Cytoprotective–Antioxidant Effect of Brunfelsia grandiflora Extract on Neuron-like Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Extract
2.3. Cell Culture
2.4. Cell Viability Evaluation (MTT)
2.5. Intracellular ROS Production
2.6. Caspase 3/7 Activity Assay
2.7. MDA Concentration
2.8. Antioxidant Defenses
2.8.1. Reduced Glutathione (GSH)
2.8.2. Antioxidant Enzymes
2.9. Real-Time PCR Assay
2.10. Statistical Analysis
3. Results
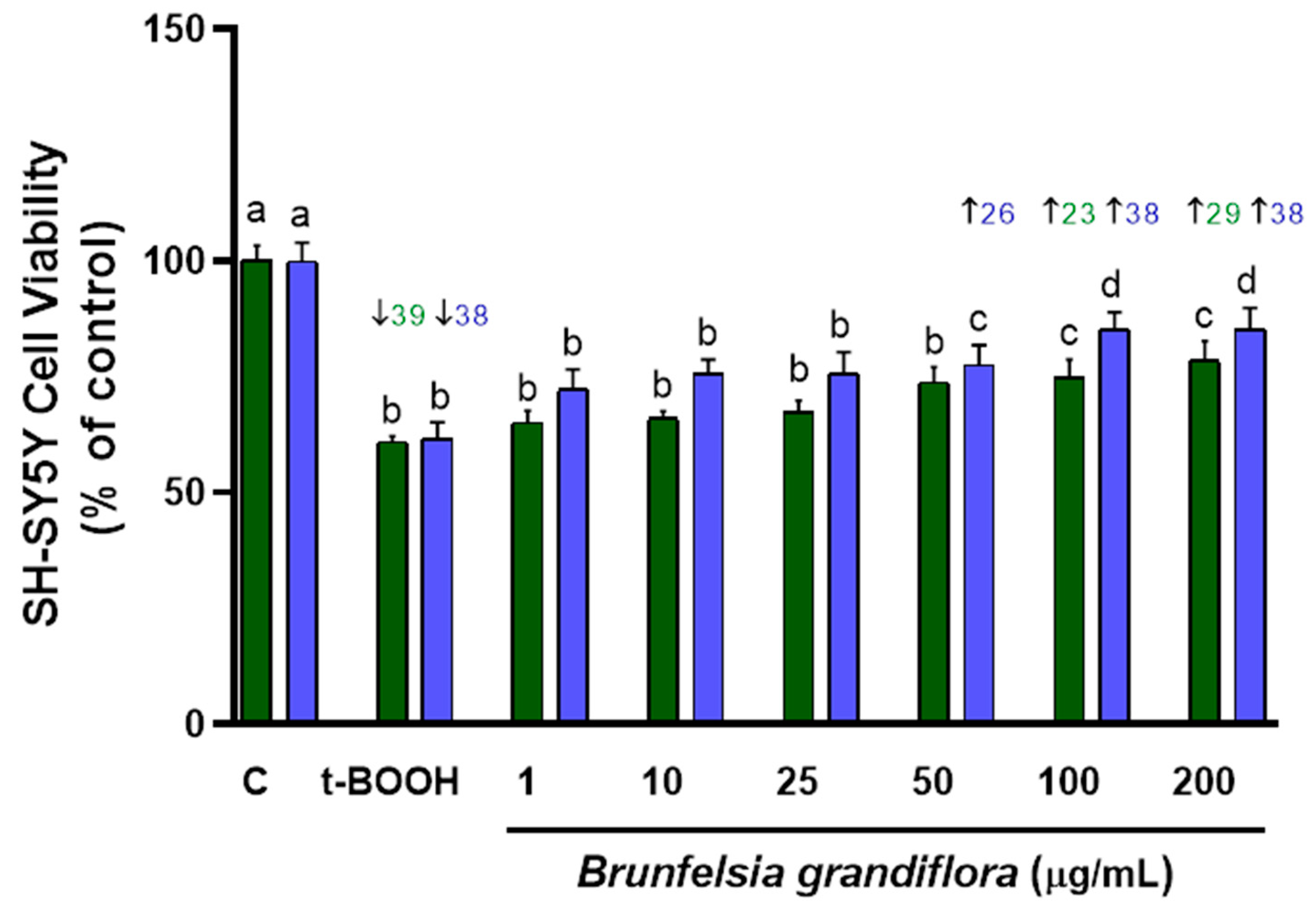
3.1. Cell Viability
3.2. Assessment of Oxidative Stress
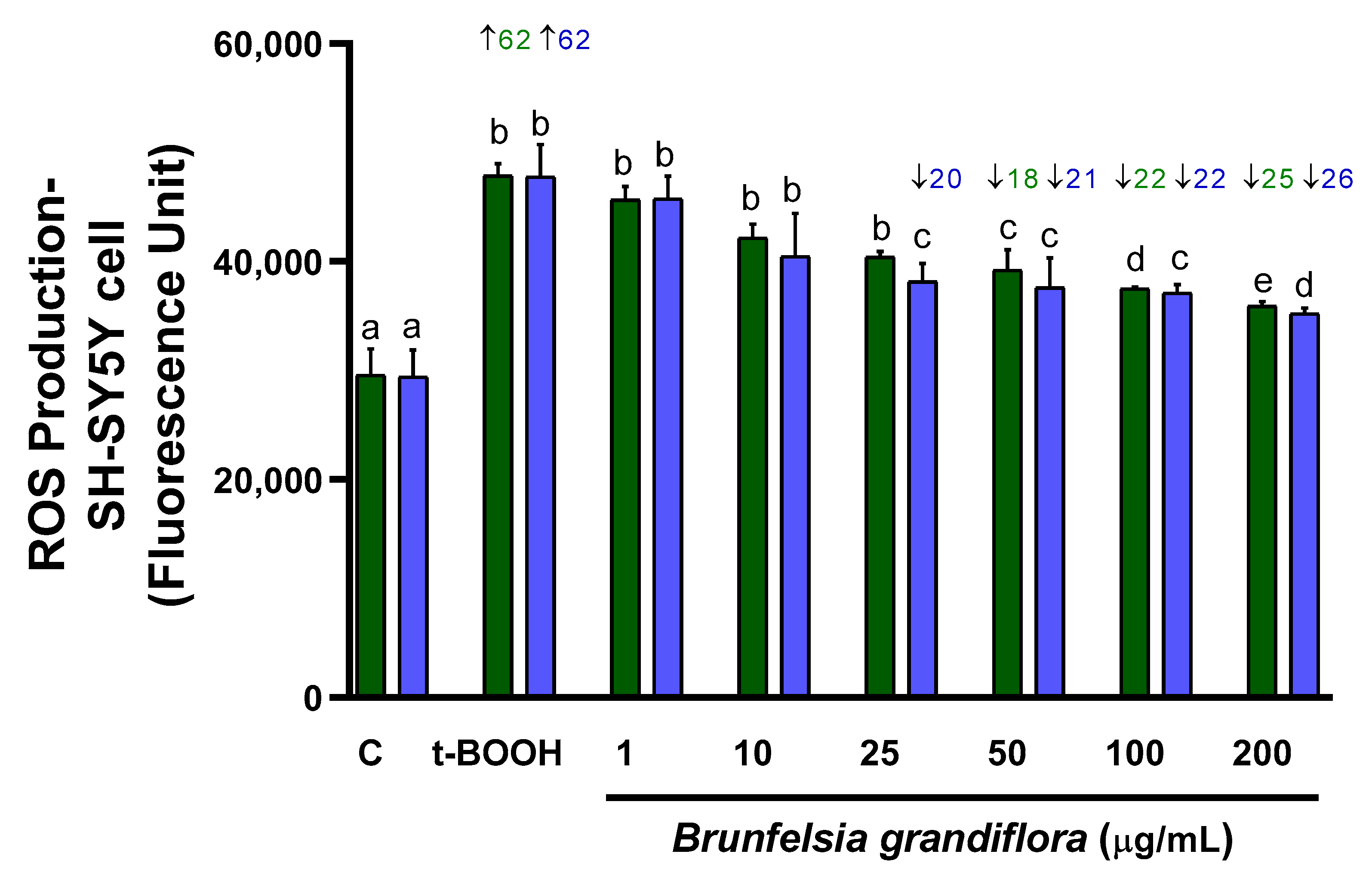
3.2.1. ROS Production
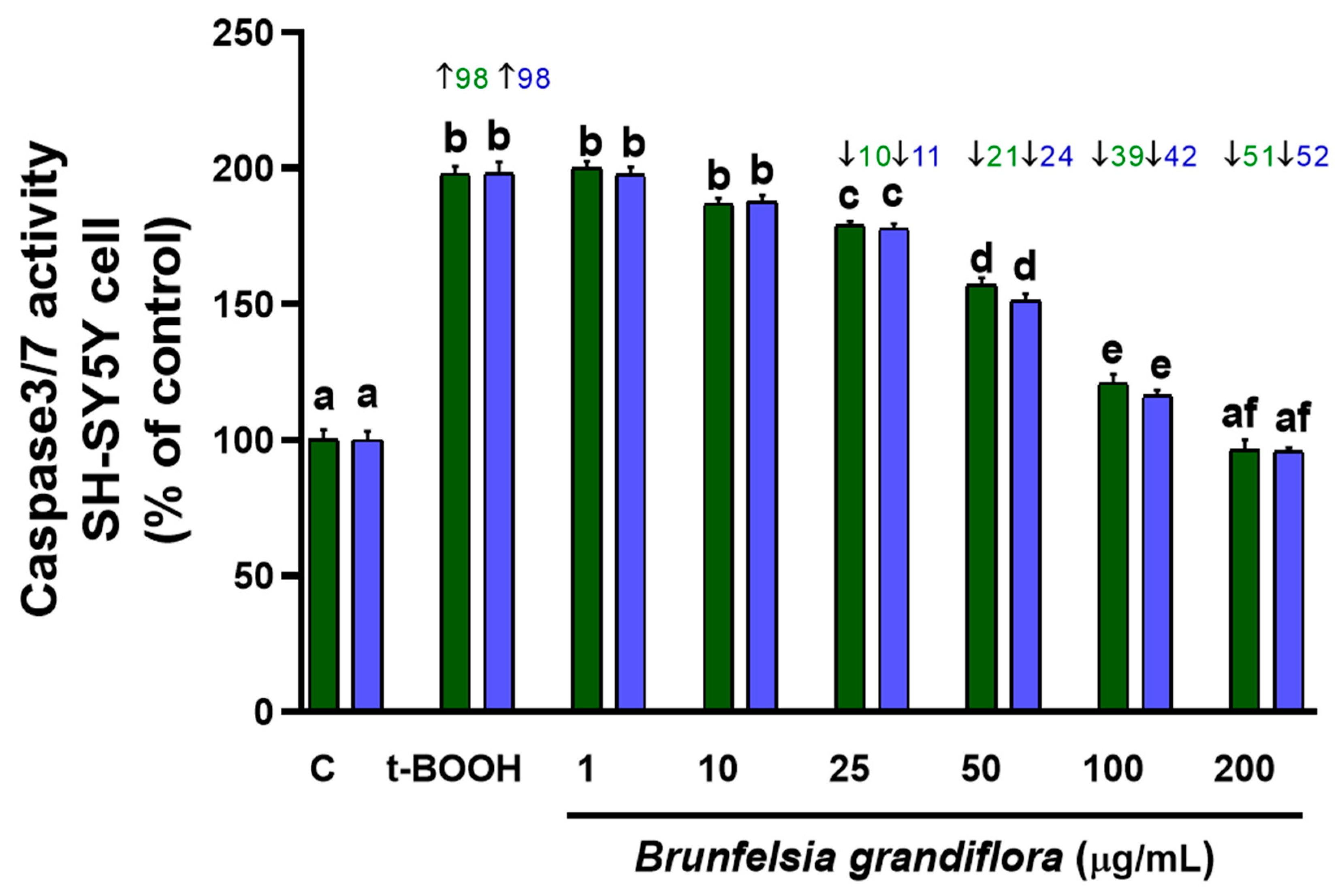
3.2.2. Caspase 3/7 Activity
3.2.3. MDA Concentration
3.3. GSH
3.4. Antioxidant Enzymes
3.4.1. GPx Activity
3.4.2. GR Activity
3.5. Molecular Assay
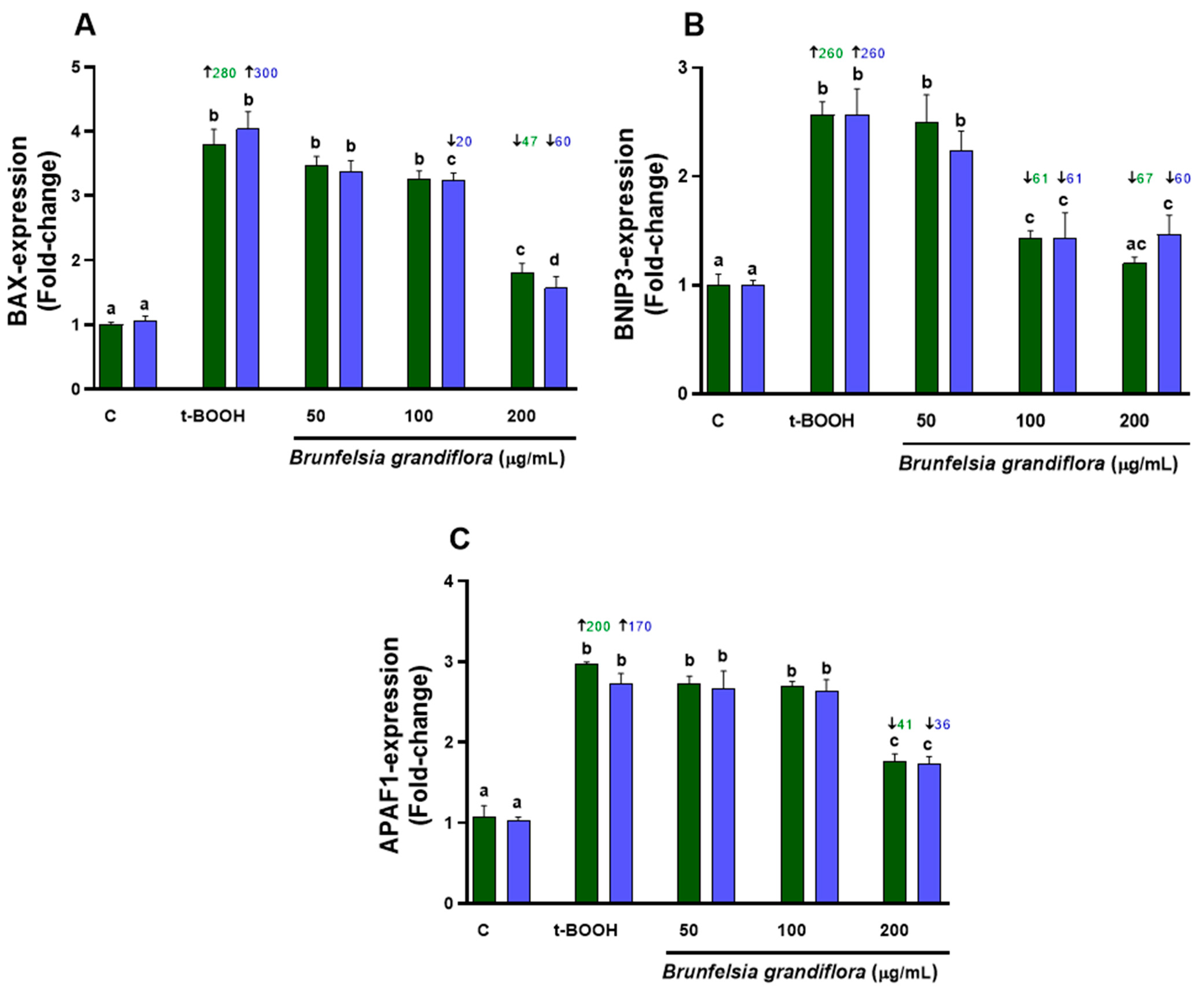
3.5.1. Biomarkers of Cell Death
3.5.2. Biomarkers of Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ionescu-Tucker, A.; Cotman, C.W. Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol. Aging 2021, 107, 86–95. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.; Poblete-Aro, C.; Pando, M.E.; Allel, M.J.; Fernandez, V.; Soto, A.; Nova, P.; Garcia-Diaz, D. Effects of Polyphenols in Aging and Neurodegeneration Associated with Oxidative Stress. Curr. Med. Chem. 2022, 29, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Strother, L.; Miles, G.B.; Holiday, A.R.; Cheng, Y.; Doherty, G.H. Long-term culture of SH-SY5Y neuroblastoma cells in the absence of neurotrophins: A novel model of neuronal ageing. Neurosci. Methods 2021, 362, 109301. [Google Scholar] [CrossRef] [PubMed]
- Carballeda Sangiao, N.; Chamorro, S.; de Pascual-Teresa, S.; Goya, L. Aqueous Extract of Cocoa Phenolic Compounds Protects Differentiated Neuroblastoma SH-SY5Y Cells from Oxidative Stress. Biomolecules 2021, 11, 1266. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.L.; Berrios, P.; Clavo, Z.M.; Marin-Bravo, M.; Inostroza-Ruiz, L.; Ramos-Gonzalez, M.; Quispe-Solano, M.; Fernández-Alfonso, M.S.; Palomino, O.; Goya, L. Chemical Characterization, Antioxidant Capacity and Anti-Oxidative Stress Potential of South American Fabaceae Desmodium tortuosum. Nutrients 2023, 15, 746. [Google Scholar] [CrossRef]
- Plowman, T. Brunfelsia in ethnomedicine. Bot. Mus. Lealf. Harv. Univ. 1977, 25, 289–320. [Google Scholar] [CrossRef]
- Polesna, L.; Polesny, Z.; Clavo, M.Z.; Hansson, A.; Kokoska, L. Ethnopharmacological inventory of plants used in Coronel Portillo Province of Ucayali Department, Peru. Pharm. Biol. 2011, 49, 125–136. [Google Scholar] [CrossRef]
- Mateos, R.; Ramos-Cevallos, N.; Castro-Luna, A.; Ramos-Gonzalez, M.; Clavo, Z.M.; Quispe-Solano, M.; Goya, L.; Rodríguez, J.L. Identification, Quantification, and Characterization of the Phenolic Fraction of Brunfelsia grandiflora: In vitro Antioxidant Capacity. Molecules 2022, 27, 6510. [Google Scholar] [CrossRef]
- Moon, P.D.; Lee, B.H.; Jeong, H.J.; An, H.J.; Park, S.J.; Kim, H.R.; Ko, S.G.; Um, J.Y.; Hong, S.H.; Kim, H.M. Use of Scopoletin to Inhibit the Production of Inflammatory Cytokines through Inhibition of the IB/NF-KB Signal Cascade in the Human Mast Cell Line HMC-1. Eur. J. Pharmacol. 2007, 555, 218–225. [Google Scholar] [CrossRef]
- Bennett, B.C. Hallucinogenic plants of the shuar and related indigenous groups in amazonian Ecuador and Peru. Brittonia 1992, 44, 483–493. [Google Scholar] [CrossRef]
- Schultes, R.E.; Hofmann, A.; Rätsch, C. Plants of the Gods. In Their Sacred, Healing and Hallucinogenic Powers; Healing Arts Press: Rochester, VT, USA, 1998. [Google Scholar]
- León-González, A.; Mateos, R.; Ramos, S.; Martín, M.A.; Sarriá, B.; Martín-Cordero, C.; López-Lázaro, M.; Bravo, L.; Goya, L. Chemo-protective activity and characterization of phenolic extracts from Corema album. Food Res. Int. 2012, 49, 728–738. [Google Scholar] [CrossRef]
- Martín, M.A.; Ramos, S.; Mateos, R.; Marais, J.; Bravo, L.; Khoo, C.; Goya, L. Chemical characterization and chemo-protective activity of cranberry phenolic extracts in a model cell culture. Response of the antioxidant defenses and regulation of signaling pathways. Food Res. Int. 2015, 71, 68–82. [Google Scholar] [CrossRef]
- Bravo, L.; Mateos, R.; Sarriá, B.; Baeza, G.; Lecumberri, E.; Ramos, S.; Goya, L. Hypocholesterolaemic and antioxidant effects of yerba mate (Ilex paraguariensis) in high-cholesterol fed rats. Fitoterapia 2014, 92, 219–229. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Zhang, Y.; Seeram, N.P.; Nair, M.G. Growth inhibition of human tumor cell lines by with anolides from Withania somnifera leaves. Life Sci. 2003, 74, 125–132. [Google Scholar] [CrossRef]
- Khan, S.; Malik, F.; Suri, K.A.; Singh, J. Molecular insight into the immune up-regulatory properties of the leaf extract of Ashwagandha and identification of Th1 immunostimulatory chemical entity. Vaccine 2009, 27, 6080–6087. [Google Scholar] [CrossRef]
- Kim, Y.C.; Kim, S.R.; Markelonis, G.J.; Oh, T.H. Ginsenosides Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J. Neurosci. Res. 1998, 53, 426–432. [Google Scholar] [CrossRef]
- Chen, X.C.; Zhu, Y.G.; Zhu, L.A.; Huang, C.; Chen, Y.; Chen, L.M.; Fang, F.; Zhou, Y.C.; Zhao, C.H. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur. J. Pharmacol. 2003, 473, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Fiala, M.; Cashman, J.; Sayre, J.; Espinosa, A.; Mahanian, M.; Zaghi, J.; Badmaev, V.; Graves, M.C.; Bernard, G.; et al. Curcuminoids enhance amyloid-beta uptake by macrophages of Alzheimer’s disease patients. J. Alzheimers Dis. 2006, 10, 1–7. [Google Scholar] [CrossRef]
- Anekonda, T.S. Resveratrol—A boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Miret, S.; De Groene, E.M.; Klaffke, W. Comparison of in vitro assays of cellular toxicity in the human hepatic cell line HepG2. J. Biomol. Screen. 2006, 11, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Lang, J.; Zadravec, S.; Slater, T.F. Detection of malonaldehyde by high-performance liquid chromatography. Methods Enzymol. 1984, 105, 319–328. [Google Scholar]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Clavo, Z.M.; Ramos-Gonzalez, M.; De La Cruz, M.; Inostroza-Ruiz, L.; Bada-Laura, W.; Palomino, O.; Fernández-Alfonso, M.S.; Mateos, R. Cytoprotective and Anti-Oxidative Stress Capacity of South American Medicinal Plant B. grandiflora in Endothelial Cells. J. Biol. Regul. Homeost. Agents 2023, unpublished. [Google Scholar]
- Sharma, A.; Kaur, G. Tinospora cordifolia as a potential neuroregenerative candidate against glutamate induced excitotoxicity: An in vitro perspective. BMC Complement. Altern. Med. 2018, 18, 268. [Google Scholar] [CrossRef]
- Gong, G.; Qi, B.; Liang, Y.T.; Dong, T.T.X.; Wang, H.Y.; Tsim, K.W.K.; Zheng, Y. Danggui Buxue Tang, an ancient Chinese herbal decoction, protects β-amyloid-induced cell death in cultured cortical neurons. BMC Complement. Altern. Med. 2019, 19, 9. [Google Scholar] [CrossRef]
- Kang, T.K.; Le, T.T.; Kim, K.A.; Kim, Y.J.; Lee, W.B.; Jung, S.H. Roots of Lithospermum erythrorhizon promotes retinal cell survival in optic nerve crush-induced retinal degeneration. Exp. Eye Res. 2021, 203, 108419. [Google Scholar] [CrossRef]
- Keilhoff, G.; Ludwig, C.; Pinkernelle, J.; Lucas, B. Effects of Gynostemma pentaphyllum on spinal cord motor neurons and microglial cells in vitro. Acta Histochem. 2021, 123, 151759. [Google Scholar] [CrossRef]
- Chen, Y.X.; Le, P.T.N.; Tzeng, T.T.; Tran, T.H.; Nguyen, A.T.; Cheng, I.H.; Huang, C.F.; Shiao, Y.J.; Ching, T.T. Graptopetalum paraguayense Extract Ameliorates Proteotoxicity in Aging and Age-Related Diseases in Model Systems. Nutrients 2021, 13, 4317. [Google Scholar] [CrossRef] [PubMed]
- Aldbass, A.; Amina, M.; Al Musayeib, N.M.; Bhat, R.S.; Al-Rashed, S.; Marraiki, N.; Fahmy, R.; El-Ansary, A. Cytotoxic and anti-excitotoxic effects of selected plant and algal extracts using COMET and cell viability assays. Sci. Rep. 2021, 11, 8512. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Pressi, G.; Bertaiola, O.; Guarnerio, C.; Mandrone, M.; Chiocchio, I.; Galeotti, N. Attenuation of neuroinflammation in microglia cells by extracts with high content of rosmarinic acid from in vitro cultured Melissa officinalis L. cells. J. Pharm. Biomed. Anal. 2022, 220, 114969. [Google Scholar] [CrossRef]
- Alía, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110–118. [Google Scholar] [CrossRef]
- Myhrstad, M.C.; Carlsen, H.; Nordström, O.; Blomhoff, R.; Moskaug, J.Ø. Flavonoids increase the intracellular glutathione level by transactivation of the gamma-glutamylcysteine synthetase catalytical subunit promoter. Free Radic. Biol. Med. 2002, 32, 386–393. [Google Scholar] [CrossRef]
- Şahin, M.; Öncü, G.; Yılmaz, M.A.; Özkan, D.; Saybaşılı, H. Transformation of SH-SY5Y cell line into neuron-like cells: Investigation of electrophysiological and biomechanical changes. Neurosci. Lett. 2021, 745, 135628. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.F.; Ferrão, R.; Silva, M.R.; Pinho, S.L.C.; Ferreira, L.; Oliveira, P.J.; Cunha-Oliveira, T. Refinement of a differentiation protocol using neuroblastoma SH-SY5Y cells for use in neurotoxicology research. Food Chem. Toxicol. 2021, 149, 111967. [Google Scholar] [CrossRef]
- Ioghen, O.C.; Ceafalan, L.C.; Popescu, B.O. SH-SY5Y Cell Line In Vitro Models for Parkinson Disease Research-Old Practice for New Trends. J. Integr. Neurosci. 2023, 22, 20. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Yang, C.L.; Wang, S.B.; He, W.P.; Liu, J.J. Anti-oxidant and Anti-inflammatory Effects of Ethanol Extract from Polygala sibirica L. var megalopha Fr. on Lipopolysaccharide-Stimulated RAW264.7 Cells. Chin. J. Integr. Med. 2023, 29, 905–913. [Google Scholar] [CrossRef]
- Puppala, E.R.; Yalamarthi, S.S.; Aochenlar, S.L.; Prasad, N.; Syamprasad, N.P.; Singh, M.; Nanjappan, S.K.; Ravichandiran, V.; Tripathi, D.M.; Gangasani, J.K.; et al. Mesua assamica (King&Prain) kosterm. Bark ethanolic extract attenuates chronic restraint stress aggravated DSS-induced ulcerative colitis in mice via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1 signaling pathways. J. Ethnopharmacol. 2023, 301, 115765. [Google Scholar] [PubMed]
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.; Kenawy, A.M.; Abd El-Wahab, A.E.; Elkaeed, E.B.; Alsfouk, B.A.; Metwaly, A.M.; Eissa, I.H. Discovery of new quinoline and isatine derivatives as potential VEGFR-2 inhibitors: Design, synthesis, antiproliferative, docking and MD simulation studies. J. Biomol. Struct. Dyn. 2023, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, X.Y.; Hao, X.Y.; Yan, Y.M.; Hong, M.; Wei, S.F.; Zhou, Y.L.; Wang, Q.; Cheng, Y.X.; Liu, Y.Q. Ethanol Extract of Centipeda minima Exerts Antioxidant and Neuroprotective Effects via Activation of the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 9421037. [Google Scholar] [CrossRef] [PubMed]

| MDA Levels (Mean ± SD) | ||
|---|---|---|
| Cotreatment | Pretreatment | |
| Control | 5.18 a ± 0.12 | 5.18 a ± 0.12 |
| t-BOOH | 8.64 b ± 0.31 | 7.63 b ± 0.29 |
| 50 µg/mL BG + t-BOOH | 0.28 c ± 0.11 | 0.50 c ± 0.21 |
| 100 µg/mL BG + t-BOOH | 2.22 d ± 0.20 | 0.68 d ± 0.17 |
| 200 µg/mL BG + t-BOOH | 3.16 e ± 0.15 | 0.88 e ± 0.18 |
| (A) | ||
|---|---|---|
| GSH Levels | ||
| nmol/mg Prot ± SD | ||
| Control | 0.47 ± 0.01 | |
| 50 µg/mL BG | 0.48 ± 0.04 | |
| 100 µg/mL BG | 0.53 ± 0.05 | |
| 200 µg/mL BG | 0.51 ± 0.01 | |
| (B) | ||
| GSH Levels | ||
| nmol/mg Prot ± SD | ||
| Cotreatment | Pretreatment | |
| Control | 0.46 a ± 0.01 | 0.47 a ± 0.01 |
| t-BOOH | 0.38 b ± 0.02 | 0.40 b ± 0.01 |
| 50 µg/mL BG + t-BOOH | 0.41 b ± 0.02 | 0.41 b ± 0.01 |
| 100 µg/mL BG + t-BOOH | 0.41 b ± 0.01 | 0.43 c ± 0.01 |
| 200 µg/mL BG + t-BOOH | 0.45 ac ± 0.01 | 0.44 c ± 0.01 |
| (A) | ||
|---|---|---|
| GPx Levels | ||
| mU GPx/mg Prot. ± SD | ||
| Control | 158.85 a ± 2.81 | |
| 50 µg/mL BG | 154.56 a ± 4.87 | |
| 100 µg/mL BG | 168.05 a ± 4.63 | |
| 200 µg/mL BG | 1922.70 b ± 3.49 | |
| (B) | ||
| mU GPx/mg Prot. (Mean ± SD) | ||
| Cotreatment | Pretreatment | |
| Control | 158.85 a ± 2.81 | 158.85 a ± 2.81 |
| t-BOOH | 305.84 b ± 4.73 | 204.24 b ± 4.31 |
| 50 µg/mL BG + t-BOOH | 185.34 c ± 4.93 | 153.24 c ± 11.41 |
| 100 µg/mL BG + t-BOOH | 181.39 d ± 3.61 | 172.35 d ± 4.63 |
| 200 µg/mL BG + t-BOOH | 203.48 b ± 4.73 | 155.79 d ± 3.83 |
| (A) | ||
|---|---|---|
| GR Levels | ||
| mU GR/mg Prot. ± SD | ||
| Control | 3.94 a ± 0.29 | |
| 50 µg/mL BG | 4.57 b ± 0.53 | |
| 100 µg/mL BG | 4.92 b ± 0.67 | |
| 200 µg/mL BG | 5.27 b ± 0.26 | |
| (B) | ||
| mU GR/mg Prot. (Mean ± SD) | ||
| Cotreatment | Pretreatment | |
| Control | 3.94 a ± 0.29 | 3.94 a ± 0.29 |
| t-BOOH | 13.69 b ± 0.78 | 7.71 b ± 1.02 |
| 50 µg/mL BG + t-BOOH | 9.35 c ± 0.49 | 5.06 c ± 0.31 |
| 100 µg/mL BG + t-BOOH | 7.00 d ± 0.30 | 3.72 a ± 0.10 |
| 200 µg/mL BG + t-BOOH | 5.25 e ± 0.44 | 4.79 ac ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez, J.-L.; Mateos, R.; Palomino, O.; Fernández-Alfonso, M.S.; Ramos-Cevallos, N.; Inostroza-Ruiz, L.; Panduro-Tenazoa, N.; Bada-Laura, W.; Ramírez-Flores, N.; Goya, L. Cytoprotective–Antioxidant Effect of Brunfelsia grandiflora Extract on Neuron-like Cells. Appl. Sci. 2023, 13, 12233. https://doi.org/10.3390/app132212233
Rodríguez J-L, Mateos R, Palomino O, Fernández-Alfonso MS, Ramos-Cevallos N, Inostroza-Ruiz L, Panduro-Tenazoa N, Bada-Laura W, Ramírez-Flores N, Goya L. Cytoprotective–Antioxidant Effect of Brunfelsia grandiflora Extract on Neuron-like Cells. Applied Sciences. 2023; 13(22):12233. https://doi.org/10.3390/app132212233
Chicago/Turabian StyleRodríguez, José-Luis, Raquel Mateos, Olga Palomino, Maria S. Fernández-Alfonso, Norma Ramos-Cevallos, Luis Inostroza-Ruiz, Nadia Panduro-Tenazoa, Wendy Bada-Laura, Noé Ramírez-Flores, and Luis Goya. 2023. "Cytoprotective–Antioxidant Effect of Brunfelsia grandiflora Extract on Neuron-like Cells" Applied Sciences 13, no. 22: 12233. https://doi.org/10.3390/app132212233
APA StyleRodríguez, J.-L., Mateos, R., Palomino, O., Fernández-Alfonso, M. S., Ramos-Cevallos, N., Inostroza-Ruiz, L., Panduro-Tenazoa, N., Bada-Laura, W., Ramírez-Flores, N., & Goya, L. (2023). Cytoprotective–Antioxidant Effect of Brunfelsia grandiflora Extract on Neuron-like Cells. Applied Sciences, 13(22), 12233. https://doi.org/10.3390/app132212233









