Abstract
The aim of this clinical study was to determine the impact of the consumption of chicken eggs enriched with n-3 polyunsaturated fatty acids, selenium, vitamin E, and lutein on micro- and macrovascular endothelium-dependent dilation, inflammation biomarkers, and oxidative stress levels in participants with chronic coronary syndrome (CCS). This was a double-blind, placebo-controlled clinical study that included 30 CCS participants (9 women, 21 men) randomized into the control group (N = 15), who ate ordinary chicken eggs (three per day), and the Nutri4 group (N = 15), who ate enriched eggs (three per day) for 21 days. Microvascular and macrovascular endothelium-dependent vasodilation was evaluated by measuring forearm skin post-occlusive reactive hyperemia (PORH) and acetylcholine-induced dilation (AChID) and the flow-mediated dilation (FMD) of the brachial artery, respectively. The serum lipid profile, anti- and proinflammatory cytokine levels, serum concentration of nitric oxide synthase (NOS) isoforms, and oxidative stress biomarkers were measured before and after the diet protocols. Enriched, but not regular, chicken eggs significantly improved microvascular PORH and AChID and macrovascular FMD, increased the serum concentration of inducible NOS, decreased serum triglyceride levels, and decreased proinflammatory cytokine IL-17A and TGF-1β levels compared to initial measurements. Patients with CCS can benefit from the consumption of enriched chicken eggs due to improved lipid biomarkers, a more favorable anti-inflammatory milieu, and improved vascular relaxation at micro- and macrovascular levels.
1. Introduction
Ischemic heart disease (IHD) can present as a chronic coronary syndrome (CCS), which is a form of stable disease, or as an acute coronary syndrome (ACS) [1]. Despite different treatment options, IHD has been the leading cause of death worldwide in the last 20 years [2]. The best-known risk factors for the development of IHD are arterial hypertension, dyslipidemia, physical inactivity, obesity, diabetes mellitus, and smoking, as the aforementioned risk factors contribute to the development of atherosclerosis, which underlies IHD [3].
A healthy lifestyle, which includes smoking cessation, physical activity, a healthy diet, and the maintenance of an optimal weight, reduces the risk of subsequent cardiovascular events and mortality [4]. The fact that diet in particular can affect cardiovascular risk was previously recognized in the Seven Countries Study, which showed that the Mediterranean diet significantly reduced the risk of IHD. The Mediterranean diet is a high-fat/low-glycemic diet in which 40% of calories come from fat; however, the fat is mainly unsaturated oils, such as olive and canola oil. In the Seven Countries Study, the coronary risk in Crete (i.e., consumption of Mediterranean diet) was only 1/15 of that in Finland, where most of the consumed fat is saturated fat (along with cholesterol), and 40% of the risk in Japan, where a low-fat diet emphasizing fish is practiced [5]. N-3 polyunsaturated fatty acids (n-3 PUFAs), but also some other nutrients, for example, selenium as a trace element, vitamin E, and the carotenoid lutein, have health-promoting effects and reduce cardiovascular risk, as shown in numerous studies [4,6,7,8]. The Mediterranean diet, rich in n-3 PUFAs, can modulate endothelial function and contribute to better vascular homeostasis balance in IHD patients, even if severe endothelial dysfunction is present. Replacing saturated fats with unsaturated fats, especially PUFAs, also reduces the risk of IHD [4]. This idea is supported by the fact that a lower risk of coronary artery disease (CAD) is linked with the consumption of fish (known for being rich in n-3 PUFAs) at least once a week [6]. N-6 PUFAs are considered proinflammatory, while n-3 PUFAs are considered anti-inflammatory; when there is a higher ratio of n-6/n-3 PUFAs, there are more mediators, such as prostaglandin series 2 (PGI2), leukotriene series 4 (LTB4), and thromboxane B series 2 (TXB2), that act as vasoconstrictors, activate platelets, and are proinflammatory. When the n-3 PUFA concentration is higher and the n-6/n-3 PUFA ratio is lower, then the production of anti-inflammatory and vasodilator mediators such as PGI3, LTB5, and TXA3 dominates. Therefore, an unbalanced n-6/n-3 PUFA ratio in favor of n-6 PUFAs has a prothrombotic and proinflammatory effect, which contributes to the development of atherosclerosis [9,10].
In addition, some micronutrients have been shown to be beneficial in maintaining health. One of them is selenium. Selenium is essential for the function of many enzymes involved in the regulation of the inflammatory response, the proliferation and differentiation of immune cells, and the migration and adhesion of leukocytes. Moreover, selenium is a part of antioxidant mechanisms; selenium increases the antioxidant capacity and affects nuclear factor kappa B (NF-κB) signaling pathways, reducing the production of tumor necrosis factor alpha (TNF-α) and proinflammatory interleukins [11]. Lutein has anti-inflammatory and atheroprotective effects in patients with CAD. Serum lutein levels are inversely proportional to the levels of proinflammatory cytokines (e.g., TNF-α, interleukin 6 (IL-6)) and oxidized low-density lipoprotein cholesterol (OxLDL) [8]. Overall, functional foods rich in these nutrients could play a role in the prevention and treatment of various cardiovascular diseases. For example, our recent study showed that the consumption of chicken eggs enriched with n-3 PUFAs (three eggs daily) for three weeks (~1053 mg n-3 PUFAs daily) had a mild anti-inflammatory effect by lowering IL-6 levels and changed the ratio of free fatty acids in the serum to a more favorable value in patients with known CADs (ACS and CCS) [12].
The first step in atherosclerosis development is endothelial dysfunction. The underlying mechanism in most cases is the impaired production or bioavailability of nitric oxide (NO) and/or the altered production of vasoactive prostaglandins/eicosanoids [13,14]. NO mediates endothelium-dependent vasodilation and inhibits platelet adhesion and aggregation [13], leukocyte adhesion/infiltration, and vascular smooth muscle cell proliferation. NO also prevents the oxidation of LDL cholesterol [15]. Oxidative stress causes reduced bioavailability of NO or impairs the production of prostaglandins, resulting in impaired microvascular vasodilation and the consequent development of atherosclerosis [16]. Antioxidant systems that combat oxidative stress primarily include three important enzymes: superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), whereas the second line of defense consists of antioxidants that neutralize or remove free radicals, and this group includes ascorbic acid, glutathione and alpha-tocopherol (vitamin E) [17]. However, data on the dietary impact, particularly the impact of functional foods (food that, in addition to being a source of energy and nutrients, also contains biologically active substances that offer health benefits beyond nutritive values) [18], on these antioxidant systems are scarce.
The hypothesis of this study is that the consumption of functional foods naturally enriched with micronutrients with antioxidant and anti-inflammatory properties could have a favorable effect on endothelial function, inflammation, lipid profiles, and levels of oxidative stress in participants with CCS. Therefore, our study aimed to investigate whether the consumption of chicken eggs with increased contents of n-3 PUFAs, selenium, vitamin E, and lutein had effects on (a) microvascular and macrovascular endothelial function and enzymes involved in the synthesis of NO; (b) oxidative stress levels and antioxidant capacity; and (c) systemic levels of pro/anti-inflammatory mediators in patients with existing CCS.
2. Materials and Methods
2.1. Study Population
Thirty patients with existing CCS of both sexes (nine women and twenty-one men) older than 18 years were included in this study. CCS is defined according to the European Society of Cardiology guidelines and includes patients with known CAD in a stable phase. They were elected at the Department for Cardiovascular Disease, University Hospital Center Osijek, Osijek, Croatia, from September to December 2020. The CONSORT flow chart of the study is depicted in Figure 1. Exclusion criteria were significant anemia (Hg < 110 g/L (male) and <100 g/L (female)), chronic or acute renal kidney disease, liver failure (chronic disease with impaired metabolic and synthetic function), chronic pulmonary disease and other conditions with chronic hypoxemia, active or chronic infections (tuberculosis, etc.), malignant diseases, hereditary systemic diseases, autoimmune diseases, hereditary metabolic diseases, untreated thyroid disease, recent surgery (last 3 months), recent severe trauma (last 6 months), neurological disorders (neurodegenerative diseases, recent stroke) (6 weeks), epilepsy, condition after resuscitation (3 months), drugs with significant effects on vascular or immune function (monoclonal antibodies, immunosuppressives, systemic corticosteroids), or active abuse of alcohol or drugs. All patients used recommended drug therapy for CCS, including statins, beta-blockers, ACE inhibitors, and antiplatelet drugs, and depending on the serum LDL concentration, some of them also used ezetimib. The study protocol was explained to all subjects, and they signed an informed consent form. The study protocol and procedures conformed to the standards set by the latest revision of the Declaration of Helsinki and were approved by the Ethical Committee of University Hospital Osijek (R2-8262/2020) and the Ethical Committee of Faculty of Medicine Osijek (Cl: 602-04/20-08/07, No.: 2158-61-07-20-118).
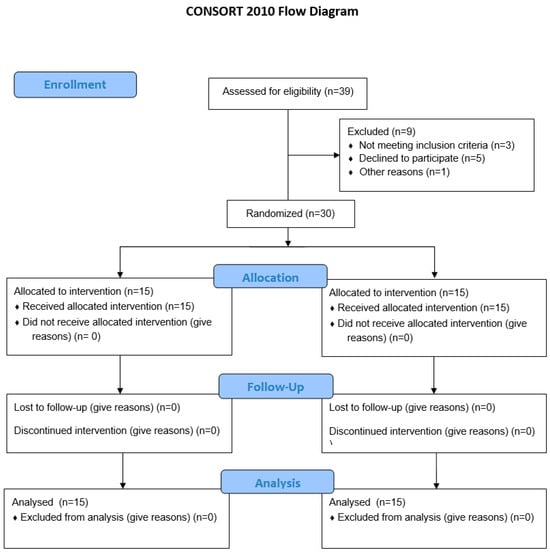
Figure 1.
CONSORT 2010 flow diagram.
2.2. The Research Procedure
This study was a double-blind, placebo-controlled, randomized, interventional study. It is registered at ClinicalTrials.gov (identifier: NCT04564690). The inclusion of participants in the study began on 1 September 2020, and subject follow-up finished on 20 December 2020. All participants ate three boiled chicken eggs daily for three weeks (a total of 63 eggs in 21 days). After recruitment, another investigator involved in the study performed the randomization by drawing A or B for each subject and divided participants into two different groups, group A (control) or B (Nutri4), according to the type of eggs they consumed (control—15 subjects: 5 women, 10 men; experimental, i.e., Nutri4—15 subjects: 4 women, 11 men). The Nutri4 group consumed chicken eggs enriched with vitamin E, lutein, selenium, and n-3 PUFAs (n-3 PUFAs 432 mg, selenium 0.0191 mg, vitamin E 1.098 mg, lutein 0.616 mg/per egg), and the control group consumed regular eggs (n-3 PUFAs 146 mg, selenium 0.0183 mg, vitamin E 0.595 mg, lutein 0.11 mg/per egg) produced on the same farm. During the study protocol, neither the researchers nor the participants knew to which group each participant belonged (eggs were labeled #1 or #2 before they were distributed to the laboratory). Both eggs (enriched and ordinary eggs) were the same commercial size—L.
The research group of the Faculty of Agrobiotechnical Sciences Osijek, University of Josip Juraj Strossmayer in Osijek, produced enriched chicken eggs according to their established protocol. All participants were instructed to eat 3 eggs daily at the same time every day (breakfast). The eggs were boiled for about 10 min. They were advised to eat only the eggs they received from the researchers for study purposes (total of 63 eggs). Also, they were advised to avoid consuming large amounts of foods that are rich in n-3 PUFAs, vitamin E, selenium, or lutein, such as fish, fish oil, nuts, and others, which would affect the results, or taking any other form of supplements during the research. They had to keep a specific diet diary during the diet protocol. To ensure compliance, subjects were called by telephone a few times to ensure maximum adherence to the diet protocol. The study was performed in the Laboratory for Clinical and Sport Physiology, Department of Physiology and Immunology at the Faculty of Medicine Osijek, University of Osijek.
All procedures mentioned in the study, including blood sampling and all other measurements, were carried out before the start of the diet and the day immediately after the end of the egg consumption protocol. All measurements were taken after overnight fasting; also, subjects were advised to avoid any strenuous activity during the 24 h preceding the visit.
2.3. Anthropometric Characteristics of the Study Population and Hemodynamic Parameters
Each subject’s height (m) and weight (kg) were measured, and from the measured values, body mass index (BMI) was calculated. Also, waist and hip circumferences were measured, and from the measured values, the waist-to-hip ratio (WHR) was calculated. The heart rate (HR) and blood pressure (BP) values were calculated as the mean values of three repeated measurements at each visit in a sitting position after a 15 min rest. An automated oscillometric sphygmomanometer (OMRON M3, OMRON Healthcare Inc., Osaka, Japan) was used to measure HR and BP.
2.4. Serum Lipid Levels and Biochemical Marker Analysis
At both study visits, blood samples were collected and analyzed for total blood count, urea, creatinine, plasma electrolytes (sodium, potassium, calcium), liver function tests (alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT)), high-sensitivity C-reactive protein (hsCRP), blood glucose, iron, and transferrin. Also, serum lipid levels were determined (including total cholesterol, low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides). All analyses were performed using standard laboratory methods at the Department of Clinical Laboratory Diagnostics, University Hospital Center Osijek, Osijek, Croatia.
2.5. Serum Free Fatty Acid, Selenium, Vitamin E, and Lutein Concentrations
In order to confirm the effect of and conformance to the dietary protocols, serum free fatty acid concentrations and the concentrations of selenium, vitamin E, and lutein were measured in the blood samples of all subjects before and after the respective protocols.
The analysis of the serum free fatty acid concentration was conducted at the BIOCentre’s Bioanalytical Laboratory, BIOCentre—Incubation Center for Biosciences, Zagreb, Croatia. A commercially available solution of a 37-component fatty acid methyl ester mix (FAME MIX, Supelco Inc., Bellefonte, PA, USA) was used for serum free fatty acid concentration detection using gas chromatography–tandem mass spectrometry (GC–MS/MS, Thermo Fisher GC Trace 1300 coupled with a TSQ 9000 Triple Quadrupole), according to an earlier described protocol [19].
The serum vitamin E concentration was determined according to a standardized protocol using absolute alcohol for protein denaturation and Xylene for the separation of the supernatant from proteins. 2, 2-Bipyridyl and FeCl2 were added to the supernatant to achieve a red color. The absorbance of the sample was measured, and measured values were proportional to the concentrations of vitamin E in serum. Absorbance was determined using an ELISA READER at 492 nm [20].
For serum selenium concentrations, samples were mixed with ultrapure HNO3 and H2O2 at 180 °C for 60 min in a closed CEM Mars 6 microwave system (CEM, Matthews, NC, USA).
To determine selenium serum concentrations, inductively coupled plasma–mass spectrometry (ICP-MS, Agilent 7500a, Agilent Technologies Inc., Santa Clara, CA, USA) was used; the analytical method was controlled with the reference material NIST 1567b (wheat flour, National Institute of Standards and Technology, Gaithersburg, ML, USA). Measurements were performed at the Faculty of Agrobiotechnical Sciences Osijek, University of Josip Juraj Strossmayer in Osijek.
Measurements of lutein serum concentrations were performed according to the protocol by Tzeng et al. using high-performance liquid chromatography (HPLC; HPLC LC-30 NEXERA, Shimadzu, Japan, 2018), which was adopted and described in earlier papers by our research group [13]. HPLC analysis was performed at the Department of Chemistry, Josip Juraj Strossmayer University of Osijek.
2.6. Microvascular Endothelium-Dependent and -Independent Vasodilation
Changes in microcirculatory blood flow were assessed by post-occlusive reactive hyperemia (PORH) and acetylcholine-induced vasodilation (AChID) as measures of endothelium-dependent vasodilation, and endothelium-independent vasodilation was determined using sodium-nitroprusside-induced vasodilation (SNPID). The laser Doppler flowmetry (LDF) technique (MoorVMS-LDF, Axminster, UK) was used to determine blood flow through the forearm skin at each study visit. Before LDF measurements, participants were placed in a lying position for 30 min to rest and to acclimatize. The temperature in the room was controlled (23.5 ± 0.5 °C). Then, the laser Doppler probe was placed on the skin of the volar forearm, 13–15 cm from the wrist, at the same place at the first and second study visits.
First, 2-min baseline measurements were taken, and then the PORH test was performed. The pneumatic cuff was positioned on the upper arm, and brachial artery occlusion was achieved by inflating the pneumatic cuff to 30–50 mmHg above systolic BP for 1 min. Changes in microcirculatory blood flow were measured before, during, and after the release of occlusion. Microcirculatory blood flow was expressed in arbitrary perfusion units, and software was used to determine and calculate the area under the curve (AUC). The result is expressed as the difference between the percentage of flow change during reperfusion and occlusion in relation to the baseline (R–O% increase) [21].
For a non-invasive transdermal application of charged substances, an iontophoretic drug-delivery electrode was positioned directly on an LDF probe. First, baseline recordings were performed for 5 min, and then a positively charged vasodilator, ACh (1%), was iontophoresed, with an anodal current applied by means of seven pulses of a direct positive electric current of 0.1 mA for 30 s, with 30 s between each dose. On the other forearm, after the baseline measurement for 5 min, the negatively charged SNP (1%) was applied by means of three pulses of a 0.1 mA negative current for 30 s and then a 30 s pulse of 0.2 mA, with 120 s between each dose. This pulsed iontophoretic protocol is adapted to obtain a stable plateau of the maximal LDF response. Microcirculatory blood flow is expressed in arbitrary perfusion units and determined by using software that compares measurements during baseline flow and during ACh or SNP application [22,23].
2.7. Macrovascular Endothelium-Dependent and -Independent Vasodilation
Brachial artery flow-mediated dilation (FMD) was used to assess macrovascular endothelium-dependent dilation, while nitroglycerine (NTG)-induced brachial artery dilation (NTG-MD) was used to assess macrovascular endothelium-independent vasodilation. To assess brachial artery FMD and NTG-MD, the appropriate vascular probe for ultrasound was used (GE Healthcare Vivid iQ R2). To determine brachial artery FMD, the brachial artery diameter and blood flow velocity were measured at baseline and following blood pressure cuff deflation. First, the baseline measurement was taken, and then 5 min vascular occlusion was performed (pneumatic cuff was inflated to 30–50 mmHg above measured systolic pressure). Nitroglycerin-induced vasodilatation was measured following sublingual NTG administration (0.4 mg), and images of the brachial artery were recorded until the dilatation reached a plateau. Brachial Analyzer for Research v.6 software (Medical Imaging Applications, Coralville, IA, USA) was used to analyze the obtained images. Brachial artery diameter changes are expressed as the percentage change relative to the vessel diameter before cuff inflation or NTG administration.
2.8. Serum Protein Concentrations of eNOS, nNOS, and iNOS
Serum protein concentrations of three different NOS isoforms, inducible NOS (iNOS), endothelial (eNOS), and neuronal NOS (nNOS), were determined using commercially available ELISA (enzyme-linked immunosorbent assay) kits (human nNOS CSB-E13872h, human eNOS CSB-E08322h, human iNOS CSB-E08148h, CUSABIO, Wuhan, China). Analyses were performed following the manufacturer’s instructions. The sample absorbance was measured with a spectrometer (PR 3100 TSC Microplate Reader, BioRad Laboratories, Hercules, CA, USA) and a standard curve.
2.9. Biomarkers of Oxidative Stress and Antioxidant Capacity
Thiobarbituric acid-reactive substances (TBARS), the serum concentration of oxidized low-density lipoprotein (OxLDL), 8-iso prostaglandin F2α (8-iso-PGF2a), and advanced oxidation protein products (AOPPs) were measured as oxidative stress biomarkers. The TBARS method measures serum malondialdehyde (MDA), which is formed during lipid peroxidation of polyunsaturated fatty acids. MDA reacts with thiobarbituric acid (TBA), forming a pink chromogen (TBARS). A Nanophotometer (P300 UV/VIS, IMPLEN) at 572 and 532 nm was used to measure the absorbance of the sample, with MDA used as a standard (µM MDA) [24]. Serum concentrations of OxLDL (MyBioSource, MyBioSource Inc., San Diego, CA, USA), 8-iso-PGF2a (MyBioSource, MyBioSource Inc., San Diego, CA, USA), and AOPPs (MyBioSource, MyBioSource Inc., San Diego, CA, USA) were measured by commercially available enzyme-linked immunosorbent assay (ELISA) kits on a compact absorbance reader for 96-well microplates (BioRad PR 3100 TSC, Bio-Rad Laboratories, CA, USA) at 450 nm.
As biomarkers of antioxidant defense, the ferric-reducing ability of plasma (FRAP) and serum antioxidant enzyme activity were measured. The FRAP method uses antioxidants as reductants, wherein ferric ions (Fe3+) at low pH are reduced to ferrous ions (Fe2+), and this reaction causes a blue-colored ferrous-tripyridyltriazine complex to form. A Nanometer (P300 UV/VIS, IMPLEN) at 593 nm with Trolox as a standard (mM/L Trolox) was used to measure the absorbance of the sample [25]. The enzyme activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT) in serum samples were determined using a Lambda 25UV-Vis spectrometer equipped with the UV WinLab 6.0 software package (PerkinElmer For the Better, Waltham, MA, USA) according to well-established protocols [26,27,28]. The measured results are expressed as U (mg protein−1). The concentration of proteins in the samples (mg mL−1) was determined using Bradford reagent at 595 nm (Bradford Reagent B6916, Sigma Aldrich), according to the manufacturer’s protocol, using bovine serum albumin as a standard. Serum antioxidant enzyme activity assays were performed at the Biochemistry Laboratory at the Department of Biology, University of Osijek.
2.10. Serum Concentration of Anti- and Proinflammatory Cytokines and Chemokines
Serum concentrations of transforming growth factor beta 1 (TGF-1β), tumor necrosis factor alpha (TNF-α), complement component 3a (C3a), interferon gamma (INF-γ), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 17A (IL-17A), interleukin 23 (IL-23), and monocyte chemoattractant protein-1 (MCP-1) were measured using ProcartaPlex Human TGF beta 1 Simplex, ProcartaPlex Human C3a Simplex, and Human ProcartaPlex Mix&Match 7-plex antibody-based, magnetic bead reagent kits and panels for multiplex protein quantitation using the Luminex 200 instrument platform. Quantitation was performed in ProcartaPlex Analyst software v1.0, and the results are expressed as concentration in picograms per milliliter. Measurements were performed in the Laboratory for Molecular and Clinical Immunology, Department of Physiology and Immunology, Faculty of Medicine Osijek, Osijek, Croatia.
2.11. Statistical Analysis
In this study, the results are expressed as the arithmetic mean and standard deviation (SD). The sample size calculation included preliminary data obtained from 10 subjects. To find differences in primary outcomes reported in our study (e.g., FMD), the calculated sample size was 14 participants in each group, with a level of significance of 0.05 and a statistical power of 80% for paired t-tests. The Kolmogorov–Smirnov normality test was applied to evaluate the normality of the data distribution. Paired t-tests were applied to assess differences within the groups (measurements taken at the beginning and the end of the study protocol). Differences between groups in the post-intervention measurement were obtained with the analysis of covariance (ANCOVA) with the baseline value (before) as the covariate. p < 0.05 was considered statistically significant. For statistical analysis, SigmaPlot, version 11.2 (Systat Software, Inc., Chicago, IL, USA), was used.
3. Results
All included participants completed the 3-week diet protocol. The initial clinical characteristics of the participants are listed in Table 1. In general, participants were overweight at baseline (defined by a BMI greater than 30 kg/m2 and WHR > 0.90). The participants’ systolic and diastolic BP values were normal, which was attributable to antihypertensive therapy (e.g., ACEi/ARB, beta-blockers, nitrates). At baseline, all subjects had normal values of blood count parameters and normal liver and kidney function, and serum electrolytes were within reference values, as were fasting total cholesterol, LDL and HDL cholesterol levels, and triglycerides. The fasting glucose level was greater than 6.4 mmol/L.

Table 1.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on anthropometric characteristics and hemodynamic and biochemical parameters in participants with chronic coronary syndrome.
3.1. Anthropometric Characteristics of the Study Population and Hemodynamic Parameters
After the consumption of Nutri4 eggs or ordinary chicken eggs, there were no significant differences in BMI, WHR, HR, systolic BP, mean BP (MBP), and diastolic BP compared with baseline measurements. No differences were observed between groups at baseline or after the dietary protocols (Table 1).
3.2. Serum Lipid Levels and Biochemical Marker Analysis
In the Nutri4 group, triglyceride levels decreased, while total cholesterol, LDL cholesterol, and HDL cholesterol levels remained unchanged (target values in CCS of LDL < 1.4 mmol/L and triglyceride levels < 1.7 mmol/L; reference values of cholesterol < 5.0 mmol/L and HDL > 1.0 mmol/L). In the control group, no significant difference in the concentration of serum lipids before and after the dietary protocol was measured. Eating eggs, both regular eggs and Nutri4 eggs, significantly lowered GGT levels. For other biochemical parameters measured, no significant differences were noted between baseline measurements and after the consumption of Nutri4 eggs. The consumption of regular chicken eggs caused a decrease in serum ferritin levels, but this was within the population reference values, so these changes were not considered physiologically relevant (Table 1).
3.3. Serum Free Fatty Acid, Selenium, Vitamin E, and Lutein Concentrations
The concentration of n-6 PUFAs (eicosadienoic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid) in serum decreased after the consumption of Nutri4 eggs, whereas the concentration of n-3 PUFAs (eicosa-5,8,11,14,17-pentaenoic acid) increased. In addition, a favorable decrease in the n6/n3 PUFA ratio was observed in the Nutri4 group after the consumption of enriched eggs (Table 2). The levels of C14:0 myristic acid and C18:0 stearic acid also decreased in the Nutri4 group after the consumption of eggs. In the control group, serum fatty acid levels were not significantly changed following the dietary protocol.

Table 2.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on serum fatty acid concentrations in participants with chronic coronary syndrome.
Serum concentrations of vitamin E and selenium increased significantly after the consumption of enriched eggs (Nutri4 group), while their concentrations in the control group remained unchanged. Serum lutein concentrations were not significantly changed after either dietary protocol in either the control group or the Nutri4 group (Table 3). There were no differences in the serum concentrations of vitamin E, selenium, and lutein between the groups either at baseline or after the dietary protocols.

Table 3.
The effect of functionally enriched (Nutri4) and regular (Control) chicken egg consumption on selenium, vitamin E, and lutein serum concentrations in participants with chronic coronary syndrome.
3.4. Microvascular Endothelium-Dependent and -Independent Vasodilation
After Nutri4 chicken egg consumption, significantly improved PORH and AChID levels in the skin microcirculation compared with baseline measurements were determined, whereas PORH- and acetylcholine-induced vasodilation were similar between baseline and after the consumption of regular chicken eggs (Figure 2A,B). SNPID remained unchanged after both dietary protocols compared with the baseline measurement (Figure 2C). There were no differences between groups in PORH, AChID, and SNPID after the dietary protocols.
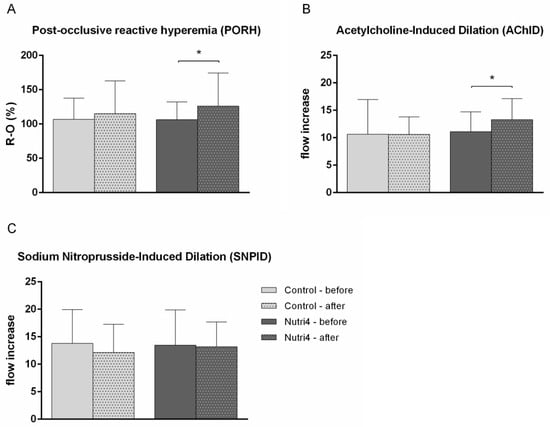
Figure 2.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on microvascular endothelium-dependent and -independent vasodilation assessed by laser Doppler flowmetry (LDF). (A) Post-occlusive reactive hyperemia (PORH), (B) acetylcholine-induced dilation (AChID), (C) sodium-nitroprusside-induced dilation (SNPID). PORH is expressed as the difference between the percentage of flow change during reperfusion and occlusion in relation to baseline (R-O%). AChID and SNPID are expressed as a flow increase between baseline flow and flow following ACh or SNP administration. Results are shown as the arithmetic mean and standard deviation (SD); * p < 0.05 before vs. after within the group (control (N = 15) or Nutri4 (N = 15))—paired t-test.
3.5. Macrovascular Endothelium-Dependent and -Independent Vasodilation
After the dietary intervention, in the Nutri4 group, the FMD of the brachial artery was significantly increased (FMD% of dilation before diet 6.84 ± 3.60 vs. after diet 9.14 ± 4.79, p = 0.015), whereas FMD in the control group remained unchanged (FMD % of dilation before diet 9.67 ± 3.60 vs. after diet 9.89 ± 3.26, p = 0.850). NTG-mediated dilation of the brachial artery was not significantly changed in either the Nutri4 (NTG-MD % of dilation before diet 13.70 ± 7.19 vs. after diet 12.38 ± 6.12, p = 0.502) or control group (NTG-MD % of dilation before diet 14.42 ± 6.40 vs. after diet 14.46 ± 5.95, p = 0.985) following the respective dietary protocols (Figure 3A,B). There were no differences in brachial artery FMD and NTG-MD between groups after the dietary protocols.
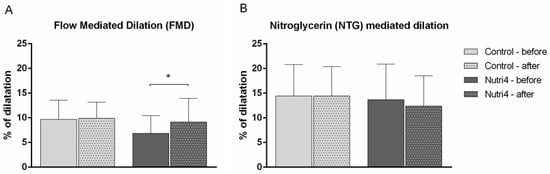
Figure 3.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on brachial artery flow (FMD, endothelium-dependent) and nitroglycerine (NTG-MD, endothelium-independent)-mediated dilation. (A) Flow-mediated dilation (FMD), (B) nitroglycerine-mediated dilation (NTG-MD). Changes in brachial artery diameter are expressed as percentage change relative to the vessel diameter before cuff inflation or NTG administration. Results are shown as the arithmetic mean and standard deviation (SD); * p < 0.05 before vs. after within the group (control (N = 15) or Nutri4 (N = 15))—paired t-test.
3.6. Serum Protein Concentrations of eNOS, nNOS, and iNOS
Nutri4 egg consumption significantly increased the serum iNOS protein concentration, while no changes in the concentrations of nNOS and eNOS were recorded in the Nutri4 group. There were no significant differences in the serum protein concentrations of eNOS, nNOS, and iNOS after the dietary protocol compared with baseline values in the control group (Figure 4).
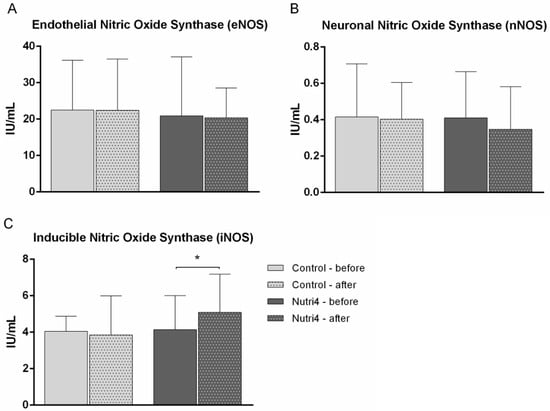
Figure 4.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on serum concentrations of three nitric oxide synthase isoforms. (A) Endothelial NOS (eNOS), (B) neuronal NOS (nNOS), (C) inducible NOS (iNOS). Results are shown as mean ± standard deviation (SD); * p < 0.05 before vs. after within the group (control (N = 15) or Nutri4 (N = 15))—paired t-test.
3.7. Oxidative Stress Biomarkers and Antioxidant Capacity
There were no significant changes in measured oxidative stress biomarkers, including TBARS, OxLDL, 8-iso-PGF2α, and AOPP, in the control or Nutri4 group after either dietary protocol in comparison with baseline measurements. There were also no significant differences in the measured biomarkers of antioxidant protection, including FRAP and the serum activities of CAT and SOD, after the consumption of Nutri4 eggs or normal eggs compared with baseline measurements or between groups (Table 4).

Table 4.
The effect of functionally enriched (Nutri4) and regular (Control) chicken egg consumption on oxidative stress biomarkers and antioxidant capacity in participants with chronic coronary syndrome.
3.8. Serum Protein Concentrations of Anti- and Proinflammatory Cytokines and Chemokines
After the consumption of Nutri4 eggs, a significant decrease in serum TGF-1β and IL-17A concentrations was observed, while INF-γ, IL-6, IL-10, IL-23, TNF-α, and MCP-1 concentrations remained unchanged compared to baseline measurements (Table 5). After regular chicken egg consumption, no significant changes in the serum concentrations of cytokines and chemokines compared to initial measurements within the control group were recorded. There were no statistically significant differences in the concentrations of measured cytokines and interleukins between groups.

Table 5.
The effect of functionally enriched (Nutri4) and regular (control) chicken egg consumption on serum concentration of anti- and proinflammatory cytokines and chemokines in participants with chronic coronary syndrome.
4. Discussion
To our knowledge, this study was the first interventional clinical experimental study to investigate the impact of the consumption of chicken eggs fortified with four nutrients on microvascular and macrovascular reactivity, serum free fatty acid and serum lipid levels, oxidative stress levels, and anti- and proinflammatory cytokine concentrations in participants with CCS. The most significant outcomes of our study are as follows: (a) the consumption of Nutri4 eggs improved endothelium-dependent vasodilation (i.e., microvascular PORH and AChID, FMD of brachial artery) in both micro- and macrocirculation and increased the serum protein concentration of iNOS; (b) the consumption of Nutri4 eggs decreased serum triglyceride levels; (c) the consumption of Nutri4 eggs reduced serum concentrations of the proinflammatory cytokines IL-17A and TGF-1β; and (d) the consumption of Nutri4 eggs did not significantly affect biomarkers of oxidative stress and antioxidant protection (TBARS, OxLDL, 8-iso-PGF2α, AOPP, FRAP, serum CAT, SOD, and GPx activity) in patients with CCS.
In our study, a significant increase in the serum concentration of n-3 PUFAs was observed in the Nutri4 group, as well as a decrease in the ratio of n-6/n-3 PUFAs. Also, increased serum concentrations of vitamin E and selenium in the Nutri4 group indicate adherence to the dietary protocol and an important opportunity for the physiological modification of serum lipid composition via diet. Overall, the consumption of nutrient-enriched chicken eggs in participants with CCS resulted in a more favorable lipid profile, improved microvascular and macrovascular endothelial function, and reduced proinflammatory potential. In addition, the consumption of chicken eggs, whether normal or Nutri4, had no deleterious effects on serum lipid levels or on other measured parameters.
Dyslipidemia stands out as a crucial risk factor in atherosclerosis development, particularly elevated levels of LDL cholesterol and triglycerides [29]. Despite improved outcomes in atherosclerotic cardiovascular disease with statin therapy, a residual risk remains. There are numerous studies with conflicting results about the role of n-3 PUFAs in changes in triglyceride and LDL levels. On the one hand, supplementation with n-3 PUFAs (EPA and DHA) can reduce serum lipids (especially levels of triglycerides) in individuals with hyperlipidemia [30]. Treatment with EPA or DHA is associated with a reduction in triglyceride levels and with differential effects on LDL and HDL cholesterol [31]. On the other hand, in patients at high cardiovascular risk treated with statins, the addition of n-3 PUFAs compared with corn oil has not resulted in a significant difference in the composite outcome of serious adverse cardiovascular events [32]. Nevertheless, the European Society of Cardiology recommends supplementation with n-3 PUFAs in combination with statins to lower triglyceride levels [33]. Our study showed that the consumption of Nutri4 eggs in participants with CCS caused a significant reduction in serum triglyceride levels, whereas the levels of total cholesterol, LDL cholesterol, and HDL cholesterol did not change significantly. It is important to state that the participants in our study had a history of CCS and were receiving optimal drug therapy, including statins and other hypolipemic drugs; therefore, triglyceride levels at the start of the dietary protocol were within the recommended levels for this group of patients. However, the consumption of Nutri4 eggs further lowered triglyceride levels, suggesting additional beneficial effects on the serum lipids of the n-3 PUFAs contained in these eggs.
N-3 PUFAs may act to reduce the risk of cardiovascular disease, and one of their vasoprotective benefits is improved endothelium-dependent vasodilation [34,35]. A previous study by our research group including healthy subjects who consumed n-3 PUFA-enriched eggs (1053 mg PUFAs daily) for three weeks showed improved PORH in the skin microcirculation. However, this effect was not observed in patients with ACS and CCS who consumed 1053 mg PUFAs per day in the form of enriched chicken eggs for three weeks [12]. On the other hand, in the present study, both microvascular and macrovascular endothelium-dependent vasodilation was improved in the group of CCS patients who consumed nutrient-enriched chicken eggs. This difference is a possible effect of the composition of the eggs; Nutri4 eggs are additionally enriched with vitamin E, selenium, and lutein, which, together with n-3 PUFAs, may have an additive effect. Also, in the previous study, the subjects were patients with both ACS and CCS, so the different results could also be related to a difference in the pathophysiological mechanism of the disease in its acute and chronic phases.
We found that the selenium plasma concentration was significantly increased after the consumption of Nutri4 eggs. This is an interesting and important finding because studies suggest that there is a link between selenium deficiency and an increased risk of developing atherosclerotic plaques. For example, endothelial cells are more likely to undergo apoptotic destruction due to selenium deficiency, and that contributes to increased atherosclerotic plaque instability [36]. Selenium deficiency negatively affects endothelium-mediated function, as selenium deficiency can lead to increased thromboxane concentration and decreased prostaglandin concentration, causing vasoconstriction [36]. There is evidence that the addition of selenium to dietary supplements containing antioxidants may reduce the risk of cardiovascular mortality [11]. For example, four years of supplementation with selenium and coenzyme Q10 reduced cardiovascular mortality risk in a group of healthy elderly participants but also in subgroups of patients with arterial hypertension, diabetes mellitus, and myocardial infarction after 12 years [37], but the mechanism is not fully known. Low soil selenium concentrations have been associated with an increased incidence of cardiomyopathy characterized by extensive myocardial fibrosis (Keshan disease) [38]. In addition, low selenium concentrations have been associated with subsequent cardiovascular death in patients with ACS [39], so supplementation of selenium in the daily diet may be an important avenue to prevent various cardiovascular diseases.
The consumption of enriched eggs in our study increased serum vitamin E concentrations and improved microvascular and macrovascular reactivity to vasodilator stimuli. It has been previously demonstrated that vitamin E can influence vascular reactivity. For example, oral vitamin E supplementation can ameliorate the transient impairment of endothelial function after smoking by acting on the oxidative status but has no effect on chronic endothelial dysfunction in healthy male smokers [40]. Furthermore, the oral application of a γ-T-rich tocopherol (TmT) mixture during short-term nicotine-replacement-therapy-assisted smoking cessation improved endothelial dysfunction measured by FMD in healthy former smokers [41]. To our knowledge, our study is the first in which vitamin E was administered in the form of a functional food and showed a significant beneficial effect on vascular relaxation in participants with CCS. Selenium supplementation can reduce oxidative stress levels (measured as MDA levels and total antioxidant capacity) [42]. Vitamin E also has antioxidant effects [43]; for example, supplementation with vitamin E (900 IU/day) in combination with vitamin C (1000 mg/day) in subjects with high-salt diets prevented increased oxidative stress [44]. However, in our study, oxidative stress biomarkers were not altered by the diet, even though eggs were fortified with vitamin E and selenium. This could be due to the long duration of CCS in the patients, in which oxidative stress plays a role at baseline but not during the course of the disease.
There is increasing evidence suggesting that n-3 PUFA supplementation improves micro- and macrovascular function [45]. In patients with peripheral arterial disease, the intake of n-3 PUFAs improved macrovascular reactivity, as measured by FMD [46]. Studies in animal models suggest that n-3 PUFAs can increase NO levels, causing ex vivo dilation of bovine coronary arteries [47,48]. In atherosclerosis, there is a disruption of the eNOS system, which decreases the bioavailability of NO in the vessel wall. Moreover, n-3 PUFAs can activate eNOS in cultured human endothelial cells [49]. We found that the consumption of enriched eggs increased the serum protein concentration of iNOS, whereas the levels of eNOS and nNOS did not change. The improvement in endothelial function in our subjects may be explained by changes in iNOS levels. An increase in the expression of the nNOS isoform was found in our previous studies in healthy young adults who consumed Nutri4 eggs [13]. Although the overexpression of iNOS has been associated with septic shock, some autoimmune diseases, and even malignancies, it also has beneficial effects and is necessary for normal cellular function [49]. Interestingly, some studies suggest that the concomitant presence or absence of oxidative stress is important in maintaining the balance between the beneficial and detrimental effects of iNOS [50]. However, in the present study, no alterations in oxidative stress biomarkers were detected in any of the dietary protocols. In transgenic mice overexpressing human endothelin-1 (ET-1), the knockout of iNOS resulted in impaired endothelium-dependent vasodilation [51]. Although the increased expression of iNOS was thought to contribute to the occurrence of cardiogenic shock in individuals with myocardial infarction, iNOS inhibitors did not show beneficial effects in this group of individuals. Moreover, NO might actually be important for better recovery after cardiac events [52]. Overall, vitamin E, selenium, and n-3 PUFAs are important in maintaining the endothelial balance, and their supplementation may contribute to better endothelial function in CCS patients [11,37,38].
An important result of the present study is the reduced levels of IL-17A and TGF-1 β after the consumption of Nutri4 eggs. IL-17A is one of the cytokines involved in the defense against a variety of microbial pathogens, as well as tissue inflammation. Its role in autoimmune diseases (for example, rheumatoid arthritis, inflammatory bowel diseases, asthma, psoriasis, and multiple sclerosis) is well known, but its influence on the pathophysiological mechanisms involved in the development of endothelial dysfunction and consequent atherosclerosis has not been fully elucidated [53]. IL-17A induces the release of chemokines that stimulate the recruitment of neutrophils and monocytes to the atherosclerotic lesion area. IL-17A also stimulates macrophages to produce other inflammatory cytokines (IL-6, TNF-α, IL-1β) and promotes a pro-atherogenic phenotype [54]. Some studies suggest that IL-17A has a proinflammatory effect, while others suggest that it is an anti-inflammatory cytokine that depends on other cytokines in its environment [55,56]. The expression of IL-17A has been associated with increased inflammation and plaque susceptibility in human atherosclerotic lesions [52]. Interestingly, statins, which are lipid-lowering agents (also taken by our patients), can downregulate inflammatory responses and stabilize atherosclerosis plaques, as well as inhibit the proinflammatory, thrombotic, and aggregation effects of IL-17 on vessels [57]. Importantly, in patients with rheumatoid arthritis, vitamin E reduces Th17 differentiation and inhibits IL-17-activated osteoclast formation, and another study suggests that n-3 PUFAs decrease IL-17A levels in vitro in stem cells derived from the adipose tissue of subjects with obesity [58,59], which may explain the decrease in IL-17A in CCS patients who consumed enriched eggs in this study (and had increased plasma vitamin E concentrations). In addition to IL-17A, the consumption of nutrient-enriched eggs resulted in lower levels of TGF-β1, an important cytokine in cell-to-cell signaling [60]. Dysregulated TGF-β1 signaling can cause arteriovenous malformations, aneurysms, atherosclerosis, cardiac fibrosis, and valvular heart disease in humans. Endothelial TGF-β1 plays an important role in signal transduction and atherosclerosis-associated vascular inflammation [61]. The effect of TGF-β on atherosclerosis depends on the balance between pro- and anti-atherogenic signaling pathways [62]. Coronary artery atherosclerotic plaques have elevated TGF-1β levels, and higher levels are also associated with stable plaques in chronic coronary syndrome [63]. In a mouse model with hyperlipidemia, after the inhibition of endothelial TGF-β signaling, there was a decrease in the inflammation of the vessel wall and further progression of atherosclerotic changes [64]. Thus, the present results suggest that enriched egg consumption may have a beneficial effect on reducing inflammation and thus may prevent the development or progression of atherosclerosis in CCS patients.
Study Limitations
In the present study, participants used the standard therapy for CCS, administered by the attending cardiologist, that was determined to be optimal for their disease and comorbidities; thus, the influence of therapy on the observed effects cannot be excluded from the study. However, the differences in the results observed between the two dietary protocols support the hypothesis that enriched chicken egg consumption can achieve a beneficial effect on cardiovascular health, despite medicinal therapy. Although throughout the study, the diets of the participants were not monitored, the respondents kept a diet diary during the protocol and received precise instructions on the food they should not consume, thus ensuring compliance with the study protocol.
5. Conclusions
The consumption of enriched eggs by CCS patients improved endothelium-depended vasodilatation, reduced triglyceride levels, increased the protein expression of iNOS, and reduced the levels of the proinflammatory cytokines IL-17A and TGF-1β. Patients with CCS may benefit from the consumption of enriched chicken eggs because of improved lipid biomarkers and improved vascular relaxation at micro- and macrovascular levels. The beneficial effects of enriched eggs occur independently of standard therapy for CCS (both groups of subjects received the same therapy). The results of this study could be useful in providing recommendations for the nutrition plans of patients with CCS.
Author Contributions
Conceptualization, A.S., A.K., K.S.-R. and I.D.; methodology, A.S., K.S.-R. and I.D.; validation, A.S., M.L., Z.M. and I.D.; formal analysis, Ž.B.Ć., A.S., A.M.M., P.Š. and D.N.; investigation, Ž.B.Ć., A.M.M., P.Š., N.K. (Nikolina Kolobarić), Z.M., I.J., N.K. (Nataša Kozina), B.J., D.N. and A.Š.; writing—original draft preparation, Ž.B.Ć., A.S., K.S.-R. and I.D.; writing—review and editing, A.S., K.S.-R. and I.D.; visualization, A.S.; supervision, I.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Structural and Investment Funds grant for the Croatian National Scientific Center of Excellence for Personalized Health Care, Josip Juraj Strossmayer University of Osijek (KK.01.1.1.01.0010).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Faculty of Medicine Osijek (Cl: 602-04/20-08/07, No.: 2158-61-07-20-118) and the Ethics Committee of University Hospital Center Osijek.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Authors Diana Nejašmić, Anita Šporec and Marija Lovrić were employed by the company BICRO BIOCENTRE, Ltd., Borongajska cesta 83 H, HR-10000 Zagreb, Croatia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [PubMed]
- Rana, J.S.; Khan, S.S.; Lloyd-Jones, D.M.; Sidney, S. Changes in Mortality in Top 10 Causes of Death from 2011 to 2018. J. Gen. Intern. Med. 2021, 36, 2517–2518. [Google Scholar] [CrossRef] [PubMed]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic Heart Disease Pathophysiology Paradigms Overview: From Plaque Activation to Microvascular Dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared with Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Lăcătușu, C.-M.; Grigorescu, E.-D.; Floria, M.; Onofriescu, A.; Mihai, B.-M. The Mediterranean Diet: From an Environment-Driven Food Culture to an Emerging Medical Prescription. Int. J. Environ. Res. Public Health 2019, 16, 942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Huang, T.; Yu, Y.; Hu, X.; Yang, B.; Li, D. Fish consumption and CHD mortality: An updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012, 15, 725–737. [Google Scholar] [PubMed]
- Violi, F.; Nocella, C.; Loffredo, L.; Carnevale, R.; Pignatelli, P. Interventional study with vitamin E in cardiovascular disease and meta-analysis. Free Radic. Biol. Med. 2022, 178, 26–41. [Google Scholar]
- Hajizadeh-Sharafabad, F.; Ghoreishi, Z.; Maleki, V.; Tarighat-Esfanjani, A. Mechanistic insights into the effect of lutein on atherosclerosis, vascular dysfunction, and related risk factors: A systematic review of in vivo, ex vivo and in vitro studies. Pharmacol. Res. 2019, 149, 104477. [Google Scholar]
- Simopoulos, A.P. Omega–3 Fatty Acids, Exercise, Physical Activity and Athletics. In Nutrition and Fitness: Cultural, Genetic and Metabolic Aspects; KARGER: Basel, Switzerland, 2008; pp. 23–50. [Google Scholar]
- Kromhout, D.; de Goede, J. Update on cardiometabolic health effects of ω-3 fatty acids. Curr. Opin. Lipidol. 2014, 25, 85–90. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef]
- Ćurić, Ž.B.; Masle, A.M.; Kibel, A.; Selthofer-Relatić, K.; Stupin, A.; Mihaljević, Z.; Jukić, I.; Stupin, M.; Matić, A.; Kozina, N.; et al. Effects of n-3 Polyunsaturated Fatty Acid-Enriched Hen Egg Consumption on the Inflammatory Biomarkers and Microvascular Function in Patients with Acute and Chronic Coronary Syndrome—A Randomized Study. Biology 2021, 10, 774. [Google Scholar] [CrossRef] [PubMed]
- Šušnjara, P.; Mihaljević, Z.; Stupin, A.; Kolobarić, N.; Matić, A.; Jukić, I.; Kralik, Z.; Kralik, G.; Miloloža, A.; Pavošević, T.; et al. Consumption of Nutritionally Enriched Hen Eggs Enhances Endothelium-Dependent Vasodilation via Cyclooxygenase Metabolites in Healthy Young People—A Randomized Study. Nutrients 2023, 15, 1599. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J. Role of Endothelial Dysfunction in Atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Trostchansky, A.; Botti, H.; Batthyány, C. Interactions of Nitric Oxide and Peroxynitrite with Low-Density Lipoprotein. Biol. Chem. 2002, 383, 547–552. [Google Scholar] [CrossRef]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative Stress in Atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Drenjančević, I.; Kralik, G.; Kralik, Z.; Mihalj, M.; Stupin, A.; Novak, S.; Grčević, M. Polyunsaturated Fatty Acids on Cardiovascular Health: Revealing Potentials of Functional Food. In Superfood and Functional Food—The Development of Superfoods and Their Roles as Medicine; InTech: Rijeka, Croatia, 2017. [Google Scholar]
- Wang, L.; Summerhill, K.; Rodriguez-Canas, C.; Mather, I.; Patel, P.; Eiden, M.; Young, S.; Forouhi, N.G.; Koulman, A. Development and validation of a robust automated analysis of plasma phospholipid fatty acids for metabolic phenotyping of large epidemiological studies. Genome Med. 2013, 5, 39. [Google Scholar] [CrossRef]
- Jargar, J.G.; Hattiwale, S.H.; Das, S.; Dhundasi, S.A.; Das, K.K. A modified simple method for determination of serum α-tocopherol (vitamin E). J. Basic Clin. Physiol. Pharmacol. 2012, 23, 45–58. [Google Scholar] [CrossRef]
- Cavka, A.; Cosic, A.; Grizelj, I.; Koller, A.; Jelakovic, B.; Lombard, J.H.; Phillips, S.A.; Drenjancevic, I. Effects of AT1 Receptor Blockade on Plasma Thromboxane A 2 (TXA 2 ) Level and Skin Microcirculation in Young Healthy Women on Low Salt Diet. Kidney Blood Press. Res. 2013, 37, 432–442. [Google Scholar] [CrossRef]
- Lenasi, H.; Štrucl, M. The effect of nitric oxide synthase and cyclooxygenase inhibition on cutaneous microvascular reactivity. Eur. J. Appl. Physiol. 2008, 103, 719–726. [Google Scholar]
- Stupin, M.; Stupin, A.; Rasic, L.; Cosic, A.; Kolar, L.; Seric, V.; Lenasi, H.; Izakovic, K.; Drenjancevic, I. Acute exhaustive rowing exercise reduces skin microvascular dilator function in young adult rowing athletes. Eur. J. Appl. Physiol. 2018, 118, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS assay in detecting oxidative stress in white sucker (Catostomus commersoni) populations exposed to pulp mill effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; pp. 121–126. [Google Scholar]
- Habig, W.H.; Jakoby, W.B. [27] Glutathione S-transferases (rat and human). In Methods in Enzymol; Academic Press: New York, NY, USA, 1981; pp. 218–231. [Google Scholar]
- Flohé, L.; Ötting, F. [10] Superoxide dismutase assays. In Methods in Enzymology; Academic Press: San Diego, CA, USA, 1984; pp. 93–104. [Google Scholar]
- Ference, B.A.; Graham, I.; Tokgozoglu, L.; Catapano, A.L. Impact of Lipids on Cardiovascular Health. J. Am. Coll. Cardiol. 2018, 72, 1141–1156. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Glickstein, S.B.; Rowe, J.D.; Soni, P.N. Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: A review. J. Clin. Lipidol. 2012, 6, 5–18. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk. JAMA 2020, 324, 2268. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar]
- Zehr, K.R.; Walker, M.K. Omega-3 polyunsaturated fatty acids improve endothelial function in humans at risk for atherosclerosis: A review. Prostaglandins Other Lipid Mediat. 2018, 134, 131–140. [Google Scholar] [CrossRef]
- Goodfellow, J.; Bellamy, M.F.; Ramsey, M.W.; Jones, C.J.; Lewis, M.J. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 35, 265–270. [Google Scholar] [CrossRef]
- Gać, P.; Czerwińska, K.; Macek, P.; Jaremków, A.; Mazur, G.; Pawlas, K.; Poręba, R. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021, 82, 103553. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Aaseth, J.; Alexander, J.; Johansson, P. Still reduced cardiovascular mortality 12 years after supplementation with selenium and coenzyme Q10 for four years: A validation of previous 10-year follow-up results of a prospective randomized double-blind placebo-controlled trial in elderly. PLoS ONE 2018, 13, e0193120. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Benstoem, C.; Goetzenich, A.; Kraemer, S.; Borosch, S.; Manzanares, W.; Hardy, G.; Stoppe, C. Selenium and Its Supplementation in Cardiovascular Disease—What do We Know? Nutrients 2015, 7, 3094–3118. [Google Scholar] [CrossRef] [PubMed]
- Neunteufl, T.; Priglinger, U.; Heher, S.; Zehetgruber, M.; Söregi, G.; Lehr, S.; Huber, K.; Maurer, G.; Weidinger, F.; Kostner, K. Effects of vitamin E on chronic and acute endothelial dysfunction in smokers. J. Am. Coll. Cardiol. 2000, 35, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mah, E.; Pei, R.; Guo, Y.; Masterjohn, C.; Ballard, K.D.; Taylor, B.A.; Taylor, A.W.; Traber, M.G.; Volek, J.S.; Bruno, R.S. Greater γ-tocopherol status during acute smoking abstinence with nicotine replacement therapy improved vascular endothelial function by decreasing 8-iso-15(S)-prostaglandin F 2α. Exp. Biol. Med. 2015, 240, 527–533. [Google Scholar] [CrossRef]
- Zakeri, N.; Kelishadi, M.R.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S. kasra Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Miyazawa, T.; Burdeos, G.C.; Itaya, M.; Nakagawa, K.; Miyazawa, T. Vitamin E: Regulatory Redox Interactions. IUBMB Life 2019, 71, 430–441. [Google Scholar] [CrossRef]
- Barić, L.; Drenjančević, I.; Mihalj, M.; Matić, A.; Stupin, M.; Kolar, L.; Mihaljević, Z.; Mrakovčić-Šutić, I.; Šerić, V.; Stupin, A. Enhanced Antioxidative Defense by Vitamins C and E Consumption Prevents 7-Day High-Salt Diet-Induced Microvascular Endothelial Function Impairment in Young Healthy Individuals. J. Clin. Med. 2020, 9, 843. [Google Scholar] [CrossRef]
- Stirban, A.; Nandrean, S.; Götting, C.; Tamler, R.; Pop, A.; Negrean, M.; Gawlowski, T.; Stratmann, B.; Tschoepe, D. Effects of n–3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am. J. Clin. Nutr. 2010, 91, 808–813. [Google Scholar] [CrossRef]
- Hammer, A.; Moertl, D.; Schlager, O.; Matschuck, M.; Seidinger, D.; Koppensteiner, R.; Steiner, S. Effects of n-3 PUFA on endothelial function in patients with peripheral arterial disease: A randomised, placebo-controlled, double-blind trial. Br. J. Nutr. 2019, 122, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Ander, B.P.; Dupasquier, C.M.; Prociuk, M.A.; Pierce, G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003, 8, 164–172. [Google Scholar] [PubMed]
- Omura, M.; Kobayashi, S.; Mizukami, Y.; Mogami, K.; Todoroki-Ikeda, N.; Miyake, T.; Matsuzaki, M. Eicosapentaenoic acid (EPA) induces Ca2+-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001, 487, 361–366. [Google Scholar] [CrossRef]
- Stebbins, C.L.; Stice, J.P.; Hart, C.M.; Mbai, F.N.; Knowlton, A.A. Effects of Dietary Decosahexaenoic Acid (DHA) on eNOS in Human Coronary Artery Endothelial Cells. J. Cardiovasc. Pharmacol. Ther. 2008, 13, 261–268. [Google Scholar] [CrossRef]
- Wilmes, V.; Scheiper, S.; Roehr, W.; Niess, C.; Kippenberger, S.; Steinhorst, K.; Verhoff, M.A.; Kauferstein, S. Increased inducible nitric oxide synthase (iNOS) expression in human myocardial infarction. Int. J. Legal Med. 2020, 134, 575–581. [Google Scholar] [CrossRef]
- Quaschning, T.; Voss, F.; Herzfeld, S.; Relle, K.; Kalk, P.; Godes, M.; Pfab, T.; Kraemer-Guth, A.; Bonz, A.W.; Theuring, F.; et al. Lack of iNOS Impairs Endothelial Function in Endothelin-1 Transgenic Mice. Kidney Blood Press. Res. 2008, 31, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Kolls, J.K. Interluekin-17A (IL17A). Gene 2017, 614, 8–14. [Google Scholar] [CrossRef]
- Gong, F.; Liu, Z.; Liu, J.; Zhou, P.; Liu, Y.; Lu, X. The paradoxical role of IL-17 in atherosclerosis. Cell. Immunol. 2015, 297, 33–39. [Google Scholar] [CrossRef]
- Allam, G.; Abdel-Moneim, A.; Gaber, A.M. The pleiotropic role of interleukin-17 in atherosclerosis. Biomed. Pharmacother. 2018, 106, 1412–1418. [Google Scholar] [CrossRef]
- Nordlohne, J.; von Vietinghoff, S. Interleukin 17A in atherosclerosis—Regulation and pathophysiologic effector function. Cytokine 2019, 122, 154089. [Google Scholar] [CrossRef] [PubMed]
- Hot, A.; Lavocat, F.; Lenief, V.; Miossec, P. Simvastatin inhibits the pro-inflammatory and pro-thrombotic effects of IL-17 and TNF-α on endothelial cells. Ann. Rheum. Dis. 2013, 72, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Ward, R.; Pestel, J.; Robert, M.; Pesenti, S.; Bendridi, N.; Michalski, M.; Laville, M.; Vidal, H.; Eljaafari, A. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-17A Secretion through Decreased ICAM-1 Expression in T Cells Co-Cultured with Adipose-Derived Stem Cells Harvested from Adipose Tissues of Obese Subjects. Mol. Nutr. Food Res. 2019, 63, 1801148. [Google Scholar] [CrossRef]
- Kim, K.-W.; Kim, B.-M.; Won, J.-Y.; Min, H.K.; Lee, S.J.; Lee, S.-H.; Kim, H.-R. Tocotrienol regulates osteoclastogenesis in rheumatoid arthritis. Korean J. Intern. Med. 2021, 36, S273–S282. [Google Scholar] [CrossRef] [PubMed]
- Hinck, A.P.; Mueller, T.D.; Springer, T.A. Structural Biology and Evolution of the TGF-β Family. Cold Spring Harb. Perspect. Biol. 2016, 8, a022103. [Google Scholar] [CrossRef]
- Xia, M.; Wu, F.; Yang, Y.; Lu, W.; Song, M.; Ma, Z. The possibility of visualizing TGF-β1 expression in ApoE−/− mice atherosclerosis using MR targeted imaging. Acta Radiol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Nurgazieva, D.; Mickley, A.; Moganti, K.; Ming, W.; Ovsyi, I.; Popova, A.; Sachindra; Awad, K.; Wang, N.; Bieback, K.; et al. TGF-β1, but Not Bone Morphogenetic Proteins, Activates Smad1/5 Pathway in Primary Human Macrophages and Induces Expression of Proatherogenic Genes. J. Immunol. 2015, 194, 709–718. [Google Scholar] [CrossRef]
- Panutsopulos, D.; Papalambros, E.; Sigala, F.; Zafiropoulos, A.; Arvanitis, D.L.; Spandidos, D.A. Protein and mRNA expression levels of VEGF-A and TGF-beta1 in different types of human coronary atherosclerotic lesions. Int. J. Mol. Med. 2005, 15, 603–610. [Google Scholar]
- Chen, P.-Y.; Qin, L.; Li, G.; Wang, Z.; Dahlman, J.E.; Malagon-Lopez, J.; Gujja, S.; Cilfone, N.A.; Kauffman, K.J.; Sun, L.; et al. Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis. Nat. Metab. 2019, 1, 912–926. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).