Influence of Nigella sativa L. Oil Addition on Physicochemical and Sensory Properties of Freezer-Stored Ground Pork for Pâté
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Colour Determination
- Sqr: the square root;
- L0*, a0*, b0*: the colour of fresh meat;
- Li*, ai*, bi*: the colour of thawed meat.
2.3. Fatty Acids Profile Analysis
2.4. Volatile Compounds Profile
2.5. Lipid Oxidation Product TBARSs
2.6. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Changes in Colour during Storage
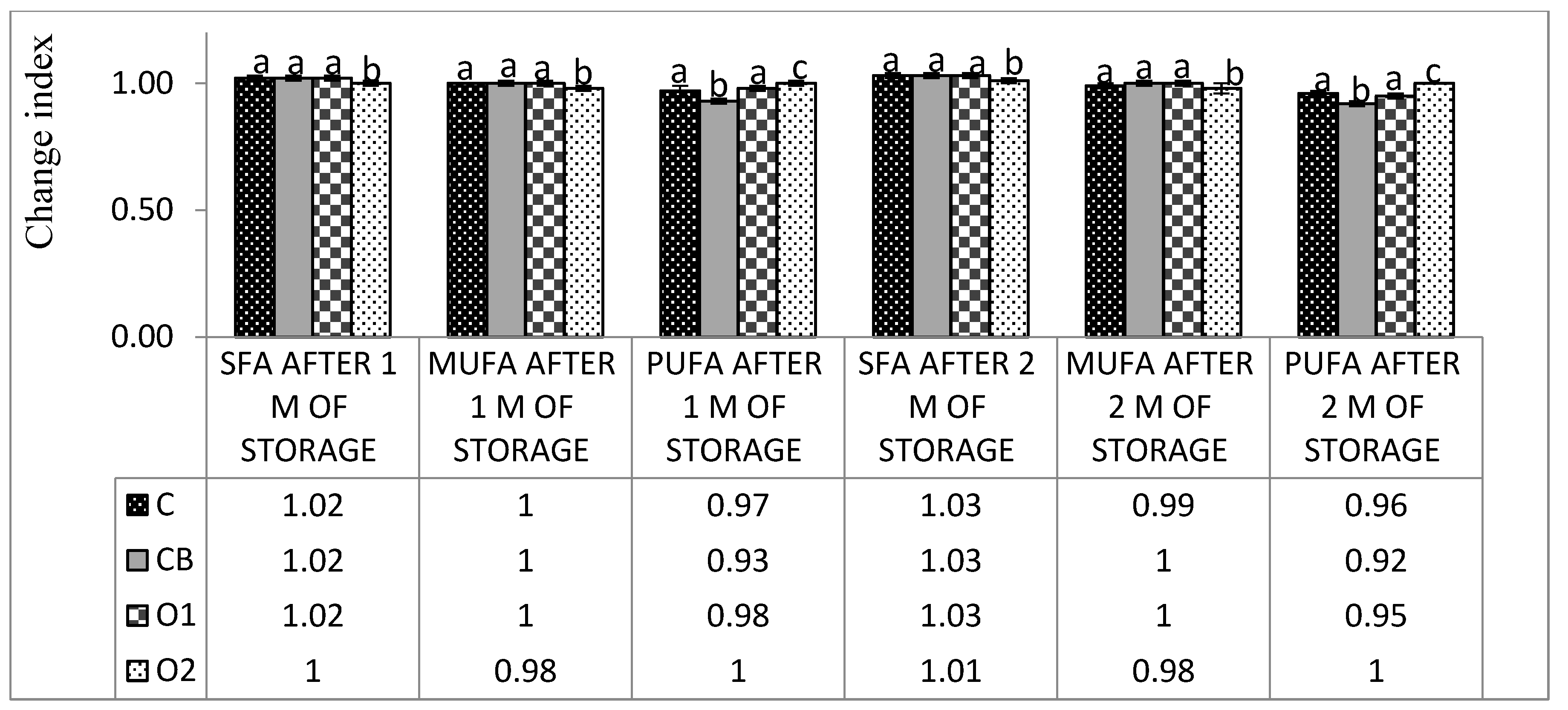
3.2. Fatty Acids
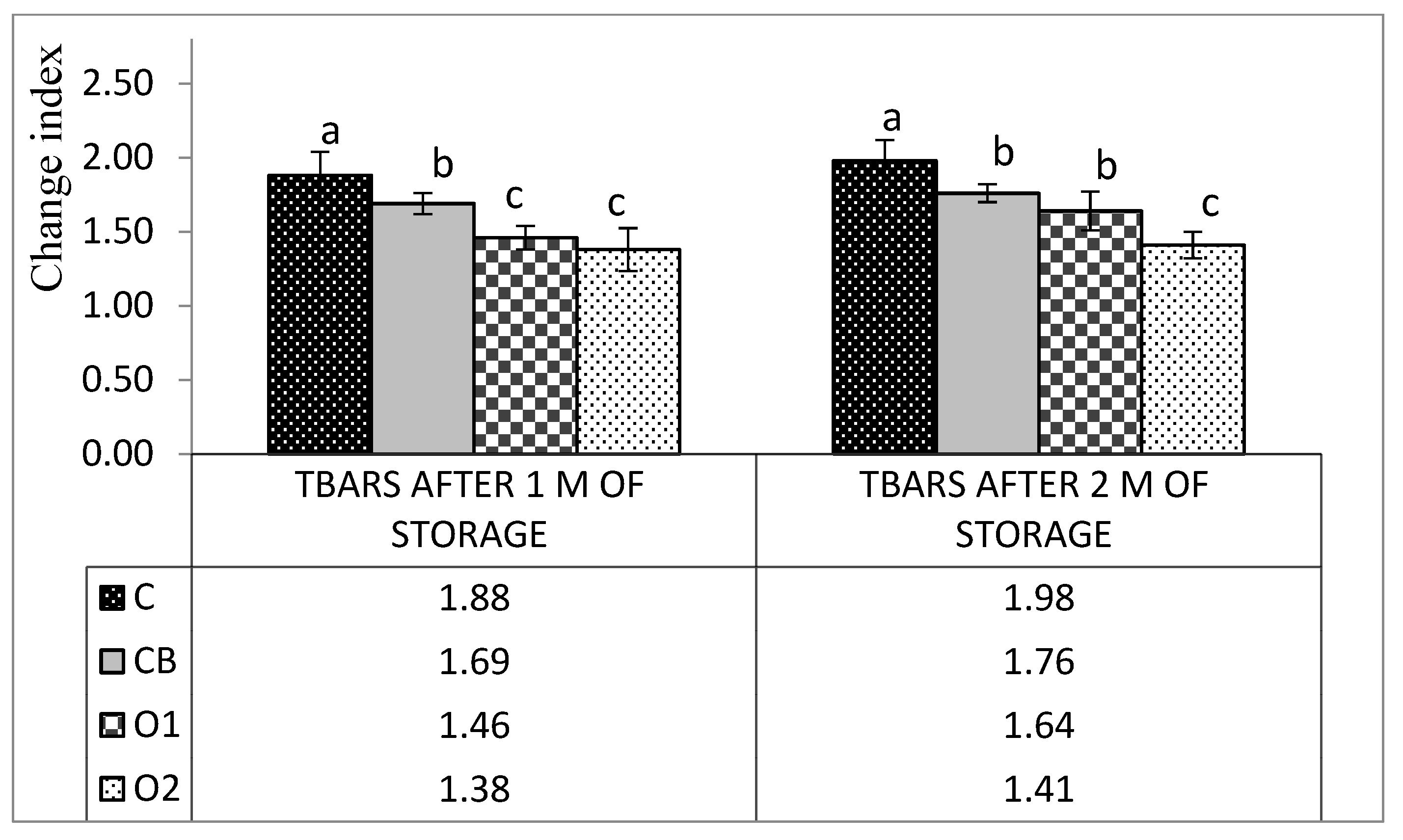
3.3. TBARSs
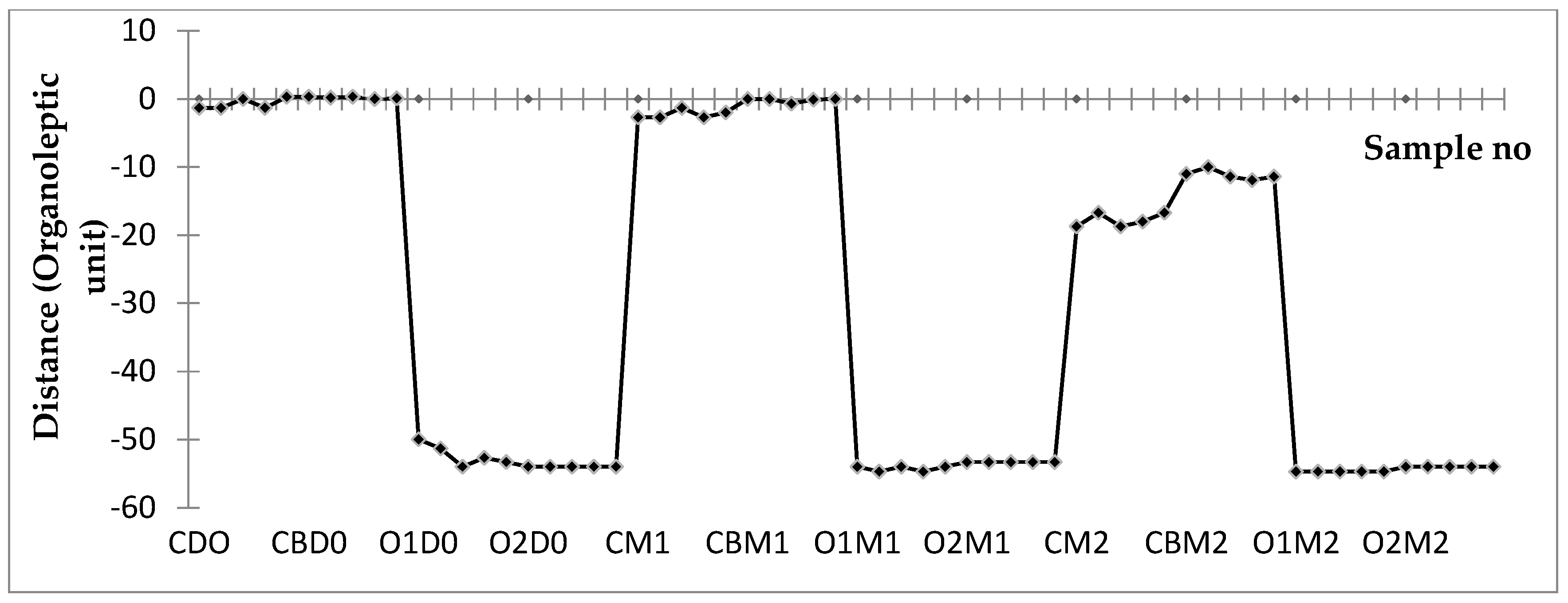
3.4. Changes in the Volatile Compounds Profile
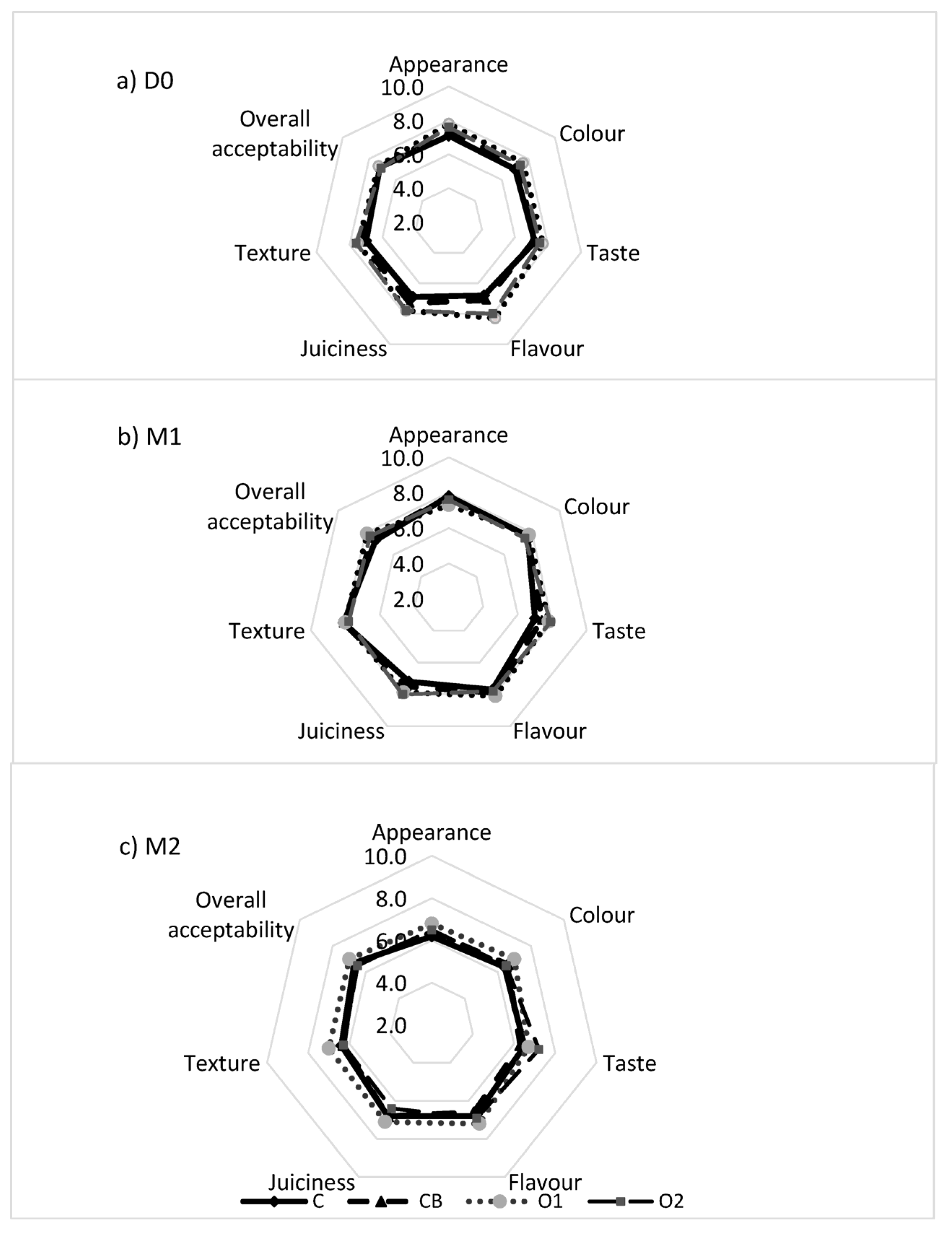
3.5. Sensory Acceptability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD; FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023; pp. 188–189. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat spoilage mechanisms and preservation techniques: A critical review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Tatiyaborworntham, N.; Oz, F.; Richards, M.P.; Wu, H. Paradoxical effects of lipolysis on the lipid oxidation in meat and meat products. Food Chem. 2022, X14, 100317. [Google Scholar] [CrossRef]
- Pikul, J. Lipidy Mięsa Drobiowego. W: Przetwórstwo Mięsa Drobiu—Podstawy Biologiczne i Technologiczne; Praca zbiorowa; Smolińskiej, T., Kopcia, W., Eds.; Wydawnictwo Uniwersytetu Przyrodniczego we Wrocławiu: Wrocław, Poland, 2009; pp. 149–177. [Google Scholar]
- Jongberg, S.; Lund, M.N.; Skibsted, L.H. Protein oxidation in meat and meat products. Challenges for antioxidative protect. In Global Food Security and Wellness; Springer: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Guzek, D.; Sun, D.-W.; Wierzbicka, A. Differentiation of chill-stored and frozen pork necks using electronic nose with ultra-fast gas chromatography. J. Food Process Eng. 2017, 40, e12540. [Google Scholar] [CrossRef]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2017, 221, 1069–1076. [Google Scholar] [CrossRef]
- Aminzare, M.; Hashemi, M.; Ansarian, E.; Bimkar, M.; Azar, H.H.; Mehrasbi, M.R.; Afshari, A. Using natural antioxidants in meat and meat products as preservatives: A review. Adv. Anim. Vet. Sci. 2019, 7, 417–426. [Google Scholar] [CrossRef]
- Manessis, G.; Kalogianni, A.I.; Lazou, T.; Moschovas, M.; Bossis, I.; Gelasakis, A.I. Plant-derived natural antioxidants in meat and meat products. Antioxidants 2020, 9, 1215. [Google Scholar] [CrossRef]
- Srinivasan, K. Cumin (Cuminum cyminum) and black cumin (Nigella sativa) seeds: Traditional uses, chemical constituents, and nutraceutical effects. Food Qual. Saf. 2018, 2, 1–16. [Google Scholar] [CrossRef]
- Burdock, G.A. Assessment of black cumin (Nigella sativa L.) as a food ingredient and putative therapeutic agent. RTP 2022, 128, 105088. [Google Scholar] [CrossRef]
- Kiralan, M. Changes in volatile compounds of black cumin (Nigella sativa L.) seed oil during thermal oxidation. Int. J. Food Prop. 2014, 17, 1482–1489. [Google Scholar] [CrossRef]
- Cakir, Y.; Cakmakci, S.; Hayaloglu, A.A. The effect of addition of black cumin (Nigella sativa L.) and ripening period on proteolysis, sensory properties and volatile profiles of Erzincan Tulum (Şavak) cheese made from raw Akkaraman sheep’s milk. Small Rumin. Res. 2016, 134, 65–73. [Google Scholar] [CrossRef]
- Khan, M.A. Chemical composition and medicinal properties of Nigella sativa Linn. Inflammopharmacology 1999, 7, 15–35. [Google Scholar] [CrossRef]
- Agarwal, R.; Kharya, M.; Shrivastava, R. Antimicrobial & anthelmintic activities of the essential oil of Nigella sativa Linn. IJEB 1979, 17, 1264–1265. [Google Scholar]
- Ali, B.H.; Blunden, G. Pharmacological and Toxicological Properties of Nigella sativa. Phytother. Res. 2003, 17, 299–305. [Google Scholar] [CrossRef]
- Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of primary and specialized metabolites in Nigella sativa seeds, a spice with vast traditional and historical uses. Molecules 2012, 17, 10159–10177. [Google Scholar] [CrossRef]
- Koshak, D.A.E.; Koshak, P.E.A. Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: A mini review of in silico studies. Curr. Ther. Res. Clin. Exp. 2020, 93, 100602. [Google Scholar] [CrossRef]
- Mukhtar, H.; Qureshi, A.S.; Anwar, F.; Mumtaz, M.W.; Marcu, M. Nigella sativa L. seed and seed oil: Potential sources of high-value components for development of functional foods and nutraceuticals/pharmaceuticals. J. Essent. Oil Res. 2019, 31, 171–183. [Google Scholar] [CrossRef]
- Pawłowska, A.; Kocur, A.; Siudem, P.; Paradowska, K. Examination of stability of linseed oil and Nigella sativa oil. Postępy Fitoter. 2018, 19, 157–163. [Google Scholar] [CrossRef]
- Ketenoglu, O.; Kiralan, S.S.; Kiralan, M.; Ozkan, G.; Ramadan, M.F. Chapter 6 Cold pressed black cumin (Nigella sativa L.) seed oil. In Cold Pressed Oils: Green Technology, Bioactive Compounds, Functionality, and Applications; Ramadan, M.F., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 53–56. [Google Scholar] [CrossRef]
- Mahgoub, S.A.M.; Osman, A.; Ramadan, M.F. Inhibitory effect of Nigella sativa oil against Listeria monocytogenes and Salmonella Enteritidis inoculated in minced beef meat. J. Food Meas. Charact. 2017, 11, 2043–2051. [Google Scholar] [CrossRef]
- Rahman, M.H.; Alam, M.S.; Monir, M.M.; Ahmed, K. Comprehensive effects of black cumin (Nigella sativa) and synthetic antioxidant on sensory and physicochemical quality of beef patties during refrigerant storage. J. Agric. Food Res. 2021, 4, 100145. [Google Scholar] [CrossRef]
- Wojtasik-Kalinowska, I.; Guzek, D.; Brodowska, M.; Godziszewska, J.; Górska-Horczyczak, E.; Pogorzelska, E.; Sadowska, A.; Gantner, M.; Wierzbicka, A. The effect of addition of Nigella sativa L. oil on the quality and shelf life of pork patties. J. Food Process. Pres. 2017, 41, e13294. [Google Scholar] [CrossRef]
- Zwolan, A.; Pietrzak, D.; Adamczak, L.; Chmiel, M.; Kalisz, S.; Wirkowska-Wojdyła, M.; Florowski, T.; Oszmiański, J. Effects of Nigella sativa L. seed extracts on lipid oxidation and color of chicken meatballs during refrigerated storage. LWT 2020, 130, 109718. [Google Scholar] [CrossRef]
- Oz E Inhibitory effects of black cumin on the formation of heterocyclic aromatic amines in meatball. PLoS ONE 2019, 14, e0221680. [CrossRef]
- Wyrwisz, J.; Półtorak, A.; Zalewska, M.; Zaremba, R.; Wierzbicka, A. Analysis of relationship between basic composition, pH, and physical properties of selected bovine muscles. Bull. Vet. Inst. Puławy 2012, 56, 403–409. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Fernández, M.; Ordóñez, J.A.; Cambero, I.; Santos, C.; Pin, C.; Hoz, L. Fatty acid compositions of selected varieties of Spanish dry ham related to their nutritional implications. Food Chem. 2007, 101, 107–112. [Google Scholar] [CrossRef]
- Górska-Horczyczak, E.; Zalewska, M.; Wierzbicka, A. Chromatographic fingerprint application possibilities in food authentication. Eur. Food Res. Technol. 2022, 248, 1–15. [Google Scholar] [CrossRef]
- Brodowska, M.; Guzek, D.; Kołota, A.; Głąbska, D.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Wierzbicka, A. Effect of diet on oxidation and profile of volatile compounds of pork after freezing storage. J. Food Nutr. Res. 2016, 55, 40–47. [Google Scholar]
- Robles-Martinez, C.; Cervantes, E.; Ke, P.J. Recommended Method for Testing the Objective Rancidity Development in Fish Based on TBARS Formation; Canadian Technical Report of Fisheries and Aquatic Sciences, No. 1089; Government of Canada, Fisheries and Oceans: Delta, BC, Canada, 1982; ISSN 1488-5379. Available online: http://publications.gc.ca/collections/collection_2013/mpo-dfo/Fs97-6-1089-eng.pdf (accessed on 15 November 2023).
- ISO 13299:2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 11132:2021; Sensory Analysis—Methodology—Guidelines for the Measurement of the Performance of a Quantitative Descriptive Sensory Panel. International Organization for Standardization: Geneva, Switzerland, 2016.
- Faustman, C.; Sun, Q.; Mancini, R.; Suman, S.P. Myoglobin and lipid oxidation interactions: Mechanistic bases and control. Meat Sci. 2010, 86, 86–94. [Google Scholar] [CrossRef]
- Este’vez, M.; Morcuende, D.; Cava, R. Oxidative and color changes in meat from three lines of free-range reared Iberian pigs slaughtered at 90 kg live weight and from industrial pig during refrigerated storage. Meat Sci. 2003, 65, 1139–1146. [Google Scholar] [CrossRef]
- Suri, K.; Singh, B.; Kaur, A. Impact of microwave roasting on physicochemical properties, Maillard reaction products, antioxidant activity and oxidative stability of nigella seed (Nigella sativa L.) oil. Food Chem. 2022, 368, 130777. [Google Scholar] [CrossRef]
- Hongxia, Z.; Zhiyang, L.; Xin, Y.; Yuan, L. Study on colour classification of pork with computer vision system based on different colour difference formula. Key Eng. Mater. 2011, 474–476, 1638–1642. [Google Scholar]
- Albakry, Z.; Karrar, E.; Ahmed, I.A.M.; Oz, E.; Proestos, C.; El Sheikha, A.F.; Oz, E.; Wu, G.; Wang, X. Nutritional composition and volatile compounds of black cumin (Nigella sativa L.) seed, fatty acid composition and tocopherols, polyphenols, and antioxidant activity of its essential oil. Horticulturae 2022, 8, 575. [Google Scholar] [CrossRef]
- Darakeh, S.A.S.S.; Weisany, W.; Diyanat, M.; Ebrahimi, R. Bio-organic fertilizers induce biochemical changes and affect seed oil fatty acids composition in black cumin (Nigella sativa Linn). Ind. Crops Prod. 2021, 164, 113383. [Google Scholar] [CrossRef]
- Alonso, V.; Muela, E.; Tenas, J.; Calanche, J.B.; Roncalés, P.; Beltrán, J.A. Changes in physicochemical properties and fatty acid composition of pork following long-term frozen storage. Eur. Food Res. Technol. 2016, 242, 2119–2127. [Google Scholar] [CrossRef]
- Medić, H.; Kušec, I.D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, L.; Rao, Z.; Feng, S.; Wang, Y.; Liu, H.; Meng, Q. Integrated Lipidomic and Metabolomics Analysis Revealing the Effects of Frozen Storage Duration on Pork Lipids. Metabolites 2022, 12, 977. [Google Scholar] [CrossRef]
- Shimizu, H.; Iwamoto, S. Problems of lipid oxidation in minced meat products for a ready-made meal during cooking, processing, and storage. Rev. Agric. Sci. 2022, 10, 24–35. [Google Scholar] [CrossRef]
- Bialek, M.; Rutkowska, J.; Bialek, A.; Adamska, A. Oxidative stability of lipid fraction of cookies enriched with chokeberry polyphenols extract. Polish J. Food Nutr. Sci. 2016, 66, 77. [Google Scholar] [CrossRef]
- Rutkowska, J.; Antoniewska, A.; Martinez-Pineda, M.; Nawirska-Olszańska, A.; Zbikowska, A.; Baranowski, D. Black chokeberry fruit polyphenols: A valuable addition to reduce lipid oxidation of muffins containing xylitol. Antioxidants 2020, 9, 394. [Google Scholar] [CrossRef]
- Barros, J.C.; Munekata, P.E.; Carvalho, F.A.L.; Domínguez, R.; Trindade, M.A.; Pateiro, M.; Lorenzo, J.M. Healthy beef burgers: Effect of animal fat replacement by algal and wheat germ oil emulsions. Meat Sci. 2021, 173, 108396. [Google Scholar] [CrossRef]
- Sallam, K.; Abd-elghany, S.; Youssef, E.; Zein, A. Antibacterial And Antioxidant Effect Of Black Seed Oil And Black Seed Powder On Beef Minced Meat. Mansoura Vet. Med. J. 2018, 19, 27–37. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, R.; Li, X.; Wang, X.; Zeng, L.; Wen, X.; Huang, Q. Effect of oil-modified crosslinked starch as a new fat replacer on gel properties, water distribution, and microstructures of pork meat batter. Food Chem. 2023, 409, 135337. [Google Scholar] [CrossRef]
- de Lima Guterres, L.; Pinton, M.B.; Alves dos Santos, B.; Correa, L.P.; Cordeiro, M.W.S.; Wagner, R.; Cichoski, A.J.; Lorenzo, J.M.; Campagnol, P.C.B. Hydrogelled emulsion from linseed oil and pea protein as a strategy to produce healthier pork burgers with high technological and sensory quality. Meat Sci. 2023, 195, 109028. [Google Scholar] [CrossRef]
- Gray, J.I.; Pearson, A.M. Rancidity and warmed-over flavor. In Advances in Meat Research; Pearson, A.M., Dutson, T.R., Eds.; Restructured Meat and Poultry Products; Van Nostrand Reinhold Co.: New York, NY, USA, 1987; Volume 3, pp. 221–269. [Google Scholar]
- Tarladgis, B.G.; Watts, B.M.; Younathan, M.T.; Dugan, L., Jr. A distillation method for the quantitative determination of malonaldehyde in rancid foods. J. Am. Oil Chem. Soc. 1960, 37, 44–48. [Google Scholar] [CrossRef]
- Carmona-Jiménez, Y.; Igartuburu, J.M.; Guillén-Sánchez, D.A.; García-Moreno, M.V. Fatty Acid and Tocopherol Composition of Pomace and Seed Oil from Five Grape Varieties Southern Spain. Molecules 2022, 27, 6980. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Niazmand, R.; Jafari, S.M. Evaluation of oxidative stability, fatty acid profile, and antioxidant properties of black cumin seed oil and extract. J. Food Measur. Character. 2019, 13, 383–389. [Google Scholar] [CrossRef]
- Bordoni, L.; Fedeli, D.; Nasuti, C.; Maggi, F.; Papa, F.; Wabitsch, M.; De Caterina, R.; Gabbianelli, R. Antioxidant and anti-inflammatory properties of Nigella sativa oil in human pre-adipocytes. Antioxidants 2019, 8, 51. [Google Scholar] [CrossRef]
- Rahim, M.A.; Shoukat, A.; Khalid, W.; Ejaz, A.; Itrat, N.; Majeed, I.; Koraqi, H.; Imran, M.; Nisa, M.; Nazir, A.; et al. A Narrative Review on Various Oil Extraction Methods, Encapsulation Processes, Fatty Acid Profiles, Oxidative Stability, and Medicinal Properties of Black Seed (Nigella sativa). Foods 2022, 11, 2826. [Google Scholar] [CrossRef]
- Tao, L. Oxidation of polyunsaturated fatty acids and its impact on food quality and human health. AFTNSOJ 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Huang, T.C.; Ho, C.T. Flavors of meat products. In Meat Science and Application, 1st ed.; Hui, Y.H., Nip, W.K., Roger, R.W., Young, O.A., Eds.; Taylor & Francis Group: New York, NY, USA, 2001; pp. 76–98. [Google Scholar] [CrossRef]
- Pignoli, G.; Bou, R.; Rodrigez-Estrada, M.T.; Decker, E.A. Suitability of saturated aldehydes as lipid oxidation markers in washed turkey meat. Meat Sci. 2009, 83, 412–416. [Google Scholar] [CrossRef]
- Kim, Y.H.; Nam, K.C.; Ahn, D.U. Volatile profiles, lipid oxidation and sensory characteristics of irradiated meat from different animal species. Meat Sci. 2002, 61, 257–265. [Google Scholar] [CrossRef]
- Olivares, A.; Dryahina, K.; Špane, P.; Flores, M. Rapid detection of lipid oxidation in beef muscle packed under modified atmosphere by measuring volatile organic compounds using SIFT-MS. Food Chem. 2012, 135, 1801–1808. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, Y.; Pan, D.; Wang, Y.; Cao, J. Effects of high pressure treatment on lipolysis-oxidation and volatiles of marinated pork meat in soy sauce. Meat Sci. 2018, 145, 186–194. [Google Scholar] [CrossRef]
- Feng, X.; Moon, S.H.; Lee, H.Y.; Ahn, D.U. Effect of irradiation on the parameters that influence quality characteristics of raw turkey breast meat. Radiat. Phys. Chem. 2017, 130, 40–46. [Google Scholar] [CrossRef]
- Madruga, M.S.; Elmore, J.S.; Oruna-Concha, M.J.; Balagiannis, D.; Mottram, D.S. Determination of some water-soluble aroma precursors in goat meat and their enrolment on flavour profile of goat meat. Food Chem. 2010, 123, 513–520. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Starowicz, M.; Zieliński, H. How Maillard Reaction Influences Sensorial Properties (Color, Flavor and Texture) of Food Products? Food Rev. Int. 2019, 35, 707–725. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.; Lei, S.; Wu, P.; Fan, G.; Xu, X.; Pan, S. Effects of lard on the formation of volatiles from the Maillard reaction of cysteine with xylose. J. Sci. Food Agric. 2011, 91, 2241–2246. [Google Scholar] [CrossRef]
- Wajs, A.; Bonikowski, R.; Kalemba, D. Composition of essential oil from seeds of Nigella sativa L. cultivated in Poland. Flavour Fragr. J. 2008, 23, 126–132. [Google Scholar] [CrossRef]
- Andaleeb, R.; Zhang, D.; Jiang, S.; Zhang, Y.; Liu, Y. Volatile profile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala. Food Sci. Hum. Wellness 2023, 12, 57–68. [Google Scholar] [CrossRef]
- Akcan, T.; Estevez, M.; Rico, R.; Ventanas, S.; Morcuende, D. Hawberry (Crataegus monogyna Jaqc.) extracts inhibit lipid oxidation and improve consumer liking of ready-to-eat (RTE) pork patties. J. Food Sci. Technol. 2017, 54, 1248–1255. [Google Scholar] [CrossRef]
- Soncu, E.D.; Özdemir, N.; Arslan, B.; Küçükkaya, S.; Soyer, A. Contribution of surface application of chitosan–thyme and chitosan–rosemary essential oils to the volatile composition, microbial profile, and physicochemical and sensory quality of dry-fermented sausages during storage. Meat Sci. 2020, 166, 108127. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, J.; Yang, F.; Wang, W.; Li, W.; Qin, C. Properties and biological activity of chitosan-coix seed starch films incorporated with nano zinc oxide and Artemisia annua essential oil for pork preservation. LWT 2022, 164, 113665. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Lorenzo, J.M.; Zamuz, S.; Montes, L.; L’opez, E.L.S.; Moreira, R.; Franco, D. Influence of pork backfat replacement by microencapsulated fish oil on physicochemical, rheological, nutritional, and sensory features of pork liver pates. LWT 2022, 163, 113522. [Google Scholar] [CrossRef]
- Morales-Irigoyen, E.E.; Severiano-Perez, P.; Rodriguez-Huezo, M.E.; Totosaus, A. Physicochemical and sensory properties compensation of fat replacing in pork liver pate’ incorporating emulsified canola oil. Food Sci. Technol. Int. 2011, 18, 413–421. [Google Scholar] [CrossRef]

| Group | L* | a* | b* | ΔE |
|---|---|---|---|---|
| D0; Fresh meat | ||||
| C | 63.37 ± 0.66 A | 11.41 ± 0.25 A | 9.06 ± 0.1 A | - |
| CB | 63.94 ± 1.33 A | 11.16 ± 0,74 A | 8.90 ± 0.58 A | 0.76 |
| O1 | 63.96 ± 0.95 A | 10.91 ± 0.17 A | 10.13 ± 0.18 B | 1.36 |
| O2 | 65.92 ± 2.16 B | 8.80 ± 1.01 B | 12.59 ± 1.02 C | 5.13 |
| M1; Meat after a month of frozen storage (−20 ± 1 °C) | ||||
| C | 65.28 ± 0.47 B | 11.35 ± 0.44 A | 11.02 ± 0.06 C | - |
| CB | 65.22 ± 1.69 B | 11.99 ± 1.13 B | 11.28 ± 1.01 C | 0.69 |
| O1 | 68.75 ± 1.44 C | 9.86 ± 0.14 B | 11.54 ± 0.44 C | 3.81 |
| O2 | 65.81 ± 0.54 B | 8.71 ± 1.69 B | 11.63 ± 0.84 C | 2.76 |
| M2; Meat after two months of frozen storage (−20 ± 1 °C) | ||||
| C | 65.51 ± 2.09 B | 10.22 ± 0.83 A | 9.86 ± 0.36 B | - |
| CB | 65.19 ± 2.37 B | 10.66 ± 1.10 A | 9.33 ± 0.82 B | 0.76 |
| O1 | 69.24 ± 0.66 C | 8.15 ± 0.69 B | 12.63 ± 0.48 D | 5.09 |
| O2 | 68.05 ± 0.9 C | 6.54 ± 0.49 D | 13.18 ± 0.45 D | 5.57 |
| Group | C | CB | O1 | O2 |
|---|---|---|---|---|
| D0; Fresh meat | ||||
| TI | 1.20 | 1.17 | 1.05 | 0.99 |
| AI | 0.52 | 0.52 | 0.46 | 0.43 |
| h/H | 2.10 | 2.12 | 2.41 | 2.55 |
| M1; Meat after a month of frozen storage (−20 ± 1 °C) | ||||
| TI | 1.22 | 1.22 | 1.07 | 1.00 |
| AI | 0.52 | 0.52 | 0.45 | 0.42 |
| h/H | 2.08 | 2.1 | 2.44 | 2.61 |
| M2; Meat after two months of frozen storage (−20 ± 1 °C) | ||||
| TI | 1.23 | 1.24 | 1.11 | 1.04 |
| AI | 0.52 | 0.52 | 0.46 | 0.43 |
| h/H | 2.10 | 2.08 | 2.35 | 2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska-Horczyczak, E.; Brodowska-Trębacz, M.; Hanula, M.; Pogorzelska-Nowicka, E.; Wierzbicka, A.; Wojtasik-Kalinowska, I.; Półtorak, A. Influence of Nigella sativa L. Oil Addition on Physicochemical and Sensory Properties of Freezer-Stored Ground Pork for Pâté. Appl. Sci. 2023, 13, 12550. https://doi.org/10.3390/app132312550
Górska-Horczyczak E, Brodowska-Trębacz M, Hanula M, Pogorzelska-Nowicka E, Wierzbicka A, Wojtasik-Kalinowska I, Półtorak A. Influence of Nigella sativa L. Oil Addition on Physicochemical and Sensory Properties of Freezer-Stored Ground Pork for Pâté. Applied Sciences. 2023; 13(23):12550. https://doi.org/10.3390/app132312550
Chicago/Turabian StyleGórska-Horczyczak, Elżbieta, Marta Brodowska-Trębacz, Monika Hanula, Ewelina Pogorzelska-Nowicka, Agnieszka Wierzbicka, Iwona Wojtasik-Kalinowska, and Andrzej Półtorak. 2023. "Influence of Nigella sativa L. Oil Addition on Physicochemical and Sensory Properties of Freezer-Stored Ground Pork for Pâté" Applied Sciences 13, no. 23: 12550. https://doi.org/10.3390/app132312550
APA StyleGórska-Horczyczak, E., Brodowska-Trębacz, M., Hanula, M., Pogorzelska-Nowicka, E., Wierzbicka, A., Wojtasik-Kalinowska, I., & Półtorak, A. (2023). Influence of Nigella sativa L. Oil Addition on Physicochemical and Sensory Properties of Freezer-Stored Ground Pork for Pâté. Applied Sciences, 13(23), 12550. https://doi.org/10.3390/app132312550








