separation and Characterization of Cellulose Fibers from Cannabis Bast Using Foamed Nickel by Cathodic Electro-Fenton Oxidation Strategy. Polymers 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Tahir, P.M.; Ahmed, A.B.; SaifulAzry, S.O.A.; Ahmed, Z. Retting Process of Some Bast Plant Fibers and Its Effect on Fibre Quality: A Review. BioResources 2011, 6, 5260–5281. [Google Scholar] [CrossRef]
- Changing Markets. Dirty Fashion: How Pollution in the Global Textiles Supply Chain Is Making Viscose Toxic. Available online: http://changingmarkets.org/wp-content/uploads/2017/06/CHANGING_MARKETS_DIRTY_FASHION_REPORT_SPREAD_WEB.pdf (accessed on 20 October 2022).
- Freitas, A.; Mathews, R. Viscose Fibers Production: An Assessment of Sustainability Issues; Water Footprint Network: Hengelo, The Netherlands, 2017. [Google Scholar]
- Council of Fashion Designers of America (CFDA) Rayon (Viscose)|Materials Index. Available online: https://cfda.com/resources/materials/detail/rayon-viscose (accessed on 23 October 2023).
- United States Environmenttal Protection Agency. Cellulose Products Manufacturing: National Emission Standards for Hazardous Air Pollutants (NESHAP). Available online: https://www.epa.gov/stationary-sources-air-pollution/cellulose-products-manufacturing-national-emission-standards (accessed on 23 October 2023).
- World Health Organization. Air Quality Guidelines for Europe, 2nd ed.; WHO Regional Publications, European Series; World Health Organization: Copenhagen, Denmark, 2000. [Google Scholar]
- Shen, L.; Worrell, E.; Patel, M.K. Environmental Impact Assessment of Man-Made Cellulose Fibres. Resour. Conserv. Recycl. 2010, 55, 260–274. [Google Scholar] [CrossRef]
- Ferreira, E.; Kalliola, R.; Ruokolainen, K. Bamboo, Climate Change and Forest Use: A Critical Combination for Southwestern Amazonian Forests? Ambio 2020, 49, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Province of Manitoba. Agriculture: Industrial Hemp Production. Available online: https://www.gov.mb.ca/agriculture/crops/crop-management/hemp.html#:~:text=The%20Industrial%20Hemp%20Industry%20is%20a%20new%20industry,nut%2C%20protein%20powder%2C%20flour%2C%20milk%20and%20other%20products. (accessed on 14 September 2022).
- Rana, S.; Pichandi, S.; Parveen, S.; Fangueiro, R. Natural Plant Fibers: Production, Processing, Properties and Their Sustainability Parameters. In Roadmap to Sustainable Textiles and Clothing: Eco-friendly Raw Materials, Technologies, and Processing Methods; Muthu, S.S., Ed.; Textile Science and Clothing Technology; Springer: Singapore, 2014; pp. 1–35. ISBN 978-981-287-065-0. [Google Scholar]
- Kaur, G.; Kander, R. The Sustainability of Industrial Hemp: A Literature Review of Its Economic, Environmental, and Social Sustainability. Sustainability 2023, 15, 6457. [Google Scholar] [CrossRef]
- Health Canada. Industrial Hemp Licensing Statistics. Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/producing-selling-hemp/about-hemp-canada-hemp-industry/statistics-reports-fact-sheets-hemp.html (accessed on 24 October 2023).
- Health Canada. List of Approved Cultivars for the 2023 Growing Season: Industrial Hemp Varieties Approved for Commercial Production. Available online: https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/producing-selling-hemp/commercial-licence/list-approved-cultivars-cannabis-sativa.html (accessed on 25 October 2022).
- Sisti, L.; Totaro, G.; Vannini, M.; Celli, A. Retting Process as a Pretreatment of Natural Fibers for the Development of Polymer Composites. In Lignocellulosic Composite Materials; Kalia, S., Ed.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 97–135. ISBN 978-3-319-68696-7. [Google Scholar]
- Baker, M.L.; Chen, Y.; Laguë, C.; Landry, H.; Peng, Q.; Zhong, W. Fiber Yield and Energy Requirement of Hemp Decortication Using a Hammermill. Appl. Eng. Agric. 2013, 29, 453–460. [Google Scholar] [CrossRef]
- Hobson, R.N.; Hepworth, D.G.; Bruce, D.M. PH—Postharvest Technology: Quality of Fibre Separated from Unretted Hemp Stems by Decortication. J. Agric. Eng. Res. 2001, 78, 153–158. [Google Scholar] [CrossRef]
- Zimniewska, M. Hemp Fibre Properties and Processing Target Textile: A Review. Materials 2022, 15, 1901. [Google Scholar] [CrossRef]
- Global Affairs Canada. Export and Import Controls, Controlled Products: Textiles & Clothing. Available online: https://www.international.gc.ca/controls-controles/textiles/index.aspx?lang=eng (accessed on 23 October 2023).
- Statistics Canada, Government of Canada. Environmental, Social and Governance (ESG) Project. Available online: https://www.statcan.gc.ca/en/trust/modernization/esg (accessed on 23 October 2023).
- Lambrechts, W. Ethical and Sustainable Sourcing: Towards Strategic and Holistic Sustainable Supply Chain Management. In Decent Work and Economic Growth; Leal Filho, W., Azul, A.M., Brandli, L., Özuyar, P.G., Wall, T., Eds.; Encyclopedia of the UN Sustainable Development Goals; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–13. ISBN 978-3-319-71058-7. [Google Scholar]


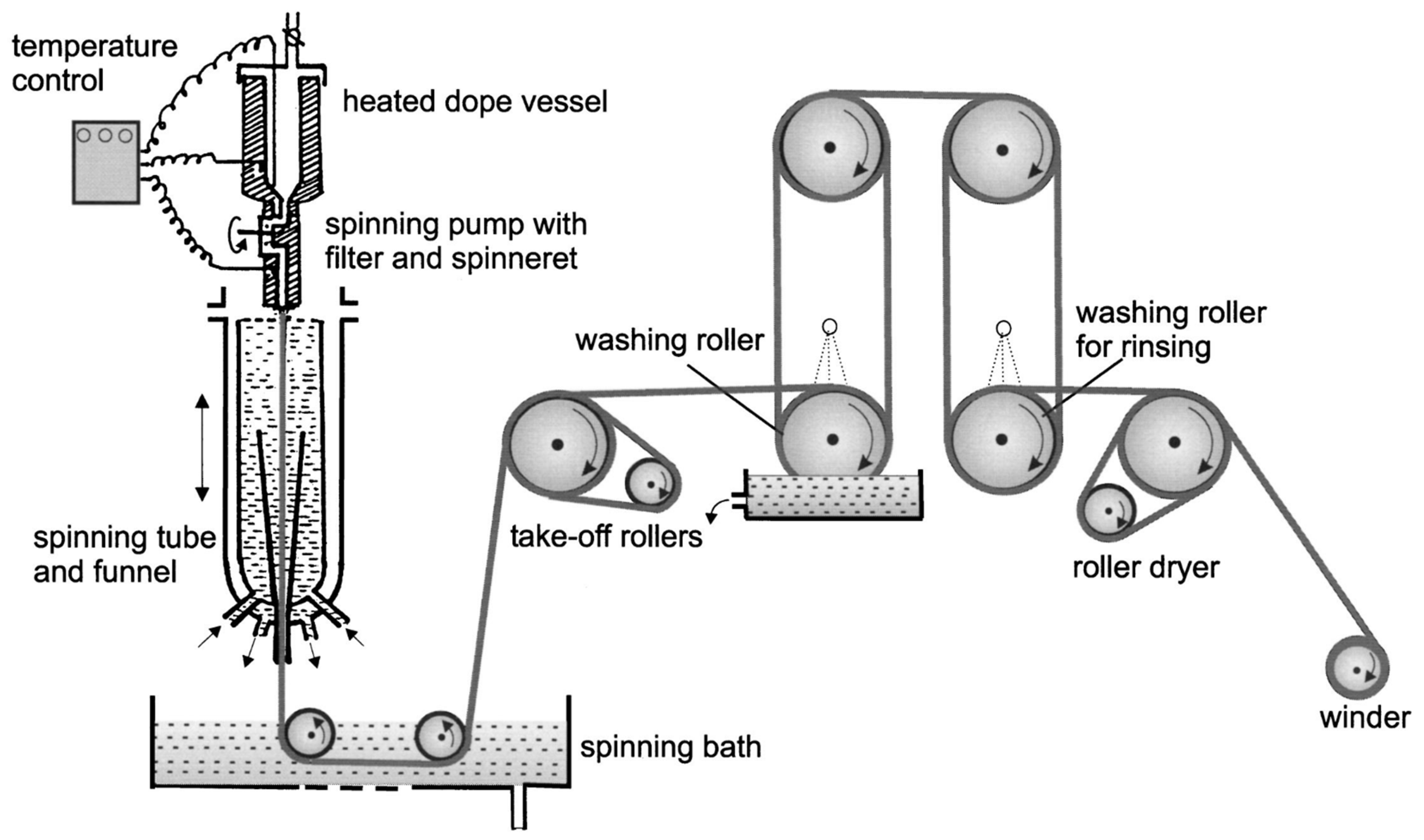
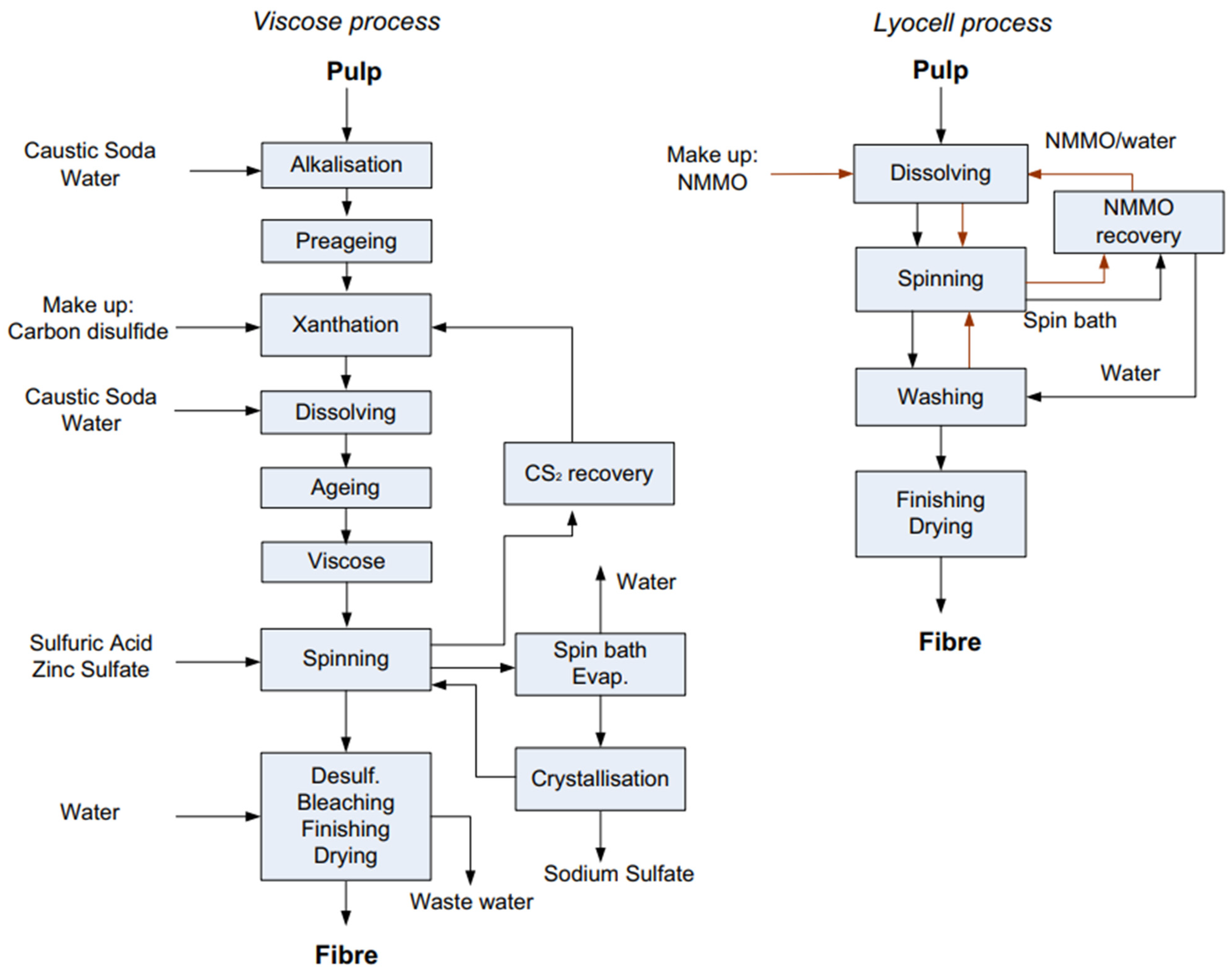
| Manufacturing Process | Solvent Process | Advantages | Drawbacks |
|---|---|---|---|
| Viscose MMCF (viscose rayon, cellulose acetate, modal, triacetate) | Derivatisation |
|
|
| L-MMCF | Direct dissolution |
|
|
| Feedstock | Cellulosic Content (%) | α-Cellulose Content (%) | Hemicellulose Content (%) | Lignin Content (%) | References |
|---|---|---|---|---|---|
| Hemp hurd | 34–46 | 77.6–89.6 | 21.5–34 | 16–25 | [7,26,41,59] |
| Hemp bast | 55–72 | 92–96.2 | 11–15 | 3–4.6 | [7,25,41,60] |
| Hardwood | 40–55 | 95.6–96 | 24–40 | 18–25 | [19,38,61] |
| Eucalyptus | 41.6–52 | 92–95 | 22 | 21.7–31.4 | [7,62,63,64] |
| Softwood | 0–50 | 92 | 24–35 | 20–35 | [37,38,61,65,66] |
| Sugar cane bagasse | 42–52.3 | 94.6–95.2 | 25–28.7 | 20–23.4 | [23,61,63,67] |
| Bamboo | 33.8–60.3 | 90 | 15–32 | 17–32 | [7,18,63,68] |
| Corn cob | 40–45 | 88.8–93.6 | 25–35 | 15–20 | [24,61,67] |
| Cotton linter | 80–95 | 95–99 | 2–20 | 0 | [11,19,61,65,67,69] |
| Wheat straw | 29–35.9 | 70.3 | 26–32 | 16–21 | [19,61,66,67,70] |
| Orange peel | 13 | n/a | 6 | <2 | [19] |
| Flax fibre/noils | 75 | 92 | 15 | <1 | [30,31,41] |
| Properties | Specification | References |
|---|---|---|
| Feedstock Fibre/Chip Length (mm) | 10–30 | [38] |
| DPCuoxam | 550–650 | [17,65,75] |
| α-cellulose (%) | >90–95 | [16,17,18,37,75] |
| Hemicellulose (%) | 1–6 | [17,18,37] |
| Pulp Yield (%) | 40–55 | [38] |
| Intrinsic Viscosity (dL/g) | 4–6 | [17,18] |
| Ash (%) | <0.1 | [16,24] |
| Lignin (%) | Trace (>0.05) | [17,18,37] |
| Kappa | <1–5 | [17,25,75] |
| S10 (%) | <10 | [17,62,84] |
| S18 (%) | <5 | [17,62,84] |
| Metal (mg/kg) [75] | Fe, Cu: <10 | [24,25,75] |
| Mn, Cr, Ni: ≤20 | ||
| Na: ≤500 | ||
| K, Ca, Mg: ≤100 |
| Metal Scan (mg/kg) | As-Received Hurd Biomass | PHK Hurd | Chelated/Bleached Hurd | As-Received Bast Biomass | PHK Bast | Chelated/Bleached Bast |
|---|---|---|---|---|---|---|
| Aluminum, Al | 30.8 | 22.3 | 9.05 | 95.5 | 95.5 | 8 |
| Arsenic, As | <0.5 | <1 | <0.5 | <1 | <1 | <0.5 |
| Barium, Ba | 20.6 | 20.2 | 7.2 | 31.3 | 31.3 | 0.779 |
| Boron, B | 12 | 8.1 | 0.3 | 9.8 | 9.8 | 1.1 |
| Cadmium, Cd | <0.05 | <0.1 | <0.02 | <0.05 | <0.05 | <0.02 |
| Calcium, Ca | 6040 | 5560 | 932 | 4200 | 4200 | 39.1 |
| Chromium, Cr | 0.18 | 0.95 | 3.96 | 0.24 | 0.24 | 0.4 |
| Cobalt, Co | <0.1 | <0.2 | 0.08 | 0.11 | 0.11 | <0.02 |
| Copper, Cu | 3.7 | 2.7 | 1.76 | 2.2 | 2.2 | 0.77 |
| Iron, Fe | 45.2 | 43.7 | 46.3 | 96.9 | 96.9 | 7.61 |
| Lead, Pb | <0.5 | 0.8 | <0.2 | 1 | 1 | <0.2 |
| Lithium, Li | 0.5 | 0.25 | 0.06 | 0.47 | 0.47 | 1.35 |
| Magnesium, Mg | 1800 | 1300 | 190 | 1530 | 1530 | 24.8 |
| Manganese, Mn | 26.2 | 22 | 1.12 | 48.4 | 48.4 | 0.116 |
| Molybdenum, Mo | <0.5 | <1 | 0.7 | <0.5 | <0.5 | <0.05 |
| Nickel, Ni | 0.7 | 1.5 | 3 | 0.8 | 0.8 | 0.22 |
| Phosphorus, P | 232 | 134 | 2.5 | 277 | 277 | 0.5 |
| Potassium, K | 3900 | 2050 | 5 | 3590 | 3590 | <2 |
| Silicon, Si | 137 | 113 | 133 | 660 | 660 | 215 |
| Sodium, Na | 40 | 126 | 50 | 111 | 111 | 8 |
| Strontium, Sr | 20.9 | 22.6 | 4.38 | 54 | 54 | 0.38 |
| Titanium, Ti | 1.5 | 0.96 | 0.921 | 4.44 | 4.44 | 0.498 |
| Vanadium, V | 0.14 | 0.06 | <0.05 | 0.28 | 0.28 | <0.05 |
| Zinc, Zn | 2.8 | 5.6 | 2.55 | 4.8 | 21 | 0.494 |
| Feedstock | Advantages | Disadvantages |
|---|---|---|
| Industrial Hemp Stalks |
|
|
| Wood and Bamboo |
|
|
| Cotton Linters |
|
|
| Other Alternative Feedstock |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, L.; Ford, M.; Hoque, M.S.; Chute, W.; Bressler, D.C.; Dolez, P.I. Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks. Appl. Sci. 2023, 13, 12759. https://doi.org/10.3390/app132312759
Lawson L, Ford M, Hoque MS, Chute W, Bressler DC, Dolez PI. Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks. Applied Sciences. 2023; 13(23):12759. https://doi.org/10.3390/app132312759
Chicago/Turabian StyleLawson, Lelia, Madison Ford, Md. Saiful Hoque, Wade Chute, David C. Bressler, and Patricia I. Dolez. 2023. "Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks" Applied Sciences 13, no. 23: 12759. https://doi.org/10.3390/app132312759
APA StyleLawson, L., Ford, M., Hoque, M. S., Chute, W., Bressler, D. C., & Dolez, P. I. (2023). Processes and Challenges for the Manufacturing of Lyocell Fibres with Alternative Agricultural Feedstocks. Applied Sciences, 13(23), 12759. https://doi.org/10.3390/app132312759






